Abstract
Metabolizable energy intake is the key determining factor for the expression of an animal’s genetic potential for growth, and current predictive growth models are not capable of accounting for all the nutritional variation that is commonly observed. The current study was designed to investigate energy transactions as lambs grow using CT scanning to assess body compositional changes at two levels of intake and two stages of maturity, and compare results to predictive equations.
A pelleted diet was provided to cross-bred lambs (n = 108) at approximately 2.5 and 3.5% of liveweight (LW) in dry matter when the lambs were approximately four (31.8 ± 0.3 kg LW) and eight (40.5 ± 0.3 kg LW) months of age. A digestibility trial was run sequentially using 10 lambs of the same genetic and nutritional history fed at the same feeding levels to determine the digestibility of the diet.
In the first feeding period, metabolizable energy intake was 15.3 ± 0.03 and 9.5 ± 0.03 MJ ME/d for high and low feeding levels respectively, resulting in higher rates of empty body gain for high feeding level lambs (197.7 ± 7.8 vs. 72.8 ± 8.2 g/d; P < 0.001). In the second feeding period, metabolizable energy intake was 15.2 ± 0.01 and 12.0 ± 0.01 MJ ME/d for high and low feeding levels respectively, resulting in higher rates of empty body gain for high feeding level lambs (176.3 ± 5.4 vs. 73.9 ± 5.3; P < 0.001).
Lambs at later stages of maturity retained proportionately more energy as fat for every unit of retained energy compared to younger lambs (95.4 ± 0.40 vs. 90.0 ± 0.42%; P < 0.001). Lambs fed the lower feeding level in period two also retained proportionately more energy as fat for every unit of retained energy than lambs at the higher feeding level (97.1 ± 0.36 vs. 94.0 ± 0.37%; P < 0.001) which is hypothesized to be because of the rapid response of visceral lean tissue to changes in nutrition. There were no significant interactions between treatments in the first and second feeding periods, indicating an absence of a compensatory gain response to a nutritional restriction in the first feeding period.
This experiment highlights the significance of a changing feed supply and the subsequent effects on body composition and the partitioning of energy to lean and fat tissue deposition. For improvements in the accuracy of predictive ruminant growth models it is necessary to gain a greater understanding of the different tissue responses over time to changes in nutrition.
Keywords: body composition, compensatory growth, digestibility, feed intake, lamb growth, nutrition
Using CT scans to assess body composition, it was identified that lambs at later stages of maturity and lower levels of feeding had increased rates of fat deposition as a proportion of empty body gain. Nutritional history had no effect on the rate or composition of gain at different feeding levels, indicating that the composition of gain is driven primarily by stage of maturity and energy intake.
Introduction
The expression of a ruminants’ genetic potential for growth is predominantly constrained by nutrition and the interactions between nutrient supply, body composition, and animal growth are complex and difficult to predict (Hegarty et al., 2006; Oddy et al., 2019). The utilization of energy for tissue deposition in ruminants has been researched extensively (Graham, 1980; Ferrell et al., 1986; Hegarty et al., 1999; Dougherty et al., 2022) but current predictive models cannot account for all the variation observed (Oddy et al., 2019). Improvements in the precision of estimating animal growth could facilitate better allocation of resources, resulting in increased productivity and profitability of farming systems (Oddy et al., 1997). The partitioning of retained energy shifts from predominantly protein deposition in favor of accretion of fat as lambs approach maturity (Searle et al., 1972; Butterfield, 1988). The energy density and protein content of the diet, level of feeding and any prior nutritional restrictions have important and lasting effects on energy partitioning (Graham et al., 1974; Ørskov et al., 1976; Graham, 1980; Rompala et al., 1988; Hegarty et al., 1999). Any resultant modifications to body composition are likely to change the proportional energy losses to heat production, thus altering the efficiency of energy utilization for gain (Oddy et al., 2019).
The partitioning of energy to protein and fat deposition is determined by the animal’s liveweight as a proportion of their mature weight, explaining why at the same liveweight, animals with greater mature weights grew faster and were more energetically efficient (Searle et al., 1972). Subsequent experiments revealed that body composition could be adjusted by nutrient supply as lambs on a rapid growth path contained more fat than those on a restricted growth path (Turgeon et al., 1986). Similarly, at the same liveweight, lambs exposed to a nutritional restriction and then re-fed contained less fat than unrestricted lambs; however, re-fed lambs appeared to gradually return to the body composition and composition of gain of lambs of uninterrupted growth (Hodge and Star, 1984; Thatcher and Gaunt, 1992).
Comparative slaughter methods were used to establish that fat deposition is principally controlled by energy intake (Hegarty et al., 1999). Despite the comprehensive nature of this research, several limitations did exist. The accuracy of a comparative slaughter study is limited by between animal variation in body composition, as the comparative slaughter method relies on the data from a representative cohort slaughtered at the commencement of the experiment. Estimating gain by this method implies a linear term for growth of components which is an oversimplification of the nonlinear nature of growth. Further to this, lambs at the same age that have been previously exposed to different nutritional regimens may utilize energy differently due to the effects of nutritional history on subsequent body composition (Fattet et al., 1984; Vipond et al., 1989). It is therefore possible that fat deposition is controlled by both energy intake and stage of maturity. Using a small number of lambs, there was some evidence that the efficiency of energy utilization for growth changes with stage of maturity (Graham, 1980), although no direct measures of body composition have been reported. The digestibility of a pelleted diet can change with level of intake and stage of maturity (Graham, 1980; Graham and Searle, 1982; Margan et al., 1982) which was not investigated in the work of Hegarty et al. (1999).
The present study was designed to investigate compositional changes at differing levels of nutrient supply and two stages of maturity. Previous research has examined compositional changes at similar stages of maturity either during or following a period of restricted growth. The concurrent investigation of diet digestibility and the use of a nondestructive method for analyzing body composition (computerized tomography (CT) scans) removes variation in gain of body composition due to inability to measure the same animal twice using serial slaughter techniques. The current study used CT scanning to investigate the composition of gain in lambs at differing levels of positive restricted growth and two stages of maturity.
Materials and Methods
The use and care of animals was approved by the CSU Animal Care and Ethics Committee (protocol number A20203 & A20305).
Site management
The experiment was conducted at the Charles Sturt University (CSU) Lamb Feeding Facility and NSW Department of Primary Industries (NSW DPI) Animal Nutrition Unit, both located in Wagga Wagga in southern NSW (30°03ʹ30.3ʺS, 147°20ʹ38.5ʺE; 219 m altitude). The CSU Lamb Feeding Facility contains 36 bare-earth pens, with each pen approximately 24 m2 including a 7 m2 shaded area. The NSW DPI Animal Nutrition Unit contains indoor individual animal pens, each approximately 2 m2, and metabolism crates for the collection of faeces and urine. The lambs were weaned onto pasture containing predominantly lucerne (Medicago sativa), subterranean clover (Trifolium subterraneum) and some annual grasses (Lolium multiflorum, Hordeum leporinum). Between the experimental periods, the lambs grazed pasture which consisted mainly of reproductive and senesced annual grasses (Lolium multiflorum, Hordeum leporinum, Vulpia myuros) and subterranean clover (Trifolium subterraneum).
Experimental design
The replicated experiment had high (H) and low (L) feeding level treatments in two feeding periods (P1 and P2). Treatments are referred to as H1 and L1 for P1 and H2 and L2 for P2. Twin born lambs (n = 108) from the single-sire joining of 18-month-old Merino × Border Leicester ewes to either one Poll Dorset (S1; 26 female and 20 castrated male lambs) or one of two White Suffolk rams (S2; 10 female and eight castrated male lambs, S3; 24 female and 20 castrated male lambs) were sourced from a commercial farm in southern NSW. Birth date, birth weight, sires and siblings were known from lambing data. Lambs were blocked by sire and sex and then stratified by age before being randomly assigned to pens containing three lambs each. Each day a weighed amount of a pelletized ration (Tables 1 and 2) was provided to each pen in a self-feeder and any refusals from the previous day were collected and weighed. The ration was designed to contain the nutrients necessary for a growing lamb (CSIRO, 2007). A separate batch was manufactured for each feeding period with wet chemistry analysis of each batch conducted prior to each feeding period (Table 2).
Table 1.
Ingredients of experimental ration provided to lambs
| Ration component | % |
|---|---|
| Barley | 46.9 |
| Cereal hay | 20.0 |
| Lucerne hay | 20.0 |
| Canola meal | 5.0 |
| Millmix | 4.9 |
| Limestone | 1.5 |
| Canola oil | 1.0 |
| Salt | 0.6 |
| Mineral mix | 0.1 |
Table 2.
Wet chemistry analysis and digestibility1 (%) of pelleted diet provided to lambs during each feeding period with calculated metabolizable energy density (M/D) (±SE)
| Feeding period 1 | Feeding period 2 | ||
|---|---|---|---|
| Composition | Low | High | |
| Dry matter (%) | 93 | 93 | |
| Organic matter (% DM2) | 92 | 92 | |
| Neutral detergent fiber (% DM2) | 32 | 26 | |
| Acid detergent fiber (% DM2) | 14 | 13 | |
| Crude protein (% DM2) | 17 | 17 | |
| Gross energy (MJ/kg DM2) | 17.8 | 18.4 | |
| M/D3 (MJ/kg DM2) | 11.3 ± 0.2 | 11.0 ± 0.1a | 10.4 ± 0.1b |
| Digestibility | |||
| Dry matter | 71.5 ± 0.9 | 71.1 ± 0.4a | 67.3 ± 0.4b |
| Organic matter | 68.3 ± 0.8 | 67.9 ± 0.4a | 64.5 ± 0.4b |
| Neutral detergent fiber | 47.3 ± 1.5 | 44.8 ± 1.3 | |
| Acid detergent fiber | 36.5 ± 1.4 | 41.9 ± 1.8 | |
| Crude protein | 72.7 ± 1.1 | 74.7 ± 0.5a | 68.8 ± 0.5b |
| Gross energy | 71.7 ± 1.1 | 69.9 ± 0.5a | 65.7 ± 0.5b |
1No significant difference in diet digestibility between low and high feeding level in period one.
2Dry matter.
3Calculated by direct measurement and using value for methane (CH4) production of 20.8 g CH4 per kg dry matter intake (Charmley et al., 2015) and gross energy of urine calculated using the equation of Blaxter et al. (1966): 9.66 kcal/g C—3.0 multiplied by 0.004184 to convert to MJ.
Lambs were weighed every 2 wk with the treatment mean liveweight (LW) used to calculate feeding levels for the subsequent 2 wk. Lambs on H feeding levels were fed the pelleted diet at a rate equivalent to 3.5% of mean LW as dry matter (DM) whereas, lambs in the L treatment were fed at a rate of 2.5% of mean LW as DM. Feeding levels were designed to generate two growth rates and it was anticipated that the digestibility of the diet may have been affected by feeding level. The H level was set just below expected ad libitum intake to ensure similar daily intake across pens. The lambs entered the feeding facility for an 8-wk feeding period at a mean age of 100 (±3.7SD) days and then again for P2 at a mean age of 230 (±3.7SD) d. Half of the pens that were previously allocated to H1 were switched to L2 and vice versa. The remaining pens remained in their allocated feeding levels for P1 and P2, thus creating four feeding treatment groups: HH, HL, LH, and LL. Lamb LW was recorded prior to CT scanning at the start and conclusion of the feeding periods and was recorded fortnightly whilst at the feeding facility to adjust daily ration allocation. All lambs were CT scanned at the Veterinary Clinical Centre (VCC) at CSU.
Prior to each feeding period, a 16-day adaptation period, starting at 100 g/head/d and increasing the daily allocation by 50 g/head/d, adapted the lambs to the ration. The first nine days of both adaptation periods occurred in the weaning paddock for P1 and the naturalized pasture paddock for P2 on the commercial farm where the lambs were located which enabled continued access to pasture. After nine days, the lambs were transported to the feeding facility for the final seven days of the adaptation period with access to barley straw in addition to the pelleted ration.
The metabolizable energy and digestibility of the diet was determined by a sequentially run animal house-based study with two six-day faeces and urine collection periods with H and L intake treatments. An additional 10 twin born lambs of mixed sexes (S1, S3; two female and two castrated male lambs, S2; one female and one castrated male lamb) were assigned to treatments so that they were balanced for sire, sex and age with each sibling allocated to different treatments for both collection periods. Lambs in the digestibility experiment were a median age of 117 (±2.5SD) and 247 (±2.5SD) days at the commencement of the first and second collection periods, respectively. These lambs were individually housed in metabolism crates and each day a weighed amount of a pelletized ration (Tables 1 and 2) was provided to each lamb in a feeder and any refusals from the previous day were collected and weighed. Each lamb in the H and L treatments were adapted to the ration and fed at the rates as outlined above.
Animal management
Male lambs were castrated using elastic rings at lamb marking on 18 August 2020 and all lambs were vaccinated at lamb marking, and again at weaning on 25 September 2020 (Ultravac 5 in 1, Zoetis, Australia). Lambs were also drenched at weaning (Triguard Triple Combination Drench for Sheep, abamectin 1.0 g/L, oxfendazole 22.5 g/L, levamisole 34 g/L, selenium 0.5 g/L and cobalt 2.2 g/L; Boehringer Ingelheim, Australia). The lambs were exposed to grain prior to weaning by trail feeding whole barley to ewes and lambs on five separate occasions.
The adaptation period for P1 commenced on 6 October 2020, with lambs (n = 136; S1, 31 female and 29 castrated male lambs; S2, 11 female and 11 castrated male lambs; S3, 30 female and 24 castrated male lambs) being fed in plastic troughs in the weaning paddock. On 15 October 2020, all lambs were brought into nearby sheep-yards and 108 were allocated to pens before being transported to the feeding facility. The remaining 28 lambs were either allocated to the digestibility experiment (n = 10) or returned to the weaning paddock to be kept as spares. Thirty-four of the 36 pens at the feeding facility contained three lambs of the same gender and sire with a birth date within six days of each other. Two pens contained three lambs of the same gender but different sires (S1 and S2).
The first feeding period commenced on 22-23 October 2020. Weighing and CT scanning of lambs was conducted before the daily ration was fed and lambs were not removed from their pens for longer than two hours. Groups of lambs were walked to nearby sheep yards and weighed using a Gallagher Sheep Auto Drafter prior to being loaded onto a trailer and taken to the VCC for CT scanning. The lambs were then returned to their pens once all lambs in the group were scanned.
On 17-18 December 2020, all lambs were weighed and CT scanned to conclude P1. The lambs were returned to the commercial farm to graze in the naturalized pasture paddock until the commencement of P2. On 20 December 2020, all lambs were shorn. The adaptation period for P2 commenced on 13 February 2021 with lambs being fed in plastic troughs in the naturalized pasture paddock. On 22 February 2021, all lambs were brought into nearby sheep-yards and 107 lambs that completed P1 and one replacement (H2 treatment), were allocated to pens and transported to the feeding facility. The remaining spare lambs took no further part in the experiment.
On 1-2 March 2021, all lambs were weighed and CT scanned as described for P1. On 5 March 2021, numerous lambs from both treatments exhibited signs of subclinical acidosis and all lambs were returned from 1000 g/head/d to a feeding level of 500 g/head/d for two days before the daily allocation was increased by 50 g/head/d. During this period the lambs were additionally provided daily with a weighed amount of lucerne hay which was included in intake calculations. Once lambs had achieved intake of 900 g/head/d, hay was withdrawn, the feeding period recommenced and was extended by 10 days. The experiment concluded on 6-7 May 2021, when all lambs were weighed and CT scanned before being returned to the commercial owner.
The ten lambs used for the determination of the digestibility of the diet were managed prior to each feeding period as outlined above. Lambs were held in one of two pens consisting of the five lambs for each treatment for seven days at the CSU Lamb Feeding Facility before being weighed and transported to the NSW DPI Animal Nutrition Unit to be housed and fed in individual pens for a further seven days. Lambs were transferred to individual metabolism crates for two days before the six-day collection periods commenced. After the first collection period, lambs were returned to the commercial farm to graze pasture until the conclusion of P1 when all lambs were reunited. Three of the lambs that were allocated to the H treatment for the first collection period were switched to the L treatment for the second collection period and vice versa. At the conclusion of the second collection period, all lambs were returned to the commercial farm and took no further part in the experiment.
Sample collection
Faeces and urine were collected daily at 0800 hours during the six-day collection periods. Ten per cent of the total weight of the faeces measured each day was collected from each lamb and stored at −20 °C. Urine was accumulated each day in a bucket containing 200 mL of sulfuric acid (1 M H2SO4). Daily urine collections were weighed and diluted to 3.5 L with water. From this, a 100 mL sample was collected and pooled to make a bulk urine sample for each lamb. All daily feed refusals were pooled to make a bulk refusals sample for each lamb. One sample of the pelletized ration from each feeding period and a single lucerne hay sample from feeding period two were also collected. All urine, feed refusals and pelletized ration and hay samples were stored at −20 °C for later chemical analysis.
Chemical analyses
All analytical procedures, with the exception of the determination of gross energy (GE) of samples from the second collection period, were undertaken at the Feed Laboratory of NSW DPI at Wagga Wagga, NSW. Analysis of GE of samples from the second collection period was undertaken at ALS Laboratory Group, Lithgow NSW.
The frozen faecal and feed samples were dried in an air-forced oven to a constant weight at 70 °C to determine their DM content (AFIA, 2014). The ash content was determined by the combustion of a 2 g sample at 600 °C for six hours, from which the organic matter (OM) as a % of DM was calculated. The N content of feed and faeces (0.2 g ground samples) were determined using a Leco CNS 2000 analyzer (Leco, St Joseph, MI, USA), from which the crude protein (CP) content was calculated by multiplying the N content by a factor of 6.25. The GE of feed and faeces was determined by measuring the heat of combustion of a pelleted sample in a bomb calorimeter (Basolo et al., 2020).
The neutral detergent fiber (NDF) content of the feed and faeces was determined by weighing approximately 0.5 g (known weight) of each sample into an ANKOM filter bag and placed in the ANKOM Fibre Analyzer (Ankom 200/220 fibre analyzer, ANKOM Technology, Macedon NY, USA) vessel along with neutral detergent solution, sodium sulphite and heat stable alpha-amylase. The bags were heated and agitated for 75 min and then rinsed three times with hot water and alpha-amylase. The bags were then soaked in acetone for 3-5 min and then dried in an oven at 105 °C for 2 h (AFIA, 2014).
The acid detergent fiber (ADF) content of the feed and faeces was determined by weighing approximately 0.5 g (known weight) of each sample into an ANKOM filter bag and placed in the ANKOM Fibre Analyzer vessel along with acid detergent solution. The bags were heated and agitated for 60 min and then rinsed three times with hot water. The bags were then soaked in acetone for 3-5 min and dried in an oven at 105 °C for 2 h (AFIA, 2014).
The frozen urine samples were thawed overnight at room temperature. The carbon (C) content of urine samples (0.2 g samples) were determined using a Leco CNS 2000 analyzer.
CT scans
Lambs were scanned live and without sedation and were individually moved through the CT scanner (16 Slice Toshiba Alexion Advance) while in a sternal recumbent position, which was secured by being strapped in a cradle. The CT scan image included legs in the scans. The X-ray tube energy setting was 120 kV. Cross-sectional slices (5 mm width) were taken between the 4th cervical vertebrae and the 1st sacral vertebrae, yielding an average of 120 images per animal. CT images were manually edited (OsiriX https://www.osirix-viewer.com/, accessed 2021; Rosset et al., 2004) to dissect reticulo-rumen, omasum, and abomasum (stomach) contents from the body.
The proportion of stomach-fill components (gas, particulate and liquid) and the fat, lean and bone composition of the empty body (stomach removed) were then determined using Image J (v1.53, https://imagej.nih.gov/ij/, accessed 2021) to create a distribution of pixels in grayscale units from 0 to 256. Component boundaries were identified in Image J by observing highlighted components at different greyscale thresholds in slices taken at 30 mm intervals from 10 randomly selected lambs at each CT scanning timepoint. Boundaries for fat, lean and bone were set to 10 to 90, 91 to 200, and 201 to 256, respectively, which correspond well with the boundaries outlined by Alston et al. (2004) and Kvame and Vangen (2006). For the stomach components, boundaries for gas, particulate and liquid were set to 0 to 1, 2 to 65, and 65 to 256, respectively.
Calculations
Calculations of empty body tissue masses and rates of gain required a combination of LW, CT scan data, an estimation of the weight of contents of the reticulo-rumen, omasum and abomasum (stomach) to correct for gut fill and an estimation of fleece weight. Fleece-free empty body weight (FFEBW) was estimated using LW less the estimated weight of stomach contents and estimated weight of the greasy fleece which was assumed to weigh 1.5 kg at the commencement of both feeding periods and 2 kg at the conclusion.
The stomach volume was calculated within the computer program OsiriX with CT images at 15 mm intervals for all lambs. The proportion of components in the empty body and stomach were calculated by allocating pixels based upon the grayscale boundaries described above. The area of components was summed across images at 30 mm intervals to calculate the component proportions which were corrected for density. Assumed tissue densities were 0.95, 1.05 and 1.45 g/cm3 for fat, lean and bone, respectively (Fullerton, 1980; Young et al., 1996; Campbell et al., 2003). For the stomach components, assumed densities were 0, 0.65, and 1 for gas, particulate and liquid, respectively (Bain et al., 2014). Component proportions were multiplied by estimated FFEBW (empty body) or stomach volume (stomach) to determine weights.
Sixteen pens were blocked by treatment and sex and randomly selected to estimate the weight of stomach contents. The stomach contents data from all four CT scans of the 48 lambs were combined to develop a linear equation using stomach volume to correct for gut fill and calculate FFEBW for all 108 lambs involved in the experiment.
Individual apparent digestibility (%) of feed was calculated on a DM basis from the difference between total faecal output and total intake less any refusals during the six-day period. Energy balances were produced from the difference between total intake and total faecal and urinary output during the six-day period. For the estimation of the metabolizable energy (ME) density of the diet, methane (CH4) production was predicted as 20.8 g CH4 per kg DM intake (Charmley et al., 2015) and GE of urine predicted using the equation of Blaxter et al. (1966): 9.66 kcal/g C—3.0 multiplied by 0.004184 to convert to MJ. Energy balance calculations to determine maintenance energy requirements and the efficiency of energy utilization for gain (Table 5) were derived from CSIRO (2007) with daily time steps and mean values reported for each feeding period.
Table 5.
Energy balance mean values using calculations by CSIRO (2007) and comparison to mean values (±SE) using CT scan data
| Treatment1 | Intake (MJ ME/d) |
Energy value fat gain (MJ/d)2 |
Energy value protein gain (MJ/d)3 | Proportion fat in retained energy (%) | Efficiency of energy utilization for gain4 | CSIRO (2007) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Maintenance energy requirement (MJ/d) | Available energy for gain (MJ/d) | Energy value fat gain (MJ/d)3 |
Energy value protein gain (MJ/d)4 | Proportion fat in retained energy (%) | ||||||
| Feeding period 1 | ||||||||||
| Low | 9.5 ± 0.03 | 1.35 ± 0.12a | 0.13 ± 0.02a | 89.8 ± 0.6 | 0.41 | 5.91 | 3.59 | 1.58 | 0.31 | 83.6 |
| High | 15.3 ± 0.03 | 3.56 ± 0.13b | 0.44 ± 0.02b | 0.47 | 6.82 | 8.48 | 3.86 | 0.63 | 86.0 | |
| Feeding period 2 | ||||||||||
| Low | 12.0 ± 0.01 | 2.27 ± 0.11a | 0.05 ± 0.02a | 97.1 ± 0.4a | 0.48 | 7.14 | 4.86 | 2.22 | 0.28 | 88.8 |
| High | 15.2 ± 0.01 | 4.35 ± 0.11b | 0.27 ± 0.02b | 94.0 ± 0.4b | 0.62 | 7.70 | 7.50 | 3.26 | 0.39 | 89.3 |
Different superscripts at each timepoint indicate treatment means differed significantly (P < 0.05).
1Low, low level of feeding; High, high level of feeding.
2Fat energy density: 39.3 MJ/kg (CSIRO, 2007).
3Protein energy density: 23.6 MJ/kg (CSIRO, 2007), protein gain: 20% of lean tissue gain (Dougherty et al., 2020).
4The proportion of retained energy from available energy for gain calculated by CSIRO (2007).
Statistical analysis
Body composition and digestibility experiment data were analyzed by generalized linear models using ASRemL (Gilmour et al., 2009). Lamb weights, growth rates, stomach volume, stomach contents, feed intake and empty body composition were modelled using a linear univariate model with fixed effects of treatment, sire, sex, birthweight, age, feeding period one start weight, appropriate two-way interactions and pen as random effect. Diet digestibility, energy balances and feed intake from the digestibility experiment were modelled using a linear univariate model with fixed effects of treatment, sire, sex, appropriate two-way interactions, and sibling as random effect.
Results
Liveweight data in Figures 1 and 2 are presented as raw (unadjusted) means (Figure 1) and values (Figure 2) by level of main effect within timepoint. Body composition, energy balance and diet digestibility results (Tables 2 to 5 and Figure 3) are presented as predicted means ± SE. There were no significant interactions between the first and second feeding period and the treatment effects from each period are therefore presented separately.
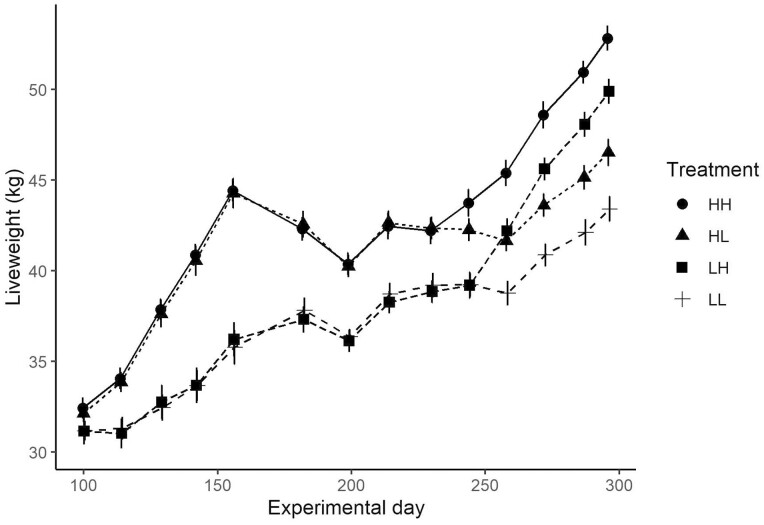
Figure 1.
Mean lamb liveweight (±SE) from the commencement of feeding period one until the conclusion of feeding period two for H (high) and L (low) feeding levels during each period. The first letter of treatment indicates feeding period one and second letter indicates feeding period two.
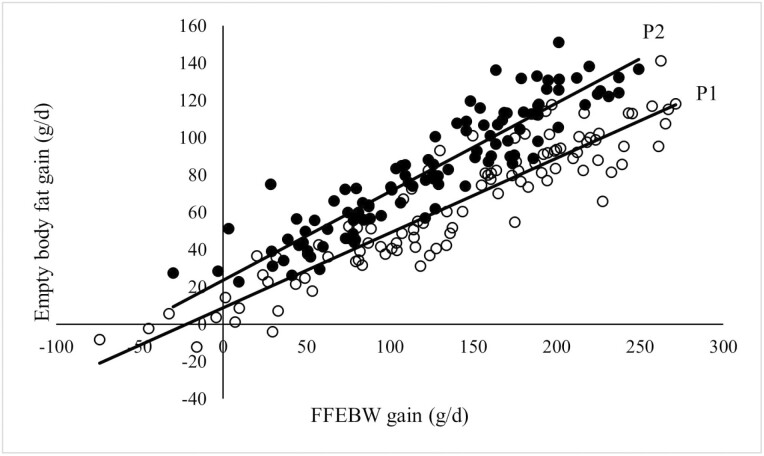
Figure 2.
Relationship between rate of empty body fat gain (g/d) and fleece-free empty body weight (FFEBW) gain (g/d) for lambs from low and high levels of intake during feeding period 1 (P1: hollow symbols) and feeding period 2 (P2: solid symbols). P1; y = 0.401x + 8.8903, r2 = 0.85; P2; y = 0.4745x + 23.704, r2 = 0.85.
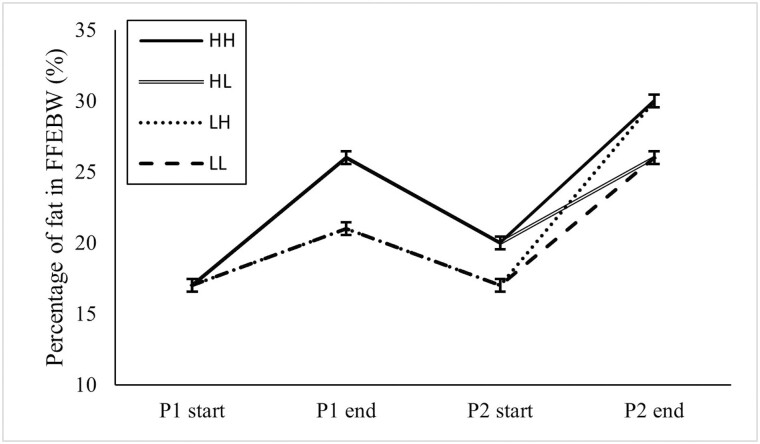
Figure 3.
Mean fat percentage of fleece free empty body weight (FFEBW) from the commencement of feeding period one (P1) until the conclusion of feeding period two (P2) for high (H) and low (L) feeding levels during each period. Treatment first letter indicates feeding period one and second letter indicates feeding period two.
Liveweight and intake
Mean LW of lambs at the commencement of P1 was 31.8 ± 0.3 kg and did not differ between treatments. The mean LW of H1 lambs was greater than L1 lambs at the conclusion of P1 (44.1 ± 0.6 vs. 36.2 ± 0.6 kg; P < 0.001), the commencement of P2 (41.8 ± 0.4 vs. 39.2 ± 0.4 kg; P < 0.001) and the conclusion of P2 (49.2 ± 0.5 vs. 46.8 ± 0.5 kg; P < 0.001; Figure 1). Mean daily ME intake was 9.5 ± 0.03 and 15.3 ± 0.03 MJ ME/d and mean DM intake was 847 ± 3 and 1357 ± 3 g/d for L1 and H1 lambs, respectively. Dry matter intake as a percentage of LW for L1 and H1 treatments was respectively 2.5 and 3.6%.
Mean LW of lambs at the commencement of P2 was 40.5 ± 0.3 kg and did not differ between P2 treatments (due to the reallocation of half of the lambs to different treatments). The mean LW of H2 lambs was greater than L2 lambs at the conclusion of P2 (51.3 ± 0.5 vs. 44.7 ± 0.5 kg; P < 0.001; Figure 1). Including hay provided during the first week of P2, mean daily ME intake was 12.0 ± 0.01 and 15.2 ± 0.01 MJ ME/d and mean DM intake was 1031 ± 1 and 1491 ± 1 g/d for L2 and H2 lambs, respectively. Dry matter intake as a percentage of LW were similar to the targeted feeding level at 2.4% for L2 and 3.3% for H2 treatments.
Empty body composition and gain
Lambs allocated to H1 or L1 did not differ in FFEBW or composition of the empty body (fat, lean and bone mass) at the commencement of P1. The H1 treatment resulted in higher rates of gain in FFEBW, fat, lean and bone than L1 lambs in P1 (Table 3). Lambs allocated to H1 had a greater FFEBW and mass of empty body components at the commencement of P2 compared to L1 despite the pasture phase between P1 and P2. The pasture phase resulted in a loss in FFEBW, empty body fat and lean mass for H1 lambs (Table 3). Lambs in L1 lost less fat (P < 0.001) than H1 lambs but gained lean mass resulting in a net FFEBW gain in the pasture phase. Lambs allocated to H1 still had greater FFEBW and mass of fat, lean and bone compared with L1 lambs at the conclusion of P2. There were no differences in rate of FFEBW, fat or lean gain during P2 due to P1 feeding level (Table 3).
Table 3.
Empty body component mass and rate of gain means (±SE) across feeding period 1 and feeding period 2 for lambs that were allocated to high or low feeding level treatments in feeding period 1
| Treatment1 | FFEBW2 (kg) |
FFEBW2 gain (g/d) | Fat (kg) |
Fat gain (g/d) |
Lean (kg) |
Lean gain (g/d) |
Bone (kg) |
Bone gain (g/d) |
|---|---|---|---|---|---|---|---|---|
| Feeding period 1 | ||||||||
| Start | 25.9 ± 0.3 | — | 4.5 ± 0.1 | — | 18.0 ± 0.2 | — | 3.4 ± 0.04 | — |
| Finish | ||||||||
| Low | 29.9 ± 0.5a | 72.8 ± 8.2a | 6.4 ± 0.2a | 34.5 ± 3.3a | 19.7 ± 0.3a | 28.6 ± 5.1a | 3.8 ± 0.1a | 9.1 ± 0.9a |
| High | 37.0 ± 0.4b | 197.7 ± 7.8b | 9.6 ± 0.2b | 90.5 ± 3.1b | 23.2 ± 0.3b | 92.2 ± 4.9b | 4.1 ± 0.1b | 13.8 ± 0.9b |
| Feeding period 2 | ||||||||
| Start | ||||||||
| Low | 31.2 ± 0.3a | 19.4 ± 5.1a | 5.9 ± 0.2a | −13.8 ± 2.2a | 21.9 ± 0.2a | 31.1 ± 3.2a | 4.0 ± 0.1a | 1.3 ± 0.4 |
| High | 33.5 ± 0.3b | −46.5 ± 5.0b | 6.3 ± 0.2b | −40.1 ± 2.2b | 22.7 ± 0.2b | −6.1 ± 3.1b | 4.2 ± 0.1b | |
| Finish | ||||||||
| Low | 39.6 ± 0.4a | 125.1 ± 4.0 | 11.1 ± 0.3a | 84.2 ± 2.1 | 24.1 ± 0.2a | 33.9 ± 2.5 | 4.4 ± 0.1a | 6.3 ± 0.5 |
| High | 41.6 ± 0.4b | 12.1 ± 0.3b | 24.9 ± 0.2b | 4.6 ± 0.1b | ||||
Different superscripts at each timepoint indicate treatment means differed significantly (P < 0.05).
1Low, low level of feeding (dry matter intake 2.5% of liveweight); High, high level of feeding (dry matter intake 3.6% of liveweight).
2Fleece free empty body weight.
Lambs in H2 had greater rates of gain in FFEBW and empty body components than L2 lambs during P2 resulting in greater mass of FFEBW and empty body fat and lean mass at the conclusion of P2 (Table 4).
Table 4.
Empty body component mass and rate of gain means (±SE) for second feeding period treatments
| Treatment1 | FFEBW2 (kg) |
FFEBW2 gain (g/d) | Fat (kg) |
Fat gain (g/d) |
Lean (kg) |
Lean gain (g/d) |
Bone (kg) |
Bone gain (g/d) |
|---|---|---|---|---|---|---|---|---|
| Start | 32.4 ± 0.2 | — | 6.1 ± 0.1 | — | 22.3 ± 0.2 | — | 4.1 ± 0.04 | — |
| Finish | ||||||||
| Low | 37.5 ± 0.4a | 73.9 ± 5.3a | 9.9 ± 0.3a | 57.8 ± 2.8a | 23.1 ± 0.2a | 9.8 ± 3.4a | 4.5 ± 0.04 | 5.0 ± 0.7a |
| High | 43.8 ± 0.4b | 176.3 ± 5.4b | 13.3 ± 0.3b | 110.7 ± 2.8b | 25.9 ± 0.2b | 57.9 ± 3.3b | 7.7 ± 0.7b |
Different superscripts at each timepoint indicate treatment means differed significantly (P < 0.05).
1Low, low level of feeding (dry matter intake 2.4% of liveweight); High, high level of feeding (dry matter intake 3.3% of liveweight).
2Fleece free empty body weight.
For any rate of fleece-free empty body gain, P1 lambs deposited less fat in the empty body than did P2 lambs (Figure 2). Empty body fat gain as a proportion of FFEBW gain was greater for lambs in P2 than P1 (0.70 vs. 0.52; P < 0.001).
The protein gain determined using CT scans for both feeding levels in both periods was less than anticipated based on calculations from CSIRO (2007) (Table 5). The anticipated fat gain corresponded reasonably well between CSIRO (2007) calculations and CT scan results with the exception of H2 lambs where fat gain was underestimated by CSIRO (2007). The proportion of fat in retained energy was greater for all treatments than anticipated from CSIRO (2007) equations, and particularly for L lambs. The proportion of fat in retained energy was greater for L2 lambs than H2 lambs (Table 5, P < 0.001).
The percentage of fat in the fleece free empty body of all lambs was 17.6 ± 0.4% at the commencement of P1 and increased to 23.9 ± 0.4% at the conclusion of P1 (P < 0.001). Fat percentage at the conclusion of P1 was greater for H1 lambs in comparison to L1 lambs (25.9 ± 0.5 vs. 21.4 ± 0.5%; P < 0.001; Figure 3). The fat percentage of all lambs decreased from 23.9 ± 0.4% at the conclusion of P1 to 18.7 ± 0.4% at the commencement of P2 (P < 0.001). At the commencement of P2, previously H1 lambs maintained a greater percentage of fat in comparison to previously L1 lambs (19.8 ± 0.6 vs. 17.3 ± 0.6%; P = 0.001; Figure 3). At the commencement of P2, there were no differences between the treatment groups due to the reallocation of half of the pens to different treatments. The empty body fat percentage of all lambs increased from 18.7 ± 0.4% at the start of P2 to 28.2 ± 0.4% at the conclusion of P2 (P < 0.001). No differences were detected at empty body fat percentage at the end of P2 due to treatment in P1. The empty body fat percentage of H2 lambs was greater than L2 lambs (30.3 ± 0.6% vs. 26.2 ± 0.6%; P < 0.001; Figure 3).
Castrated male lambs had greater FFEBW (P = 0.001) and lean tissue mass (P < 0.001) at the commencement of P1 and females had greater fat mass (P = 0.01) at the conclusion of P2. Sex had no other significant effects throughout the experiment. Sire type had effects on lean tissue mass (P < 0.001) at the conclusion of P1 and P2 and commencement of P2 but no other effects of sire were detected.
Stomach volume and mass
Mean reticulo-rumen, omasum, and abomasum (stomach) volume did not differ between treatments within feeding periods but differed between all CT scan timepoints (P < 0.001). At the commencement of P1 the mean stomach volume was 5.1 ± 0.2 L and at the conclusion was 5.5 ± 0.2 L. At the commencement of P2 the mean stomach volume was 7.6 ± 0.1 L and at the conclusion was 6.1 ± 0.1 L.
The weight of stomach contents from 48 lambs at the commencement and conclusion of each feeding period was described by the equation with an r2 of 0.93. No differences were detected for the proportion of particulate (22.2 ± 2.1%) and liquid (73.1 ± 2.0%) content of the stomach between treatments or feeding periods. No differences were detected for gas content of the stomach between treatments within feeding period; however, gas content was lower at the commencement of both feeding periods in comparison to the conclusion of both feeding periods (2.5 ± 1.0 vs. 7.0 ± 1.0%; P < 0.001).
Diet digestibility
The mean LW of the ten lambs used to determine diet digestibility was 30.5 ± 1.1 kg at the commencement of the first collection period and 40.1 ± 1.0 kg at the commencement of the second collection period. Dry matter intake was for L and H intake treatments respectively 0.84 ± 0.00 and 1.38 ± 0.02 kg DM/d in the first feeding period and 1.02 ± 0.00 and 1.57 ± 0.02 kg DM/d in the second feeding period.
Diet digestibility in the first collection period did not differ significantly between the high and low feeding levels (Table 2). Mean diet DM, OM, and GE digestibility was greater (P < 0.05) in the first collection period compared to the second collection period. Crude protein and NDF digestibility did not differ significantly between collection periods; however, ADF digestibility was greater (P < 0.05) in the second collection period. The low feeding level in the second collection period had greater DM, OM, CP, and GE digestibility compared to the high feeding level, with no differences in NDF or ADF digestibility (Table 2).
Discussion
As evidenced by the current study, the rate of fat gain in lambs is principally determined by stage of maturity and rate of empty body gain. It was expected that fat deposition as a proportion of retained energy would also demonstrate a similar pattern; however, lambs at lower levels of feeding deposited proportionately greater amounts of fat. The changes in body composition between the feeding periods highlights the sensitivity of lean tissue mass to changing nutrition, something that current predictive growth models are not capable of anticipating (see Table 5). The present results apply only to the current breed types, diets used, feeding levels and stages of development and may not be representative of the response in general.
In the current experiment, lambs were not fed differing levels of nutrition prior to the commencement of P2 and there was no response in rate or composition of gain during P2 due to P1 feeding level. The absence of a response is indicative that what has previously been referred to as compensatory gain (Drew and Reid, 1975; Turgeon et al., 1986; Sainz et al., 1995) may be primarily due to a change in feeding level for previously restricted animals rather than a change in the efficiency of energy utilization for gain. The efficiency of energy utilization for gain did appear to increase with stage of maturity and also with level of feeding; however, this result relies on the accuracy of the estimation of maintenance energy requirements (Hegarty et al., 1999). The finding that the higher level of feeding in P1 had no effect on the digestibility of the diet but decreased diet digestibility in P2 possibly indicates that the effects of level of feeding on diet digestibility are more closely related to intake as a proportion of stomach capacity rather than animal maturity.
Empty body composition and gain
Fat deposition was a greater proportion of retained energy at a later stage of maturity, which is consistent with previous research (Searle et al., 1972; Butterfield, 1988); however, fat gain was also proportionately greater at lower levels of feeding which contrasts current expectations for ruminant growth (Hegarty et al., 1999; Oddy et al., 2019). The proportion of fat in retained energy is dependent on the changes in lean tissue mass, which means that if there is a restriction or reduction in the rate of lean tissue deposition, fat deposition increases proportionately. Lean tissue mass in the empty body is comprised of both carcass muscle and the combined internal organs which are referred to as the viscera. Visceral lean mass is highly sensitive to changes in nutrition (Ferrell et al., 1986; Rompala et al., 1988; Burrin et al., 1990; Sainz et al., 1995; Sainz and Bentley, 1997) and is reduced with decreasing levels of feed intake (Hegarty et al., 1999), and at similar levels of intake by increasing the energy density of the diet (Dougherty et al., 2022).
In the current study, all lambs commenced P1 having previously been held on a lucerne pasture estimated to be of high energy density with no restrictions on intake. Lambs then commenced P2 after being held on dry naturalized pasture of high availability but predicted low energy density. The lucerne pasture would have likely resulted in a larger liver and gastrointestinal tract, whilst dry pasture may have decreased the liver mass, especially for the H1 lambs, but increased the weight of the gastrointestinal tract (Hegarty et al., 1999; Dougherty et al., 2022). This might explain the lower than expected lean tissue gain for L lambs in comparison to H lambs in the current study as a restricted diet during the feeding periods would likely have reduced lean tissue mass in the viscera. This effect is most notable in P2; although, it is not possible to confirm with the currently available CT scan data as lean tissue mass in the viscera has not been separately represented. Although it may be possible that on the lower feeding level the amount of protein available from the diet constrained protein deposition, there was more than sufficient dietary protein to maximize microbial protein supply. Under such conditions, Hegarty et al. (1999) showed no additional carcass protein deposition due to increased rumen escape protein in the diet. They concluded that at a comparable stage of maturity, protein deposition of lambs was proportional to ingested metabolizable energy supply. Further to this, lambs at zero or negative empty body gain during the feeding periods were able to continue to gain fat which can only be explained by a loss in lean tissue mass, most likely in the viscera; however, data on visceral protein change is incomplete.
Another potential explanation for fat deposition being proportionately greater at lower levels of feeding is that a reduction in viscera lean mass at lower levels of intake reduces maintenance energy requirements (Ortigues and Durand, 1995) potentially resulting in more energy availability for gain and increased fat deposition (Oddy et al., 1997). The diet could have also been limiting in protein supply or excess amino acids may have been catabolized and used as an energy source for fat deposition at lower levels of feeding (Andrews and Ørskov, 1970; Ørskov and Fraser, 1973). It is unlikely that the diet was protein deficient (Hegarty et al., 1999), or that the catabolism of amino acids resulted in substantially increased energy availability for fat deposition (Dougherty et al., 2022). It is also possible that CT scan data is not perfectly calibrated for chemical composition (fat and lean) potentially confounding results (Alston et al., 2009).
In the pasture phase between the feeding periods, previously L lambs continued to gain lean tissue mass while losing fat, see also Fattet et al. (1984). The naturalized pasture paddock contained what was assumed to be low quality forage with high fiber content. Visceral mass for the previously L lambs likely increased due to increased DM intake (Sainz et al., 1995; Dougherty et al., 2022) which is supported by the observed increase in stomach volume at the commencement of P2. The fat loss demonstrated by both treatments indicates the lambs were in negative energy balance and both the H1 and L1 lambs would have likely lost or maintained carcass lean tissue (Ball et al., 1997). This highlights the significance of the changes in the viscera for the previously L lambs as they gained in empty body lean mass. The fat loss between feeding periods could be due to one or a combination of factors that resulted in a reduction in available energy for tissue deposition. These include the insufficient intake of energy due to lower energy density feed and gut fill constraints (Allen, 2014), the increased activity energy due to grazing (CSIRO, 2007; De Brito et al., 2017) and the increased protein mass and consequent greater energy expenditure of a larger stomach and gastrointestinal tract (Koong et al., 1985; Ortigues and Durand, 1995; Oddy et al., 1997).
The lesser empty body lean tissue mass of L1 lambs at the conclusion of P1 and start of P2 may have reduced energy losses to heat production which has shown to be closely related to whole body protein mass (Graham et al., 1974). If the dry pasture between the feeding periods was insufficient to meet maintenance energy requirements for all lambs, then lambs with lower maintenance energy requirements would have mobilized less fat to meet the deficit (Fattet et al., 1984).
One limitation of CT scans is that it is not possible to remove all the intestinal contents, thus lean tissue mass may be overestimated, especially when a diet of low M/D is fed (Dougherty et al., 2022), which may partially explain results in the current study. The lack of difference in stomach volume or weight of stomach contents irrespective of feeding level or LW is consistent with the findings of Dougherty et al. (2022) and Sainz et al. (1995) where the size of the rumen was more responsive to physical characteristics of the feed and less responsive to level of intake. It is clear that the response of visceral organs to a range of diet types, nutritional restrictions and subsequent re-feeding needs further investigation.
Energetic efficiency of growth
Current feeding systems use separate constant values for the efficiency of the utilization of energy for maintenance and growth. In the current study, when the energy available for gain is calculated from estimated maintenance requirements (CSIRO, 2007), the efficiency of energy use for gain improved in older lambs and at higher levels of intake (Table 5). This is despite a reduction in the energy density of the diet at the higher feeding level in P2 (Table 2). These results are consistent with the findings of Graham (1980); however, the effects of changing levels of nutrition on viscera mass and consequent heat production (Koong et al., 1985; Ortigues and Durand, 1995; Oddy et al., 1997) are not accounted for in current maintenance energy calculations. In addition, any reduction in lean tissue mass because of nutritional history would reduce the theoretical efficiency of energy use for gain; protein deposition was expected to make up a greater proportion of empty body gain for all treatments (CSIRO, 2007; Table 5).
The results of the current study correspond reasonably well with predicted rates of fat gain (CSIRO, 2007; Table 5) with the greatest discrepancy being an underestimation of the rates of fat gain for H2 lambs. Fat deposition in the current study demonstrated a similar pattern to the data of Hegarty et al. (1999) which indicates that maturity, in terms of stage of growth, is more important than absolute age as lambs subjected to a period of weight stasis have similar priorities for fat deposition as less mature lambs.
Compensatory gain
A period of nutritional restriction has previously been shown to improve the energetic efficiency of liveweight gain and even carcass protein deposition (Drew and Reid, 1975; Turgeon et al., 1986; Sainz et al., 1995; Hegarty et al., 1999); however, there was no evidence of compensatory growth in the current study. The difference between the current experiment and that of many others that have provided evidence for compensatory growth is that irrespective of P1 treatment, lambs remained together with access to the same diet for over 10 wk prior to P2. This would have resulted in similar visceral organ mass at the commencement of P2 and similar energy losses to heat production (Graham et al., 1974; Koong et al., 1985; Ortigues and Durand, 1995). Visceral mass of sheep achieves a steady state within approximately 6 wk after a change in diet (Burrin et al., 1990).
Despite L1 lambs commencing P2 at an FFEBW that was 2.3 kg less than their H1 counterparts, they concluded the experiment 2.0 kg lighter irrespective of P2 treatment. It may be that the restriction during P1 was not great enough or that the difference in FFEBW at the commencement of P2 was too small to detect any effects; however, the current results indicate that an improvement in the energetic efficiency of growth did not occur.
Diet digestibility
The effects of level of feeding on DM, OM, CP, and GE digestibility observed in P2 are consistent with previous researchers (Ørskov et al., 1969; Graham and Searle, 1982; Margan et al., 1982). Differences in digestibility due to level of feeding were only detected during the second collection period which contrasts the results of Graham (1980) where the greatest differences were in younger, lighter lambs.
Additionally, the diet DM, OM, and GE digestibility was reduced in the second feeding period; however, the diet in P2 was a different batch (see Table 2 for difference in chemical constituents despite similar ingredients to P1). The diets differed slightly in NDF content from P1 to P2, although, the increased NDF content in the first feeding period would have more likely decreased apparent digestibility of the diet (Graham, 1980). The finding that calculated stomach volumes using CT scans were not vastly different at the end of both feeding periods might indicate that the residence time of digesta in the rumen and digestive tract was faster in lambs with higher intake, reducing DM digestibility in the second feeding period (Graham and Searle, 1982; Margan et al., 1982).
Implications and conclusion
Accurate growth predictions have significant implications for the intensification of lamb production systems to better determine the amount of time and feeding requirements to reach desired weights and body composition. For predictive growth models to better account for the variability observed in commercial production systems, it is likely that the effects of changes in nutrition on the rate of gain and mass of empty body tissues will need to be accounted for. A single term for the efficiency of energy utilization for gain was unable to account for the changes in lean tissue mass observed in the current study. The ability to predict the response of visceral organs to changes in nutrient supply and level of intake is likely integral to the development of accurate energy transaction models in growing ruminants. The commonly reported phenomenon of compensatory growth was not observed in the current experiment further highlighting the potential importance of the energetic requirements of visceral organs to the prediction of growth responses.
The deposition of fat as lambs matured appeared to follow a similar pattern to the one described by Hegarty et al. (1999) in lambs that had previously undergone a growth restriction. Whilst the efficiency of energy utilization for growth increased with stage of maturity and level of feeding, previous researchers have identified that current methods for the prediction of maintenance energy requirements are problematic for such an assessment (Hegarty et al., 1999). The loss of fat and gain in lean tissue mass observed for previously L lambs whilst grazing pasture between the feeding periods is not well understood and requires further investigation.
There was no evidence to suggest that diet digestibility increased with age in the current study possibly indicating that lower digestibility levels reported for less mature animals may be associated with LW and the capacity of the stomach rather than age.
The use of CT scans of live lambs for repeat measures on body composition removed several limitations of comparative slaughter studies (Hegarty et al., 1999). Individual animals could be represented more than once creating larger datasets from fewer animals, and there was no reliance on a representative cohort slaughtered at the commencement of the experiment. CT scanning of live animals will likely be a valuable resource in the future development and testing of more precise ruminant feeding systems. The separation of tissues into both carcass and visceral components will likely be necessary for further model development. The difficulty in manually removing intestinal contents is one clear limitation in the capability of CT scans to accurately predict lean tissue mass.
ACKNOWLEDGMENTS
The authors acknowledge and thank the technical staff for their hard work and dedication, specifically Amy Bates for assistance feeding and CT scanning lambs, Kelsey Jensen for CT scanning lambs, John Piltz for assistance with the digestibility trial, Kathleen Bernie, Peter Hawkins, Ed Clayton, and Richard Meyer for assistance with sample analysis, technical officers of Charles Sturt University; Greg Roberts and Quinten Flanagan. Additionally, we thank Sharon Nielsen Statistical Consulting for analysis assistance and Keogh Agriculture for supplying the animals and for use of paddocks. The study was supported financially by the Meat and Livestock Australia Donor Company, the Fred Morley Centre and an Australian Government Research Training Program Scholarship awarded to T.K.
Glossary
Abbreviations
- ADF
acid detergent fiber
- C
carbon
- CH4
methane
- CP
crude protein
- DM
dry matter
- OM
organic matter
- EBW
empty body weight
- FFEBW
fleece-free empty body weight
- GE
gross energy
- LW
liveweight
- M/D
energy density of feed/diet (MJ ME/kg DM)
- ME
metabolizable energy
- NDF
neutral detergent fiber
Contributor Information
Thomas P Keogh, Fred Morley Centre, School of Agricultural, Environmental and Veterinary Sciences, Charles Sturt University, Wagga Wagga, NSW 2650, Australia; Gulbali Institute, Charles Sturt University, Wagga Wagga, NSW 2650, Australia.
Shawn R McGrath, Fred Morley Centre, School of Agricultural, Environmental and Veterinary Sciences, Charles Sturt University, Wagga Wagga, NSW 2650, Australia; Gulbali Institute, Charles Sturt University, Wagga Wagga, NSW 2650, Australia.
Maxwell B Allworth, Fred Morley Centre, School of Agricultural, Environmental and Veterinary Sciences, Charles Sturt University, Wagga Wagga, NSW 2650, Australia; Gulbali Institute, Charles Sturt University, Wagga Wagga, NSW 2650, Australia.
Victor H Oddy, NSW Department of Primary Industries, Livestock Industries Centre, University of New England, Armidale, NSW 2351, Australia.
AUTHOR CONTRIBUTIONS
TK (Conceptualization, Methodology, Investigation, Formal analysis, Writing—original draft), SM (Investigation, Project administration, Funding acquisition, Writing—review and editing, Supervision), MA (Funding acquisition, Writing—review and editing, Supervision), VO (Conceptualization, Methodology, Writing—review and editing, Supervision).
Conflict of Interest Statement
The authors declare that they do not have any conflict of interest.
Literature Cited
- AFIA. 2014. Laboratory methods manual: a reference manual of standard methods for the analysis of fodder. Version 8. Melbourne, VIC: Australian Fodder Industry Association Limited. [Google Scholar]
- Allen, M. S. 2014. Drives and limits to feed intake in ruminants. Anim. Prod. Sci. 54:1513–1524. doi: 10.1071/an14478 [DOI] [Google Scholar]
- Alston, C. L., Mengersen K. L., and Gardner G. E.. . 2009. A new method for calculating the volume of primary tissue types in live sheep using computed tomography scanning. Anim. Prod. Sci. 49:1035–1042. doi: 10.1071/an09038 [DOI] [Google Scholar]
- Alston, C. L, Mengersen K. L., Thompson J. M., Littlefield P. J., Perry D., and Ball A. J.. . 2004. Statistical analysis of sheep CAT scan images using a Bayesian mixture model. Aust. J. Agric. Res. 55:57–68. doi: 10.1071/ar03017 [DOI] [Google Scholar]
- Andrews, R. P., and Ørskov E. R.. . 1970. The nutrition of the early weaned lamb: II. The effect of dietary protein concentration, feeding level and sex on body composition at two live weights. J. Agric. Sci. 75:19–26. doi: 10.1017/s0021859600026009. [DOI] [Google Scholar]
- Bain, W. E., Bezuidenhout L., Jopson N. B., Pinares-Patiño C. S., and McEwan J. C.. . 2014. Rumen differences between sheep identified as being low or high methane emitters. In: Proceedings World Congress of Genetics Applied to Livestock Production, vol. 10; p. 39. [Google Scholar]
- Ball, A. J., Oddy V. H., and Thompson J. M.. . 1997. Nutritional manipulation of body composition and efficiency in ruminants. Rec. Adv. Anim. Nutr. Australia. 13:192–208. [Google Scholar]
- Basolo, A., Parrington S., Ando T., Hollstein T., Piaggi P., and Krakoff J.. . 2020. Procedures for measuring excreted and ingested calories to assess nutrient absorption using bomb calorimetry. Obesity. 28:2315–2322. doi: 10.1002/oby.22965. [DOI] [PubMed] [Google Scholar]
- Blaxter, K., Clapperton J., and Martin A.. . 1966. The heat of combustion of the urine of sheep and cattle in relation to its chemical composition and to diet. Br. J. Nutr. 20:449–460. doi: 10.1079/BJN19660046. [DOI] [PubMed] [Google Scholar]
- Burrin, D., Ferrell C., Britton R., and Bauer M.. . 1990. Level of nutrition and visceral organ size and metabolic activity in sheep. Br. J. Nutr. 64:439–448. doi: 10.1079/BJN19900044. [DOI] [PubMed] [Google Scholar]
- Butterfield, R. M. 1988. New concepts of sheep growth. Sydney, NSW, Australia: The Department of Veterinary Anatomy, University of Sydney. [Google Scholar]
- Campbell, A., Bain W., McRae A., Broad T., Johnstone P., Dodds K., Veenvliet B., Greer G., Glass B., Beattie A., . et al. 2003. Bone density in sheep: genetic variation and quantitative trait loci localisation. Bone. 33:540–548. doi: 10.1016/s8756-3282(03)00228-x. [DOI] [PubMed] [Google Scholar]
- Charmley, E., Williams S. R. O., Moate P. J., Hegarty R. S., Herd R. M., Oddy V. H., Reyenga P., Staunton K. M., Anderson A., and Hannah M. C.. . 2015. A universal equation to predict methane production of forage-fed cattle in Australia. Anim. Prod. Sci. 56:169–180. doi: 10.1071/AN15365. [DOI] [Google Scholar]
- CSIRO. 2007. Nutrient requirements of domesticated ruminants. Melbourne, VIC, Australia: CSIRO Publishing. [Google Scholar]
- De Brito, G. F., Ponnampalam E. N., and Hopkins D. L.. . 2017. The effect of extensive feeding systems on growth rate, carcass traits, and meat quality of finishing lambs. Compr. Rev. Food Sci. Food Saf. 16:23–38. doi: 10.1111/1541-4337.12230. [DOI] [PubMed] [Google Scholar]
- Dougherty, H. C., Evered M., Oltjen J. W., Hegarty R. S., Neutze S. A., and Oddy V. H.. . 2022. Effects of dietary energy density and supplemental rumen undegradable protein on intake, viscera, and carcass composition of lambs recovering from nutritional restriction. J. Anim. Sci. 100:1–16. doi: 10.1093/jas/skac158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty, H. C., Evered M., Oltjen J. W., Oddy V. H., and Hegarty R. S.. . 2020. Crude protein-content of fat-free muscle and viscera in sheep. J. Anim. Sci. 98:144. doi: 10.1093/jas/skaa278.262. [DOI] [Google Scholar]
- Drew, K., and Reid J.. . 1975. Compensatory growth in immature sheep: I. The effects of weight loss and realimentation on the whole body composition. J. Agric. Sci. 85:193–204. doi: 10.1017/S0021859600061980. [DOI] [Google Scholar]
- Fattet, I., Hovell F. D., Ørskov E. R., Kyle D. J., Pennie K., and Smart R. I.. . 1984. Undernutrition in sheep. The effect of supplementation with protein on protein accretion. Br. J. Nutr. 52:561–574. doi: 10.1079/bjn19840123. [DOI] [PubMed] [Google Scholar]
- Ferrell, C. L., Koong L. J., and Nienaber J. A.. . 1986. Effect of previous nutrition on body composition and maintenance energy costs of growing lambs. Br. J. Nutr. 56:595–605. doi: 10.1079/bjn19860140. [DOI] [PubMed] [Google Scholar]
- Fullerton, G. D. 1980. Tissue imaging and characterisation. In: Fullerton, G. D., and Zagzebski J. A., editors. Medical physics of CT and ultrasound. Medical physics monograph, vol. 6. New York: American Institute of Physics; p. 125–162. [Google Scholar]
- Gilmour, A., Cullis B., Thompson R., Street W., and Hempstead H.. . 2009. ASReml update. What’s new in Release 3.00. Hemel Hempstead, UK: VSN International. [Google Scholar]
- Graham, N. M. 1980. Variation in energy and nitrogen utilization by sheep between weaning and maturity. Aust. J. Agric. Res. 31:335–345. doi: 10.1071/AR9800335. [DOI] [Google Scholar]
- Graham, N., and Searle T.. . 1982. Energy and nitrogen utilization for body growth in young sheep from two breeds with differing capacities for wool growth. Aust. J. Agric. Res. 33:607–615. doi: 10.1071/ar9820607. [DOI] [Google Scholar]
- Graham, N., Searle T., and Griffiths D.. . 1974. Basal metabolic rate in lambs and young sheep. Aust. J. Agric. Res. 25:957–971. doi: 10.1071/ar9740957. [DOI] [Google Scholar]
- Hegarty, R. S., Neutze S. A., and Oddy V. H.. . 1999. Effects of protein and energy supply on the growth and carcass composition of lambs from differing nutritional histories. J. Agric. Sci. 132:361–375. doi: 10.1017/s0021859698006315. [DOI] [Google Scholar]
- Hegarty, R. S., Shands C., Marchant R., Hopkins D. L., Ball A. J., and Harden S.. . 2006. Effects of available nutrition and sire breeding values for growth and muscling on the development of crossbred lambs. 1: growth and carcass characteristics. Aust. J. Agric. Res. 57:593–603. doi: 10.1071/ar04275. [DOI] [Google Scholar]
- Hodge, R. W., and Star M.. . 1984. Comparison of the fat status of lambs during continuous growth and following nutritional restriction and subsequent re-alimentation. Aust. J. Exp. Agric. 24:150. doi: 10.1071/ea9840150. [DOI] [Google Scholar]
- Koong, L. J., Ferrell C. L., and Nienaber J. A.. . 1985. Assessment of interrelationships among levels of intake and production, organ size and fasting heat production in growing animals. J. Nutr. 115:1383–1390. doi: 10.1093/jn/115.10.1383. [DOI] [PubMed] [Google Scholar]
- Kvame, T., and Vangen O.. . 2006. In-vivo composition of carcass regions in lambs of two genetic lines, and selection of CT positions for estimation of each region. Small Ruminant Res. 66:201–208. doi: 10.1016/j.smallrumres.2005.09.014. [DOI] [Google Scholar]
- Margan, D., Faichney G., Graham N., and Donnelly J.. . 1982. Digestion of a ground and pelleted diet in the stomach and intestines of young sheep from two breeds. Aust. J. Agric. Res. 33:617–627. doi: 10.1071/ar9820617. [DOI] [Google Scholar]
- Oddy, V. H., Ball A. J., and Pleasants A. B.. . 1997. Understanding body composition and efficiency in ruminants: a non-linear approach. Rec. Adv. Anim. Nutr. Australia. 11:209–222. [Google Scholar]
- Oddy, V. H., Dougherty H. C., and Oltjen J. W.. . 2019. Integration of energy and protein transactions in the body to build new tools for predicting performance and body composition of ruminants. Anim. Prod. Sci. 59:1970–1979. doi: 10.1071/an19229. [DOI] [Google Scholar]
- Ørskov, E. R., and Fraser C.. . 1973. The effect of level of feeding and protein concentration on disappearance of protein in different segments of the gut in sheep. Proc. Nutr. Soc. 32:68A–69A. [PubMed] [Google Scholar]
- Ørskov, E., Fraser C., and Kay R.. . 1969. Dietary factors influencing the digestion of starch in the rumen and small and large intestine of early weaned lambs. Br. J. Nutr. 23:217–226. doi: 10.1079/BJN19690029. [DOI] [PubMed] [Google Scholar]
- Ørskov, E. R., McDonald I., Grubb D. A., and Pennie K.. . 1976. The nutrition of the early weaned lamb. IV. Effects on growth rate, food utilization and body composition of changing from a low to a high protein diet. J. Agric. Sci. 86:411–423. doi: 10.1017/s0021859600054897. [DOI] [Google Scholar]
- Ortigues, I., and Durand D.. . 1995. Adaptation of energy metabolism to undernutrition in ewes. Contribution of portal-drained viscera, liver and hindquarters. Br. J. Nutr. 73:209–226. doi: 10.1079/bjn19950024. [DOI] [PubMed] [Google Scholar]
- Rompala, R. E., Hoagland T. A., and Meister J. A.. . 1988. Effect of dietary bulk on organ mass, fasting heat production, and metabolism of the small and large intestine in sheep. J. Nutr. 118:1553–1557. doi: 10.1093/jn/118.12.1553. [DOI] [PubMed] [Google Scholar]
- Rosset, A., Spadola L., and Ratib O.. . 2004. OsiriX: an open-source software for navigating in multidimensional DICOM images. J. Digit Imaging. 17:205–216. doi: 10.1007/s10278-004-1014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainz, R., and Bentley B.. . 1997. Visceral organ mass and cellularity in growth-restricted and refed beef steers. J. Anim. Sci. 75:1229–1236. doi: 10.2527/1997.7551229x. http://livestocklibrary.com.au/handle/1234/19837 [DOI] [PubMed] [Google Scholar]
- Sainz, R. D., Torre F. D., and Oltjen J. W.. . 1995. Compensatory growth and carcass quality in growth-restricted and refed beef steers. J. Anim. Sci. 73:2971–2979. doi: 10.2527/1995.73102971x. [DOI] [PubMed] [Google Scholar]
- Searle, T. W., Graham N. M., and O’Callaghan M.. . 1972. Growth in sheep. I. The chemical composition of the body. J. Agric. Sci. 79:371–382. doi: 10.1017/s0021859600025727. [DOI] [Google Scholar]
- Thatcher, L. P., and Gaunt G. M.. . 1992. Effects of growth path and post-slaughter chilling regime on carcass composition and meat quality of ewe lambs. Aust. J. Agric. Res. 43:819. doi: 10.1071/ar9920819. [DOI] [Google Scholar]
- Turgeon, O. A., Brink D. R., Bartle S. J., Klopfenstein T. J., and Ferrell C. L.. . 1986. Effects of growth rate and compensatory growth on body composition in lambs. J. Anim. Sci. 63:770–780. doi: 10.2527/jas1986.633770x. [DOI] [PubMed] [Google Scholar]
- Vipond, J. E., King M. E., Ørskov E. R., and Wetherill G. Z.. . 1989. Effects of fish-meal supplementation on performance of overfat lambs fed on barley straw to reduce carcass fatness. Anim. Prod. 48:131–138. doi: 10.1017/s000335610000386x. [DOI] [Google Scholar]
- Young, M. J., Nsoso S. J., Ogan C. M., and Beatson P. R.. . 1996. Prediction of carcass tissue weight in vivo using live weight, ultrasound or X-ray CT measurements. Proc. N. Z. Soc. Anim. Prod. 56:205–211. [Google Scholar]