Abstract
The perfluorosulfonic acid (PFSA) proton exchange membrane (PEM) is the key component for hydrogen fuel cells (FCs). We used in situ synchrotron scattering to investigate the PEM morphology evolution and found a “stream-reservoir” morphology, which enables efficient proton transport. The short-side-chain (SSC) PFSA PEM is fabricated under the guidance of morphology optimization, which delivered a proton conductivity of 193 milliSiemens per centimeter [95% relativity humidity (RH)] and 40 milliSiemens per centimeter (40% RH) at 80°C. The improved glass transition temperature, water permeability, and mechanical strength enable high-temperature low-humidity FC applications. Performance improvement by 82.3% at 110°C and 25% RH is obtained for SSC-PFSA PEM FCs compared to Nafion polymer PEM devices. The insights in chain conformation, packing mechanism, crystallization, and phase separation of PFSAs build up the structure-property relationship. In addition, SSC-PFSA PEM is ideal for high-temperature low-humidity FCs that are needed urgently for high-power-density and heavy-duty applications.
The SSC-PFSA PEM with hierarchical morphology is developed and successfully used in high-temperature low-humidity fuel cells.
INTRODUCTION
The polymer electrolytes have been widely used as the ionic conducting membrane to enable energy applications in lithium ion batteries and hydrogen fuel cells (FCs) (1–3). The state-of-the-art Nafion perfluorosulfonic acid (PFSA) polymer displays high proton mobility and good stability owing to its fully fluorinated chemical structure and is widely used in proton exchange membrane (PEM) FCs. Despite the successes, the requirement of higher power density, longer lifetime, and more compact device calls for better PEM that can undergo higher operation temperature and afford faster ion mobility (4–8). To address these necessities, PFSA polymer with higher ion exchange capacity (IEC) and better thermal stability is key.
We shift our focus from Nafion polymers to short-side-chain PFSA (SSC-PFSA) polymer using perfluoro-2-sulfonic acid ethoxy ion-conducting side chains. This fundamental change in chemical structure leads to a higher weight ratio of sulfonic acid moiety in PFSA, and the IEC value can be elevated to a high level (9). However, SSC-PFSA PEM in FC is less studied, which is due to the difficulty in obtaining the high–molecular weight polymer and the complexity in PEM fabrication and morphology optimization. The key issue in making high-performance PEMs is to manage the nanomorphology of the ion-conducting channel, which is a challenging task because multiple interchain and intrachain interactions need to be balanced for polymer electrolyte in solution and membrane (10–12). It is known that Nafion polymer assembles into rigid coil in solution and forms interconnecting micelle ion-conducting channels in membrane (13, 14). The high sulfonic acid content in SSC-PFSA can result in different chain solubility and assembly behaviors. The polymer chain assembly and electrostatic aggregation in solution need to be well controlled and transformed into the ionic conducting channel phase. Preliminary results suggest that water/alcohol solvent mixture can be an advantageous system to better balance the intra- and intermolecular interactions, which are thus applied in our study to optimize the bicontinuous ionic channel morphology.
To better understand the morphology control mechanism, in situ grazing incidence wide-angle/small-angle x-ray scattering (GIWAXS/GISAXS) methods are applied to probe the solution behavior and structure transformation from solution to film. A rod micelle chain assembly with polytetrafluoroethylene (PTFE) as backbone and pendant side chains arranged outside was identified, along with a hierarchical assembly upscales. The PFSA polymer chain crystallization and ionic channel formation occur in a small length scale, which is accompanied by a larger size phase separation to induce interconnected ionic channels in SSC-PFSA PEM. A “stream-reservoir” ionic channel morphology is constructed, which is integrated into a PFSA crystalline phase matrix. The drying material depletion led to shrink-sized nanopores with sulfonic acid walls to enhance mass water permeability and proton conductivity. This morphology results in high ion transport capability and excellent moisture permeability, which, together with the high glass transition temperature (Tg) of SSC-PFSA, afford high-temperature low-humidity FC application. The excellent proton conductivity of 193 mS/cm at 95% relativity humidity (RH) and 40 mS/cm at 40% RH is achieved, and superior mechanical strength is recorded. High-temperature low-humidity FC devices delivered a power density of 0.279 W/cm2 (at 0.9 A/cm2) under 110°C and 25% RH condition, which is an 82.3% increase compared to Nafion polymer PEM FCs. Besides, improved operation stability is recorded under the open circuit voltage (OCV) durability test. These advances originate from improved ion moiety density, higher glass transition temperature (Tg), superior morphology, and lower transport activation energy of SSC-PFSA, which serves as an ideal material platform for high-temperature low-humidity and high-power-density FCs.
RESULTS
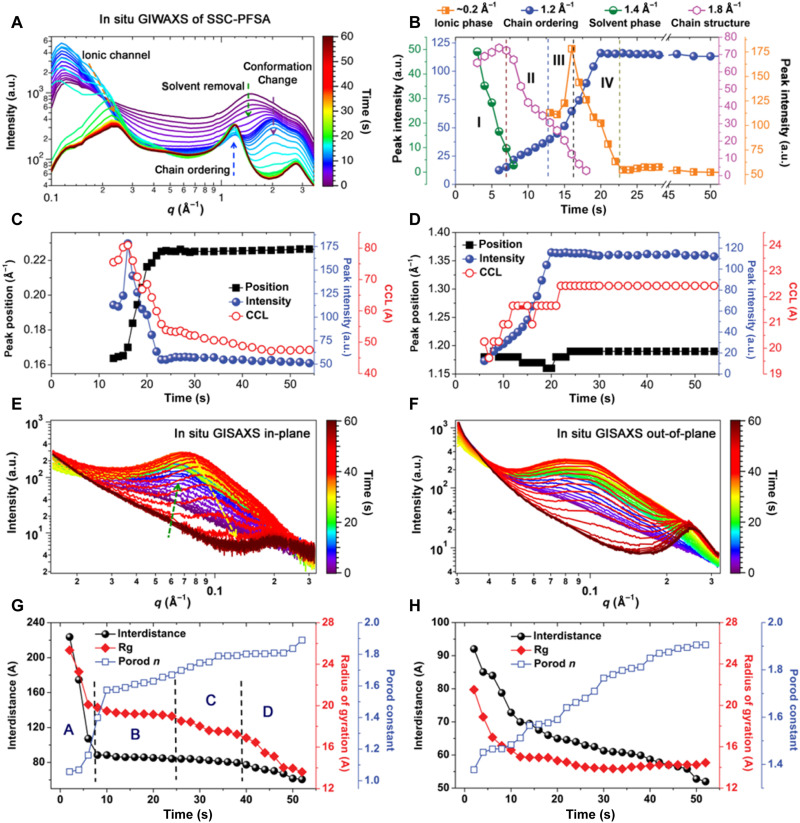
The polymer chain assembly process affords the most valuable information to understand the morphology of PEMs since the formation of ionic channels is key in determining PEM performance. We carried out in situ GIWAXS/GISAXS experiments using a synchrotron scattering beamline that is equipped with a mini slot die coater to allow PEM coating with simultaneous x-ray scattering signal collection. The SSC-PFSA polymer was dissolved in water/ethanol mixture (1:1 volume ratio) in 5 wt % concentration, and in situ experiments were done in a coating and probing manner. Figure 1A shows the in situ GIWAXS profiles, where the distinctive structure transformation is observed. A few key peak series can be clearly noted from the two-dimensional (2D) GIWAXS patterns selected at different time points (fig. S1). The 1.4-Å−1 diffraction peak represents the solvent content. The peak (1.8 to 2.0 Å−1) shows the PFSA chain conformation change and reorganization. The 1.2-Å−1 peak is associated with chain ordering, which is close to that in PTFE polymer (15). The 0.2-Å−1 peak represents the formation of close-packed ionic phase (16, 17). The detailed morphology evolution covers the transition of these major structure changes, as shown in Fig. 1B. We separate the morphology evolution into four main stages. In stage I, the solvent vaporizes quickly, which leads to the growth and saturation of the 1.8-Å−1 diffraction peak. For water-ethanol/solvent mixture, ethanol is expected to leave quicker, resulting in the solubility parameter change (18, 19), and the polymer chain conformation changes, leading to the formation of a helical perfluorochain conformation similar to PTFE structure features (20, 21). In addition, the 1.8-Å−1 diffraction peak originates from the intrachain F-F distance. In stages II and III, a continuous decrease of the 1.8-Å−1 peak is seen, indicating a structure transformation. The trend is associated with the increased intensity of the 1.2-Å−1 chain-ordering peak. Thus, it is expected that polymer chains get closer and assembled, and the specific F-F in-chain correlation is disturbed by neighboring F atoms. Such a process takes place in acceleration during film drying, which is commonly seen as the nucleation and growth in polymer crystallization (22). In the intermediate of the chain-ordering process (stage III), the 0.2-Å−1 peak appears with maximum intensity, and then its intensity decreases in stage IV. This peak corresponds to the assembly of SSC-PFSA primitive assemblies, and it contains a fraction of solvent initially to afford a large scattering volume cross section and thus a high intensity. Further removal of solvent results in a closer packing of these assemblies, and thus, the packing distance and scattering intensity reduce simultaneously. In the end, a stabilized membrane with fixed ordering (1.2-Å−1 peak) and ionic channel packing (0.2-Å−1 peak) is achieved. The detailed peak position, intensity, and crystalline coherence length [CCL; calculated by Scherrer’s analysis (23)] of 0.2- and 1.2-Å−1 peaks are summarized in Fig. 1 (C and D). The comparison of the intensity profiles with respect to time scales features that the ionic phase formation is an after-chain-ordering process. In addition, the CCL of 0.2-Å−1 peak is about twice to that of 1.2-Å−1 peak, revealing the chain bundle assembly (primitive assembly) toward the smaller-sized ion-conducting channels.
Fig. 1. In situ GIWAXS and GISAXS profiles of SSC-PFSA with the fitting results.
(A) In situ GIWAXS profiles for the SSC-PFSA film formation process. (B) Diffraction peaks of critical structure formations. Evolution processes of (C) ionic phase diffraction peak (~0.2 Å−1) and (D) chain-ordering peak (1.2 Å−1). In situ GISAXS profiles of SSC-PFSA film in (E) in-plane (IP) and (F) out-of-plane (OOP) directions. Guinier-Porod fitting plots of GISAXS profiles in (G) IP and (H) OOP directions. a.u., arbitrary units.
In situ GISAXS was used to probe the phase separation of SSC-PFSA during membrane formation. The 2D GISAXS patterns selected at different time points are shown in fig. S2, and the in-plane (IP) and out-of-plane (OOP) scattering profiles are extracted and shown in Fig. 1 (E and F). The initial IP GISAXS profiles show a global decay with a small hump around 0.05 Å−1, which is ascribed to the presence of SSC-PFSA chain assembly. The evaporation of solvent induces the development of a broad peak at around 0.06 Å−1 (Fig. 1E), whose intensity first increases, then decreases, and gradually shifts toward the higher q region. This peak indicates the formation of a defined nano-sized phase separation in SSC-PFSA PEM. The decrease and shift of the scattering peak indicate that this ionic channel get closer, which then disappear when the solvent is fully removed. The peak located around 0.2 Å−1 originates from the primitive PFSA assembly that defines the small-sized ionic channel, which appears in the late state of membrane formation and is in good agreement with in situ GIWAXS results. The 0.06 Å−1 is ascribed to a larger-sized ionic channel, which is formed due to the hydrophilic-hydrophobic phase separation. The OOP GISAXS shows a similar trend with IP GISAXS (Fig. 1F). However, a much more obvious and closer-packed ionic phase peak (0.2 Å−1) is seen, which is due to a more tightly packed nanostructure in the surface layer. Guinier-Porod fitting with interference model was applied to analyze the in situ GISAXS results (Fig. 1, G and H). On the basis of these changes, four stages can be rationally defined. Stage A is associated with initial solvent removal and the formation of SSC-PFSA assembly, which is in accordance with stage I in GIWAXS. Stage B summarizes the II, III, and IV stages of GIWAXS, where the interdistance stabilizes at around 9 nm in IP and reduces in OOP, indicating the vertical shrinkage condensation process. This stage (B) is associated with chain-ordering and small-sized ionic channel formation. Stages C and D reveal the internal structure reorganization (in IP and OOP) due to the removal of residual water. Note that the distance of small-sized ionic channel (0.2-Å−1 peak) remains stable, and the large ionic channel (0.06-Å−1 peak) reduces in this region. Thus, the large ionic channel can better host water in membrane. In analytical results, the radius of gyration (Rg) shows a quick drop in stage A and then reduces in small values in B and C. The Porod constant (n) is in-between 1 and 1.8 during membrane drying. These results suggest that SSC-PFSA PEM formation is a rod-to-mass fractal transition, and the condensed membrane is a mesoporous structure that could be effectively swelled by water and transport protons.
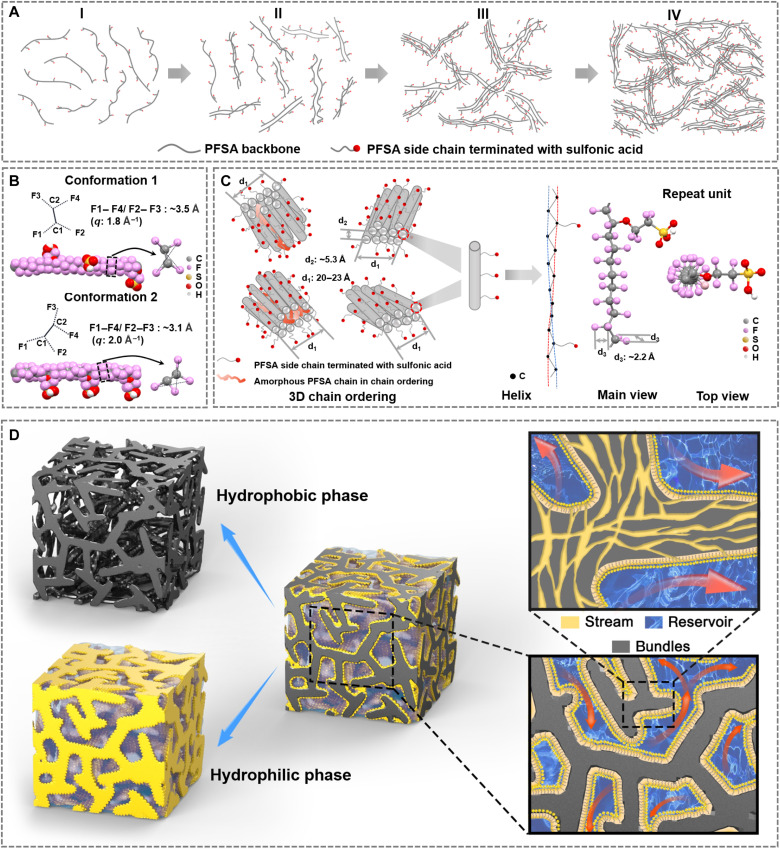
The in situ GIWAXS/GISAXS results afford a clear picture of the SSC-PFSA membrane morphology formation. A four-stage assembly process is developed and schematically illustrated in Fig. 2A. The PFSA chain conformation change and ordering are shown in Fig. 2 (B and C). On the basis of the PTFE backbone structure and sulfonic acid side chains, the twisted polymer chain assembly is probably the key structure formed in stage I, where the sulfonic acid group would distribute randomly outward to provide solubility. The solution concentration is moderate (5 wt % initially), and quick solvent evaporation leads to electrostatic induced weak aggregations. These aggregation displays a size and interdistance as that visualized by GISAXS (0.06-Å−1 peak). Further solvent removal leads to a stabilized phase separation and the ordering of PFSA chains, which is then followed by the formation of the 0.2-Å−1 ionic channel peak. Thus, large-scaled phase separation and the small-sized ionic channel formation take place in a similar time window. The SSC-PFSA would take a twisted conformation as shown in Fig. 2B. In addition, the interchain ordering would be in a chain-chain packing via hydrophobic interactions (Fig. 2C) that bilayer assembly (primitive assembly) takes place (24). These bilayer assemblies would then assemble to form the 0.2-Å−1 ionic channel. The real-space morphology of SSC-PFSA PEM is investigated by transmission electron microscopy (TEM). The well-defined polymer assembly (light) and ionic phase (dark, stained) are observed in the cross section (OOP) and lateral plane (IP) TEM images, and a major phase separation ~15 nm is observed with blurred polymer phase image (fig. S3) (25, 26). Note that the feature size for SSC-PFSA PEM is larger than that in Nafion polymer PEM; thus, improved transport is expected. A zoom-in view of the SSC-PFSA polymer phase reveals the smaller phase separation around 2 to 3 nm (fig. S4), which images the small-sized ionic channels. The combination of in situ scattering and TEM results suggested a 3D hierarchical morphology for SSC-PFSA PEM, and a stream-reservoir model is proposed (Fig. 2D). The “stream” is the small-sized ionic channel of primitive assembles with a typical distance of about 2 to 3 nm, which is buried in polymer bundle phase. The “reservoir” is the large-sized voids induced by phase separation with a typical feature size of ~10 nm when water swelled. The streams and reservoirs are interconnected to the phase separation mechanism and form a 3D interconnected structure to facilitate proton transport. Both streams and reservoirs can absorb water moisture quickly, and under high moisture condition, the reservoirs can expand their size to host extra water. The material crystallization acts as a physical cross-linking center to prohibit overswelling to maintain mechanical strength. These features can be expected by looking into the in situ GIWAXS/GISAXS results reversely.
Fig. 2. Nanostructure of SSC-PFSA.
(A) Four-step PFSA chain self-assembly during solvent evaporation. (B) Chain conformation changes (q of 1.8 to 2.0 Å−1) during solvent evaporation and (C) chain orderings. (D) Three-dimensional scheme of the SSC-PFSA membrane structure: The gray area represents hydrophobic phase, and the yellow area represents hydrophilic phase.
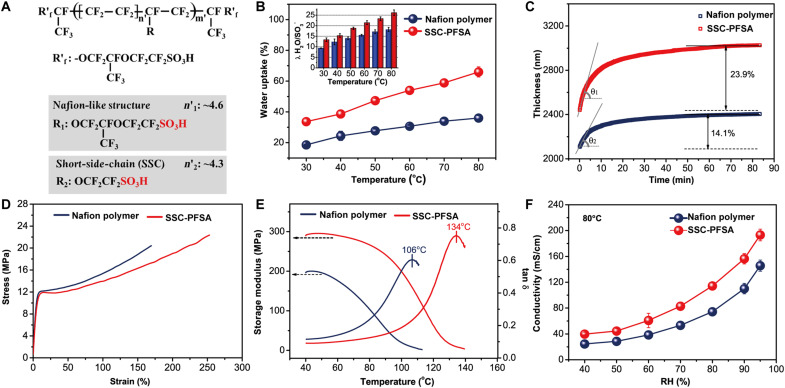
The physical properties of SSC-PFSA are systematically studied, and compared with Nafion polymer, Fig. 3A shows the chemical structure of perfluoro-2-(2-sulfonic acid ethoxy) propoxy end-capped Nafion polymer and SSC-PFSA in this study. The water uptake of SSC-PFSA membranes at different temperatures is shown in Fig. 3B. Much higher water uptake is evident for the SSC-PFSA membrane, which is due to the higher ionic content and better nanostructure. The dynamic water moisture swelling process of SSC-PFSA membrane was measured through in situ membrane thickness change by an optical profile meter (Fig. 3C). The SSC-PFSA membrane demonstrates a swelling ratio of 23.9%, which is much higher than that of the Nafion polymer membrane (14.1%). A moisturizing rate of 78.6 nm/min is obtained for the SSC-PFSA membrane, which is 93% larger than that of the Nafion polymer membrane (40.8 nm/min). The quick moisturizing in SSC-PFSA membrane can be an important advantage, indicating a better water-absorbing ability. This improvement can be traced back to the hierarchical morphology of SSC-PFSA membrane. The mechanical properties of SSC-PFSA membrane are further studied (Fig. 3D). The SSC-PFSA membrane shows a tensile strength of 22.4 MPa, which is 9.8% higher compared to that of the Nafion polymer membrane (20.4 MPa). Dynamic mechanical analysis (DMA) is used to investigate the material dynamic properties. A much higher storage modulus is seen for SSC-PFSA, which indicates a better elastic property of SSC-PFSA membrane and better capability to host external deformation (Fig. 3E). The tanδ in DMA is related to the α relaxation, which is commonly used to determine the glass transition temperature (Tg) for membranes. The SSC-PFSA shows a Tα of 134°C, which is much higher than that of the Nafion polymer (106°C). The improvement in mechanical properties in SSC-PFSA membrane can be attributed to the high–molecular weight and the strong interchain crystallization and the stronger electrostatic interactions within the ionic aggregates, which serve as cross-links to suppress the long-range movement of chain segments (27, 28).
Fig. 3. Chemical structures and physical properties.
(A) Chemical structure of PFSA ionomers. (B) Water uptake and corresponding water content (the inset) of PFSA membranes. (C) Swelling ratio of PFSA membranes (~2 μm) in the thickness direction at room temperature with saturated water vapor environment. (D) Stress-strain curves of membranes at 23 ± 2°C with 50% RH. (E) Dynamic mechanical analysis spectra of PFSA membranes. (F) Proton conductivity of PFSA membranes at 80°C with different RH values.
The proton conductivity of the Nafion polymer membrane and SSC-PFSA membrane is measured in IP direction at 80°C under different RH (40 to 95%) using a two-probe ac impedance method (Fig. 3F). The proton conductivities of the Nafion polymer membrane and SSC-PFSA membrane both increase upon the humidity increasing, and the conductivity of SSC-PFSA membrane is apparently higher compared to Nafion polymer membrane at all testing conditions. The highest proton conductivity of the SSC-PFSA membrane reaches 193 mS/cm at 95% RH, which is among the highest values for PFSA PEM to the best of our knowledge. Even at a low 40% RH, a conductivity of 40 mS/cm is obtained, which is still high enough for FC applications. The higher proton conductivity at high temperatures and lower humidity condition, as well as the higher thermal-mechanical property, indicates the potential use of SSC-PFSA membrane in heavy-duty FCs. The charge (proton) transfer resistance (Rct) for SSC-PFSA membrane decreases as the humidity increases, as evidenced by the Nyquist plots (figs. S5 and S6). The proton conductivity of PFSA membrane is also measured at different temperatures in water (fig. S7A), which shows that SSC-PFSA exhibits a 30 to 40% higher proton conductivity than Nafion polymer between 30° and 80°C. The activation energy (Ea) of proton transport is calculated using the conductivity-temperature measurement (fig. S7B), which is found to be 0.069 eV for SSC-PFSA PEM and 0.084 eV for Nafion polymer PEM. According to the Ea values, both the SSC-PFSA and Nafion polymer PEMs follow the Grotthuss proton transport mechanism (29). The 17.8% lower activation energy for SSC-PFSA guarantees high mobility over wide temperature and humidity conditions (30, 31). Such a result is attributed to the hierarchical proton transport pathway and high IEC of SSC-PFSA, where protons can find better connected multiple channels to jump over, achieving a high proton conductivity.
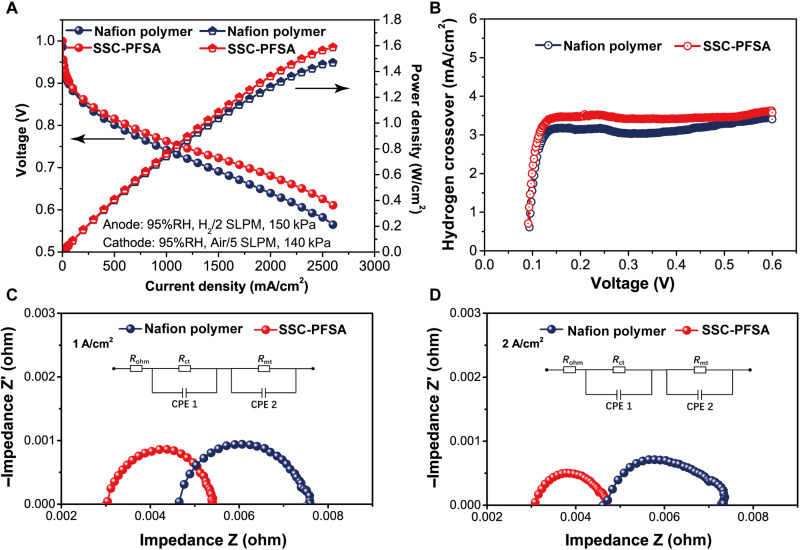
The performance of SSC-PFSA PEM is evaluated in a single-stack FC device, using Nafion polymer PEM for comparison. The process of preparing membrane electrode assemblies (MEAs) and testing of FC are shown in fig. S8. Under conventional operation condition (80°C, 95% RH), the SSC-PFSA MEA exhibits a similar performance with the Nafion polymer MEA when the current density is below 700 mA/cm2. However, in higher current density region, SSC-PFSA MEA displays improved performances with reduced resistance. The SSC-PFSA MEA shows a power density of 1.588 W/cm2 at 2600 mA/cm2, which is higher than that of the Nafion polymer MEA (1.469 at 2600 mA/cm2) by 8.1% (Fig. 4A). The enhanced power output of SSC-PFSA MEA highlights the advantage of SSC-PFSA PEM. The improvement in power density is benefited from the improved proton conductivity and the better water and heat management of the SSC-PFSA PEM. The improved water uptake and thermal and mechanical stability are less sensitive to electrode dehydration or flooding, shrinkage, and cracking, making the SSC-PFSA PEM a better choice for the high-power-density FCs.
Fig. 4. FC properties at 80°C.
(A) Polarization curves of PFSA membranes at 80°C and 95% RH under H2/air. (B) Hydrogen crossover current in MEAs with LSV of the membranes at 80°C and 95% RH without back pressure. EIS measurement: Nyquist plots of the MEAs operated in (C) 1 A/cm2 and (D) 2 A/cm2 at 80°C and 95% RH. The equivalent circuit models are shown the inset of (C) and (D). SLPM, standard liters per minute.
The hydrogen crossover of the PEMs at 80°C is investigated by using the linear sweep voltammetry (LSV) method with a scanning range from 0 to 0.6 V. As shown in Fig. 4B, the SSC-PFSA MEA hydrogen crossover is 3.41 mA/cm2 without back pressure, which is slightly larger than that of the Nafion polymer MEA (3.10 mA/cm2). This character is due to the high porosity and high water uptake of SSC-PFSA PEM (6, 32). It should be mentioned that the increase of hydrogen crossover value in percentage (10%) is much smaller compared with the IEC increase for SSC-PFSA compared to Nafion polymer (27%). Thus, the hierarchical ionic channels could decouple the proton conductivity with gas permeability, which is an unexpected advantage in FC operation. Electrochemical impedance spectroscopy (EIS) measurements are used to evaluate the resistance of MEAs under 1 A/cm2 (Fig. 4C) and 2 A/cm2 (Fig. 4D) testing conditions. The resistance components for MEAs are listed in table S1. The ohmic resistance (Rohm) for SSC-PFSA MEA is 3.02 milliohms at 1 A/cm2, which is lower than that for Nafion polymer MEA (4.63 milliohms at 1 A/cm2). A similar trend is kept at 2 A/cm2. The Rct values for SSC-PFSA MEA are smaller than the Nafion polymer MEA at both 1 and 2 A/cm2, which indicates a more efficient oxygen reduction reaction in the SSC-PFSA MEA (33, 34). The mass transfer resistance (Rmt) is 0.0031 milliohms for the SSC-PFSA MEA at 1 A/cm2, which is only 4.8% of the Nafion polymer MEA. It is benefited from the markedly improved water management in SSC-PFSA PEM, which reduces water accumulation and facilitates water back diffusion through the hierarchical ionic phase. At higher current density (2 A/cm2), the Rmt for SSC-PFSA MEA is estimated to be 0.0737 milliohms, which is only 58% of the Nafion polymer MEA value. Considering the same thickness of the Nafion polymer and SSC-PFSA PEMs in FC devices, the difference in water transportation for the two cases is mainly caused by the improved ionic phase morphology (stream-reservoir morphology) and water uptake capability of the SSC-PFSA PEM.
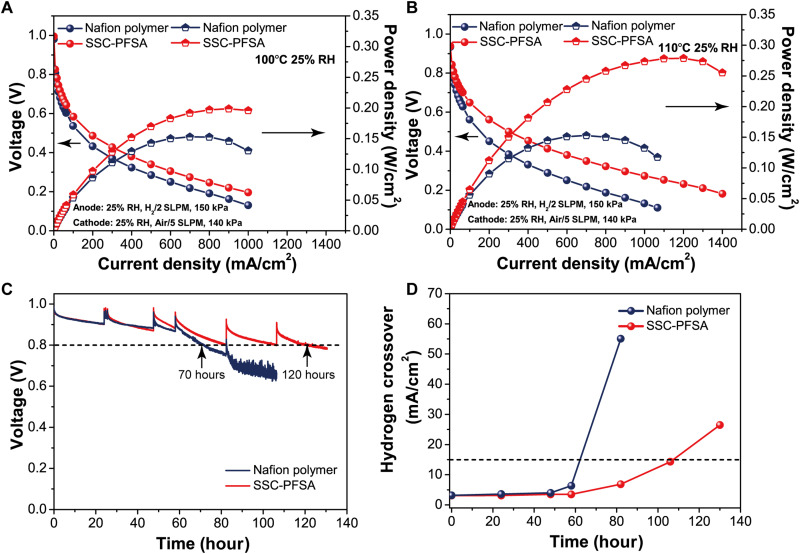
We then investigate the SSC-PFSA MEA FC performance at a higher-temperature and low-humidity condition to further evaluate the advantage of SSC-PFSA PEM. The polarization curves of SSC-PFSA and Nafion polymer MEA at 100° and 110°C under reduced relative humidity (25% RH) are shown in Fig. 5 (A and B). Under 100°C and 25% RH condition, the peak power density for SSC-PFSA and Nafion polymer MEAs is 0.200 W/cm2 (at 0.9 A/cm2) and 0.153 W/cm2 (at 0.7 A/cm2), respectively. The performance for Nafion polymer MEA under 110°C and 25% RH shows a similar value with that of 100°C and 25% RH condition. The SSC-PFSA MEA shows a peak power density of 0.279 W/cm2 (at 0.9 A/cm2) under 110°C and 25% RH condition. Numerically, the peak power density for the SSC-PFSA MEA FC is 30.7% higher than the Nafion polymer MEA FC at 100°C and 25% RH; this value further elevates to 82.3% at 110°C and 25% RH. Such a big leap in performance fully visualizes the advantage of SSC-PFSA PEM for high-temperature low-humidity FC application, which originates from the mechanical improvement and material basis. Thus, SSC-PFSA is a superior material platform for the next generation high-temperature low-humidity FCs, which can also deliver an improved performance under conventional operation conditions. The PEM stability is evaluated by an OCV durability test under 90°C and 30% RH condition, and the hydrogen crossover is also monitored during the OCV test. The results are shown in Fig. 5 (C and D). It can be seen that the SSC-PFSA MEA can stand 120 hours test before the OCV reached 80% of the initial voltage (Voc) value, and the Nafion polymer MEA can only last 70 hours. The SSC-PFSA MEA showed a higher operation lifetime than the Nafion polymer MEA, which is due to the combination of better thermal stability, water/heat management, and higher conductivity for SSC-PFSA at PEMFC operating condition. The hydrogen crossover values for SSC-PFSA and Nafion polymer during the OCV test show a similar trend, and better durability is reached for SSC-PFSA MEA with ~74.2% higher value than that of the Nafion polymer. The much higher performance and better stability for SSC-PFSA MEA at higher temperatures and low relative humidity indicated its high potential for heavy-duty PEM FC applications.
Fig. 5. FC properties and durability at a higher temperature.
Polarization curves of PFSA membranes at (A) 100°C and 25% RH and (B) 110°C and 25% RH under H2/air. (C) OCV test at 90°C and 30% RH and (D) hydrogen crossover at 75°C and 100% RH. SLPM, standard liters per minute.
DISCUSSION
To conclude, we have fully explored the structure formation and structure-property relationship for SSC-PFSA PEM, for which the stream-reservoir hierarchical morphology sets up the basis for fast proton transport and better water/heat management. Comprehensive results demonstrated that the high–molecular weight SSC-PFSA is an excellent material platform that bears high Tg and high IEC for high-power-density FC applications. The proton conductivity of 193 mS/cm was obtained for SSC-PFSA PEM, which is among the highest values for PEMs. The Tg of 134°C enabled high-temperature FC operation for medium and heavy-duty transport applications; meanwhile, the conventional condition is not hampered and even improved. The laboratory demo FC devices exhibit a power density of 1.588 W/cm2 at the current density of 2600 mA/cm2 at 80°C and 95% RH, which was among the top performance for PFSA-based FCs. At higher temperature and lower humidity, the advantage of SSC-PFSA PEM is fully demonstrated, as seen by the 30.7% performance increase at 100°C and 25% RH and 82.3% performance increase under 110°C and 25% RH. The chemical structure and nanomorphology together induce improved operation stability, as seen by the higher operation lifetime in the OCV durability test. We expect the SSC-PFSA PEM to be a key product to address key challenges in vehicle FCs in the current stage, which also serves as a bridge toward the development of even higher-temperature FC PEMs.
MATERIALS AND METHODS
Materials availability
Nafion-structured PFSA (Nafion polymer, Mn = 335,000) (fig. S9A) and SSC-PFSA (Mn = 339,000) (fig. S9B) polymers were synthesized by Dongyue Shenzhou New Materials Co. Ltd., China, without further treatment. The solid-state 19F nuclear magnetic resonance was used to identify the chemical structure of the ionomers (fig. S9C). The IECs of the Nafion polymer and SSC polymer are 1.1 mmol/g (equivalent weight of 909 g/mol) and 1.4 mmol/g (equivalent weight of 714 g/mol), respectively (fig. S9D). The synthesis of SSC polymer was shown in fig. S10. The gas diffusion layer (GDL; JNT) was supplied by JNTG company from South Korea. Pt/C catalyst (TEC10E50) was supplied by Tanaka company from Japan. Isopropanol and ethanol were purchased from Sigma-Aldrich Chemical Reagent Co., Ltd. Deionized water was used throughout the experiments. Poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PVP AI 4083) was purchased from Heraeus Clevios (Germany) and used as received.
Preparation of the Nafion polymer and SSC-PFSA membranes
The procedures for preparation of membranes were previously described in (35). The polymers were dissolved into water/alcohol mixed solvent to form ~10 wt % solutions. The membranes were fabricated by solution casting on a glass plate using the doctor blade with certain thicknesses. The membranes were dried and annealed on a glass plate in the drying oven for which the temperature had been calibrated. The solvents were allowed to evaporate at 80°C for 30 min, and the membranes were annealed at 140° to 150°C for 30 min. During the heating process, the heated air was blown into the oven. Then, the membrane was further annealed. After cooling down to room temperature, the membrane was peeled off from the glass substrate. The thickness of the prepared membranes is in the range of 17 to 20 μm.
In situ grazing incidence x-ray scattering (GISAXS/GIWAXS)
In situ grazing incidence x-ray scattering characterization was performed at Beamline 7.3.3, Advanced Light Source, Lawrence Berkeley National Laboratory. The incidence angle is 0.16°, and the beam energy is 10 keV. The charge-coupled device (CCD) distance is ~280 mm for in situ GIWAXS and ~ 2.5 m for in situ GISAXS; thus, the q range of 0.1 to 3.5 Å−1 and 0.01 to 0.32 Å−1 are obtained, respectively. The experiment was performed in the helium chamber to avoid air scatterings and also to provide a controllable environment. In the experiment, a motor speed (20 mm/s) was used to coat the film, and the head to substrate height is 200 μm. The PFSA solutions were injected into the slot die head using a syringe pump with the speed of 0.2 ml/min. A surveillance camera was used to monitor the solvent flow from the slot die head. When the solution went out, the coating process directly started. Before the coating process, the substrate was prealigned; thus, the x-ray could be applied to the sample immediately after the coating process, with 1-s exposure time. The signals were recorded with a Pilatus 1M CCD (172 μm–by–172 μm pixel size) and analyzed with IGOR Pro software.
MEA preparation and FC evaluation
The catalyst layer was fabricated on PTFE substrate (supplied by Shanghai Maxim Fuel Cell Co., Ltd., China) first, which was then transferred printed (1.0 MPa, 145°C for 105 s) onto PEM to make the catalyst-coated membranes (CCMs). The catalyst loading is 0.4 mg/cm2 on both anode and cathode. The MEAs were prepared by sandwiching the CCM between two sheets of GDLs. The assembled single cells with an effective area of 25 cm2 were tested on a 100-W FC test station (850Es, Hephas Energy Co., Ltd., Taiwan). The cell was conditioned for 6 hours with a voltage of 0.40- to 0.55-V conditions before the polarization curves were recorded.
The polarization curves were obtained at three conditions: 80°C (95% RH), 100°C (25% RH), and 110°C (25% RH) using hydrogen [2 standard liters per minute (SLPM)] and air (5 SLPM) with a back pressure of 150 and 140 kPa for anode and cathode, respectively. The cell voltage was obtained at each current load, which was held 3 min to reach a steady state.
EIS was conducted after polarization curve collections by imposing an ac signal to the FC and the amplitude for the ac impedance was 5% of the direct current over a frequency range of 10 kHz to 0.1 Hz. The EIS curves were recorded under 80°C and 95% RH using hydrogen (2 SLPM) and air (5 SLPM) with backpressure of 150 and 140 kPa for anode and cathode, respectively.
The H2 crossover current density was measured by LSV under constant 100/200 standard cubic centimeters per minute H2/N2 using a scan rate (2 mV/s) between OCV and 0.6 V. The test condition is 80°C and 95% RH, without back pressure for both anode and cathode.
The chemical durability of the Nafion polymer and SSC-PFSA membrane was evaluated by the OCV test with 50-cm2 MEA at 90°C and 30% RH using hydrogen (3.4 SLPM) and air (8.2 SLPM) without back pressure on HS-1000s FC test station (Hephas Energy Co., Ltd., Taiwan). During the OCV test, hydrogen crossover was measured every 24 hours. The test condition of LSV for hydrogen crossover during the OCV test is 75°C and 100% RH using hydrogen (3.936 SLPM) and nitrogen (8.2 SLPM) without back pressure.
Acknowledgments
Funding: F.L. and Y.Zh. acknowledge the support by the National Key R&D Program of China under the grant nos. 2020YFB1505500, 2020YFB1505502, and 2020YFB1505503. X-ray scattering experiments were carried out at Beamline 7.3.3 at the Advanced Light Source, Lawrence Berkeley National Laboratory, which was supported by the DOE, Office of Science, and Office of Basic Energy Sciences. We are grateful for TEM tests supported by the Instrumental Analysis Center of Shanghai Jiao Tong University. We thank P. He for the guidance of FC testing.
Author contributions: Y.Zh. and F.L. acted as the roles of supervision, project administration, and funding acquisition. P.G. prepared the membranes, MEAs, and all the test and characterizations of samples. J.X., M.Z., and W.Z. conducted the x-ray scattering experiments and related analysis. J.L., Y.Zo., H.D., and W.F. contributed to membrane property characterization and device fabrication and testing. All authors discussed the results and commented on the manuscript.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Supplementary Text
Figs. S1 to S10
Table S1
REFERENCES AND NOTES
- 1.H. Zhang, P. K. Shen, Advances in the high performance polymer electrolyte membranes for fuel cells. Chem. Soc. Rev. 41, 2382–2394 (2012). [DOI] [PubMed] [Google Scholar]
- 2.D. W. Shin, M. D. Guiver, Y. M. Lee, Hydrocarbon-based polymer electrolyte membranes: Importance of morphology on ion transport and membrane stability. Chem. Rev. 117, 4759–4805 (2017). [DOI] [PubMed] [Google Scholar]
- 3.X. Liu, D. Wu, X. Liu, X. Luo, Y. Liu, Q. Zhao, J. Li, D. Dong, Perfluorinated comb-shaped cationic polymer containing long-range ordered main chain for anion exchange membrane. Electrochim. Acta 336, 135757 (2020). [Google Scholar]
- 4.M. A. Hickner, H. Ghassemi, S. K. Yu, B. R. Einsla, J. E. Mcgrath, Alternative polymer systems for proton exchange membranes (PEMs). Chem. Rev. 104, 4587–4611 (2004). [DOI] [PubMed] [Google Scholar]
- 5.A. Kusoglu, A. Z. Weber, New insights into perfluorinated sulfonic-acid ionomers. Chem. Rev. 117, 987–1104 (2017). [DOI] [PubMed] [Google Scholar]
- 6.J. Zhang, Z. Xie, J. Zhang, Y. Tang, C. Song, T. Navessin, Z. Shi, D. Song, H. Wang, D. P. Wilkinson, Z.-S. Liu, S. Holdcroft, High temperature PEM fuel cells. J. Power Sources 160, 872–891 (2006). [Google Scholar]
- 7.J. M. Andújar, F. Segura, Fuel cells: History and updating. A walk along two centuries. Renew. Sustain. Energy Rev. 13, 2309–2322 (2009). [Google Scholar]
- 8.M. P. Rodgers, L. J. Bonville, H. R. Kunz, D. K. Slattery, J. M. Fenton, Fuel cell perfluorinated sulfonic acid membrane degradation correlating accelerated stress testing and lifetime. Chem. Rev. 112, 6075–6103 (2012). [DOI] [PubMed] [Google Scholar]
- 9.N. Zhao, D. Edwards, L. Chao, K. Wang, L. Jing, Y. Zhang, S. Holdcroft, Z. Shi, The importance of water transport on short-side chain perfluorosulfonic acid membrane fuel cells operating under low relative humidity. J. Power Sources 242, 877–883 (2013). [Google Scholar]
- 10.W. Wu, Y. Li, J. Liu, J. Wang, Y. He, K. Davey, S. Z. Qiao, Molecular-level hybridization of Nafion with quantum dots for highly enhanced proton conduction. Adv. Mater. 30, 1707516 (2018). [DOI] [PubMed] [Google Scholar]
- 11.P. J. Dudenas, A. Kusoglu, Evolution of ionomer morphology from dispersion to film: An in situ X-ray study. Macromolecules 52, 7779–7785 (2019). [Google Scholar]
- 12.X. Liu, N. Xie, J. Xue, M. Li, C. Zheng, J. Zhang, Y. Qin, Y. Yin, D. R. Dekel, M. D. Guiver, Magnetic-field-oriented mixed-valence-stabilized ferrocenium anion-exchange membranes for fuel cells. Nat. Energy 7, 329–339 (2022). [Google Scholar]
- 13.M. Bass, A. Berman, A. Singh, O. Konovalov, V. Freger, Surface-induced micelle orientation in Nafion films. Macromolecules 44, 2893–2899 (2011). [Google Scholar]
- 14.K. Schmidt-Rohr, Q. Chen, Parallel cylindrical water nanochannels in Nafion fuel-cell membranes. Nat. Mater. 7, 75–83 (2008). [DOI] [PubMed] [Google Scholar]
- 15.M. Fujimura, T. Hashimoto, H. Kawai, Small-angle x-ray scattering study of perfluorinated ionomer membranes. 1. Origin of two scattering maxima. Macromolecules 14, 1309–1315 (1981). [Google Scholar]
- 16.W. Y. Hsu, T. D. Gierke, Ion transport and clustering in Nafion perfluorinated membranes. J. Membr. Sci. 13, 307–326 (1983). [Google Scholar]
- 17.T. D. Gierke, G. E. Munn, F. C. Wilson, The morphology in Nafion perfluorinated membrane products, as determined by wide- and small-angle x-ray studies. J. Polym. Sci. Polym. Phys. 19, 1687–1704 (1981). [Google Scholar]
- 18.C. H. Ma, T. L. Yu, H. L. Lin, Y. T. Huang, Y. L. Chen, U. S. Jeng, Y. H. Lai, Y. S. Sun, Morphology and properties of nafion membranes prepared by solution casting. Polymer 50, 1764–1777 (2009). [Google Scholar]
- 19.C. Welch, A. Labouriau, R. Hjelm, B. Orler, C. Johnston, Y. S. Kim, Nafion in dilute solvent systems: Dispersion or solution? ACS Macro Lett. 1, 1403–1407 (2012). [DOI] [PubMed] [Google Scholar]
- 20.C. W. Bunn, E. R. Howells, Structures of molecules and crystals of fluoro-carbons. Nature 174, 549–551 (1954). [Google Scholar]
- 21.D. Lentz, A. Bach, J. Buschmann, P. Luger, M. Messerschmidt, Crystal and molecular structures and experimentally determined charge densities of fluorinated ethenes. Chem. A Eur. J. 10, 5059–5066 (2004). [DOI] [PubMed] [Google Scholar]
- 22.F. Liu, S. Ferdous, E. Schaible, A. Hexemer, M. Church, X. Ding, C. Wang, T. P. Russell, Fast printing and in situ morphology observation of organic photovoltaics using slot-die coating. Adv. Mater. 27, 886–891 (2015). [DOI] [PubMed] [Google Scholar]
- 23.M. Zhang, L. Zhu, C. Qiu, Y. Zhang, F. Liu, Printing and in situ morphology detection of organic thin film photovoltaic devices. Acta Polym. Sin. 50, 352–358 (2019). [Google Scholar]
- 24.A. Kusoglu, K. Vezzù, G. A. Hegde, G. Nawn, A. R. Motz, H. N. Sarode, G. M. Haugen, Y. Yang, S. Seifert, M. A. Yandrasits, S. Hamrock, C. M. Maupin, A. Z. Weber, V. Di Noto, A. M. Herring, Transport and morphology of a proton exchange membrane based on a doubly functionalized perflourosulfonic imide side chain perflourinated polymer. Chem. Mater. 32, 38–59 (2020). [Google Scholar]
- 25.J. Lin, P. H. Wu, R. Wycisk, P. N. Pintauro, Z. Shi, Properties of water in prestretched recast Nafion. Macromolecules 41, 4284–4289 (2008). [Google Scholar]
- 26.F. I. Allen, L. R. Comolli, A. Kusoglu, M. A. Modestino, A. M. Minor, A. Z. Weber, Morphology of hydrated as-cast Nafion revealed through cryo electron tomography. ACS Macro Lett. 4, 1–5 (2015). [DOI] [PubMed] [Google Scholar]
- 27.K. A. Page, K. M. Cable, R. B. Moore, Molecular origins of the thermal transitions and dynamic mechanical relaxations in perfluorosulfonate ionomers. Macromolecules 38, 6472–6484 (2005). [Google Scholar]
- 28.V. Di Noto, M. Piga, G. A. Giffin, K. Vezzù, T. A. Zawodzinski, Interplay between mechanical, electrical, and thermal relaxations in nanocomposite proton conducting membranes based on nafion and a [(ZrO2)·(Ta2O5)0.119] core–shell nanofiller. J. Am. Chem. Soc. 134, 19099–19107 (2012). [DOI] [PubMed] [Google Scholar]
- 29.P. Ramaswamy, N. E. Wong, G. K. H. Shimizu, MOFs as proton conductors-challenges and opportunities. Chem. Soc. Rev. 43, 5913–5932 (2014). [DOI] [PubMed] [Google Scholar]
- 30.X. Zhang, Y. Li, X. Liu, J. Zhang, Y. Yin, M. D. Guiver, A paradigm shift for a new class of proton exchange membranes with ferrocyanide proton-conducting groups providing enhanced oxidative stability. J. Membr. Sci. 616, 118536 (2020). [Google Scholar]
- 31.S. Mukhopadhyay, J. Debgupta, C. Singh, R. Sarkar, O. Basu, S. K. Das, Designing UiO-66-based superprotonic conductor with the highest metal–organic framework based proton conductivity. ACS Appl. Mater. Interfaces 11, 13423–13432 (2019). [DOI] [PubMed] [Google Scholar]
- 32.A. Kusoglu, T. J. Dursch, A. Z. Weber, Nanostructure/swelling relationships of bulk and thin-film PFSA ionomers. Adv. Funct. Mater. 26, 4961–4975 (2016). [Google Scholar]
- 33.C. Yang, N. Han, Y. Wang, X. Z. Yuan, J. Xu, H. Huang, J. Fan, H. Li, H. Wang, A novel approach to fabricate membrane electrode assembly by directly coating the Nafion ionomer on catalyst layers for proton exchange membrane fuel cells. ACS Sustain. Chem. Eng. 8, 9803–9812 (2020). [Google Scholar]
- 34.Y. Garsany, R. W. Atkinson, M. B. Sassin, R. Hjelm, B. D. Gould, K. E. Swider-Lyons, Improving PEMFC performance using short-side-chain low-equivalent-weight PFSA ionomer in the cathode catalyst layer. J. Electrochem. Soc. 165, F381–F391 (2018). [Google Scholar]
- 35.P. Guan, J. Lei, X. Liu, K. Xu, S. Pei, H. Ding, Y. Zou, W. Feng, F. Liu, Y. Zhang, Origins of water state and ionic cluster morphology for high proton conductivity of short side-chain perfluorinated sulfonic acid membranes. Chem. Mater. 34, 7845–7857 (2022). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text
Figs. S1 to S10
Table S1