Abstract
Background:
The humerus is the second most common site of metastatic disease involving long bones, yet it is still unclear which patients are at high risk for a fracture and may require prophylactic surgical fixation. The goal of this study was to assess the validity of the Mirels score to predict fractures of metastatic lesions in the humerus.
Methods:
We performed a retrospective electronic chart review of patients with humeral metastases at our institution (2005 to 2021), with 188 patients meeting the inclusion criteria. Sixty-one of the patients developed a fracture during follow-up. The metastatic humeral lesions were scored according to the Mirels rating system and additional radiographic criteria (cortical breach, location within the humerus, number of lesions). The predictive value of each Mirels score cutoff for fracture was assessed using sensitivity, specificity, area under the receiver operating characteristic curve (AUC), and multivariate logistic regression. Survivorship until fracture was analyzed for each Mirels score cutoff using Kaplan-Meier curves and the log-rank test. Significance was set at p < 0.01.
Results:
There were no significant differences in age, sex, side of the lesion, type of malignancy, and radiation dose between the groups with and without fracture (all p > 0.01). A Mirels score of ≥8 points had the best predictive profile, with sensitivity of 83.6%, specificity of 79.5%, and AUC of 0.82 (95% confidence interval [CI], 0.75 to 0.88, p < 0.01). A logistic regression model also demonstrated that a Mirels score of ≥8 (odds ratio = 5.8, 95% CI = 1.9 to 18.2, p < 0.01) and a cortical breach (odds ratio = 21.0, 95% CI = 5.7 to 77.2, p < 0.01) were significant predictors of pathological fracture. No other radiographic characteristics were found to be significant predictors of fracture.
Conclusions:
This study indicated that a Mirels score of ≥8 points had the best predictive profile for anticipating fractures at a metastasis in the humerus. This is in contrast to the traditional Mirels definition of an impending pathological fracture that is used for the lower extremity, a score of ≥9. Additionally, the presence of a cortical breach was a significant predictor of fracture risk.
Level of Evidence:
Prognostic Level III. See Instructions for Authors for a complete description of levels of evidence.
In 1989, Mirels developed a scoring system for predicting the risk of impending pathological fractures of long bones on the basis of 4 components of the metastatic lesion: anatomical location, size, radiographic appearance, and pain. Each component was given a score of 1 to 3, yielding an aggregate score of 4 to 121. In that study, a Mirels score of <7 was found to indicate a low risk of fracture (4% risk, with a 22% false-positive rate), and these patients were recommended not to undergo prophylactic fixation. A score of 8 indicated a higher risk of fracture, 15% (with a false-positive rate of 6%), and those with a score of 9 had an even higher risk, 33% (with a false-positive rate of 0%). Therefore, the study findings suggested that a lesion with a score of ≥9 points warranted prophylactic surgical stabilization.
Several subsequent studies have investigated the validity and reliability of the Mirels score to predict fracture risk2-4. Each of the 4 risk factors, when considered independently, performed poorly for predicting pathological fracture risk; however, when they were used in aggregate, a score of ≥9 identified impending pathological fractures with a sensitivity of 91% and a specificity of 33%4. The low specificity is a known drawback of the Mirels score. A specificity of 33% means that the score would overestimate the risk of fracture and potentially result in unnecessary procedures in 2 out of 3 patients with a score indicating that prophylactic fixation should be performed5. The morbidity associated with unnecessary surgery in these patients can be detrimental, especially if they have a prolonged postoperative recovery time during an already short projected life expectancy3. Nevertheless, clinical use of the Mirels score is widespread for long bones with pathological metastases.
Mirels’ original study involved a group of patients who predominantly had femoral lesions from breast cancer metastases, yet its results have been extrapolated to the prediction of fracture risk in the upper extremity. The humerus is the second most common site of metastatic disease involving a long bone6. Long bones in the upper extremity, such as the humerus, have different load-bearing requirements, undergo torsional and bending forces rather than compression forces, and have cortical densities that may confer differences in fracture risk compared with bones in the lower limb7. To our knowledge, only 2 studies have investigated the validity of the Mirels scoring system in the upper extremity3,8. Both studies showed that a Mirels score of ≥9 had a low predictive value, with an area under the receiver operating characteristic (ROC) curve (AUC) of 0.55 (95% confidence interval [CI], 0.51 to 0.59, p = 0.057), sensitivity of 14%, and specificity of 73%3,8. However, those studies were underpowered as a result of their small sample sizes. Therefore, the validity of the Mirels scoring system in the upper extremity deserves further investigation.
The purpose of the present study was to evaluate the validity of the Mirels score for fracture prediction in a larger cohort of patients with metastatic lesions in the humerus. We also considered whether other radiographic features of the lesion, including location within the humerus and a cortical breach, influenced the risk of fracture. A better understanding of pathological fracture risk can help to guide management of upper extremities with metastases to improve outcomes and decrease morbidity9,10.
Materials and Methods
We performed a retrospective electronic chart review of patients treated at our institution over a 16-year period between 2005 and 2021, using our radiation oncology patient database.
Patients who were ≥18 years of age and had a diagnosis of metastatic bone disease involving the humerus were eligible for inclusion. We searched our database for humeral metastases using the search terms SHOR (indicating the right shoulder), SHOL (left shoulder), HUMR (right humerus), HUML (left humerus), ARUL (left upper arm), and ARUR (right upper arm). Primary disease included bladder, brain, breast, gastrointestinal, lung, oropharyngeal, renal, skin, and thyroid cancer, lymphoma, and multiple myeloma. Patients were included regardless of their prognosis and prior and expected medical treatment, including chemotherapy, immunotherapy, and radiotherapy. All patients in this study received radiotherapy. Patients who had a primary bone tumor, those who received prophylactic surgical stabilization, and those with <6 months of follow-up were excluded.
Baseline characteristics, including age, sex, side of the metastasis (left/right), primary disease diagnosis, and radiation dose, were extracted from patient charts. The Mirels score is calculated on the basis of the clinical history and radiographic features, and has 4 components: location (upper extremity, lower extremity, pertrochanteric), pain (mild, moderate, severe, or functional), lesion size (0 to <1/3, 1/3 to 2/3, >2/3 of the diameter of the bone), and lesion type (osteoblastic, mixed, osteolytic). The corresponding score of 1 to 3 is assigned for each component, and the scores are added to yield an aggregate score. Since the present study involved humeral lesions, the aggregate score could range from 4 to 10 rather than 4 to 12. Pain scores in the present study were based on either a visual analogue scale (VAS) score or the clinical history in the patient’s chart. A VAS score of 0 to 3 was mild; 4 to 6, moderate; and 7 to 10, severe. Any pain with limb function was deemed functional pain and assigned a Mirels score of 3.
Additional radiographic features were also assessed in the study: location within the long bone (epiphysis, metaphysis, diaphysis), diaphyseal location (proximal, middle, distal 1/3 of the shaft, for diaphyseal lesions), mediolateral location (central, medial, lateral), number of lesions, and presence of a cortical breach. A cortical breach was characterized by any disruption in the humeral cortex; the presence of any cortical erosion was scored as 1, and its absence was scored as 0 (Fig. 1). Unlike previous studies that used computed tomography (CT) imaging11,12, the degree of cortical involvement was not characterized as a percentage. For diaphyseal location, we defined the humeral diaphysis as the length between the surgical neck of the humerus to the supracondylar ridge as defined in the AO/OTA fracture classification13. Using this measurement, we were able to define the percent of shaft involved by dividing the measured length of the lesion by the length of the shaft. Based on the total length of the shaft, the regions comprising the proximal (0% to 33%), middle (33% to 67%) and distal (67% to 100%) thirds of the diaphysis were defined. All radiographic features were assessed using radiographs of the humerus made in orthogonal planes (anteroposterior and lateral views). All measurements were performed by 2 independent reviewers (J.T. and D.B.).
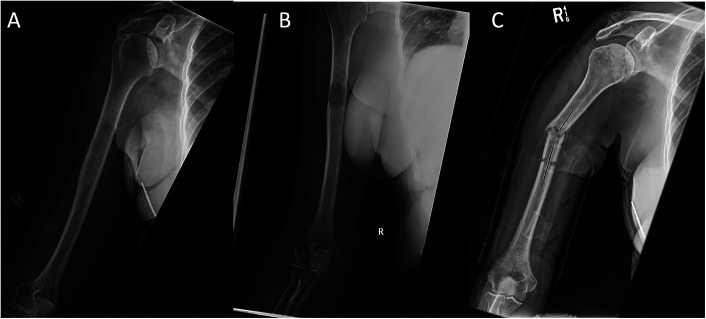
Fig. 1.
Fig. 1-A Anteroposterior radiograph showing a metastatic humeral lesion. The lesion is characterized as diaphyseal and having a midshaft location, a size of >2/3 of the diameter of the bone, and evidence of a cortical breach (in the lateral cortex). The lesion was assigned a Mirels score of 10. Fig. 1-B Anteroposterior radiograph showing progression of the lesion, with increased size and greater cortical disruption. Fig. 1-C Anteroposterior radiograph showing a pathological fracture involving the lesion.
The occurrence and timing of pathological fractures (Fig. 1) were extracted from the patient records. Times were calculated from the date at which the humeral metastasis was first detected on radiographs. Patients who did not develop a fracture during follow-up of at least 6 months (from detection of the metastasis until the date of the last clinic note) were classified in the non-fracture group. Patients without a fracture who had inadequate follow-up or who did not survive for at least 6 months following presentation were excluded. Six months was chosen as the surveillance cutoff because of the typical progressive natural history of metastatic disease and the resulting short median life expectancy2,14.
Statistical Analysis
Patient baseline characteristics were compared using the independent-samples t test for ordinal data and the chi-square test for categorical data. The predictive value of the Mirels score was assessed using the sensitivity, specificity, and AUC. A forward stepwise multivariate logistic regression analysis was used to determine if any radiographic variables were significant predictors of fracture. The regression model was designed to control for age, sex, side (left/right), primary disease diagnosis, and radiation dose. Survivorship free of fracture was analyzed on the basis of a cutoff Mirels score using Kaplan-Meier curves and the log-rank test. All analyses were performed using SPSS Statistics (version 13.0; IBM). A p value of <0.01 was considered significant.
Source of Funding
No external funding was received for this study.
Results
We screened 1,369 patients identified by our initial search strategy. Of these patients, 188 met our inclusion criteria regarding metastatic humeral lesions; 61 developed a fracture and 127 did not. Pain scores were retrospectively determined from VAS scores (34 patients) or clinical scoring from chart review (n = 154). A cohort of patients (n = 119) were excluded as they had a pathological humeral fracture but no baseline radiographs showing the metastatic lesion prior to the fracture were available. We also excluded 120 patients who had other upper-extremity lesions (clavicle, scapula, ulna, radius), 150 patients who died or were lost to follow-up before 6 months, and 9 patients who had prophylactic stabilization because they were not able to tolerate nonoperative treatment. The remaining exclusions were due to inadequate imaging or represented duplicates (patients who received >1 radiation treatment).
There were no significant differences in age, sex, side of the lesion, primary disease diagnosis, or radiation dose between the fracture and non-fracture groups (p > 0.01) (Table I). Nonoperative management, which involved a period of non-weight-bearing immobilization, was the most common treatment (39 patients, 63.9%) for individuals who developed fractures. The other individuals who sustained fractures were treated with open reduction and internal fixation (13 patients, 21.3%) and intramedullary nailing (9 patients, 14.8%).
TABLE I.
Baseline Characteristics*
| Characteristic | No Fracture (N = 127) | Fracture (N = 61) | P Value† |
|---|---|---|---|
| Side | 0.062 | ||
| Right | 72 (56.7%) | 30 (49.2%) | |
| Left | 55 (43.3%) | 31 (50.8%) | 0.037 |
| Age (yr) | 69.0 ± 14.1 | 73.5 ± 12.9 | |
| Sex | 0.75 | ||
| Female | 75 (59.1%) | 34 (55.7%) | |
| Male | 52 (40.9%) | 27 (44.3%) | |
| Disease | 0.022 | ||
| Bladder | 0 | 1 (1.6%) | |
| Brain | 2 (1.6%) | 0 | |
| Breast | 29 (22.8%) | 8 (13.1%) | |
| Gastrointestinal | 13 (10.2%) | 8 (13.1%) | |
| Lung | 13 (10.2%) | 9 (14.8%) | |
| Lymphoma | 1 (0.8%) | 1 (1.6%) | |
| Multiple myeloma | 3 (2.4%) | 1 (1.6%) | |
| Oropharyngeal | 39 (30.7%) | 14 (23.0%) | |
| Renal | 6 (4.7%) | 14 (23.0%) | |
| Skin | 1 (0.8%) | 1 (1.6%) | |
| Thyroid | 2 (1.6%) | 0 | |
| Unspecified | 18 (14.2%) | 4 (6.6%) | |
| Radiation dose (cGy) | 1,552 ± 796 | 1,350 ± 657 | 0.12 |
The values are given as the number with the percentage in parentheses or as the mean ± standard deviation.
The independent-samples t test was used for ordinal data, and the chi-square test was used for categorical data. No values were significant at p <0.01.
Table II compares the radiographic features of the non-fracture and fracture groups. The main differences between these groups were the clinical and radiographic features pertaining to the Mirels score (pain, lesion type, and lesion size) and the presence of a cortical breach. Patients with a fracture had significantly greater scores for all 3 of the Mirels score components (p < 0.01) as well as a greater likelihood of a cortical breach in their baseline radiographs (91.8% in the fracture group versus 22.4% in the non-fracture group, p < 0.01).
TABLE II.
Fracture Characteristics*
| Feature | No Fracture (N = 127) | Fracture (N = 61) | P Value† |
|---|---|---|---|
| Pain | <0.01 | ||
| Mild | 53 (43.1%) | 11 (18.0%) | |
| Moderate | 44 (35.8%) | 26 (42.6%) | |
| Severe | 26 (21.1%) | 24 (39.3%) | |
| Lesion type | <0.01 | ||
| Blastic | 56 (44.4%) | 1 (1.6%) | |
| Mixed | 22 (17.5%) | 6 (9.8%) | |
| Lytic | 48 (38.1%) | 54 (88.5%) | |
| Size relative to bone diameter | <0.01 | ||
| <1/3 | 51 (42.9%) | 5 (8.5%) | |
| 1/3 to 2/3 | 34 (28.6%) | 12 (20.3%) | |
| >2/3 | 34 (28.6%) | 42 (71.2%) | |
| Location | 0.08 | ||
| Epiphysis | 22 (17.6%) | 4 (6.6%) | |
| Metaphysis | 29 (23.2%) | 11 (18.0%) | |
| Diaphysis | 74 (59.2%) | 46 (75.4%) | |
| Diaphysis location | 0.053 | ||
| Proximal 1/3 | 39 (53.4%) | 28 (59.6%) | |
| Middle 1/3 | 29 (39.7%) | 15 (31.9%) | |
| Distal 1/3 | 5 (6.9%) | 4 (8.5%) | |
| Mediolateral location | 0.25 | ||
| Central | 57 (47.1%) | 33 (54.1%) | |
| Lateral | 28 (23.1%) | 14 (23.0%) | |
| Medial | 36 (29.8%) | 14 (23.0%) | |
| Breach | <0.01 | ||
| Yes | 28 (22.4%) | 56 (91.8%) | |
| No | 97 (76.6%) | 5 (8.2%) | |
| No. of lesions | 1.8 ± 2.7 | 1.3 ± 0.9 | 0.19 |
The Mirels score includes pain, lesion type, and lesion size. We also assessed other radiographic features including location within the long bone, diaphyseal location, mediolateral location, breaching, and number of lesions. The values are given as the number with the percentage in parentheses or as the mean ± standard deviation.
The independent-samples t test was used for ordinal data, and the chi-square test was used for categorical data. Significant p values are boldfaced.
The predictive value of different Mirels score cutoffs is shown in Table III. A Mirels score of ≥8 points had the best predictive profile, with sensitivity of 83.6%, specificity of 79.5%, and an AUC of 0.82 (95% CI, 0.75 to 0.88, p < 0.01 for all) (Table III). The multivariate logistic regression model also demonstrated that a Mirels score of ≥8 (odds ratio = 5.8, 95% CI = 1.9 to 18.2, p < 0.01) and a cortical breach (odds ratio = 21.0, 95% CI = 5.7 to 77.2, p < 0.01) were both significant predictors of pathological fracture after controlling for age, sex, side of the lesion, primary diagnosis, and radiation dose. Using the model with a Mirels score of ≥8 as a predictor, we were able to correctly predict 77.0% of the pathological fractures. After adding a cortical breach as an additional predictor in the model, we were able to increase the accuracy to 83.6% of the pathological fractures. No other radiographic characteristics were found to be significant predictors of fracture (p > 0.01).
TABLE III.
Ability of Mirels Score to Predict Fractures*
| Mirels Score | Sensitivity | Specificity | AUC (95% CI) | P Value† |
|---|---|---|---|---|
| ≥6 | 98.4% | 28.3% | 0.63 (0.55-0.71) | <0.01 |
| ≥7 | 96.7% | 56.7% | 0.77 (0.70-0.83) | <0.01 |
| ≥8 | 83.6% | 79.5% | 0.82 (0.75-0.88) | <0.01 |
| ≥9 | 57.4% | 86.6% | 0.72 (0.64-0.80) | <0.01 |
| 10 | 26.2% | 96.1% | 0.61 (0.52-0.70) | <0.01 |
AUC = area under the receiver operating characteristic curve.
For the comparison of the AUC value with 0.5 (the value that would result from chance). Significant p values are boldfaced.
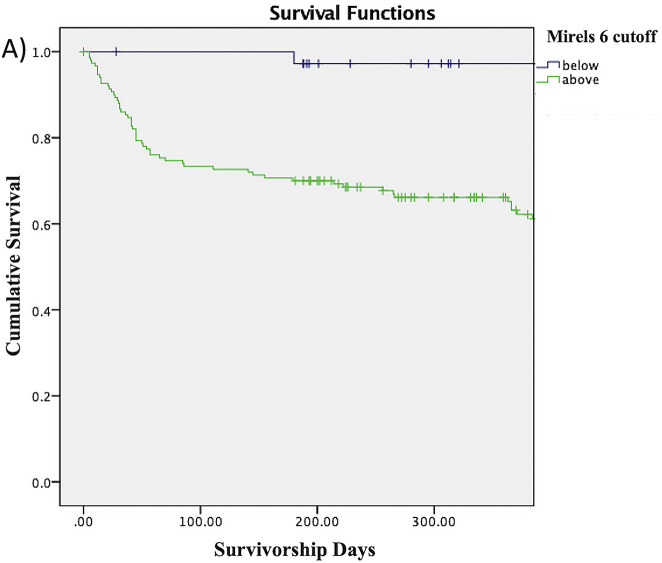
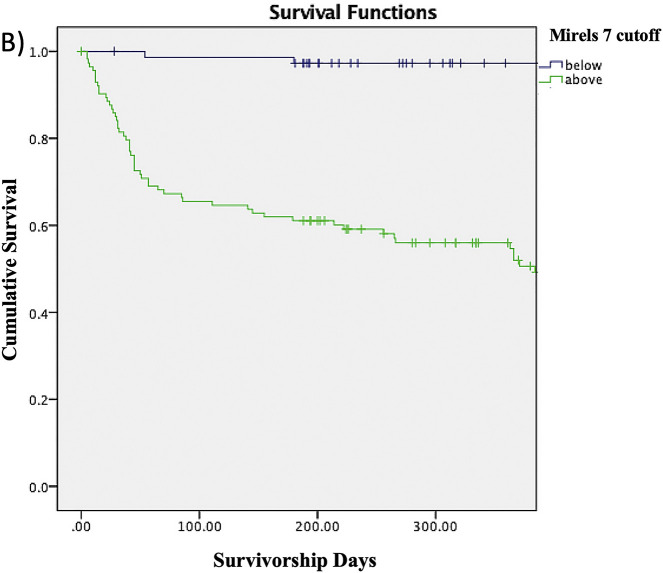
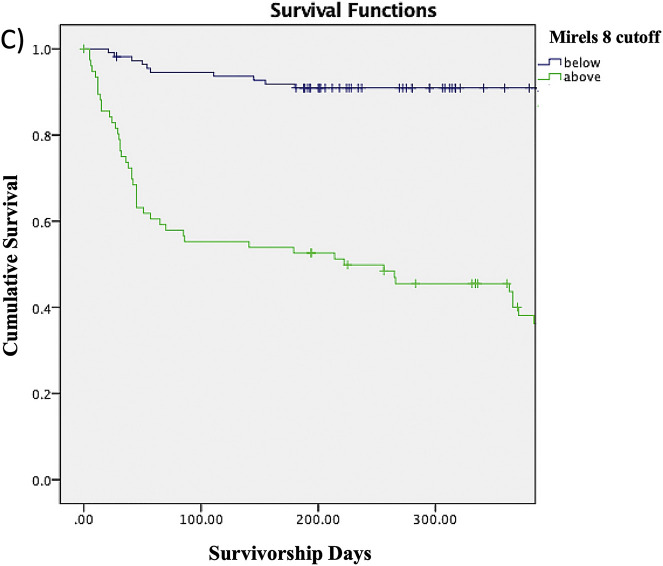
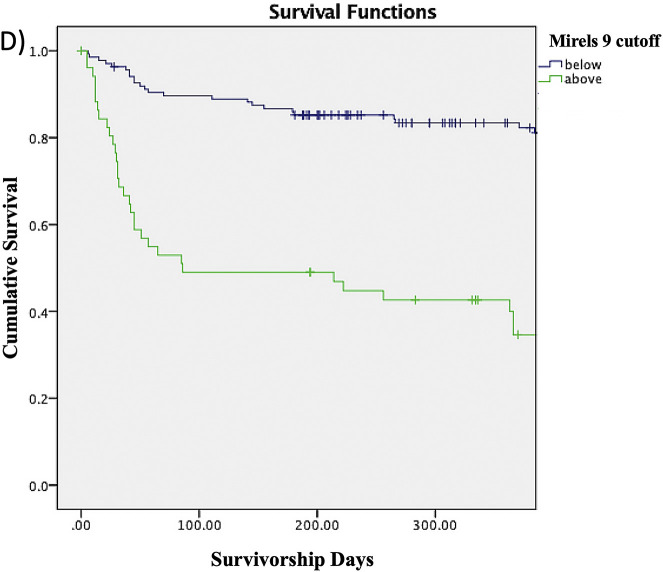
The Kaplan-Meier survivorship rates shown in Table IV represent the proportions of patients without a fracture at any given time point. For example, the proportion of patients with a Mirels score of ≥8 who survived without a fracture was 55.3% at 3 months, 52.6% at 6 months, 45.5% at 9 months, and 40% at 12 months. The proportion of patients without a fracture at any given time point decreased as the Mirels score cutoff increased from 6 to 10. The log-rank test similarly showed that patients with a Mirels score at or above each of the cutoffs had significantly lower survivorship free of fracture compared with patients scoring below the cutoff (Figs. 2-A through 2-E).
Fig. 2-A.
Figs. 2-A through 2-E Kaplan-Meier survivorship curves for Mirels scores of ≥6 (Fig. 2-A), ≥7 (Fig. 2-B), ≥8 (Fig. 2-C), ≥9 (Fig. 2-D), and 10 (Fig. 2-E). The blue line represents patients who have a Mirels score less than the cutoff, and the green line represents patients with a Mirels score greater than or equal to the cutoff. Each line shows the proportion of patients who have not developed a pathological fracture at the given time interval. The log-rank test indicated that there was a significant difference (p < 0.01) between the 2 curves for each cutoff.
Fig. 2-A.
Fig. 2-B.
Fig. 2-C.
Fig. 2-D.
Fig. 2-E.
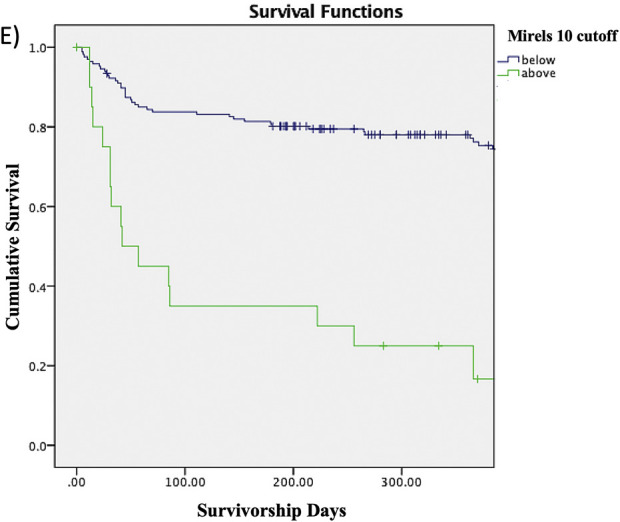
TABLE IV.
Survivorship Rates for Patients Free of Pathologic Fracture
| Mirels Score | 3 Months | 6 Months | 9 Months | 12 Months | P Value* |
|---|---|---|---|---|---|
| ≥6 | 73.3% | 70.0% | 66.1% | 65.2% | <0.01 |
| ≥7 | 65.5% | 61.1% | 56.0% | 52.0% | <0.01 |
| ≥8 | 55.3% | 52.6% | 45.5% | 40.0% | <0.01 |
| ≥9 | 49.0% | 46.9% | 42.6% | 34.6% | <0.01 |
| 10 | 35.0% | 30.0% | 25.0% | 16.7% | <0.01 |
Significant p values are boldfaced and represent the log-rank test comparison between survivorship curves.
Discussion
This study represents the largest cohort of patients with metastatic humeral lesions used to validate the Mirels rating system for predicting pathological fractures, to our knowledge. A Mirels score of ≥8 points yielded the best profile for predicting fractures involving humeral metastases, with a high sensitivity of 83.6%, specificity of 79.5%, and AUC of 0.82 (95% CI, 0.75 to 0.88, p < 0.01 for all). In contrast, the traditional Mirels definition of impending pathological fracture (≥9 points) that was developed on the basis of lower-extremity lesions had lower sensitivity and specificity. Hoban et al.8 also suggested using a lower Mirels cutoff for the upper extremity, but recommended a score of ≥7. We suspect that the difference in recommended cutoffs is due to the populations studied. Hoban et al. examined all upper-extremity pathological fractures (humerus, ulna, radius, clavicle, scapula), whereas the present study examined only the humerus. Furthermore, the patients studied by Hoban et al. were referred to their orthopaedic department for a surgical opinion, whereas the patients in the present study were referred to our oncology department for initial medical management of the humeral metastasis. Thus, it is not surprising that the fracture rate of 76% (35 of 46) reported by Hoban et al. was higher than the rate of 32% (61 of 188) in the present study. Until a more valid scoring system has been developed for the upper extremity, the Mirels score is a valid tool for use in metastatic humeral lesions, and we have demonstrated good predictive accuracy for Mirels scores of ≥7, ≥8, and ≥9.
A cortical breach was a significant predictor when used in conjunction with the Mirels score to predict pathological humeral fractures. The presence of a cortical breach was first introduced in the 1980s as part of the Harrington criteria to predict fracture risk15. It was defined as a lesion involving >50% of the cortical bone. However, the Harrington criteria have limitations: they apply only to the proximal femur, and they have yet to be validated. Use of the Harrington criteria is still debated. A cortical breach alone, based on a different definition of “axial cortical involvement >30 mm,” has since been validated as an independent predictor of fracture involving femoral metastases11, but its role in fracture prediction has not been well established. In the present study, we quantitatively demonstrated the utility of a cortical breach as a predictor of fracture using radiographs. Thus, our findings may not be accurate to extrapolate to the use of other imaging, such as CT or magnetic resonance imaging (MRI). A cortical breach had a high odds ratio of 21.0 (95% CI = 5.7 to 77.2, p < 0.01); in other words, patients with a cortical breach were 21 times more likely to sustain a fracture compared with those without a breach. We also found that adding a cortical breach to our regression model increased the model’s ability to correctly predict humeral fractures from 77.0% to 83.6%. Therefore, we propose that a cortical breach may be useful as an adjunct to the Mirels score to predict fracture risk with better accuracy.
Clinicians such as orthopaedic surgeons and radiation oncologists frequently need to make treatment recommendations based on radiographs of humeral metastases. Prophylactic surgical fixation of impending pathological fractures is preferred, as it provides better outcomes (improved function and fewer complications)16,17. Moreover, we know from previous studies that pathological fractures decrease quality of life and are associated with more advanced disease18. Therefore, accurately predicting the risk of impending pathological fractures is critical to identify those patients who would benefit from prophylactic fixation. The survivorship profiles that we present in this study (Table IV, Figs. 2-A through 2-E) provide another useful tool to help guide clinicians in understanding fracture risk over time and could ultimately help guide decision-making regarding prophylactic fixation for impending fractures.
The present study has several limitations, including its single-center retrospective cohort design. Although we suggest that patients with a high fracture risk may benefit from early prophylactic stabilization to prevent morbidity associated with fractures, we did not analyze clinical outcomes following prophylactic fixation or fracture. Recommendations on optimal timing of surgery are beyond the scope of this study. In practice, the decision to proceed with prophylactic fixation depends on many factors, including radiographic features and patient factors (health, ambulatory status, chemotherapy, need for upper-extremity weight-bearing, expected duration of survival, cancer type). There is also interobserver variability in the Mirels grading system. Previous studies have demonstrated only moderate interobserver variability (kappa = 0.580, 95% CI = 0.395 to 0.765) for grading upper-extremity lesions3,8, which may have affected our overall Mirels scores. Thus, our recommendation to use a Mirels score of ≥8 for metastatic humeral lesions should be viewed with caution. Lastly, our study relied on retrospective assessment of pain from patient electronic records, and this is a potential source of bias in the total Mirels score. The retrospective nature also resulted in exclusion of a large proportion of our initial 1,369 patients due to inadequate imaging (preoperative imaging was not always available).
In conclusion, this study indicated that a Mirels score of ≥8 points had the best profile for predicting fractures involving humeral metastases. That cutoff is higher than the value used in the traditional Mirels definition of impending pathological fracture (≥9 points). Additionally, a cortical breach was a significant predictor and may be a useful adjunct variable for predicting fractures.
Footnotes
Investigation performed at the Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada
Disclosure: The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJSOA/A509).
References
- 1.Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989. Dec; (249):256-64. [PubMed] [Google Scholar]
- 2.Damron TA, Morgan H, Prakash D, Grant W, Aronowitz J, Heiner J. Critical evaluation of Mirels’ rating system for impending pathologic fractures. Clin Orthop Relat Res. 2003. Oct; (415)(Suppl):S201-7. [DOI] [PubMed] [Google Scholar]
- 3.Evans AR, Bottros J, Grant W, Chen BY, Damron TA. Mirels’ rating for humerus lesions is both reproducible and valid. Clin Orthop Relat Res. 2008. Jun; 466(6):1279-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hipp JA, Springfield DS, Hayes WC. Predicting pathologic fracture risk in the management of metastatic bone defects. Clin Orthop Relat Res. 1995. Mar; (312):120-35. [PubMed] [Google Scholar]
- 5.Piccioli A, Spinelli MS, Maccauro G. Impending fracture: A difficult diagnosis. Injury. 2014. Dec; 45(Suppl 6):S138-41. [DOI] [PubMed] [Google Scholar]
- 6.Frassica FJ, Frassica DA. Evaluation and treatment of metastases to the humerus. Clin Orthop Relat Res. 2003. Oct; (415)(Suppl):S212-8. [DOI] [PubMed] [Google Scholar]
- 7.Voskuil RT, Mayerson JL, Scharschmidt TJ. Management of Metastatic Disease of the Upper Extremity. J Am Acad Orthop Surg. 2021. Feb 1; 29(3):e116-25. [DOI] [PubMed] [Google Scholar]
- 8.Hoban KA, Downie S, Adamson DJA, MacLean JG, Cool P, Jariwala AC. Mirels’ score for upper limb metastatic lesions: do we need a different cutoff for recommending prophylactic fixation? JSES Int. 2022. Apr 25; 6(4):675-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El Abiad JM, Raad M, Puvanesarajah V, Rao SS, Morris CD, Levin AS. Prophylactic Versus Postfracture Stabilization for Metastatic Lesions of the Long Bones: A Comparison of 30-day Postoperative Outcomes. J Am Acad Orthop Surg. 2019. Aug 1; 27(15):e709-e716. [DOI] [PubMed] [Google Scholar]
- 10.Jawad MU, Scully SP. In brief: classifications in brief: Mirels’ classification: metastatic disease in long bones and impending pathologic fracture. Clin Orthop Relat Res. 2010. Oct; 468(10):2825-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van der Linden YM, Dijkstra PD, Kroon HM, Lok JJ, Noordijk EM, Leer JW, Marijnen CA. Comparative analysis of risk factors for pathological fracture with femoral metastases. J Bone Joint Surg Br. 2004. May; 86(4):566-73. [PubMed] [Google Scholar]
- 12.Damron TA, Nazarian A, Entezari V, Brown C, Grant W, Calderon N, Zurakowski D, Terek RM, Anderson ME, Cheng EY, Aboulafia AJ, Gebhardt MC, Snyder BD. CT-based structural rigidity analysis is more accurate than Mirels scoring for fracture prediction in metastatic femoral lesions. Clin Orthop Relat Res. 2016. Mar; 474(3):643-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meinberg EG, Agel J, Roberts CS, Karam MD, Kellam JF. Fracture and dislocation classification compendium—2018. J Orthop Trauma. 2018. Jan; 32(Suppl 1):S1-170. [DOI] [PubMed] [Google Scholar]
- 14.Rades D, Haus R, Schild SE, Janssen S. Prognostic factors and a new scoring system for survival of patients irradiated for bone metastases. BMC Cancer. 2019. Nov 28; 19(1):1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrington KD. Impending pathologic fractures from metastatic malignancy: evaluation and management. Instr Course Lect. 1986;35:357-81. [PubMed] [Google Scholar]
- 16.Ward WG, Holsenbeck S, Dorey FJ, Spang J, Howe D. Metastatic disease of the femur: surgical treatment. Clin Orthop Relat Res. 2003. Oct; (415)(Suppl):S230-44. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal MG, Nayak P. Management of skeletal metastases: An orthopaedic surgeon’s guide. Indian J Orthop. 2015. Jan-Feb; 49(1):83-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saad F, Lipton A, Cook R, Chen YM, Smith M, Coleman R. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer. 2007. Oct 15; 110(8):1860-7. [DOI] [PubMed] [Google Scholar]