Abstract
Interoception refers to the processing, integration, and interpretation of bodily signals by the brain. Interoception is key to not only basic survival, but also motivational and affective functioning. There is emerging evidence suggesting altered interoception in schizophrenia, but few studies have explored potential neural underpinnings. The current study aims to investigate the anatomical connectivity of a previously identified interoception network in individuals with schizophrenia, and the relationship between network structural connectivity and both emotional functioning and clinical symptoms. Thirty-five participants with schizophrenia (SZ) and 36 healthy control participants (HC) underwent diffusion tensor imaging (DTI) and performed tasks measuring emotional functioning. Probabilistic tractography was used to identify white matter tracts connecting key hubs in an interoception network. Microstructural integrity of these tracts was compared across groups and correlated with measures of emotional functioning and symptom severity. Compared with HC, SZ exhibited altered structural connectivity in the interoception network. In HC, the structural connectivity of the network was significantly correlated with emotion recognition, supporting a link between the interoception network and emotional functioning. However, this correlation was much weaker in SZ. These findings suggest that altered interoception may have implications for illness mechanisms of schizophrenia, especially in relation to emotional deficits.
Keywords: DTI, facial emotion recognition, amygdala, insula, anterior cingulate cortex, allostasis
1. Introduction
Research into the physiological mechanisms of schizophrenia has traditionally focused almost exclusively on how the brain processes and interprets information from the outside world. However, there is growing evidence that internal signals from the body can influence a wide range of cognitive and emotional functions (Makowski et al., 2020; Pramme et al., 2014, 2016; Tsakiris & De Preester, 2019). The processing of bodily signals by the central nervous system is called interoception. Disturbances in interoception may underlie a wide range of schizophrenia symptoms (see Yao & Thakkar, 2022 for review).
Interoception is closely linked to allostasis (Schulkin & Sterling, 2019; Sterling, 2012): a predictive regulation process wherein the brain adjusts and prepares the body based on predicted metabolic needs (e.g., drink before we become dehydrated). According to the interoceptive inference theory (Barrett & Simmons, 2015; Seth et al., 2012; Seth & Friston, 2016), the brain maintains an internal model of the body and is constantly trying to minimize discrepancies between the predicted and actual signals from the body (i.e., prediction error signals). To minimize prediction errors, the brain will either shift the prediction to match the incoming sensory data (i.e., update the model of the body), or shift the sensory data towards the prediction (i.e., active inference; Seth, 2015). The latter can be achieved by changing the sensory input through hormonal, visceral, immunological, autonomic, and behavioral mechanisms (e.g., taking a break during exercise when heart rate is too fast; Pezzulo et al., 2015).
Emotion has been theorized as a crucial component of the interoceptive inference process (Barrett, 2017; Seth, 2013). It is a conscious “label” that helps us better understand our everchanging bodily state, so that we can prepare for incoming bodily sensations and take adaptive actions to resolve interoceptive prediction errors when needed. For example, we may interpret the same bodily sensations experienced while riding a rollercoaster as either “afraid” or “excited” and decide to rest or ride again as a result of those attributions. We may assign different emotion labels to similar patterns of bodily signals because emotion results from dynamically incorporating interoception, past experience, current context, and attentional focus.
According to the Embodied Predictive Interoceptive Coding (EPIC) model, the computational model of interoceptive inference discussed above is instantiated by a specific brain network. Namely, the visceromotor cortices generate predictions regarding the state of the body depending on past experience and the external environment, while the body sends signals of actual physiological states to mid-posterior insula, the interoceptive sensory cortex (Barrett & Simmons, 2015). Tract-tracing studies in monkeys and neuroimaging studies in humans provide support for this allostatic-interoceptive network (Kleckner et al., 2017). Moreover, individuals with stronger functional connectivity within this network exhibit higher interoceptive accuracy (Kleckner et al., 2017).
Although few studies have directly investigated interoception in schizophrenia (Ardizzi et al., 2016; Critchley et al., 2019; Koreki et al., 2021; Torregrossa et al., 2022), a larger body of work investigating various bodily systems further supports altered interoception in schizophrenia (Yao & Thakkar, 2022). Furthermore, there is a robust evidence base for structural and functional abnormalities in a key node in the aforementioned interoception network--the insula--that are associated with symptom severity and emotion recognition (Sheffield et al., 2020; Takahashi et al., 2020; Wylie & Tregellas, 2010).
The current study aimed to extend this previous neuroimaging work by investigating the structural connectivity of a putative interoception network and its relationship with emotional functioning and clinical symptoms in schizophrenia. White matter microstructural abnormalities are widespread in schizophrenia (Kelly et al., 2018). Such abnormalities may lead to disrupted communications within the interoception network, resulting in altered allostasis and/or emotional functioning. Therefore, we examined the white matter microstructural integrity within this network in the current study. We predicted that relative to healthy controls, participants with schizophrenia would have reduced structural connectivity. We also predicted that structural connectivity within this network would be correlated with emotional functioning across participants and with clinical symptom severity in schizophrenia.
2. Methods
2.1. Participants
We recruited 37 individuals with a diagnosis of schizophrenia or schizoaffective disorder (SZ) from an outpatient psychiatric facility and through community and internet advertisements. Thirty-six HC were recruited through community and internet advertisements and existing subject pools. Diagnoses were determined using the Structured Clinical Interview for DSM-IV. All participants gave written informed consent and were reimbursed for participation. The study was approved by the University of Michigan Medical School Institutional Review Board. After excluding two participants due to poor imaging data quality, the remaining participants (35 SZ, 36 HC) were well matched in demographics (Table 1). See Supplementary Methods for exclusion criteria.
Table 1.
Demographic and clinical information.
| SZ (N = 35) | HC (N = 36) | Statistic | p | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| Age (Years) | 33.7 (10.4) | 33.1 (9.8) | t = 0.25 | .80 |
| Sex (Female/Male) | 18/17 | 19/17 | X2 = 0.013 | .91 |
| Education (Years) | 14.2 (2.1) | 16.1 (1.8) | t = − 4.18 | < .001 |
| Parental education | 15.3 (2.1) | 15.8 (3.1) | t = − 0.83 | .41 |
| Childhood SESa: medium-high | 11 | 23 | X2 = 8.08 | .04 |
| medium | 17 | 9 | ||
| medium-low | 5 | 3 | ||
| low | 0 | 1 | ||
| WRAT3-R | 50.1 (5.4) | 51.5 (4.1) | t = −1.23 | .22 |
| Race: Asian | 0 | 3 | X2 = 7.9 | .095 |
| Black | 15 | 9 | ||
| Native American | 0 | 1 | ||
| White | 18 | 22 | ||
| Multiracial | 2 | 0 | ||
| Unknown | 0 | 1 | ||
| Hispanic/Latino (Y/N) | 3/32 | 3/33 | X2 = .001 | .97 |
| ER40 accuracy | 31.88 (2.99) | 34.42 (2.61) | t = − 3.74 | < .001 |
| MSCEIT standardized score | 94.10 (19.24) | 114.16 (16.99) | t = −4.60 | < .001 |
| BDI | 15.7 (12.1) | 1.8 (2.8) | t = 6.42 | < .001 |
| STAI - state anxiety | 45.6 (7.8) | 37.5 (5.0) | t = 5.12 | < .001 |
| SAPS: Total scoreb | 16.5 (14.9) | - | - | - |
| Global summaryb | 4.7 (3.5) | - | - | - |
| SANSc: Total score | 20.9 (12.5) | - | - | - |
| Global summary | 6.0 (3.6) | - | - | - |
| Illness Duration (Years) | 13.3 (10.9) | - | - | - |
| Number of hospitalizations | 4.3 (4.9) | - | - | - |
| BPRS | 29.5 (7.5) | - | - | - |
| Antipsychotics (Y/N) | 29/6 | - | - | - |
| CPZ Equivalent (mg) | 304.40 (353.15) | - | - | - |
Notes: BDI, Beck Depression Inventory; BPRS, Brief Psychiatric Rating Scale; CPZ, chlorpromazine; ER40, Penn Emotion Recognition - 40 Task; MSCEIT, Mayer-Salovey-Caruso Emotional Intelligence Test (standardized using age- and gender- corrected normative data); SANS, Scale for the Assessment of Negative Symptoms; SAPS, Scale for the Assessment of Positive Symptoms; STAI, State-Trait Anxiety Inventory; WRAT3-R, Wide Range Achievement Test 3 - Reading.
Childhood SES: medium-high: professional or high-level managerial position, adults hold college or advanced degrees; medium: small businessmen, white collar and skilled workers, high school grads; medium-low: semi-skilled workers, laborers, education below secondary level; low: unskilled and semi-skilled workers, elementary education.
Total score: sum of individual symptom ratings; Global summary: sum of global item ratings.
SANS scores did not include the 2 items on attention due to their poor reliability.
2.2. Assessments
Positive and negative symptoms were assessed using the Scale for the Assessment of Positive Symptoms (SAPS; Andreasen, 1984b) and Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1984a), respectively. Overall psychiatric symptom severity was measured by the Brief Psychiatric Rating Scale (BPRS; Overall & Gorham, 1962). All participants completed the Penn Emotion Recognition - 40 Task (ER40; Gur et al., 2002) and Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT; Mayer et al., 1999). The ER40 measures facial emotion recognition by asking participants to judge the emotion of photographs of faces. The MSCEIT was used as a broad general measure of emotional functioning and measures participants’ emotional intelligence. See Supplementary Methods for more details on these measures.
2.3. Image acquisition
All DTI data were acquired at the University of Michigan on a 3.0 T GE Discovery MR750 scanner (LX [8.3] release, General Electric Healthcare, Buckinghamshire, United Kingdom) with a 32-channel receiver array head coil (Nova Medical, Wilmington, MA) and a multi-band slice accelerated echo-planar imaging (EPI) sequence (96 noncollinear gradient directions). For registration purposes, a whole-brain three-dimensional T1-weighted scan was acquired. See Supplementary Methods for detailed imaging parameters.
2.4. Preprocessing
The neuroimaging data was organized in the BIDS format (Gorgolewski et al., 2016). Preprocessing and quality control of the diffusion-weighted scans were performed using MRtrix3 (Tournier et al., 2019). Group differences in percent of outlier slices and head movement parameters were examined using independent T-tests. See Supplementary Methods for preprocessing and quality assurance details.
2.5. Regions of interest
Based on the EPIC model (Barrett & Simmons, 2015; Kleckner et al., 2017), the current study included the following six regions of interest (ROIs) in each hemisphere: rostral anterior cingulate cortex (rACC), caudal anterior cingulate cortex (cACC), amygdala, ventral anterior insula (vaIns), dorsal mid insula (dmIns), and dorsal posterior insula (dpIns). According to the EPIC model, interoceptive prediction signals travel from rACC, cACC, amygdala, and vaIns to dmIns and dpIns, while interoceptive prediction error signals travel the opposite direction (Figure 1). Therefore, the white matter pathways connecting these ROIs are the key structures supporting the dynamic process of interoceptive inference. See Supplementary Methods for details on generation and transformation of ROI masks.
Figure 1. A schematic of the interoceptive network.
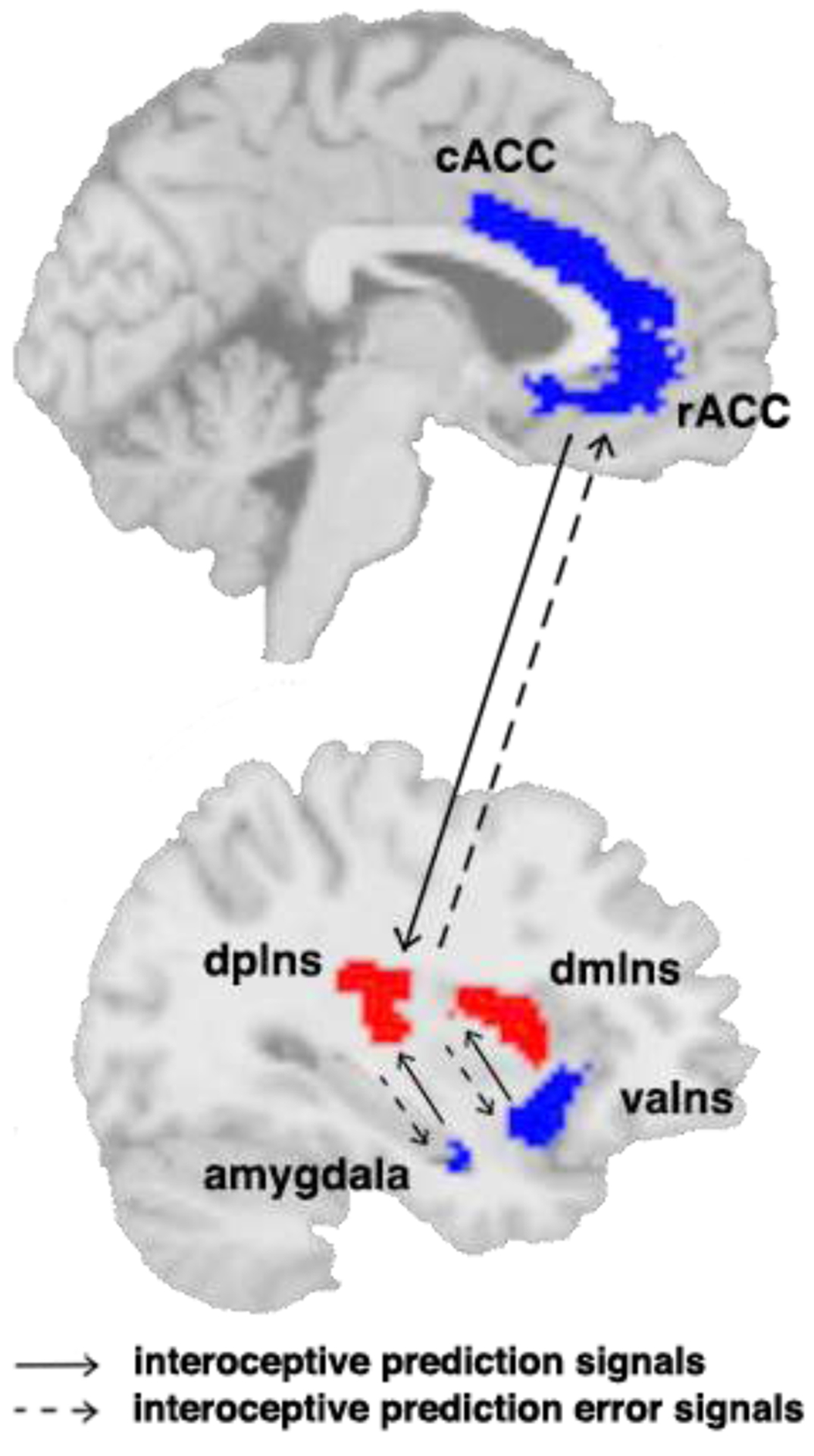
The red regions are the interoceptive sensory regions (dmIns and dpIns). The blue regions are the visceromotor control regions (rACC, cACC, amygdala, and vaIns). According to the EPIC model (Barrett & Simmons, 2015), interoceptive prediction signals travel from rACC, cACC, amygdala, and vaIns to dmIns and dpIns, and interoceptive prediction error signals travel the opposite direction. cACC: caudal anterior cingulate cortex; dmIns: dorsal mid insula; dpIns: dorsal posterior insula; rACC: rostral anterior cingulate cortex.
2.6. Probabilistic tractography
Probabilistic tractography was performed using FSL 6.0.3 (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). Probabilistic tractography is an analysis technique that reconstructs anatomical pathways, presumably white matter tracts, between any given brain regions based on a distribution profile of probable fiber orientations in each voxel (Behrens et al., 2003, 2007). Tracts were constructed based on the connectivity value at each voxel of the brain. Voxels with higher numbers indicate a higher probability that this voxel is part of the tract between the seed and target regions. For each participant, white matter tracts were constructed between visceromotor control and interoceptive sensory ROIs. Because DTI cannot distinguish the direction of axonal fibers, measures generated from both directions (e.g., rACC to dmIns and dmIns to rACC) were considered equally accurate and combined to achieve a single tract for each ROI pair. This resulted in 8 tracts in total (i.e., vaIns – dmIns, vaIns – dpIns, amygdala – dmIns, amygdala – dpIns, rACC – dmIns, rACC – dpIns, cACC – dmIns, cACC – dpIns) for each hemisphere.
After applying white matter masks, we then corrected the connectivity values within each voxel of the tract by dividing the value by the sum of total streamlines between the corresponding pair of ROIs. These tracts were then transformed to MNI space and averaged within each group. Tracts were thresholded using the 99th percentile of all voxels within the tract. These thresholded connectivity maps for each ROI pair were then binarized into masks within each group for later analysis and extraction of diffusivity measures (see Supplementary Methods for details on tract construction and white matter mask generation).
2.7. Diffusivity measures of microstructural integrity
Each participant’s diffusion-weighted images were fit to a tensor model using FSL 6.0.3 (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl) to generate four diffusivity maps, each with one of the following diffusivity measures at each voxel: fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD). FA quantifies the overall anisotropy within a voxel (i.e., how prominent the principal diffusion direction is). MD quantifies the average diffusivity along all directions within a voxel. AD characterizes diffusion along the principal axis, while RD characterizes average diffusion along the directions perpendicular to the principal (axial) direction. FA is currently the most widely used measure of diffusivity, highly sensitive to microstructural integrity of white matter tracts. Higher FA value indexes higher anisotropy and lower diffusivity. However, FA value contains no information on the orientation of anisotropy and thus can be difficult to interpret alone. A decrease in FA could be due to neuropathology, fiber crossing, or aging (Alexander et al., 2007). Therefore, using multiple diffusivity measures can provide a clearer understanding. Specifically, increased RD indicates demyelination (Budde et al., 2011; Kronlage et al., 2017; Song et al., 2003; Sun et al., 2006), and AD captures axonal damage (Song et al., 2003; Sun et al., 2006). MD seems to be most sensitive at assessing white matter maturation and aging (Abe et al., 2002; Snook et al., 2007).
For each participant, diffusivity maps were normalized to MNI space so that diffusivity measures could be extracted from each of the binarized group tracts to be averaged and used in later analysis. See Supplementary Methods for examinations of whole-brain white matter integrity.
2.8. Statistical analyses
Statistical analyses were performed using IBM SPSS Statistics 25.0 (IBM, Armonk, NY) and R (R Core Team, Vienna, Austria). Age was included as a covariate in all group comparisons as white matter integrity decreases with age (Snook et al., 2007).
First, to make sure that the group tracts occupy similar locations in the brain between HC and SZ, we computed η2 for all pairs (HC and SZ) of binarized group tract masks, as well as for the network mask that combines all tract masks in one image (Cohen et al., 2008). η2 measures the similarity between two images on a voxel-by-voxel basis. The value ranges from 0 (no similarity) to 1 (identical).
Next, to examine group differences in structural connectivity of the interoception network, we conducted MANCOVAs on FA and MD extracted from the 16 tracts because FA and MD are the most widely used diffusivity measures. Follow-up exploratory analyses on AD and RD are reported in Supplementary Methods and Results. To protect against inflated Type I error rate, we included all 16 tracts in the initial MANCOVAs (i.e., one for FA and one for MD; Tabachnick & Fidell, 2013). Group was included as a between-subjects variable, and hemisphere as a within-subjects variable. Significant main effects were then followed up by univariate tests. Specifically, individual group comparisons between each ROI pair were evaluated with a repeated-measures ANCOVA, including diagnostic group as a between-subjects variable, and hemisphere as a within-subjects variable. Significant effects of group were followed up with independent t-tests. Significant effects of hemisphere were followed up with paired t-tests. We hypothesized that SZ would have lower FA and higher MD than HC, reflecting compromised white matter microstructure.
Finally, to assess whether structural connectivity of the interoception network is associated with emotional functioning, we investigated the relationship between FA and ER40 accuracy and MSCEIT total score in all participants. To reduce the number of statistical tests, we did not include the other diffusivity measures in this analysis. Since we were interested in the relationship between emotional functioning and structural connectivity specifically within the interoception network, mean FA over the whole brain was regressed out of the tract FA measurements.* These standardized residuals were used in the remaining analyses. These “standardized FA values” are 0 when equal to the whole brain FA average, higher for more anisotropic tracts and lower for more isotropic tracts. We then ran linear regression models using standardized FA from each tract and diagnosis to predict participants’ performance on each emotion task. The linear regression model can be expressed in the following equation, where i stands for the tract, and j stands for the emotional functioning task (ER40 accuracy score or MSCEIT standardized score):
Group was dummy coded as 0 = HC and 1 = SZ. In other words, HC served as the implicit reference group in the model. Significant interaction effects were followed up by rerunning the same model with the opposite dummy coding (i.e., 0 = SZ and 1 = HC) to obtain significance testing of β2 in the SZ group. To control for family-wise error rate, the alpha level for these regressions was Bonferroni-corrected to 0.05/15 = 0.003 (i.e., 15 regressions per emotional functioning task). We hypothesized that lower FA within a putative interoception network would predict worse emotional functioning.
Using SZ data only, we examined the relationship between clinical symptom severity and structural connectivity of the interoception network. See Supplementary Methods for analysis details and Supplementary Results for results.
Because most participants with schizophrenia recruited for the study were taking antipsychotics, we also examined potential confounding effects of antipsychotic use by correlating the normalized (chlorpromazine equivalent) dose (Andreasen et al., 2010; Danivas & Venkatasubramanian, 2013; Rothe et al., 2018) with diffusivity measures and emotional functioning measures (reported in Supplementary Results).
3. Results
3.1. Group differences in structural connectivity
The thresholded group tracts within the putative interoception network can be seen in Figure 2. We computed η2 for all pairs of binarized group tracts and the entire network to examine overall similarities between the location of the tracts. The tracts share very similar locations between the two groups (network η 2=.88; Table S3), indicating no gross anatomical differences in the most probable connections between nodes in the interoception network between groups.
Figure 2. Probabilistic tractography results in both groups.
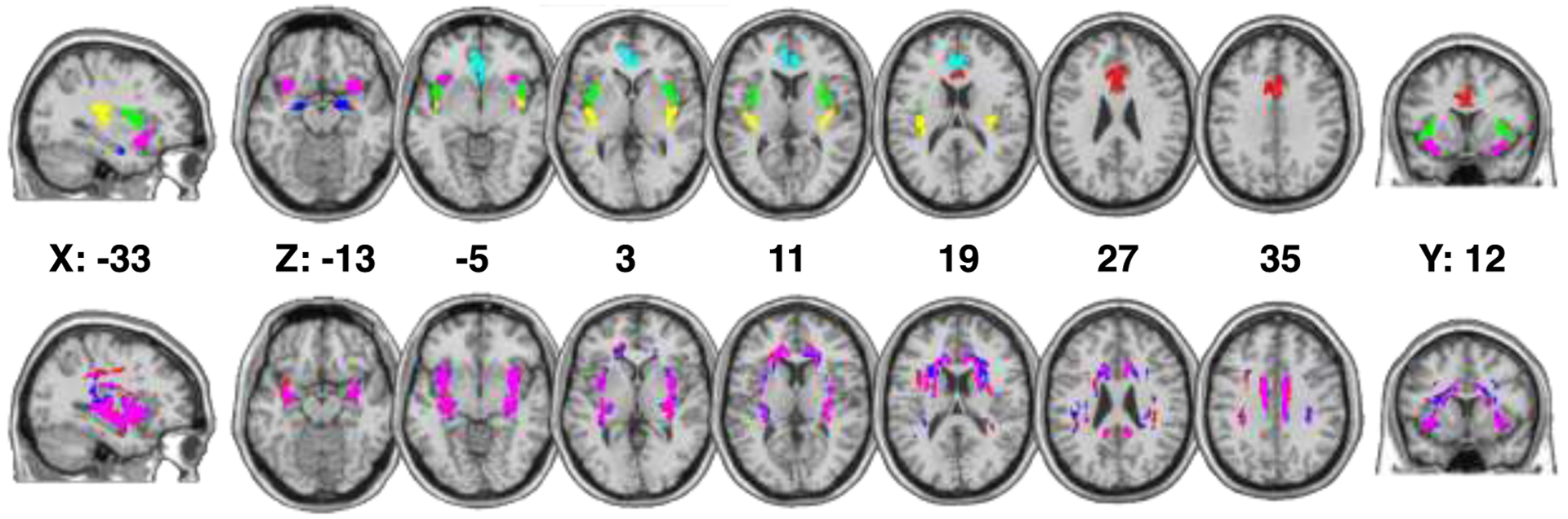
Top panel: ROI masks projected onto a standard-space brain in multi-slice horizontal view; Bottom panel: group-averaged and thresholded white matter tracts in standard space. ROIs are color-coded as follows: amygdala – blue, caudal anterior cingulate cortex – red, rostral anterior cingulate cortex – cyan, ventral anterior insula – pink, dorsal mid insula – green, dorsal posterior insula – yellow. Group-tracts are color-coded as follows: healthy control tracts – blue, schizophrenia participants tracts – red, overlapping tracts – pink. MNI coordinates of each slice are presented between the two rows.
Next, to compare microstructural integrity of the white matter tracts, we conducted two MANCOVAs on FA and MD extracted from the 16 tracts (Figure 3). The MANCOVA on FA revealed significant effects of group (F(8,61)=12.23, p<.001), hemisphere (F(8,62)=103.34, p<.001), group × hemisphere interaction (F(8,62)=45.41, p<.001), and age (F(8,61)=3.26, p=.004). The MANCOVA on MD also revealed significant effects of group (F(8,61)=9.96, p<.001), hemisphere (F(8,62)=108.14, p<.001), and group × hemisphere interaction (F(8,62)=173.70, p<.001); however, there was no significant age effect on MD (F(8,61)=1.56, p=.16). Because hemisphere effects are not the focus of the current study, follow-up analyses on main effects of hemisphere and interactions driven by hemisphere effects (i.e., when the lateralized effect is significantly different between HC and SZ, but the group difference does not differ across hemispheres) are detailed in the Supplementary Results. We now go through results from follow-up univariate ANCOVA tests on FA and MD.
Figure 3. Group differences in the microstructural integrity measures extracted from ROI pairs by hemisphere.
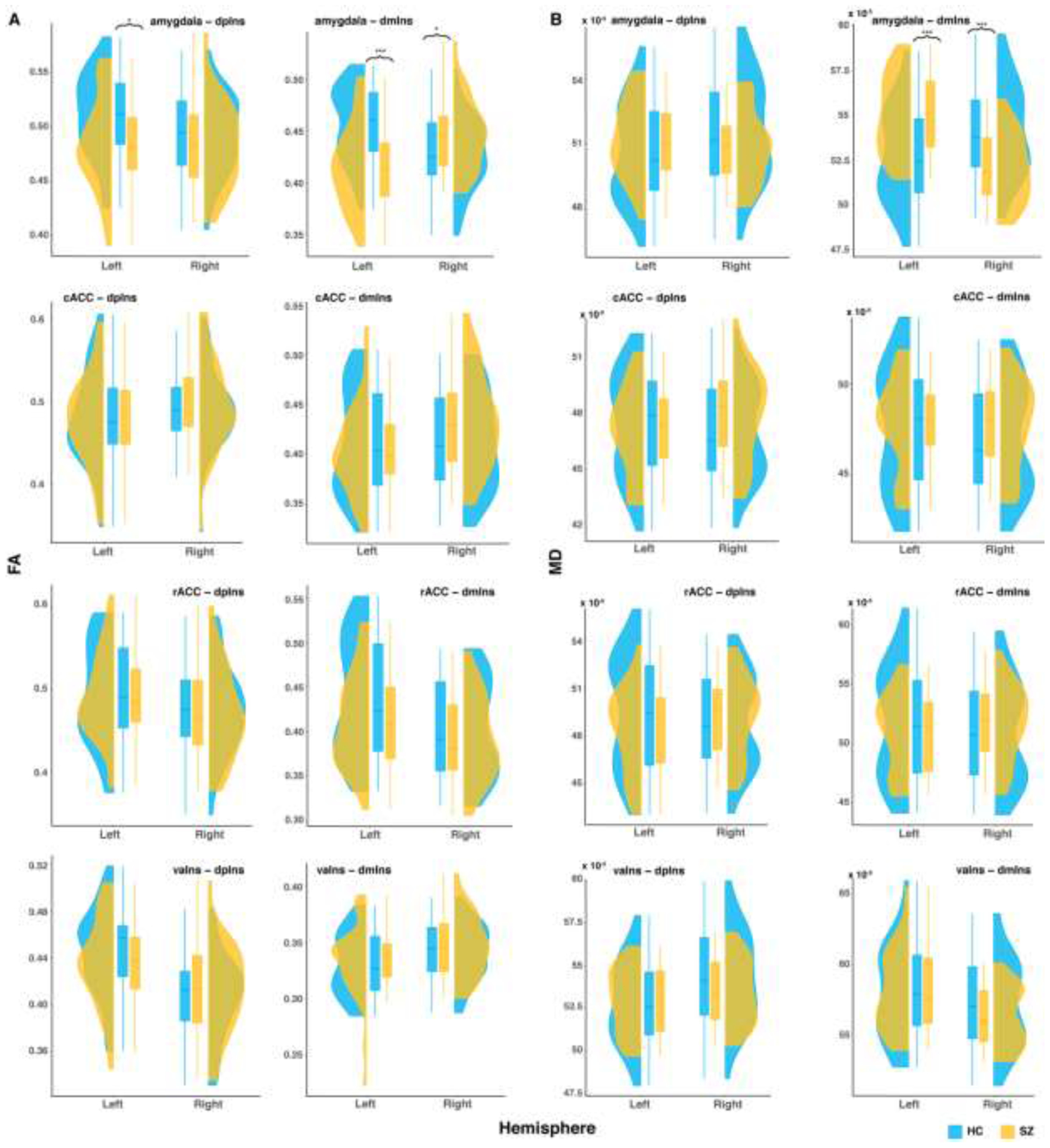
A: fractional anisotropy (FA); B: mean diffusivity (MD). cACC: caudal anterior cingulate cortex; dmIns: dorsal mid insula; dpIns: dorsal posterior insula; rACC: rostral anterior cingulate cortex. * p < .05, ** p < .01, *** p < .001.
For the amygdala-dpIns tract, there was a significant group effect on FA (F(1,68)=4.04, p=.048), such that SZ had lower FA than HC (t(69)=−2.01, p=.049). There was also a significant group × hemisphere effect on FA (F(1,69)=8.13, p=.006); the group difference in FA was only statistically significant in the left hemisphere (t(69)=−2.63, p=.01). For MD, there was no group effect (F(1,68) = 0.02, p = .88). In summary, SZ had reduced white matter microstructural integrity than HC in the amygdala-dpIns tract.
For the amygdala-dmIns tract, there was no group effect on FA (F(1,68)=1.69, p=.20), but a significant group × hemisphere effect (F(1,69)=185.10, p<.001). HC had higher FA than SZ in the left hemisphere (t(69)=−4.42, p<.001), but lower FA than SZ in the right hemisphere (t(69)=2.24, p=.03). Similarly for MD, there was no group effect (F(1,68)=0, p=.997), but a significant group × hemisphere effect (F(1,69)=694.08, p<.001). HC had lower MD than SZ in the left hemisphere (t(69)=3.74, p<.001), but higher MD than SZ in the right hemisphere (t(69)=−3.93, p<.001). In summary, there were group differences in the structural connectivity within the amygdala-dmIns tract, the direction of which depending on hemisphere.
For the rest of the ROI pairs, there were no significant group or group × hemisphere interaction effects that were driven by group differences (see Supplementary Results).
3.2. Relationship between structural connectivity and emotional functioning
As expected, HC performed significantly better than SZ on both emotion tasks (Table 1). To assess the associations between the structural connectivity of the interoception network and emotional functioning, we conducted linear regressions using diagnosis, standardized FA from each tract, and their interaction to predict emotional functioning. Here we only present significant main and interaction effects involving standardized FA. See Table S4 & S5 for model coefficients.
Standardized FA from the right vaIns-dmIns tract was a significant predictor of MSCEIT total score in HC (FA: b=−6.30, t(65)=−2.10, p=.04), and this association was not significantly different in SZ (group × FA: b=3.99, t(65)=0.91, p=.37), R2=.26, F(3,65)=9, p<.0001. In other words, diffusivity of the right vaIns-dmIns tract was related with general emotional functioning across both groups. Standardized FA from the right rACC-dmIns tract was a significant predictor of ER40 accuracy in HC (FA: b=−1.11, t(64)=−2.26, p=.03), and this association was significantly different in SZ (group × FA: b=1.77, t(64)=2.61, p=.01; Figure 4), R2=.22, F(3,64)=7.39, p=.00025. Follow-up analysis revealed that standardized FA from the right rACC-dmIns tract was not a significant predictor of ER40 accuracy in SZ (FA: b=0.66, t(64)=1.41, p=.16). Similarly, standardized FA from the right rACC-dpIns (FA: b=−1.03, t(64)=−2.05, p=.04) and right cACC-dmIns (FA: b=−1.23, t(64)=−2.34, p=.02) tracts were significant predictors of ER40 accuracy in HC (right rACC-dpIns: R2=.19, F(3,64)=6.28, p=.00084; right cACC-dmIns: R2=.21, F(3,64)=6.94, p=.0004). Though the group × FA interaction effects were not statistically significant (right rACC-dpIns: b=1.21, t(64)=1.73, p=.09; right cACC-dmIns: b=0.86, t(64)=1.16, p=.25), standardized FA was similarly not a significant predictor of ER40 accuracy in SZ (right rACC-dpIns: b=0.18, t(64)=0.37, p=.71; right cACC-dmIns: b=−0.37, t(64)=−0.71, p=.48). In sum, diffusivity of multiple tracts between ACC and insula were related with facial emotion recognition, and the relationship with right rACC was specific to HC. To ensure that group differences were not confounded by education, we ran follow-up models that included years of education. Adding education to these models did not change these effects, and education was not a significant predictor of either ER40 or MSCEIT performance (see Supplementary Results).
Figure 4. Scatterplots of emotion recognition against tract-specific indices of microstructural integrity.
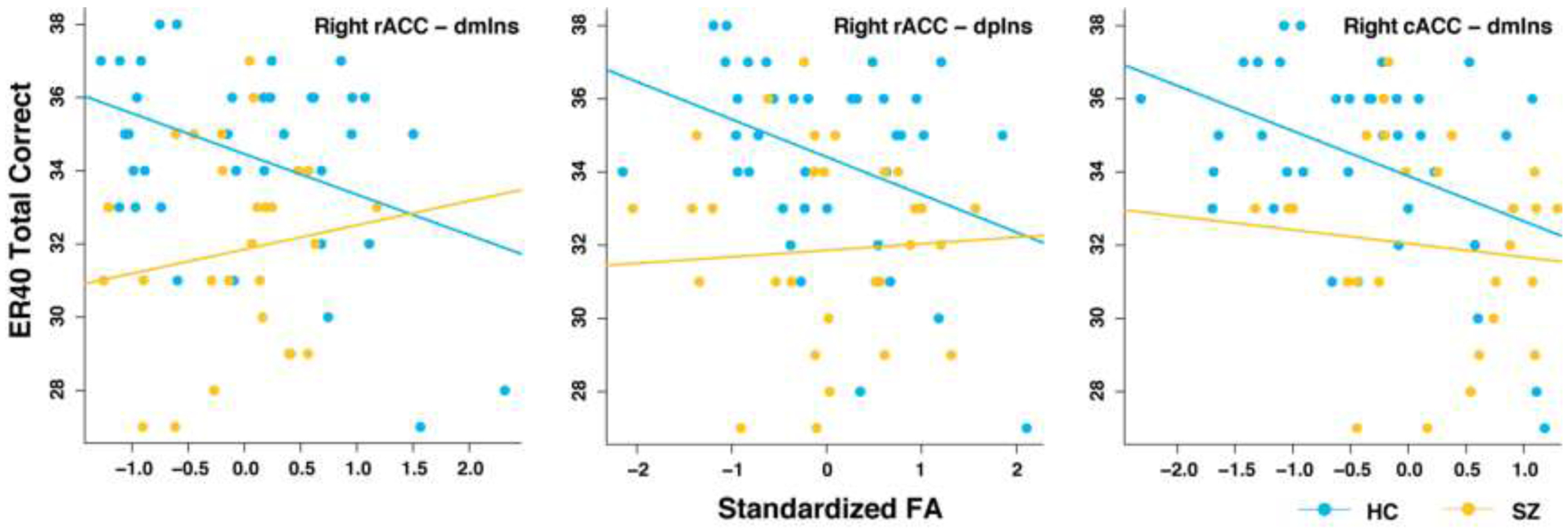
cACC: caudal anterior cingulate cortex; dmIns: dorsal mid insula; dpIns: dorsal posterior insula; ER40, Penn Emotion Recognition - 40 Task; FA: fractional anisotropy; rACC: rostral anterior cingulate cortex.
4. Discussion
In this study, we examined the structural connectivity of an interoception network, defined on the basis of prior studies, in participants with schizophrenia (SZ) and healthy controls (HC). Given the crucial role interoception plays in supporting emotion, we also examined the relationship between interoception network connectivity and emotional functioning. We observed a lateralized pattern of white matter alterations within this network in SZ. Group differences in network anatomical connectivity were largely specific to the connections between the amygdala and insula (dmIns and dpIns). Moreover, we found a correlation between emotion recognition and network anatomical connectivity in HC, indirectly supporting the involvement of interoception network in emotional functioning. However, this relationship was significantly weaker in SZ, potentially suggesting less reliance on this network.
The current finding of increased right amygdala-dmIns structural connectivity in SZ is consistent with a previous finding of increased right amygdala-insula functional connectivity in persons with schizophrenia, compared with their unaffected relatives (Goswami et al., 2020). The finding of decreased left amygdala-dmIns connectivity in SZ is consistent with a previous finding of reduced left amygdala-vaIns structural connectivity (indexed by lower FA) in persons with schizophrenia (Amodio et al., 2018). As Amodio and colleagues divided the insula into anterior and posterior portions (i.e., with no mid-insula) using a different atlas, it is possible that there are overlaps between their anterior insula and the mid insula in this study. Given the same directionality in group differences (i.e., reduced connectivity in SZ) in left amygdala-dmIns and amygdala-dpIns tracts, and similar patterns in functional connectivity (Kleckner et al., 2017), we will first discuss the amygdala-insula connection broadly, and then return to discuss the lateralized group differences.
To understand the implications of altered amygdala-insula connectivity in schizophrenia, we will first revisit the role of this connection alongside the ACC-insula connection within the interoception network. The amygdala has numerous reciprocal connections to cortical and subcortical structures that allow it to immediately translate emotionally important input into autonomic and endocrine physiological responses before processed and integrated information can reach conscious awareness (Šimić et al., 2021). It is associated with biological instincts (e.g., thirst, hunger, libido), motivational states, and the activation of fight-or-flight responses. On the other hand, ACC seems to be involved in higher-level processes like using context and event-specific information to regulate autonomic and emotional states (Seamans & Floresco, 2022). Therefore, the combination of decreased amygdala-insula connectivity in participants with schizophrenia and no group difference in ACC-insula connectivity may indicate compromised capacity to rapidly respond to emotionally salient stimuli in an automatic fashion, but intact capacity to use contextual clues to modulate emotional states in schizophrenia. In other words, this potentially indicates an imbalance in the interoceptive inferential process where the bottom-up interoceptive prediction error signals are underweighted compared with the top-down prediction signals. However, within this conceptualization, it should be acknowledged that it is unclear whether altered white matter integrity would engender altered communication of interoceptive prediction signals, interoceptive prediction errors, or both, as DTI cannot distinguish the direction of axonal fibers.
The lateralized group differences in amygdala-insula structural connectivity (i.e., decreased connectivity on the left and increased connectivity on the right in SZ) warrant further discussion. Our finding that compromised amygdala-insula connectivity in individuals with schizophrenia was specific to the left hemisphere is consistent with a recent activation-likelihood estimation meta-analysis that found disrupted activation only in the left dmIns using different interoceptive probes (e.g., pain, hunger, interoceptive attention) among multiple psychiatric disorders, including schizophrenia (Nord et al., 2021), suggesting both functional and structural alterations in the left hemisphere in processing basic interoceptive signals. Conventionally, it was thought that to optimize homeostasis, the right insula was associated with sympathetic activities and energy expenditure, while the left insula was associated with parasympathetic activities and energy enrichment (Craig, 2005; Strigo & Craig, 2016). However, recent studies suggest that this may be too simplistic a view of lateralized interoception and emotion functions in the brain. When consciously attending to interoceptive signals like heartbeat and breathing, both sides of insula activated in healthy participants (Failla et al., 2020; Haase et al., 2016; Wiebking et al., 2010) with no difference in level of activation (Haruki & Ogawa, 2021). However, there does seem to be a potential lateralization in interoceptive precision regulation (i.e., assigning more weight to particular interoceptive signals and less weight to “noise”) as interoceptive accuracy on a heartbeat counting task was correlated with right insula only (Haruki & Ogawa, 2021). In other words, both right and left insula are involved in processing and interpreting bodily states, but the right side may have a stronger role in assigning importance to (i.e., weighting) specific interoceptive signals. Therefore, the lateralized group differences in amygdala-insula connections may suggest disruptions in processing bottom-up interoceptive signals in people with schizophrenia, leading to an over reliance on top-down precision regulation as a compensatory response, not unlike the imbalance between amygdala-insula connection and ACC-insula connection discussed above. See Supplementary Discussion for interpretations of group differences in different diffusivity indices.
Our finding that emotion recognition was correlated with ACC-insula structural connectivity in healthy participants is consistent with previous functional neuroimaging literature demonstrating the involvement of ACC and insula in emotion experience and perception (Lindquist & Barrett, 2012). Moreover, stronger ACC-dpIns functional connectivity is correlated with better interoceptive ability in healthy participants, supporting the involvement of this connection in interoceptive inference and autonomic regulation (Kleckner et al., 2017). Our finding that ACC-insula structural connectivity also relates to emotional functioning adds to this existing literature by strengthening the triangulation between emotion, interoception, and ACC-insula connectivity. However, the direction of the brain-behavior association in the current study was opposite to our prediction (i.e., lower standardized FA was correlated with better emotion recognition in healthy participants). The interpretation of this relationship is complicated by the fact that multiple cellular mechanisms can contribute to differences in FA. In addition to localized tissue damage or neuropathology, lower FA may also reflect more crossing fibers (orthogonal to the principal diffusion direction) within a specific region (Figley et al., 2022), lighter axonal packing or smaller axonal diameters (Feldman et al., 2010), and lower cohesiveness of the fiber tracts (Beaulieu, 2002). Exploring these brain-behavior relationships using multi-modal imaging methods in future investigations (e.g., measuring structural and functional connectivity in the same study) would aid in clarifying the nature of the relationship among emotion, interoception, and ACC-insula connectivity. See Supplementary Discussion for a discussion on how interoceptive inference may support emotion recognition.
Several limitations should be considered when interpreting the current findings. First, we would expect that compromised structural connectivity of the interoception network should most directly relate to interoceptive functioning; however, we did not have access to direct interoception measures. Future studies should utilize reliable behavioral paradigms and self-report measures of interoception (Torregrossa et al., 2022) to establish a more direct correlation between brain structures and interoception. Second, though the sample size in the current study is adequate for detecting medium-sized effects in linear regressions (Jenkins & Quintana-Ascencio, 2020; Wilson Van Voorhis & Morgan, 2007), it is possible that some of the brain-behavior associations have a small effect size that can only be detected by a larger sample. Lastly, we were not able to rule out potential confounding effects of antipsychotic treatment because current study used a medicated sample. It will be crucial to replicate the findings in a medication-naïve and/or clinical high-risk sample to rule out the effects of antipsychotic treatment completely. See Supplementary Discussion for suggested future directions.
In conclusion, we found evidence of altered structural connectivity in a putative interoception network in persons with schizophrenia. The structural connectivity of this network was correlated with emotion recognition in healthy participants, supporting a link between the interoception network and emotional functioning. However, this association was much weaker in participants with schizophrenia, suggesting less reliance on this network in emotional functioning. These findings add to our understanding of emotional deficits in schizophrenia and suggest a promising new direction for further research into the role of interoception in illness mechanisms.
Supplementary Material
Highlights.
Interoception refers to the processing of bodily signals by the brain
Interoception is crucial to basic survival and cognition, especially emotion
Altered structural connectivity within an interoception network in schizophrenia
Network connectivity related with emotion recognition in healthy participants
Much weaker connectivity-emotion association in schizophrenia
Acknowledgments
This work was supported in part by the National Institutes of Health [5KL2TR000434]; and the National Institute of Mental Health [R01-MH112644, R01MH121417, 5K23MH108823, R01MH122491, R01MH118634]. This material is also based upon work supported by the National Science Foundation Graduate Research Fellowship (Grant No. DGE-1841052 to CAL). Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors(s) and do not necessarily reflect the views of the National Science Foundation. The funding agencies have no further role in the preparation and submission of the manuscript. The authors would like to thank Tristan Greathouse for his assistance with data preprocessing and quality assurance, Mike Angstadt for his technical support, and participants for their participation in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare that they have no known financial, personal, or other relationships that could have inappropriately influenced the work reported in this paper.
When no hemisphere effect was found in group comparisons, standardized FA values were computed by regressing whole-brain FA out of the tract FA averaged across both hemispheres; when lateralized effects were observed, mean FA within each hemisphere was regressed out of the corresponding tract FA.
References
- Abe O, Aoki S, Hayashi N, Yamada H, Kunimatsu A, Mori H, Yoshikawa T, Okubo T, & Ohtomo K (2002). Normal aging in the central nervous system: Quantitative MR diffusion-tensor analysis. Neurobiology of Aging, 23(3), 433–441. 10.1016/S0197-4580(01)00318-9 [DOI] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, & Field AS (2007). Diffusion tensor imaging of the brain. Neurotherapeutics, 4(3), 316–329. 10.1016/j.nurt.2007.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio A, Quarantelli M, Mucci A, Prinster A, Soricelli A, Vignapiano A, Giordano GM, Merlotti E, Nicita A, & Galderisi S (2018). Avolition-Apathy and White Matter Connectivity in Schizophrenia: Reduced Fractional Anisotropy Between Amygdala and Insular Cortex. Clinical EEG and Neuroscience, 49(1), 55–65. 10.1177/1550059417745934 [DOI] [PubMed] [Google Scholar]
- Andreasen NC (1984a). Scale for the Assessment of Negative Symptoms (SANS). University of Iowa. [PubMed] [Google Scholar]
- Andreasen NC (1984b). Scale for the Assessment of Positive Symptoms (SAPS). University of Iowa. [Google Scholar]
- Andreasen NC, Pressler M, Nopoulos P, Miller D, & Ho B-C (2010). Antipsychotic Dose Equivalents and Dose-Years: A Standardized Method for Comparing Exposure to Different Drugs. Biological Psychiatry, 67(3), 255–262. 10.1016/j.biopsych.2009.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardizzi M, Ambrosecchia M, Buratta L, Ferri F, Peciccia M, Donnari S, Mazzeschi C, & Gallese V (2016). Interoception and Positive Symptoms in Schizophrenia. Frontiers in Human Neuroscience, 10, 379. 10.3389/fnhum.2016.00379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF (2017). The theory of constructed emotion: An active inference account of interoception and categorization. Social Cognitive and Affective Neuroscience, 12(1), 1–23. 10.1093/scan/nsw154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, & Simmons WK (2015). Interoceptive predictions in the brain. Nature Reviews Neuroscience, 16(7), 419–429. 10.1038/nrn3950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C (2002). The basis of anisotropic water diffusion in the nervous system—A technical review. NMR in Biomedicine, 15(7–8), 435–455. 10.1002/nbm.782 [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Berg HJ, Jbabdi S, Rushworth MFS, & Woolrich MW (2007). Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? NeuroImage, 34(1), 144–155. 10.1016/j.neuroimage.2006.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TEJ, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, & Smith SM (2003). Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magnetic Resonance in Medicine, 50(5), 1077–1088. 10.1002/mrm.10609 [DOI] [PubMed] [Google Scholar]
- Budde MD, Janes L, Gold E, Turtzo LC, & Frank JA (2011). The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: Validation in the rat using Fourier analysis of stained tissue sections. Brain, 134(8), 2248–2260. 10.1093/brain/awr161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AL, Fair DA, Dosenbach NUF, Miezin FM, Dierker D, Van Essen DC, Schlaggar BL, & Petersen SE (2008). Defining functional areas in individual human brains using resting functional connectivity MRI. NeuroImage, 41(1), 45–57. 10.1016/j.neuroimage.2008.01.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A. D. (Bud). (2005). Forebrain emotional asymmetry: A neuroanatomical basis? Trends in Cognitive Sciences, 9(12), 566–571. 10.1016/j.tics.2005.10.005 [DOI] [PubMed] [Google Scholar]
- Critchley HD, Ewing DL, van Praag CG, Habash-Bailey H, Eccles JA, Meeten F, & Garfinkel SN (2019). Transdiagnostic expression of interoceptive abnormalities in psychiatric conditions. MedRxiv, 19012393. 10.1101/19012393 [DOI] [Google Scholar]
- Danivas V, & Venkatasubramanian G (2013). Current perspectives on chlorpromazine equivalents: Comparing apples and oranges! Indian Journal of Psychiatry, 55(2), 207–208. 10.4103/0019-5545.111475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Failla MD, Bryant LK, Heflin BH, Mash LE, Schauder K, Davis S, Gerdes MB, Weitlauf A, Rogers BP, & Cascio CJ (2020). Neural Correlates of Cardiac Interoceptive Focus Across Development: Implications for Social Symptoms in Autism Spectrum Disorder. Autism Research, 13(6), 908–920. 10.1002/aur.2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman HM, Yeatman JD, Lee ES, Barde LHF, & Gaman-Bean S (2010). Diffusion Tensor Imaging: A Review for Pediatric Researchers and Clinicians. Journal of Developmental & Behavioral Pediatrics, 31(4), 346–356. 10.1097/DBP.0b013e3181dcaa8b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figley CR, Uddin MN, Wong K, Kornelsen J, Puig J, & Figley TD (2022). Potential Pitfalls of Using Fractional Anisotropy, Axial Diffusivity, and Radial Diffusivity as Biomarkers of Cerebral White Matter Microstructure. Frontiers in Neuroscience, 15, 799576. 10.3389/fnins.2021.799576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski KJ, Auer T, Calhoun VD, Craddock RC, Das S, Duff EP, Flandin G, Ghosh SS, Glatard T, Halchenko YO, Handwerker DA, Hanke M, Keator D, Li X, Michael Z, Maumet C, Nichols BN, Nichols TE, Pellman J, … Poldrack RA (2016). The brain imaging data structure, a format for organizing and describing outputs of neuroimaging experiments. Scientific Data, 3(1), 160044. 10.1038/sdata.2016.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami S, Beniwal RP, Kumar M, Bhatia T, Gur RE, Gur RC, Khushu S, & Deshpande SN (2020). A preliminary study to investigate resting state fMRI as a potential group differentiator for schizophrenia. Asian Journal of Psychiatry, 52, 102095. 10.1016/j.ajp.2020.102095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Sara R, Hagendoorn M, Marom O, Hughett P, Macy L, Turner T, Bajcsy R, Posner A, & Gur RE (2002). A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. Journal of Neuroscience Methods, 115(2), 137–143. 10.1016/S0165-0270(02)00006-7 [DOI] [PubMed] [Google Scholar]
- Haase L, Stewart JL, Youssef B, May AC, Isakovic S, Simmons AN, Johnson DC, Potterat EG, & Paulus MP (2016). When the brain does not adequately feel the body: Links between low resilience and interoception. Biological Psychology, 113, 37–45. 10.1016/j.biopsycho.2015.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruki Y, & Ogawa K (2021). Role of anatomical insular subdivisions in interoception: Interoceptive attention and accuracy have dissociable substrates. European Journal of Neuroscience, 53(8), 2669–2680. 10.1111/ejn.15157 [DOI] [PubMed] [Google Scholar]
- Jenkins DG, & Quintana-Ascencio PF (2020). A solution to minimum sample size for regressions. PLOS ONE, 15(2), e0229345. 10.1371/journal.pone.0229345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S, Jahanshad N, Zalesky A, Kochunov P, Agartz I, Alloza C, Andreassen OA, Arango C, Banaj N, Bouix S, Bousman CA, Brouwer RM, Bruggemann J, Bustillo J, Cahn W, Calhoun V, Cannon D, Carr V, Catts S, … Donohoe G (2018). Widespread white matter microstructural differences in schizophrenia across 4322 individuals: Results from the ENIGMA Schizophrenia DTI Working Group. Molecular Psychiatry, 23(5), 1261–1269. 10.1038/mp.2017.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner IR, Zhang J, Touroutoglou A, Chanes L, Xia C, Simmons WK, Quigley KS, Dickerson BC, & Feldman Barrett L (2017). Evidence for a large-scale brain system supporting allostasis and interoception in humans. Nature Human Behaviour, 1(5), 0069. 10.1038/s41562-017-0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koreki A, Funayama M, Terasawa Y, Onaya M, & Mimura M (2021). Aberrant interoceptive accuracy in patients with schizophrenia performing a heartbeat counting task. Schizophrenia Bulletin Open, 2(1), sgaa067. 10.1093/schizbullopen/sgaa067 [DOI] [Google Scholar]
- Kronlage M, Pitarokoili K, Schwarz D, Godel T, Heiland S, Yoon M-S, Bendszus M, & Bäumer P (2017). Diffusion Tensor Imaging in Chronic Inflammatory Demyelinating Polyneuropathy: Diagnostic Accuracy and Correlation With Electrophysiology. Investigative Radiology, 52(11), 701–707. 10.1097/RLI.0000000000000394 [DOI] [PubMed] [Google Scholar]
- Lindquist KA, & Barrett LF (2012). A functional architecture of the human brain: Emerging insights from the science of emotion. Trends in Cognitive Sciences, 16(11), 533–540. 10.1016/j.tics.2012.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makowski D, Sperduti M, Blondé P, Nicolas S, & Piolino P (2020). The heart of cognitive control: Cardiac phase modulates processing speed and inhibition. Psychophysiology, 57(3), 1–11. 10.1111/psyp.13490 [DOI] [PubMed] [Google Scholar]
- Mayer JD, Salovey P, & Caruso DR (1999). Mayer-Salovey-Caruso Emotional Intelligence Test. Multi-Health Systems Inc. [Google Scholar]
- Nord CL, Lawson RP, & Dalgleish T (2021). Disrupted Dorsal Mid-Insula Activation During Interoception Across Psychiatric Disorders. American Journal of Psychiatry, 178(8), 761–770. 10.1176/appi.ajp.2020.20091340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JE, & Gorham DR (1962). The Brief Psychiatric Rating Scale. Psychological Reports, 10(3), 799–812. 10.2466/pr0.1962.10.3.799 [DOI] [Google Scholar]
- Pezzulo G, Rigoli F, & Friston KJ (2015). Active Inference, homeostatic regulation and adaptive behavioural control. Progress in Neurobiology, 134, 17–35. 10.1016/j.pneurobio.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramme L, Larra MF, Schächinger H, & Frings C (2014). Cardiac cycle time effects on mask inhibition. Biological Psychology, 100, 115–121. 10.1016/j.biopsycho.2014.05.008 [DOI] [PubMed] [Google Scholar]
- Pramme L, Larra MF, Schächinger H, & Frings C (2016). Cardiac cycle time effects on selection efficiency in vision. Psychophysiology, 53(11), 1702–1711. 10.1111/psyp.12728 [DOI] [PubMed] [Google Scholar]
- Rothe PH, Heres S, & Leucht S (2018). Dose equivalents for second generation long-acting injectable antipsychotics: The minimum effective dose method. Schizophrenia Research, 193, 23–28. 10.1016/j.schres.2017.07.033 [DOI] [PubMed] [Google Scholar]
- Schulkin J, & Sterling P (2019). Allostasis: A Brain-Centered, Predictive Mode of Physiological Regulation. Trends in Neurosciences. 10.1016/j.tins.2019.07.010 [DOI] [PubMed] [Google Scholar]
- Seamans JK, & Floresco SB (2022). Event-based control of autonomic and emotional states by the anterior cingulate cortex. Neuroscience & Biobehavioral Reviews, 133(December 2021), 104503. 10.1016/j.neubiorev.2021.12.026 [DOI] [PubMed] [Google Scholar]
- Seth AK (2013). Interoceptive inference, emotion, and the embodied self. Trends in Cognitive Sciences, 17(11), 565–573. 10.1016/j.tics.2013.09.007 [DOI] [PubMed] [Google Scholar]
- Seth AK (2015). The Cybernetic Bayesian Brain: From Interoceptive Inference to Sensorimotor Contingencies. In Metzinger TK & Windt JM (Eds.), Open MIND. MIND Group. 10.15502/9783958570108 [DOI] [Google Scholar]
- Seth AK, & Friston KJ (2016). Active interoceptive inference and the emotional brain. Philosophical Transactions of the Royal Society B: Biological Sciences, 371(1708), 20160007. 10.1098/rstb.2016.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth AK, Suzuki K, & Critchley HD (2012). An Interoceptive Predictive Coding Model of Conscious Presence. Frontiers in Psychology, 2. 10.3389/fpsyg.2011.00395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield JM, Rogers BP, Blackford JU, Heckers S, & Woodward ND (2020). Insula functional connectivity in schizophrenia. Schizophrenia Research, 220, 69–77. 10.1016/j.schres.2020.03.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šimić G, Tkalčić M, Vukić V, Mulc D, Španić E, Šagud M, Olucha-Bordonau FE, Vukšić M, & R. Hof P (2021). Understanding Emotions: Origins and Roles of the Amygdala. Biomolecules, 11(6), 823. 10.3390/biom11060823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snook L, Plewes C, & Beaulieu C (2007). Voxel based versus region of interest analysis in diffusion tensor imaging of neurodevelopment. NeuroImage, 34(1), 243–252. 10.1016/j.neuroimage.2006.07.021 [DOI] [PubMed] [Google Scholar]
- Song S-K, Sun S-W, Ju W-K, Lin S-J, Cross AH, & Neufeld AH (2003). Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage, 20(3), 1714–1722. 10.1016/j.neuroimage.2003.07.005 [DOI] [PubMed] [Google Scholar]
- Sterling P (2012). Allostasis: A model of predictive regulation. Physiology & Behavior, 106(1), 5–15. 10.1016/j.physbeh.2011.06.004 [DOI] [PubMed] [Google Scholar]
- Strigo IA, & Craig A. D. (Bud). (2016). Interoception, homeostatic emotions and sympathovagal balance. Philosophical Transactions of the Royal Society B: Biological Sciences, 371(1708), 20160010. 10.1098/rstb.2016.0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S-W, Liang H-F, Le TQ, Armstrong RC, Cross AH, & Song S-K (2006). Differential sensitivity of in vivo and ex vivo diffusion tensor imaging to evolving optic nerve injury in mice with retinal ischemia. NeuroImage, 32(3), 1195–1204. 10.1016/j.neuroimage.2006.04.212 [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, & Fidell LS (2013). Using Multivariate Statistics. Pearson Education, Inc. [Google Scholar]
- Takahashi T, Kido M, Sasabayashi D, Nakamura M, Furuichi A, Takayanagi Y, Noguchi K, & Suzuki M (2020). Gray Matter Changes in the Insular Cortex During the Course of the Schizophrenia Spectrum. Frontiers in Psychiatry, 11. 10.3389/fpsyt.2020.00659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa LJ, Amedy A, Roig J, Prada A, & Park S (2022). Interoceptive functioning in schizophrenia and schizotypy. Schizophrenia Research, 239(July 2021), 151–159. 10.1016/j.schres.2021.11.046 [DOI] [PubMed] [Google Scholar]
- Tournier J-D, Smith R, Raffelt D, Tabbara R, Dhollander T, Pietsch M, Christiaens D, Jeurissen B, Yeh C-H, & Connelly A (2019). MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage, 202(January), 116137. 10.1016/j.neuroimage.2019.116137 [DOI] [PubMed] [Google Scholar]
- Tsakiris M, & De Preester H (Eds.). (2019). The Interoceptive Mind: From homeostasis to awareness. Oxford University Press. [Google Scholar]
- Wiebking C, Bauer A, de GRECK M, Duncan NW, Tempelmann C, & Northoff G (2010). Abnormal body perception and neural activity in the insula in depression: An fMRI study of the depressed “material me.” The World Journal of Biological Psychiatry, 11(3), 538–549. 10.3109/15622970903563794 [DOI] [PubMed] [Google Scholar]
- Wilson Van Voorhis CR, & Morgan BL (2007). Understanding Power and Rules of Thumb for Determining Sample Sizes. Tutorials in Quantitative Methods for Psychology, 3(2), 43–50. 10.20982/tqmp.03.2.p043 [DOI] [Google Scholar]
- Wylie KP, & Tregellas JR (2010). The role of the insula in schizophrenia. Schizophrenia Research, 123(2–3), 93–104. 10.1016/j.schres.2010.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao B, & Thakkar K (2022). Interoception abnormalities in schizophrenia: A review of preliminary evidence and an integration with Bayesian accounts of psychosis. Neuroscience & Biobehavioral Reviews, 132, 757–773. 10.1016/j.neubiorev.2021.11.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


