Abstract
Background:
REBOA is a life-saving therapy for hemorrhagic shock following pelvic/lower extremity injuries in military settings. However, Zone-1 aortic occlusion (AO, above the celiac artery), while providing brain/cardiac perfusion, may induce/worsen visceral ischemia and organ dysfunction. In contrast, AO Zone-3 (below the renal arteries) provides abdominal perfusion potentially minimizing ischemia/reperfusion injury. We hypothesized that compared to AO Zone-1, AO Zone-3 provides neuro/cardio-protection while minimizing visceral ischemia and reperfusion coagulopathy post severe traumatic hemorrhage due to pelvic/lower extremity injuries.
Methods:
50kg male Yorkshire swine underwent a blast polytrauma series of injuries followed by a resuscitation protocol with randomization to No-AO (No-AO, n=6) or AO with REBOA at Zone 1 (AO-Zone-1; n=6), or Zone 3 (AO-Zone-3; n=4). Vital signs and intracranial pressure were monitored for 240 minutes. Citrate native (CN) and tissue plasminogen activator (tPA) challenge Thrombelastography (TEG), prothrombin time (PT), creatinine, lipase, total bilirubin, troponin, and ELISA protein levels were measured at set intervals.
Results:
Both AO groups had significant increases in mean arterial pressure during aortic occlusion. All three groups had significant increases in ICP, but final ICP in the No-AO group (26 ±5.8mmHg) was significantly elevated compared to AO-Zone-1 (17 ±5.2mmHg) and AO-Zone-3 (16±4.2mmHg), p<0.01. Final mean troponin in the No-AO group (4.10 ±5.67ng/mL) was significantly higher than baseline (0.03 ± 0.02ng/mL, p<0.05), while the two AO groups had no significant changes (p>0.05). AO-Zone-1 was the only group associated with hyperfibrinolysis (p<0.05) and significantly increased PT (p<0.05). Only AO-Zone-1 group had significantly higher markers of organ damage.
Conclusions:
Compared to AO Zone-1, AO Zone-3 provided similar neuro/cardioprotection but with less organ dysfunction and coagulopathy. This study suggests Zone 3 REBOA may be preferable over Zone 1 for treating military relevant blast polytraumas with minimal intrabdominal and chest trauma, but further clinical investigation is warranted.
Level of Evidence:
N/A
Keywords: DCBI, REBOA, trauma resuscitation, trauma induced coagulopathy, fibrinolysis, animal models
Media Summary:
In a polytrauma swine model, compared to AO Zone-1, Zone-3 provided similar neuro/cardioprotection but with less organ dysfunction and coagulopathy. This suggests Zone 3 REBOA may be preferable over Zone 1 for treating military relevant DCBIs with minimal intrabdominal and chest trauma.
Introduction
In a combat environment heavily burdened by improvised explosive devices (IED), such as Iraq and Afghanistan, explosions account for nearly 80% of all injuries on the battlefields (1). The energy from IEDs, referred to as primary blast injury, is due to the blast over pressure wave and results in multiple amputations, pelvic fractures with extensive soft tissue damage, and blast traumatic brain injury (bTBI). The number one cause of death on the battlefield following IED injuries is hemorrhage(2). However, IED related bTBI is the number one cause of morbidity in warfare, and blast casualties are commonly comorbid with bTBI(1). Thus, innovative solutions to treat blast injuries and IED-related hemorrhage should not only prioritize hemorrhage control, but also optimize cerebral perfusion in the setting of potential bTBI.
Resuscitative endovascular balloon occlusion of the aorta (REBOA) remains a relatively new technique for trauma resuscitation and is most commonly used in civilian clinical settings as an adjunct to trauma resuscitation and as an alternative to resuscitative thoracotomy to achieve temporary aortic occlusion (AO). Furthermore, its potential as a life-saving intervention to control hemorrhage after blast polytrauma and other combat injuries makes it an important intervention for military medicine(3), particularly for use in austere combat environments(4, 5). Among the few military studies describing REBOA use, IEDs were the most common injury mechanism encountered and balloon occlusion was most often deployed in Zone 1 (inflated above the celiac artery)(6). While Zone 1 aortic occlusion (AO Zone 1), provides cerebral and cardiac perfusion, the resulting visceral ischemia may lead to delayed complications such as acute kidney injury, multiple organ failure, and coagulopathy(7). Animal models employing AO Zone 1 have shown that ischemic injury occurs in as little as 20 minutes(8–10). In contrast, AO Zone 3, in which the balloon is inflated below the renal arteries, maintains perfusion to abdominal organs minimizing ischemia/reperfusion injury(11–13). Zone 3 inflation is controversial for intra-abdominal or retroperitoneal bleeding(14); specifically, Zone 3’s ability to double the proximal intraarterial pressure(15) raises concern if such an increase in pressure could exacerbate bleeding from hepatic or splenic injuries. However, the effects of Zone 1 and Zone 3 AO on blast over pressure visceral organ injury have not yet been elucidated.
In a combat environments heavily burdened by IEDs resulting in blast injuries, the use of protective body gear can provide protection from penetrating injuries to the chest and abdomen, but such body armor does not prevent the energy of the blast wave from transmitting through the body(16). In these scenarios where major bleeding is due to extensive blast pelvic damage compared to thoracic and intraabdominal bleeding, AO Zone 3 may be preferred over Zone 1. We hypothesized that for polytrauma injuries comorbid with bTBI, but free from penetrating thoracic and abdominal injuries, AO Zone 3 compared to Zone 1 provides cerebral and cardiac perfusion without the risks of visceral ischemia and reperfusion coagulopathy. To address this hypothesis, we used a blast polytrauma swine model to compare the effects of AO Zone-1 vs AO Zone-3 vs No-AO on cerebral and cardiac perfusion as well as on abdominal organ function and coagulopathy.
Methods
The Institutional Animal Care and Use Committee approved this animal study under protocol #1050. The facility where the research occurred is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). Research was conducted in compliance with the Animal Welfare Act, implementing Animal Welfare Regulations, and the principles of the Guide for the Care and Use of Laboratory Animals, National Research Council. The results are reported in accordance with the ARRIVE guidelines (SDC 1). Healthy adolescent male Yorkshire swine weighing between 45–58 kg were used for all experiments.
The experimental protocol composing the blast polytauma injury series has been described previously in detail(17). Briefly, the injuries consisted of 1) bTBI(18), 2) bilateral, open femur fractures, and 3) fixed-pressure hemorrhagic shock (HS). The bTBI injury was induced using a whole-body blast over pressure energy wave of 55 psi (Applied Research Associates, Inc., Littleton, CO) targeted at the swine’s cranium. As the swine’s whole body is placed within the blast chamber, they wear a National Institute of Justice Level II vest which helps to protect their visceral organs from the blast-over pressure insult and models the protective combat gear worn by warfighters. Open femur fractures were induced using large muscle cutdown to the femur, then a captive bolt stunner (Blitz-Kerner, Turbocut JOBB GmbH, Germany) was fired directly onto the femur. Fracture was confirmed by digital and visual inspection. HS was induced by bleeding from the femoral arteries to a target mean arterial pressure (MAP) of 15 mmHg, end-tidal carbon dioxide (EtCO2) of 20 mmHg, and base excess (BE) of −10mEq/l. During HS, animals were randomized to one of three groups: No-AO, AO Zone-1, or AO Zone-3. AO was accomplished with a 6-French balloon occlusion catheter (ER-REBOA, Prytime Medical, Lakewood, CO), with complete AO for 30 minutes during HS. Bleeding was discontinued for all animals during the last 30 minutes of the hemorrhagic shock phase when AO was employed.
For AO Zone-1, the balloon was inflated at 50cm with 6ml of contrast, while for AO Zone-3 the balloon was inflated at 26cm with 5ml. Location of the balloon was confirmed by X-ray. Swine in the No-AO group continued did not undergo any AO. After the 30 minutes of HS with AO (or without), resuscitation was initiated using a protocol that emphasizes whole blood. Resuscitation was initiated with 500mL of 5% human albumin (Grifols Biologicals Inc., Los Angeles, CA), followed by one unit of shed blood, followed by one more unit of albumin (Figure 1). After the 2nd unit of albumin, all swine received fresh whole blood only until each swine received the entirety of their shed blood volume. For the AO Zone-1 and AO Zone-3 groups, the balloon was progressively deflated over the first 15 minutes of resuscitation.
Figure 1.
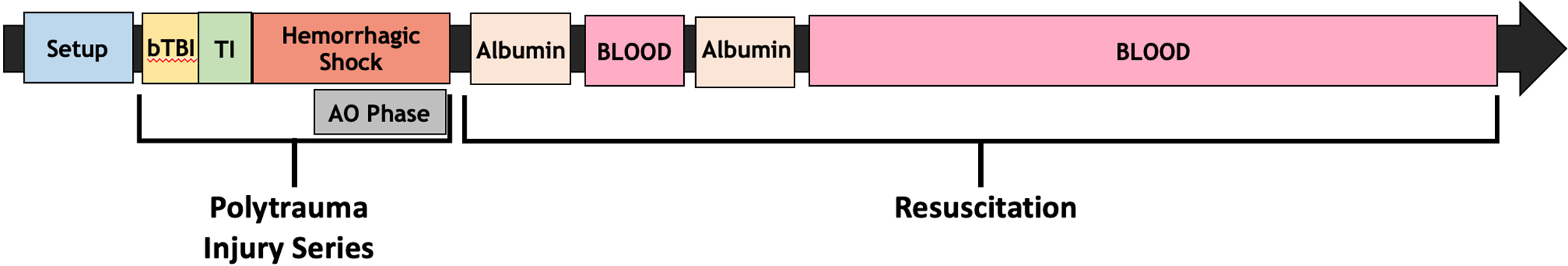
Graphical representation of experiment. Following instrumentation and anesthesia swine underwent an injury series comprised of bTBI, TI (bilateral, open femur fractures), and HS. AO Zone 1, AO Zone 3, or No-AO was employed during the last 30 minutes of HS. Resuscitation was initiated following HS utilizing 500mL of 5% albumin, followed by 1 unit of fresh whole blood, 500mL of 5% albumin, and then the remainder of each swine’s volume of shed fresh whole blood. Swine were euthanized 240 minutes after the conclusion of HS.
Swine were monitored for a total of 240 minutes after HS conclusion. Vital signs, intracranial pressure (ICP) and calculated cerebral perfusion pressure (CPP=MAP-ICP) were recorded every 5 minutes. Arterial blood was collected to assess coagulation (citrated native thrombelastography [TEG], tissue plasminogen activator challenge TEG [tPA-TEG](19), prothrombin time [PT]), base excess (BE), and organ function (lipase, creatinine, total bilirubin, and troponin) using iSTAT-1 whole blood analyzers with CG8+ and PT/INR Cartridges (Abbott Point of Care Inc., Princeton, NJ). Blood was collected at baseline, end of HS phase/injury, 30 minutes post injury, 60 minutes post injury, and then every hour until 240 minutes post injury. Citrated anticoagulated plasma from whole blood samples were collected on ice, centrifuged to yield platelet-free plasma, and flash frozen in liquid nitrogen for Enzyme-Linked Immunosorbent Assays (ELISA) analysis of tissue plasminogen activator levels (tPA; Innovative Research, Inc, Novi, MI, PTPAKT), plasminogen activator inhibitor 1 (PAI-1; Innovative Research, Inc, Novi, MI, POPAIKT), and PAI-1-tPA complex (Innovative Research, Inc, Novi, MI, POPAITPAKT-COM).
Statistical analysis: All analyses were conducted with SAS vs9.4 (SAS Institute, Cary, NC). Baseline statistical tests are not presented as any differences in baseline values in a randomized study are per definition by chance. Linear mixed models for repeated measures were used to compare temporal trends, with contrasts between groups adjusted by false discovery rate. For skewed variables a Box-Cox power transformation was performed to approximate normality. Data are presented as mean and standard deviation or median (interquartile range) or number (percentage). A p-value of <0.05 was considered statistically significant.
Results
Six swine were randomized to the No-AO group, six to AO Zone-1, and five to AO Zone-3. One AO Zone-3 swine expired during instrumentation due to cardiac dysfunction and was excluded from analysis. Baseline values were comparable amongst the groups (Table 1). The length of time that animals remained in HS (50min [IQR: 45, 60], for all groups) and percentage of total blood volume removed (49 ± 9.4%) to achieve the target HS level were similar between groups (p>0.05). During HS, all groups had similar levels of mean arterial pressure (MAP) and EtCO2. Both AO Zone-1 and AO Zone-3 groups had significant increases in SBP after 15 minutes of AO (Figure 2, Panel A).
Table 1.
Swine blast polytrauma randomized experiment: Baseline variables. Data are presented as mean (standard deviation) or N (%) unless otherwise indicated.
| No-AO | AO Zone-1 | AO Zone-3 | |
|---|---|---|---|
| Heart rate (bpm) | 68 (17.8) | 84 (17.2) | 96 (29.0) |
| End tidal CO2 (mmHg) | 36.0 (4.6) | 37.0 (5.6) | 34.8 (1.7) |
| Hemoglobin (g/dl) | 9.8 (0.6) | 9.8 (0.8) | 9.7 (0.7) |
| Base Excess (mEq/l) | 10.0 (1.7) | 9.8 (2.5) | 10.8 (3.1) |
| Platelet count (× 109/l) | 368.3 (60.0) | 311.3 (99.1) | 254.0 (49.5) |
| Sodium (mEq/l) | 142.0 (3.9) | 141.8 (2.1) | 140.8 (0.5) |
| Potassium (mEq/l) | 4.2 (0.2) | 4.2 (0.1) | 4.2 (0.4) |
| Ionized Calcium (mg/dl) | 1.3 (0.1) | 1.2 (0.3) | 1.4 (0.2) |
| Troponin (ng/l) | 0.0 (0) | 0.0 (0) | 0.0 (0) |
| Creatinine (mg/dl) | 1.7 (0.2) | 1.6 (0.2) | 1.5 (0.2) |
| Total Bilirubin (mg/dl) | 0.12 (0.04) | 0.30 (0.49) | 0.23 (0.25) |
| Lipase (U/L) | 32.83 (1.94) | 32.17 (2.32) | 32.50 (4.51) |
| TEG Angle (degrees) | 68.68 (2.85) | 72.72 (6.40) | 63.33 (16.89) |
| TEG Maximum Amplitude (mm) | 76.58 (1.74) | 78.00 (4.79) | 75.13 (5.25) |
| Prothrombin Time (sec) | 12.53 (0.73) | 12.51 (0.71) | 12.45 (1.19) |
Figure 2.
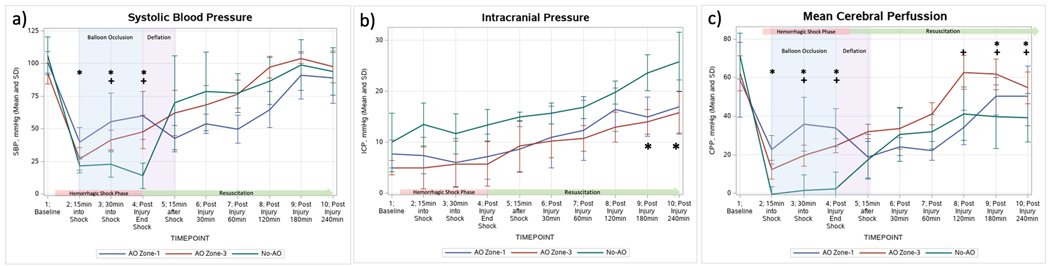
Physiology temporal trends. Panel a) Mean Systolic Blood Pressure: * indicates p<0.05 in the comparison No-AO vs AO Zone-1; + indicates p<0.05 in the comparison of No-AO vs AO Zone-3; Panel b) Mean Intracranial Pressure: * indicates p<0.05 in the comparison of No AO vs both AO Zone-1 and AO Zone-3; c) Mean Cerebral Perfusion Pressure: * indicates p<0.05 in the comparison No-AO vs AO Zone-1; + indicates p<0.05 in the comparison of No-AO vs AO Zone-3.
Cerebral Hemodynamics
ICP remained stable throughout HS in all groups (Figure 2, Panel B). Upon initiation of resuscitation, ICPs increased significantly in all groups (p<0.05), however, at the end of the experiment (240min) the No-AO group had a significantly higher ICP compared to both AO Zone-1 and AO Zone-3 groups (25.8±5.8mmHg vs 17±5.2mmHg and 15.8±4.2mmHg, respectively, p<0.0001). There were no significant differences between the final ICP values in the AO Zone-1 group vs AO Zone-3 group. CPP decreased in all groups with the initiation of HS (Figure 2, Panel C); however, in the AO Zone-1 and AO Zone-3 groups the 30 minutes of AO resulted in significantly elevated CPP compared to the No-AO group (p<0.05). There were no significant differences in the CPPs between the AO Zone-1 and the AO Zone-3 group during AO; however at 60min and 120min post injury the AO Zone-3 did have significantly higher CPPs compared to the AO Zone-1 group (p<0.05). At the conclusion of the model, both AO Zone-1 and AO Zone-3 groups had CPPs that returned to baseline levels, but the No-AO group never recovered back to baseline.
Organ Function
Troponin increased significantly compared to baseline in the No-AO group (4.1 vs 0.0 ng/mL, p=0.03), but not in the AO Zone-1 and AO Zone-3 groups. Total bilirubin increased significantly in all groups, but final total bilirubin was significantly higher in the AO Zone-1 group than AO Zone-3 and No-AO groups (0.9 vs 0.5 vs 0.6 mg/dL, p<0.05). Similarly, the AO Zone-1 group had significantly higher final lipase (49.4 vs 32.3 vs 27.8 U/L, p<0.05) and creatinine (2.5 vs 1.9 vs 2.0 mg/dL, p<0.005) at 240 minutes compared to the AO Zone-3 and No-AO groups.
Coagulation
Compared to AO Zone-3 and No-AO groups, the AO Zone-1 group experienced significant coagulation abnormalities which were most pronounced at 60min postinjury. The AO Zone-1 group was the only group that experienced a significantly prolonged R-Time, peaking at 60min post-injury (Figure 3). Compared to the AO Zone-3 and the No-AO groups, at 60min postinjury the AO Zone-1 group also had significantly lower maximum amplitude (MA) (59.2 vs 70.1 vs 66.4 mm, p<0.05) and angle (55.2 vs 74.9 vs 70.2 degrees, p<0.05). The tPA-challenge TEG LY30% (Figure 3) was significantly higher in AO Zone-1 group than the AO Zone-3 and No-AO groups. PT was significantly elevated in AO Zone-1 compared to the AO Zone-3 and the No-AO groups (19.6 vs 13.45 vs 14.97 sec, p<0.05); AO Zone-1group was the only group in which PT did not return to the baseline level at the end of the experiment.
Figure 3.
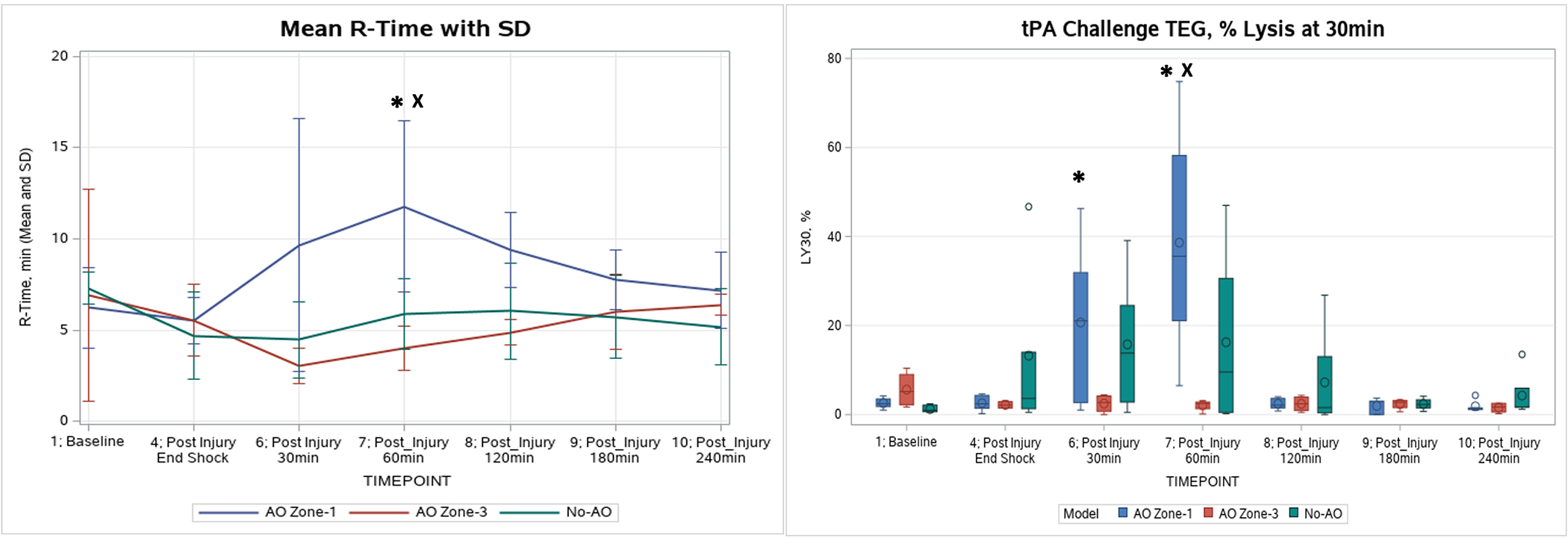
Coagulation changes in TEG R time and LY30% throughout the experiment. Left image, Thrombelastogram (TEG) R-time (min): AO Zone-1 had a significant increase in R-time above baseline at 60min postinjury (indicted by *), and significantly higher than No-AO’s and AO Zone-3’s (indicted by X). Right image, tPA Challenge TEG LY30: Only AO Zone-1 showed a significant change from baseline at 30 and 60min postinjury (indicted by *). At 60min postinjury, the AO Zone-1 tPA Challenge LY30 was significantly higher than AO Zone-3’s and No-AO groups (indicted by X).
All groups showed significant increases in active PAI-1 levels at the completion of the model compared to baseline (AO Zone-1: 63.0 vs 0.4 ng/mL; AO Zone-3: 46.6 vs 1.8 ng/mL; No-AO: 87.0 vs 3.9 ng/mL, p<0.05). The AO Zone-1 group was the only group that had a significant increase in active tPA levels (Figure 4) above baseline (0.39 ng/mL), which peaked at 30 minutes (1.43 ng/mL, p=0.0006) and remained significantly elevated at 60 minutes (1.30 ng/mL, p=0.001). PAI-1-tPA complex levels increased significantly compared to baseline in both the AO Zone-1group (at 240 minutes: 136.4 vs 10.2 ng/mL, p<0.05) and the AO Zone-3 group (at 60 minutes: 55.8 vs 8.8 ng/mL, p<0.05), while in the No-AO group the complex levels trended up but did not reach significance.
Figure 4.
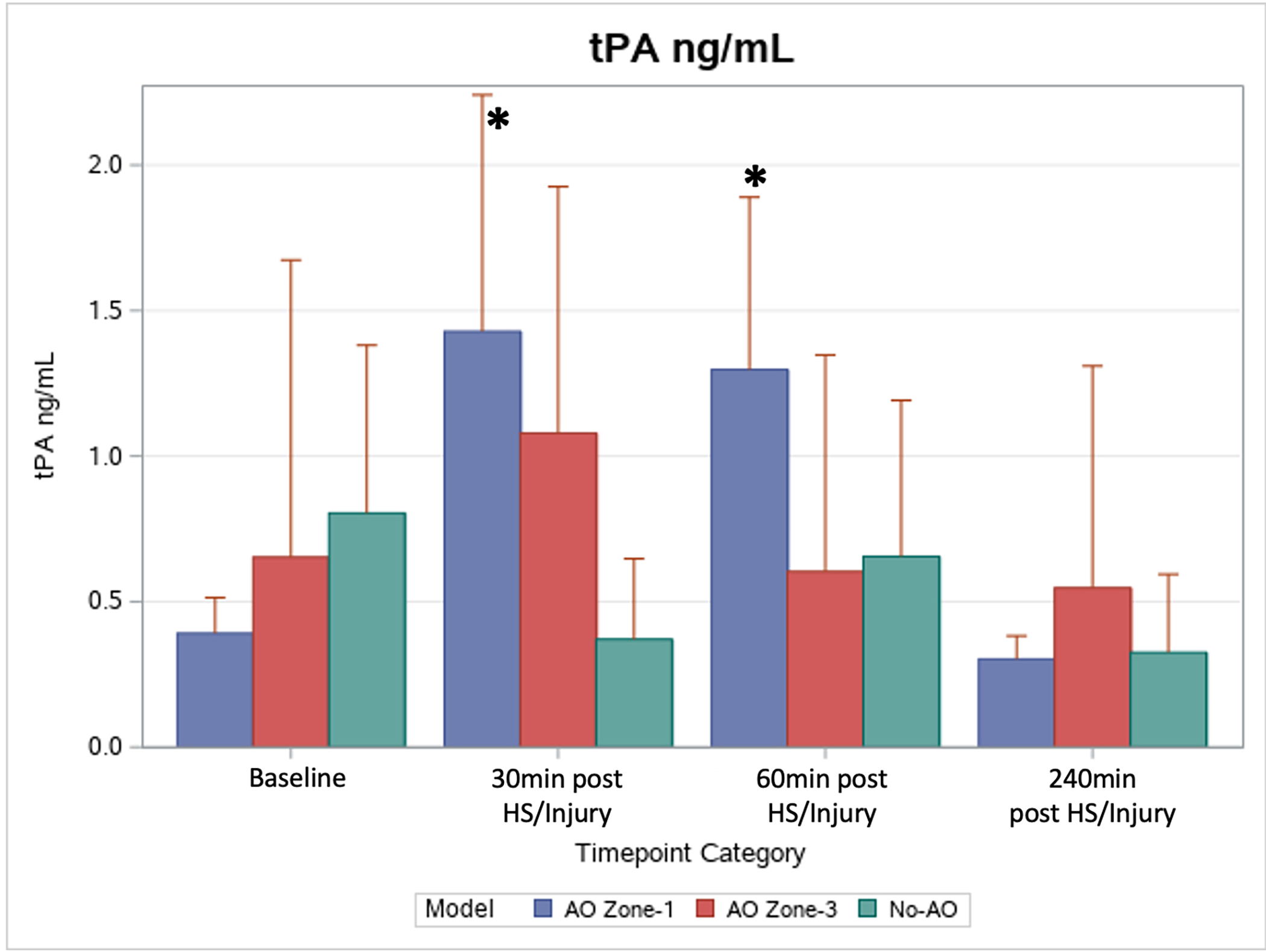
Changes in levels of active tPA. At 30 and 60min postinjury, AO Zone-1 active tPA levels were significantly elevated above the group’s mean baseline (indicted by *).
Discussion
The results of this animal study indicated that AO Zone-1 and AO Zone-3 provide similar adequate cerebral and cardiac perfusion; however, AO Zone-1 was associated with greater elevations in abdominal organ ischemia/reperfusion injury and greater reperfusion coagulopathy compared to AO Zone-3.
Currently REBOAs are most often placed in Zone 1 in the military setting are for the treatment of IED related hemorrhage (43%)(4, 6). A retrospective analysis of United Kingdom military warfighters who were severely injured in combat identified that of the patients with injury patterns amendable to REBOA, 40% met the indications for Zone 3 inflation(20). Additionally, for blast injuries and IED-related trauma, pelvic vascular injury has been identified as the greatest risk factor for mortality(21). However, the most recent review of REBOA use for treatment of combat casualties reports that only 12.8% of REBOAs were inflated in Zone 3 and all others utilized Zone 1(6). This suggests that Zone 3 use may be underutilized, and the preference for Zone 1 inflation could be introducing unnecessary visceral ischemia. Based on our animal findings, for polytrauma injuries or blast casualties with comorbid bTBI, and without secondary penetrating injuries to the abdomen or chest, AO Zone-3 REBOA for non-compressible hemorrhage from the pelvis may be an efficacious and safe alternative that deserves further investigation.
Our study results conflict with experiments in swine by Markov et al (22), which indicated that 30 minutes of Zone 1 occlusion did not result in discernable visceral injury, and with Reva et al(9), although the latter work was in an ovine model. While military combat shows the clinical diagnosis of blast injury to the lungs or visceral organs is infrequent (incidence of blast lung ranging from 7–11% in UK casualties(23), and in US casualties incidence of blast lung is <5% and incidence of intestinal blast is <1% (24)), even minimal, microscopic damage caused by blast over pressure waves could potentially enhance ischemic complications provoked by AO Zone-1 REBOA. This could explain the increase in organ function labs seen in our swine model employing 30 minutes of Zone 1 REBOA compared to previous animal models(22, 25). While the 55psi blast over pressure injury is primarily used to induce a bTBI in our model, we are unable to completely eliminate the effects to the remainder of the animal’s body. The protective gear worn by warfighters is mimicked by the military vest worn by our swine; however, such gear provides only some protection for the lungs and visceral organs against the blast(16). Other animal model investigators have found adverse effects associated with 30 minutes of AO Zone-1. Li et al6 reported significantly increased lactate, renal IL-6, intestinal TNA-α, and hepatic IL-6 in pigs undergoing REBOA for HS, while Sadeghi et al (10) detected prolonged elevations in lactate, potassium, creatinine, aspartate aminotransferase, and lipase in normovolemic pigs with REBOA.
Clinical studies have also shown that AO Zone-1 may increase susceptibility to organ dysfunction and failure. In the prospective observational trial the Emergent Truncal Hemorrhage Control study, the rate of multiple organ failure (MOF) in surviving Zone 1 patients was 29% compared to 4% in Zone 3 survivors(26). In a retrospective review of Zone 1 REBOA use in severely injured patients in Japan, the MOF rate in the surviving patients 64%(27). Additionally, the Emergent Truncal Hemorrhage Control Study investigators evaluated a commonly accepted REBOA algorithm to determine adherence to optimal zone placement(28), and the investigators found that of the 36 AO Zone-1 REBOAs placed, 4 (11.1%) should have been AO Zone-3 and of those patients 3 ultimately died due to MOF(29).
To our knowledge, our swine experiment is the first to comprehensively characterize the significant coagulopathic disparities between Zone 1 and Zone 3 occlusion. Compared to AO Zone-3, AO Zone-1 was associated with significant TEG-measured coagulation abnormalities, including prolonged time to clot formation, poor clot strength, and increased occult fibrinolysis. Only AO Zone-1 was associated with significant increases in tPA. Post-injury tPA release has been linked to the development of hyperfibrinolysis in trauma patients(30) and the increase in active tPA seen in the AO Zone-1 group could explain the significant increases in fibrinolysis in this group. Coagulopathy was also observed in conventional coagulation assays as well, with persistently abnormal PT in the AO Zone-1 animals contrasting with the recovery experienced by AO Zone-3 animals. The Cowley Shock Trauma group was one of the first to explore coagulation abnormalities associated with REBOA in their model of intermittent AO Zone-1 occlusion; they identified that one of eight swine developed severe coagulopathy requiring re-intervention(31). The literature on coagulation effects of AO in clinical practice is scarce, with only one case report highlighting an intracranial hemorrhage expansion following Zone 1 REBOA and the authors also noting that the patient became coagulopathic during resuscitation(32).
This single case report has raised concerns regarding the safety profile of REBOA for treating concomitant HS and TBI. Several animal models have begun exploring the effects of AO on brain physiology. To our knowledge this is the only model that includes bTBI, while others rely on cortical impact devices which create a unilateral, focal injury site. Additionally, this is the only swine model that has evaluated both Zone 1 and Zone 3 AO on ICPs and CPPs. Previous work has shown that partial and complete AO in Zone 1 is not associated with increases in cerebral edema(33, 34) or TBI lesion progression(35) within 6 hours after injury; however, results should be interpreted according to the magnitude of HS and targeted blood pressure resuscitation ranges. In our model, final ICPs for the AO Zone-1 and AO Zone-3 groups were similar, while the No-AO group reached a significantly higher ICP of 26 mmHg. Notably, the Brain Trauma Foundation advocates for treatment of ICPs above 22 mmHg, as this cutoff is associated with increased mortality(36). Thus, it is important to note that both the Zone 1 and Zone 3 AO groups had final ICP values below this threshold. This suggests that for treatment of polytrauma or blast injuries with severe Class IV HS and comorbid with bTBI both Zone 1 and Zone 3 may be capable of providing a comparable neuroprotective benefit, and this benefit extends beyond the AO phase leading to lower ICPs and subsequently higher cerebral perfusion hours after injury. However, it should be noted that our model involved a severe blood volume loss of 50% and thus supraphysiologic proximal pressures were not encountered.
Limitations
Our swine model used complete REBOA occlusion, and we have not yet tested alternative Zone 1 REBOA strategies that may produce less organ ischemia but similar hemodynamic support. These include intermittent or partial REBOA, which include either intermittently or partially deflating the balloon to allow some distal flow while still supporting proximal perfusion partial. There is also a new semi-compliant balloon device, recently approved by the FDA that allows more precise control of aortic blood pressure distal to a partial occlusion(37–39). Second, the 240-minute monitoring time may be insufficient to evaluate the long-term effects of either AO zone, however, longer observation periods are costly and resource intensive. For this blast injury focused model, we were primarily interested in understanding the effects of the primary blast injury only (and not secondary or tertiary blast injury), thus the only injury to the visceral organs was the blast wave and this model is not representative of clinical polytrauma or blast injuries that are comorbid with penetrating or blunt abdominal/thoracic injury. Additionally, histology findings are not included in our results, and this is a significant limitation in grading and understanding the total morbidity that the blast wave and polytrauma injuries created on the brain and visceral organs. While our resuscitation protocol emphasizes whole blood, the addition of albumin does not replicate current Tactical Combat Casualty Care guidelines that would be used to treat combat casualties. Additionally, the neuroprotective effects of REBOA should be interpreted within the context of HS severity, blast induced TBI, the specified resuscitation protocol, and the short observation time. Specifically in this model, AO occurred prior to volume resuscitation and the protocol did not produce supraphysiologic pressures. Other models have shown that REBOA can potentially induce such pressures(40), especially when using different HS thresholds and resuscitation methods.
Conclusions
In summary, our swine model which compares AO Zone-1 and AO Zone-3 for use in treating military relevant blast polytrauma injuries suggests Zone 3 REBOA may be preferable in treating certain blast casualties without abdominal or thoracic injury. Further clinical investigation should focus on prehospital abilities to rule out abdominal and thoracic hemorrhage, prior to deploying Zone 3 AO.
Supplementary Material
Acknowledgments:
Icons utilized in the visual abstract are provided by Flaticon, a Freepik Company, Málaga, Spain (https://www.flaticon.com/).
Support:
This research is funded by the Department of Defense contract number W81XWH2010205. This contract provides salary support and research support for animal and laboratory costs. Research support is also provided by the National Institute of General Medical Sciences of the National Institutes of Health (T32 GM008315). The current major funding source is an RM-1grant (1RM1GM131968-01). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Department of Defense.
Footnotes
Conflict of interest/Disclosure: E.E.M. has patents pending related to coagulation and fibrinolysis diagnostics and therapeutic fibrinolytics and is a cofounder with stock options in ThromboTherepeutics. E.E.M. has received grant support from Haemonetics, Inc., Stago, Hemosonics, Instrumentation Laboratories, Inc, and Diapharma outside the submitted work. C.J.F is a on the Clinical Advisory Board for Prytime Medical Devices.
Supplemental Digital Content
Included with this manuscript is a checklist of the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. This checklist has been uploaded as supplemental digital content 1.
I, Alexis Cralley, MD, have reviewed and edited the submission to omit any identifying information. I hereby submit this self-blinded manuscript for consideration in The Journal of Trauma and Acute Care Surgery.
References
- 1.Cannon JW, Hofmann LJ, Glasgow SC, Potter BK, Rodriguez CJ, Cancio LC, et al. Dismounted Complex Blast Injuries: A Comprehensive Review of the Modern Combat Experience. J Am Coll Surg. 2016;223(4):652–64 e8. [DOI] [PubMed] [Google Scholar]
- 2.Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, et al. Death on the battlefield (2001–2011): implications for the future of combat casualty care. J Trauma Acute Care Surg. 2012;73(6 Suppl 5):S431–7. [DOI] [PubMed] [Google Scholar]
- 3.Rasmussen TE, Eliason JL. Military-civilian partnership in device innovation: Development, commercialization and application of resuscitative endovascular balloon occlusion of the aorta. J Trauma Acute Care Surg. 2017;83(4):732–5. [DOI] [PubMed] [Google Scholar]
- 4.Northern DM, Manley JD, Lyon R, Farber D, Mitchell BJ, Filak KJ, et al. Recent advances in austere combat surgery: Use of aortic balloon occlusion as well as blood challenges by special operations medical forces in recent combat operations. J Trauma Acute Care Surg. 2018;85(1S Suppl 2):S98–S103. [DOI] [PubMed] [Google Scholar]
- 5.Glaser J, Stigall K, Jensen CS, Morrison UJJ, Snyder S, & Russo MR. Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) for Hemorrhagic Shock (CPG ID:38) JOINT TRAUMA SYSTEM CLINICAL PRACTICE GUIDELINE (JTS CPG). 31 Mar 2020:1–28. [Google Scholar]
- 6.Stokes SC, Theodorou CM, Zakaluzny SA, DuBose JJ, Russo RM. Resuscitative endovascular balloon occlusion of the aorta in combat casualties: The past, present, and future. J Trauma Acute Care Surg. 2021;91(2S Suppl 2):S56–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joseph B, Zeeshan M, Sakran JV, Hamidi M, Kulvatunyou N, Khan M, et al. Nationwide Analysis of Resuscitative Endovascular Balloon Occlusion of the Aorta in Civilian Trauma. JAMA surgery. 2019;154(6):500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Dubick MA, Yang Z, Barr JL, Gremmer BJ, Lucas ML, et al. Distal organ inflammation and injury after resuscitative endovascular balloon occlusion of the aorta in a porcine model of severe hemorrhagic shock. PLoS One. 2020;15(11):e0242450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reva VA, Matsumura Y, Horer T, Sveklov DA, Denisov AV, Telickiy SY, et al. Resuscitative endovascular balloon occlusion of the aorta: what is the optimum occlusion time in an ovine model of hemorrhagic shock? European journal of trauma and emergency surgery : official publication of the European Trauma Society. 2018;44(4):511–8. [DOI] [PubMed] [Google Scholar]
- 10.Sadeghi M, Dogan EM, Karlsson C, Jansson K, Seilitz J, Skoog P, et al. Total resuscitative endovascular balloon occlusion of the aorta causes inflammatory activation and organ damage within 30 minutes of occlusion in normovolemic pigs. BMC Surg. 2020;20(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tibbits EM, Hoareau GL, Simon MA, Davidson AJ, DeSoucy ES, Faulconer ER, et al. Location is everything: The hemodynamic effects of REBOA in Zone 1 versus Zone 3 of the aorta. The journal of trauma and acute care surgery. 2018;85(1):101–7. [DOI] [PubMed] [Google Scholar]
- 12.Brannstrom A, Dahlquist A, Gustavsson J, Arborelius UP, Gunther M. Increased crystalloid fluid requirements during zone 3 Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) versus Abdominal Aortic and Junctional Tourniquet (AAJT) after class II hemorrhage in swine. Eur J Trauma Emerg Surg. 2022;48(1):335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beyer CA, Johnson MA, Galante JM, DuBose JJ. Zones matter: Hemodynamic effects of zone 1 vs zone 3 resuscitative endovascular balloon occlusion of the aorta placement in trauma patients. Injury. 2019;50(4):855–8. [DOI] [PubMed] [Google Scholar]
- 14.Brenner M, Bulger EM, Perina DG, Henry S, Kang CS, Rotondo MF, et al. Joint statement from the American College of Surgeons Committee on Trauma (ACS COT) and the American College of Emergency Physicians (ACEP) regarding the clinical use of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA). Trauma Surg Acute Care Open. 2018;3(1):e000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pieper A, Thony F, Brun J, Rodiere M, Boussat B, Arvieux C, et al. Resuscitative endovascular balloon occlusion of the aorta for pelvic blunt trauma and life-threatening hemorrhage: A 20-year experience in a Level I trauma center. J Trauma Acute Care Surg. 2018;84(3):449–53. [DOI] [PubMed] [Google Scholar]
- 16.Plurad DS. Blast injury. Mil Med. 2011;176(3):276–82. [DOI] [PubMed] [Google Scholar]
- 17.Cralley AL, Moore EE, Kissau D, Coleman JR, Vigneshwar N, DeBot M, et al. A Combat Casualty Relevant Dismounted Complex Blast Injury Model In Swine. Shock. 2022, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNeil E, Walilko T, Hulbert LE, VanMeter JW, LaConte S, VandeVord P, et al. Development of a Minipig Model of BINT From Blast Exposure Using a Repeatable Mobile Shock Expansion Tube. Mil Med. 2021. [DOI] [PubMed] [Google Scholar]
- 19.Moore HB, Moore EE, Gonzalez E, Hansen KC, Dzieciatkowska M, Chapman MP, et al. Hemolysis exacerbates hyperfibrinolysis, whereas platelolysis shuts down fibrinolysis: evolving concepts of the spectrum of fibrinolysis in response to severe injury. Shock. 2015;43(1):39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrison JJ, Ross JD, Rasmussen TE, Midwinter MJ, Jansen JO. Resuscitative endovascular balloon occlusion of the aorta: a gap analysis of severely injured UK combat casualties. Shock. 2014;41(5):388–93. [DOI] [PubMed] [Google Scholar]
- 21.Rankin IA, Webster CE, Gibb I, Clasper JC, Masouros SD. Pelvic injury patterns in blast: Morbidity and mortality. J Trauma Acute Care Surg. 2020;88(6):832–8. [DOI] [PubMed] [Google Scholar]
- 22.Markov NP, Percival TJ, Morrison JJ, Ross JD, Scott DJ, Spencer JR, et al. Physiologic tolerance of descending thoracic aortic balloon occlusion in a swine model of hemorrhagic shock. Surgery. 2013;153(6):848–56. [DOI] [PubMed] [Google Scholar]
- 23.Smith JE. The epidemiology of blast lung injury during recent military conflicts: a retrospective database review of cases presenting to deployed military hospitals, 2003–2009. Philos Trans R Soc Lond B Biol Sci. 2011;366(1562):291–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ritenour AE, Blackbourne LH, Kelly JF, McLaughlin DF, Pearse LA, Holcomb JB, et al. Incidence of primary blast injury in US military overseas contingency operations: a retrospective study. Annals of surgery. 2010;251(6):1140–4. [DOI] [PubMed] [Google Scholar]
- 25.Avaro JP, Mardelle V, Roch A, Gil C, de Biasi C, Oliver M, et al. Forty-minute endovascular aortic occlusion increases survival in an experimental model of uncontrolled hemorrhagic shock caused by abdominal trauma. J Trauma. 2011;71(3):720–5; discussion 5–6. [DOI] [PubMed] [Google Scholar]
- 26.Moore LJ, Fox EE, Meyer DE, Wade CE, Podbielski JM, Xu X, et al. Prospective Observational Evaluation of the ER-REBOA Catheter at 6 U.S. Trauma Centers. Annals of Surgery. 9000;Publish Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 27.Saito N, Matsumoto H, Yagi T, Hara Y, Hayashida K, Motomura T, et al. Evaluation of the safety and feasibility of resuscitative endovascular balloon occlusion of the aorta. J Trauma Acute Care Surg. 2015;78(5):897–903; discussion 4. [DOI] [PubMed] [Google Scholar]
- 28.Brenner M, Hoehn M, Pasley J, Dubose J, Stein D, Scalea T. Basic endovascular skills for trauma course: bridging the gap between endovascular techniques and the acute care surgeon. The journal of trauma and acute care surgery. 2014;77(2):286–91. [DOI] [PubMed] [Google Scholar]
- 29.Johnson NL, Wade CE, Fox EE, Meyer DE, Fox CJ, Moore EE, et al. Determination of optimal deployment strategy for REBOA in patients with non-compressible hemorrhage below the diaphragm. Trauma Surg Acute Care Open. 2021;6(1):e000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chapman MP, Moore EE, Moore HB, Gonzalez E, Gamboni F, Chandler JG, et al. Overwhelming tPA release, not PAI-1 degradation, is responsible for hyperfibrinolysis in severely injured trauma patients. J Trauma Acute Care Surg. 2016;80(1):16–23; discussion -5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrison JJ, Ross JD, Houston Rt, Watson JD, Sokol KK, Rasmussen TE. Use of resuscitative endovascular balloon occlusion of the aorta in a highly lethal model of noncompressible torso hemorrhage. Shock. 2014;41(2):130–7. [DOI] [PubMed] [Google Scholar]
- 32.Uchino H, Tamura N, Echigoya R, Ikegami T, Fukuoka T. “REBOA” - Is it Really Safe? A Case with Massive Intracranial Hemorrhage Possibly due to Endovascular Balloon Occlusion of the Aorta (REBOA). Am J Case Rep. 2016;17:810–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams AM, Bhatti UF, Dennahy IS, Graham NJ, Nikolian VC, Chtraklin K, et al. Traumatic brain injury may worsen clinical outcomes after prolonged partial resuscitative endovascular balloon occlusion of the aorta in severe hemorrhagic shock model. J Trauma Acute Care Surg. 2019;86(3):415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cralley AL, Moore EE, Fox CJ, Kissau D, DeBot M, Schaid TR, et al. Zone 1 REBOA in a combat DCBI swine model does not worsen brain injury. Surgery. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson MA, Williams TK, Ferencz SE, Davidson AJ, Russo RM, O’Brien WT, Sr., et al. The effect of resuscitative endovascular balloon occlusion of the aorta, partial aortic occlusion and aggressive blood transfusion on traumatic brain injury in a swine multiple injuries model. J Trauma Acute Care Surg. 2017;83(1):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GW, Bell MJ, et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery. 2017;80(1):6–15. [DOI] [PubMed] [Google Scholar]
- 37.Russo RM, Franklin CJ, Davidson AJ, Carlisle PL, Iancu AM, Baer DG, et al. A new, pressure-regulated balloon catheter for partial resuscitative endovascular balloon occlusion of the aorta. J Trauma Acute Care Surg. 2020;89(2S Suppl 2):S45–S9. [DOI] [PubMed] [Google Scholar]
- 38.Russo RM, White JM, Baer DG. Partial Resuscitative Endovascular Balloon Occlusion of the Aorta: A Systematic Review of the Preclinical and Clinical Literature. J Surg Res. 2021;262:101–14. [DOI] [PubMed] [Google Scholar]
- 39.Russo RM, Neff LP, Lamb CM, Cannon JW, Galante JM, Clement NF, et al. Partial Resuscitative Endovascular Balloon Occlusion of the Aorta in Swine Model of Hemorrhagic Shock. J Am Coll Surg. 2016;223(2):359–68. [DOI] [PubMed] [Google Scholar]
- 40.Hoehn MR, Teeter WA, Morrison JJ, Gamble WB, Hu P, Stein DM, et al. Aortic branch vessel flow during resuscitative endovascular balloon occlusion of the aorta. J Trauma Acute Care Surg. 2019;86(1):79–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


