Abstract
The treatment of calcific coronary lesions is still a major interventional issue in haemodynamics laboratories. The prevalence of the disease is even increasing, considering the general ageing of the population undergoing coronarography, as well as the often associated comorbidities. In recent years, new devices have been developed that allow both better identification and also better treatment of these lesions. The aim of this review is to summarize both imaging modalities and dedicated techniques and materials, thus providing a kind of compendium for the treatment approach.
Keywords: Coronary calcified lesions, Intracoronary imaging, Rotational and orbital atherectomy
General consideration and epidemiology
Calcified coronary lesions (CCL) constitute one of the main challenges in the cardiac catheterization laboratory, being present in up to one-third of patients undergoing percutaneous coronary intervention (PCI). This high prevalence is even increasing, considering the trend towards ageing of the population, and the presence of comorbidities such as diabetes and chronic kidney disease.1,2 Calcified coronary lesions reduce the success rate of PCI, increasing the possibility of stent malapposition and under-expansion, which predispose to intrastent restenosis (ISR) and thrombosis, and are associated with an increase in procedural complications (e.g. edge dissection and coronary ruptures).
Moreover, it has been clearly demonstrated that extent of coronary artery calcification strongly correlates with atherosclerosis and increases the number of future cardiac major adverse cardiac events even in asymptomatic patients.3–5
Considering the aforementioned reasons, appropriate identification and procedural planning, with the use of the right techniques and dedicated materials, are the most important points for the correct treatment of CCL.
Imaging for calcified coronary lesions identification
The presence of calcium in the coronary arteries can be suspected already with simple angiography, and in this case calcified lesions will appear as an area of attenuation of the X-rays, visible already before the contrast injection, arranged along the contour of the vessel, which moves with heart movement. CCL are generally defined as severe if present on both sides of the vessel, and moderate if visible only during the cardiac cycle prior to contrast injection.6
Since angiography alone has been shown to have low sensitivity in identifying calcified lesions, it is necessary to use modern intravascular imaging methods to assess the degree and extent of the aforementioned. Indeed, not only the presence and the length, but even the depth of calcium is important in order to choose the right instruments and to plan the PCI strategy. At this regard, calcium is defined superficial when present at the intimal–lumen interface or closer to the lumen than to the adventitia, and deep when it is located at the media/adventitia border or closer to the adventitia than to the lumen.
Intravascular ultrasound
Intravascular ultrasound (IVUS) is the first and most used intracoronary imaging method used to investigate the disease within the vessel wall. There are two types of IVUS system: a solid-state one, externally mounted on a catheter and controlled electronically, and a mechanical system that relies on a rotating internal cable, the latter one capable to measure the length of the lesion as well as to detect the other characteristics of the coronary plaque. The image produced by the IVUS represents the various components of the plaque in grayscale and offers a colour conversion algorithm, based on tissue density, called virtual histology (VH). Calcified lesion is defined as the presence of hyperechogenic structure that shadows underlying ultrasound anatomy, or white spots at the VH, and it is quantified by measuring the circumferential extent in degrees location calcium is classified like ‘superficial’ if is closer to the lumen than to adventitia or ‘deep’ if it is closer to the adventitia than to the lumen.7,8 This technology is fundamental not only for pre-implantation diagnosis but also for evaluating the effects of calcium ablation/rupture techniques (e.g. shock-wave fracture in the calcified plaque or thinning the calcium thickness with a rotational ateherectomy), thus allowing to establish whether it is possible to perform a stent implantation.
Optical coherence tomography
Optical coherence tomography (OCT) is a light-based imaging modality which shows outstanding accuracy in the setting of coronary imaging. This technology is based on the emission of infrared light waves which reflect off the internal microstructure within the biological tissues. Compared to IVUS, OCT has a 10-fold higher image resolution and is able to remarkably characterize the individual structures of the coronary wall, the composition of the plaque (presence or absence of thrombus, calcium, macrophages, dissections, etc.) and the correct stent placement, e.g. identifying edge dissection or malapposition/underexpansion. Therefore, in the setting of calcified disease, it is really important to note that calcium deposits are characterized by the presence of well-delineated, low backscattering, signal-poor heterogeneous regions.
Optical coherence tomography helps to classify calcium into deep, superficial and nodular, as well as eccentric or concentric, allowing a perfect definition of the length and the circumferential extension in degrees.9,10 The use of this technology finds its best use in the detection of ssuperficial calcium deposits, which can be studied and measured by OCT unless thicker than 1.0–1.5 mm (infrared penetration limit threshold).11 In conclusion, the use of OCT imaging plays a fundamental role in the treatment of calcified lesions determining the optimal therapeutic approach because different types of calcium require a different treatment technique.12
Calcified coronary lesions treatment techniques
Balloon-based techniques
Cutting balloon
Cutting balloon (CB) (Flextome and Wolverine, Boston Scientific) are special non-compliant balloon with three surgical blades attached on the surface. These devices are 6 Fr compatible, and should be use in a 1:1 ratio with the vessel reference diameter. They act creating small calcium fracture, reducing elastic recoil after predilation, thus allowing a major lumen gain when compared to conventional non-compliant balloon angioplasty (percutaneous transluminal coronary angioplasty [PTCA]). Moreover, the lower nominal pressure helps in minimizing intimal trauma and reduces the risk of dissection in the adjacent vessel’s zones. Despite this premises, in the GLOBAL trial13, CB was not superior to PTCA in reducing the rate of angiographic restenosis after angioplasty without stent implantation. Analogue findings were described by Albiero et al.,14 with CB unable to guarantee a reduction in recurrent ISR when compared with PTCA. However, in the REDUCE III trial, Ozaky et al demonstrated that a strategy based on CB followed by bare metal stent (BMS) implantation, especially when IVUS guided, was superior than conventional PTCA followed BMS implantation in terms of restenosis and target lesion revascularization.15
Scoring balloon
Scoring balloon are semi-compliant balloon covered by nitinol scoring elements.
Scoreflex (OrbusNeich) balloon is characterized by a very short rapid exchange portion, so that the conventional guidewire is used as a scoring element, in concert with a further nitinol guidewire that runs from the distal to the proximal portion of the balloon.
The NSE Alpha (Bbraun) balloon is characterized by the presence of three triangular-shaped scoring elements, arranged at the proximal and distal portion of the balloon. This particularity guarantees a very low crossing profile and a good trackability, reducing at the same time the phenomenon of the slippage during the inflation.
The Angiosculpt (Angiosculpt, Biotronik) balloon is covered by three spiral nitinol wires, which slide and rotate during the inflation, exerting a scoring effect on the plaque.
Due to their conformation, which allows the prevalent transmission of the radial force to the nitinol elements, scoring balloon are very useful for the preparation of CCL, especially if ostial, reducing the possibility of barotrauma, perforation and dissection of the portion of the vessel adjacent to the lesion. In de novo lesions, pre-dilation with scoring balloon has been demonstrated to help in reaching optimal stent expansion,16 and more recently to prepare lesions before drug-coated balloon angioplasty, allowing better procedural outcomes and reducing the target lesion failure rates.17
The difference in terms of lesion crossability and preparation between cutting and scoring balloon was investigated recently by Ishihara et al., who found that Wolverine cutting balloon was smoother in lesion crossing, nevertheless being similar to NSE Alpha in terms of acute cross-sectional area gain.18
Ultrahigh pressure balloon
Super high-pressure non-compliant balloon (OPN NC, Sis-Medical) are newly designed devices with a two-layer structure, able to be inflated up to 35–40 atm reducing the dog-boning effect. They are 6F compatible, with a better crossing profile compared with cutting and scoring balloon. Early studies demonstrated their safety when compared with conventional non-compliant balloon in dilating lesions avoiding the risk of vessel perforation,19 whereas more recently in the ISAR-CALC trial Rheude et al. showed how OPN balloon performed as good as scoring balloon in terms of lesion preparation, allowing a better, although not statistically significant, angiographic result.20
Lithotripsy
Coronary lithotripsy (Shockwave Coronary Rx Lithoplasty System, Shockwave Medical) is the most modern technique developed for the treatment of CCL. The system is 6 Fr compatible, and consists of a semi-compliant balloon equipped with two emitters placed both at proximal and distal level. When the balloon is inflated (at 4 atm in order to touch the vessel) and the system is activated, the two emitters vapourize the balloon saline/contrast solution creating micro-bubbles which swell and explode, generating shock waves (1 per second) which propagate through the coronary wall, fracturing the calcium located at the level of the coronary artery, with minimal interaction with the adventitia. In the DISRUPT CAD III, a large single-arm multicentre trial, Shockwave has been demonstrated to be safe in terms of MACE at 30 days and very effective for lesion preparation before stent implantation.21 The short-learning curve, combined with the ease of use, have ensured its ever-increasing diffusion over the last 2 years, not only as a lonely technique, but even in a complementary way with the calcium ablation techniques.
Ablative techniques
Rotational atherectomy
Rotational atherectomy (Rotablator, Boston Scientific) is the first calcium ablation system to be put on the market (1993), and consists of a diamond-coated burr, which, through rotation between 135 000 and 180 000 rpm, succeeds in pulverizing the calcium present along the vascular walls, interacting only minimally with the elastic tissue. The burr is available in sizes ranging from 1.25 to 2.5 mm, compatible with 6–8 Fr guide catheters, and is advanced on a dedicated 0.009 in. guide (Rotawire).
More recently, a new device was developed (RotaPro, Boston Scientific), with the aim of making the procedure executable by a single operator, eliminating the use of pedals to activate the system and moving all controls to the table-top advancer.
The choice of the right burr size, which must have a ratio to the vessel diameter between 0.5 and 0.7, is one of the crucial factors to be taken into account in order to avoid complications related to the procedure, first and foremost possible perforation and flow limiting dissection. During the advancement, which usually takes place with a ‘picket’ motion, the calcium is reduced into particles of between 5 and 10 µm in diameter, which then end up in the microcirculation; to avoid no-reflow and slow-flow phenomena, the system should constantly flushed with a solution containing heparin, nitrates and calcium channel blockers. From an anatomical point of view, the extreme tortuosity of the vessel or the eccentric disposition of the calcium are factors that limit the effectiveness of this device, which on the other hand is very useful in treating uncrossable lesions, when the residual lumen is less than the burr diameter.
Several studies have investigated rotational atherectomy (RA) superiority over balloon angioplasty in calcified lesions. In the ROTAXUS trial, patients were randomized to PCI with first generation DES implantation with or without RA lesion modification, and even if RA was associated with higher procedural success, there was no evidence of reduced late lumen loss at follow-up.22 Analogue results were found in the PREPARE-CALC trial, where RA was compared with cutting and scoring balloon angioplasty (modified balloon, MB) both followed by third-generation DES implantation. Once again, RA strategy resulted in a higher procedural success, with 16% crossover strategy in the MB-treated group, but at the expense of a significantly longer fluoroscopy time; furthermore, at the 9-month follow-up there was no statistically significant difference in terms of late lumen loss.23
Excimer laser atherectomy
Excimer Laser Coronary Atherectomy (ELCA, Spectranetics CVX-300, Philips) uses high-power ultraviolet pulses (308 nm wavelength) generated through a xenon–chlorine fibre-optic catheter and acts modifying the plaque in three different ways: photochemical (breaking the molecular carbon bonds), photothermal (elevation of the temperature of intracellular water), and photomechanical (expansion and implosion of vapour bubbles). Usually, it is performed in cycles with a duration of 5–10 s, during intracoronary saline irrigation, followed by the same period of rest. In order to avoid the formation of vapour bubbles, continuous intracoronary irrigation is useful in flushing blood and dye from vessels.. The system is available in four different diameters, ranging from 0.9 to 2 mm, compatible with 6–8 Fr guide catheter, and can be used with a workhorse 0.014 in. wire, thus resulting very useful in lesions uncrossable with a microcatheter or with the dedicated Rotawire and VipeWire. ELCA has been demonstrated to be not superior to RA and conventional angioplasty in terms of safety and efficacy,24 while it could be very useful for the treatment of uncrossable lesions and severe ISR, where the laser explosion technique (injection of dye during laser pulses) could be the last chance.25
Orbital atherectomy
Recently, DIAMONDBACK 360°® Coronary Orbital Atherectomy System (OAS) developed by CSI has been released on the market.
The system is intended to modify coronary calcium through a mechanism of action based on centrifugal force and differential sanding. The Diamondback is equipped with a 1.25 mm eccentrically mounted diamond-coated crown that reduces superficial calcification and allows the healthy tissue to flex-away. The kit also includes a dedicated 0.012 in. wire (ViperWire®) with a 0.014 in. tip, with features of workhorse wire enabling easier maneuverability and steerability. The intimal calcium is sanded in small particles of 2 µm in diameter on average—smaller than a capillary vessel—minimizing the risk of slow-flow/no-reflow phenomenon and transient heart block. Moreover, the pulsatile impact of the crown against the vessel’s wall can create microfractures that modify deep calcium. Furthermore, the blood flow is always maintained during orbital atherectomy. Vessels from 2.5 to 4 mm in diameter may be treated with a single crown through a 6 Fr guiding catheter, by both regulating the speed of rotation or the rate of advancement (the slower the burr is advanced, the larger is the orbit). Given the ability of OA to ablate bi-directionally, the likelihood of burr entrapment should be limited, whereas the GlideAssist® allows safe removal and delivery of the device.
Usually, multiple burr passes of less than 30 s with a maximum of 5 min are recommended, until audible and tactile changes are obtained. Both intracoronary imaging to assess calcium fracture and non-compliant balloon dilatation to ensure adequate lesion preparation should be performed prior to stent implantation.
The safety and feasibility of orbital atherectomy in treating CCL have been addressed in the ORBIT I study, whereas the ORBIT II trial assessed the rate of MACE at 30 days, 1 year, and 3 years after orbital atherectomy therapy. Of note, there was a lower rate of MACE at 3 years in ORBIT II, compared to 2 years outcome in Rotaxus. The ongoing ECLIPSE trial will further clarify the role of orbital atherectomy compared to conventional angioplasty in coronary calcific lesions.27–29
Conclusions
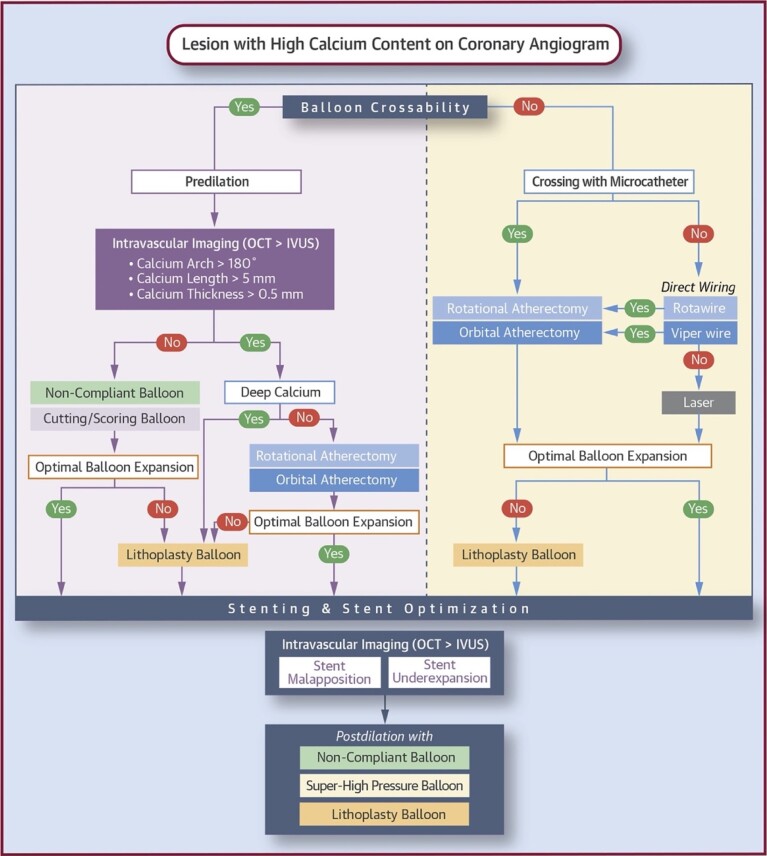
As we have discussed in this review, there are several methods for identifying and correctly treating calcific lesions. As some of these are complementary to each other, we believe that, depending on the availability in the cath-lab, they should be integrated so that the best can be made of each one. In this regard, the algorithm proposed by De Maria et al. in 2019 (Figure 1) seems to us the most valid. Once again, we would like to stress the concept that the use of imaging methods, if the lesion is crossable, is mandatory because it allows to obtain fundamental information about the type of calcific lesion we are treating (extension, depth and thickness) and consequently to plan the use of atherectomy (RA, OA, ECLA), lithotripsy or scoring/cutting balloon PCI.
Figure 1.
Treatment algorithm for calcified coronary lesions. Courtesy by De Maria et al.26
Contributor Information
Pio Cialdella, Department of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169 Rome, Italy.
Sonia Cristina Sergi, Department of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169 Rome, Italy.
Giuseppe Zimbardo, Department of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169 Rome, Italy.
Michael Donahue, Department of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169 Rome, Italy.
Giovanni Paolo Talarico, Department of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169 Rome, Italy.
Umberto Maria Lombardi d’Aquino, Department of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169 Rome, Italy.
Paolo Di Fusco, Department of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169 Rome, Italy.
Leonardo Calò, Department of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169 Rome, Italy.
Funding
None declared.
Data availability
No new data were generated or analysed in support of this research.
References
- 1. Madhavan MV, Tarigopula M, Mintz GS, Maehara A, Stone GW, Généreux P. Coronary ar- tery calcification: pathogenesis and prognostic implications. J Am Coll Cardiol 2014;63:1703–1714. [DOI] [PubMed] [Google Scholar]
- 2. Kwok CS, Martinez SC, Pancholy S, Ahmed W, Al-Shaibi K, Potts Jet al. Effect of comorbidity on unplanned readmissions after percutaneous coronary intervention (from the nationwide readmission database). Sci Rep 2018;8:11156–11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huisman J, van der Heijden LC, Kok MM, Danse PW, Jessurun GA, Stoel MGet al. Impact of severe lesion calcification on clinical outcome of patients with stable angina, treated with newer generation permanent polymer-coated drug-eluting stents: a patient-level pooled analysis from TWENTE and DUTCH PEERS (TWENTE II). Am Heart J 2016;175:121–129. [DOI] [PubMed] [Google Scholar]
- 4. Copeland-Halperin RS, Baber U, Aquino M, Rajamanickam A, Roy S, Hasan Cet al. Prevalence, correlates, and impact of coronary calcification on adverse events following PCI with newer-generation DES: findings from a large multiethnic registry. Catheter Cardiovasc Interv 2018;91:859–866. [DOI] [PubMed] [Google Scholar]
- 5. Kawaguchi R, Tsurugaya H, Hoshizaki H, Toyama T, Oshima S, Taniguchi K. Impact of lesion calcification on clinical and angiographic outcome after sirolimus-eluting stent implantation in real-world patients. Cardiovasc Revasc Med 2008;9:2–8. [DOI] [PubMed] [Google Scholar]
- 6. Onuma Y, Tanimoto S, Ruygrok P, Neuzner J, Piek JJ, Seth Aet al. Efficacy of everolimus eluting stent implantation in patients with calcified coronary culprit lesions: two-year angiographic and three-year clinical results from the SPIRIT II study. Catheter Cardiovasc Interv 2010;76:634–642. [DOI] [PubMed] [Google Scholar]
- 7. Mintz GS, Popma JJ, Pichard AD, Kent KM, Satler LF, Chuang YCet al. Patterns of calcification in coronary artery disease. A statistical analysis of intravascular ultrasound and coronary angiography in 1155 lesions. Circulation 1995;91:1959–1965. [DOI] [PubMed] [Google Scholar]
- 8. Friedrich GJ, Moes NY, Mühlberger VA, Gabl C, Mikuz G, Hausmann Det al. Detection of intralesional calcium by intracoronary ultrasound depends on the histologic pattern. Am Heart J 1994;128:435–441. [DOI] [PubMed] [Google Scholar]
- 9. Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Hg Bet al. International Working Group for Intravascular Optical Coherence Tomography (IWG-IVOCT). Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol 2012;59:1058–1072. Erratum in: J Am Coll Cardiol 2012;(18):1662. Dudeck, Darius [corrected to Dudek, Darius]; Falk, Erlin [corrected to Falk, Erling]; Garcia, Hector [corrected to Garcia-Garcia, Hector M]; Sonada, Shinjo [corrected to Sonoda, Shinjo]; Troels, Thim [corrected to Thim, Troels]; van Es, Gerrit-Ann [correct. PMID: 22421299. [DOI] [PubMed] [Google Scholar]
- 10. Mehanna E, Bezerra HG, Prabhu D, Brandt E, Chamié D, Yamamoto Het al. Volumetric characterization of human coronary calcification by frequency-domain optical coherence tomography. Circ J 2013;77:2334–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maehara A, Mintz GS, Stone GW. OCT versus IVUS: accuracy versus clinical utility. JACC Cardiovasc Imaging 2013;6:1105–1107. [DOI] [PubMed] [Google Scholar]
- 12. Fujino A, Mintz GS, Matsumura M, Lee T, Kim SY, Hoshino Met al. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention 2018;13:e2182–e2189. [DOI] [PubMed] [Google Scholar]
- 13. Mauri L, Bonan R, Weiner BH, Legrand V, Bassand JP, Popma JJet al. Cutting balloon angioplasty for the prevention of restenosis: results of the cutting balloon global randomized trial. Am J Cardiol 2002;90:1079–1083. [DOI] [PubMed] [Google Scholar]
- 14. Albiero R, Silber S, Di Mario C, Cernigliaro C, Battaglia S, Reimers Bet al. Cutting balloon versus conventional balloon angioplasty for the treatment of in-stent restenosis: results of the restenosis cutting balloon evaluation trial (RESCUT). J Am Coll Cardiol 2004;43:943–949. [DOI] [PubMed] [Google Scholar]
- 15. Ozaki Y, Lemos PA, Yamaguchi T, Suzuki T, Nakamura M, Ismail TFet al. A quantitative coronary angiography-matched comparison between a prospective randomised multicentre cutting balloon angioplasty and bare metal stent trial (REDUCE III) and the Rapamycin-Eluting Stent Evaluation at Rotterdam Cardiology Hospital (RESEARCH) study. EuroIntervention 2010;6:400–406. [DOI] [PubMed] [Google Scholar]
- 16. de Ribamar Costa J Jr, Mintz GS, Carlier SG, Mehran R, Teirstein P, Sano Ket al. Nonrandomized comparison of coronary stenting under intravascular ultrasound guidance of direct stenting without predilation versus conventional predilation with a semi-compliant balloon versus predilation with a new scoring balloon. Am J Cardiol 2007;100:812–817. [DOI] [PubMed] [Google Scholar]
- 17. Bonaventura K, Schwefer M, Yusof AKM, Waliszewski M, Krackhardt F, Steen Pet al. Systematic scoring balloon lesion preparation for drug-coated balloon angioplasty in clinical routine: results of the PASSWORD observational study. Adv Ther 2020;37:2210–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ishihara T, Iida O, Takahara M, Tsujimura T, Okuno S, Kurata Net al. Improved crossability with novel cutting balloon versus scoring balloon in the treatment of calcified lesion. Cardiovasc Interv Ther 202;36:198–207. [DOI] [PubMed] [Google Scholar]
- 19. Secco GG, Ghione M, Mattesini A, Dall’Ara G, Ghilencea L, Kilickesmez Ket al. Very high-pressure dilatation for undilatable coronary lesions: indications and results with a new dedicated balloon. EuroIntervention 2016;12:359–365. [DOI] [PubMed] [Google Scholar]
- 20. Rheude T, Rai H, Richardt G, Allali A, Abdel-Wahab M, Sulimov DSet al. Super high-pressure balloon versus scoring balloon to prepare severely calcified coronary lesions: the ISAR-CALC randomised trial. EuroIntervention 2021;17:481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hill JM, Kereiakes DJ, Shlofmitz RA, Klein AJ, Riley RF, Price MJet al. Disrupt CAD III investigators. Intravascular lithotripsy for treatment of severely calcified coronary artery disease. J Am Coll Cardiol 2020;76:2635–2646. [DOI] [PubMed] [Google Scholar]
- 22. de Waha S, Allali A, Büttner HJ, Toelg R, Geist V, Neumann FJet al. Rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: two-year clinical outcome of the randomized ROTAXUS trial. Catheter Cardiovasc Interv 2016;87:691–700. [DOI] [PubMed] [Google Scholar]
- 23. Hemetsberger R, Toelg R, Mankerious N, Allali A, Traboulsi H, Sulimov DSet al. Impact of calcified lesion complexity on the success of percutaneous coronary intervention with upfront high-speed rotational atherectomy or modified balloons – a subgroup-analysis from the randomized PREPARE-CALC trial. Cardiovasc Revasc Med 2021;33:26–31. [DOI] [PubMed] [Google Scholar]
- 24. Lee T, Shlofmitz RA, Song L, Tsiamtsiouris T, Pappas T, Madrid Aet al. The effectiveness of excimer laser angioplasty to treat coronary in-stent restenosis with peri-stent calcium as assessed by optical coherence tomography. EuroIntervention 2019;15:e279–e288. [DOI] [PubMed] [Google Scholar]
- 25. Latib A, Takagi K, Chizzola G, Tobis J, Ambrosini V, Niccoli Get al. Excimer Laser LEsion modification to expand non-dilatable stents: the ELLEMENT registry. Cardiovasc Revasc Med 2014;15:8–12. [DOI] [PubMed] [Google Scholar]
- 26. De Maria GL, Scarsini R, Banning AP. Management of calcific coronary artery lesions: is it time to change our interventional therapeutic approach? JACC Cardiovasc Interv 2019;12:1465–1478. [DOI] [PubMed] [Google Scholar]
- 27. Parikh K, Chandra P, Choksi N, Khanna P, Chambers J. Safety and feasibility of orbital atherectomy for the treatment of calcified coronary lesions: the ORBIT I trial. Catheter Cardiovasc Interv 2013;81:1134–1139. [DOI] [PubMed] [Google Scholar]
- 28. Lee M, Généreux P, Shlofmitz R, Phillipson D, Anose BM, Martinsen BJet al. Orbital atherectomy for treating de novo, severely calcified coronary lesions: 3-year results of the pivotal ORBIT II trial. Cardiovasc Revasc Med 2017;18:261–264. [DOI] [PubMed] [Google Scholar]
- 29. Généreux P, Kirtane AJ, Kandzari DE, Armstrong EJ, Krucoff MW, Redfors Bet al. Randomized evaluation of vessel preparation with orbital atherectomy prior to drug-eluting stent implantation in severely calcified coronary artery lesions: design and rationale of the ECLIPSE trial. Am Heart J 2022;249:1–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.