Abstract
Importance:
The impact of a persistently enlarged GH after vaginal hysterectomy with uterosacral ligament suspension on prolapse outcomes is currently unclear.
Objectives:
This secondary analysis of the SUPeR (Study of Uterine Prolapse Procedures Randomized) trial was conducted among participants who underwent vaginal hysterectomy with uterosacral ligament suspension. We hypothesized that women with a persistently enlarged genital hiatus (GH) size would have a higher proportion of prolapse recurrence.
Study Design:
Women who underwent vaginal hysterectomy with uterosacral ligament suspension as part of the SUPeR trial (NCT01802281) were divided into three groups based on change in their preoperative to 4–6 week postoperative GH measurement: 1) Persistently Enlarged GH 2) Improved GH, or 3) Stably Normal GH. Baseline characteristics and 2-year surgical outcomes were compared across groups. A logistic regression model for composite surgical failure controlling for advanced anterior wall prolapse and genital hiatus group was fitted.
Results:
This secondary analysis included 81 women. The proportion with composite surgical failure was significantly higher among those with a Persistently Enlarged genital hiatus (50%) compared to a Stably Normal genital hiatus (12%) with unadjusted risk difference of 38% (95% CI: 4% to 68%). When adjusted for advanced prolapse in the anterior compartment at baseline, the odds of composite surgical failure was 6 times higher in the Persistently Enlarged genital hiatus group compared to the Stably Normal group (95% CI: 1.0–37.5; p=0.06).
Conclusions:
A persistently enlarged GH after vaginal hysterectomy with uterosacral ligament suspension for pelvic organ prolapse may be a risk factor for recurrent prolapse.
Keywords: genital hiatus, prolapse, uterosacral ligament suspension
Plain Language Summary:
Study Objectives:
To investigate whether or not a smaller vaginal opening after surgery for pelvic organ prolapse affects the future recurrence of pelvic organ prolapse.
Brief Description of Study:
We utilized data from women who had previously undergone pelvic surgery for prolapse in the SUPeR (Study of Uterine Prolapse Procedures Randomized) trial. We divided women into three groups based on the size of their vaginal opening before and after surgery. We then compared the chance that the women in each group had a recurrence of their prolapse either by symptoms or findings of prolapse on at 2 years after surgery.
Primary Findings:
Among 81 women in the study, there was more recurrence of prolapse in the group with a larger vaginal opening before and after surgery, but there are also likely other factors that impact the chance of prolapse recurrence
Introduction
Uterosacral ligament suspension and sacrospinous ligament fixation are commonly performed at the time of total vaginal hysterectomy as native tissue apical suspensions for uterovaginal prolapse.[1], [2] Given that recurrent pelvic organ prolapse (POP) after native tissue apical suspensions increases over time,[3] several bodies of research have sought to delineate both modifiable and non-modifiable risk factors for recurrent prolapse.[4]–[6] Numerous studies suggest that an enlarged preoperative and postoperative genital hiatus is a risk factor for recurrent anterior or overall POP after native tissue repair,[7], [8], [9] This may be due to the fact that an enlarged postoperative genital hiatus could be accompanied by a downward shift in the pelvic organs that might put increased stresses on the attachments of the vagina to the pelvic walls.[10], [11] These studies were limited by their retrospective nature and short-term follow-up. They additionally lacked subjective report of vaginal bulge symptoms.
The SUPeR (Study of Uterine Prolapse Procedures – Randomized) trial was a randomized trial of vaginal mesh hysteropexy compared with vaginal hysterectomy with uterosacral ligament suspension [14] and unlike the aforementioned retrospective studies, included subjective measures of prolapse outcomes. Our objective was to evaluate the efficacy of the vaginal hysterectomy with uterosacral ligament suspension among groups with different surgical reduction of GH size among participants who underwent this prolapse repair in the SUPeR (Study of Uterine Prolapse Procedures Randomized) trial. We hypothesized that those with a persistently enlarged GH size would have higher proportions of prolapse recurrence as compared to those with both a smaller GH pre- and post-operatively along with those with an enlarged GH pre-operatively, but a smaller GH postoperatively.
Material and Methods:
Study Population
This was an ancillary analysis of the SUPeR trial conducted by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Institutional review board approval was obtained for the SUPeR trial at each the participating sites and all participants gave informed consent. The study design, methods, and results have been published previously. [16],[14]
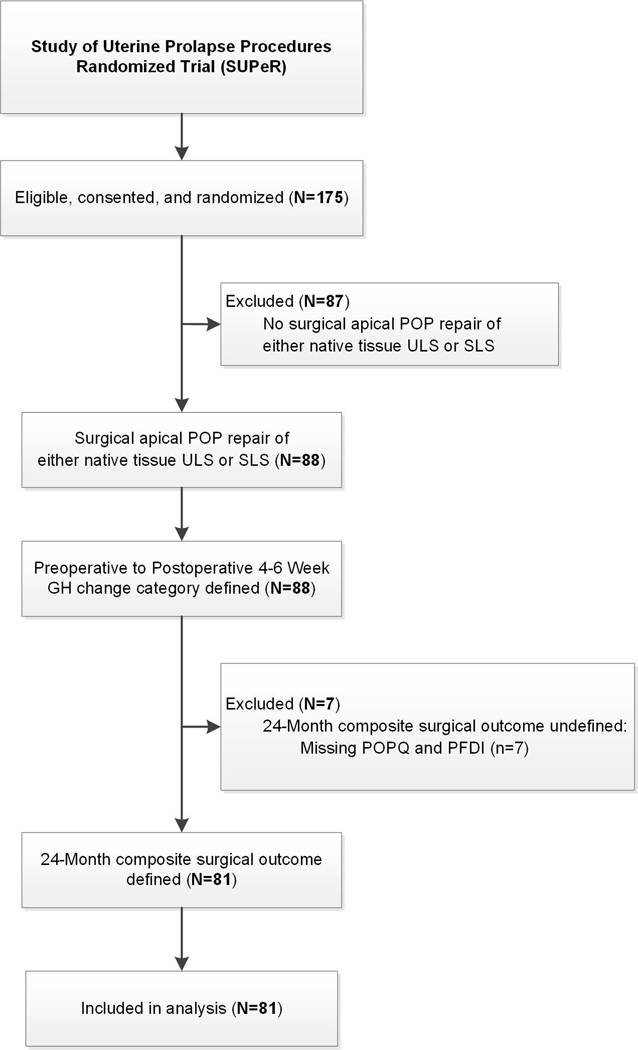
This ancillary analyses included participants who underwent vaginal hysterectomy with uterosacral ligament suspension in the SUPeR trial with 2-year follow-up. (Figure 1) Participants were divided into three groups based on the change in their preoperative to 4–6 weeks postoperative genital hiatus measured with strain on the pelvic organ quantification (POPQ) exam. Based on previous literature, a genital hiatus of ≥4 cm was considered enlarged and <4 cm was considered normal.[12], [13] Groups were: 1) Persistently Enlarged genital hiatus defined as enlarged genital hiatus at both time points, 2) Improved genital hiatus defined as an improvement in genital hiatus from enlarged to normal, or 3) Stably Normal genital hiatus defined as normal genital hiatus at both time points. Advanced pelvic organ prolapse (POP) beyond the hymen in any compartment was defined as a prolapse beyond the hymen of greater than 1 cm in any compartment (i.e. POPQ point C, Ba, or Bp > 1), where POPQ point C > 1, POPQ point Ba > 1, and POPQ point Bp > 1 corresponds to advanced POP beyond the hymen in the apical, anterior, and posterior compartments respectively.
Figure 1:
Study Flow Diagram
Outcomes
The primary aim was to compare composite surgical failure, as defined in the SUPeR trial, across the genital hiatus groups at 24 months. This primary outcome of composite surgical failure was any of the following: (1) re-treatment for prolapse (pessary or surgery); (2) anatomic failure, defined as any POP-Q measure beyond the hymen; and (3) bothersome vaginal bulge symptoms, defined as a positive response (and any degree of bother other than “not at all”) to the Pelvic Floor Distress Inventory-20[17] question “Do you usually have a bulge or something falling out that you can see or feel in your vaginal area?”.[18] Secondary outcomes included the subcomponents of composite surgical failure, POPQ measurements, post-operative complications, improvement defined as a response of “much better” or “very much better” on the Patient Global Impression of Improvement (PGII), and dyspareunia defined as pain during sex experienced ‘usually’ or ‘always’ among sexually active women or fear of pain during sex experienced ‘usually’ or ‘always’ among those who were sexually inactive based on data collected on the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-IR).[19]
Statistical Analysis
Baseline characteristics of the population were described as percentages if categorical, or with median and interquartile range (P25, P75) if continuous. For categorical measures, unadjusted p-values comparing all 3 genital hiatus groups were obtained from Fisher’s exact test, and exact pairwise risk differences and 95% confidence intervals (CI) were obtained by exact methods based on the score statistic. For continuous measures, unadjusted p-values comparing all 3 genital hiatus groups were obtained using Kruskal-Wallis test, and pairwise location shifts and 95% confidence intervals were obtained using Wilcoxon Rank-Sum test with a Hodges-Lehmann estimation. Unadjusted analyses of outcomes were performed using the same methods. Baseline characteristics that differed between genital hiatus groups at an alpha level of 0.2 were further assessed for inclusion in adjusted models based on clinical relevance with consideration for the small sample size (limiting the number of covariates), low frequencies of some characteristics, and correlation between potential covariates. Adjusted analyses were conducted for composite surgical failure and bothersome vaginal bulge via logistic regression models controlling for selected covariates.
The correlation between intraoperative genital hiatus and the first postoperative genital hiatus with strain at 4–6 weeks was explored using the Spearman rank correlation test and 95% confidence interval via Fisher’s transformation. Using the same methods, the correlations between 4–6 weeks postoperative GH and POPQ point C and total vaginal length (TVL) were also explored. All tests were conducted at a 0.05 significance level and no adjustments for multiple comparisons were made. All analyses were completed in SAS 9.4.
Results
This secondary analysis included 81 women who were primarily white (86%), with a median age of 65.6 years (P25, P75: 61.3, 71.4). There were 14 (17%) in the Persistently Enlarged group, 50 (62%) in the Improved group, and 17 (21%) in the Stably Normal group. Almost all participants underwent a vaginal hysterectomy with uterosacral ligament suspension (n=79, 98%) except for two in the Stably Normal group who underwent vaginal hysterectomy with sacrospinous ligament fixation. Advanced anterior compartment prolapse (defined as prolapse beyond the hymen) at baseline was more common in the Persistently Enlarged group compared to the Stably Normal [100% vs. 71%, risk difference 29% (95% CI: 3% to 56%)] and the Improved groups [100% vs. 70%, risk difference 30% (95% CI: 1% to 45%)]. Proportion of patients who underwent concomitant posterior repair/perineorrhaphy varied across the 3 genital hiatus groups (p=0.03) with a significant difference only between the Improved and Stably Normal groups [94% vs. 71%, risk difference 23% (95% CI: 3% to 50%)] (Table 1).
Table 1:
Preoperative and Intraoperative Characteristics by Genital Hiatus Group based on Change from Preoperative Genital Hiatus (with strain) to Postoperative 4–6 Week Genital Hiatus (with strain)
| Characteristics | Genital Hiatus Group | Pairwise Risk Difference or Location Shift (95% CI) a | p-value a | |||||
|---|---|---|---|---|---|---|---|---|
| Total (N=81) | Persistently Enlarged (N=14) | Improved (N=50) | Stably Normal (N=17) | Persistently Enlarged vs. Stably Normal | Improved vs. Stably Normal | Persistently Enlarged vs. Improved | ||
| Demographics | ||||||||
| Age (years) | 65.6 (61.3, 71.4) | 61.7 (55.2, 65.6) | 65.4 (61.9, 71.9) | 68.0 (66.2, 72.6) | −7.5 (−12.8 to −2.3) | −2.2 (−6.3 to 1.1) | −4.6 (−10.2 to −0.1) | 0.03 |
| White race | 70/81 (86) | 12/14 (86) | 43/50 (86) | 15/17 (88) | −3 (−31 to 24) | −2 (−19 to 23) | 0 (−29 to 18) | >0.99 |
| Hispanic/Latina | 7/79 (9) | 1/14 (7) | 4/49 (8) | 2/16 (13) | −5 (−32 to 22) | −4 (−31 to 11) | −1 (−15 to 26) | 0.85 |
| Higher education after high school | 49/79 (62) | 6/13 (46) | 32/49 (65) | 11/17 (65) | −19 (−52 to 19) | 1 (−24 to 30) | −19 (−48 to 12) | 0.46 |
| Married/Living as married | 53/81 (65) | 10/14 (71) | 36/50 (72) | 7/17 (41) | 30 (−6 to 61) | 31 (0 to 56) | −1 (−31 to 23) | 0.07 |
| Medical History | ||||||||
| Body mass index (kg/m2) | 27.6 (24.3, 31.0) | 29.0 (25.7, 31.0) | 27.6 (24.7, 31.0) | 25.0 (23.3, 30.5) | 2.2 (−0.8 to 6.0) | 1.4 (−0.8 to 3.7) | 0.9 (−1.5 to 3.8) | 0.28 |
| Vaginal parity | 2.0 (2.0, 3.0) | 2.0 (1.0, 2.0) | 2.0 (2.0, 3.0) | 3.0 (2.0, 3.0) | −1.0 (−1.0 to 0.0) | 0.0 (−1.0 to 0.0) | −1.0 (−1.0 to 0.0) | 0.08 |
| Post-Menopausal | 79/81 (98) | 13/14 (93) | 49/50 (98) | 17/17 (100) | 7 (−13 to 35) | 2 (−18 to 11) | 5 (−6 to 32) | 0.36 |
| Currently using vaginal estrogen | 23/81 (28) | 4/14 (29) | 13/50 (26) | 6/17 (35) | −7 (−41 to 29) | −9 (−37 to 15) | 3 (−21 to 33) | 0.78 |
| Prior prolapse surgery | 3/81 (4) | 0/14 (0) | 3/50 (6) | 0/17 (0) | 6 (−15 to 17) | −6 (−17 to 18) | 0.76 | |
| Prior urinary incontinence surgery | 4/81 (5) | 0/14 (0) | 2/50 (4) | 2/17 (12) | −12 (−36 to 12) | −8 (−33 to 6) | −4 (−14 to 21) | 0.32 |
| Current smoker | 1/81 (1) | 0/14 (0) | 1/50 (2) | 0/17 (0) | 2 (−18 to 11) | −2 (−11 to 23) | >0.99 | |
| Diabetes | 14/80 (18) | 2/13 (15) | 9/50 (18) | 3/17 (18) | −2 (−31 to 30) | 0 (−27 to 19) | −3 (−22 to 28) | >0.99 |
| Connective tissue disease | 1/81 (1) | 1/14 (7) | 0/50 (0) | 0/17 (0) | 7 (−13 to 35) | 7 (−3 to 34) | 0.17 | |
| Pelvic Floor Characteristics | ||||||||
| Pelvic Organ Prolapse Quantification (POPQ) Measurements (cm) b | ||||||||
| GH (at strain) | 4.5 (4.0, 5.0) | 5.0 (4.5, 6.0) | 4.5 (4.0, 5.0) | 3.0 (3.0, 3.5) | 2.0 (1.5 to 2.5) | 1.5 (1.0 to 2.0) | 0.5 (0.0 to 1.0) | <0.001 |
| TVL | 9.0 (8.0, 10.0) | 9.0 (9.0, 10.0) | 9.0 (8.0, 9.0) | 9.0 (9.0, 10.0) | 0.0 (−1.0 to 1.0) | −0.5 (−1.0 to 0.0) | 0.5 (0.0 to 1.0) | 0.15 |
| C | 0.0 (−2.0, 3.0) | 1.5 (−2.0, 5.0) | 0.0 (−3.0, 3.0) | 0.0 (−2.0, 2.0) | 2.0 (−1.0 to 5.0) | 0.0 (−1.5 to 2.0) | 1.0 (−1.5 to 4.0) | 0.56 |
| Ba | 3.0 (1.5, 4.0) | 4.0 (3.0, 5.0) | 3.0 (1.0, 4.0) | 2.0 (1.0, 2.0) | 2.0 (1.0 to 3.0) | 1.0 (0.0 to 2.0) | 1.0 (0.0 to 2.5) | 0.006 |
| Bp | 1.0 (−1.0, 3.0) | 1.0 (−2.0, 4.5) | 0.5 (−1.0, 3.0) | 1.0 (−1.0, 2.0) | 1.0 (−1.0 to 3.0) | 0.5 (−1.0 to 2.0) | 0.5 (−2.0 to 3.0) | 0.71 |
| Advanced Pelvic Organ Prolapse (POP) Beyond the Hymen c | ||||||||
| Any compartment | 62/81 (77) | 14/14 (100) | 36/50 (72) | 12/17 (71) | 29 (3 to 56) | 1 (−22 to 29) | 28 (1 to 43) | 0.06 |
| Apical | 33/81 (41) | 7/14 (50) | 20/50 (40) | 6/17 (35) | 15 (−22 to 48) | 5 (−25 to 29) | 10 (−19 to 39) | 0.69 |
| Anterior | 61/81 (75) | 14/14 (100) | 35/50 (70) | 12/17 (71) | 29 (3 to 56) | −1 (−24 to 27) | 30 (1 to 45) | 0.04 |
| Posterior | 31/81 (38) | 6/14 (43) | 19/50 (38) | 6/17 (35) | 8 (−28 to 42) | 3 (−26 to 27) | 5 (−23 to 35) | 0.90 |
| Intraoperative Characteristics | ||||||||
| ULS and hysterectomy (vs. SLS and hysterectomy) | 79/81 (98) | 14/14 (100) | 50/50 (100) | 15/17 (88) | 12 (−12 to 36) | 12 (0 to 36) | 0.07 | |
| Anterior colporrhaphy | 59/81 (73) | 10/14 (71) | 39/50 (78) | 10/17 (59) | 13 (−23 to 47) | 19 (−6 to 46) | −7 (−37 to 17) | 0.33 |
| Posterior colporrhaphy and/or perineorrhaphy | 71/81 (88) | 12/14 (86) | 47/50 (94) | 12/17 (71) | 15 (−17 to 45) | 23 (3 to 50) | −8 (−37 to 8) | 0.03 |
| Urinary incontinence repair | 42/81 (52) | 7/14 (50) | 26/50 (52) | 9/17 (53) | −3 (−38 to 33) | −1 (−29 to 27) | −2 (−31 to 27) | >0.99 |
| Estimated blood loss (cc) | 100 (100, 200) | 150 (100, 300) | 138 (100, 200) | 100 (75, 150) | 50 (0 to 125) | 25 (0 to 50) | 0 (−50 to 100) | 0.14 |
| Intraoperative POPQ GH (at rest) (cm) b | 3.0 (3.0, 4.0) | 3.8 (3.5, 4.0) | 3.5 (3.0, 4.0) | 3.0 (3.0, 3.0) | 0.5 (0.5 to 1.0) | 0.0 (0.0 to 0.5) | 0.5 (0.0 to 1.0) | 0.009 |
Data are median (P25, P75) for continuous measures and n/N (%) for categorical measures unless otherwise specified. CI=Confidence Interval, P25=25th Percentile, P75=75th Percentile, ULS=Uterosacral Ligament Suspension, SLS=Sacrospinous Ligament Suspension
. For categorical measures, the p-values were obtained from Fisher’s exact test and exact pairwise risk difference and 95% CI limits were obtained by exact methods based on the score statistic. For continuous measures p-values were obtained using Kruskal-Wallis test and pairwise location shift and 95% confidence intervals were obtained using Wilcoxon Rank-Sum test with a Hodges-Lehmann estimation of location shift.
In the Pelvic Organ Prolapse Quantification (POPQ) system, TVL (total vaginal length) and GH (genital hiatus) are measured as positive values. The positions of C, Ba, and Bp are measured at the most dependent location (the point of greatest prolapse) of the apex, anterior vaginal wall, and posterior vaginal wall respectively during a straining. Values are measured in cm and are negative if above the hymen, and positive if below the hymen.
Advanced pelvic organ prolapse (POP) beyond the hymen in any compartment is defined as a prolapse beyond the hymen of greater than 1 cm in any compartment (i.e. POPQ point C, Ba, or Bp > 1), where POPQ point C > 1, POPQ point Ba > 1, and POPQ point Bp > 1 corresponds to advanced POP beyond the hymen in the apical, anterior, and posterior compartments respectively.
Regarding the primary outcome, in unadjusted analysis, the proportion with composite surgical failure at 2 years was significantly higher in the Persistently Enlarged group (7/14, 50%) compared to the Stably Normal group (2/17, 12%) with unadjusted risk difference of 38% (95% CI: 4% to 68%). There was no difference between the Persistently Enlarged group compared to the Improved group (22%) with unadjusted risk difference of 28% (95% CI: −2% to 56%). Recurrent prolapse beyond the hymen in any compartment varied across the GH groups (p=0.02), the highest in the Persistently Enlarged group (5/14, 36%), followed by 6/49 (12%) in the Improved group, and 0/17 in the Stably Normal group with most prolapses occurring in the anterior compartment (10/11, 91%). Both any- and anterior-compartment prolapse were significantly different between only the Persistently Enlarged and Stably Normal groups with an unadjusted risk difference of 36% (95% CI: 10% to 65%). (Table 2) There was no significant difference in bothersome vaginal bulge symptoms (p=0.75) or retreatment for prolapse (p=0.51) across the GH groups. When adjusting for advanced prolapse in the anterior compartment at baseline, the adjusted odds of composite surgical failure in the Persistently Enlarged GH group was 6.0 times the odds in the Stably Normal group (95% CI: 1.0 to 37.5), but this did not meet statistical significance (p=0.06). In adjusted analysis, there was no difference between groups for bothersome vaginal bulge. (Table 3)
Table 2:
Unadjusted Analyses of 24-Month Outcomes by Genital Hiatus Group based on Change from Preoperative Genital Hiatus (with strain) to Postoperative 4–6 Week Genital Hiatus (with strain)
| Outcomes | Genital Hiatus Group | Pairwise Risk Difference or Location Shift (95% CI) a | p-value a | |||||
|---|---|---|---|---|---|---|---|---|
| Total (N=81) | Persistently Enlarged (N=14) | Improved (N=50) | Stably Normal (N=17) | Persistently Enlarged vs. Stably Normal | Improved vs. Stably Normal | Persistently Enlarged vs. Improved | ||
| Composite surgical failure b | 20/81 (25) | 7/14 (50) | 11/50 (22) | 2/17 (12) | 38 (4 to 68) | 10 (−16 to 28) | 28 (−2 to 56) | 0.05 |
| Anatomic outcomes | ||||||||
| Pelvic Organ Prolapse Quantification (POPQ) Measurements (cm) c | ||||||||
| GH (at strain) | 3.0 (3.0, 4.0) | 4.0 (4.0, 5.0) | 3.0 (3.0, 4.0) | 3.0 (2.5, 3.0) | 1.5 (1.0 to 2.0) | 0.5 (0.0 to 1.0) | 1.0 (0.5 to 1.5) | <0.001 |
| TVL | 8.0 (7.0, 8.5) | 8.0 (8.0, 9.0) | 8.0 (7.0, 9.0) | 7.0 (6.5, 8.0) | 1.0 (0.0 to 2.0) | 1.0 (0.0 to 1.0) | 0.5 (0.0 to 1.0) | 0.02 |
| C | −6.0 (−7.0, −5.0) | −5.3 (−7.0, −4.5) | −6.0 (−7.0, −5.0) | −6.0 (−7.0, −5.0) | 1.0 (0.0 to 2.0) | 0.0 (−0.5 to 1.0) | 0.5 (−0.5 to 1.5) | 0.35 |
| Ba | −1.0 (−2.0, 0.0) | −0.8 (−1.0, 1.0) | −1.0 (−2.0, 0.0) | −2.0 (−2.0, −1.0) | 1.0 (0.5 to 2.5) | 0.5 (0.0 to 1.0) | 1.0 (0.0 to 2.0) | 0.04 |
| Bp | −2.0 (−3.0, −1.0) | −2.3 (−3.0, −1.0) | −2.0 (−3.0, −1.0) | −2.5 (−3.0, −1.5) | 0.0 (0.0 to 1.0) | 0.0 (0.0 to 1.0) | 0.0 (−0.5 to 0.5) | 0.48 |
| Pelvic Organ Prolapse (POP) Beyond the Hymen d | ||||||||
| Any compartment | 11/80 (14) | 5/14 (36) | 6/49 (12) | 0/17 (0) | 36 (10 to 65) | 12 (−10 to 25) | 23 (−2 to 52) | 0.02 |
| Apical | 0/80 (0) | 0/14 (0) | 0/49 (0) | 0/17 (0) | ||||
| Anterior | 10/80 (13) | 5/14 (36) | 5/49 (10) | 0/17 (0) | 36 (10 to 65) | 10 (−11 to 23) | 26 (0 to 54) | 0.01 |
| Posterior | 1/80 (1) | 0/14 (0) | 1/49 (2) | 0/17 (0) | 2 (−19 to 12) | −2 (−12 to 22) | >0.99 | |
| Subjective outcomes | ||||||||
| Bothersome vaginal bulge symptoms e | 8/80 (10) | 2/13 (15) | 5/50 (10) | 1/17 (6) | 10 (−16 to 40) | 4 (−20 to 18) | 5 (−13 to 35) | 0.75 |
| Patient Global Impression of Improvement (PGII) of much better or very much better | 72/80 (90) | 13/13 (100) | 44/50 (88) | 15/17 (88) | 12 (−15 to 36) | 0 (−16 to 25) | 12 (−15 to 25) | 0.57 |
| Dyspareunia f | 4/72 (6) | 1/13 (8) | 2/43 (5) | 1/16 (6) | 1 (−24 to 30) | −2 (−27 to 12) | 3 (−11 to 32) | 0.81 |
| Retreatment for prolapse (surgery or pessary) | 6/81 (7) | 2/14 (14) | 3/50 (6) | 1/17 (6) | 8 (−17 to 37) | 0 (−23 to 13) | 8 (−8 to 37) | 0.51 |
Data are median (P25, P75) for continuous measures and n/N (%) for categorical measures unless otherwise specified. CI=Confidence Interval, P25=25th Percentile, P75=75th Percentile
For categorical measures, the p-values were obtained from Fisher’s exact test and exact pairwise risk difference and 95% CI limits were obtained by exact methods based on the score statistic. For continuous measures p-values were obtained using Kruskal-Wallis test and pairwise location shift and 95% confidence intervals were obtained using Wilcoxon Rank-Sum test with a Hodges-Lehmann estimation of location shift.
Composite surgical failure is defined as the occurrence of an anatomic failure (i.e. pelvic organ prolapse beyond the hymen d), subjective failure (i.e. bothersome vaginal bulge symptoms e), or retreatment failure (i.e. surgery or pessary retreatment for pelvic organ prolapse).
In the Pelvic Organ Prolapse Quantification (POPQ) system, TVL (total vaginal length) and GH (genital hiatus) are measured as positive values. The positions of C, Ba, and Bp are measured at the most dependent location (the point of greatest prolapse) of the apex, anterior vaginal wall, and posterior vaginal wall respectively during a straining. Values are measured in cm and are negative if above the hymen, and positive if below the hymen.
Pelvic organ prolapse (POP) beyond the hymen in any compartment is defined as a prolapse beyond the hymen in any compartment (i.e. POPQ point C, Ba, or Bp > 0), where POPQ point C > 0, POPQ point Ba > 0, and POPQ point Bp > 0 corresponds to POP beyond the hymen in the apical, anterior, and posterior compartments respectively.
Bothersome vaginal bulge symptoms is defined as a positive response to any vaginal bulge symptoms (i.e. PFDI-20 item 3) with a degree of bother greater than not at all indicated.
Dyspareunia is defined as pain during sex experienced usually or always among the sexually active or fear of pain during sex experienced usually or always among the sexually inactive based on data collected on the PISQ-IR for SUPeR study participants. No dyspareunia is defined as pain during sex experienced never, seldom, or sometimes among the sexually active or fear of pain during sex experienced never, seldom, or sometimes among the sexually inactive based on data collected on the PISQ-IR for SUPeR study participants
Table 3:
Adjusted Analyses of Composite Surgical Failure and Bothersome Vaginal Bulge Symptoms a
| Model to Predict Composite Surgical Failure a | |||
|---|---|---|---|
| Variable | Mean Log Estimate (Standard Error) b | Adjusted Odds Ratio (95% Confidence Interval) b | p-value b |
| GH group: Improved vs Stably Normal | 0.8 (0.83) | 2.2 (0.4, 11.1) | 0.36 |
| GH group: Persistently Enlarged vs Stably Normal | 1.8 (0.94) | 6.0 (1.0, 37.5) | 0.06 |
| Advanced POP in anterior compartment e: Yes vs No | 1.0 (0.83) | 2.8 (0.6, 14.1) | 0.21 |
| Model to Predict Bothersome Vaginal Bulge Symptoms d | |||
| Variable | Mean Log Estimate (Standard Error) b | Adjusted Odds Ratio (95% Confidence Interval) b | p-value b |
| GH group: Improved vs Stably Normal | 0.6 (1.14) | 1.8 (0.2, 16.6) | 0.61 |
| GH group: Persistently Enlarged vs Stably Normal | 0.9 (1.30) | 2.4 (0.2, 31.0) | 0.50 |
| Advanced POP in anterior compartment c: Yes vs No | 0.8 (1.13) | 2.3 (0.2, 20.9) | 0.47 |
Composite surgical failure is defined as the occurrence of an anatomic failure (i.e. pelvic organ prolapse beyond the hymen c), subjective failure (i.e. bothersome vaginal bulge symptoms d), or retreatment failure (i.e. surgery or pessary retreatment for pelvic organ prolapse)
Mean log estimates, standard errors, adjusted odds ratios, 95% Wald confidence intervals, and p-values were obtained from the adjusted logistic regression model adjusting for categorized change from preoperative genital hiatus (strain) to postoperative 4–6 week genital hiatus (strain) GH groups and advanced prolapse beyond the hymen in the anterior compartment e.
Pelvic organ prolapse (POP) beyond the hymen in any compartment is defined as a prolapse beyond the hymen in any compartment (i.e. POPQ point C, Ba, or Bp > 0).
Bothersome vaginal bulge symptoms is defined as a positive response to any vaginal bulge symptoms (i.e. PFDI-20 item 3) with a degree of bother greater than not at all.
Advanced pelvic organ prolapse (POP) beyond the hymen in the anterior compartment is defined as a prolapse beyond the hymen of greater than 1 cm in the anterior compartment (i.e. POPQ point Ba > 1).
There were no differences in post-operative complications among the GH groups. (Supplemental Table 1) There was also no difference in dyspareunia among GH groups. (Table 2)
Finally, there was a statistically significant moderate Spearman-rank correlation of 0.30 between the intraoperative GH at rest measurement and the first postoperative GH measurement with strain at 4–6 weeks (95% CI: 0.09 to 0.49; p=0.006). At 4–6 weeks postoperative, the correlation between GH and POPQ point C was not statistically significant (r=0.03, 95% CI: −0.19 to 0.24; p=0.81) or TVL (r=0.06, 95% CI: −0.16 to 0.27; p=0.60). (Supplemental Figures 1-3)
Discussion
In a secondary analysis of the SUPeR trial, women with a Persistently Enlarged genital hiatus 4 to 6 weeks after vaginal hysterectomy with uterosacral ligament suspension were not at a higher risk of composite surgical failure 2-years after surgery when compared to other groups, but there was more recurrent anatomic prolapse in the anterior vaginal compartment between those with a Persistently Enlarged genital hiatus and those with a Stably Normal genital hiatus. There were no anatomic recurrences in the Stably Normal group. This is similar to other studies[9], [13] and continues to highlight that, even in a prospective population from a randomized controlled trial, women with a persistently enlarged genital hiatus may have a higher risk of recurrent prolapse. We did not detect a difference in vaginal bulge between the persistently enlarged and stably normal groups, but we were limited in our ability to find significant differences given the small group sizes and the fact that only 10% of participants reported bothersome postoperative vaginal bulge.
It appears that women with a normal pre-operative GH measure are at the lowest risk of recurrent prolapse after vaginal hysterectomy with uterosacral ligament suspension. Although we found that the Persistently Enlarged group was not associated with future bothersome vaginal bulge or retreatment, other studies have shown that bulge symptom bother severity was significantly associated with genital hiatus size.[20] Longitudinal studies also show that prolapse incidence is strongly associated with genital hiatus size and that a more enlarged genital hiatus is an important predictor of future prolapse risk.[7], [21] It could be that as patients are followed into the future we would see a more frequent report of bother among women with a persistently enlarged GH.[22]. We did find that there was more anterior compartment recurrence in the Persistently Enlarged group, but of note, there was also more advanced anterior wall prolapse in this group at baseline. We were limited in our ability to control for this factor in logistic regression given small size, but we did adjust for advanced prolapse in general. Future studies that continue to investigate the relationship of GH size and prolapse severity should be continued.
Other studies have found that concomitant posterior repair at sacrospinous ligament fixation or uterosacral ligament suspension is not associated with surgical success after adjusting for baseline covariates using propensity scores or unadjusted comparison.[5] We also found those that underwent a posterior repair and/or perineorrhaphy did not universally have a reduction in their genital hiatus and there are likely many factors such as underlying levator avulsion[23] or posterior repair technique that underly these findings. We also did not see a correlation between postoperative genital hiatus and POPQ point C so it does not seem to necessarily be the “quality” of one’s apical suspension that relates to modulation of genital hiatus size. It seems to be the unfortunate current reality that we do not know how best to modulate the genital hiatus to prevent prolapse recurrence after native tissue apical suspension. We did find that zero participants in the Stably Normal group had anatomic recurrence at 2 years and this points to the fact that it may be important for patient counseling and selection that these women have a lower chance of prolapse recurrence than their counterparts with an enlarged preoperative GH size.
Previous literature has shown that an immediate post-operative GH measurement >3.5 cm is also related to recurrent prolapse after native tissue apical suspension.[9] For purposes of this study, we chose to use the 4 to 6-week postoperative measurement according to the conventional definition,[18] which is performed during Valsalva as this is similar to the categorization from previous literature. Additionally, we did find moderate correlation between intraoperative genital hiatus measurement (taken after suspension at rest) and the 4–6 week genital hiatus measurement with strain. Surgeons can use our data, and the aforementioned recent literature showing an enlarged genital hiatus immediately following surgery is also associated with prolapse recurrence after apical suspension,[9] to help guide their practice.
The main strengths of our study include inclusion of a well-characterized study population from a rigorous, multi-center randomized control trial with consistent surgical techniques and standardized questionnaires. Additionally, we used a novel categorization from previous evidence[12], [13] that considers the surgical reduction of an individual patient’s genital hiatus size. We based our primary outcome of composite surgical failure on a rigorous definition that was used in the original SUPeR trial which accounts for subjective outcomes that were lacking from previous evidence.[16] Finally, we have significantly longer follow-up of our cohort as compared to the previous literature that had average follow-up of less than a year.
In terms of study limitations, we recognize that our study has an overall small study size. The fact that we did not find a statistically significant difference between prolapse recurrence in women in the Persistently Enlarged and Improved groups despite an unadjusted risk difference of 28% could have been due to the small number of participants in each group. Future research is necessary to determine if these groups do have differential recurrence as time goes on. We chose to exclude the vaginal mesh hysteropexy group as a part of our analysis do the inherent differences in outcomes and surgical technique.[23] Additionally, the decision to add a posterior repair or perineorrhaphy at the time of vault suspension was not standardized among surgeons, but there was still a similar proportion with these repairs in the Persistently Enlarged and Improved groups. Other studies have used alternative cut-offs including greater than 3.75 cm[21] and greater than or equal to 3.5 cm[9] for an enlarged GH. Although we used greater than or equal to 4 cm in our study, the median GH for the Improved and Stably Normal groups was 3.0 cm which would fall below the enlarged GH cut-off for both other studies.
Our present work builds on the association between pre- and post-operative genital hiatus size and vaginal prolapse recurrence.[9], [12], [13] Our study expands on these investigations by confirming that an enlarged genital hiatus is associated with risk of anatomic prolapse recurrence, specifically in the anterior compartment. Future research utilizing MRI, transperineal ultrasound,[23] and biomarkers must continue to investigate the underlying mechanism of prolapse recurrence after native tissue apical suspension with specific attention paid towards how to optimize modulation of the genital hiatus.
In conclusion, baseline genital hiatus size itself is not a modifiable risk factor, but a persistently enlarged genital hiatus does appear to be associated with more anterior vaginal wall prolapse recurrence after vaginal hysterectomy with uterosacral ligament suspension when compared to those with a normal genital hiatus measurement before and after surgery. We do emphasize that at this current time, modulation of GH size, via a posterior repair/perineorrhaphy or levator plication, is not universally successful in terms of prevention of future prolapse given our data and the current literature. Our information may add to the growing body of research surrounding the predictive nature of GH size to help providers with patient selection and counseling.
Supplementary Material
Supplemental Figure 1. Correlation between Intraoperative and Postoperative 4–6 Week Genital Hiatus
Supplemental Figure 2. Correlation between Postoperative 4–6 Week POPQ C and Genital Hiatus
Supplemental Figure 3. Correlation between Postoperative 4–6 Week Total Vaginal Length and Genital Hiatus
“Why this matters?”.
Previous studies investigating the impact of a reduction in genital hiatus (GH) size before and after pelvic organ prolapse surgery have lacked subjective data. Utilizing data from women who underwent vaginal hysterectomy with uterosacral ligament suspension in the SUPeR (Study of Uterine Prolapse Procedures Randomized) trial, we sought to incorporate both objective and subjective as we investigated the impact on pre- and post-operative GH size on prolapse outcomes. Although we found that there was a higher proportion of composite prolapse recurrence in those with a persistently enlarged GH after surgery, this impact was somewhat mitigated in models controlling for pre-operative advanced prolapse in general. Future research to investigate how to improve prolapse outcomes by surgical methods, including addressing GH size, are still necessary.
Acknowledgements:
Primary authors of the SUPeR trial paper including Charles W. Nager, MD; Anthony G. Visco; Holly E. Richter, PhD, MD; Charles R. Rardin, MD; Rebecca G. Rogers, MD; Heidi S. Harvie, MD, MSCE, MBA; Halina M. Zyczynski, MD; Marie Fidela R. Paraiso, MD; Scott Grey, PhD; Dennis Wallace, PhD. Additionally, Cassandra Carberry, Nicole Korbly, Ann S Meers, Deborah L Myers, Vivian W Sung, Ly Pung, Beri Ridgeway, Annette Graham, Cecile Ferrando, Matthew Barber, Amie Kawasaki, Shantae McLean, Alison Weidner, Nazema Y. Siddiqui, Gouri B. Diwadkar, Linda M Mackinnon, Katrina Burson, Marie Gantz, Amaanti Sridhar, Ryan Whitworth, Kendra Glass, Kathy Carter, David Ellington, Ryanne Johnson, Sunita Patel, Robin Willingham, Velria Willis, Keisha Dyer, Kimberly Ferrante, Kyle Herrala, Emily S Lukacz, Yuko Komesu, Gena Dunivan, Peter Jeppson, Lorraine Flick, Heidi S Harvie, Michelle Kingslee, Ariana Smith, Judy Gruss, Michael Bonidie, Pamela Moalli, Jonathan Shepherd, Gary Sutkin and Donna Mazloomdoost whom were all central for the success of the SUPeR trial.
Funding:
The study was conducted by the Eunice Kennedy Shriver National Institute of Child Health and Development-sponsored Pelvic Floor Disorders Network (grant numbers U10 HD054214, U10 HD041267, U10 HD041261, U10 HD069013, U10 HD069025, U10 HD069010, U10 HD069006, U10 HD054215, and U01 HD069031). The project was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institutes of Health Office of Research on Women’s Health. Boston Scientific Corporation provided partial study support through an unrestricted grant.
Footnotes
Conflicts of Interest:
Bradley, MS: none. Disclosure: Hologic, Axonics – research support
Sridhar A: none.
Ferrante KL: none. Disclosure: Valencia Technologies and BlueWind Medical- grant support
Andy UU: none.
Visco AG: none. Disclosure: Stock ownership in Ninomed.
Florian-Rodriguez M: none,
Myers D: none.
Varner E: none,
Mazloomdoost D: none.
Gantz MG: Research grant to RTI as the PFDN DCC from Boston Scientific.
Accepted as a scientific salon presentation for the annual American Urogynecologic Society meeting Oct 12–15, 2021
Trial Registry: http://www.clinicaltrials.gov (NCT01802281)
References:
- [1].Olsen AL, Smith VJ, Bergstrom JO, Colling JC, and Clark AL, “Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence,” Obstet Gynecol, vol. 89, pp. 501–506, 1997, doi: 10.1016/s0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- [2].Margulies RU, Rogers MA, and Morgan DM, “Outcomes of transvaginal uterosacral ligament suspension: systematic review and metaanalysis,” Am J Obstet Gynecol, vol. 202, pp. 124–134, 2010, doi: 10.1016/j.ajog.2009.07.052. [DOI] [PubMed] [Google Scholar]
- [3].Jelovsek JE et al. , “Effect of uterosacral ligament suspension vs sacrospinous ligament fixation with or without perioperative behavioral therapy for pelvic organ vaginal prolapse on surgical outcomes and prolapse symptoms at 5 years in the OPTIMAL randomized clinical trial,” JAMA - Journal of the American Medical Association, vol. 319, no. 15, pp. 1554–1565, Apr. 2018, doi: 10.1001/jama.2018.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jakus-Waldman S et al. , “Risk Factors for Surgical Failure and Worsening Pelvic Floor Symptoms Within 5 Years After Vaginal Prolapse Repair,” Obstetrics and gynecology, vol. 136, no. 5, pp. 933–941, Nov. 2020, doi: 10.1097/AOG.0000000000004092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sutkin G. et al. , “Association between adjuvant posterior repair and success of native tissue apical suspension,” in American Journal of Obstetrics and Gynecology, Feb. 2020, vol. 222, no. 2, pp. 161.e1–161.e8. doi: 10.1016/j.ajog.2019.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vergeldt TF, Weemhoff M, IntHout J, and Kluivers KB, “Risk factors for pelvic organ prolapse and its recurrence: a systematic review,” Int Urogynecol J, vol. 26, pp. 1559–1573, 2015, doi: 10.1007/s00192-015-2695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vakili B, Zheng YT, Loesch H, Echols KT, Franco N, and Chesson RR, “Levator contraction strength and genital hiatus as risk factors for recurrent pelvic organ prolapse,” Am J Obstet Gynecol, vol. 192, pp. 1592–1598, 2005, doi: 10.1016/j.ajog.2004.11.022. [DOI] [PubMed] [Google Scholar]
- [8].Medina CA, Candiotti K, and Takacs P, “Wide genital hiatus is a risk factor for recurrence following anterior vaginal repair,” Int J Gynaecol Obstet, vol. 101, pp. 184–187, 2008, doi: 10.1016/j.ijgo.2007.11.008. [DOI] [PubMed] [Google Scholar]
- [9].Siff LN et al. , “Immediate Postoperative Pelvic Organ Prolapse Quantification Measures and 2-Year Risk of Prolapse Recurrence,” Obstetrics and gynecology, vol. 136, no. 4, pp. 792–801, Oct. 2020, doi: 10.1097/AOG.0000000000004043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Delancey JO and Hurd WW, “Size of the urogenital hiatus in the levator ani muscles in normal women and women with pelvic organ prolapse,” Obstet Gynecol, vol. 91, pp. 364–368, 1998. [DOI] [PubMed] [Google Scholar]
- [11].Chen L, Ashton-Miller JA, Hsu Y, and DeLancey JO, “Interaction among apical support, levator ani impairment, and anterior vaginal wall prolapse,” Obstet Gynecol, vol. 108, pp. 324–332, 2006, doi: 10.1097/01.AOG.0000227786.69257.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bradley MS, Askew AL, Vaughan MH, Kawasaki A, and Visco AG, “Robotic-assisted sacrocolpopexy: early postoperative outcomes after surgical reduction of enlarged genital hiatus,” Am J Obstet Gynecol, vol. 218, pp. 514.e1–514.e8, 2018, doi: 10.1016/j.ajog.2018.01.046. [DOI] [PubMed] [Google Scholar]
- [13].Vaughan MH et al. , “Surgical Alteration of Genital Hiatus Size and Anatomic Failure After Vaginal Vault Suspension,” Obstet Gynecol, vol. 131, pp. 1137–1144, 2018, doi: 10.1097/aog.0000000000002593. [DOI] [PubMed] [Google Scholar]
- [14].Nager CW et al. , “Effect of vaginal mesh hysteropexy vs vaginal hysterectomy with uterosacral ligament suspension on treatment failure in women with uterovaginal prolapse: A randomized clinical trial,” JAMA - Journal of the American Medical Association, vol. 322, no. 11, pp. 1054–1065, Sep. 2019, doi: 10.1001/jama.2019.12812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nager CW et al. , “Effect of sacrospinous hysteropexy with graft vs vaginal hysterectomy with uterosacral ligament suspension on treatment failure in women with uterovaginal prolapse: 5-year results of a randomized clinical trial.,” American journal of obstetrics and gynecology, vol. 322, no. 11, pp. 1054–1065, Mar. 2021, doi: 10.1016/j.ajog.2021.03.012. [DOI] [Google Scholar]
- [16].Nager CW et al. , “The Design of a Randomized Trial of Vaginal Surgery for Uterovaginal Prolapse: Vaginal Hysterectomy With Native Tissue Vault Suspension Versus Mesh Hysteropexy Suspension (The Study of Uterine Prolapse Procedures Randomized Trial),” Female Pelvic Med Reconstr Surg, 2016, doi: 10.1097/spv.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Barber MD, Kuchibhatla MN, Pieper CF, and Bump RC, “Psychometric evaluation of 2 comprehensive condition-specific quality of life instruments for women with pelvic floor disorders,” Am J Obstet Gynecol, vol. 185, pp. 1388–1395, 2001, doi: 10.1067/mob.2001.118659. [DOI] [PubMed] [Google Scholar]
- [18].Bump RC et al. , “The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction,” Am J Obstet Gynecol, vol. 175, pp. 10–17, 1996. [DOI] [PubMed] [Google Scholar]
- [19].Lukacz ES et al. , “Sexual Activity and Dyspareunia 1 Year After Surgical Repair of Pelvic Organ Prolapse,” Obstetrics and gynecology, vol. 136, no. 3, pp. 492–500, Sep. 2020, doi: 10.1097/AOG.0000000000003992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Muñiz KS, Voegtline K, Olson S, and Handa V, “The role of the genital hiatus and prolapse symptom bother,” International Urogynecology Journal, vol. 32, no. 4, pp. 829–834, Apr. 2021, doi: 10.1007/s00192-020-04569-x. [DOI] [PubMed] [Google Scholar]
- [21].Lowder JL, Oliphant SS, Shepherd JP, Ghetti C, and Sutkin G, “Genital hiatus size is associated with and predictive of apical vaginal support loss,” Am J Obstet Gynecol, vol. 214, pp. 718.e1–8, 2016, doi: 10.1016/j.ajog.2015.12.027. [DOI] [PubMed] [Google Scholar]
- [22].Handa VL, Blomquist JL, Carroll MK, and Muñoz A, “Genital Hiatus Size and the Development of Prolapse Among Parous Women,” Female pelvic medicine & reconstructive surgery, vol. 27, no. 2, pp. e448–e452, Feb. 2021, doi: 10.1097/SPV.0000000000000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chen L, Xie B, Fenner DE, Duarte Thibault ME, Ashton-Miller JA, and DeLancey JO, “Structural failure sites in posterior vaginal wall prolapse: stress 3D MRI-based analysis,” International Urogynecology Journal, 2021, doi: 10.1007/s00192-021-04685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].CW N. et al. , “Effect of sacrospinous hysteropexy with graft vs vaginal hysterectomy with uterosacral ligament suspension on treatment failure in women with uterovaginal prolapse: 5-year results of a randomized clinical trial,” American journal of obstetrics and gynecology, vol. 225, no. 2, pp. 153.e1–153.e31, Aug. 2021, doi: 10.1016/J.AJOG.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Correlation between Intraoperative and Postoperative 4–6 Week Genital Hiatus
Supplemental Figure 2. Correlation between Postoperative 4–6 Week POPQ C and Genital Hiatus
Supplemental Figure 3. Correlation between Postoperative 4–6 Week Total Vaginal Length and Genital Hiatus