Abstract
Background:
The oral commensal bacterial species Streptococcus gordonii has been reported to regulate the inflammation of oral epithelial cells stimulated by the periodontal pathogen Porphyromonas gingivalis. This study investigated the activities of S. gordonii metabolites in S. gordonii spent culture supernatants (Sg-SCS) on periodontal-related bacterial growth and periodontitis-associated inflammatory cytokines.
Methods:
Sg-SCS was collected from S. gordonii cultures grown in Dulbecco’s Modified Eagle Medium (DMEM) and added to the growth media of representative health- and disease-related oral species: S. gordonii, Streptococcus sanguinis, Streptococcus mitis, Streptococcus oralis, P. gingivalis, Tannerella forsythia, and Treponema denticola. The Sg-SCS was also tested for its ability to regulate expression of proinflammatory cytokines by human macrophages, epithelial cells, and gingival fibroblasts upon stimulation with P. gingivalis-derived lipopolysaccharide (Pg-LPS).
Results:
Sg-SCS significantly reduced transcript and protein levels of interleukin (IL)-1β, 6, and 8 induced by Pg-LPS stimulation in multiple types of periodontal cells. mRNA sequencing and bioinformatics analyses indicated that Sg-SCS significantly affects 10 inflammatory pathways. Additionally, Sg-SCS exhibited suppression of the growth of periodontal disease-related bacteria, including T. denticola and P. gingivalis, along with the primary plaque colonizing species S. oralis. At a low concentration, Sg-SCS also inhibits P. gingivalis adhesion.
Conclusions:
These results strongly suggest that S. gordonii-derived SCS contains metabolites that have anti-inflammatory properties and an ability to inhibit periodontitis-associated pathogenic bacteria. Further investigation will be needed to identify the individual metabolites within the Sg-SCS in order to develop a novel metabolite-based approach to treating and preventing periodontitis. (244 words)
Keywords: Streptococcus gordonii, Culture Media, Conditioned, Microbiology, Inflammation, Cytokines
A one-sentence summary:
S. gordonii-derived spent culture supernatant contains metabolites that have anti-inflammatory properties and an ability to inhibit periodontitis-associated pathogenic bacteria.
Introduction
Periodontitis is a chronic inflammatory disease with a complex etiology acting at multiple levels, including microbial and host contributions, and may lead to tooth loss. Nearly 50% of adults have periodontitis which also is closely linked to systemic diseases, including cardiovascular disease, gastrointestinal and colorectal cancer, diabetes and insulin resistance, and Alzheimer’s disease 1–4. While oral hygiene, scaling and cleaning, and antibiotics have achieved relative success in arresting the progression of early stage periodontitis without systemic disease association, surgical intervention is needed for patients with advanced periodontitis. However, the success rate of the current surgical treatment for moderate to advanced periodontitis is relatively low 5. Recent studies have demonstrated that systemic conditions such as metabolic syndrome altered the microbial profile of both healthy subjects and periodontitis patients 6. Further, microbial dysbiosis may only initiate periodontal disease in the oral tissues of susceptible individuals who have other risk factors associated with host genotype, stress, diet or risk-related behavior such as smoking, obesity and diabetes 7. A poorly controlled host immune response has been postulated to generate a self-perpetuating pathogenic cycle where dysbiosis and inflammation reinforce each other by forming a positive feedback loop in periodontitis 8.
Commensal bacteria are known to serve as an interface between host metabolism and the immune system via nutrient- and metabolite-dependent mechanisms 9, 10. Many of these nutrients and metabolites are implicated in the immune system’s development, homeostasis and function. Oral commensals, including Streptococcus mitis and Streptococcus oralis, have been reported to protect the middle ear against inflammation induced by respiratory pathogens 11. Streptococcus gordonii, a Gram-positive bacterium, is commensal bacterial colonizer of the oral cavity where it can be found attached to the tooth surface 12. Although S. gordonii can be an opportunistic pathogen that may cause local or systemic diseases under specific circumstances 13, a recent study demonstrated that S. gordonii can reprogram epithelial cell global transcriptional patterns following Porphyromonas gingivalis-induced gingival epithelial cell proliferation, highlighting the potential for S. gordonii to be used in periodontitis prevention and treatment 14. S. gordonii also effectively prevented the invasion of P. gingivalis into oral epithelial cells and reprogrammed the cells to resist P. gingivalis-induced Zinc finger E-box-binding homeobox 2 (Zeb2) by suppressing Forkhead Box O1 (FOXO1) 15. Based on this evidence, we hypothesize that the beneficial effect of S. gordonii resides in metabolites it produces and that they act to modulate the immune responses of host cells in gingival tissues. In this study, we investigated the properties of S. gordonii spent culture supernatant (Sg-SCS) on the proliferation of periodontal pathogens and the expression of proinflammatory cytokines.
Interleukin (IL)-1β, 6, and 8 are proinflammatory cytokines that are elevated and involved in the progression of periodontitis and subsequent tissue destruction 16–19. IL-1β upregulates the expression of matrix metalloproteinases (MMPs) and receptor activator of nuclear factor kappa-Β ligand (RANKL) which promotes extracellular matrix and bone destruction 16. It also goes on to modulate the secretion of secondary cytokines such as IL-6 and IL-8 17. IL-6, on the other hand, regulates adaptive immune system by promoting the differentiation of B cells to plasma cells. The chemokine IL-8 attracts neutrophil to infiltrate the diseased area which further progresses periodontitis 20. These three proinflammatory cytokines are markers we have used to compare the inflammatory state of the host when treated with Sg-SCS. In addition, oral epithelial cells and oral fibroblasts have been shown to respond differently to proinflammatory stimulus 21, therefore both cells were investigated in our study. Monocyte-derived macrophage were also investigated because they have shown a high relevance to the severity of alveolar bone destruction in periodontal disease 22.
In this study, we observed that Sg-SCS significantly reduced proinflammatory cytokines, including IL-1β, 6, and 8 induced by P. gingivalis-derived lipopolysaccharide (Pg-LPS), Gram-negative bacterial components capable of initiating inflammation through the activation of both TLR2 and TLR423. mRNA-seq and bioinformatics analysis further demonstrated that Sg-SCS regulated periodontitis-associated inflammatory signaling pathways. Additionally, Sg-SCS selectively suppressed the growth of periodontitis-related bacterial species, including Treponema denticola and P. gingivalis, and inhibited P. gingivalis adhesion. These results support the hypothesis that the beneficial effects of S. gordonii on inflammation and microbial ecology reside in specific metabolites that S. gordonii produces and that these may be a source of novel agents for the treatment and prevention of periodontitis.
Materials and Methods
1. Preparation of the SCS of health-related oral Streptococcus species
S. gordonii (ATCC 33399 and 10558), Streptococcus sanguinis (ATCC 10556), S. mitis (ATCC 49456), and S. oralis (ATCC 35037) were cultured individually in a total of 40 ml Dulbecco’s Modified Eagle Medium (DMEM) * at 5% CO2, 37 °C. After cultures reached an OD600 of 0.2±0.05, the supernatant were collected by centrifugation for 6 min at 16000g and then filtered through a 0.22μm filter. The SCS was aliquoted into 1.5 ml tubes and stored at −80°C.
2. Culture conditions and growth measurement of health-related bacterial strains
S. gordonii (ATCC 33399), S. sanguinis, S. mitis, and S. oralis were grown on Brain Heart Infusion (BHI)† agar at 37°C 5% CO2. Each strain was then subcultured in 4 ml BHI medium for 30 min at 37°C before inoculating 60 μl into each well of a 24-well plate containing 2 ml of BHI medium with different concentrations of Sg-SCS from S. gordonii (ATCC 33399) or DMEM. 1% horse serum ‡ was added to the medium for S. mitis. The bacteria were then incubated at 37°C 5% CO2 for up to 48 hrs. At intervals of 4, 12, and 24 hrs, OD measurement was performed in duplicate using a microplate reader § at a wavelength of 600 nm.
3. Culture conditions and growth measurement of pathogenic bacterial strains
P. gingivalis (ATCC 49417), Tannerella forsythia (ATCC 43037), and T. denticola (ATCC 35405) were inoculated in 5 ml thioglycolate medium enriched with Vitamin K1 and Hemin †. The bacteria were incubated at 37°C in an anaerobic atmosphere until they reached stationary phase. For P. gingivalis, 300 μl was inoculated into each culture tube containing 5 ml of BHI media with 5% horse serum and with different concentrations of Sg-SCS or DMEM. The bacteria were then incubated at 37°C anaerobically for up to 96 hrs. At intervals of 6 and 12 hrs, OD measurement was performed in duplicate using a microplate reader at a wavelength of 600 nm. For T. forsythia and T. denticola, 150 μl of each was then inoculated into wells of a 24-well plate containing 2 ml of BHI media supplemented with 5% horse serum and with different concentrations of Sg-SCS or DMEM. The bacteria were then incubated at 37°C anaerobically for up to 48 hrs. At intervals of 6, 12, and 24 hrs, OD measurement was performed in duplicate using a microplate reader at a wavelength of 600 nm.
4. Calculation of maximal growth rate and growth yield
The maximal growth rate (GR) was calculated using OD readings from two time points during the exponential growth phase where the OD readings doubled. The slope of the growth between those two time points were calculated as the maximal growth rate. Growth yield (GY) was calculated by subtracting the OD reading at time zero from the highest OD reading as the growth curve transitioned to stationary phase.
5. Attachment measurement of P. gingivalis
Analysis of P. gingivalis attachment was performed based on previous publication 24. Briefly, 150 μl of P. gingivalis was inoculated into a 24-well plate containing 2 ml of BHI media with 5% horse serum and different concentrations of Sg-SCS or DMEM. The bacteria were then incubated at 37°C anaerobically until they reached stationary phase. Culture media was removed and the plate was immersed in deionized water four times to wash-off unattached bacteria. After the plate was dried in a 37°C incubator, each well was stained with 0.2% crystal violet for 5 min. After the stain was removed and plates were rinsed, dried, and destained with 80% ethanol, OD was measured at 595 nm to quantify the attached bacteria. The effect of Sg-SCS at 1% on P. gingivalis attachment was further investigated using a serum-coated plate as an attachment substrate. Each experimental well was coated with diluted horse serum to PBS at a ratio of 1:5 overnight at 4°C prior to the experiment25.
6. Comparison of Streptococcus species SCS on proinflammatory cytokines
Mouse macrophages (RAW 264.7) were used to test SCS from different Streptococcus species for effects on markers of inflammation. The mouse macrophages were cultured in DMEM medium in 24-well plates (2×104 cells/per well) and treated with Pg-LPS ‖ (100ng/ml) supplemented (5% v/v) with cell-free SCS. The transcripts of proinflammatory cytokines, including IL-1β, IL-6, and IL-8, were measured up to 24 hrs using two-step real-time quantitative reverse-transcription polymerase chain reaction (qRT-PCR).
7. Function of Sg-SCS on human cell viability
THP-1 cells (human monocyte) (ATCC TIB-202) and primary human gingival fibroblasts (HGF) were purchased commercially. Human oral epithelial cells, non-tumor-derived GMSM-K cells established by Dr. Valerie A. Murrah at the University of North Carolina School of Dentistry 26, were kindly obtained from Dr. Brad Amendt at the University of Iowa. Human monocyte-derived macrophages (MDM) were differentiated from THP-1 cells using 8 ng/ml phorbol 12-myristate 13-acetate ¶ over 24 hrs. THP-1 is cultured in Roswell Park Memorial Institute (RPMI) 1640 ‡ supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. After differentiation, MDM was cultured in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin. HGF and GMSM-K were cultured using DMEM with 10% FBS and 1% penicillin-streptomycin. An MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5- diphenyltetrazolium bromide) assay was used to test the effect of Sg-SCS on the viability of human macrophages, epithelial cells, and HGFs according to the manufacturer’s protocol # 27.
8. Proinflammatory cytokines regulated by Sg-SCS
Human MDM were seeded at 105 cells/per well in a 6 well-plate and cultured with RPMI 1640 medium. GMSM-K cells and HGF were seeded in a 24-well plate at 2×104 cells/per well and cultured with DMEM with 1% FBS. The cells were pretreated with Sg-SCS at 1%, 5%, and 10% (v/v) for 24 hrs before LPS challenge at 100 ng/ml. Because the timelines of proinflammatory cytokine secretions are different after Pg-LPS exposure, multiple time points were used to comprehensively investigate the inhibitory function of Sg-SCS. The transcripts of proinflammatory cytokines, including IL-1β, IL-6, and IL-8, were measured after 6 and 24 hrs. The protein levels of IL-1β, IL-6, and IL-8 in the culture supernatant were quantified after 24 hrs using enzyme-linked immunosorbent assay (ELISA) ** according to the manufacturer’s instructions17. The protein levels of inflammatory cytokines of HGF with different treatments were also quantified using a Human Inflammation Array C1 kit †† according to the manufacturer’s instructions.
9. Quantitative gene analysis
Total RNA from cultured mouse macrophage, human MDM, HGF and GMSM-K cells was extracted using a miRNeasy Mini Kit ‡‡. The concentration and purity of total RNA were quantified using a NanoDrop One spectrophotometer §§. mRNA expression was measured by qRT-PCR using the PrimeScript Reagent Kit ‖‖ to carry out reverse transcription and amplification reactions using primers with SYBR Green PCR Master Mix §§. The forward and reverse primers and real-time probes for human IL-1β were: 5’-AATGGATTTGGACGCATTGGT-3’, 5’-TTTGCACTGGTACGTGTTGAT-3’; human IL-6: 5’-CCATCTTTGGAAGGTTCAGGTTG-3’, 5’-ACTCACCTCTTCAGAACGAATTG-3’; human IL-8: 5’-AACCCTCTGCACCCAGTTTTC-3’, 5’- ACTGAGGATTGAGAGTGGAC-3’; human GAPDH: 5’-TGTGGGCATCAATGGATTTGG-3’, 5’-ACACCATGTATTCCGGGTCAAT-3; mouse IL-1β: 5’-ATGATGGCTTATTACAGTGGCAA-3’, 5’-GTCGGAGATTCGTAGCTGGA-3’; mouse IL-6: 5’-TAGTCCTTCCTACCCCAATTTCC-3’, 5’-TTGGTCCTTAGCCACTCCTTC-3’; mouse IL-8: 5’-CAAGGCTGGTCCATGCTCC-3’, 5’-TGCTATCACTTCCTTTCTGTTGC-3’; mouse GAPDH: 5’-GAAATGCCACCTTTTGACAGTG-3’, 5’-TGGATGCTCTCATCAGGACAG-3’. All samples were normalized to GAPDH.
10. mRNA sequencing
mRNA-seq was performed to compare untreated primary HGFs and HGFs treated with 5% Sg-SCS. HGFs were seeded in a 24-well plate and treated with Sg-SCS and DMEM at 5% (v/v). Total RNA from HGFs was extracted after 24 hrs using the miRNeasy Mini kit and mRNA-seq was performed commercially ¶¶. RNA degradation and contamination was monitored on 1% agarose gels, RNA purity was checked using a Nanophotometer® spectrophotometer, and RNA integrity and quantitation were assessed using the RNA Nano 6000 Assay Kit of the Bioanalyzer 2100 system ##. A total of 1 μg RNA per sample was used as input material for the RNA sample preparations. Sequencing libraries were generated using NEBNext® UltraTM RNA Library Prep Kit for Illumina® *** following manufacturer’s recommendations and index codes were added to attribute sequences to each sample. The clustering of the index-coded samples was performed on a cBot Cluster Generation System using PE Cluster Kit cBot-HS ††† according to the manufacturer’s instructions. After cluster generation, the library preparations were sequenced on an Illumina platform and paired-end reads were generated. RNA samples with an RNA integrity number (RIN) > 7 were used for the mRNA sequencing executed.
11. Statistical Analyses
A one-way ANOVA with post-hoc Tukey’s HSD test was utilized to evaluate whether there was a significant difference in each measurement among different treatments at each time point. Student’s T-test was utilized to evaluate the difference between the attachment of P. gingivalis on a serum-coated plate. All statistical analyses were performed using commercially available SPSS 25 Statistical Software ‡‡‡ utilizing a significance level of 0.05. For mRNA seq data, differential expression analysis between two conditions/groups (three biological replicates per condition) was performed using DESeq2 R package28. DESeq2 provides statistical routines for determining differential expression in digital gene expression data using a model based on the negative binomial distribution. The resulting P values were adjusted using the Benjamini and Hochberg’s approach for controlling the False Discovery Rate (FDR)29. Genes with an adjusted P value < 0.05 identified by DESeq2 were assigned as differentially expressed. To identify enriched pathways, pathway analysis using IPA software was performed on differentially expressed genes.
Results
Sg-SCS has only minor effects on the growth of health-related streptococci.
We tested the effects of Sg-SCS on the growth of four health-related commensal species. Figure 1 summarizes the growth profiles and measurements of maximal growth rate (GR) and growth yield (GY) with different concentrations of Sg-SCS. The Sg-SCS was collected from S. gordonii cultured in DMEM and thus the treatment of DMEM was used as a control. Sg-SCS and DMEM at different concentrations showed no significant changes in GR and GY for S. mitis (Fig. 1A). Sg-SCS significantly increased the GR and GY of S. gordonii and S. sanguinis compared to the untreated control, but the difference compared to growth in DMEM was not statistically significant (Figure 1B and C). For S. oralis, Sg-SCS at 10% was no different compared to the untreated control but had a significantly decreased GY compared to DMEM (Figure 1D), indicating a potential inhibitory function of Sg-SCS on S. oralis growth at high concentrations.
Figure 1.
Proliferation of health-related streptococci treated with Sg-SCS. A-D: Growth curve (top) and calculated maximal growth rates (GR), and growth yields (GY) of S. mitis (A), S. gordonii (B), S. sanguinis (C) and S. oralis (D) after treatment with different concentrations of Sg-SCS, compared to the untreated control and DMEM. Column means that do not share a letter are statistically significantly different using the post-hoc Tukey’s HSD test (p<0.05; performed in triplicate).
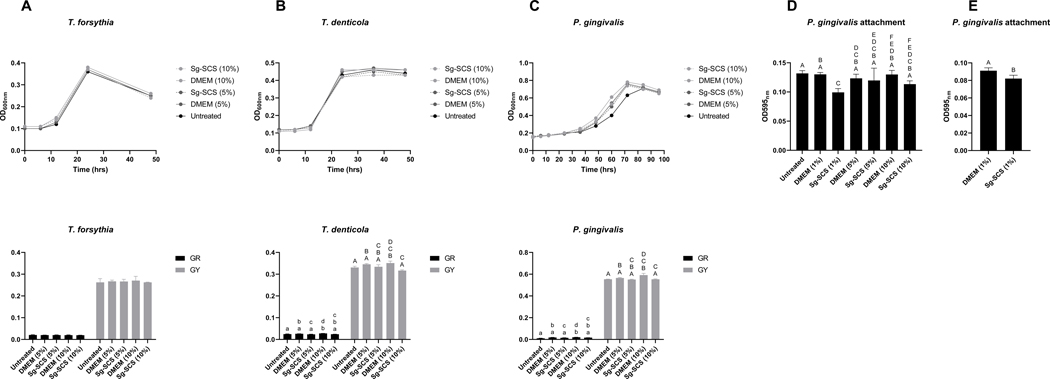
Sg-SCS selectively inhibits periodontal pathogenic bacterial growth.
The effects of Sg-SCS were also tested on the growth of three periodontal pathogen species. Figure 2 summarizes the regulation of Sg-SCS on the growth of T. forsythia, T. denticola, and P. gingivalis, and on the attachment of P. gingivalis. No effect of the SCS and DMEM on T. forsythia growth was observed (Figure 2A). DMEM at 10% significantly increased the GR and GY of T. denticola and P. gingivalis, whereas Sg-SCS at 10% was similar to the untreated control but significantly reduced the GR and GY compared to DMEM at the same concentrations (Figure 2B, C). In addition, Sg-SCS at 1% significantly reduced attachment of P. gingivalis compared to the untreated control and the same concentration of DMEM. However, this effect was not observed at higher concentrations of Sg-SCS (Figure 2D). Upon testing on serum-coated plates as an attachment substrate to mimic the serum-rich subgingival environment, Sg-SCS at 1% still showed a significantly reduced attachment of P. gingivalis (Figure 2E).
Figure 2.
Proliferation and attachment of periodontal pathogenic bacteria treated with Sg-SCS. A-C: Growth curves (top) and calculated GR and GY of T. forsythia (A), T. denticola (B), and P. gingivalis (C) after treatment with Sg-SCS and controls at different concentrations. D: P. gingivalis attachment to uncoated-plate after different treatment of Sg-SCS as measured by optical density. E: P. gingivalis attachment to serum-coated plate after treatment with 1% Sg-SCS as measured by optical density. Column means that do not share a letter are statistically significantly different using the post-hoc Tukey’s HSD test or Student’s T-Test (p<0.05; performed in triplicate).
SCS from select Streptococcus species vary in their effects on Pg-LPS-induced expression of IL-1β, 6 and 8
Mouse macrophages were used to test SCS from different Streptococcus species for effects on inflammation. Figure 3 highlights the superior anti-inflammatory effects of Sg-SCS on mouse macrophages induced by Pg-LPS, compared to SCS from other oral Streptococcus species. Specifically, after 6 and 24 hrs, macrophages exposed to Pg-LPS highly increased the transcripts of IL-1β, 6 and 8 (Figure 3A-C). SCS from both strains of S. gordonii (ATCC 33399 and 10558) shared similar abilities in effectively and significantly downregulating transcripts of IL-1β, 6 and 8 compared to Pg-LPS treatment after 6 and 24 hrs. The SCS from S. mitis and S. sanguinis showed similar anti-inflammatory abilities as S. gordonii on IL-6 and 8 but did not affect IL-1β. The SCS of S. oralis was not anti-inflammatory as it significantly increased transcripts of IL-8.
Figure 3.
Comparison of Streptococcus species on regulation of proinflammatory cytokines. A-C: Relative fold changes in the transcripts of IL-1β (A), IL-6 (B), and IL-8 (C) in mouse macrophages treated with SCS from different Streptococcus species after Pg-LPS challenge. Column means that do not share a letter are statistically significantly different using the post-hoc Tukey’s HSD test (p<0.05; performed in triplicate). Sg1: S. gordonii ATCC 33399, Sg2: S. gordonii ATCC 10558.
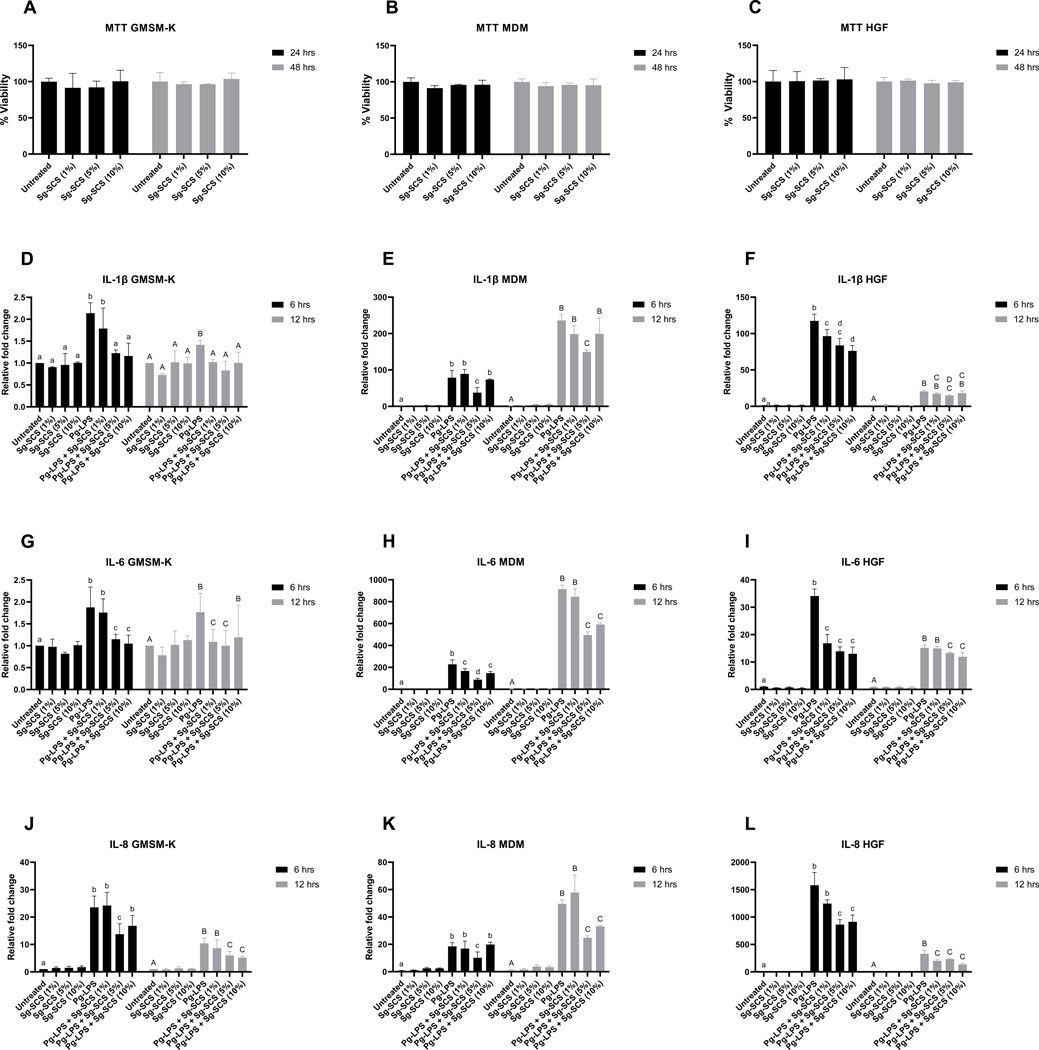
Sg-SCS is biocompatible and potentially reduces proinflammatory cytokine transcripts in human macrophages, epithelial cells, and HGFs.
We tested the toxicity and the anti-inflammation properties of Sg-SCS. Compared to untreated controls, no significant variation of Sg-SCS treatment at different concentrations on cell viabilities was observed in MDM, GMSM-K and HGF after 24 and 48 hrs (Figure 4A-C). To investigate the anti-inflammatory properties of Sg-SCS, cells were cultured using 1% FBS and supplemented with Sg-SCS at 1%, 5%, and 10% (v/v) for 24 hrs before challenge with Pg-LPS (100 ng/ml). Transcripts of IL-1β, 6, and 8 were quantitatively measured using qRT-PCR after different periods. Figure 4D-L summarizes the variations of proinflammatory cytokine transcripts after treatment with Sg-SCS and Pg-LPS. Whereas transcripts of IL-1β, 6 and 8 are comparable to the untreated controls after treatment with Sg-SCS at different concentrations, Pg-LPS significantly increased the transcripts of IL-1β, 6, and 8 in MDM, GMSM-K cells, and HGF after 6 and 12 hrs. Interestingly, pretreatment with Sg-SCS at 5% significantly downregulated transcripts of IL-1β, 6, and 8 in the cells 6 and 12 hrs after Pg-LPS challenge. Sg-SCS at 10% significantly downregulated the majorities of IL-1β, 6, and 8 transcripts in the cells after the Pg-LPS challenge. However, 1% SCS has limited protective effect.
Figure 4.
Effects of Sg-SCS on viabilities and transcripts of IL-1β, IL-6, and IL-8 of periodontal cells. A-C: MTT assay of epithelial cells (A), macrophages (B), and HGFs (C); D-L: Relative fold changes in the transcripts of IL-1β (D-F), IL-6 (G-I), and IL-8 (J-L) in human epithelial cells (D, G, J), macrophages (E, H, K) and HGFs (F, I, L) treated with Sg-SCS after Pg-LPS challenge. Column means that do not share a letter are statistically significantly different using the post-hoc Tukey’s HSD test (p<0.05; performed in triplicate).
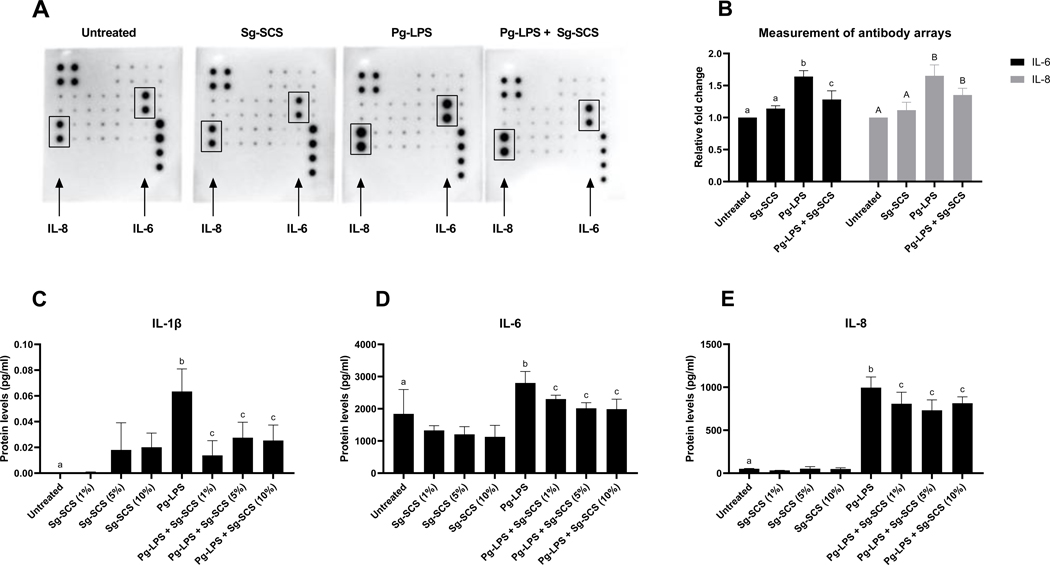
Sg-SCS reduces IL-1β, 6 and 8 proteins in HGF after Pg-LPS stimulation
The protein levels of IL-1β, 6 and 8 in HGF 24 hrs after treatment with Sg-SCS and challenge with Pg-LPS were quantified using the Human Inflammation Array C1 and ELISA. Figure 5A and B represents images and signal intensity measurements obtained with RayBio C-series Antibody Array. Notably, treatment with 5% Sg-SCS significantly downregulated the signal intensity of IL-6 protein (p<0.05) and marginally reduced IL-8 (p=0.081). Measured by ELISA, there were no significant differences among the cells treated with Sg-SCS at different concentrations, but treatment with Sg-SCS significantly reduced the amount of IL-1β, 6 and 8 after Pg-LPS challenge. (Figure 5 C-E).
Figure 5.
Effects of Sg-SCS on proteins of IL-1β, IL-6, and IL-8 from HGFs. A-B: Representative images (A) and signal intensities (B) obtained with RayBio C-Series Antibody Arrays. C-E: Protein levels of IL-1β (C), IL-6 (D), and IL-8 (E) in HGF treated with Sg-SCS after Pg-LPS challenge. Column means that do not share a letter are statistically significantly different using the post-hoc Tukey’s HSD test (p<0.05; performed in triplicate).
Sg-SCS upregulates anti-inflammatory signaling and down-regulates pro-inflammatory signaling.
We performed mRNA sequencing to compare untreated primary HGF and HGF treated with 5% Sg-SCS (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE211078). Figure 6 summarizes the enriched canonical pathways of the differentially expressed immune genes using Ingenuity Pathway Analysis (IPA). Significant canonical pathways were determined by setting the P value threshold 0.05(–log (p-value)>1.33). There were 10 pathways identified in Sg-SCS regulation, including inflammatory signaling pathways of IL-6, IL-8, NF-ĸB (Figure 6A). mRNA-seq analysis also identified the associated genes that are involved in the respective canonical pathways (Figure 6B). Notably, proinflammatory cytokines and mediators of periodontitis, including MMP16, IL6ST, and EGFR, were down-regulated after Sg-SCS treatment.
Figure 6.
Immune signaling pathways and associated genes regulated by Sg-SCS in HGF.
Discussion
The oral microbiome is comprised of hundreds of different species that coexist within a complex network of interactions, among themselves and with the host. The nature of these interactions and the dominant players differ between health and disease. Certain health-associated species have been found to potently modulate interactions with the host by regulating signaling pathways in host epithelial cells. Elucidating the mechanisms by which select microbes promote a healthy oral environment offers opportunities to apply this knowledge for the treatment and prevention of disease. The current investigation focused on S. gordonii due to its reported ability to modulate the inflammatory response of epithelial cells to the periodontal pathogen P. gingivalis 15. Periodontitis is characterized by a dysbiotic oral microbiome to which the host strongly responds with an imbalanced expression of proinflammatory cytokine that can further disease progression 7, 8, 30. S. gordonii products of metabolism, present in SCS, were hypothesized to be the source of the health-promoting activities.
The culture supernatant of P. gingivalis has been shown to induce an increase in the transcripts and protein levels of IL-1β, 6, and 8 in periodontal ligament stem cells while the culture supernatant of two oral Streptococcus species, Streptococcus mutans and Streptococcus anginosus, did not significantly increase the transcripts of those proinflammatory cytokines 18. We have demonstrated that following Pg-LPS challenge, an increase in IL-1β, 6, and 8 transcripts of GMSM-K, MDM, and HGF and protein levels in HGF was observed. In contrast, groups treated with Sg-SCS showed a significant decrease in transcripts and protein levels of the three proinflammatory markers compared to LPS challenge alone. This shows that Sg-SCS contains anti-inflammatory potential, unlike culture supernatant from P. gingivalis, S. mutans, and S. anginosus. Upregulation of these proinflammatory cytokines in periodontal tissues and cells exaggerates the inflammation of periodontitis and leads to activation of osteoclasts and subsequent bone resorption 31. Our RNA-seq analysis further clarified that the regulation mediated by Sg-SCS in HGFs involves multiple periodontitis-associated inflammatory signaling pathways, including NF-ĸB, IL-6 and IL-8, and downregulates associated gene expression32–34. These findings further support the idea that metabolites within Sg-SCS may fine tune periodontal inflammation by mitigating proinflammatory cytokines and mediators.
The SCS from S. gordonii cultured in DMEM also had inhibitory activity directed towards the growth of periodontal pathogens. Sg-SCS significantly inhibited the maximal growth rate and growth yield of specific strains of T. denticola and P. gingivalis. In addition, our current study also observed that Sg-SCS reduced the ability of P. gingivalis to adhere and form an in vitro biofilm. These outcomes indicate that the metabolite components within the Sg-SCS may also function to maintain symbiosis of the microbiome by suppressing the shift to a periodontitis-associated dysbiosis.
Metabolite components within Sg-SCS vary based on the culture medium. Although it is not an optimal medium for the growth of streptococcal species, we found that S. gordonii, S. sanguinis, S. mitis, and S. oralis grow well in DMEM (see Figure S1 in online Journal of Periodontology). The SCS from DMEM cultures can easily be tested for inflammatory functions induced by SCS components. We found that Sg-SCS exhibits superior anti-inflammatory properties compared to the SCS from other common oral Streptococcus species. We have begun the process of identifying the differential components within the Sg-SCS responsible for the effects on inflammation and oral microbes. These will form the basis for developing a novel approach to periodontitis treatment and prevention. Our future work will focus on the investigation of the differential components as it is likely that distinct metabolites harbor the properties observed in this study.
Commensal organisms are known to antagonize pathogens through colonization resistance in polymicrobial communities. They engage in antimicrobial activities by producing bacteriocins or by competing for niches and nutrients. However, not all interactions are solely antimicrobial. For example, interactions between commensal S. gordonii and the periodontal pathogen P. gingivalis affect colonization and proliferation in a complex and cascading manner that can have variable effects on virulence 30. It may one day be possible to positively manipulate the microbiome on a grand scale in a ‘natural’ manner through consumption of prescribed substrates. But before this becomes reality, reductionist approaches with select metabolites offer an opportunity to both gain insight into the metabolic networks within polymicrobial communities and exploit these associations to promote the health of the host.
Conclusions
Sg-SCS inhibits the growth and attachment of periodontal bacteria and reduces proinflammatory cytokine transcripts and protein levels upon Pg-LPS challenge. Certain proinflammatory cytokines and mediators of periodontitis were downregulated in an mRNA differential expression analysis.
Supplementary Material
Acknowledgments
This study was supported by the National Institute of Dental and Craniofacial Research (Grant No. R01DE026433 to LH; Grant No. R01DE026716 to JB) of the National Institutes of Health (NIH).
Footnotes
Conflict of Interests: All authors have no conflicts of interest to declare that are relevant to the content of this article.
HyClone, Cytiva, Logan, UT
Becton, Dickinson and Company, MD
Gibco, ThermoFisher Scientific, Waltham, MA
SpectraMax iD3, Molecular Devices, San Jose, CA
InvivoGen, San Diego, CA
Sigma-Aldrich, St Louis, MO
Biotium, Fremont, CA
R&D systems, Minneapolis, MN
RayBio C-Series, RayBiotech, Peachtree Corners, CA
Qiagen, Germantown, MD
Thermo Scientific, Waltham, MA
Takara Bio USA, San Jose, CA
Novogen, Sacramento, CA
Agilent Technologies, Santa Clara, CA
New England Biolabs, Ipswich, MA
Illumina, San Diego, CA
IBM, Armonk, NY
References
- 1.Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ, Cdc Periodontal Disease Surveillance workgroup: James Beck GDRP. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res 2012;91:914–920. [DOI] [PubMed] [Google Scholar]
- 2.Akram Z, Abduljabbar T, Abu Hassan MI, Javed F, Vohra F. Cytokine Profile in Chronic Periodontitis Patients with and without Obesity: A Systematic Review and Meta-Analysis. Dis Markers 2016;2016:4801418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapple IL, Bouchard P, Cagetti MG, et al. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J Clin Periodontol 2017;44 Suppl 18:S39–S51. [DOI] [PubMed] [Google Scholar]
- 4.Iwasaki M, Kimura Y, Ogawa H, et al. Periodontitis, periodontal inflammation, and mild cognitive impairment: A 5-year cohort study. J Periodontal Res 2019;54:233–240. [DOI] [PubMed] [Google Scholar]
- 5.Lundgren D, Asklow B, Thorstensson H, Harefeldt AM. Success rates in periodontal treatment as related to choice of evaluation criteria. Presentation of an evaluation criteria staircase for cost-benefit use. J Clin Periodontol 2001;28:23–30. [DOI] [PubMed] [Google Scholar]
- 6.Pirih FQ, Monajemzadeh S, Singh N, et al. Association between metabolic syndrome and periodontitis: The role of lipids, inflammatory cytokines, altered host response, and the microbiome. Periodontol 2000 2021;87:50–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol 2015;15:30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers 2017;3:17038. [DOI] [PubMed] [Google Scholar]
- 9.Blacher E, Levy M, Tatirovsky E, Elinav E. Microbiome-Modulated Metabolites at the Interface of Host Immunity. J Immunol 2017;198:572–580. [DOI] [PubMed] [Google Scholar]
- 10.Brestoff JR, Artis D. Commensal bacteria at the interface of host metabolism and the immune system. Nat Immunol 2013;14:676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan R, Petersen FC, Shekhar S. Commensal Bacteria: An Emerging Player in Defense Against Respiratory Pathogens. Front Immunol 2019;10:1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rath H, Feng D, Neuweiler I, Stumpp NS, Nackenhorst U, Stiesch M. Biofilm formation by the oral pioneer colonizer Streptococcus gordonii: an experimental and numerical study. FEMS Microbiol Ecol 2017;93. [DOI] [PubMed]
- 13.Mans JJ, von Lackum K, Dorsey C, et al. The degree of microbiome complexity influences the epithelial response to infection. BMC Genomics 2009;10:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanel AN, Herzog HM, James MG, Cuadra GA. Effects of Oral Commensal Streptococci on Porphyromonas gingivalis Invasion into Oral Epithelial Cells. Dent J (Basel) 2020;8. [DOI] [PMC free article] [PubMed]
- 15.Ohshima J, Wang Q, Fitzsimonds ZR, et al. Streptococcus gordonii programs epithelial cells to resist ZEB2 induction by Porphyromonas gingivalis. Proc Natl Acad Sci U S A 2019;116:8544–8553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng R, Wu Z, Li M, Shao M, Hu T. Interleukin-1β is a potential therapeutic target for periodontitis: a narrative review. Int J Oral Sci 2020;12:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yee M, Kim S, Sethi P, Düzgüneş N, Konopka K. Porphyromonas gingivalis stimulates IL-6 and IL-8 secretion in GMSM-K, HSC-3 and H413 oral epithelial cells. Anaerobe 2014;28:62–67. [DOI] [PubMed] [Google Scholar]
- 18.Ramenzoni LL, Russo G, Moccia MD, Attin T, Schmidlin PR. Periodontal bacterial supernatants modify differentiation, migration and inflammatory cytokine expression in human periodontal ligament stem cells. PLoS One 2019;14:e0219181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunsch C, Rosen CA. NF-kappa B subunit-specific regulation of the interleukin-8 promoter. Mol Cell Biol 1993;13:6137–6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mikolajczyk-Pawlinska J, Travis J, Potempa J. Modulation of interleukin-8 activity by gingipains from Porphyromonas gingivalis: implications for pathogenicity of periodontal disease. FEBS Lett 1998;440:282–286. [DOI] [PubMed] [Google Scholar]
- 21.Cvikl B, Lussi A, Moritz A, Sculean A, Gruber R. Sterile-filtered saliva is a strong inducer of IL-6 and IL-8 in oral fibroblasts. Clin Oral Investig 2015;19:385–399. [DOI] [PubMed] [Google Scholar]
- 22.Lam RS, O’Brien-Simpson NM, Lenzo JC, et al. Macrophage depletion abates Porphyromonas gingivalis-induced alveolar bone resorption in mice. J Immunol 2014;193:2349–2362. [DOI] [PubMed] [Google Scholar]
- 23.Darveau RP, Pham TT, Lemley K, et al. Porphyromonas gingivalis lipopolysaccharide contains multiple lipid A species that functionally interact with both toll-like receptors 2 and 4. Infect Immun 2004;72:5041–5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merritt JH, Kadouri DE, O’Toole GA. Growing and analyzing static biofilms. Curr Protoc Microbiol 2005;Chapter 1:Unit 1B.1. [DOI] [PMC free article] [PubMed]
- 25.Davies JR, Kad T, Neilands J, et al. Polymicrobial synergy stimulates Porphyromonas gingivalis survival and gingipain expression in a multi-species subgingival community. BMC Oral Health 2021;21:639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilchrist EP, Moyer MP, Shillitoe EJ, Clare N, Murrah VA. Establishment of a human polyclonal oral epithelial cell line. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000;90:340–347. [DOI] [PubMed] [Google Scholar]
- 27.Kouokam JC, Huskens D, Schols D, et al. Investigation of griffithsin’s interactions with human cells confirms its outstanding safety and efficacy profile as a microbicide candidate. PLoS One 2011;6:e22635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014;15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res 2001;125:279–284. [DOI] [PubMed] [Google Scholar]
- 30.Hajishengallis G, Lamont RJ. Polymicrobial communities in periodontal disease: Their quasi-organismal nature and dialogue with the host. Periodontol 2000 2021;86:210–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.AlQranei MS, Chellaiah MA. Osteoclastogenesis in Periodontal Diseases: Possible Mediators and Mechanisms. J Oral Biosci 2020. [DOI] [PMC free article] [PubMed]
- 32.Hiscott J, Marois J, Garoufalis J, et al. Characterization of a functional NF-kappa B site in the human interleukin 1 beta promoter: evidence for a positive autoregulatory loop. Mol Cell Biol 1993;13:6231–6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Libermann TA, Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol 1990;10:2327–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee YS, Lee CH, Bae JT, et al. Inhibition of skin carcinogenesis by suppression of NF-κB dependent ITGAV and TIMP-1 expression in IL-32γ overexpressed condition. J Exp Clin Cancer Res 2018;37:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.