Abstract
Background:
Young age has been associated with poorer control of hypertension in children with chronic kidney disease (CKD). Using data from the Chronic Kidney Disease in Children (CKiD) Study, we examined the relationship between age, hypertensive blood pressure (BP) recognition, and pharmacologic BP control in children with non-dialysis dependent CKD.
Methods:
Participants included 902 CKiD Study participants with CKD stages 2–4. A total of 3550 annual study visits met inclusion criteria and participants were stratified by age (0 to <7 years, ≥7 to <13 years, ≥13 to ≤18 years). Generalized estimating equations to account for repeated measures were applied to logistic regression analyses to evaluate the association of age with unrecognized hypertensive BP and medication use.
Results:
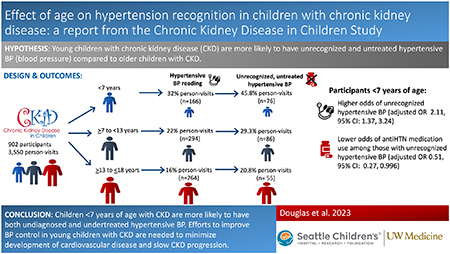
Children <7 years of age had a higher prevalence of hypertensive BP and a lower prevalence of antihypertensive medication use compared to older children. At visits where participants <7 years of age had hypertensive BP readings, 46% had unrecognized, untreated hypertensive BP compared to 21% of visits for children ≥13 years of age. The youngest age group was associated with higher odds of unrecognized hypertensive BP (adjusted OR 2.11, 95% CI: 1.37, 3.24) and lower odds of antihypertensive medication use among those with unrecognized hypertensive BP (adjusted OR 0.51, 95% CI: 0.27, 0.996).
Conclusions:
Children younger than 7 years of age with CKD are more likely to have both undiagnosed and undertreated hypertensive BP. Efforts to improve BP control in young children with CKD are needed to minimize development of cardiovascular disease and slow CKD progression.
Keywords: blood pressure, hypertension, chronic kidney disease, pediatric, cardiovascular disease
Graphical Abstract
Introduction
Although hypertension is a known modifiable risk factor for cardiovascular disease and chronic kidney disease (CKD) progression, the role of age in the recognition and treatment of hypertension in pediatric CKD remains poorly understood. Younger age has been associated with unrecognized hypertension (HTN) in both the general pediatric population and in children on dialysis1, 2. Large cohort studies have noted increased prevalence of persistent hypertension in those of younger age with mild to moderate CKD and end-stage kidney disease (ESKD)2, 3. This suggests that hypertension may be both underdiagnosed and undertreated in young children with CKD despite an increased lifetime risk of cardiovascular morbidity and mortality, although current literature is lacking4–7.
The primary objective of this study was to determine the prevalence of unrecognized hypertension for differing age groups of children with CKD. We hypothesized that among children with CKD with hypertensive BP readings, young children would be less likely to carry a diagnosis of hypertension despite hypertensive BP readings compared to older children. In addition, we sought to evaluate the association between participant age and the prevalence of pharmacologic treatment of stage I and II HTN, with the hypothesis that young children with hypertensive BP are less likely to undergo pharmacologic treatment. Finally, we evaluated the association of age with BP control among hypertensive participants receiving antihypertensive medications and hypothesized that hypertensive BP is more often poorly controlled in young children compared to older children despite antihypertensive medication administration.
Methods
Study Population
Data were obtained from the Chronic Kidney Disease in Children (CKiD) study, a multi-center prospective cohort study dedicated to longitudinal follow-up of children and adolescents with mild to moderate CKD living in North America. Anonymized data and materials have been made publicly available upon request at the NIDDK Central Repository (https://repository.niddk.nih.gov/studies/ckid/). Details regarding inclusion criteria and CKiD study designs have been previously published8. Children 0–16 years of age with an estimated glomerular filtration rate (eGFR) of 30–90 ml/min/1.73m2 based on the bedside Schwartz estimating equation were eligible for enrollment9. Enrollment began in 2005 and concluded in 2020. For this analysis, we utilized data from participants with complete BP data and covariate data including eGFR per the CKiD Under 25 (U25) estimating equation10, urine protein to creatinine ratio (UPC), and body mass index (BMI) at initial and follow-up study visits. Any participants without BP available at the initial visit were excluded. In addition, follow-up study visits with missing BP or covariate data were excluded.
Blood Pressure Measurements
Standardized clinic BP measurements were obtained at all annual study visits by auscultation using aneroid sphygmomanometers (Mabis MedicKit 5, Mabis Healthcare, Waukegan, IL) provided by the CKiD Clinical Coordinating Centers. Prior to BP measurement, trained study staff measured the child’s mid-arm circumference and selected the appropriate cuff size. The child remained at rest for at least 5 minutes before cuff inflation and three measurements were obtained, with 30 seconds between each measurement. The recorded BP for the study visit was the average of the three auscultated measurements. Further details of the CKiD clinic BP protocol have been previously published3.
Ambulatory blood pressure monitoring (ABPM) was conducted one year after the baseline visit, i.e., at the second annual visit and every two years thereafter in study participants 6 years of age and older using the Spacelabs 90217 monitor (Spacelabs Healthcare, Issaquah, WA)11. All ABPMs were placed on the participant at the home institution, but data were sent to a single center for analysis. All study participants were provided with a patient diary to record sleep and wake times as well as medication administration times or unusual activities. Monitors were programmed to obtain BP readings every 20 minutes throughout the 24-hour period. Once the monitor was returned and data downloaded, each ABPM recording was assessed for adequacy and study participants were offered a second recording in cases of inadequate readings. Monitors worn for ≥24 hours, with ≥18 hours with ≥1 reading per hour were considered adequate for interpretation. Additionally, a balance of readings during both the sleep and wake periods was required (≥1 BP per hour in ≥75% of each of the two periods).
Blood Pressure Status Classification
Blood pressure status for clinic BP was categorized using current American Academy of Pediatrics (AAP) criteria as normal, elevated, stage 1 hypertensive BP, or stage 2 hypertensive BP12. Blood pressure status for ABPM data was defined utilizing the 2014 American Heart Association (AHA) guidelines that were in effect at the time of data analysis13. ABPM hypertension was defined as greater than or equal to the sex-age-specific 95th percentile for wake or sleep index, or wake or sleep load > 25%.
Participants completed a medical history form that includes a field for diagnosis of hypertension (“Child has hypertension YES/NO”). This information was used to define self-reported hypertension diagnosis as a participant was diagnosed with hypertension versus not diagnosed with hypertension. Medication use was also collected at each annual study visit. Antihypertensive medication use was categorized as a participant currently taking an antihypertensive medication versus not currently taking an antihypertensive medication.
Primary Outcomes
Unrecognized hypertensive BP was defined as a participant with BP measurements that were classified as hypertensive (stage 1 or stage 2) without a reported diagnosis of hypertension, versus recognized hypertensive BP where a diagnosis of hypertension was provided. We also categorized blood pressure as uncontrolled when participants had a measured BP reading in hypertensive range despite the use of antihypertensive medication. Treated versus untreated hypertensive BP was defined as participants with hypertensive BP readings currently using antihypertensive medications versus not currently using antihypertensive medications.
Primary Exposure
Age category was the primary exposure of interest. Age was categorized into three groups: 0 to <7 years, ≥7 to <13 years, ≥13 to ≤18 years. These groups were chosen to reflect early, middle, and late childhood. In all analyses, the oldest group, ≥13 to ≤18 years of age, was considered the reference.
Additional Covariates
Demographic and clinical characteristics were measured at baseline and at follow-up visits. Age-sex-specific BMI percentiles were determined based on the 2000 Centers for Disease Control (CDC) growth charts14. Obesity was defined as BMI >95th percentile. Additional characteristics of interest included low birth weight (<2500 grams) and premature birth (delivery at <36 weeks gestation). Kidney related variables included CKD etiology (characterized as glomerular and non-glomerular), as well as UPC (characterized as nephrotic range if UPC >2 mg/mg) and GFR, both iohexol (iGFR) and estimated GFR (eGFR)10. Blood and urine samples for laboratory data were collected at the same visit as the clinic BP measurement and were analyzed in the central study lab (University of Rochester, Rochester, NY). Participant demographic and medical history information, including medication administration, were gathered by self-report and caregiver report at each study visit. Covariates of interest in adjusted analyses included sex, glomerular diagnosis, current eGFR, nephrotic-range UPC, and obesity as they were thought to be potential confounders.
Statistical Analysis
Demographic and clinical characteristics of the study population at the first (baseline) study visit were described overall and by age group. Median [interquartile range (IQR)] and percent (n) were used for continuous and categorical variables, respectively. BP related characteristics including BP percentiles and BP status were also described across all person-visits by age group. The relationship between self-reported hypertension diagnosis and antihypertensive medication use was explored among study participants with stages 1 and 2 hypertensive BP readings using descriptive statistics. Univariate and multivariable logistic regression were performed to quantify the relationship between unrecognized hypertensive BP and age among participants with hypertensive BP stages 1 and 2. Among participants with unrecognized hypertension, the relationship of treatment use with age was quantified using similar logistic regression models. Generalized estimating equations (GEE) were used to account for repeated measurements within individuals across follow-up study visits. Similar descriptive tables of BP-related characteristics of participants with adequate ABPM data, including ABPM indices as well as the relationship of reported hypertension diagnosis and medication use, are included in the Supplemental Material section. All analyses were done using SAS version 9.4 and figures were produced in R version 4.0.0.
Results
At the time of our analysis, 1098 children had completed 5033 CKiD annual study visits prior to initiation of kidney replacement therapy (KRT) and reaching 18 years of age. Participants with complete BP (stage, hypertension diagnosis, and antihypertensive medication use) and covariate (eGFR, UPC, and BMI) data at baseline and follow-up visits were included in our study population. After applying the exclusion criteria, 902 CKiD study participants contributed 3550 study visits to the analyses (Figure 1). Table 1 describes the baseline characteristics of the study cohort, overall and stratified by age: 0 to <7 years of age (n=264), ≥7 to 13 years of age (n=317), and ≥13 to ≤18 years of age (n=321). A majority of the participants were male (64%), and a majority had normal BMI (82%). The median age of the cohort was 10.73 years (IQR: 6.21, 14.40), with the median age of the <7 years of age group being 4.62 years (IQR: 3.62, 5.60). The median iGFR was 49.0 ml/min/1.73m2 (IQR: 37.0–65.1) and was similar across all age groups. Glomerular diagnosis was a less common etiology of CKD (28%, n=252), with the highest prevalence in the eldest age group (47%) compared to the youngest (9%).
Figure 1.
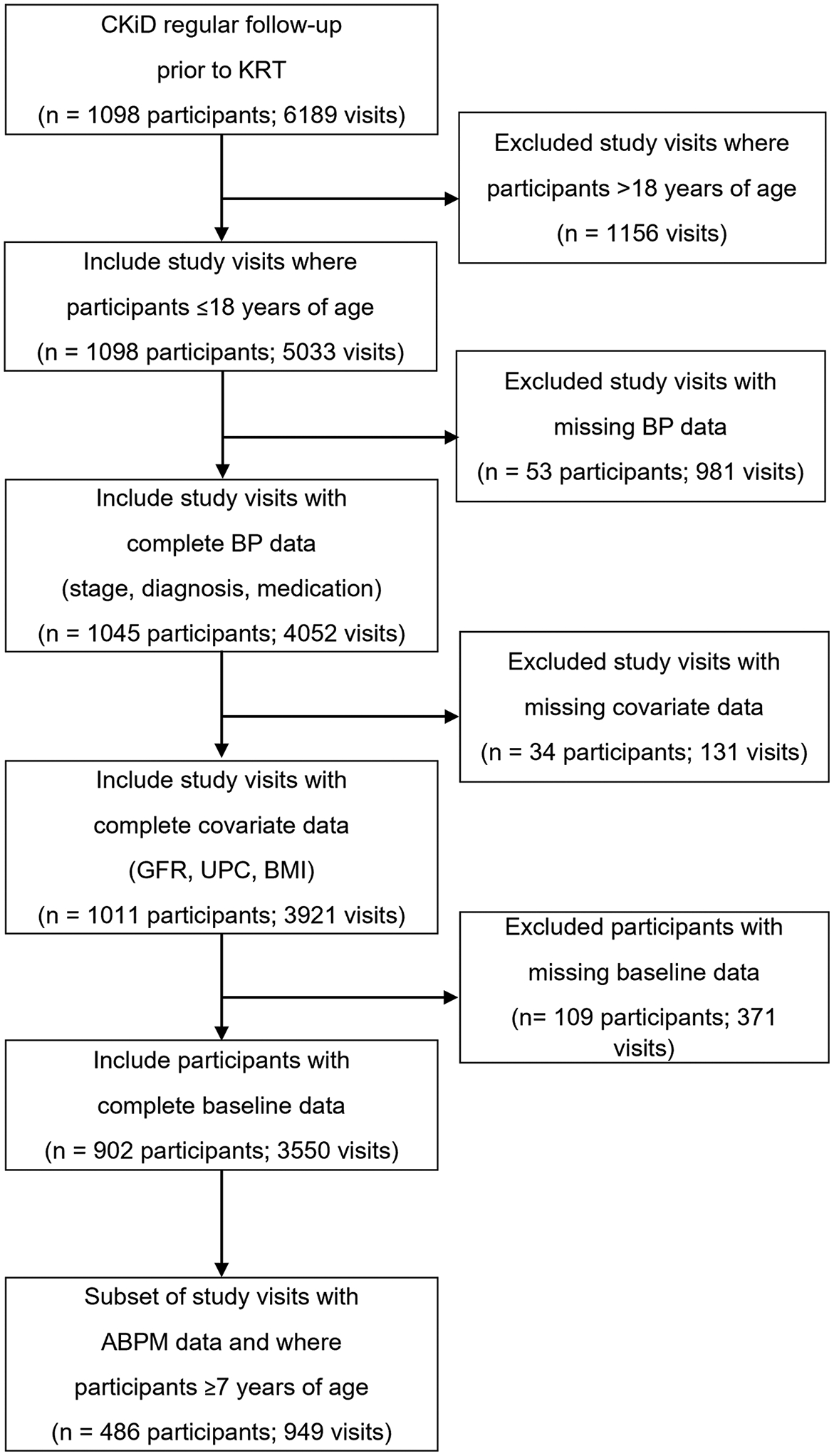
Flow diagram of study design. KRT kidney replacement therapy, BP blood pressure, GFR glomerular filtration rate, BMI body mass index, ABPM ambulatory blood pressure monitoring.
Table 1:
Baseline clinical characteristics by age for 902 participants.
| Characteristics* | Overall, n=902 | 0 to <7 yrs, n=264 | ≥7 to <13 yrs, n=317 | ≥13 to ≤18 yrs, n=321 |
|---|---|---|---|---|
| Median age, years Male sex | 10.73 [6.21, 14.40] 64% (573) | 4.62 [3.62, 5.60] 70% (184) | 10.19 [8.73, 11.40] 62% (195) | 15.25 [14.23, 16.16] 60% (194) |
| BMI percentile | 69 [39, 91] | 66 [38, 86] | 69 [41, 91] | 72 [42, 93] |
| Obesity (BMI >95th percentile) | 18% (162) | 14% (37) | 18% (58) | 21% (67) |
| Low birth weight (<2500 grams) | 18% (155) | 22% (54) | 18% (53) | 16% (48) |
| Premature birth (<36 weeks) | 12% (108) | 19% (49) | 10% (29) | 10% (30) |
| iGFR, ml/min/1.73m2 | 49.0 [37.0, 65.1] | 49.0 [35.9, 60.8] | 47.7 [37.3, 61.6] | 52.9 [37.3, 71.5] |
| eGFR, ml/min/1.73m2 | 49.9 [36.6, 64.4] | 49.7 [36.0, 62.4] | 48.5 [37.6, 62.0] | 51.5 [36.8, 68.4] |
| Proteinuria (UPC) | 0.34 [0.13, 0.95] | 0.33 [0.15, 0.72] | 0.24 [0.10, 0.93] | 0.45 [0.15, 1.35] |
| Glomerular diagnosis | 28% (252) | 9% (24) | 24% (77) | 47% (151) |
| CKD duration at enrollment, years | 7 [4, 12] | 4 [3, 5] | 9 [7, 11] | 13 [3, 15] |
Reported as median [IQR] for continuous variables and percent (n) for categorical variables. Missing data includes birth weight, n=61; premature, n=33; iGFR, n=112; CKD onset date, n=8. BMI body mass index, iGFR iohexol glomerular filtration rate, eGFR estimated glomerular filtration rate, UPC urine protein to creatinine ratio, CKD chronic kidney disease.
The BP status of study participants determined by clinic BP measurements at annual study visits is shown in Table 2. Thirty two percent (n=166) of children <7 years of age had clinic BP readings consistent with stage 1 or stage 2 hypertensive BP, while 22% (n=294) and 16% (n=264) of the ≥7 to 13 and ≥13 to ≤18 years of age sub-groups had hypertensive BP readings, respectively. Self-reported use of antihypertensive medication was lowest in the youngest age group (45%, n=234), with progressive increase in medication use with increasing age, measuring 72% (n=1201) in the ≥13 years of age group. Less children in the youngest age group (31%, n=162) were prescribed angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (ACEi/ARB) compared to the oldest age group (61%, n=1023). In addition, fewer participants in the <7 years of age group (32%, n=165) reported a known diagnosis of hypertension compared to older participants (46% and 47% in ages ≥7 to 13 and ≥13 to ≤18 years, respectively). Of the 2410 study visits at which clinic BP was normal, 38% (n=906) were of participants with known diagnosis of hypertension on medication, overall consistent with well-controlled hypertension. However, fewer participants in the <7 years of age group (25%, n=71) demonstrated well-controlled hypertension, versus 39% in the ≥13 years of age group (n=480).
Table 2:
Blood pressure status and characteristics by age for 3550 person-visits contributed by 902 participants. Hypertensive status was defined using clinic data, based on current American Academy of Pediatrics (AAP) guidelines12. Clinic BP was classified as normal, elevated, stage 1 hypertensive BP, or stage 2 hypertensive BP.
| Characteristics* | 0 to <7 yrs, n=524 | ≥7 to <13 yrs, n=1354 | ≥13 to ≤18 yrs, n=1672 |
|---|---|---|---|
| Systolic BP percentile | 76 [50, 91] | 67 [38, 89] | 52 [24, 76] |
| Systolic BP status | |||
| <90th percentile | 71% (374) | 77% (1037) | 87% (1457) |
| ≥90th to <95th percentile | 10% (50) | 8% (106) | 5% (89) |
| ≥95th percentile | 19% (100) | 15% (211) | 8% (126) |
| Diastolic BP percentile | 82 [60, 94] | 65 [44, 86] | 57 [31, 82] |
| Diastolic BP status | |||
| <90th percentile | 64% (336) | 80% (1078) | 84% (1403) |
| ≥90th to <95th percentile | 13% (66) | 7% (99) | 6% (96) |
| ≥95th percentile | 23% (122) | 13% (177) | 10% (173) |
| Blood pressure status | |||
| Normal | 54% (282) | 67% (909) | 73% (1219) |
| Elevated | 14% (76) | 11% (151) | 11% (189) |
| Stage 1 hypertensive BP | 28% (144) | 18% (246) | 12% (199) |
| Stage 2 hypertensive BP | 4% (21) | 4% (48) | 4% (65) |
| Hypertensive BP (stages 1 & 2) | 32% (166) | 22% (294) | 16% (264) |
| Current use of antihypertensive medication | 45% (234) | 65% (878) | 72% (1201) |
| Current use of ACEi/ARB | 31% (162) | 55% (748) | 61% (1023) |
| Self-reported hypertension | 32% (165) | 46% (624) | 47% (783) |
Reported as median [IQR] for continuous variables and percent (n) for categorical variables. ACEi angiotensin-converting enzyme inhibitor, ARB angiotensin receptor blocker.
There were 410 CKiD study participants across 724 visits with stage 1 or 2 hypertensive BP based on clinic BP. Within this group, thirty-nine percent (n=65) of visits that occurred in which children were <7 years of age reported a diagnosis of hypertension, versus 55% (n=161) in the ≥7 to <13 years and 63% (n=167) in the ≥13 years age groups. Figure 2 displays the hypertensive status for participants with stages 1 and 2 hypertensive BP by age group. Individuals reporting a diagnosis of hypertension were labeled as recognized and those who did not report a diagnosis as unrecognized. Hypertensive participants who were prescribed antihypertensive medication were labeled as uncontrolled while those not on medication were labeled as untreated. The highest incidence of unrecognized, untreated hypertensive BP was in children <7 years of age (46%) compared to children ≥13 years of age (21%).
Figure 2.
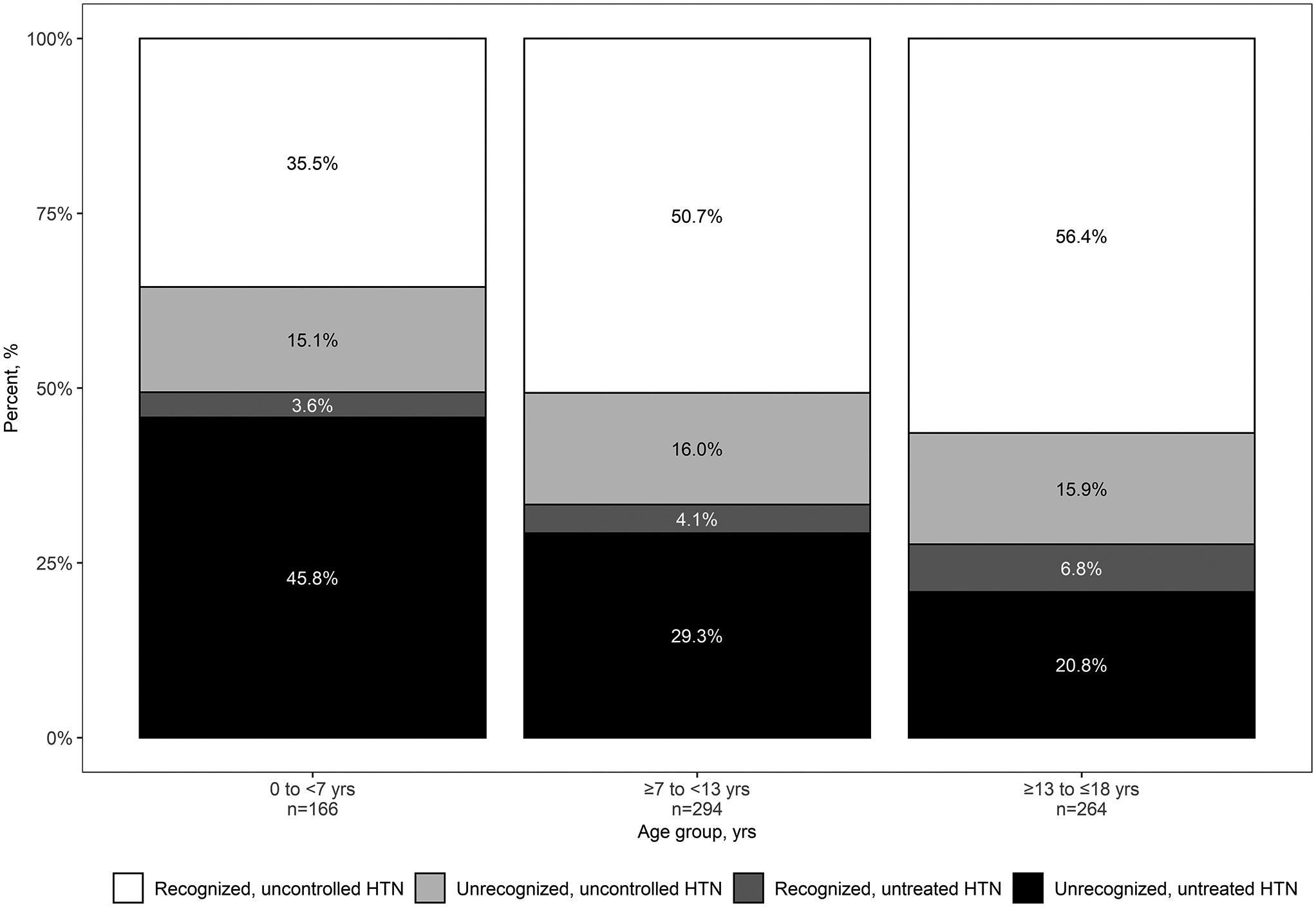
Hypertensive status by age from 724 person-visits contributed by 410 participants with stages 1 and 2 hypertensive BP readings. Those who reported diagnosis of hypertension were labeled as recognized versus those who did not as unrecognized. Participants who were prescribed antihypertensive medication but demonstrated hypertensive BP readings were labeled as uncontrolled, while those not on medication were labeled as untreated.
Figure 3 provides the unadjusted and adjusted odds ratios (OR) (95% confidence interval [CI]) of unrecognized hypertensive BP among participants with stages 1 and 2 hypertensive BP (Figure 3A) as well as OR (95% CI) of antihypertensive medication use among participants with unrecognized hypertensive BP (Figure 3B). The reference population was children ≥13 to ≤18 years of age. The youngest age group was associated with higher odds of unrecognized hypertensive BP (adjusted OR 2.11, 95% CI: 1.37, 3.24) and lower odds of antihypertensive medication use among those with unrecognized hypertensive BP (adjusted OR 0.51, 95% CI: 0.27, 0.996).
Figure 3.
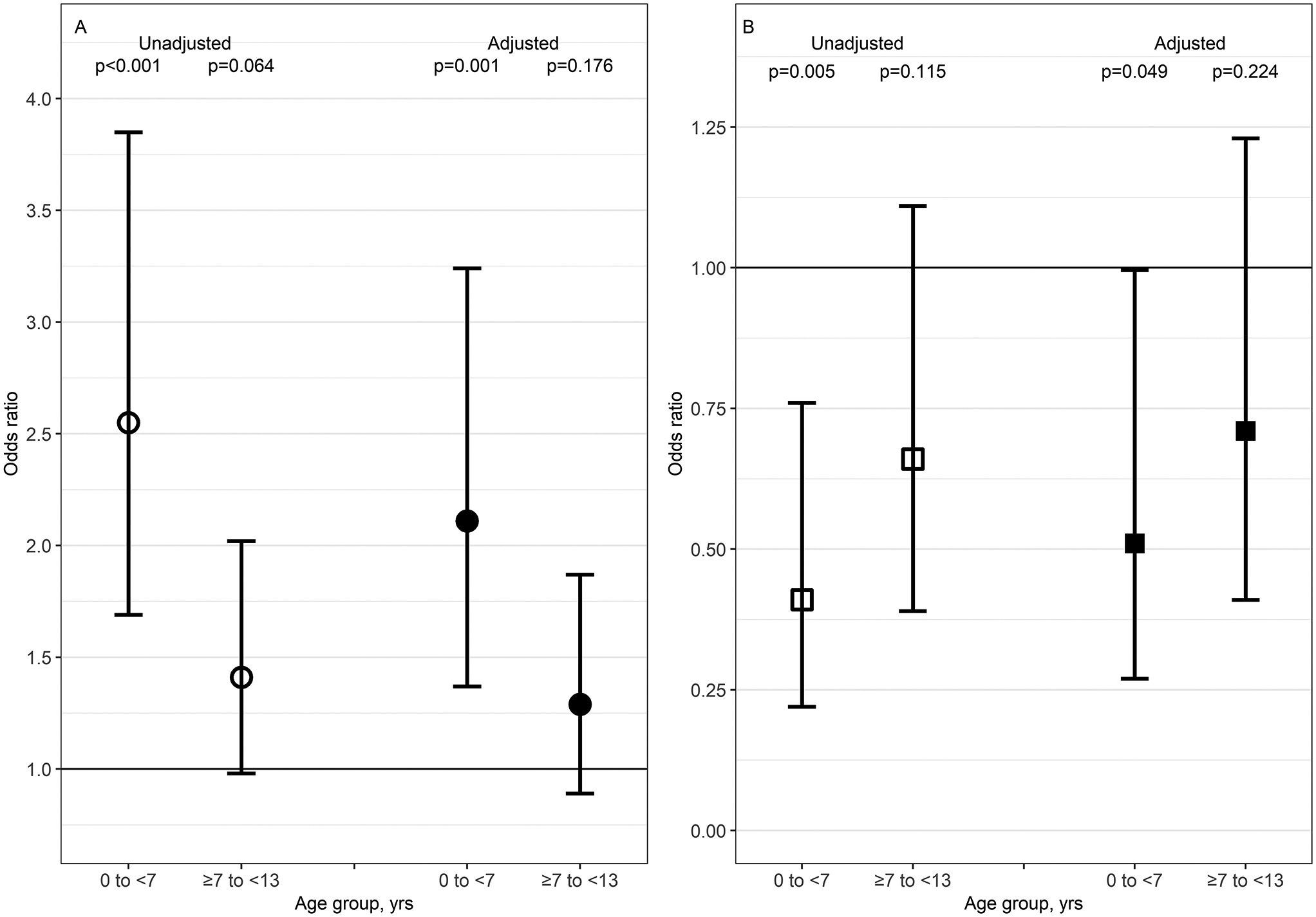
Odds ratios (OR) of unrecognized hypertensive BP and pharmacologic treatment of unrecognized hypertensive BP. Panel A represents OR of unrecognized hypertensive BP among 410 participants contributing 724 person-visits with hypertensive BP readings (A). Panel B represents OR of pharmacologic treatment use among 227 participants contributing 331 person-visits with unrecognized hypertensive BP (B). Unrecognized hypertensive BP was defined as having stage 1 or 2 hypertensive BP per 2017 BP guidelines without a self-reported diagnosis of hypertension12. The reference population was children ≥13 to ≤18 years of age. Open shapes are unadjusted OR and closed shapes are adjusted OR. Circles represent OR for unrecognized hypertensive BP and squares depict OR for treatment use. Adjusted models included male sex, glomerular diagnosis, and concurrent values of BMI, eGFR, and UPC. Body mass index BMI, estimated glomerular filtration rate eGFR, urine protein to creatine ratio UPC.
A total of 486 participants ages ≥7 to ≤18 years of age contributed 949 ABPM person-visits. Children <7 years of age were excluded from this sub-analysis given the small number of person-visits (52) available for this age group. Supplement Table S1 details hypertensive status of this cohort as determined by clinic BP measurements. The cohort was again stratified by age: ≥7 to 13 years of age (n=403), and ≥13 to ≤ 18 years of age (n=546). Nineteen percent (n=77) of visits occurred when children ≥7 to 13 years of age had stage 1 or 2 hypertensive clinic BP, while in 14% (n=77) of the visits for the ≥13 to ≤18 years of age group, BP readings met hypertensive criteria. Using ABPM hypertension criteria, 57% (n=229) of person-visits for children ≥7 to 13 years demonstrated hypertension, with a similar prevalence in the oldest age group (52%, n=285) (Supplement Table S2). Forty-five percent (n=104) of children ≥7 to <13 years with hypertensive BP readings on ABPM reported a diagnosis of hypertension, with a similar prevalence in the oldest age group (46%, n=131). However, the ≥7 to <13 years of age group had a higher proportion of hypertensive children who did not report both a diagnosis of hypertension and antihypertensive medication use (i.e. unrecognized, untreated HTN), totaling 63% (n=79) versus 50% (n=77) in the ≥13 to ≤18 years of age group (Supplement Table S3).
Discussion
We found that young children with mild to moderate CKD in the CKiD cohort were more likely to have unrecognized and untreated hypertensive BP readings compared to older children with CKD. Children <7 years of age had a higher prevalence of hypertensive BP readings based on age, sex, and height normative values using the 2017 AAP clinical practice guidelines12. Additionally, caregivers of children <7 years of age were less likely to report a hypertension diagnosis and current antihypertensive medication use despite hypertensive BP readings, suggesting that a proportion of young children with hypertension have inadequate BP management. We found that with increasing age, the prevalence of hypertensive BP readings decreased while report of hypertension diagnosis and antihypertensive medication use increased, suggesting a discrepancy between younger and older children in the recognition and management of hypertension. Children aged ≥7 to <13 years with hypertensive BP readings had lower odds of unrecognized hypertensive BP, in addition to a higher prevalence of treatment with antihypertensive medications, compared to younger children. Thus, hypertensive BP readings were not adequately recognized in young children in our cohort, and when recognized and treated, were often uncontrolled.
Unrecognized hypertension is a known phenomenon in pediatrics despite routine screening15–17. Many contributing factors have been identified in the general patient population, such as lack of provider familiarity with normative BP thresholds, leading to missed diagnoses12, 18. Similarly, despite knowledge of increased cardiovascular risk among children with CKD and the association of hypertension with CKD progression, hypertensive BP is often poorly recognized and uncontrolled in both CKD and ESKD patients19–22. Baseline data from the CKiD cohort at study entry demonstrated that 39% of children with hypertensive systolic or diastolic BP were not receiving antihypertensive agents3. Among treated participants, nearly half had persistently hypertensive BPs, suggesting that hypertension is both underdiagnosed and undertreated in children with CKD, which this subsequent analysis validates using age as a risk factor. Similar to the general pediatric population, this may be secondary to lack of familiarity with normative BP values. Specifically for young children with CKD, other factors may include difficulty in obtaining accurate BP measurements in this age group and provider lack of familiarity of BP targets. In addition, the use of potent antihypertensive agents, such as renin-angiotensin-aldosterone-system (RAAS) inhibitors, may be less frequent in young children. Abraham et al. demonstrated in the CKiD cohort that users of RAAS inhibitors were more likely to be of older age, less likely to have hypertensive blood pressure, and had a lower risk of KRT compared to non-RAAS users23. Despite this, only 31% of children <7 years of age in our study were prescribed ACEi/ARB, compared to larger proportions in the older age groups, suggesting that young children are not prescribed the most effective class of antihypertensive medications.
Our study has several limitations. The diagnosis of hypertension was based on participant or caregiver report on a medical history intake form rather than a provider-issued diagnosis documented in the medical record. However, even if erroneous caregiver omission of hypertension diagnosis resulted in underestimating the prevalence of recognized hypertension, a mismatch between caregiver and provider knowledge of hypertension diagnosis suggests a patient education barrier that is important to address. Additionally, longitudinal trends for individual participants were not assessed in this analysis as person-visits for each participant were viewed as independent events. However, examination of the association between duration of CKD and antihypertensive medication administration with regards to CKD progression over time could be a worthwhile focus of future work. Antihypertensive medication adherence was also not assessed, which may be a contributor to uncontrolled BP in all age groups. In addition, it is unknown whether participants were prescribed ACEi/ARB for reasons other than blood pressure control, such as treatment of proteinuria. Future investigations should focus on specific barriers to both clinical guideline implementation and medication adherence.
This study primarily utilized clinic BP measurements to ascertain BP status, as ABPM data was limited in children <7 years of age. While ABPM is the current gold standard approach to confirm a diagnosis of hypertension, there are clinical barriers to successful completion of ABPM in children with younger developmental age. In addition, normative ABPM values are lacking in those with height <120cm, making this data difficult to interpret. Thus, we cannot exclude white-coat effect or white-coat hypertension (WCH) as a diagnosis in those with hypertensive clinic BP readings. However, prior analyses of the CKiD cohort have shown that the prevalence of WCH is relatively low among those participants who were able to successfully complete ABPM, while masked hypertension was common11. Although the benefits of ABPM have been clearly demonstrated in children with CKD in predicting target organ effects and CKD progression, a recent CKiD analysis found that systolic clinic BP was not inferior to mean wake SBP on ABPM in their ability to predict these important outcomes24, 25.
Perspectives
Given the impact of hypertension on CKD progression and cardiovascular disease, efforts to improve BP control in young children with CKD are needed. Increased provider recognition of hypertensive BP in young children with chronic kidney disease is integral to minimizing future cardiovascular morbidity. Further investigation should include a focused evaluation of young children with CKD for subsequent cardiac remodeling and target organ damage, with the goal of decreasing the morbidity and mortality associated with both childhood and adult CKD.
Supplementary Material
Novelty and Relevance.
“What is new?”:
This study is the largest prospective cohort of children with mild to moderate CKD to demonstrate that young children are less likely to be diagnosed with hypertension compared to older children despite hypertensive BP readings.
“What is relevant?”:
Provider recognition of hypertension improves as children approach adulthood. This suggests that practitioners have barriers to accurate diagnosis of hypertensive BP in daily practice.
“Clinical/pathophysiological implications?”:
Our hope is that these findings will increase provider awareness of hypertension in young children with CKD and underline the need to address obstacles to clinical practice guideline implementation, leading to improved lifelong outcomes in this high-risk population.
Acknowledgements
Data in this manuscript were collected by the Chronic Kidney Disease in children prospective cohort study (CKiD) with clinical coordinating centers (Principal Investigators) at Children’s Mercy Hospital and the University of Missouri – Kansas City (Bradley Warady, MD) and Children’s Hospital of Philadelphia (Susan Furth, MD, PhD), Central Biochemistry Laboratory (George Schwartz, MD) at the University of Rochester Medical Center, and data coordinating center (Alvaro Muñoz, PhD and Derek Ng, PhD) at the Johns Hopkins Bloomberg School of Public Health. The CKiD website is located at https://statepi.jhsph.edu/ckid and a list of CKiD collaborators can be found at https://statepi.jhsph.edu/ckid/site-investigators/, in addition to the Supplemental Material.
Sources of Funding
The CKiD Study is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (U01 DK066143, U01 DK066174, U24 DK082194, U24 DK066116). Dr. Chloe Douglas is supported by the National Institute of Health training grant (5T32DK007467-39, PI Bansal).
Non-standard Abbreviations and Acronyms:
- HTN
hypertension
- CKD
chronic kidney disease
- CKiD
Chronic Kidney Disease in Children
- BP
blood pressure
- ESKD
end stage kidney disease
- eGFR
estimated glomerular filtration rate
- AAP
American Academy of Pediatrics
- AHA
American Heart Association
- CDC
Center for Disease Control
- UPC
urine protein to creatinine ratio
- BMI
body mass index
- ABPM
ambulatory blood pressure monitoring
- iGFR
iohexol glomerular filtration rate
- GEE
generalized estimating equations
- KRT
kidney replacement therapy
- ACEi
angiotensin-converting enzyme inhibitor
- ARB
angiotensin receptor blocker
- RAAS
renin-angiotensin-aldosterone system
- WCH
white-coat hypertension
Footnotes
References
- 1.Halbach SM, Martz K, Mattoo T, Flynn J. Predictors of blood pressure and its control in pediatric patients receiving dialysis. J Pediatr. 2012;160:621–625.e621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kramer AM, van Stralen KJ, Jager KJ, Schaefer F, Verrina E, Seeman T, et al. Demographics of blood pressure and hypertension in children on renal replacement therapy in europe. Kidney Int. 2011;80:1092–1098 [DOI] [PubMed] [Google Scholar]
- 3.Flynn JT, Mitsnefes M, Pierce C, Cole SR, Parekh RS, Furth SL, et al. Blood pressure in children with chronic kidney disease: A report from the chronic kidney disease in children study. Hypertension. 2008;52:631–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parekh RS, Carroll CE, Wolfe RA, Port FK. Cardiovascular mortality in children and young adults with end-stage kidney disease. J Pediatr. 2002;141:191–197 [DOI] [PubMed] [Google Scholar]
- 5.Groothoff JW, Gruppen MP, Offringa M, Hutten J, Lilien MR, Van De Kar NJ, et al. Mortality and causes of death of end-stage renal disease in children: A dutch cohort study. Kidney Int. 2002;61:621–629 [DOI] [PubMed] [Google Scholar]
- 6.Mitsnefes M, Flynn J, Cohn S, Samuels J, Blydt-Hansen T, Saland J, et al. Masked hypertension associates with left ventricular hypertrophy in children with ckd. J Am Soc Nephrol. 2010;21:137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urbina EM, Khoury PR, McCoy C, Daniels SR, Kimball TR, Dolan LM. Cardiac and vascular consequences of pre-hypertension in youth. J Clin Hypertens (Greenwich). 2011;13:332–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, et al. Design and methods of the chronic kidney disease in children (ckid) prospective cohort study. Clin J Am Soc Nephrol. 2006;1:1006–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate gfr in children with ckd. J Am Soc Nephrol. 2009;20:629–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pierce CB, Muñoz A, Ng DK, Warady BA, Furth SL, Schwartz GJ. Age- and sex-dependent clinical equations to estimate glomerular filtration rates in children and young adults with chronic kidney disease. Kidney Int. 2021;99:948–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samuels J, Ng D, Flynn JT, Mitsnefes M, Poffenbarger T, Warady BA, et al. Ambulatory blood pressure patterns in children with chronic kidney disease. Hypertension. 2012;60:43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140. [DOI] [PubMed] [Google Scholar]
- 13.Flynn JT, Daniels SR, Hayman LL, Maahs DM, McCrindle BW, Mitsnefes M, et al. Update: Ambulatory blood pressure monitoring in children and adolescents: A scientific statement from the american heart association. Hypertension. 2014;63:1116–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000. cdc growth charts for the united states: Methods and development. Vital Health Stat 11. 2002:1–190 [PubMed] [Google Scholar]
- 15.Gunn PW, Hansen ML, Kaelber DC. Underdiagnosis of pediatric hypertension-an example of a new era of clinical research enabled by electronic medical records. AMIA Annu Symp Proc. 2007:966. [PubMed] [Google Scholar]
- 16.Brady TM, Solomon BS, Neu AM, Siberry GK, Parekh RS. Patient-, provider-, and clinic-level predictors of unrecognized elevated blood pressure in children. Pediatrics. 2010;125:e1286–1293 [DOI] [PubMed] [Google Scholar]
- 17.McNiece KL, Poffenbarger TS, Turner JL, Franco KD, Sorof JM, Portman RJ. Prevalence of hypertension and pre-hypertension among adolescents. J Pediatr. 2007;150:640–644, 644.e641 [DOI] [PubMed] [Google Scholar]
- 18.Rea CJ, Brady TM, Bundy DG, Heo M, Faro E, Giuliano K, et al. Pediatrician adherence to guidelines for diagnosis and management of high blood pressure. J Pediatr. 2022;242:12–17.e11 [DOI] [PubMed] [Google Scholar]
- 19.Wühl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, Zurowska A, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639–1650 [DOI] [PubMed] [Google Scholar]
- 20.Fathallah-Shaykh SA, Flynn JT, Pierce CB, Abraham AG, Blydt-Hansen TD, Massengill SF, et al. Progression of pediatric ckd of nonglomerular origin in the ckid cohort. Clin J Am Soc Nephrol. 2015;10:571–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barletta GM, Pierce C, Mitsnefes M, Samuels J, Warady BA, Furth S, et al. Is blood pressure improving in children with chronic kidney disease? A period analysis. Hypertension. 2018;71:444–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warady BA, Abraham AG, Schwartz GJ, Wong CS, Muñoz A, Betoko A, et al. Predictors of rapid progression of glomerular and nonglomerular kidney disease in children and adolescents: The chronic kidney disease in children (ckid) cohort. Am J Kidney Dis. 2015;65:878–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abraham AG, Betoko A, Fadrowski JJ, Pierce C, Furth SL, Warady BA, et al. Renin-angiotensin ii-aldosterone system blockers and time to renal replacement therapy in children with ckd. Pediatr Nephrol. 2017;32:643–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guzman-Limon ML, Jiang S, Ng D, Flynn JT, Warady B, Furth SL, et al. Nocturnal hypertension in children with chronic kidney disease is common and associated with progression to kidney replacement therapy. Hypertension. 2022:101161HYPERTENSIONAHA12118101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ku E, McCulloch CE, Warady BA, Furth SL, Grimes BA, Mitsnefes MM. Twenty-four-hour ambulatory blood pressure versus clinic blood pressure measurements and risk of adverse outcomes in children with ckd. Clin J Am Soc Nephrol. 2018;13:422–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


