Abstract
Preterm birth and intra-partum related complications account for a substantial amount of mortality and morbidity in the neonatal period despite significant advancements in neonatal-perinatal care. Currently, there is a noticeable lack of curative or preventative therapies available for any of the most common complications of prematurity including bronchopulmonary dysplasia, necrotizing enterocolitis, intraventricular hemorrhage, periventricular leukomalacia and retinopathy of prematurity or hypoxic-ischemic encephalopathy, the main cause of perinatal brain injury in term infants. Mesenchymal stem/stromal cell-derived therapy has been an active area of investigation for the past decade and has demonstrated encouraging results in multiple experimental models of neonatal disease. It is now widely acknowledged that mesenchymal stem/stromal cells exert their therapeutic effects via their secretome, with the principal vector identified as extracellular vesicles. This review will focus on summarizing the current literature and investigations on mesenchymal stem/stromal cell-derived extracellular vesicles as a treatment for neonatal diseases and examine the considerations to their application in the clinical setting.
Introduction
Neonatal diseases including preterm birth complications and intrapartum-related events are the main causes of pediatric mortality < 5 years per World Health Organization 2000-20191. Survival of premature infants has improved significantly over the past half century, most notably the extremely low gestational age neonates (ELGAN) who are at the highest risk for developing serious complications of prematurity that impact the central nervous system, cardiorespiratory system, and gastrointestinal tract2,3. The morbidities associated with prematurity unfortunately remain high despite advancement in neonatal-perinatal medicine and the use of antenatal steroids and pulmonary surfactant therapy2,3. In addition, the burden of the complications of prematurity are long-lasting and span into childhood and even adulthood4-7. At present, there are no curative therapies available for the most common complications of prematurity such as bronchopulmonary dysplasia (BPD), necrotizing enterocolitis (NEC), intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL) and retinopathy of prematurity (ROP). The mainstay of treatment for these prematurity complications includes mechanical ventilation, supportive medical therapy and in extreme scenarios, surgical therapy. In contrast, the most common cause of morbidity in term infants is due to perinatal brain injury such as hypoxic ischemic encephalopathy (HIE), which does have a standard of care treatment but is non-curative and has a limited time frame for application. Most of these neonatal pathologies are challenging to develop treatments for given that the diseases occur in immature and developing organs and have complex underlying pathophysiological injury mechanisms (Figure 1). Therefore, there is an urgent need to develop new, safe, and effective therapies for the treatment and prevention of neonatal diseases. Stem cell, specifically mesenchymal stem/stromal cell (MSC) based therapies have emerged as a promising therapeutic option due to their inherent regenerative and immunomodulatory properties. Numerous preclinical studies of MSCs in several neonatal diseases have demonstrated favorable results and it is now known that the therapeutic effect of MSCs is mediated by a paracrine mechanism via the secretome with various studies identifying extracellular vesicles (EVs) as the therapeutic vector. EVs are lipid-bilayer enclosed microparticles, endogenously produced by cells that mediate intercellular communication via transfer of their diverse bioactive cargo. In this review, we will summarize recent advances in stem cell secretome therapy for intractable neonatal diseases in preclinical models, the ways in which we can harness their therapeutic potential and discuss the considerations and challenges to clinical applications in this vulnerable patient population.
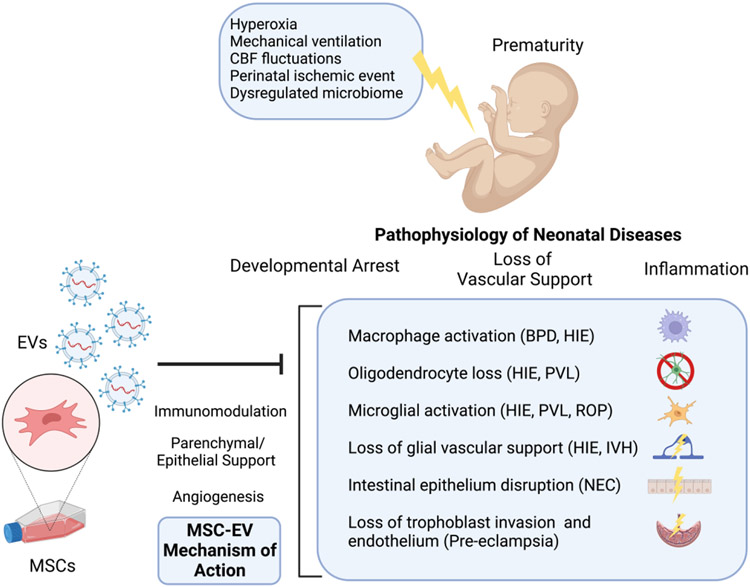
Figure 1. The common pathophysiology shared by neonatal diseases and the mechanism of action of MSC-EV treatment.
Prematurity results in under-development of the lungs, brain, gastrointestinal tract, retina and other vital organs that are susceptible to injury from a variety of sources (such as hyperoxia, mechanical ventilation, cerebral blood flow (CBF) fluctuations, perinatal ischemic events, dysregulated microbiome) that subsequently result in inflammation, developmental arrest and loss of vascular support. MSC-EVs have been shown to exert their therapeutic effects by modulating the immune response and phenotype, maintaining parenchymal and epithelial support and promoting angiogenesis.
The Evolution of Stem Cell Secretome Therapy
Stem cell therapy has been a promising field of investigation in medical science since the initial discovery in 1963 by Till and McCulloch of stem cells as undifferentiated cells with self-renewal and differentiation properties8. Since then, stem cells have found their way into clinical application with bone marrow (BM) and hematopoietic stem cell (HSC) transplantation as routine therapies for oncologic, hematologic, and immunologic disorders. Stem cells can be broadly divided into two categories: pluripotent stem cells (PSCs) and adult (somatic) stem cells. PSCs include embryonic stem cells and induced pluripotent stem cells, which have unlimited renewal capabilities and expansive repertoire of differentiation potential9,10. Adult stem cells include HSCs, endothelial progenitor cells, human amnion epithelial cells and mesenchymal stem/stromal cells (MSCs), which have a more limited renewal and differentiation potential. To date this vast array of stem cell types have had limited clinical use with the notable exception of HSCs and most of the therapeutic approaches have been focused on MSCs.
MSCs were first described in the 1970s as fibroblasts derived from the BM with colony-forming capabilities11,12. MSCs have been shown to differentiate into a variety of mesoderm-derived cells such as adipocytes, osteocytes, chondrocytes, fibroblasts, and skeletal muscle cells. MSCs can be derived from a variety of tissues including BM, adipose tissue, amniotic fluid (AF), umbilical cord (UC) Wharton’s jelly, umbilical cord blood (UCB) and placenta. The ease of access, isolation, expansion, and in vitro differentiation potential of MSCs as well as their immunoprivileged nature have made them a popular stem cell choice in preventive and regenerative preclinical studies of human disease. Considering the heightened research interest in MSCs, the International Society for Cellular Therapy (ISCT) provided a minimal criteria for defining human MSCs, including (1) tissue culture plastic adherence, (2) expression of CD105, CD73 and CD90, (3) lacking expression of CD45 (pan-leukocyte), CD34 (hematopoietic and endothelial cells), CD14 or CD11b (monocytes and macrophages), CD79α or CD19 (B cells) and HLA-DR surface molecules, and (4) ability to differentiate into osteoblasts, adipocytes and chondroblasts in vitro13. This definition was updated in 2019 to recommend (1) including the tissue origin of cells, (2) use of stromal cell nomenclature unless rigorous evidence for stemness is shown and (3) including functional assays to define therapeutic mechanism of action, but no tissue- specific guidelines were addressed14.
Transplantation of different adult stem cell types (predominantly MSCs) in preclinical models of neonatal disease has produced very promising results including immunomodulatory, anti-inflammatory, neuroprotective, angiogenic and regenerative effects15-34. Researchers initially assumed that MSCs exerted their therapeutic effects by homing to damaged tissues and differentiating into resident cells, however studies uncovered that the transplanted MSC cells neither underwent long-term engraftment nor differentiation35-37. Instead, the therapeutic action of MSCs has been determined to be mainly through paracrine mechanisms15,38-41. A multitude of studies have demonstrated that MSCs secrete a broad spectrum of bioactive compounds into the extracellular fluid and subsequently the bloodstream including growth factors, cytokines as well as EVs36,42. Multiple groups of investigators have gone on to prove that EVs are the key component that mediates the therapeutic effects of MSCs in numerous preclinical models of neonatal disease (Figure 1)40,43-45. EVs are a heterogeneous class of lipid-bilayer enclosed microparticles containing bioactive cargo without a nucleus that can be generated by almost all cell types. EVs were initially thought to be part of the cellular “garbage disposal” mechanism, through which corrupted or unwanted macromolecules are packaged and ejected from the cell. However, this mechanism has apparently evolved to also produce EV subpopulations designed for broadcasting signals and representing vectors of intercellular communication, arguably occasionally induced by environmental triggers 46-48. The bioactive cargo of each EV subpopulation is highly variable and dependent on the type and state of the parent cells and their biogenetic pathway. EV cargo can be composed of any combination of lipids, proteins (enzymes, growth factors, receptors and cytokines), nucleotides (DNA, messenger RNA, long non-coding RNA and microRNA) and metabolites46,49.
The mechanisms by which EVs mediate cell-cell signaling are still in the process of being discovered. Studies have shown that EVs can act on target cells by fusing with the target cell membrane to enter the cell and release the vesicle contents, interacting with the receptor-ligand and inducing target cells to ingest EVs via endocytosis. Upon absorption from the environment, the EVs’ cargo has the potential to impact a multitude of cellular processes including gene transcription, antigen presentation, cell proliferation, differentiation or apoptosis, and many others50,51. Even though the full definition of EV sub-types and their biogenesis are still being elucidated, in general EVs can be classified based on their size into 3 main types: (1) small EVs (including exosomes), defined as vesicles of approximately 30-150 nm in diameter and secreted through the endosomal pathway, (2) microvesicles, defined as vesicles of approximately 150-1000 nm in diameter that bud directly from the plasma membrane and usually have a membrane composition similar to that of the cell membrane (3) apoptotic bodies or blebs, defined as vesicles greater than 1 μm in diameter which are released by cells undergoing apoptosis46-49. For the remainder of this review, the term EVs will encompass all classes of EVs as endorsed by the International Society for Extracellular Vesicles (ISEV)47. There are several advantages of the stem cell secretome (such as EVs) over cellular approaches in clinical applications for the treatment of neonatal disease. First is the inherent nature of EVs being biologically inert and therefore less impacted by the environment. EVs also have a decreased risk of immunogenicity (such as rejection, tumor formation or autoimmune responses) and toxicity. Lastly, EV production, sterilization and storage are less complex compared with cell therapy49. Ultimately, all these EV-specific features are thought to facilitate the generation of a robust, ready-to-use, clinical-grade product for use in humans.
Bronchopulmonary Dysplasia
BPD is the most common respiratory complication of prematurity that affects 42% of very low birth weight (VLBW) infants in the United States2. Over the past few decades with the increasing survival of VLBW infants due to the use of antenatal steroids, surfactant supplementation and advancements of neonatal care, BPD remains the only complication of prematurity that continues to rise in prevalence3. BPD is the result of a complex multifactorial injury process in premature lungs that occurs in multiple stages and impacts lung development. Preterm birth disrupts late lung development and infants are born with lungs in the saccular stage of development prior to distal airway formation (alveolarization). In addition, both antenatal and postnatal insults can cause inflammation that impairs lung repair and can result in long-term remodeling in the lungs52. BPD is the most common chronic disease in children and can result in long-term complications such as increased risk of respiratory illnesses (viral infections, asthma, and emphysema), growth and neurodevelopmental impairment53-58. Despite progress in perinatal care and non-invasive respiratory management, the therapeutic options for BPD remain mostly supportive and minimally efficacious. In recent years, the stem cell secretome has emerged as a promising field of investigation for clinical application in BPD given its anti-inflammatory, immunomodulatory and angiogenic effects (Figure 1).
The interest in stem cell therapy originated from encouraging initial studies that showed beneficial effects of MSC therapy in pre-clinical models of BPD, which included improved survival, inhibition of inflammation, and attenuation in alveolar and lung vascular injury 15-17. Aslam et al., from our group first revealed that cell-free conditioned media (CM) provided better protection than MSCs in a neonatal hyperoxia murine model of BPD, which was subsequently confirmed by other groups15,38,59-62. Lee et al. then went on to demonstrate that MSC-CM mediated its therapeutic effects via a paracrine mechanism by identifying exosomes as the vehicle of protection in a model of hypoxia induced pulmonary hypertension, a known complication of severe BPD43. Subsequently, in Willis et al., our group were the first to demonstrate that a single dose of human UC derived-MSC-EVs (hUC-MSC-EVs) promoted alveolarization, decreased lung fibrosis, ameliorated pulmonary vascular remodeling, improved pulmonary function and alleviated associated pulmonary hypertension in the neonatal hyperoxia model39. Numerous studies from other groups have reproduced similar findings of MSC-EV’s protective effects, including improved alveolarization, lung function, lung vascularization and pulmonary hypertension, using a variety of routes of administration in different models of BPD63-71. Our group has also gone on to show that MSC-EV treatment was not only capable of preventing hyperoxic lung injury but also contribute to partial reversal of established injury upon serial administration 72.
In terms of the mechanism of action of MSC-EVs, different hypotheses have been proposed with a core theme surrounding anti-inflammatory and immunomodulatory pathways. Willis et al. first proposed immunomodulation as the mechanism of action whereby MSC-EVs suppressed the pro-inflammatory “M1-like” macrophage state and enhanced the anti-inflammatory “M2-like” state39. Further studies by our group revealed that MSC-EVs localized to the lung, interacted with pulmonary myeloid cells, and restored alveolar macrophages in the injured lung. The interaction between MSC-EVs and myeloid cells promoted an immunosuppressive, Ly6C+ (lymphocyte antigen 6 complex, locus C1)/ LyG+ (lymphocyte antigen 6 complex, locus G), CX3CR1+ (C-X3-C motif chemokine receptor 1) and CCR2− (C-C motif chemokine receptor 2) myeloid cell phenotype. Additionally, transplantation of MSC-EV-educated BM-derived myeloid cells paralleled the therapeutic effects as MSC-EV treatment. Functional studies helped elucidate that MSC-EVs induced transcriptomic and epigenetic reprogramming of the monocytes via modulation of the CCR2/CCL2 (C-C motif chemokine ligand 2) axis, which is involved in the infiltration and recruitment of monocyte-derived suppressive cells with high levels of anti-inflammatory activities such as Arginase 1 and interleukin 10 (IL-10)73.
Similarly, Chaubey et al. proposed that TSG-6 (tumor necrosis factor-stimulated gene-6), an immunomodulatory glycoprotein detected in MSC-EVs was responsible for their mechanism of action. TSG-6 induced a macrophage phenotypic shift from “M1-like” towards “M2-like” profile64. The group showed that administration of TSG-6 in vivo attenuated BPD and its associated pathologies in the lung (alveolar-capillary leakage and alveolar simplification), heart (pulmonary hypertension and right ventricular hypertrophy) and brain (neuronal apoptosis and hypomyelination). They also demonstrated that knocking down TSG-6 in MSC-EVs abolished their therapeutic effects almost entirely64. Porzionato et al. also demonstrated that hyperoxia exposure reduced “M2-like” macrophages in interstitial, alveolar and perivascular populations, which were prevented by hUC-MSC-EV treatment and suggestive that M2 macrophage polarization could play a role in MSC-EVs’ anti-inflammatory effects68. Lithopoulos et al. also demonstrated the immunomodulatory capabilities of hUC-MSC-EVs in a multifactorial neonatal lung injury model induced by endotoxin and ventilation whereby treatment resulted in decreased expression of pro-inflammatory marker IP-10 (interferon-γ induced protein 10) and increased expression of anti-inflammatory markers (IL-4, IL-13) in the lung71.
Other groups have been proponents of a different MSC-EV mechanism of action, namely its pro-angiogenic and pulmonary vascularization effects mediated via VEGF (vascular endothelial growth factor)63,65,67. Ahn et al. first demonstrated this by knocking down VEGF in hUC-MSC-EVs, which impaired their therapeutic effects in neonatal hyperoxic lung injury both in vitro and in vivo. Interestingly, they also showed that the MSC-EVs were internalized by vascular pericytes, macrophages, type 2 epithelial cells and fibroblasts but not by endothelial cells, which suggests paracrine crosstalk between these cells mediating the pro-angiogenic effects of MSC-EVs63. Braun et al. also showed that murine BM derived-MSC-EVs (mBM-MSC-EVs) had VEGF-dependent pro-angiogenic effects using an in vitro human umbilical vein endothelial cell tube formation assay to demonstrate increased angiogenic capabilities, which were reversed with anti-VEGF antibody treatments. They also used in vivo rodent hyperoxia model to show that MSC-EVs improved alveolar growth, increased small blood vessel number and size as well as inhibited right ventricular hypertrophy65. You et al. demonstrated similar findings of hUC-MSC-EVs improving alveolarization and angiogenesis by protecting type II alveolar epithelial cells and pulmonary vascular endothelial cells via the PTEN (phosphatase and tensin homolog)/Akt (protein kinase B) signaling pathway67.
MSC-EVs are known to carry a variety of bioactive cargo, which can include microRNAs (miRNAs). miRNAs are small non-coding RNAs that modulate gene expression on the post-transcriptional level. Some investigators have postulated that miRNAs may be a potential mechanism of action for MSC-EVs. Hyperoxia exposure in rodents results in a reduction of miR21-5p74. Wu et al. showed that murine adipose tissue-derived MSC-EVs attenuated the effects of hyperoxia in neonatal mice by transferring miR-21-5p into lung cells, which resulted in modulation of C/EBPα (CCAAT-enhancer-binding protein α) expression, a key transcription factor in perinatal lung maturation and lung epithelial repair75,76. The same group has also shown that miR-425 in rodent BM-MSC-EVs (rBM-MSC-EVs) attenuated hyperoxic injury by targeting PTEN and activating the PI3K (phosphatidylinositol-3-kinase)/Akt axis69. Other proposed mechanisms of action of MSC-EVs include differentiation of lung cell types and protein regulation. Ai et al. demonstrated that hUC-MSC-EVs suppressed transdifferentiation of alveolar type 2 cells into type 1 cells (which is increased in hyperoxic neonatal lung injury) through downregulation of Wnt5 (Wingless/Integrase 1 family member 5)70,77. Li et al. reported that human amnion derived-MSC-EVs ameliorated neonatal hyperoxic lung injury through increased APEH (acylaminoacyl-peptide hydrolase) expression, which is a proteasome that facilitates clearance of oxidatively damaged proteins as part of the cellular response to DNA damage78.
Hyperoxia exposure also has multi-organ effects beyond the cardio-pulmonary system including the thymus, brain, and eye (which will be discussed in a separate section). From our group, Reis et al. reported that hyperoxia induced involution of the thymic medulla, disruption of FoxP3+ (forkhead box P3) T regulatory cells and increased T cell autoreactivity, all of which suggests disruption of central tolerance and adaptive immunity79. hUC-MSC-EV treatment restored thymic architecture and functionality via the thymic medullary antigen presentation axis with enrichment of antigen presentation and anti-oxidative-stress related genes in dendritic cells and medullary thymic epithelial cells79. Fernandez-Gonzalez et al. demonstrated that hyperoxia resulted in multiorgan effects in the brain and retina. Regarding the neurologic impacts of hyperoxia, the group demonstrated decreased myelination and increased astrogliosis, which induced activation of microglial cells in the brain, all of which was prevented by a single dose of hUC-MSC-EVs80. Lithopoulos similarly used a multifactorial neonatal mouse model of lung injury to demonstrate neurologic impacts with impaired neural progenitor cell function, which was mitigated by hUC-MSC-EVs71.
To date, there have been several published clinical trials of stem cell therapy in BPD including different MSC sources such as the UC and amnion. Chang et al. were the first group to publish a Phase I dose-escalation trial of allogeneic hUC-MSCs administered intratracheally in 9 extremely preterm infants (<30 weeks gestation) at risk for BPD. They showed that MSC treatment was well-tolerated with no serious adverse effects and that it resulted in a reduction of pro-inflammatory cytokines IL-6 and tumor necrosis factor-α (TNF-α) in tracheal aspirates and reduced BPD severity81. They also demonstrated long-term safety (growth, respiratory and neurodevelopment) with MSC administration in the same group of patients82. Powell et al. recapitulated similar findings of safety and feasibility in another Phase I dose-escalation trial of allogeneic hUC-MSCs in 12 extremely low birth weight (<28 weeks gestation and < 1000 g) infants 83. Ahn et al. conducted the first Phase II double-blind, randomized, placebo-controlled trial of hUC-MSCs in 33 extremely preterm infants (<29 weeks gestation) receiving mechanical ventilation with respiratory deterioration between postnatal days 5-14. There was no statistical significance in the primary outcome of death or moderate-severe BPD however there was a significant reduction in severe BPD (19% in treatment group versus 53% in control group) in the most extremely preterm infants at 23-24 weeks in the subgroup analysis84. The same group is currently conducting an additional larger and controlled phase II clinical trial focused on infants between 23-24 weeks gestation (NCT003392467).
Ren et al. performed a Phase I placebo-control trial of autologous umbilical cord mononuclear cells in 15 preterm infants (< 35 weeks gestation) and showed no difference in mortality or preterm complication rates however there was a reduced duration of mechanical ventilation and oxygen therapy85. Lim et al. conducted a Phase I trial of allogeneic human amnion epithelial cells (which are stem-like cells derived from placental membranes) in 6 preterm infants with BPD, that showed safety of the IV infusion and no adverse effects86. The two-year outcome data similarly showed no evidence of long-term adverse effects87. To our knowledge, there has only been one clinical trial utilizing the stem cell secretome, a Phase I study to evaluate the safety of MSC-EVs in BPD, which was discontinued due to business decisions (NCT03857841). The clinical trials listed above have promising safety and feasibility results in small cohorts of preterm infants and preliminary signs of efficacy in the extremely preterm infants, which is encouraging for the field and at present there are 8 ongoing trials of stem cell therapy in BPD88.
Hypoxic-Ischemic Encephalopathy & Perinatal Arterial Ischemic Stroke
HIE is a form of perinatal brain injury that occurs due to oxygen deprivation to the brain that can result in neonatal encephalopathy. The incidence of HIE is estimated to be 1-3 per 1000 live term births in developed countries and accounts for 15-35% of all cases of neonatal encephalopathy89,90. HIE is a significant cause of mortality and neurologic morbidity including neurodevelopment disability, neurosensory impairment, epilepsy, and cerebral palsy (CP) in term infants. HIE may occur by one or more of the following mechanisms: impaired oxygenation or inadequate perfusion of the maternal placenta, placental pathology and impaired fetal oxygenation or perfusion91. HIE injury occurs through 3 phases including the initial hypoxic-ischemic insult, followed by secondary effects, such as mitochondrial deficiency, oxidative stress, excitotoxicity inflammation and early stages of neuronal necrosis and apoptosis and the third phase, which encompasses long-term cell death, inflammation, cell turnover and repair and gliosis92,93. Currently the only therapy with proven efficacy for HIE is therapeutic hypothermia (TH), which is hypothesized to reduce the negative consequences of secondary injury and inflammation94,95. Multiple TH trials have demonstrated improved outcomes (survival, neurodevelopmental disability, CP, and neurosensory deficits) but it does not completely alleviate the effects of HIE94,96-99. In addition, TH must be initiated promptly (within 6 hours of life) to be beneficial. Researchers continue to search for other agents and strategies to improve HIE treatment and outcomes and one of the encouraging pursuits is stem cell therapy.
There have been numerous pre-clinical studies of hypoxic brain injury demonstrating that MSC therapy results in significant neuroprotection, increased neuro-regeneration, attenuated neuroinflammation and improved functional outcomes18-20,100. Investigators have also evaluated strategies to augment the therapeutic capabilities of MSCs including thrombin preconditioning and genetic modification101-103. Other groups have combined MSC therapy with standard of care treatment, TH and found that the combination was capable of augmenting neuroprotective activity and lengthening the therapeutic time window104,105. Recently there has been interest in evaluating other sources of stem cells. Huang et al. demonstrated that human PSC-derived ectomesenchymal stromal cells were capable of inducing more functional recovery in hypoxic-ischemic (HI) brain damage than hUC-MSCs106. With regards to the stem cell secretome, there have been multiple studies demonstrating similar beneficial effects to MSCs (as listed above)40,107-115. Wei et al. published the first study to suggest therapeutic effects of the stem cell secretome by demonstrating that CM from adipose-derived MSCs conferred neuroprotection in a rodent model of HIE107. Ophelders et al. went on to become the first group to demonstrate that human BM MSC-EVs (hBM-MSC-EVs) could provide neuroprotection by improving brain function, reducing total number and duration of seizures and restoring subcortical white matter myelination in an ovine model of HIE40. Interestingly, they reported that cerebral inflammation was unaffected by MSC-EV administration, but subsequent studies from independent groups have demonstrated MSC-EV therapy has an anti-inflammatory effect on the brain108,109,111-113,115.
One of the main hypotheses proposed regarding the mechanism of action of the MSC-EVs is their anti-inflammatory effects modulated by the MAPK (mitogen-activated protein kinase) and NFκB (nuclear factor kappa-light-chain-enhancer of activated B cells) signaling pathway. Kaminski et al. showed that hBM-MSC-EVs resulted in anti-inflammatory effects via significant downregulation of pro-inflammatory cytokine, TNFα and upregulation of anti-inflammatory cytokine, TGFβ (transforming growth factor β)109. In contrast, Drommelschmidt showed that hBM-MSC-EVs ameliorated inflammation-induced cellular damage but did not affect systemic and cerebral pro-inflammatory cytokine expression108. Thomi et al. proposed that hUC-MSC-EVs reduced microglia mediated neuroinflammation by interfering with TLR-4 (Toll-like receptor 4) signaling pathway and preventing degradation of NFκB inhibitor, IκBα (nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha) and phosphorylation of the MAPK family111. Shu et al. demonstrated that hBM-MSC-EVs’ anti-inflammatory effects on microglia were mediated by inhibition of P38MAPK/P65NFκB signaling pathway resulting in dampened transcription of inflammation-related genes115.
There have also been multiple groups that have postulated that the neuroprotective effects of MSC-EVs are mediated via the delivery of miRNAs and subsequent immunomodulation. Joerger-Messerli et al. showed in an in vitro model of oxygen/glucose deprivation/reoxygenation (OGD/R) injury that hUC-MSC-EVs contained miRs let-7a and let-7e and released their contents into the neuronal cells41. Given that miRs let-7a and let-7e are known regulators of Casp3 (caspase 3), the group proposed that MSC-EV treatment inhibited Casp3 expression and subsequent HI induced apoptosis of neuronal cells41. Xin et al. used an in vivo murine model of HIE to demonstrate that mBM-MSC-EVs transferred miR-21a-5q to neurons and microglia therefore conferring neuroprotection via the Timp3 (tissue inhibitor of metalloproteinase 3) pathway113. In addition, they also showed that MSC-EV treatment had immunomodulatory effects by skewing both microglia and brain monocyte/macrophage towards an anti-inflammatory phenotype113. The same group also went on to show that delivery of miR-21a-5p by mBM-MSC-EVs induced microglial M2 polarization via the STAT3 (signal transducer and activator of transcription 3) pathway114. Chu et al. showed that hydrogen sulfide preconditioned mBM-MSC-EVs had increased therapeutic efficacy (neuroprotection, anti-inflammation and improved long-term cognitive and memory outcomes) and that MSC-EVs transferred miR-7b-5p into the ipsilateral cortex, which further induced its expression therefore promoting an anti-inflammatory microglia and mononuclear phagocyte phenotype116. Han et al. proved successful delivery of miR-410 by hUC-MSC-EVs to neurons which inhibited neuronal apoptosis via histone deacetylase dependent EGR2 (early growth response 2)/Bcl2 (B-cell lymphoma 2) axis117.
Other proposed mechanisms of action of MSC-EVs’ neuroprotection in HI induced brain injury include strengthening of the blood-brain-barrier (BBB), enhancing mitophagy and increasing growth factor expression. Gussenhoven et al. demonstrated that hBM-MSC-EVs contained Annexin A1 (ANXA1) and that both MSC-EVs and human recombinant ANXA1 could improve the integrity of the BBB via the formyl peptide receptor pathway in both a fetal ovine HIE model and an in vitro OGD/R injury model118. Hu et. al used the same OGD/R model to prove that hUC-MSC-EVs protected microglia from ischemia/reperfusion injury by enhancing mitophagy via an upregulation of FOXO3a (Forkhead box O3a) expression119. Kaminski et al. has demonstrated that hBM-MSC-EVs lead to an increase in neuronal growth factor expression, which mediates neuro-regenerative responses109. At present, there have been three Phase I trials demonstrating safety and feasibility of autologous UCB cells for neonates with HIE120-122. Studies from Cotten et al., suggested potential efficacy with 74% of recipients of cell infusion versus 41% of cooled controls obtaining normal neurodevelopmental scores in 3 domains of the Bayley-III, however this finding did not reach statistical significance121. There have also been two Phase I trials revealing the feasibility of allogeneic UCB-derived MSCs123,124. Currently there are 9 clinical trials of stem cell therapy in neonatal HIE88. With regards to clinical trials of stem cell secretome in HIE, thus far there has only been one study exploring the safety and efficacy of MSC’s paracrine effects and unfortunately the status of study is unknown (NCT02854579).
The MSC secretome has also been studied in other pre-clinical models of perinatal brain injury such as arterial ischemic stroke (AIS) and brain injuries related to prematurity, which will be discussed in the following section. Perinatal AIS is an occlusive cerebral arterial event, typically thromboembolic in nature however the exact pathogenesis remains unknown and unlike adult stroke, there are minimal interventions available for perinatal AIS. There have been a few studies that have revealed beneficial effects of MSC therapy in pre-clinical models of perinatal AIS125-128. Erythropoietin enhanced rodent adipose tissue-derived MSC improved long-term neurobehavioral outcomes in an in vivo rodent model of neonatal stroke129. Pathipathi et al. used the same model to show that mBM-MSC-EVs accumulated in activated microglia and macrophage in the injury site and attenuated a subset of cytokines and chemokines induced by transient ischemia, which may contribute to a reduction in Casp3-dependent apoptosis130. Recently, the first clinical trial of intranasal hBM-MSCs in neonates with perinatal AIS stroke was published, which demonstrated both feasibility and safety131.
Intraventricular Hemorrhage and Periventricular Leukomalacia
IVH is the most common neurologic complication of prematurity and estimated to occur in 25-30% of VLBW infants132. IVH is an important cause of morbidity as it places infants at risk for adverse neurodevelopment outcomes, which increases with IVH severity2,133,134. The pathophysiology of IVH relates to the inherent germinal matrix fragility in premature infants and the interplay with disturbances in cerebral blood flow (CBF)135-137. This is further compounded by the impaired cerebral vascular autoregulation in the immature brain, which leads to an inability to maintain constant CBF during changes in systemic pressure138. The clinical presentation of IVH is often silent and typically only detected on screening cranial ultrasounds. Prevention of brain injury in preterm infants has been predominantly focused on maintaining constant CBF and avoiding any fluctuations, which can be challenging given the lack of autoregulation and lack of CBF measurement technology. Currently there is only supportive therapy available for IVH with no active therapies or strategies to prevent the development of its complications. The incidence of severe IVH has changed minimally in the past two decades despite neonatal advancements therefore new therapies are needed to help mitigate morbidity2.
After the development of IVH, the most common complications that arise are posthemorrhagic hydrocephalus (PHH) and PVL. There are interventions available for progressive and symptomatic PHH that focus on cerebrospinal fluid drainage such as serial lumbar punctures, ventricular access devices or more permanent ventricular shunts. PVL consists of periventricular focal necrosis with subsequent cystic formation and more diffuse cerebral white matter injury139. PVL is a result of secondary brain injury induced by IVH and the exact mechanism remains unknown however there are several factors involved including microglia and astrocyte activation, degradation of blood components with release of “toxic” products, infiltration of the brain by immune cells, death of neuronal and glial cells and arrest of preoligodendrocyte maturation, which ultimately results in BBB dysfunction140. PVL injury is also often silent in presentation and can best be detected on magnetic resonance imaging whereby its pattern of injury can be described as either cystic or diffuse139. Cystic PVL tends to damage deeper, more medial tracts that control lower extremity motor function, resulting in spastic diplegia whereas injury to the more lateral tracts may cause upper extremity impairment and extensive white matter injury may result in quadriplegia. On the other hand, diffuse PVL is the most common form of brain injury and the major cause of cognitive deficits and chronic development impairment in preterm infants141. Unfortunately, there is no treatment available to prevent or treat PVL and current standard of care is symptomatic management and rehabilitation.
There have been multiple studies reporting that MSC therapy attenuated further cortical injuries and progressive hydrocephalus in a rodent neonate model of severe IVH induced by intracerebroventricular (IC) injection of blood22-27. Ahn et al. demonstrated that human UCB-MSC (hUCB-MSC) treatment significantly attenuated impairment on behavioral tests, sensorimotor coordination and neuronal loss, as well as promoted neurogenesis in the hippocampus24,27,142. The group also proposed a paracrine mechanism of action of MSCs and identified brain-derived neurotropic factor (BDNF) as a factor secreted by hUCB-MSCs that mediated its neuroprotective effects via the BDNF/TrkB (tyrosine kinase B)/CREB (cAMP response element-binding protein) signaling axis activation23,27. The same group also conducted numerous optimization studies of hUCB-MSC therapy in IVH and found that MSCs conferred neuroprotection only with early administration (2 days after induction of IVH) and that both IC and IV routes had equivalent therapeutic efficacy in protecting against severe IVH22,25. More recently, they have also demonstrated that thrombin preconditioning of hUCB-MSCs significantly improved their therapeutic efficacy compared to naïve MSCs and was more effective at reducing brain injury including progressive PHH, astrogliosis, neuronal cell death, inflammation, and neurobehavioral impairment143. Moreover, the first study evaluating the application of stem cell secretome in IVH was published by the same group whereby they found that a single IC injection of hUCB-MSC-EVs conveyed equivalent neuroprotection to MSC therapy via BDNF signaling44.
At present, there have been a few clinical trials investigating the safety and feasibility of stem cell therapy in IVH and its complications but no trials of the secretome yet. Ahn et al. conducted a Phase I dose escalation clinical trial of allogeneic UCB-MSC therapy in 9 extremely preterm infants with severe IVH, which showed that the IC injection procedure was well-tolerated with no significant adverse effects reported by term-corrected age144. Sun et al. performed a phase I trial of autologous UCB for congenital hydrocephalus, which showed that repeated IV infusions of UCB were both safe and feasible145. Currently, there are two ongoing clinical trials for IVH: first is a Phase II randomized controlled trial of UC-MSCs assessing efficacy, safety and feasibility in preterm infants with severe IVH that is in the active stage of recruiting (NCT02890953), second is a Phase I trial of autologous UCB cells in extremely preterm infants (< 28 weeks gestation prior to development of IVH) to evaluate for safety and feasibility with the eventual goal to assess for neuroprotection (ACTRN12619001637134)146.
There have also been some promising studies of MSCs and their secretome utilized as therapy in pre-clinical models of PVL but no reports of any clinical trials yet. Zhu et al. demonstrated that a single intraperitoneal injection of hUC-MSCs increased mature oligodendrocytes, decreased reactive astrocytes and activated microglia cells and markedly improved functional outcomes (sensorimotor and cognitive function) in a rodent model of PVL28. Oppliger et al. used an in vitro model to demonstrate that hUC-MSCs caused increased oligodendrocyte differentiation in neural progenitor cells (NPCs) via cell-to-cell contact and that trophic factors secreted in CM were capable of promoting astrogenesis. They also found that term hUC-MSCs caused a stronger oligodendroglial differentiation effect in NPCs than pre-term hUC-MSCs and proteomic analysis of CM identified the laminin-α2-subunit as a potential factor for the difference29. Morioka et al. showed that CM from interferon γ (IFNγ) preconditioned hUC-MSCs decreased pro-inflammatory cytokines in the brain and improved myelination in a rodent model of PVL induced by lipopolysaccharide (LPS) injection30.
Necrotizing Enterocolitis
NEC is a devastating neonatal gastrointestinal disease that occurs in 1-3 per 1000 live births every year in the United States with high rates of morbidity and mortality3. NEC is characterized by extensive intestinal inflammation, ranging from mucosal injury to full thickness necrosis and perforation that often leads to systemic inflammation affecting distal organs147-149. The etiology of NEC is multifactorial with numerous risk factors that have been identified, the most widely recognized are low gestational age, low birth weight and formula feeding. The pathogenesis of NEC stems from the susceptibility and hyperreactivity of the preterm gut, which predisposes the intestine to altered immune response, vascular injury, and dysregulated microbiome150. The clinical presentation of NEC ranges widely from subtle non-specific symptoms to severe hemodynamic instability. Diagnosis of NEC is typically made with abdominal radiographic findings such as gas filled bowel loops, pneumatosis intestinalis, portal venous gas or pneumoperitoneum. NEC prevention includes cautious advancement of enteral feedings and the exclusive use of breastmilk in high-risk patient populations. The mainstay of NEC treatment includes bowel rest by withholding enteral feedings, gastric decompression, broad spectrum antibiotics and in severe cases, surgical resection. Despite medical and surgical advances, mortality of NEC remains high between 20-40% with younger gestational age infants and infants requiring NEC surgery demonstrating higher mortality151. There are also long-term sequelae of NEC including short gut syndrome, intestinal stricture, intestinal failure and neurodevelopmental delays152. Researchers have been exploring different strategies for NEC treatment such as stem cell therapy.
There have been various studies from different groups demonstrating the efficacy of MSC therapy in preclinical rodent models of NEC. Primary effects of MSC therapy reported include improved survival and weight gain, decreased mucosal damage and improved gut barrier function78,153-159. Interestingly, there have been two separate reports of AF-MSCs conferring protective effects in NEC but not BM-MSCs155,160. There have been different mechanisms proposed regarding the mechanism of action of MSC therapy in NEC including different signaling pathways such as COX-2 (cyclooxygenase 2), Wnt and NFκB-IGF-1 (insulin growth factor 1)-TGFβ78,155,159. Drucker et al. demonstrated that hUC-MSCs may exert their beneficial effects in NEC via the production of paracrine mediator hydrogen sulfide at low levels, which conferred cytoprotective, antioxidant and anti-inflammatory functions158. The myenteric plexus ganglia are damaged in NEC patients and neural stem cells (NSCs) are responsible for repairing and renewing neurons in the enteric nervous system hence there has been an interest in investigating their therapeutic effects161. Investigators have utilized both in vivo and in vitro models of NEC and found NSC therapy to be effective in decreasing NEC incidence and mortality as well as improving the enteric nervous system, gut barrier function and intestinal motility162-164. One group demonstrated that heparin-binding epidermal growth factor-like factor promoted NSC proliferation and enhanced nitric oxide production via increasing expression of neuronal nitric oxide synthase, which conferred protection on the neurons from degeneration and damage163,164. Considering the future clinical applications of this therapy, collecting NSCs from fetal sources is not feasible hence AF has been studied as an alternative source of NSCs. McCulloh et al. used a rodent model of NEC to directly compare different sources for stem cells including BM-MSC, AF-MSC, AF-NSC and enteric NSC (E-NSC). They found that all stem cell treatments resulted in a significant decrease in intestinal permeability and increase in gut barrier function with no difference between the different types157,165.
Rager et al. was the first group to demonstrate therapeutic efficacy of stem cell derived EVs and its equivalence to stem cell therapy in NEC. They found that rBM-MSC-EVs increased wound healing in vitro and significantly decreased gut permeability and incidence of NEC in vivo166. McCulloh et al. performed a similar comparison study between different rodent sources of EVs including BM-MSC, AF-MSC, AF-NSC and E-NSC and found that all four demonstrated equivalent reductions in NEC incidence and that EVs were equivalent to direct stem cell therapy as well167. Li et al. also demonstrated in a murine model of NEC that rodent AF-MSC-EVs increased cellular proliferation, reduced inflammation and regenerated normal intestinal epithelium via increasing intestinal stem cell activity and Wnt pathway activation, which ultimately reduced the incidence of NEC. The group also showed that EVs failed to prevent injury if administered prior to NEC induction highlighting the importance of timing of therapy78. Majority of the studies to date utilized rodent sources of stem cells, more recently the same group showed that both CM and EVs from human AF-MSC (hAF-MSC) were also effective in reducing NEC incidence, intestinal inflammation and injury while increasing intestinal stem cell activity168,169. Proteomic analysis of the hAF-MSC-CM revealed several protein clusters including immune-regulation, cell cycle and stem cell regulation, which may further elucidate their mechanism of action169. Thus far, there has yet to be any clinical trials of stem cell or secretome therapy in NEC and only one case report of a 22-day old patient with NEC who underwent a laparotomy and had 60 cm of necrotic bowel resected, and subsequently received IV allogeneic UC-MSCs who demonstrated improvement in intestinal blood supply and did not develop short bowel syndrome170.
Retinopathy of Prematurity
ROP is a vision-threatening disease characterized by abnormal retinal vascular development that predominantly affects premature infants and is the leading cause of avoidable childhood blindness worldwide171,172. ROP is also a lifetime disease with deleterious impacts on other ocular diseases such as glaucoma, amblyopia, strabismus, myopia and retinal detachment173.The two major factors in the pathogenesis of ROP are the immaturity of retinal vessels and oxygen exposure resulting in oxidative injury174. ROP progression occurs in two phases: in phase 1 there is cessation of normal retinal vascular development due to a loss of growth factors (IGF-1 and VEGF) secondary to hyperoxia and loss of the maternal-fetal interface however the retina continues to mature with increasing metabolic demands therefore resulting in relative hypoxia, and in phase 2 the hypoxic retina stimulates the expression of oxygen-regulated factors such as erythropoietin and VEGF, which induce retinal neovascularization resulting in disorganized and leaky retinal vessels, predisposing to retinal detachment174,175. ROP prevention strategies include limiting oxygen exposure and toxicity by establishing oxygen saturation target parameters and routine ROP screening examinations to monitor for disease development and progression176. Current therapy options for ROP include laser photocoagulation, anti-VEGF agents and in severe cases, scleral buckling and/or vitrectomy. Laser photocoagulation is the standard of treatment for threshold (condition with 50% risk of retinal detachment) or severe ROP (partial or complete retinal detachment), whereby the non-vascularized retina is ablated to prevent further inflammation and detachment177. Laser therapy is a lengthy procedure, which requires sedation or general anesthesia and can result in serious vision-threatening complications177. Anti-VEGF therapies are a recent development in the treatment of threshold ROP either as a single treatment or as an adjunct to laser therapy. Inhibiting VEGF prevents abnormal vasoproliferation and facilitates physiologic retinal vascular development. Anti-VEGF therapy appears to be as effective as laser therapy however at present there is a lack of long-term outcome and safety data178. Anti-VEGF agents are administered by intravitreal injection, which is an invasive procedure with the risk of vision-threatening complications hence there is a need for less invasive and potentially curative therapies for ROP 178.
There have been several groups that have shown promising results of MSC therapy in pre-clinical ROP models such as retinal ischemia/reperfusion injury and oxygen-induced retinopathy (OIR). MSC treatment was able to reduce retinal neovascularization, preserve retinal thickness and prevent the loss of retinal ganglion cells31,33,34,179. In terms of the therapeutic effects of the MSC secretome, Moisseiev et al showed that hBM-MSC-EVs partially preserved retinal vascular flow, reduced retinal thinning and retinal neovascularization in an in vivo OIR model. Proteomic analysis of MSC-EVs demonstrated the presence of pro-survival associated proteins from the CREB pathway. They also demonstrated that MSC-EV treatment did not induce any immunogenicity or adverse effects45. Mathew et al. demonstrated that hBM-MSC-EVs attenuated cell death in an in vitro OGD model. They also showed significantly improved functional recovery, decreased neuro-inflammation and apoptosis in an in vivo rodent model of retinal ischemia180. Noueihed et al showed that both mBM-MSCs and their CM could inhibit neovascularization and diminish vasoobliteration in the OIR model by restoring neural Sema3E (semaphorin 3E) levels, which in turn reduced levels of IL17A and -other pro-inflammatory factors34. More recently Fernandez-Gonzalez et al., demonstrated that hyperoxia induced multi-organ damage including retinal thinning, induction of gliosis and microglial activation and invasion into the outer nuclear layer, which were all prevented by a single IV dose of hUC-MSC-EVs80.
Placental Diseases: Preeclampsia and Chorioamnionitis
The placenta serves as the maternal-fetal interface essential for the delivery of nutrients and oxygen from mother to fetus to maintain normal fetal growth181. Placental diseases such as preeclampsia (PE) and chorioamnionitis can have significant impact on the well-being of the fetus during pregnancy such as fetal growth restriction (FGR), preterm birth and in severe cases, stillbirth. In addition, these placental diseases can also have longer-lasting effects on neonatal health that are just starting to be understood. Sequelae of placental diseases such as growth restriction and an inflammatory intrauterine environment are both known to have an adverse effect on the developing lung and are significant risk factors for the development of BPD. PE is a gestational-specific maternal systemic vasculopathy affecting 3-5% of all pregnancies182. The pathogenesis of PE is characterized by impaired spiral arteriole remodeling resulting in hypoperfusion of the placenta and an imbalance in pro- and anti-angiogenic factors, which when released in the maternal circulation alter systemic endothelial function resulting in the clinical manifestations of PE. PE presents most commonly with maternal symptoms of hypertension, proteinuria, and if left untreated liver dysfunction, cerebral edema, and seizures. Current PE treatment is focused on alleviating maternal preeclamptic symptoms but there are no current available therapies to prevent PE, its progression, or adverse fetal effects. Chorioamnionitis is an inflammatory disorder of the chorion, amnion or both that can be due to an intra-amniotic infection, sterile inflammation, environmental pollutants, cigarette smoke and other toxicants183. Chorioamnionitis is a frequent cause of preterm birth with 40-70% of cases attributed to it184-186. The inflammatory mediators released in chorioamnionitis are indicated as the causative agents of preterm labor and/or premature rupture of membranes. Chorioamnionitis is also associated with a multitude of serious neonatal complications including perinatal death, early-onset neonatal sepsis, septic shock, pneumonia, meningitis, IVH, cerebral white matter damage, ROP, NEC, respiratory distress syndrome and BPD183. Current treatments for chorioamnionitis and/or preterm labor include antibiotics, progesterone, and antenatal corticosteroids however none of these treatments ameliorate the neonatal morbidities.
Recently there has been an emerging interest in studying the effects of the MSC secretome in placental diseases. Xiong et al. showed that intra-abdominal administration of hUC-MSC-EVs on gestational day 14 for 6 days total was able to decrease blood pressure and urinary protein levels in a rodent model of PE induced by injections of a nitric oxide synthase inhibitor, NG-nitro-L-arginine methyl ester. Their group also illustrated that MSC-EVs were capable of improving placental quality and promoting angiogenesis187. Wang et al. used an in vitro model of human extravillous cytotrophoblast derived transformed cell lines to demonstrate that hUC-MSC-EVs were capable of boosting trophoblast cell proliferation, migration and invasion via restoration of miRNA 133b, which subsequently restricted SGK1 (serum/glucocorticoid regulated kinase 1)188. Recently, our group led by Taglauer et al. has demonstrated that a single antenatal dose of hUC-MSC-EVs can prevent pregnancy loss, FGR and PE physiology in a preclinical in vivo model of PE (heme oxygenase null mouse). Mass cytometric analysis of utero-placental leukocytes demonstrated that antenatal MSC-EV treatment resulted in intrauterine immunomodulation impacting abundance, surface marker repertoire and cytokine profiles189. They also demonstrated that the newborn pups of preeclamptic mothers exhibited significant alveolar simplification, altered bronchial epithelial morphology and alterations in lung developmental genes, which is consistent with the clinical impact of PE on the developing lung. Weekly antenatal administration of MSC-EVs normalized fetal lung branching morphogenesis and prevented the prior deleterious lung effects by altering the expression of multiple inflammatory mediators in preeclamptic AF190. Abele et al. utilized a rat model of endotoxin-induced chorioamnionitis and demonstrated that it resulted in reduced alveolarization and pulmonary vessel density, increased right ventricular hypertrophy and decreased lung mechanics in rat pups, which are findings similar to BPD. They went on to show that intrauterine administration of hBM-MSC-EVs was able to reduce placental inflammatory gene signaling NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3) and IL-1β , improve trophoblast cell invasion and normalize spiral artery architecture. Additionally, the pups treated with MSC-EVs demonstrated preserved distal lung growth and mechanics. Further in vitro studies of fetal lung explants showed that EV treatment had a direct effect on the lung by enhancing distal lung branching and restoring VEGF and SPC (surfactant protein C) gene expression191.
Challenges to Therapeutic Applications of Stem Cell Secretome in Neonatal Diseases
The promising results of stem cell secretome therapy (mostly MSC-EVs) in preclinical models of neonatal disease has contributed to the growing interest in its clinical applications in patients. The majority of stem cell based clinical trials in neonates have focused on stem cell transplantation itself81-85,121-124,131,144,145. Currently there have only been 2 reported clinical trials of stem cell secretome therapy (NCT03857841, a safety study of MSC-EVs in severe BPD and NCT02854579, a study to evaluate the safety and efficacy of MSC’s paracrine effects in HIE), which were both sadly unable to yield results for different reasons. The demonstrated safety and feasibility of stem cell therapy in both term and preterm neonates and the promising results from preclinical studies of MSV-EV therapy is highly suggestive of the clinical potential of the stem cell secretome. The field of stem cell secretome research is still in its early stages and there are various challenges ahead of its clinical applications, ranging from mechanistic to technical.
First off, further preclinical studies are required to identify the mechanism of action of EVs in each neonatal disease process. EVs are diverse molecules that carry a multitude of bioactive cargo including protein, lipids, genetic material, and metabolites, which can exert molecular and epigenetic effects on target cells that can be difficult to tease apart. Several studies mentioned in this review have identified different singular miRNAs or proteins as the effector molecule of EV function, however given the complex effects of EVs and their vastly heterogeneous cargo this makes a single moiety being responsible for their therapeutic action highly unlikely. It is more likely that a combination of different active elements orchestrates their therapeutic efficacy. Given that EV cargo is highly dependent on its parent cell, there has been some consideration to exposing parent cells to varying culture conditions such as low pH or low oxygen concentrations to influence EV secretion and its subsequent cargo192,193. Furthermore, there are various techniques being explored to load cargo into EVs therefore allowing us to influence their bioactive properties and enhance their effects for specific therapeutic applications. There are two different approaches to influencing EV cargo, exogenous and endogenous loading. Exogenous therapeutic cargo can be loaded into EVs via co-incubation, electroporation or sonication whereas endogenous loading is achieved by genetic modification of the parent cell in order to overexpress a specific nucleotide or protein, which is then incorporated into the secreted EVs49,194. Bioengineered MSC-EVs have not yet been explored in preclinical models of neonatal disease however there are good examples of modified MSCs demonstrating heightened therapeutic effects in preclinical models of HIE and PVL such as genetically modified, IFNγ primed and thrombin preconditioned MSCs30,44,101,103. There has also been demonstration of good manufacturing practice (GMP) manufactured IFN-γ primed MSCs for potential clinical use with no evidence of toxicity or heightened anti-inflammatory effects195.
One of the major technical challenges is the absence of standardized criteria of EV production. The ISEV has published a field-consensus standardization of the minimal information for the studies of extracellular vesicles (MISEV) guideline that provides criteria for MSC-EV nomenclature, isolation, purification, and characterization47. There continues to be room for improvement with existing isolation methods to yield more homogeneous EV preparations in terms of particle number, potency, and purity, in addition to optimizing the duration and cost of EV production49. Another area of concern in production is the need to develop more sensitive tools requiring less of this limited product to quantify and characterize EVs (both phenotypically and functionally). The next MISEV guidelines are currently being established to help provide advice on the pros and cons of different isolation techniques and to develop a panel of markers to differentiate EV subtypes196. The optimal source of EVs also needs to be determined given that access to human stem cell sources is limited and there are numerous different types of stem cells that could be utilized to harvest EVs. MSCs have garnered the most attention as they are easily extracted, exert anti-inflammatory effects, have low immunogenicity and self-renewal properties197. Most preclinical studies of neonatal disease have demonstrated efficacy utilizing MSCs which can be sourced from a wide range of adult and perinatal tissues. MSCs originating from perinatal tissues (UCB, UC and placenta) have demonstrated increased secretion of chemokines, pro-inflammatory proteins, and growth factors with a higher rate of cellular proliferation compared to MSCs obtained from adult tissues (adipose, BM)198-200.
Optimal timing and dosing of EVs is also another major query, depending on the clinical scenario single versus multiple doses of MSC-EVs may be required to achieve therapeutic effect. There have been several preclinical studies reporting positive effects with a single dose administered early in the disease process39,72,73,79,189, while there have been other studies that have shown the need for multiple doses at later time points to ameliorate other disease processes72,190. Other technical challenges in the application of MSC-EVs relates to their administration and storage for clinical purposes. Multiple routes of administration for MSC-EVs have been proposed in pre-clinical models of neonatal diseases with some routes being more high-risk due to their invasive nature such as IC or intravitreal administration. IV administration would be the easiest route from an application standpoint but requires further confirmation of successful delivery to protected organ sites such as the brain and eye. Given that EVs can contain a variety of bioactive molecules in a lipid bilayer, the storage and preservation of the product is essential to maintain its optimal biologic activity201. Finally, prior to application in a clinical setting, there needs to be a regulatory framework in place including guidelines regarding GMP, production compliance, quality control criteria, sterilization methods and standard operating procedures for reproducibility49. The next MISEV plans to implement further guidelines regarding the safety, efficacy, clinical grade manufacturing, and regulatory issues196,202. In addition, the concern regarding scalability of MSC-EV production for use in clinical trials is also being addressed by an increase in biotechnology companies venturing into EV production.
In conclusion, the stem cell secretome (such as MSC-EVs) hold an immense amount of promise as a therapeutic avenue for numerous intractable neonatal pathologies given their immunomodulatory, anti-inflammatory, neuroprotective, angiogenic and regenerative effects (Figure 1). However, further research needs to be done as illustrated above to help develop our understanding of the exact mechanism of action of EVs and refine isolation, characterization, storage, and administration techniques prior to the generation of a robust and clinical-grade EV product for utilization in neonatal patients. Lastly, rigorous clinical trials are still needed to determine both safety and feasibility of EV therapy prior to eventual determination of efficacy and long-term effects.
Grant support
This work was supported by NIH R01 HL146128 (SK), R21 AI134025 (SK), Hood Foundation Major Grants Initiative to Advance Child Health (SK), and United Therapeutics Research Grant (SK & SAM).
Abbreviations:
- AF
amniotic fluid
- AIS
arterial ischemic stroke
- Akt
protein kinase B
- APEH
acylaminoacyl-peptide hydrolase
- ANXA1
annexin A1
- BBB
blood-brain-barrier
- Bcl2
B-cell lymphoma 2
- BDNF
brain-derived neurotropic factor
- BM
bone marrow
- BPD
bronchopulmonary dysplasia
- Casp3
caspase 3
- CBF
cerebral blood flow
- CCL2
C-C motif chemokine ligand 2
- CCR2
C-C motif chemokine receptor 2
- C/EBPα
CCAAT-enhancer-binding protein α
- CM
conditioned media
- COX-2
cyclooxygenase 2
- CP
cerebral palsy
- CREB
cAMP response element-binding protein
- CX3CR1
C-X3-C motif chemokine receptor 1
- EGR2
early growth response 2
- ELGAN
extremely low gestational age neonates
- EVs
extracellular vesicles
- FGR
fetal growth restriction
- FOXO3a
forkhead box O3a
- FoxP3
forkhead box P3
- hAF-MSC
human AF-MSC
- hBM-MSC-EVs
human BM MSC-EVs
- HI
hypoxic-ischemic
- HIE
hypoxic-ischemic encephalopathy
- HSCs
hematopoietic stem cells
- hUC-MSC-EVs
human UC derived-MSC-EVs
- hUCB-MSC
human UCB-MSC
- IC
intracerebroventricular
- IFNγ
interferon γ
- IGF-1
insulin growth factor
- κBα
nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha
- IL
interleukin
- IP-10
(interferon-γ induced protein 10
- ISCT
International Society for Cellular Therapy
- ISEV
International Society for Extracellular Vesicles
- IV
intravenous
- IVH
intraventricular hemorrhage
- LPS
lipopolysaccharide
- Ly6C
lymphocyte antigen 6 complex, locus C1
- Ly6G
lymphocyte antigen 6 complex, locus G
- MAPK
mitogen-activated protein kinase
- mBM-MSC-EVs
murine BM derived-MSC-EVs
- miR
microRNAs
- MISEV
minimal information for the studies of extracellular vesicles
- MSC
mesenchymal stem/stromal cell
- NEC
necrotizing enterocolitis
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NLRP3
NOD-, LRR- and pyrin domain-containing protein 3
- NPCs
neural progenitor cells
- NSCs
neural stem cells
- OGD/R
oxygen/glucose deprivation/reoxygenation
- OIR
oxygen-induced retinopathy
- PE
preeclampsia
- PHH
posthemorrhagic hydrocephalus
- PI3K
phosphatidylinositol-3-kinase
- PSC
pluripotent stem cells
- PTEN
phosphatase and tensin homolog
- PVL
periventricular leukomalacia
- rBM-MSC-EVs
rodent BM-MSC-EVs
- ROP
retinopathy of prematurity
- Sema3E
semaphorin 3E
- SGK1
serum/glucocorticoid regulated kinase 1
- SPC
surfactant protein C
- TGFβ
transforming growth factor β
- TH
therapeutic hypothermia
- Timp3
tissue inhibitor of metalloproteinase 3
- TLR-4
Toll-like receptor 4
- TNF-α
tumor necrosis factor-a
- TrkB
tyrosine kinase B
- TSG-6
tumor necrosis factor-stimulated gene-6
- UC
umbilical cord
- UCB
umbilical cord blood
- VEGF
vascular endothelial growth factor
- VLBW
very low birth weight
- Wnt
Wingless/Integrase 1 family member
Footnotes
Declaration of Competing Interest
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perin J, Mulick A, Yeung D, et al. Global, regional, and national causes of under-5 mortality in 2000-19: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet Child Adolesc Health. Feb 2022;6(2):106–115. doi: 10.1016/S2352-4642(21)00311-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. Sep 2010;126(3):443–56. doi: 10.1542/peds.2009-2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoll BJ, Hansen NI, Bell EF, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993-2012. JAMA. Sep 8 2015;314(10):1039–51. doi: 10.1001/jama.2015.10244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stocks J, Hislop A, Sonnappa S. Early lung development: lifelong effect on respiratory health and disease. Lancet Respir Med. Nov 2013;1(9):728–42. doi: 10.1016/S2213-2600(13)70118-8 [DOI] [PubMed] [Google Scholar]
- 5.Serenius F, Ewald U, Farooqi A, et al. Neurodevelopmental Outcomes Among Extremely Preterm Infants 6.5 Years After Active Perinatal Care in Sweden. JAMA Pediatr. Oct 1 2016;170(10):954–963. doi: 10.1001/jamapediatrics.2016.1210 [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Kingsford RA, Horwood J, et al. Lung Function of Adults Born at Very Low Birth Weight. Pediatrics. Feb 2020;145(2)doi: 10.1542/peds.2019-2359 [DOI] [PubMed] [Google Scholar]
- 7.Collaco JM, McGrath-Morrow SA. Bronchopulmonary dysplasia as a determinant of respiratory outcomes in adult life. Pediatr Pulmonol. Nov 2021;56(11):3464–3471. doi: 10.1002/ppul.25301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Till JE, Mc CE. Early repair processes in marrow cells irradiated and proliferating in vivo. Radiat Res. Jan 1963;18:96–105. [PubMed] [Google Scholar]
- 9.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. Nov 6 1998;282(5391):1145–7. doi: 10.1126/science.282.5391.1145 [DOI] [PubMed] [Google Scholar]
- 10.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. Aug 25 2006;126(4):663–76. doi: 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- 11.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. Oct 1970;3(4):393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x [DOI] [PubMed] [Google Scholar]
- 12.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. Sep 1976;4(5):267–74. [PubMed] [Google Scholar]
- 13.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. doi: 10.1080/14653240600855905 [DOI] [PubMed] [Google Scholar]
- 14.Viswanathan S, Shi Y, Galipeau J, et al. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT(R)) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy. Oct 2019;21(10):1019–1024. doi: 10.1016/j.jcyt.2019.08.002 [DOI] [PubMed] [Google Scholar]
- 15.Aslam M, Baveja R, Liang OD, et al. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med. Dec 1 2009;180(11):1122–30. doi: 10.1164/rccm.200902-0242OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang YS, Choi SJ, Sung DK, et al. Intratracheal transplantation of human umbilical cord blood-derived mesenchymal stem cells dose-dependently attenuates hyperoxia-induced lung injury in neonatal rats. Cell Transplant. 2011;20(11-12):1843–54. doi: 10.3727/096368911X565038 [DOI] [PubMed] [Google Scholar]
- 17.van Haaften T, Byrne R, Bonnet S, et al. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med. Dec 1 2009;180(11):1131–42. doi: 10.1164/rccm.200902-0179OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ. Mesenchymal stem cell treatment after neonatal hypoxic-ischemic brain injury improves behavioral outcome and induces neuronal and oligodendrocyte regeneration. Brain Behav Immun. Mar 2010;24(3):387–93. doi: 10.1016/j.bbi.2009.10.017 [DOI] [PubMed] [Google Scholar]
- 19.Xia G, Hong X, Chen X, Lan F, Zhang G, Liao L. Intracerebral transplantation of mesenchymal stem cells derived from human umbilical cord blood alleviates hypoxic ischemic brain injury in rat neonates. J Perinat Med. Mar 2010;38(2):215–21. doi: 10.1515/jpm.2010.021 [DOI] [PubMed] [Google Scholar]
- 20.Jellema RK, Wolfs TG, Lima Passos V, et al. Mesenchymal stem cells induce T-cell tolerance and protect the preterm brain after global hypoxia-ischemia. PLoS One. 2013;8(8):e73031. doi: 10.1371/journal.pone.0073031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hegyi B, Környei Z, Ferenczi S, et al. Regulation of mouse microglia activation and effector functions by bone marrow-derived mesenchymal stem cells. Stem Cells Dev. Nov 1 2014;23(21):2600–12. doi: 10.1089/scd.2014.0088 [DOI] [PubMed] [Google Scholar]
- 22.Ahn SY, Chang YS, Sung DK, et al. Optimal Route for Mesenchymal Stem Cells Transplantation after Severe Intraventricular Hemorrhage in Newborn Rats. PLoS One. 2015;10(7):e0132919. doi: 10.1371/journal.pone.0132919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahn SY, Chang YS, Sung DK, Sung SI, Ahn JY, Park WS. Pivotal Role of Brain-Derived Neurotrophic Factor Secreted by Mesenchymal Stem Cells in Severe Intraventricular Hemorrhage in Newborn Rats. Cell Transplant. Jan 24 2017;26(1):145–156. doi: 10.3727/096368916X692861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahn SY, Chang YS, Sung DK, et al. Mesenchymal stem cells prevent hydrocephalus after severe intraventricular hemorrhage. Stroke. Feb 2013;44(2):497–504. doi: 10.1161/STROKEAHA.112.679092 [DOI] [PubMed] [Google Scholar]
- 25.Park WS, Sung SI, Ahn SY, et al. Optimal Timing of Mesenchymal Stem Cell Therapy for Neonatal Intraventricular Hemorrhage. Cell Transplant. 2016;25(6):1131–44. doi: 10.3727/096368915X689640 [DOI] [PubMed] [Google Scholar]
- 26.Mukai T, Mori Y, Shimazu T, et al. Intravenous injection of umbilical cord-derived mesenchymal stromal cells attenuates reactive gliosis and hypomyelination in a neonatal intraventricular hemorrhage model. Neuroscience. Jul 4 2017;355:175–187. doi: 10.1016/j.neuroscience.2017.05.006 [DOI] [PubMed] [Google Scholar]
- 27.Ko HR, Ahn SY, Chang YS, et al. Human UCB-MSCs treatment upon intraventricular hemorrhage contributes to attenuate hippocampal neuron loss and circuit damage through BDNF-CREB signaling. Stem Cell Res Ther. Nov 21 2018;9(1):326. doi: 10.1186/s13287-018-1052-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu LH, Bai X, Zhang N, Wang SY, Li W, Jiang L. Improvement of human umbilical cord mesenchymal stem cell transplantation on glial cell and behavioral function in a neonatal model of periventricular white matter damage. Brain Res. May 14 2014;1563:13–21. doi: 10.1016/j.brainres.2014.03.030 [DOI] [PubMed] [Google Scholar]
- 29.Oppliger B, Joerger-Messerli MS, Simillion C, Mueller M, Surbek DV, Schoeberlein A. Mesenchymal stromal cells from umbilical cord Wharton's jelly trigger oligodendroglial differentiation in neural progenitor cells through cell-to-cell contact. Cytotherapy. Jul 2017;19(7):829–838. doi: 10.1016/j.jcyt.2017.03.075 [DOI] [PubMed] [Google Scholar]
- 30.Morioka C, Komaki M, Taki A, et al. Neuroprotective effects of human umbilical cord-derived mesenchymal stem cells on periventricular leukomalacia-like brain injury in neonatal rats. Inflamm Regen. 2017;37:1. doi: 10.1186/s41232-016-0032-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li N, Li XR, Yuan JQ. Effects of bone-marrow mesenchymal stem cells transplanted into vitreous cavity of rat injured by ischemia/reperfusion. Graefes Arch Clin Exp Ophthalmol. Apr 2009;247(4):503–14. doi: 10.1007/s00417-008-1009-y [DOI] [PubMed] [Google Scholar]
- 32.Kim YE, Park WS, Sung DK, et al. Intratracheal transplantation of mesenchymal stem cells simultaneously attenuates both lung and brain injuries in hyperoxic newborn rats. Pediatr Res. Sep 2016;80(3):415–24. doi: 10.1038/pr.2016.88 [DOI] [PubMed] [Google Scholar]
- 33.Wang JD, An Y, Zhang JS, et al. Human bone marrow mesenchyamal stem cells for retinal vascular injury. Acta Ophthalmol. Sep 2017;95(6):e453–e461. doi: 10.1111/aos.13154 [DOI] [PubMed] [Google Scholar]
- 34.Noueihed B, Rivera JC, Dabouz R, et al. Mesenchymal Stromal Cells Promote Retinal Vascular Repair by Modulating Sema3E and IL-17A in a Model of Ischemic Retinopathy. Front Cell Dev Biol. 2021;9:630645. doi: 10.3389/fcell.2021.630645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang OD, Mitsialis SA, Chang MS, et al. Mesenchymal stromal cells expressing heme oxygenase-1 reverse pulmonary hypertension. Stem Cells. Jan 2011;29(1):99–107. doi: 10.1002/stem.548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keating A Mesenchymal stromal cells: new directions. Cell Stem Cell. Jun 14 2012;10(6):709–716. doi: 10.1016/j.stem.2012.05.015 [DOI] [PubMed] [Google Scholar]
- 37.Prockop DJ. The exciting prospects of new therapies with mesenchymal stromal cells. Cytotherapy. Jan 2017;19(1):1–8. doi: 10.1016/j.jcyt.2016.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansmann G, Fernandez-Gonzalez A, Aslam M, et al. Mesenchymal stem cell-mediated reversal of bronchopulmonary dysplasia and associated pulmonary hypertension. Pulm Circ. Apr-Jun 2012;2(2):170–81. doi: 10.4103/2045-8932.97603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willis GR, Fernandez-Gonzalez A, Anastas J, et al. Mesenchymal Stromal Cell Exosomes Ameliorate Experimental Bronchopulmonary Dysplasia and Restore Lung Function through Macrophage Immunomodulation. Am J Respir Crit Care Med. Jan 1 2018;197(1): 104–116. doi: 10.1164/rccm.201705-0925OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ophelders DR, Wolfs TG, Jellema RK, et al. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Protect the Fetal Brain After Hypoxia-Ischemia. Stem Cells Transl Med. Jun 2016;5(6):754–63. doi: 10.5966/sctm.2015-0197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joerger-Messerli MS, Oppliger B, Spinelli M, et al. Extracellular Vesicles Derived from Wharton's Jelly Mesenchymal Stem Cells Prevent and Resolve Programmed Cell Death Mediated by Perinatal Hypoxia-Ischemia in Neuronal Cells. Cell Transplant. Jan 2018;27(1):168–180. doi: 10.1177/0963689717738256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Y, Tang Y, Long W, Zhang C. Stem Cell-Released Microvesicles and Exosomes as Novel Biomarkers and Treatments of Diseases. Stem Cells Int. 2016;2016:2417268. doi: 10.1155/2016/2417268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee C, Mitsialis SA, Aslam M, et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. Nov 27 2012;126(22):2601–11. doi: 10.1161/circulationaha.112.114173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahn SY, Sung DK, Kim YE, Sung S, Chang YS, Park WS. Brain-derived neurotropic factor mediates neuroprotection of mesenchymal stem cell-derived extracellular vesicles against severe intraventricular hemorrhage in newborn rats. Stem Cells Transl Med. Mar 2021;10(3):374–384. doi: 10.1002/sctm.20-0301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moisseiev E, Anderson JD, Oltjen S, et al. Protective Effect of Intravitreal Administration of Exosomes Derived from Mesenchymal Stem Cells on Retinal Ischemia. Curr Eye Res. Oct 2017;42(10):1358–1367. doi: 10.1080/02713683.2017.1319491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yeung V, Willis GR, Taglauer E, Mitsialis SA, Kourembanas S. Paving the Road for Mesenchymal Stem Cell-Derived Exosome Therapy in Bronchopulmonary Dysplasia and Pulmonary Hypertension. Stem Cell-Based Therapy for Lung Disease. 2019:131–152. doi: 10.1007/978-3-030-29403-8_8 [DOI] [Google Scholar]
- 47.Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. doi: 10.1080/20013078.2018.1535750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. Feb 18 2013;200(4):373–83. doi: 10.1083/jcb.201211138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lesage F, Thebaud B. Mesenchymal Stromal Cell-Derived Extracellular Vesicles for Neonatal Lung Disease: Tiny Particles, Major Promise, Rigorous Requirements for Clinical Translation. Cells. Mar 31 2022;11(7)doi: 10.3390/cells11071176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lo Cicero A, Stahl PD, Raposo G. Extracellular vesicles shuffling intercellular messages: for good or for bad. Curr Opin Cell Biol. Aug 2015;35:69–77. doi: 10.1016/j.ceb.2015.04.013 [DOI] [PubMed] [Google Scholar]
- 51.van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. Apr 2018;19(4):213–228. doi: 10.1038/nrm.2017.125 [DOI] [PubMed] [Google Scholar]
- 52.Thebaud B, Goss KN, Laughon M, et al. Bronchopulmonary dysplasia. Nat Rev Dis Primers. Nov 14 2019;5(1):78. doi: 10.1038/s41572-019-0127-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paes B, Fauroux B, Figueras-Aloy J, et al. Defining the Risk and Associated Morbidity and Mortality of Severe Respiratory Syncytial Virus Infection Among Infants with Chronic Lung Disease. Infect Dis Ther. Dec 2016;5(4):453–471. doi: 10.1007/s40121-016-0137-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kurzner SI, Garg M, Bautista DB, Sargent CW, Bowman CM, Keens TG. Growth failure in bronchopulmonary dysplasia: elevated metabolic rates and pulmonary mechanics. J Pediatr. Jan 1988;112(1):73–80. doi: 10.1016/s0022-3476(88)80126-4 [DOI] [PubMed] [Google Scholar]
- 55.Vom Hove M, Prenzel F, Uhlig HH, Robel-Tillig E. Pulmonary outcome in former preterm, very low birth weight children with bronchopulmonary dysplasia: a case-control follow-up at school age. J Pediatr. Jan 2014;164(1):40–45.e4. doi: 10.1016/j.jpeds.2013.07.045 [DOI] [PubMed] [Google Scholar]
- 56.Wong PM, Lees AN, Louw J, et al. Emphysema in young adult survivors of moderate-to-severe bronchopulmonary dysplasia. Eur Respir J. Aug 2008;32(2):321–8. doi: 10.1183/09031936.00127107 [DOI] [PubMed] [Google Scholar]
- 57.Fawke J, Lum S, Kirkby J, et al. Lung function and respiratory symptoms at 11 years in children born extremely preterm: the EPICure study. Am J Respir Crit Care Med. Jul 15 2010;182(2):237–45. doi: 10.1164/rccm.200912-1806OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Treluyer L, Jarreau PH, Marchand-Martin L, et al. Bronchopulmonary Dysplasia and Risk of Developmental Delay: An EPIPAGE-2 Cohort Study. Neonatology. 2022;119(1):124–128. doi: 10.1159/000520451 [DOI] [PubMed] [Google Scholar]
- 59.Sutsko RP, Young KC, Ribeiro A, et al. Long-term reparative effects of mesenchymal stem cell therapy following neonatal hyperoxia-induced lung injury. Pediatr Res. Jan 2013;73(1):46–53. doi: 10.1038/pr.2012.152 [DOI] [PubMed] [Google Scholar]
- 60.Zhang X, Wang H, Shi Y, et al. Role of bone marrow-derived mesenchymal stem cells in the prevention of hyperoxia-induced lung injury in newborn mice. Cell Biol Int. Jun 1 2012;36(6):589–94. doi: 10.1042/CBI20110447 [DOI] [PubMed] [Google Scholar]
- 61.Chang YS, Oh W, Choi SJ, et al. Human umbilical cord blood-derived mesenchymal stem cells attenuate hyperoxia-induced lung injury in neonatal rats. Cell Transplant. 2009;18(8):869–86. doi: 10.3727/096368909X471189 [DOI] [PubMed] [Google Scholar]
- 62.Pierro M, Ionescu L, Montemurro T, et al. Short-term, long-term and paracrine effect of human umbilical cord-derived stem cells in lung injury prevention and repair in experimental bronchopulmonary dysplasia. Thorax. May 2013;68(5):475–84. doi: 10.1136/thoraxjnl-2012-202323 [DOI] [PubMed] [Google Scholar]
- 63.Ahn SY, Park WS, Kim YE, et al. Vascular endothelial growth factor mediates the therapeutic efficacy of mesenchymal stem cell-derived extracellular vesicles against neonatal hyperoxic lung injury. Exp Mol Med. Apr 13 2018;50(4):1–12. doi: 10.1038/s12276-018-0055-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chaubey S, Thueson S, Ponnalagu D, et al. Early gestational mesenchymal stem cell secretome attenuates experimental bronchopulmonary dysplasia in part via exosome-associated factor TSG-6. Stem Cell Res Ther. Jun 26 2018;9(1):173. doi: 10.1186/s13287-018-0903-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Braun RK, Chetty C, Balasubramaniam V, et al. Intraperitoneal injection of MSC-derived exosomes prevent experimental bronchopulmonary dysplasia. Biochem Biophys Res Commun. Sep 18 2018;503(4):2653–2658. doi: 10.1016/j.bbrc.2018.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Porzionato A, Zaramella P, Dedja A, et al. Intratracheal administration of clinical-grade mesenchymal stem cell-derived extracellular vesicles reduces lung injury in a rat model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. Jan 1 2019;316(1):L6–l19. doi: 10.1152/ajplung.00109.2018 [DOI] [PubMed] [Google Scholar]
- 67.You J, Zhou O, Liu J, et al. Human Umbilical Cord Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Alleviate Lung Injury in Rat Model of Bronchopulmonary Dysplasia by Affecting Cell Survival and Angiogenesis. Stem Cells Dev. Dec 1 2020;29(23):1520–1532. doi: 10.1089/scd.2020.0156 [DOI] [PubMed] [Google Scholar]
- 68.Porzionato A, Zaramella P, Dedja A, et al. Intratracheal administration of mesenchymal stem cell-derived extracellular vesicles reduces lung injuries in a chronic rat model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. May 1 2021;320(5):L688–l704. doi: 10.1152/ajplung.00148.2020 [DOI] [PubMed] [Google Scholar]
- 69.Wu Y, Li J, Yuan R, Deng Z, Wu X. Bone marrow mesenchymal stem cell-derived exosomes alleviate hyperoxia-induced lung injury via the manipulation of microRNA-425. Arch Biochem Biophys. Jan 15 2021;697:108712. doi: 10.1016/j.abb.2020.108712 [DOI] [PubMed] [Google Scholar]
- 70.Ai D, Shen J, Sun J, et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Suppress Hyperoxia-Induced Transdifferentiation of Rat Alveolar Type 2 Epithelial Cells. Stem Cells Dev. Feb 2022;31(3-4):53–66. doi: 10.1089/scd.2021.0256 [DOI] [PubMed] [Google Scholar]
- 71.Lithopoulos MA, Strueby L, O'Reilly M, et al. Pulmonary and Neurologic Effects of Mesenchymal Stromal Cell Extracellular Vesicles in a Multifactorial Lung Injury Model. Am J Respir Crit Care Med. Mar 14 2022;doi: 10.1164/rccm.202012-4520OC [DOI] [PubMed] [Google Scholar]
- 72.Willis GR, Fernandez-Gonzalez A, Reis M, et al. Mesenchymal stromal cell-derived small extracellular vesicles restore lung architecture and improve exercise capacity in a model of neonatal hyperoxia-induced lung injury. J Extracell Vesicles. Jul 13 2020;9(1):1790874. doi: 10.1080/20013078.2020.1790874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Willis GR, Reis M, Gheinani AH, et al. Extracellular Vesicles Protect the Neonatal Lung from Hyperoxic Injury through the Epigenetic and Transcriptomic Reprogramming of Myeloid Cells. Am J Respir Crit Care Med. Dec 15 2021;204(12):1418–1432. doi: 10.1164/rccm.202102-0329OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qin S, Chen M, Ji H, et al. miR215p regulates type II alveolar epithelial cell apoptosis in hyperoxic acute lung injury. Mol Med Rep. Apr 2018;17(4):5796–5804. doi: 10.3892/mmr.2018.8560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu Y, Zhang Z, Li J, et al. Mechanism of Adipose-Derived Mesenchymal Stem Cell-Derived Extracellular Vesicles Carrying miR-21-5p in Hyperoxia-Induced Lung Injury. Stem Cell Rev Rep. Mar 2022;18(3):1007–1024. doi: 10.1007/s12015-021-10311-x [DOI] [PubMed] [Google Scholar]
- 76.Xu Y, Saegusa C, Schehr A, Grant S, Whitsett JA, Ikegami M. C/EBP{alpha} is required for pulmonary cytoprotection during hyperoxia. Am J Physiol Lung Cell Mol Physiol. Aug 2009;297(2):L286–98. doi: 10.1152/ajplung.00094.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abdelwahab EMM, Rapp J, Feller D, et al. Wnt signaling regulates trans-differentiation of stem cell like type 2 alveolar epithelial cells to type 1 epithelial cells. Respir Res. Sep 6 2019;20(1):204. doi: 10.1186/s12931-019-1176-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li B, Lee C, O'Connell JS, et al. Activation of Wnt signaling by amniotic fluid stem cell-derived extracellular vesicles attenuates intestinal injury in experimental necrotizing enterocolitis. Cell Death Dis. Sep 14 2020;11(9):750. doi: 10.1038/s41419-020-02964-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reis M, Willis GR, Fernandez-Gonzalez A, et al. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Restore Thymic Architecture and T Cell Function Disrupted by Neonatal Hyperoxia. Front Immunol. 2021;12:640595. doi: 10.3389/fimmu.2021.640595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fernandez-Gonzalez A, Willis GR, Yeung V, et al. Therapeutic Effects of Mesenchymal Stromal Cell-Derived Small Extracellular Vesicles in Oxygen-Induced Multi-Organ Disease: A Developmental Perspective. Front Cell Dev Biol. 2021;9:647025. doi: 10.3389/fcell.2021.647025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chang YS, Ahn SY, Yoo HS, et al. Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trial. J Pediatr. May 2014;164(5):966–972.e6. doi: 10.1016/j.jpeds.2013.12.011 [DOI] [PubMed] [Google Scholar]
- 82.Ahn SY, Chang YS, Kim JH, Sung SI, Park WS. Two-Year Follow-Up Outcomes of Premature Infants Enrolled in the Phase I Trial of Mesenchymal Stem Cells Transplantation for Bronchopulmonary Dysplasia. J Pediatr. Jun 2017;185:49–54.e2. doi: 10.1016/j.jpeds.2017.02.061 [DOI] [PubMed] [Google Scholar]
- 83.Powell SB, Silvestri JM. Safety of Intratracheal Administration of Human Umbilical Cord Blood Derived Mesenchymal Stromal Cells in Extremely Low Birth Weight Preterm Infants. The Journal of Pediatrics. 2019/July/01/ 2019;210:209–213.e2. [DOI] [PubMed] [Google Scholar]
- 84.Ahn SY, Chang YS, Lee MH, et al. Stem cells for bronchopulmonary dysplasia in preterm infants: A randomized controlled phase II trial. Stem Cells Transl Med. Aug 2021;10(8):1129–1137. doi: 10.1002/sctm.20-0330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ren Z, Xu F, Zhang X, et al. Autologous cord blood cell infusion in preterm neonates safely reduces respiratory support duration and potentially preterm complications. Stem Cells Transl Med. Feb 2020;9(2):169–176. doi: 10.1002/sctm.19-0106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lim R, Malhotra A, Tan J, et al. First-In-Human Administration of Allogeneic Amnion Cells in Premature Infants With Bronchopulmonary Dysplasia: A Safety Study. Stem Cells Translational Medicine. 2018;7(9):628–635. doi: 10.1002/sctm.18-0079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Malhotra A, Lim R, Mockler JC, Wallace EM. Two-year outcomes of infants enrolled in the first-in-human study of amnion cells for bronchopulmonary dysplasia. Stem Cells Translational Medicine. 2019;9(3):289–294. doi: 10.1002/sctm.19-0251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou L, McDonald C, Yawno T, Jenkin G, Miller S, Malhotra A. Umbilical Cord Blood and Cord Tissue-Derived Cell Therapies for Neonatal Morbidities: Current Status and Future Challenges. Stem Cells Transl Med. Mar 17 2022;11(2):135–145. doi: 10.1093/stcltm/szab024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vannucci RC. Hypoxic-ischemic encephalopathy. Am J Perinatol. 2000;17(3):113–20. doi: 10.1055/s-2000-9293 [DOI] [PubMed] [Google Scholar]
- 90.Nelson KB, Bingham P, Edwards EM, et al. Antecedents of neonatal encephalopathy in the Vermont Oxford Network Encephalopathy Registry. Pediatrics. Nov 2012;130(5):878–86. doi: 10.1542/peds.2012-0714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martinez-Biarge M, Diez-Sebastian J, Wusthoff CJ, Mercuri E, Cowan FM. Antepartum and intrapartum factors preceding neonatal hypoxic-ischemic encephalopathy. Pediatrics. Oct 2013;132(4):e952–9. doi: 10.1542/peds.2013-0511 [DOI] [PubMed] [Google Scholar]
- 92.Ferriero DM. Neonatal brain injury. N Engl J Med. Nov 4 2004;351(19):1985–95. doi: 10.1056/NEJMra041996 [DOI] [PubMed] [Google Scholar]
- 93.Hagberg H, David Edwards A, Groenendaal F. Perinatal brain damage: The term infant. Neurobiol Dis. Aug 2016;92(Pt A):102–12. doi: 10.1016/j.nbd.2015.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. Jan 31 2013;(1):CD003311. doi: 10.1002/14651858.CD003311.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.FETUS CO, NEWBORN. Hypothermia and Neonatal Encephalopathy. Pediatrics. 2014;133(6):1146–1150. doi: 10.1542/peds.2014-0899 [DOI] [PubMed] [Google Scholar]
- 96.Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch Pediatr Adolesc Med. Jun 1 2012;166(6):558–66. doi: 10.1001/archpediatrics.2011.1772 [DOI] [PubMed] [Google Scholar]
- 97.Shankaran S, Pappas A, McDonald SA, et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med. May 31 2012;366(22):2085–92. doi: 10.1056/NEJMoa1112066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pappas A, Shankaran S, McDonald SA, et al. Cognitive outcomes after neonatal encephalopathy. Pediatrics. Mar 2015;135(3):e624–34. doi: 10.1542/peds.2014-1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee-Kelland R, Jary S, Tonks J, Cowan FM, Thoresen M, Chakkarapani E. School-age outcomes of children without cerebral palsy cooled for neonatal hypoxic-ischaemic encephalopathy in 2008-2010. Arch Dis Child Fetal Neonatal Ed. Jan 2020;105(1):8–13. doi: 10.1136/archdischild-2018-316509 [DOI] [PubMed] [Google Scholar]
- 100.Li F, Zhang K, Liu H, Yang T, Xiao DJ, Wang YS. The neuroprotective effect of mesenchymal stem cells is mediated through inhibition of apoptosis in hypoxic ischemic injury. World J Pediatr. Apr 2020;16(2):193–200. doi: 10.1007/s12519-019-00310-x [DOI] [PubMed] [Google Scholar]
- 101.Kim YE, Sung SI, Chang YS, Ahn SY, Sung DK, Park WS. Thrombin Preconditioning Enhances Therapeutic Efficacy of Human Wharton's Jelly-Derived Mesenchymal Stem Cells in Severe Neonatal Hypoxic Ischemic Encephalopathy. Int J Mol Sci. May 20 2019;20(10)doi: 10.3390/ijms20102477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ahn SY, Sung DK, Chang YS, et al. BDNF-Overexpressing Engineered Mesenchymal Stem Cells Enhances Their Therapeutic Efficacy against Severe Neonatal Hypoxic Ischemic Brain Injury. Int J Mol Sci. Oct 22 2021;22(21)doi: 10.3390/ijms222111395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Noh JH, Jeong JS, Park SJ, et al. Preclinical assessment of thrombin-preconditioned human Wharton's jelly-derived mesenchymal stem cells for neonatal hypoxic-ischaemic brain injury. J Cell Mol Med. Nov 2021;25(22):10430–10440. doi: 10.1111/jcmm.16971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Park WS, Sung SI, Ahn SY, et al. Hypothermia augments neuroprotective activity of mesenchymal stem cells for neonatal hypoxic-ischemic encephalopathy. PLoS One. 2015;10(3):e0120893. doi: 10.1371/journal.pone.0120893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ahn SY, Chang YS, Sung DK, Sung SI, Park WS. Hypothermia broadens the therapeutic time window of mesenchymal stem cell transplantation for severe neonatal hypoxic ischemic encephalopathy. Sci Rep. May 16 2018;8(1):7665. doi: 10.1038/s41598-018-25902-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang J, U KP, Yang F, et al. Human pluripotent stem cell-derived ectomesenchymal stromal cells promote more robust functional recovery than umbilical cord-derived mesenchymal stromal cells after hypoxic-ischaemic brain damage. Theranostics. 2022;12(1):143–166. doi: 10.7150/thno.57234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wei X, Du Z, Zhao L, et al. IFATS collection: The conditioned media of adipose stromal cells protect against hypoxia-ischemia-induced brain damage in neonatal rats. Stem Cells. Feb 2009;27(2):478–88. doi: 10.1634/stemcells.2008-0333 [DOI] [PubMed] [Google Scholar]
- 108.Drommelschmidt K, Serdar M, Bendix I, et al. Mesenchymal stem cell-derived extracellular vesicles ameliorate inflammation-induced preterm brain injury. Brain Behav Immun. Feb 2017;60:220–232. doi: 10.1016/j.bbi.2016.11.011 [DOI] [PubMed] [Google Scholar]
- 109.Kaminski N, Köster C, Mouloud Y, et al. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Reduce Neuroinflammation, Promote Neural Cell Proliferation and Improve Oligodendrocyte Maturation in Neonatal Hypoxic-Ischemic Brain Injury. Front Cell Neurosci. 2020;14:601176. doi: 10.3389/fncel.2020.601176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thomi G, Joerger-Messerli M, Haesler V, Muri L, Surbek D, Schoeberlein A. Intranasally Administered Exosomes from Umbilical Cord Stem Cells Have Preventive Neuroprotective Effects and Contribute to Functional Recovery after Perinatal Brain Injury. Cells. Aug 8 2019;8(8)doi: 10.3390/cells8080855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thomi G, Surbek D, Haesler V, Joerger-Messerli M, Schoeberlein A. Exosomes derived from umbilical cord mesenchymal stem cells reduce microglia-mediated neuroinflammation in perinatal brain injury. Stem Cell Res Ther. Mar 21 2019;10(1):105. doi: 10.1186/s13287-019-1207-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xin D, Li T, Chu X, Ke H, Liu D, Wang Z. MSCs-extracellular vesicles attenuated neuroinflammation, synapse damage and microglial phagocytosis after hypoxia-ischemia injury by preventing osteopontin expression. Pharmacol Res. Feb 2021;164:105322. doi: 10.1016/j.phrs.2020.105322 [DOI] [PubMed] [Google Scholar]
- 113.Xin D, Li T, Chu X, et al. Mesenchymal stromal cell-derived extracellular vesicles modulate microglia/macrophage polarization and protect the brain against hypoxia-ischemic injury in neonatal mice by targeting delivery of miR-21a-5p. Acta Biomater. Sep 1 2020;113:597–613. doi: 10.1016/j.actbio.2020.06.037 [DOI] [PubMed] [Google Scholar]
- 114.Xin DQ, Zhao YJ, Li TT, et al. The delivery of miR-21a-5p by extracellular vesicles induces microglial polarization via the STAT3 pathway following hypoxia-ischemia in neonatal mice. Neural Regen Res. Oct 2022;17(10):2238–2246. doi: 10.4103/1673-5374.336871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shu J, Jiang L, Wang M, et al. Human bone marrow mesenchymal stem cells-derived exosomes protect against nerve injury via regulating immune microenvironment in neonatal hypoxic-ischemic brain damage model. Immunobiology. Jan 10 2022;227(3):152178. doi: 10.1016/j.imbio.2022.152178 [DOI] [PubMed] [Google Scholar]
- 116.Chu X, Liu D, Li T, et al. Hydrogen sulfide-modified extracellular vesicles from mesenchymal stem cells for treatment of hypoxic-ischemic brain injury. J Control Release. Dec 10 2020;328:13–27. doi: 10.1016/j.jconrel.2020.08.037 [DOI] [PubMed] [Google Scholar]
- 117.Han J, Yang S, Hao X, et al. Extracellular Vesicle-Derived microRNA-410 From Mesenchymal Stem Cells Protects Against Neonatal Hypoxia-Ischemia Brain Damage Through an HDAC1-Dependent EGR2/Bcl2 Axis. Front Cell Dev Biol. 2020;8:579236. doi: 10.3389/fcell.2020.579236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gussenhoven R, Klein L, Ophelders D, et al. Annexin A1 as Neuroprotective Determinant for Blood-Brain Barrier Integrity in Neonatal Hypoxic-Ischemic Encephalopathy. J Clin Med. Jan 24 2019;8(2)doi: 10.3390/jcm8020137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hu Z, Yuan Y, Zhang X, et al. Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Attenuate Oxygen-Glucose Deprivation/Reperfusion-Induced Microglial Pyroptosis by Promoting FOXO3a-Dependent Mitophagy. Oxid Med Cell Longev. 2021;2021:6219715. doi: 10.1155/2021/6219715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jiun L Autologous cord blood cell therapy for newborn infants with hypoxic ischemic encephalopathy. Cell Organ Transplantol. 2013;1(1):27–28. [Google Scholar]
- 121.Cotten CM, Murtha AP, Goldberg RN, et al. Feasibility of autologous cord blood cells for infants with hypoxic-ischemic encephalopathy. J Pediatr. May 2014;164(5):973–979 e1. doi: 10.1016/j.jpeds.2013.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tsuji M, Sawada M, Watabe S, et al. Autologous cord blood cell therapy for neonatal hypoxic-ischaemic encephalopathy: a pilot study for feasibility and safety. Sci Rep. Mar 12 2020;10(1):4603. doi: 10.1038/s41598-020-61311-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cotten CM, Fisher K, Kurtzberg J, Simmons R. Phase I Trial of Allogeneic Umbilical Cord Tissue-Derived Mesenchymal Stromal Cells in Neonates with Hypoxic-Ischemic Encephalopathy. Cytotherapy. 2020/May/01/ 2020;22(5, Supplement):S192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kabatas S, Civelek E, Savrunlu EC, et al. Feasibility of allogeneic mesenchymal stem cells in pediatric hypoxic-ischemic encephalopathy: Phase I study. World J Stem Cells. May 26 2021;13(5):470–484. doi: 10.4252/wjsc.v13.i5.470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim ES, Ahn SY, Im GH, et al. Human umbilical cord blood-derived mesenchymal stem cell transplantation attenuates severe brain injury by permanent middle cerebral artery occlusion in newborn rats. Pediatr Res. Sep 2012;72(3):277–84. doi: 10.1038/pr.2012.71 [DOI] [PubMed] [Google Scholar]
- 126.van Velthoven CT, Sheldon RA, Kavelaars A, et al. Mesenchymal stem cell transplantation attenuates brain injury after neonatal stroke. Stroke. May 2013;44(5):1426–32. doi: 10.1161/STROKEAHA.111.000326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wei ZZ, Gu X, Ferdinand A, et al. Intranasal delivery of bone marrow mesenchymal stem cells improved neurovascular regeneration and rescued neuropsychiatric deficits after neonatal stroke in rats. Cell Transplant. 2015;24(3):391–402. doi: 10.3727/096368915X686887 [DOI] [PubMed] [Google Scholar]
- 128.van Velthoven CT, Dzietko M, Wendland MF, et al. Mesenchymal stem cells attenuate MRI-identifiable injury, protect white matter, and improve long-term functional outcomes after neonatal focal stroke in rats. J Neurosci Res. May 2017;95(5):1225–1236. doi: 10.1002/jnr.23954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Larpthaveesarp A, Pathipati P, Ostrin S, Rajah A, Ferriero D, Gonzalez FF. Enhanced Mesenchymal Stromal Cells or Erythropoietin Provide Long-Term Functional Benefit After Neonatal Stroke. Stroke. Jan 2021;52(1):284–293. doi: 10.1161/STROKEAHA.120.031191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pathipati P, Lecuyer M, Faustino J, Strivelli J, Phinney DG, Vexler ZS. Mesenchymal Stem Cell (MSC)-Derived Extracellular Vesicles Protect from Neonatal Stroke by Interacting with Microglial Cells. Neurotherapeutics. Jul 2021;18(3):1939–1952. doi: 10.1007/s13311-021-01076-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Baak LM, Wagenaar N, van der Aa NE, et al. Feasibility and safety of intranasally administered mesenchymal stromal cells after perinatal arterial ischaemic stroke in the Netherlands (PASSIoN): a first-in-human, open-label intervention study. Lancet Neurol. Jun 2022;21(6):528–536. doi: 10.1016/S1474-4422(22)00117-X [DOI] [PubMed] [Google Scholar]
- 132.Christian EA, Jin DL, Attenello F, et al. Trends in hospitalization of preterm infants with intraventricular hemorrhage and hydrocephalus in the United States, 2000-2010. J Neurosurg Pediatr. Mar 2016;17(3):260–9. doi: 10.3171/2015.7.PEDS15140 [DOI] [PubMed] [Google Scholar]
- 133.Bolisetty S, Dhawan A, Abdel-Latif M, et al. Intraventricular hemorrhage and neurodevelopmental outcomes in extreme preterm infants. Pediatrics. Jan 2014;133(1):55–62. doi: 10.1542/peds.2013-0372 [DOI] [PubMed] [Google Scholar]
- 134.Shankaran S, Bajaj M, Natarajan G, et al. Outcomes Following Post-Hemorrhagic Ventricular Dilatation among Infants of Extremely Low Gestational Age. J Pediatr. Nov 2020;226:36–44 e3. doi: 10.1016/j.jpeds.2020.07.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ballabh P Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. Jan 2010;67(1):1–8. doi: 10.1203/PDR.0b013e3181c1b176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tsuji M, Saul JP, du Plessis A, et al. Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics. Oct 2000;106(4):625–32. doi: 10.1542/peds.106.4.625 [DOI] [PubMed] [Google Scholar]
- 137.O'Leary H, Gregas MC, Limperopoulos C, et al. Elevated cerebral pressure passivity is associated with prematurity-related intracranial hemorrhage. Pediatrics. Jul 2009;124(1):302–9. doi: 10.1542/peds.2008-2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.du Plessis AJ. Cerebrovascular injury in premature infants: current understanding and challenges for future prevention. Clin Perinatol. Dec 2008;35(4):609–41, v. doi: 10.1016/j.clp.2008.07.010 [DOI] [PubMed] [Google Scholar]
- 139.Bass WT. Periventricular Leukomalacia. NeoReviews. 2011;12(2):e76–e84. doi: 10.1542/neo.12-2-e76 [DOI] [Google Scholar]
- 140.Romantsik O, Bruschettini M, Ley D. Intraventricular Hemorrhage and White Matter Injury in Preclinical and Clinical Studies. NeoReviews. 2019;20(11):e636–e652. doi: 10.1542/neo.20-11-e636 [DOI] [PubMed] [Google Scholar]
- 141.BEAINO G, KHOSHNOOD B, KAMINSKI M, et al. Predictors of cerebral palsy in very preterm infants: the EPIPAGE prospective population-based cohort study. Developmental Medicine & Child Neurology. 2010;52(6):e119–e125. [DOI] [PubMed] [Google Scholar]
- 142.Ahn SY, Jie H, Jung WB, et al. Stem cell restores thalamocortical plasticity to rescue cognitive deficit in neonatal intraventricular hemorrhage. Exp Neurol. Aug 2021;342:113736. doi: 10.1016/j.expneurol.2021.113736 [DOI] [PubMed] [Google Scholar]
- 143.Jung SY, Kim YE, Park WS, et al. Thrombin Preconditioning Improves the Therapeutic Efficacy of Mesenchymal Stem Cells in Severe Intraventricular Hemorrhage Induced Neonatal Rats. Int J Mol Sci. Apr 18 2022;23(8)doi: 10.3390/ijms23084447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ahn SY, Chang YS, Sung SI, Park WS. Mesenchymal Stem Cells for Severe Intraventricular Hemorrhage in Preterm Infants: Phase I Dose-Escalation Clinical Trial. Stem Cells Transl Med. Dec 2018;7(12):847–856. doi: 10.1002/sctm.17-0219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sun JM, Grant GA, McLaughlin C, et al. Repeated autologous umbilical cord blood infusions are feasible and had no acute safety issues in young babies with congenital hydrocephalus. Pediatr Res. Dec 2015;78(6):712–6. doi: 10.1038/pr.2015.161 [DOI] [PubMed] [Google Scholar]
- 146.Malhotra A, Novak I, Miller SL, Jenkin G. Autologous transplantation of umbilical cord blood-derived cells in extreme preterm infants: protocol for a safety and feasibility study. BMJ Open. May 11 2020;10(5):e036065. doi: 10.1136/bmjopen-2019-036065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. Jan 20 2011;364(3):255–64. doi: 10.1056/NEJMra1005408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mutanen A, Pierro A, Zani A. Perioperative Complications Following Surgery for Necrotizing Enterocolitis. Eur J Pediatr Surg. Apr 2018;28(2):148–151. doi: 10.1055/s-0038-1636943 [DOI] [PubMed] [Google Scholar]
- 149.Biouss G, Antounians L, Li B, et al. Experimental necrotizing enterocolitis induces neuroinflammation in the neonatal brain. J Neuroinflammation. May 10 2019;16(1):97. doi: 10.1186/s12974-019-1481-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Denning TL, Bhatia AM, Kane AF, Patel RM, Denning PW. Pathogenesis of NEC: Role of the innate and adaptive immune response. Semin Perinatol. Feb 2017;41(1):15–28. doi: 10.1053/j.semperi.2016.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Clark RH, Gordon P, Walker WM, Laughon M, Smith PB, Spitzer AR. Characteristics of patients who die of necrotizing enterocolitis. J Perinatol. Mar 2012;32(3):199–204. doi: 10.1038/jp.2011.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rich BS, Dolgin SE. Necrotizing Enterocolitis. Pediatrics In Review. 2017;38(12):552–559. doi: 10.1542/pir.2017-0002 [DOI] [PubMed] [Google Scholar]
- 153.Tayman C, Uckan D, Kilic E, et al. Mesenchymal stem cell therapy in necrotizing enterocolitis: a rat study. Pediatr Res. Nov 2011;70(5):489–94. doi: 10.1203/PDR.0b013e31822d7ef2 [DOI] [PubMed] [Google Scholar]
- 154.Yang J, Watkins D, Chen CL, Bhushan B, Zhou Y, Besner GE. Heparin-binding epidermal growth factor-like growth factor and mesenchymal stem cells act synergistically to prevent experimental necrotizing enterocolitis. J Am Coll Surg. Oct 2012;215(4):534–45. doi: 10.1016/j.jamcollsurg.2012.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zani A, Cananzi M, Fascetti-Leon F, et al. Amniotic fluid stem cells improve survival and enhance repair of damaged intestine in necrotising enterocolitis via a COX-2 dependent mechanism. Gut. Feb 2014;63(2):300–9. doi: 10.1136/gutjnl-2012-303735 [DOI] [PubMed] [Google Scholar]
- 156.Zani A, Cananzi M, Lauriti G, et al. Amniotic fluid stem cells prevent development of ascites in a neonatal rat model of necrotizing enterocolitis. Eur J Pediatr Surg. Feb 2014;24(1):57–60. doi: 10.1055/s-0033-1350059 [DOI] [PubMed] [Google Scholar]
- 157.McCulloh CJ, Olson JK, Wang Y, Vu J, Gartner S, Besner GE. Evaluating the efficacy of different types of stem cells in preserving gut barrier function in necrotizing enterocolitis. J Surg Res. Jun 15 2017;214:278–285. doi: 10.1016/j.jss.2017.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Drucker NA, Te Winkel JP, Shelley WC, Olson KR, Markel TA. Inhibiting hydrogen sulfide production in umbilical stem cells reduces their protective effects during experimental necrotizing enterocolitis. J Pediatr Surg. Jun 2019;54(6):1168–1173. doi: 10.1016/j.jpedsurg.2019.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chen H, Zhang H, Zheng Y, et al. Prolyl hydroxylase 2 silencing enhances the paracrine effects of mesenchymal stem cells on necrotizing enterocolitis in an NF-kappaB-dependent mechanism. Cell Death Dis. Mar 16 2020;11(3):188. doi: 10.1038/s41419-020-2378-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li B, Lee C, Cadete M, et al. Amniotic fluid stem cell administration can prevent epithelial injury from necrotizing enterocolitis. Pediatr Res. Jan 2022;91(1):101–106. doi: 10.1038/s41390-021-01657-6 [DOI] [PubMed] [Google Scholar]
- 161.Zeng R, Wang J, Zhuo Z, Luo Y, Sha W, Chen H. Stem cells and exosomes: promising candidates for necrotizing enterocolitis therapy. Stem Cell Res Ther. Jun 5 2021;12(1):323. doi: 10.1186/s13287-021-02389-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhou Y, Yang J, Watkins DJ, et al. Enteric nervous system abnormalities are present in human necrotizing enterocolitis: potential neurotransplantation therapy. Stem Cell Res Ther. 2013;4(6):157. doi: 10.1186/scrt387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wei J, Zhou Y, Besner GE. Heparin-binding EGF-like growth factor and enteric neural stem cell transplantation in the prevention of experimental necrotizing enterocolitis in mice. Pediatr Res. Jul 2015;78(1):29–37. doi: 10.1038/pr.2015.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhou Y, Wang Y, Olson J, Yang J, Besner GE. Heparin-binding EGF-like growth factor promotes neuronal nitric oxide synthase expression and protects the enteric nervous system after necrotizing enterocolitis. Pediatr Res. Sep 2017;82(3):490–500. doi: 10.1038/pr.2017.68 [DOI] [PubMed] [Google Scholar]
- 165.McCulloh CJ, Olson JK, Zhou Y, Wang Y, Besner GE. Stem cells and necrotizing enterocolitis: A direct comparison of the efficacy of multiple types of stem cells. J Pediatr Surg. Jun 2017;52(6):999–1005. doi: 10.1016/j.jpedsurg.2017.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rager TM, Olson JK, Zhou Y, Wang Y, Besner GE. Exosomes secreted from bone marrow-derived mesenchymal stem cells protect the intestines from experimental necrotizing enterocolitis. J Pediatr Surg. Jun 2016;51(6):942–7. doi: 10.1016/j.jpedsurg.2016.02.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.McCulloh CJ, Olson JK, Wang Y, et al. Treatment of experimental necrotizing enterocolitis with stem cell-derived exosomes. J Pediatr Surg. Jun 2018;53(6):1215–1220. doi: 10.1016/j.jpedsurg.2018.02.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.O'Connell JS, Lee C, Farhat N, et al. Administration of extracellular vesicles derived from human amniotic fluid stem cells: a new treatment for necrotizing enterocolitis. Pediatr Surg Int. Mar 2021;37(3):301–309. doi: 10.1007/s00383-020-04826-6 [DOI] [PubMed] [Google Scholar]
- 169.O'Connell JS, Li B, Zito A, et al. Treatment of necrotizing enterocolitis by conditioned medium derived from human amniotic fluid stem cells. PLoS One. 2021;16(12):e0260522. doi: 10.1371/journal.pone.0260522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Akdmnan H, Dilli D, Ergun E, et al. Successful Mesenchymal Stem Cell Application in Supraventricular Tachycardia-Related Necrotizing Enterocolitis: A Case Report. Fetal Pediatr Pathol. Jun 2021;40(3):250–255. doi: 10.1080/15513815.2019.1693672 [DOI] [PubMed] [Google Scholar]
- 171.Blencowe H, Lawn JE, Vazquez T, Fielder A, Gilbert C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res. Dec 2013;74 Suppl 1:35–49. doi: 10.1038/pr.2013.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Darlow BA, Gilbert C. Retinopathy of prematurity - A world update. Semin Perinatol. Oct 2019;43(6):315–316 doi: 10.1053/j.semperi.2019.05.001 [DOI] [PubMed] [Google Scholar]
- 173.Rothschild MI, Russ R, Brennan KA, et al. The Economic Model of Retinopathy of Prematurity (EcROP) Screening and Treatment: Mexico and the United States. Am J Ophthalmol. Aug 2016;168:110–121. doi: 10.1016/j.ajo.2016.04.014 [DOI] [PubMed] [Google Scholar]
- 174.Hellstrom A, Hard AL. Screening and novel therapies for retinopathy of prematurity - A review. Early Hum Dev. Nov 2019;138:104846. doi: 10.1016/j.earlhumdev.2019.104846 [DOI] [PubMed] [Google Scholar]
- 175.Hartnett ME, Penn JS. Mechanisms and management of retinopathy of prematurity. N Engl J Med. Dec 27 2012;367(26):2515–26. doi: 10.1056/NEJMra1208129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Higgins RD. Oxygen Saturation and Retinopathy of Prematurity. Clin Perinatol. Sep 2019;46(3):593–599. doi: 10.1016/j.clp.2019.05.008 [DOI] [PubMed] [Google Scholar]
- 177.Bancalari A, Schade R. Update in the Treatment of Retinopathy of Prematurity. Am J Perinatol. Jan 2022;39(1):22–30. doi: 10.1055/s-0040-1713181 [DOI] [PubMed] [Google Scholar]
- 178.VanderVeen DK, Cataltepe SU. Anti-vascular endothelial growth factor intravitreal therapy for retinopathy of prematurity. Semin Perinatol. Oct 2019;43(6):375–380. doi: 10.1053/j.semperi.2019.05.011 [DOI] [PubMed] [Google Scholar]
- 179.Kim KS, Park JM, Kong T, et al. Retinal Angiogenesis Effects of TGF-β1 and Paracrine Factors Secreted From Human Placental Stem Cells in Response to a Pathological Environment. Cell Transplant. 2016;25(6):1145–57. doi: 10.3727/096368915x688263 [DOI] [PubMed] [Google Scholar]
- 180.Mathew B, Ravindran S, Liu X, et al. Mesenchymal stem cell-derived extracellular vesicles and retinal ischemia-reperfusion. Biomaterials. Mar 2019;197:146–160. doi: 10.1016/j.biomaterials.2019.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sun C, Groom KM, Oyston C, Chamley LW, Clark AR, James JL. The placenta in fetal growth restriction: What is going wrong? Placenta. Jul 2020;96:10–18. doi: 10.1016/j.placenta.2020.05.003 [DOI] [PubMed] [Google Scholar]
- 182.Chappell LC, Cluver CA, Kingdom J, Tong S. Pre-eclampsia. Lancet. Jul 24 2021;398(10297):341–354. doi: 10.1016/S0140-6736(20)32335-7 [DOI] [PubMed] [Google Scholar]
- 183.Jain VG, Willis KA, Jobe A, Ambalavanan N. Chorioamnionitis and neonatal outcomes. Pediatr Res. Jan 2022;91(2):289–296. doi: 10.1038/s41390-021-01633-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. Jan 5 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. May 18 2000;342(20):1500–7. doi: 10.1056/NEJM200005183422007 [DOI] [PubMed] [Google Scholar]
- 186.DiGiulio DB, Romero R, Amogan HP, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. Aug 26 2008;3(8):e3056. doi: 10.1371/journal.pone.0003056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Xiong Z-H, Wei J, Lu M-Q, Jin M-Y, Geng H-L. Protective effect of human umbilical cord mesenchymal stem cell exosomes on preserving the morphology and angiogenesis of placenta in rats with preeclampsia. Biomedicine & Pharmacotherapy. 2018/September/01/ 2018;105:1240–1247. [DOI] [PubMed] [Google Scholar]
- 188.Wang D, Na Q, Song GY, Wang L. Human umbilical cord mesenchymal stem cell-derived exosome-mediated transfer of microRNA-133b boosts trophoblast cell proliferation, migration and invasion in preeclampsia by restricting SGK1. Cell Cycle. 2020/August/02 2020;19(15):1869–1883. doi: 10.1080/15384101.2020.1769394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Taglauer ES, Fernandez-Gonzalez A, Willis GR, et al. Mesenchymal stromal cell-derived extracellular vesicle therapy prevents preeclamptic physiology through intrauterine immunomodulation†. Biol Reprod. Feb 11 2021;104(2):457–467. doi: 10.1093/biolre/ioaa198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Taglauer ES, Fernandez-Gonzalez A, Willis GR, et al. Antenatal Mesenchymal Stromal Cell Extracellular Vesicle Therapy Prevents Preeclamptic Lung Injury in Mice. Am J Respir Cell Mol Biol. Jan 2022;66(1):86–95. doi: 10.1165/rcmb.2021-0307OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Abele AN, Taglauer ES, Almeda M, et al. Antenatal mesenchymal stromal cell extracellular vesicle treatment preserves lung development in a model of bronchopulmonary dysplasia due to chorioamnionitis. Am J Physiol Lung Cell Mol Physiol. Feb 1 2022;322(2):L179–l190. doi: 10.1152/ajplung.00329.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ban JJ, Lee M, Im W, Kim M. Low pH increases the yield of exosome isolation. Biochem Biophys Res Commun. May 22 2015;461(1):76–9. doi: 10.1016/j.bbrc.2015.03.172 [DOI] [PubMed] [Google Scholar]
- 193.Shao C, Yang F, Miao S, et al. Role of hypoxia-induced exosomes in tumor biology. Mol Cancer. Aug 11 2018;17(1):120. doi: 10.1186/s12943-018-0869-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wiklander OPB, Brennan MA, Lotvall J, Breakefield XO, El Andaloussi S. Advances in therapeutic applications of extracellular vesicles. Sci Transl Med. May 15 2019;11(492)doi: 10.1126/scitranslmed.aav8521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Guess AJ, Daneault B, Wang R, et al. Safety Profile of Good Manufacturing Practice Manufactured Interferon gamma-Primed Mesenchymal Stem/Stromal Cells for Clinical Trials. Stem Cells Transl Med. Oct 2017;6(10):1868–1879. doi: 10.1002/sctm.16-0485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Witwer KW, Goberdhan DC, O'Driscoll L, et al. Updating MISEV: Evolving the minimal requirements for studies of extracellular vesicles. J Extracell Vesicles. Dec 2021;10(14):e12182. doi: 10.1002/jev2.12182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wang Y, Long W, Cao Y, Li J, You L, Fan Y. Mesenchymal stem cell-derived secretomes for therapeutic potential of premature infant diseases. Biosci Rep. May 29 2020;40(5)doi: 10.1042/BSR20200241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. May 2006;24(5):1294–301. doi: 10.1634/stemcells.2005-0342 [DOI] [PubMed] [Google Scholar]
- 199.Amable PR, Teixeira MV, Carias RB, Granjeiro JM, Borojevic R. Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton's jelly. Stem Cell Res Ther. Apr 16 2014;5(2):53. doi: 10.1186/scrt442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hass R, Kasper C, Bohm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. May 14 2011;9:12. doi: 10.1186/1478-811X-9-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Gelibter S, Marostica G, Mandelli A, et al. The impact of storage on extracellular vesicles: A systematic study. J Extracell Vesicles. Feb 2022;11(2):e12162. doi: 10.1002/jev2.12162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lener T, Gimona M, Aigner L, et al. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J Extracell Vesicles. 2015;4:30087. doi: 10.3402/jev.v4.30087 [DOI] [PMC free article] [PubMed] [Google Scholar]