ABSTRACT
Background and Aims:
Intraoperative dexmedetomidine infusion decreases the concurrent anaesthetic and analgesic requirement. However, because of slow onset and offset, it is often used with other drugs. Opioids have a depressant effect on the cardiorespiratory system while ketamine has the opposite pharmacodynamics. Hence, it was hypothesised that ketamine will have a better intraoperative haemodynamic profile compared to fentanyl. This study compared the clinical effects and recovery characteristics of ketamine versus fentanyl when used as an adjuvant along with dexmedetomidine infusion intraoperatively.
Methods:
A total of 80 patients (18-60 years) undergoing major surgeries were divided into two groups: Group (D + K) received an intraoperative infusion of ketamine 0.5 mg/kg/h, while group (D + F) received fentanyl 0.5 μg/kg/h along with intravenous dexmedetomidine 0.5 μg/kg/h. Intraoperative heart rate (HR), mean blood pressure, and oxygen saturation were recorded at 0 min, 10 min of induction, and thereafter every 30 min throughout the procedure. Ramsay sedation score (RSS) and visual analogue scale (VAS) score were measured at the end of the surgery, at 2 hours, 4 hours, and 6 hours.
Results:
Reduction in HR and mean blood pressure was more with a tendency of developing hypotension in the fentanyl group compared to the ketamine group. Post-anaesthesia care unit (PACU) stay, need for muscle relaxant and VAS score for pain were also significantly lesser in the ketamine group.
Conclusion:
Dexmedetomidine with ketamine provided better haemodynamic stability and reduced PACU stay compared to dexmedetomidine with fentanyl.
Keywords: Analgesia, dexmedetomidine, fentanyl, ketamine, sedation
INTRODUCTION
Perioperative use of dexmedetomidine as an adjunct to general anaesthesia has been shown to have a smooth and haemodynamically stable intraoperative and post-operative course because of its analgesic and anaesthetic sparing effect and cardiovascular stabilising effects without respiratory depression.[1,2] However, the slow onset and offset of action of dexmedetomidine is the major limitation of its use.[3] Hence, several drugs have been studied to be used as adjuvant to perioperative dexmedetomidine infusion. Fentanyl with dexmedetomidine has explicated a better clinical outcome compared to dexmedetomidine alone.[4] But the cardiorespiratory depressant effect of opioids can aggravate the well-known side effects of dexmedetomidine like bradycardia and hypotension.[5] On the other hand, ketamine [an N-Methyl-D-aspartate (NMDA) receptor antagonist] causes tachycardia, hypertension, salivation, nausea and emergence delirium but has a faster onset of action.[6,7] Thus, the antagonising adverse effect profile of the two drugs can have a synergistic combination allowing smoother anaesthesia and recovery profile. The use of ketamine allows faster onset of action, thereby eliminating the slower onset seen with dexmedetomidine alone.[8]
Dexmedetomidine has been studied for its effects via several routes like intravenous, intranasal, intrathecal, intramuscular and oral.[9] Also, it has been studied with several adjuvants for effect potentiation. However, there are no studies directly comparing the intraoperative and post-operative effects of fentanyl versus ketamine, when used with dexmedetomidine infusion. One recent study, compared dexmedetomidine and ketamine combination with dexmedetomidine, midazolam and fentanyl combination for procedural sedation in the adult population. This failed to show any significant difference between onset time, recovery time and cardiorespiratory variables. In that study, local anaesthetic was also infiltrated, probably requiring a lesser dose of intravenous drugs. Hence, the cardiorespiratory effects of adjuvants could not have been evident in that study.[8]
Therefore, this study was conducted to compare the clinical effects and recovery characteristics of intraoperative dexmedetomidine with ketamine versus dexmedetomidine with fentanyl infusion. The primary objectives were to compare haemodynamic parameters and recovery characteristics [emergence and agitation using Ramsay sedation scale (RSS), duration in post-anaesthesia care unit (PACU)] when using intraoperative dexmedetomidine infusion with ketamine versus fentanyl as an adjuvant. Secondary objectives were to compare the anaesthetic sparing effect, the need for post-operative rescue analgesia and adverse effects in post-operative periods like bradycardia, hypotension, nausea and vomiting between the two groups.
METHODS
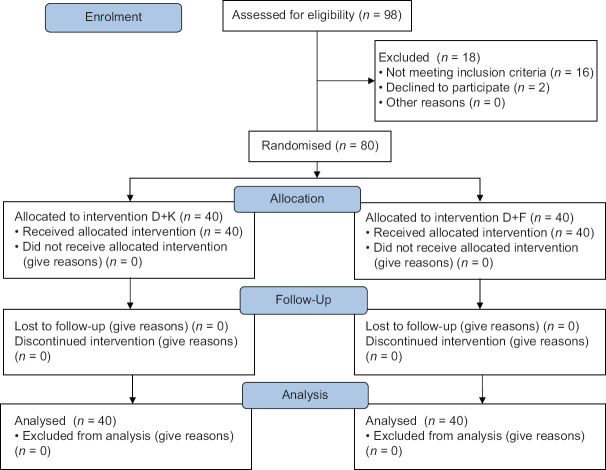
After approval from the institutional research and ethical committee (ECR/Bhu/Inst/UP/2013/Re-registration-2017 dated 2018 Feb 23), this double-blinded, prospective, randomised controlled trial was conducted from July 2018 to December 2019 at a tertiary care hospital. The study was conducted according to the Declaration of Helsinki principles. After taking written informed consent, American Society of Anesthesiologists (ASA) physical status I and II patients, aged between 18 and 60 years, undergoing major abdominal or head and neck onco-surgeries were included. Patients with ASA grade III or more; heart block, arrhythmias, basal heart rate less than 60/min, patients on beta-blockers or calcium channel blockers, known allergy to study drug, lack of consent, known chronic use of analgesics and sedatives, renal and hepatic dysfunction; psychiatric disorders; and respiratory disorder and sleep apnoea were excluded [Figure 1].
Figure 1.
Consolidated standards of reporting trials (CONSORT) flow diagram
A sample size of 34 in each group was calculated at 90% power with a significance level (α) of 0.05, and an effect size of 0.8, using a two-tailed independent t-test (G*Power software ver. 3.1.9.4; Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany). Several previous similar studies have effect sizes between 0.4 and 0.9. Hence, we chose the large effect size (0.8) for sample size calculation, so that even if a statistically significant result is obtained, it must be large enough to be of practical significance to be implied in our clinical applications. The sample size was increased to 40 patients per group assuming 15% intraoperative exclusions/dropouts. Patients were randomly divided into two study groups using computerised randomisation using a 1:1 allocation ratio. Allocation concealment was done using white sealed envelopes containing the study drug for the unblinded team. To maintain uniformity throughout the study, each drug was from one brand – ketamine hydrochloride (Ketamine 50 mg/ml, Themis Medicare Limited, India), fentanyl (Fent 50 μg/ml, Neon Laboratories Ltd) and dexmedetomidine hydrochloride (Dexem 100 μg/ml, Themis Medicare Limited, India). Randomly allocated coded syringes containing either fentanyl or ketamine were prepared by an anaesthesiologist not involved in either conducting the case, data recording or for monitoring during the intra-and post-operative periods. The method of drug dilution was as follows: In one group, intravenous (IV) fentanyl (50 μg per ml) at 0.5 μg/kg/h was started (2 μg per ml). The other group received ketamine IV infusion at 0.5 mg/kg/h (50 mg per ml and 2 mg per ml). Dexmedetomidine was prepared in a standardised manner in the concentration of 4 μg/ml (200 μg diluted in 50 ml normal saline) for both groups. Baseline demographic and cardiorespiratory parameters were noted in both groups upon arrival of the patient in the operating room. The patients were pre-oxygenated and pre-medicated with intravenous fentanyl 1.5 μg/kg and midazolam 30 μg/kg IV, induced by administering injection propofol (till loss of verbal command) and injection vecuronium 0.1 mg/kg as muscle relaxant. The patient was intubated when there was a disappearance of three (T1) or all twitches (T0) after train of four (TOF) stimuli, and clinical conditions were satisfactory like relaxed jaw, absence of coughing, gagging, swallowing, etc. Maintenance of anaesthesia was done with isoflurane and nitrous oxide in both groups. Immediately after intubation, intraoperative infusion of the following agents was started: Group 1: D + K Group – Intraoperative infusion of intravenous (IV) dexmedetomidine 0.5 μg /kg/h in combination with ketamine 0.5 mg/kg/h. Group 2: D + F Group - Intraoperative infusion of IV dexmedetomidine 0.5 μg/kg/h in combination with fentanyl 0.5 μg/kg/h.
Intermittent muscle relaxant requirement was assessed by a TOF response. Mechanical ventilation was maintained by volume control mode to keep the end-tidal carbon dioxide (EtCO2) between 30 and 35 mmHg. After the induction of anaesthesia, mean arterial pressure (MAP) and heart rate (HR) were maintained at 20% above or below the baseline values. Hypotension (MAP value <60 mmHg), not responding to a decrease in the inspired isoflurane concentration, was managed with fluid challenges or mephentermine 5 mg IV boluses. Bradycardia (HR <60 beats per min) persisting for >2 min was treated with atropine 20 μg/kg. Infusion of study drugs was terminated after skin closure and neuromuscular block were antagonised with neostigmine (50 μg/kg) and glycopyrrolate (20 μg/kg). The patient was extubated once appropriate extubation criteria were met. The duration between the termination of anaesthetic gases and the extubation was defined as ‘extubation time’.
The primary outcome measured has intraoperative HR, MAP, peripheral capillary oxygen saturation (SpO2), EtCO2, which were recorded at 0 min, 10 min of induction and thereafter every 30 min throughout the procedure; and also measured after extubation, 5 min after extubation and 2 h in the post-operative period. On arrival at the PACU, RSS was assessed at admission to PACU as well as 60 min later. Secondary outcomes included were pain assessment on 10-point visual analogue scale (VAS) at the end of the surgery, at 2 hours, 4 hours and 6 hours. IV tramadol (1 mg/kg, maximum three times a day) was administered for rescue analgesia. It was administered if the VAS score was ≥4 at rest or ≥5 at movement. After arrival in the PACU, oxygen saturation, MAP and HR were noted. Duration of PACU stay, time of administration of first analgesic for each patient, emergence delirium and any adverse events like post-operative nausea and vomiting (PONV), bradycardia, hypotension, hypertension, pain, shivering and delirium were noted.
Qualitative data were analysed with Chi-square test, while quantitative data were presented as mean ± standard deviation (SD), or as the median for skewed data. For normally distributed data, t-test was used, while for skewed data, Mann–Whitney U-test was used for assessing the significance. All statistical analyses were performed with Statistical Package for Social Sciences (SPSS) version 21.0 (International Business Machines, Armonk, NY, USA).
RESULTS
Both groups had comparable demographic and baseline clinical profiles [Table 1]. In both groups, HR increased immediately after intubation and then decreased gradually below the baseline value at 10, 40, 70, 100 and 130 minutes. However, there was a greater reduction in HR in the D + F group at all points of time post-intubation (P = 0.000) [Figure 2].
Table 1.
Baseline demographic and clinical profile
| Mean±SD n=40 | P | ||
|---|---|---|---|
|
| |||
| Group 1 | Group 2 | ||
| Age (years) | 45.72±10.643 | 43.30±11.567 | 0.332 |
| Height (cm) | 156.62±3.705 | 157.48±3.850 | 0.317 |
| Weight (kg) | 59.25±6.159 | 60.98±4.532 | 0.158 |
| M: F (ratio) | 5:3 | 2.3:1.7 | 0.648 |
| ASA grade 1:2 (ratio) | 7:3 | 2.1:1.9 | 0.108 |
| Baseline heart rate (beats/min) | 72.68±8.11 | 74.85±5.52 | 0.165 |
| Baseline MBP (mmHg) | 84.68±8.33 | 82.58±2.05 | 0.121 |
| Baseline SpO2 (%) | 99.35±0.7 | 99.5±0.5 | 0.275 |
| Baseline EtCO2 (mmHg) | 31.68±1.51 | 31.12±1.34 | 0.09 |
ASA: American Society of Anesthesiologists; SpO2: Peripheral oxygen saturation; EtCO2: End-tidal carbon dioxide; M: Male; F: Female; SD: Standard deviation; n: Number
Figure 2.
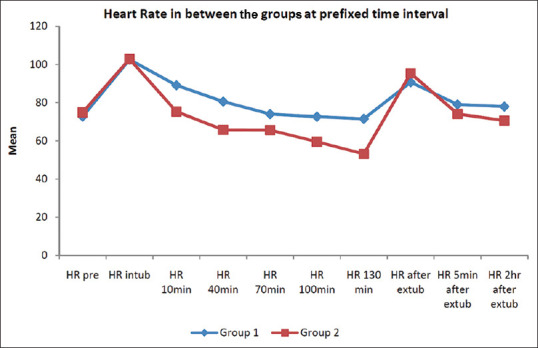
Heart rate variability in the two study groups at different points of time. Pre = pre-operatively; intub = intubation; HR = heart rate; min = minutes; extub = extubation; hr = hours
After intubation, the mean blood pressure (BP) was 96.62 ± 12.04 and 97.28 ± 5.22 mm Hg in group D + K and D + F, respectively, and both were comparable and statistically insignificant (P = 0.755). In both the groups, mean BP increased immediately after intubation, and then it gradually reduced below the baseline value at 10, 40, 70, 100 and 130 min. However, there was a tendency for the development of hypotension in the D + F group thus giving it a lesser favourable haemodynamic profile as compared to D + K group. Mean BP showed a significant difference between the two groups (P < 0.001) at all points of time post-intubation [Figure 3].
Figure 3.
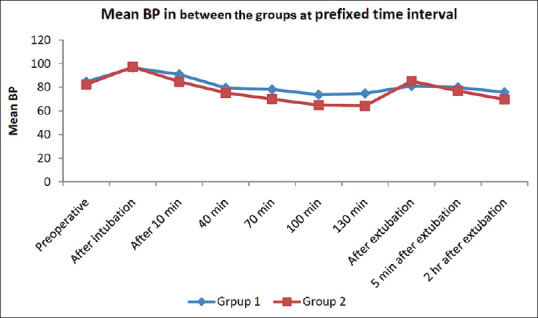
Blood pressure variability in the two study groups at different points of time. BP = blood pressure; min = minutes; hr = hours
Group D + F showed statistically significant lower mean BP as compared to D + K group, and hypotension was observed in four patients in D + F group as compared to 1 patient in D + K group (P < 0.05). Bradycardia was seen in three patients in D + F group as compared to none in D + K group, which was statistically insignificant (P = 0.077). The incidence of nausea and vomiting was also comparable between the two study groups (one in D + K versus three in the D + F group). No differences in the SpO2 or sedation score among the two groups were observed.
The total intraoperative requirement of muscle relaxants in D + K group (3.50 ± 0.60 mg) was significantly lesser than in D + F group (4.05 ± 0.85 mg) [P < 0.001] [Figure 4]. There was no significant difference between the analgesic requirements among the two study groups during the first 6 hours in the post-operative period, but the pain score was significantly lesser in D + K group than D + F group at 4 and 6 hours post-operatively [P < 0.001]. Extubation time was 7.66 ± 2.22 minutes in the ketamine group and 7.25 ± 2.65 min in the fentanyl group which was statistically insignificant.
Figure 4.
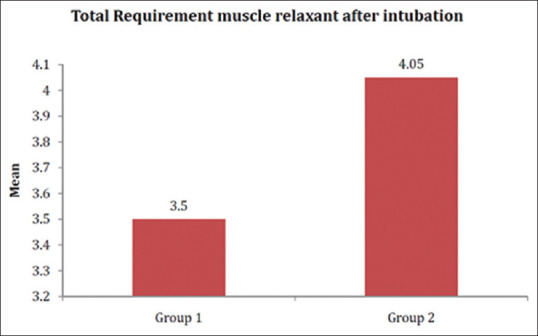
Muscle relaxant (vecuronium) requirement in milligrams (P < 0.001)
RSS in all the patients at admission to PACU was either 2 or 3 which became either 1 or 2, 60 minutes post-operatively. D + K group had 13 patients at score 2 and 27 patients at score 3, while D + F group had 19 patients at score 2 and 21 patients at score 3 (χ2 = 1.875a; P = 0.171). The mean PACU stay in D + K group (1.12 ± 0.20 hours) was significantly lesser than D + F group (1.45 ± 0.14 hours) [P < 0.001].
DISCUSSION
Balanced anaesthesia with minimal haemodynamic variations is always desirable in anaesthesia practice. In our study, there was a decrease in intraoperative HR and BP in both groups, but a greater decrease was seen in the D + F group. Haemodynamic variations were easily managed in our study, with IV fluid therapy. One study comparing the haemodynamic effects of ketamine (1 mg/kg IV followed by 0.25 mg/kg/h infusion)-dexmedetomidine (1.0 μg/kg over 20 min and then 0.2–0.7 μg/kg/h) with ketamine (1 mg/kg IV followed by 0.25 mg/kg/h infusion)-propofol (1 mg/kg bolus followed by 25–50 μg/kg/min) for sedation in post-coronary artery bypass grafting (CABG) patients showed no significant difference in the haemodynamic stability among the two groups, probably because ketamine which antagonises the hypotensive and bradycardic effect of dexmedetomidine, and propofol was common to both the groups.[10] Our findings were contrary to another study where there was no difference in cardiorespiratory variables, onset time, recovery time and rescue analgesia requirement among the dexmedetomidine (1 μg/kg dexmedetomidine over 10 min followed by 0.2–1.0 μg/kg/h) with ketamine (0.5 mg/kg) group versus dexmedetomidine (same dose) with midazolam (0.05 mg/kg) and fentanyl (0.5 μg/kg) group when used for chemo port insertion. Rather, sedation quality and patient satisfaction were more in the latter group. This might be possibly due to lesser opioid requirement as midazolam was also used in that group, and local anaesthetic was also infiltrated at the insertion site in both the groups. The use of opioids in very low doses might have stopped its cardiorespiratory side effects from being overtly manifested.[8]
Dexmedetomidine causes sedation, analgesia, inhibition of central sympathetic outflow by activation of receptors in the vasomotor centre, reducing catecholamine levels, reducing norepinephrine turnover, resulting in alterations in sympathetic function enhanced vagal activity and decreased HR and BP through stimulation of the presynaptic α2 receptors.[11] The cause of the bradycardia in the D + F group can be direct stimulation of the central vagal nucleus by fentanyl, as dexmedetomidine was common in both groups. Ketamine prevented bradycardia in the D + K Group. Similarly, the D + F group had a significant decrease in mean BP compared to the D + K group. Fentanyl is also known to cause hypotension indirectly by decreasing central sympathetic outflow, whereas ketamine may antagonise the bradycardia and hypotension, caused by dexmedetomidine. Thus, the dexmedetomidine with ketamine group of patients had better haemodynamic stability with easy arousability.[12]
Dexmedetomidine enhances specific components of anaesthesia requiring lesser doses of general anaesthetics with fewer side effects.[13-15] However, when the add-on effect of ketamine versus fentanyl with dexmedetomidine infusion was compared, the requirement of the muscle relaxant was lesser in the D + K group. Earlier cases have been reported where dexmedetomidine (1 μg/kg loading dose followed by infusion 0.5 μg/kg/h) and ketamine (2 mg/kg) were used along with inhalational anaesthetic agents without muscle relaxants for surgery.[16] Ketamine allows good intubating conditions without requiring muscle relaxants, and this has been shown in earlier studies also.[17] This finding is contrary to an earlier study where the investigators could not find the difference in muscle relaxation and intubating conditions between the ketamine (0.5 mg/kg) and alfentanil (20 μg/kg) groups.[18] Dexmedetomidine, ketamine and opioids are known for their analgesic effects. In our study, we found that the pain score was lesser in the D + K group compared to the D + F group, suggesting a better analgesic profile of dexmedetomidine with ketamine. A similar study comparing pain scores in patients undergoing lower limb surgeries under spinal anaesthesia between two groups receiving dexmedetomidine with ketamine (1 mg/kg) or dexmedetomidine with fentanyl (1 μg/kg) found better analgesia in the ketamine group.[19,20]
No significant differences in the incidence of emergence delirium and extubation time were observed between the two groups in our study. Our findings were consistent with earlier studies where dexmedetomidine significantly reduced the incidence of emergence delirium with no significant effect on the extubation time.[21-23] However, another study has shown a longer recovery time with the dexmedetomidine–ketamine combination compared to the propofol–ketamine combination.[23] This difference in findings can be attributed to the differing pharmacodynamics and pharmacokinetics of propofol and dexmedetomidine as ketamine was common in both groups.
The limitations of our study were that we did not have an accurate measure of the degree of possible fall in BP and HR because in case more than desired fluctuation (>20%) was observed, corrective measures were taken in the form of decreasing the inhalation anaesthetic, fluid boluses, IV mephentermine or atropine. However, not taking corrective measures would have been ethically inappropriate in the study. Also, we did not use the bispectral index/entropy for assessing intraoperative awareness and the quality of sedation during anaesthesia due to non-availability. Another limitation of our study was that we could not compare the respiratory depressant effect of both drug combinations as the patient was on controlled mechanical ventilation intraoperatively with a target EtCO2 between 30 and 35 mmHg.
CONCLUSION
Both ketamine and fentanyl when co-administered with dexmedetomidine attenuate the haemodynamic response to surgical stress, but dexmedetomidine along with ketamine provides better haemodynamic stability, and faster recovery in the post-operative period compared to dexmedetomidine and fentanyl group. A significant decrease in the requirement of muscle relaxants, analgesics and reduced PACU stay was seen in the dexmedetomidine with ketamine group. Hence, ketamine is recommended over fentanyl to be used as an adjuvant along with dexmedetomidine infusion.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Basantwani S, Patil M, Govardhane B, Magar J, Tendolkar B. Effect of dexmedetomidine infusion on hemodynamic responses in microsurgery of larynx. J Anaesthesiol Clin Pharmacol. 2018;34:51–7. doi: 10.4103/joacp.JOACP_136_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garg A, Kamal M, Mohammed S, Singariya G, Chouhan DS, Biyani G. Efficacy of dexmedetomidine for prevention of emergence agitation in patients posted for nasal surgery under desflurane anaesthesia:A prospective double-blinded randomised controlled trial. Indian J Anaesth. 2018;62:524–30. doi: 10.4103/ija.IJA_788_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parashchanka A, Schelfout S, Coppens M. Role of novel drugs in sedation outside the operating room:Dexmedetomidine, ketamine and remifentanil. Curr Opin Anaesthesiol. 2014;27:442–7. doi: 10.1097/ACO.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 4.Abdul Hadi B, Sbeitan SM, Shakya AK. Fentanyl vs fentanyl-dexmedetomidine in lumbar foraminotomy surgery. Ther Clin Risk Manag. 2019;15(15):885–90. doi: 10.2147/TCRM.S195108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldo BA. Toxicities of opioid analgesics:Respiratory depression, histamine release, hemodynamic changes, hypersensitivity, serotonin toxicity. Arch Toxicol. 2021;95:2627–42. doi: 10.1007/s00204-021-03068-2. [DOI] [PubMed] [Google Scholar]
- 6.Natoli S. The multiple faces of ketamine in anaesthesia and analgesia. Drugs Context. 2021;10 doi: 10.7573/dic.2020-12-8. 2020-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JG, Lee HB, Jeon SB. Combination of dexmedetomidine and ketamine for magnetic resonance imaging sedation. Front Neurol. 2019;10:416. doi: 10.3389/fneur.2019.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chun EH, Han MJ, Baik HJ, Park HS, Chung RK, Han JI, et al. Dexmedetomidine-ketamine versus dexmedetomidine-midazolam-fentanyl for monitored anesthesia care during chemoport insertion:A prospective randomized study. BMC Anesthesiol. 2016;16:49. doi: 10.1186/s12871-016-0211-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X, Li Y, Kang L, Wang Q. Recent advances in the clinical value and potential of dexmedetomidine. J Inflamm Res. 2021;14:7507–27. doi: 10.2147/JIR.S346089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahran MS, Elbaradi GF. Safety and efficacy of ketamine-dexmedetomidine versus ketamine-propofol combinations for sedation in patients after coronary artery bypass graft surgery. Ann Card Anaesth. 2017;20:182–7. doi: 10.4103/aca.ACA_254_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vora KS, Baranda U, Shah VR, Modi M, Parikh GP, Butala BP. The effects of dexmedetomidine on attenuation of hemodynamic changes and there effects as adjuvant in anesthesia during laparoscopic surgeries. Saudi J Anaesth. 2015;9:386–92. doi: 10.4103/1658-354X.159461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manne GR, Upadhyay MR, Swadia V. Effects of low dose dexmedetomidine infusion on haemodynamic stress response, sedation and post-operative analgesia requirement in patients undergoing laparoscopic cholecystectomy. Indian J Anaesth. 2014;58:726–31. doi: 10.4103/0019-5049.147164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ravipati P, Reddy PN, Kumar C, Pradeep P, Pathapati RM, Rajashekar ST. Dexmedetomidine decreases the requirement of ketamine and propofol during burns debridement and dressings. Indian J Anaesth. 2014;58:138–42. doi: 10.4103/0019-5049.130813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan ZP, Munday IT, Jones RM, Thornton C, Mant TG, Amin D. Effects of dexmedetomidine on isoflurane requirements in healthy volunteers. 1:Pharmacodynamic and pharmacokinetic interactions. Br J Anaesth. 1999;83:372–80. doi: 10.1093/bja/83.3.372. [DOI] [PubMed] [Google Scholar]
- 15.Bulow NM, Barbosa NV, Rocha JB. Opioid consumption in total intravenous anesthesia is reduced with dexmedetomidine:A comparative study with remifentanil in gynecologic videolaparoscopic surgery. J Clin Anesth. 2007;19:280–5. doi: 10.1016/j.jclinane.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Varma S, Kalingarayar S. Anaesthesia for thymectomy:Use of ketamine-dexmedetomidine without muscle relaxant. Indian J Anaesth. 2013;57:315–6. doi: 10.4103/0019-5049.115590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim KS, Kwak HJ, Min SK, Lee SY, Kim KM, Kim JY. The effect of ketamine on tracheal intubating conditions without neuromuscular blockade during sevoflurane induction in children. J Anesth. 2011;25:195–9. doi: 10.1007/s00540-011-1092-9. [DOI] [PubMed] [Google Scholar]
- 18.Kim JY, Lee JS, Park HY, Kim YB, Kwon Y, Kwak HJ. The effect of alfentanil versus ketamine on the intubation condition and hemodynamics with low-dose rocuronium in children. J Anesth. 2013;27:7–11. doi: 10.1007/s00540-012-1485-4. [DOI] [PubMed] [Google Scholar]
- 19.Lee KH, Lee SJ, Park JH, Kim SH, Lee H, Oh DS, et al. Analgesia for spinal anesthesia positioning in elderly patients with proximal femoral fractures:Dexmedetomidine-ketamine versus dexmedetomidine-fentanyl. Medicine (Baltimore) 2020;99:e20001. doi: 10.1097/MD.0000000000020001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.García-Henares JF, Moral-Munoz JA, Salazar A, Del Pozo E. Effects of ketamine on postoperative pain after remifentanil-based anesthesia for major and minor surgery in adults:A systematic review and meta-analysis. Front Pharmacol. 2018;9:921. doi: 10.3389/fphar.2018.00921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hauber JA, Davis PJ, Bendel LP, Martyn SV, McCarthy DL, Evans MC, et al. Dexmedetomidine as a rapid bolus for treatment and prophylactic prevention of emergence agitation in anesthetized children. Anesth Analg. 2015;121:1308–15. doi: 10.1213/ANE.0000000000000931. [DOI] [PubMed] [Google Scholar]
- 22.Kwon SY, Joo JD, Cheon GY, Oh HS, In JH. Effects of dexmedetomidine infusion on the recovery profiles of patients undergoing transurethral resection. J Korean Med Sci. 2016;31:125–30. doi: 10.3346/jkms.2016.31.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joshi VS, Kollu SS, Sharma RM. Comparison of dexmedetomidine and ketamine versus propofol and ketamine for procedural sedation in children undergoing minor cardiac procedures in cardiac catheterization laboratory. Ann Card Anaesth. 2017;20:422–6. doi: 10.4103/aca.ACA_16_17. [DOI] [PMC free article] [PubMed] [Google Scholar]