Background:
Although infection following anterior cruciate ligament (ACL) reconstruction is rare, these cases have potentially catastrophic ramifications. Nontuberculous mycobacteria (NTM) are ubiquitous, located in the water supply and soil. Our objective was to review the literature to characterize NTM infection following ACL reconstruction.
Methods:
A database search was performed for arthroscopic ACL reconstructions that resulted in a postoperative diagnosis of NTM infection.
Results:
The literature search returned 6 case reports that met the inclusion criteria. The initial postoperative presentation occurred after 3 to 52 weeks, which is consistent with reports of postoperative NTM infection.
Conclusions:
The common indolent course of NTM infection hinders prompt diagnosis and treatment. Organism susceptibility testing is crucial for effective treatment and limiting unnecessary antibiotic exposure.
Clinical Relevance:
The duration of medical treatment for NTM infection is 4 to 6 months, depending on the severity of disease and species. Based on the aggregate literature, we recommend removal of local implants if the infection is localized and without articular involvement. However, in cases of articular involvement, all fixation and graft material should be removed.
Nontuberculous mycobacteria (NTM) are mycobacteria species other than the Mycobacterium tuberculosis complex and M. leprae. These pathogens are ubiquitous, located in the water supply and soil. Although a majority do not infect humans, recent literature has reported an increasing number of infectious NTM species1. This may be due in part to advancements in testing that provide more rapid and precise identification of NTM species2.
The growth rate is a characteristic used to classify NTM. Slow-growing species are most common3, fast-growing species have been described as ancestral4,5, and an intermediate-growth species has been recently described5. The slow growth of some NTM is a major contributing factor to the difficulty in their detection and treatment in surgical site infections (SSIs).
Although infection following anterior cruciate ligament (ACL) reconstruction is rare, these cases have potentially catastrophic ramifications6,7. Cases of NTM infection often have an indolent course with nonspecific characteristics, and the low index of suspicion for NTM can lead to delayed diagnosis. Cases of NTM in ACL SSI are indeed rare, but aggregate data can provide an understanding of commonalities across cases. This is especially important given the current rise in reported NTM infections.
Our objective was to review the literature to characterize NTM infection following ACL reconstruction. We will describe the emerging data regarding NTM, including appropriate surgical and antimicrobial treatment.
Materials and Methods
Queries of PubMed and Google Scholar were conducted on February 21, 2023, in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines for systematic reviews. The following search terms were utilized: mycobacterium AND knee OR ACL OR ligament. The search was further narrowed by excluding tuberculous infections. Cases in which arthroscopic ACL reconstruction resulted in a postoperative diagnosis of NTM SSI were included. Cases in which SSI following ACL reconstruction involved diagnosed pathogens other than NTM were excluded, as were NTM infections that occurred following procedures other than ACL reconstruction.
Results
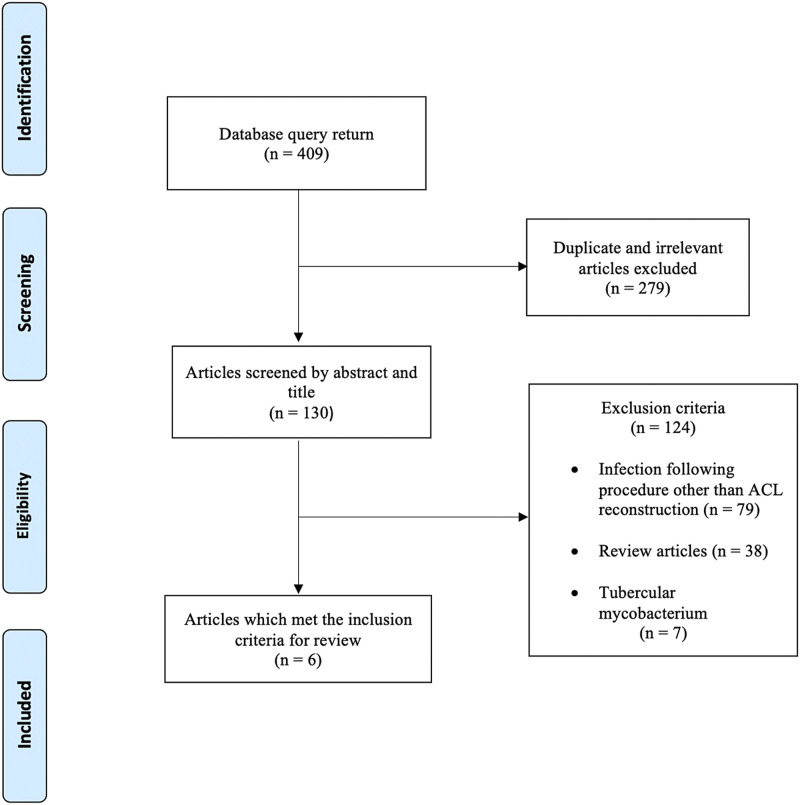
The literature search identified 6 case reports that met the inclusion criteria (Fig. 1, Table I). The reported NTM species were M. fortuitum8,9, M. farcinogenes10, and M. abscessus11-13 (Table II). M. fortuitum and M. abscessus are rapidly growing species, and M. farcinogenes is slow-growing.
Fig. 1.
Flowchart depicting the literature search process and its results.
TABLE I.
Anterior Cruciate Ligament Reconstructions*
| Study | Patient Age* (yr) | Graft | Femoral Fixation | Tibial Fixation | Concomitant Procedures |
|---|---|---|---|---|---|
| Oh, 20108 | 15 | 4-strand hamstring autograft | Cortical suspension button | PLA and tricalcium phosphate sheath and interference screw | Medial bucket-handle meniscal tear repair |
| Umprai, 201412 | 21 | Quadrupled hamstring autograft | PLLA interference screw | PLLA interference screw | All-inside medial meniscal repair |
| Grantham, 20159 | 14 | Quadrupled hamstring autograft | Cortical suspension button | PLLA interference screw, washer, post | All-inside lateral meniscal repair |
| Ng, 201511 | 22 | “Hamstring grafts” | Cortical suspension button | PLLA/HA screw, metal staples | None |
| Al Farsi, 202110 | 49 | NR | NR | “Tibial screw” | Medial meniscal repair |
| Emerson, 202113 | 67 | Quadriceps tendon | Suspensory fixation | PEEK screw | Partial medial meniscectomy |
PLLA = poly-L-lactic-acid, PLA = polylactic acid, HA = hydroxyapatite, PEEK = polyetheretherketone, and NR = not reported.
TABLE II.
Nontuberculous Mycobacterium Infections Following Anterior Cruciate Ligament Reconstruction
| Study | Presentation* | Arthrocentesis* | Initial Laboratory Values* | Initial Antibiotics* | NTM Species | No. of I&Ds | Surgical Treatment | Final Antibiotic Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Oh, 20108 | At 11 weeks, with wound dehiscence | NR | Within normal range | Cephalexin | M. fortuitum | 2 | Removal of tibial hardware | 10 weeks of linezolid, 6 months of moxifloxacin | At 7 months after ACL reconstruction, no evidence of recurrent infection and a normal Lachman test |
| Umprai, 201412 | At 1 year, with painless mass and mild redness | No pus or discharge | Within normal range | Cefazolin, gentamicin, dicloxacillin | M. abscessus | 2 | Open debridement with retention of hardware | 3 months of moxifloxacin and clarithromycin | At 6 months after the most recent surgical intervention, no evidence of recurrent infection |
| Grantham, 20159 | At 3 weeks, with fever, abdominal pain, no concerning knee or wound symptoms | 11,880 nucleated cells | ESR, 55 mm/hr; CRP, 95.5 mg/L | Cephalexin | M. fortuitum | 3 | Removal of tibial hardware, filling of the tibial tunnel | 6 months of sulfamethoxazole/trimethoprim and levofloxacin | At 17 months, no evidence of recurrent infection and mild increase in excursion on Lachman test |
| Ng, 201511 | At 7 weeks, with knee pain and swelling, fever, chills, dehiscence of the tibial wound | Turbid fluid | ESR, 71 mm/hr; CRP, 139 mg/L | “Multiple courses of antibiotics” | M. abscessus | 2 | Removal of tibial hardware | 6 weeks of clarithromycin, 2 weeks of cloxacillin | At 14 months after the last surgical intervention, no evidence of recurrent infection and a positive Lachman test |
| Al Farsi, 202110 | At 8 weeks, with septic arthritis | NR | Within normal range | “Oral antibiotics” | M. farcinogenes | “Multiple” | Removal of tibial hardware | 6 months of ciprofloxacin and doxycycline | At 10 months after completion of antibiotic regimen, no evidence of recurrent infection |
| Emerson, 202113 | At 8 weeks, with septic arthritis | Dry tap | ESR, 105 mm/hr; CRP, 3.6 mg/L | Ciprofloxacin, vancomycin | M. abscessus | 2 | Removal of all hardware, tibial tunnel debridement | 3 months of azithromycin, tigecycline, and cefoxitin, then 7 months of azithromycin, clofazimine, and bedaquiline | After completion of antibiotic regimen, the patient continued to progress through standard rehabilitation |
NTM = nontuberculous mycobacteria, I&D = irrigation and debridement (with antimicrobial treatment following the final surgical intervention), and NR = not reported.
Case 1
Oh et al.8 reported on a 15-year-old girl who underwent repair of a bucket-handle meniscal tear and 4-strand hamstring autograft ACL reconstruction. Dehiscence of the tibial wound was seen at 11 weeks postoperatively, and the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level were measured and found to be normal. The patient was started on cephalexin, followed by irrigation and debridement (I&D) and primary closure. There was no evidence of wound communication with the tibial tunnel. Intraoperative cultures revealed no growth, and cephalexin was continued. At 1 week, antibiotics were changed to amoxycillin with clavulanic acid. At 4 weeks, the wound had persistent exudate, and roxithromycin was added. At 4 months postoperatively, magnetic resonance imaging (MRI) showed fluid collection around the tibial screw and tunnel. A second I&D revealed a sinus tract communicating with the tibial tunnel. The polylactic acid and tricalcium phosphate tibial sheath and screw were removed, and a tibial tunnel curettage was performed. After 1 week of management with a negative-pressure dressing, primary closure was performed. M. fortuitum was identified, and the patient was treated with linezolid for 10 weeks and moxifloxacin for 6 months, based on the susceptibility results. At 7 months following the initial surgery, there was no evidence of recurrent infection and the Lachman test was normal.
Case 2
Umprai and Yuktanadana12 reported on a 21-year-old man who underwent ACL reconstruction with a quadrupled hamstring autograft. At 1 year, the patient presented with a painless rubbery mass on the posterolateral aspect of the operatively treated knee. There was no swelling, effusion, or anteroposterior laxity. Needle aspiration yielded no pus or discharge suggestive of infection. MRI demonstrated a high-signal-intensity mass around the posterolateral aspect of the knee that communicated with a high-signal-intensity area around the femoral tunnel fixation. ESR and CRP were described as normal. Arthroscopic debridement and open drainage of the posterolateral mass were performed. The patient was started on cefazolin, gentamicin, and dicloxacillin. Gram staining and culture results from the debridement were negative. The pathology report described necrotic tissue with numerous histiocytes and neutrophils, with a diagnosis of acute necrotizing granulomatous inflammation. Five weeks following the first debridement, the wound demonstrated evidence of recurrent infection. An open debridement was performed with retention of hardware, by choice of the patient. At 6 weeks following the second debridement, M. abscessus was identified on culture, with sensitivity to amikacin and clarithromycin. After discharge, the patient completed a 2-week course of amikacin, moxifloxacin, and clarithromycin followed by a 3-month course of clarithromycin and moxifloxacin. At 6 months following the second debridement, the patient had no evidence of recurrent infection.
Case 3
Grantham et al.9 reported on a 14-year-old boy who underwent a quadrupled autologous hamstring ACL reconstruction. At 3 weeks postoperatively, he presented with constitutional symptoms of fever and abdominal pain and had a normal postoperative knee appearance. Arthrocentesis yielded 11,880 nucleated cells and a negative Gram stain. A blood test revealed a white blood-cell (WBC) count within the normal range (9,900 cells/µL) and elevated ESR (55 mm/hr) and CRP (95.5 mg/L). An arthroscopic I&D yielded no notable findings. An open exploration of the tibial tunnel demonstrated necrotic tissue. The patient was discharged on clindamycin and levofloxacin. The cultures “ultimately” grew M. fortuitum, and the antibiotics were changed to a 6-month course of clarithromycin and levofloxacin. Five months following the I&D, the patient presented with erythema and fluctuance at the tibial incision, normal laboratory values, and an absence of constitutional symptoms. A second I&D was performed with tunnel curettage and removal of all tibial fixation devices. The graft was noted to be intact. Six weeks later, the patient returned with wound drainage and no additional symptoms. MRI showed increased signal at the tibial tunnel and an articular sinus tract. A third I&D, involving repeat curettage of the tibial tunnel, was performed. The tibial tunnel was filled with antibiotic-laden bone graft, and the patient completed a 6-month course of sulfamethoxazole/trimethoprim and levofloxacin. At 17 months, the patient had no recurrent infection; however, a Lachman test revealed a mild increase in excursion compared with the contralateral side.
Case 4
Ng and Yee Han11 reported on a 22-year-old man who underwent “hamstring graft” ACL reconstruction. At 7 weeks postoperatively, the patient presented with pain and swelling of the operatively treated knee, dehiscence of the tibial wound, and fever and chills. Laboratory values were elevated (CPR, 139 mg/L; ESR, 71 mm/hr). Arthrocentesis yielded turbid fluid with no bacterial growth. The patient was started on oral antibiotics and declined surgical debridement. Three months postoperatively, the patient had no clinical improvement and underwent debridement with retention of the graft and tibial fixation devices. A 5-day incubation on blood agar revealed NTM, and subsequent DNA probe testing identified M. abscessus. Based on organism sensitivity testing, the patient received 2 weeks of inpatient amikacin and clarithromycin. Following a gradual decline in CRP and ESR values, the patient was treated with 4 months of clarithromycin and Bactrim (sulfamethoxazole/trimethoprim). Two years later, the patient presented with tibial wound discharge of 6-month duration. Operative investigation showed granulomatous tissue and a sinus tract over the tibial tunnel and attenuation of the ACL graft. All tibial fixation was removed, and initial cultures demonstrated moderate growth of Staphylococcus aureus. The patient received intravenous amikacin and cefoxitin and oral clarithromycin during 17 days of hospitalization. Following discharge, he was treated with clarithromycin for 6 weeks and cloxacillin for 2 weeks. Delayed cultures at 6 weeks were positive for M. abscessus. At 14 months following the last surgery, the patient demonstrated no signs of recurrent infection and had full return to activities, but did have a positive Lachman test.
Case 5
Al Farsi et al.10 reported on a 49-year-old diabetic male animal farm owner who developed an infection following ACL reconstruction. At 8 weeks postoperatively, the patient was diagnosed with septic arthritis and underwent multiple washout procedures and oral antibiotic treatment, without resolution of symptoms. All cultures were negative. Approximately 4 months postoperatively, the patient developed a sinus tract at the proximal aspect of the tibia, and MRI findings suggested tibial osteomyelitis. The tibial screw and ACL graft were removed, and cultures were positive for M. farcinogenes, which was identified by mass spectrometry and confirmed by polymerase chain reaction (PCR). Following drug susceptibility testing, the patient completed a 6-month course of ciprofloxacin and doxycycline. At 10 months after completion of the antimicrobial treatment, there was no indication of recurrent infection.
Case 6
Emerson et al.13 reported on a 67-year-old man who underwent ACL reconstruction with a quadriceps tendon autograft, femoral suspensory fixation, and a polyetheretherketone (PEEK) interference screw for tibial fixation. At 8 weeks, the knee range of motion had not demonstrated improvement and arthroscopic lysis of adhesions and manipulation under anesthesia were performed. Two weeks following the manipulation, the patient developed a fever with swelling and erythema of the operatively treated knee. Arthrocentesis yielded a dry tap, and the patient was given ciprofloxacin and vancomycin for 2 weeks. MRI demonstrated evidence of septic arthritis. Laboratory investigation yielded no leukocytosis, an ESR of 105 mm/hr, and a CRP level of 3.6 mg/L. Two months after the manipulation, the patient underwent open debridement of the tibial tunnel with partial removal of hardware. Cultures were positive for M. abscessus with sensitivity to amikacin and clarithromycin. The patient was started on azithromycin, amikacin, and imipenem and subsequently demonstrated clinical improvement. One month following the open debridement, MRI indicated recurrent infection. A subsequent procedure involving removal of the retained tibial hardware and debridement of the tibial tunnel was performed. The patient completed a 3-month course of azithromycin, during which time the amikacin was switched to tigecycline due to ototoxicity and the imipenem was switched to cefoxitin. Subsequently, the cefoxitin was discontinued due to adverse effects. Two months following this medication change, the patient completed a 7-month course of azithromycin, clofazimine, and bedaquiline. Following cessation of all medications, the patient was progressing through a standard rehabilitation protocol.
Discussion
Infection following ACL reconstruction has been reported in up to 2.6% of cases14. Recent data have indicated increasing recognition of NTM infection in orthopaedic surgery, including after peri-articular injections and in periprosthetic joint infection15,16. NTM infection has been associated with environmental exposure, and water is a common mode of transmission. NTM are intracellular organisms that can replicate within the macrophages of the host17. Importantly, some species of NTM have demonstrated resistance to disinfectants, including 2% aqueous formaldehyde and 2% alkaline glutaraldehyde18-20. Additionally, commonly used surgical antiseptics such as chlorhexidine and povidone-iodine may have inadequate mycobactericidal effects21,22. Although the literature displays the rarity of NTM infection following ACL reconstruction, there is a reasonable expectation of an increasing trend, as all 6 of the cases identified in this review were reported between 2010 and 2021. This finding is also reflective of the reported global trend of increasing NTM infection diagnosis23.
In aggregate, the included case reports display 4 important elements that characterize NTM infection following ACL reconstruction. First, there is a common indolent and atypical course of infection. Across the reviewed articles, initial postoperative presentation occurred between 3 to 52 weeks, which is consistent with reports on the temporal presentation of NTM infection following a variety of surgical interventions24. In 2 of the 6 cases, initial laboratory values were within the normal range and the patient did not demonstrate symptoms suggestive of deep infection8,13. Second, NTM are notoriously difficult to grow and identify in the laboratory. Positive identification of NTM occurred between 3 and 17 weeks after presentation for postoperative SSI8,10-13. In 5 of the 6 cases, the initial cultures (2 arthrocentesis and 3 surgical) failed to identify NTM. Routine cultures are often ineffective at identifying NTM because of the length of time and specific parameters needed for NTM growth25. The difficulty and delay in NTM identification may result in additional procedures and inappropriate medical treatment. All reviewed patients required a minimum of 2 additional interventions following the index ACL reconstruction. Third, the resolution of symptoms required removal of tibial hardware. All 6 patients underwent at least 1 surgical procedure prior to removal of hardware. Fourth, successful management required a prolonged antimicrobial treatment, with 4 of the 6 patients completing ≥6 months of treatment. All patients were initially treated with empirical antibiotics prior to identification of NTM. In totality, these common elements contributed to substantial morbidity resulting from NTM SSI following ACL reconstruction. Notably, there are similarities between NTM and tuberculous mycobacterial infections following ACL reconstruction, including the variable postoperative presentation with unremarkable symptoms and inconclusive laboratory findings26.
Five of the 6 reports provided recommendations for management of NTM infection following ACL reconstruction based on their individual case experiences. Grantham et al.9 suggested a high index of suspicion for infection in patients with persistent elevation of CRP beyond 2 weeks and/or of ESR beyond 1 month. Furthermore, given the variable growth rate of NTM, all cultures should be held for a minimum of 2 weeks. Similarly, Al Farsi et al.10 reported that the primary culture required 7 days to positivity, and removal of prosthetic material played an integral role in achieving resolution. Ng and Yee Han11 noted that a chronic draining sinus at the surgical site with negative cultures should raise suspicion for NTM infection. Oh et al.8 recommended mycobacterial culture for all cases of infection after ACL surgery that had a sinus tract or abscess. Emerson et al.13 recommended early removal of all implants and a prolonged course of antibiotics following positive identification of NTM.
The majority of evidence for management of NTM infection is for pulmonary disease, which represents approximately 90% of NTM infections27. Thus, there is sparse empirical evidence for NTM management in musculoskeletal infection. NTM infections are heterogeneously distributed across the globe, with identifiable geographic trends in clusters of infections28-30. Although debated, the literature demonstrates an increase in the reported incidence of NTM infections across certain regions31,32. However, improved detection capability may heavily contribute to the trend of increasing incidence of NTM infection24.
A hindrance to prompt NTM diagnosis is the laborious nature of NTM culture growth. Most NTM will grow within 2 to 3 weeks, but even rapidly growing species may take up to 7 days2. The preferred method of NTM diagnosis is molecular identification using nucleic acid probes or gene sequencing. Probe assays, which are simpler than gene sequencing, have the capability of identifying a large variety of species but have limitations in differentiating closely related species. Gene sequencing is a more complex and expensive method but provides more discriminatory results. It is recommended that samples be sent to entities with appropriate capability and experience in mycobacterium sequencing33. An emerging method of NTM identification is matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS), which has demonstrated clinical efficacy without substantial financial burden34,35.
Treatment of M. abscessus using a macrolide has been recommended (with the supporting evidence rated as strong), with a conditional preference for azithromycin over clarithromycin because of its lower risk of drug interactions and less frequent dosing regimen36. M. abscessus can develop macrolide resistance through mutations (in the erm gene) and is generally more resistant to antibiotics than other species33. Mutations of the erm gene alter the macrolide binding site, which renders the antibiotic ineffective. Furthermore, other commonly administered antibiotics demonstrate little bactericidal activity in M. abscessus strains37. Therefore, susceptibility testing is especially critical for M. abscessus diagnoses. The multi-drug recommendations for NTM treatment amplify the importance of susceptibility testing because of the variable pattern of resistance that characterizes NTM36. The duration of medical treatment for NTM infections is 4 to 6 months, depending on severity of disease and species27,38. Six months or more may be required in patients with osseous involvement2,39.
Based on the aggregate literature, we recommend removal of local implants if the infection is localized and without articular involvement. However, in cases of articular involvement, all fixation and graft material should be removed. These steps are critical and potentially essential for infection resolution.
Limitations of the present study include the small sample of cases, which is indicative of the rarity of NTM infection following ACL reconstruction. It is reasonable to expect that these infections may be more common than was indicated by our literature review, as NTM cases are not systematically reported within local jurisdictions or global databases. Additionally, there are limitations that are inherent to systematic reviews, namely the reliance on the quality of reporting in the published literature. The current study presents an aggregation of details regarding NTM infection following ACL reconstruction. Although statistical analysis was not feasible, the commonalities among cases provide understanding regarding the identification of NTM and recommendations for subsequent management.
Conclusions
The aggregate literature displays common elements that characterize NTM infection following ACL reconstruction. The common indolent course of infection hinders prompt diagnosis and treatment. Organism susceptibility testing is crucial for effective treatment and limiting unnecessary antibiotic exposure. Resolution may require complete hardware removal. In totality, these elements contribute to substantial morbidity resulting from NTM infection following ACL reconstruction.
Note
The authors appreciate the continued support of Amy and Steve Heifner.
Footnotes
Investigation performed at the Department of Orthopaedic Surgery, Larkin Hospital, Coral Gables, Florida
Disclosure: The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJSOA/A511).
References
- 1.Brode SK, Daley CL, Marras TK. The epidemiologic relationship between tuberculosis and non-tuberculous mycobacterial disease: a systematic review. Int J Tuberc Lung Dis. 2014 Nov;18(11):1370-7. [DOI] [PubMed] [Google Scholar]
- 2.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ, Jr, Winthrop K; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007 Feb 15;175(4):367-416. [DOI] [PubMed] [Google Scholar]
- 3.Johnson MM, Odell JA. Nontuberculous mycobacterial pulmonary infections. J Thorac Dis. 2014 Mar;6(3):210-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devulder G, de Montclos MP, Flandrois JP. A multigene approach to phylogenetic analysis using the genus Mycobacterium as a model. Int J Syst Evol Microbiol. 2005 Jan;55(Pt 1):293-302. [DOI] [PubMed] [Google Scholar]
- 5.Fedrizzi T, Meehan CJ, Grottola A, Giacobazzi E, Fregni Serpini G, Tagliazucchi S, Fabio A, Bettua C, Bertorelli R, De Sanctis V, Rumpianesi F, Pecorari M, Jousson O, Tortoli E, Segata N. Genomic characterization of Nontuberculous Mycobacteria. Sci Rep. 2017 Mar 27;7:45258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marom N, Kapadia M, Nguyen JT, Ammerman B, Boyle C, Wolfe I, Halvorsen KC, Miller AO, Henry MW, Brause BD, Hannafin JA, Marx RG, Ranawat AS. Factors Associated With an Intra-articular Infection After Anterior Cruciate Ligament Reconstruction: A Large Single-Institution Cohort Study. Am J Sports Med. 2022 Apr;50(5):1229-36. [DOI] [PubMed] [Google Scholar]
- 7.Eisenberg MT, Block AM, Vopat ML, Olsen MA, Nepple JJ. Rates of Infection After ACL Reconstruction in Pediatric and Adolescent Patients: A MarketScan Database Study of 44,501 Patients. J Pediatr Orthop. 2022 Apr 1;42(4):e362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oh HL, Chen DB, Seeto BG, Macdessi SJ. Mycobacterium fortuitum infection after anterior cruciate ligament reconstruction using a polylactic acid bioabsorbable screw: Case report. Knee. 2010 Mar;17(2):176-8. [DOI] [PubMed] [Google Scholar]
- 9.Grantham WJ, Raynor MB, Martus JE. Articular Sinus Tract with Mycobacterium fortuitum Osteomyelitis After Anterior Cruciate Ligament Reconstruction: A Case Report. JBJS Case Connect. 2015 Oct-Dec;5(4):e105. [DOI] [PubMed] [Google Scholar]
- 10.Al Farsi F, Al Adawi B, Ba Tahir H, Al Mutani M, Adikaram C, Al Siyabi T, Al Busaidi I. Mycobacterium farcinogenes osteomyelitis of the proximal tibia: A case report. IDCases. 2021 Jun 15;25:e01194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng SW, Yee Han DL. Lessons learnt from an atypical mycobacterium infection post-anterior cruciate ligament reconstruction. Clin Orthop Surg. 2015 Mar;7(1):135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Umprai V, Yuktanadana P. Non-tuberculous Mycobacterial Infection (Mycobacterium Abscessus) after Anterior Cruciate Ligament Reconstruction: A case report. JseaOrtho. 2014;38(3-4):77-83. [Google Scholar]
- 13.Emerson CP, Brecount H, Quintero D, Greif DN, Jose J, Kaplan LD. Mycobacterium abscessus subsp. massiliense infection secondary to traumatic anterior cruciate ligament reconstruction surgery. Brazilian Journal of Case Reports. 2021;01(4):22-30. [Google Scholar]
- 14.Judd D, Bottoni C, Kim D, Burke M, Hooker S. Infections following arthroscopic anterior cruciate ligament reconstruction. Arthroscopy. 2006 Apr;22(4):375-84. [DOI] [PubMed] [Google Scholar]
- 15.Peng SH, Lee SH, Chen CC, Lin YC, Chang Y, Hsieh PH, Shih HN, Ueng SWN, Chang CH. Nontuberculous mycobacteria peri-prosthetic joint infection: An outcome analysis for two stage revision arthroplasty. J Orthop Surg (Hong Kong). 2022 Sep-Dec;30(3):10225536221140610. [DOI] [PubMed] [Google Scholar]
- 16.Lee BI, Jang BW, Choi HS, Lee JS, Kim YB. Arthroscopic treatment of Mycobacterium massiliense septic arthritis outbreak after intra-articular injection: A case-series report and literature review. Medicine (Baltimore). 2021 Feb 5;100(5):e23839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shamaei M, Mirsaeidi M. Nontuberculous Mycobacteria, Macrophages, and Host Innate Immune Response. Infect Immun. 2021 Jul 15;89(8):e0081220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallace RJ, Jr, Brown BA, Griffith DE. Nosocomial outbreaks/pseudo-outbreaks caused by nontuberculous mycobacteria. Annu Rev Microbiol. 1998;52:453-90. [DOI] [PubMed] [Google Scholar]
- 19.Soto LE, Bobadilla M, Villalobos Y, Sifuentes J, Avelar J, Arrieta M, Ponce de Leon S. Post-surgical nasal cellulitis outbreak due to Mycobacterium chelonae. J Hosp Infect. 1991 Oct;19(2):99-106. [DOI] [PubMed] [Google Scholar]
- 20.Carson LA, Petersen NJ, Favero MS, Aguero SM. Growth characteristics of atypical mycobacteria in water and their comparative resistance to disinfectants. Appl Environ Microbiol. 1978 Dec;36(6):839-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee YH, Kim HK, Kim MS, You HJ, Kim DW, Lee TY. Chemical Sterilization of Lipoplasty Cannula and Nontuberculous Mycobacteria Disinfection: An Experimental Study. J Craniofac Surg. 2022 Mar-Apr 01;33(2):719-22. [DOI] [PubMed] [Google Scholar]
- 22.Cheng A, Sun HY, Tsai YT, Wu UI, Chuang YC, Wang JT, Sheng WH, Hsueh PR, Chen YC, Chang SC. In Vitro Evaluation of Povidone-Iodine and Chlorhexidine against Outbreak and Nonoutbreak Strains of Mycobacterium abscessus Using Standard Quantitative Suspension and Carrier Testing. Antimicrob Agents Chemother. 2017 Dec 21;62(1):e01364-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abubakar I, Gupta RK, Rangaka MX, Lipman M. Update in Tuberculosis and Nontuberculous Mycobacteria 2017. Am J Respir Crit Care Med. 2018 May 15;197(10):1248-53. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein N, St Clair JB, Kasperbauer SH, Daley CL, Lindeque B. Nontuberculous Mycobacterial Musculoskeletal Infection Cases from a Tertiary Referral Center, Colorado, USA. Emerg Infect Dis. 2019 Jun;25(6):1075-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stephenson D, Perry A, Appleby MR, Lee D, Davison J, Johnston A, Jones AL, Nelson A, Bourke SJ, Thomas MF, De Soyza A, Lordan JL, Lumb J, Robb AE, Samuel JR, Walton KE, Perry JD. An evaluation of methods for the isolation of nontuberculous mycobacteria from patients with cystic fibrosis, bronchiectasis and patients assessed for lung transplantation. BMC Pulm Med. 2019 Jan 21;19(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nag HL, Neogi DS, Nataraj AR, Kumar VA, Yadav CS, Singh U. Tubercular infection after arthroscopic anterior cruciate ligament reconstruction. Arthroscopy. 2009 Feb;25(2):131-6. [DOI] [PubMed] [Google Scholar]
- 27.Piersimoni C, Scarparo C. Extrapulmonary infections associated with nontuberculous mycobacteria in immunocompetent persons. Emerg Infect Dis. 2009 Sep;15(9):1351-8, quiz 1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baldwin SL, Larsen SE, Ordway D, Cassell G, Coler RN. The complexities and challenges of preventing and treating nontuberculous mycobacterial diseases. PLoS Negl Trop Dis. 2019 Feb 14;13(2):e0007083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buser GL, Laidler MR, Cassidy PM, Moulton-Meissner H, Beldavs ZG, Cieslak PR. Outbreak of Nontuberculous Mycobacteria Joint Prosthesis Infections, Oregon, USA. Emerg Infect Dis. 2019 May;25(5):849-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou L, Xu D, Liu H, Wan K, Wang R, Yang Z. Trends in the Prevalence and Antibiotic Resistance of Non-tuberculous Mycobacteria in Mainland China, 2000-2019: Systematic Review and Meta-Analysis. Front Public Health. 2020 Jul 28;8:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schildkraut JA Gallagher J Morimoto K Lange C Haworth C Floto RA Hoefsloot W Griffith DE Wagner D Ingen JV; NTM-NET. Epidemiology of nontuberculous mycobacterial pulmonary disease in Europe and Japan by Delphi estimation. Respir Med. 2020 Nov;173:106164. [DOI] [PubMed] [Google Scholar]
- 32.Griffith DE, Marras TK. Nontuberculous Mycobacterial Disease Epidemiology: You Can See the Stars and Still Not See the Light. Clin Infect Dis. 2021 Jul 15;73(2):e327-9. [DOI] [PubMed] [Google Scholar]
- 33.Brown-Elliott BA, Philley JV. Rapidly Growing Mycobacteria. Microbiol Spectr. 2017 Jan;5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez-Temporal D, Alcaide F, Mareković I, O’Connor JA, Gorton R, van Ingen J, Van den Bossche A, Héry-Arnaud G, Beauruelle C, Orth-Höller D, Palacios-Gutiérrez JJ, Tudó G, Bou G, Ceyssens PJ, Garrigó M, González-Martin J, Greub G, Hrabak J, Ingebretsen A, Mediavilla-Gradolph MC, Oviaño M, Palop B, Pranada AB, Quiroga L, Ruiz-Serrano MJ, Rodríguez-Sánchez B. Multicentre study on the reproducibility of MALDI-TOF MS for nontuberculous mycobacteria identification. Sci Rep. 2022 Jan 24;12(1):1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tran A, Alby K, Kerr A, Jones M, Gilligan PH. Cost Savings Realized by Implementation of Routine Microbiological Identification by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry. J Clin Microbiol. 2015 Aug;53(8):2473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ, Jr, Andrejak C, Böttger EC, Brozek J, Griffith DE, Guglielmetti L, Huitt GA, Knight SL, Leitman P, Marras TK, Olivier KN, Santin M, Stout JE, Tortoli E, van Ingen J, Wagner D, Winthrop KL. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J. 2020 Jul 7;56(1):2000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maurer FP, Bruderer VL, Ritter C, Castelberg C, Bloemberg GV, Böttger EC. Lack of antimicrobial bactericidal activity in Mycobacterium abscessus. Antimicrob Agents Chemother. 2014 Jul;58(7):3828-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wallace RJ, Jr, Swenson JM, Silcox VA, Bullen MG. Treatment of nonpulmonary infections due to Mycobacterium fortuitum and Mycobacterium chelonei on the basis of in vitro susceptibilities. J Infect Dis. 1985 Sep;152(3):500-14. [DOI] [PubMed] [Google Scholar]
- 39.Kasperbauer S, Huitt G. Management of extrapulmonary nontuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013 Feb;34(1):143-50 [DOI] [PubMed] [Google Scholar]