Abstract
Atrial fibrillation (AF) is a common and harmful arrhythmia. Its complex pathogenesis can be outlined using Coumel’s Triangle, that considers at the base of AF three different factors: substrate, trigger, and catalyst factor. The triangle can serve as a guide to understand the mechanism of action of the different possible treatments. Anti-arrhythmic drug therapies have a modest efficacy and no proven benefit on prognosis. Interventional therapy is more effective, especially if employed in the first stages of the disease, and can reduce mortality in selected populations. Ablative schemes must be different depending on the type of AF (paroxysmal, persistent) and the presence or absence of atrial dilation.
Keywords: Atrial fibrillation, Transcatheter ablation
Atrial fibrillation (AF) is the most common arrhythmia in Western world. Its prevalence has grown over the years, probably for the increasing ageing of general population.
Once regarded as a benign arrhythmia, it is now considered harmful not only for thromboembolic risk, but also for symptoms, quality of life, hospitalization rate, cognitive impairment,1 and risk of death.2
Atrial fibrillation mechanism
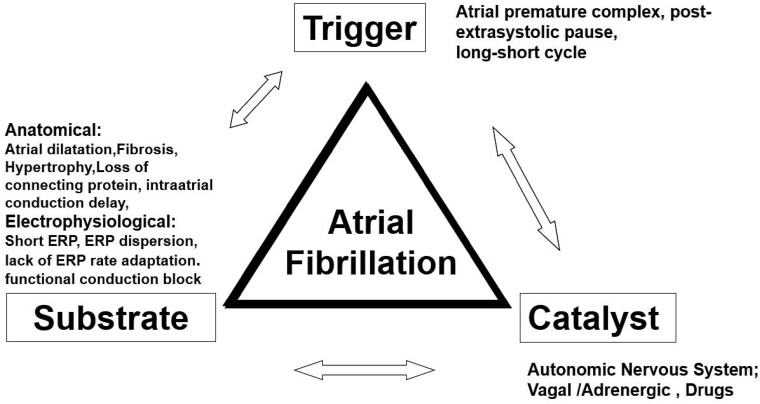
The main electrophysiological concepts at its base can be outlined using Coumel’s Triangle (Figure 1). Originally, it was created to analyse ventricular fibrillation. The triangle’s vertexes are substrate, trigger, and catalyst agent: AF is caused by a trigger event that acts on a vulnerable substrate; their interaction can be facilitated by a catalyst agent.
Figure 1.
Coumel’s Triangle.
The most important anatomic substrate is atrial dilation: volume augmentation creates non-homogenous fibrotic zones that facilitate re-entry. Dilation extent is correlated to the concomitant disease: mild dilation in hypertension, more severe, for example, in mitral valve disease or hypertrophic cardiomyopathy.
The trigger event is normally an extra beat, which must have peculiar characteristics to trigger AF. The more premature and repetitive the extra beats, the more likely is arrhythmia induction. Wall stretching associated with dilation promotes extra beats, more frequently where myocardial sleeves enter the venous system, mainly pulmonary veins, but also superior vena cava.
Autonomic system and other factors (illicit drugs, thyroid hormone) can act on both substrate and trigger as a catalyst, modifying refractory periods, and increasing automatic activity.
Trigger, substrate, and catalyst are not static entities. They can evolve with the progression of a concomitant cardiopathy, and with prolonged exposure to AF.
The substrate can be deeply modified by AF itself. In 1995, Allessie demonstrated that AF begets AF3 with a beautiful experiment on an animal model. He used goats, that have a relatively small atrium, and consequently a low vulnerability to AF.
He artificially created AF pacing with a cycle length ranging from 80 to 100 ms for a variable amount of time. Then he performed burst pacing, to induce atrial fibrillation. Induced arrhythmia lasted only seconds in animals previously stimulated for 24 h, but was sustained in animals stimulated for 2 weeks. That was the demonstration that AF begets AF. The base of this phenomenon is atrial remodelling due to AF itself: electrical (refractory period shortening), functional (contractility reduction), and more important anatomical (increasing of connexin and fibrosis).
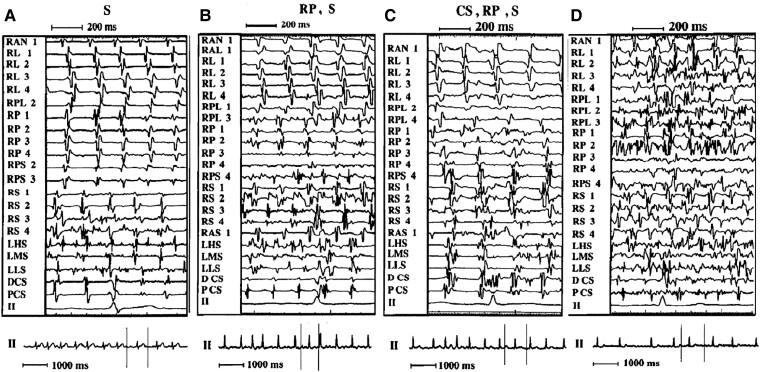
One of the first observations of electrical remodelling in human comes from our group,4 showing that electrical activity recorded with a basket catheter tends to be faster and less organized in persistent than in paroxysmal AF. Notably, the shortest atrial intervals were recorded on interatrial septum and inside pulmonary veins (Figure 2).
Figure 2.
Examples of different patterns of AF. Left panel (A S): The atrial activation is regular in the lateral and posterior wall in the right atrium and in the CS, with a definite single electrogram. The activation sequence is clearly craniocaudal in the RL wall. The septal region, both right (especially mid and low) and left, shows disorganized atrial activity with double, fragmented electrograms. Middle left panel (B RP, S): Atrial activation is regular in the lateral wall, where a craniocaudal sequence is evident, and in the CS. In contrast, the RS, LS, and RP wall show irregular atrial activation with fragmented, double, and low-amplitude atrial electrograms. Right middle panel (C CS, RP, S): The activation sequence is almost regular in the RL wall, with a craniocaudal sequence, whereas in the other sites (RP wall, septum, and CS), fragmented and double electrograms are present. Right panel (D): All recording sites show very irregular, disorganized atrial activation. RAN = right anterior wall; RL = right lateral wall; RPL = right posterolateral wall; RP = right posterior wall; RPS = right posteroseptal wall; RS = right septum; RAS = right anteroseptal wall; LHS = left high septum; LMS = left mid septum; LLS = left low septum; DCS = distal coronary sinus; PCS = proximal coronary sinus; II = lead II electrocardiogram; S = septum (both right and left); LS = left septum. Each pair of electrodes of the basket catheter is identified by a number: 1 = most distal (superior); 4 = most proximal (inferior) on the spline. At the bottom of each panel, a 7-s electrocardiographic strip is shown; the space between the vertical lines corresponds to the endocavitary signals. Source: Gaita et al.4 (Granted Licence no. 5485360497672).
Atrial fibrillation treatment
Atrial fibrillation treatment, pharmacologic or interventional, can be addressed on each vertex of Coumel’s Triangle, individually or on several at the same time. The most effective target can be different at the different stages of the disease.
The first instrument employed to treat AF is pharmacologic therapy.
Atrial fibrillation drug treatment started with rhythm control in 18th century with quinidine, discovered in 1633 for its antipyretic properties, and recognized as an antiarrhythmic in 1749. Digitalis for rate control came shortly after. Other antiarrhythmic drugs were synthetized in the last century.
Antiarrhythmic drugs can act on trigger, substrate, and autonomic tone, and have a variable efficacy in maintaining sinus rhythm, grossly ranging from 50% to 70% after 1 year of treatment. They bear a risk of collateral effects, some minor and some serious, as torsade de pointes which ranges from 0.4% to 4.6% of cases. All the studies testing antiarrhythmic drugs from 2000 to 2009 showed a reduction of AF episodes, an improvement in quality of life, but no mortality reduction compared with rate control therapy. This result can be explained by the proarrhythmic risk of antiarrhythmic drugs (Table 1).
Table 1.
Main studies testing antiarrhytmic drugs from 2000 to 2009
| Trial | Inclusion criteria | Primary outcome parameter | Patients reaching primary outcome (n) | ||
|---|---|---|---|---|---|
| Rate ctrl | Rhytm ctrl | P | |||
| PIAF (2000) 252 Patients |
Persistent AF (7–360 days) | Symptomatic improvement | 76/125 (60.8%) | 70/127 (55.1%) | 0.32 |
| AFFIRM (2002) 4060 Patients |
Paroxysmal AF or persistent AF, age ≥ 65 years, or risk of stroke or death | All-cause mortality | 310/2027 (25.9%) | 356/2033 (26.7%) | 0.08 |
| RACE (2002) 522 Patients |
Persistent AF or flutter for <1 year and 1–2 cardioversions over 2 years and oral anticoagulation | Composite: cardiovascular death, chronic heart failure, severe bleeding, pacemaker implantation, thrombo-embolic events, severe adverse effects of antiarrhyt. drugs | 44/256 (17.2%) | 60/266 (22.6%) | 0.11 |
| STAF (2003) 200 Patients |
Persistent AF (>4 weeks and <2 years), LA size >45 mm, CHF NYHA II-IV, LVEF <45% | Composite: overall mortality, cerebrovascular complications, CPR, embolic events | 10/100 (10.0%) | 9/100 (9.0%) | 0.99 |
| HOT CAFè (2004) 205 Patients |
First clinically overt persistent AF (≥7 days and <2 years), age 50–75 years | Composite: death, thrombo-embolic events; intracranial/major haemorrhage | 1/101 (1.0%) | 4/104 (3.9%) | >0.71 |
| AF-CHF (2008) 1376 Patients |
LVEF ≤ 35%, symptoms of CHF, history of AF (≥6 h or DCC < last 6 months) | Cardiovascular death | 175/1376 (25%) | 182/1376 (27%) | 0.59 |
| J-RHYTHM (2009) 823 Patients |
Paroxysmal AF | Composite of total mortality, symptomatic cerebral infarction, systemic embolism, major bleeding, hospitalization for heart failure, or physical/psychological disability | 89/405 (22.0%) | 64/418 (15.3%) | 0.012 |
Due to the relatively low efficacy and to the absence of mortality reduction, other techniques for the rhythm control were explored.
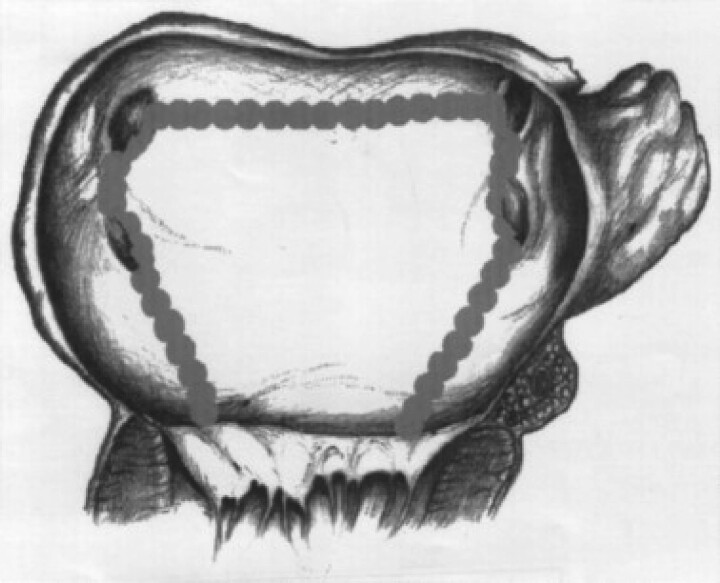
The first non-pharmacologic approach was surgical, aiming to substrate modification, with several incisions in left and right atrium (the Maze procedure5). The procedure showed very good results in rhythm control but a significant number of complications, also related to prolongation of the operation time. With the purpose to simplify the procedure, different schemes were tested. Our group proposed a surgical posterior cryoablation scheme6 consisting in a posterior box including pulmonary veins and prolonged to the mitral annulus along the septum and along the left isthmus (Figure 3).
Figure 3.
A scheme of the surgical lesions created in left atrium in Gaita et al.6 (Granted Licence no. 5485361081525).
All the patients treated were in permanent AF but the results were extremely good. Ninety per cent of patients were in sinus rhythm 1 year after the procedure, and 73% after a very long follow-up of 10 years.7
Surgical procedures proved extremely effective, but bore significant procedural risk, so many efforts were made to employ alternative techniques, as transcatheter ablation. The first attempts of transcatheter ablation had poor results since, for technical reasons, were limited to right atrium substrate modification. A slightly better effect was observed if rapid fractionated potential were identified and ablated (especially in atrial septum) and if a post procedural increase in vagal tone was documented.8
1998 was the pivotal year for transcatheter ablation, related to two important discoveries: the first is that the majority of AF triggers come from pulmonary veins9; the second is creation of an irrigated ablation catheter, with a lower char formation risk, allowing to ablate in the left side of the heart. The target then shifted from substrate to the triggers, and AF transcatheter ablation became a feasible and relatively easy procedure, with the precise endpoint of electrical pulmonary vein isolation.
Due to its high effectiveness, superior to pharmacologic therapy, and its low complication rate, the procedure spread. An enormous and still growing number of publication appeared. All the studies performed in the next 20 years confirmed that transcatheter ablation lowers AF recurrences,10 thus reducing symptoms and improving quality of life more than drugs, but were not designed to study effects on mortality. The population studied was young with no or mild cardiac disease (the most common comorbidity was hypertension), and had, therefore, a mortality rate too low to draw any conclusion.
The first study evaluating hard endpoints was CABANA,11 in which a very large number of AF patients (more than 2000) were randomized to radiofrequency ablation vs. drug therapy, and evaluated in composite endpoint (death, disabling stroke, serious bleeding, or cardiac arrest) five years after randomization. Although patients in ablation group had less recurrences and better quality of life, no statistical difference in primary endpoint was found in the total population. To understand this result, we have to consider some limitations of the study: a high number of crossovers (27%) from drug treatment to ablation was recorded; paroxysmal, persistent, and long-standing persistent arrhythmias, representing different stages of arrhythmic disease, with different response to treatment were enrolled; and, more important, the majority of patients had just mild cardiopathy, hence with low mortality rate. When the subgroup of patients with heart failure was analysed,12 a net benefit in mortality in ablation patients became evident (hazard ratio 0.57).
This finding is consistent with the CASTLE AF trial,13 designed ad hoc to study AF ablation effects in heart failure patients: after three years of follow-up mortality was 53% lower in ablation arm.
Twenty years after the first AF publications, CABANA and CASTLE AF finally showed that ablative treatment not only improves symptoms and quality of life, but also reduces mortality in heart failure patients. As suggested by Eugene Braunwald, AF and heart failure are the epidemics of the 21st century. These diseases are strictly interconnected, almost 40% of heart failure patients have AF, and AF worsens hospitalization rate and mortality in heart failure patients.14
Despite this result, in 2020 European Society of Cardiology Guidelines, AF ablation has a II level of recommendation in heart failure patients, and raises to class I only in cases of suspected tachycardiomyopathy.
Two other points are still debated: in which moment of AF evolution suggest ablation and which type of ablation employ in patients with long-standing AF or more advanced cardiomyopathy.
A large number of patients with AF diagnosed less than a year earlier were randomised to usual care or rhythm control in the EAST AFNET 415 trial, published in 2020. The trial was stopped for rhythm control efficacy at the third interim analysis, for a benefit in composite endpoint of death from cardiovascular causes, stroke, or hospitalization with worsening of heart failure or acute coronary syndrome.
Although a small number of patients were treated with transcatheter ablation, considering the physiopathological concept that ‘AF begets AF’ causing atrial remodelling, it would seem sage to consider ablation at an early phase, when AF is still paroxysmal. In this phase, the triggers are still the main drivers of arrhythmia, while in long-standing AF, trigger abolition is not sufficient and substrate modification is needed with a more aggressive ablation (linear ablation, fragmented signals, rotors mapping, different energy forms). We confirmed this hypothesis comparing the previously described surgical linear lesions to pulmonary vein deconnection alone in patents with long-standing AF16: if only pulmonary veins were treated, the prevalence of sinus rhythm after 2 years was only 20%. Moreover, many tools for pulmonary veins deconnection have been developed by industry over the years; on the contrary transcatheter substrate modification is performed in the majority of cases with point by point ablation. For this reason, we have high expectations of new catheters and energy sources, able to treat trigger and substrate in a single procedure. But at the same time we hope that an earlier treatment of AF will lower the incidence of persistent forms.
Conclusions
Atrial fibrillation is a common and harmful arrhythmia, not only for thromboembolic risk but also for symptoms, quality of life, hospitalization rate, and risk of death.
The electrophysiological mechanisms at the base of AF are the presence of a substrate, generally atrial dilation, of a trigger, generally a short coupled extra beat, and the influence of catalyst agents such as autonomic nervous system or drugs.
Trigger and substrate can be targeted by transcatheter ablation. Trigger elimination with pulmonary veins deconnection is a simple procedure, with a unique anatomic target and proven efficacy on arrhythmias recurrences, symptoms, quality of life, and, in heart failure patients, mortality reduction. For these reasons, together with drug therapy, it should be considered in the early phases of the disease, to achieve a better result and to prevent further substrate modification.
When fibrillation has progressed to persistent form, trigger elimination is not sufficient. Substrate modification must be pursued with a more aggressive intervention; several approaches have been proposed (linear ablation, fragmented signals, rotors mapping, different energy forms), but none of them has reached the incontrovertible results of pulmonary veins deconnection for paroxysmal form.
Contributor Information
Fiorenzo Gaita, Department of Medical Sciences, University of Torino, Turin, Italy; Maria Pia Hospital, GVM Care & Research, Torino, Italy.
Federico Ferraris, Division of Cardiology, Cardiovascular and Thoracic Department, Città della Salute e della Scienza di Torino, Turin, Italy.
Matteo Anselmino, Division of Cardiology, Department of Medical Sciences, Città della Salute e della Scienza di Torino, University of Turin, Turin, Italy.
Leonardo Calò, Division of Cardiology, Policlinico Casilino, Roma, Italy.
Funding
None declared.
Data availability
No new data were generated or analysed in support of this research.
References
- 1. Saglietto A, Scarsoglio S, Canova D, Roatta S, Gianotto N, Piccotti Aet al. Increased beat-to-beat variability of cerebral microcirculatory perfusion during atrial fibrillation: a near-infrared spectroscopy study. Europace 2021;23:1219–1226. [DOI] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98:946–952. [DOI] [PubMed] [Google Scholar]
- 3. Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995;92:1954–1968. [DOI] [PubMed] [Google Scholar]
- 4. Gaita F, Calò L, Riccardi R, Garberoglio L, Scaglione M, Licciardello Get al. Different patterns of atrial activation in idiopathic atrial fibrillation: simultaneous multisite atrial mapping in patients with paroxysmal and chronic atrial fibrillation. J Am Coll Cardiol 2001;37:534–541. [DOI] [PubMed] [Google Scholar]
- 5. Cox JL, Schuessler RB, D'Agostino HJ Jr, Stone CM, Chang BC, Cain ME, et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg 1991;101:569–583. [PubMed] [Google Scholar]
- 6. Gaita F, Gallotti R, Calò L, Manasse E, Riccardi R, Garberoglio Let al. Limited posterior left atrial cryoablation in patients with chronic atrial fibrillation undergoing valvular heart surgery. J Am Coll Cardiol 2000;36:159–166. [DOI] [PubMed] [Google Scholar]
- 7. Gaita F, Ebrille E, Scaglione M, Caponi D, Garberoglio L, Vivalda L, et al. Very long-term results of surgical and transcatheter ablation of long-standing persistent atrial fibrillation. Ann Thorac Surg 2013;96:1273–1278. [DOI] [PubMed] [Google Scholar]
- 8. Gaita F, Riccardi R, Calò L, Scaglione M, Garberoglio L, Antolini Ret al. Atrial mapping and radiofrequency catheter ablation in patients with idiopathic atrial fibrillation. Electrophysiological findings and ablation results. Circulation 1998;97:2136–2145. [DOI] [PubMed] [Google Scholar]
- 9. Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou Get al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659–666. [DOI] [PubMed] [Google Scholar]
- 10. Calkins H, Reynolds MR, Spector P, Sondhi M, Xu Y, Martin Aet al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol 2009;2:349–361. [DOI] [PubMed] [Google Scholar]
- 11. Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JEet al. Lee KL; CABANA investigators. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321:1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Packer DL, Piccini JP, Monahan KH, Al-Khalidi HR, Silverstein AP, Noseworthy PAet al. Ablation versus drug therapy for atrial fibrillation in heart failure: results from the CABANA trial. Circulation 2021;143:1377–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens Let al. Bänsch D; CASTLE-AF investigators. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417–427. [DOI] [PubMed] [Google Scholar]
- 14. Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PAet al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation 2003;107:2920–2925. [DOI] [PubMed] [Google Scholar]
- 15. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan Aet al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–1316. [DOI] [PubMed] [Google Scholar]
- 16. Gaita F, Riccardi R, Caponi D, Shah D, Garberoglio L, Vivalda Let al. Linear cryoablation of the left atrium versus pulmonary vein cryoisolation in patients with permanent atrial fibrillation and valvular heart disease: correlation of electroanatomic mapping and long-term clinical results. Circulation 2005;111:136–142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.