Abstract
Reproduction is the biological process by which new individuals are produced by their parents. It is the fundamental feature of all known life and is required for the existence of all species. All mammals reproduce sexually, a process that involves the union of two reproductive cells, one from a male and one from a female. Sexual behaviors are a series of actions leading to reproduction. They are composed of appetitive, action, and refractory phases, each supported by dedicated developmentally-wired neural circuits to ensure high reproduction success. In rodents, successful reproduction can only occur during female ovulation. Thus, female sexual behavior is tightly coupled with ovarian activity, namely the estrous cycle. This is achieved through the close interaction between the female sexual behavior circuit and the hypothalamic-pituitary-gonadal (HPG) axis. In this review, we will summarize our current understanding, learned mainly in rodents, regarding the neural circuits underlying each phase of the female sexual behaviors and their interaction with the HPG axis, highlighting the gaps in our knowledge that require future investigation.
Introduction
Reproduction is crucial for all species. An efficient, robust, and well-timed reproduction strategy ensures the existence and prevalence of a species from generation to generation. Given that productive copulation requires fertilization in mammals, many species, including rodents, employ a peri-ovulation mating strategy; that is, females become only sexually receptive when mature eggs are released from the ovary into the oviduct, a period known as estrus (Gutierrez-Castellanos et al., 2022; Inoue, 2022). After a male ejaculates, for one time or several times depending on the species, both the male and female enter a refractory period with low interest in the opposite sex (Bermant, 1961; Seizert, 2018; Yang and Clemens, 1996; Yin et al., 2022). Meanwhile, the fertilized eggs undergo many cell divisions to form blastocysts, and sex hormones prepare the uterus for accepting blastocysts for implantation (Wang and Dey, 2006). Thus, female reproduction in rodents is an intricate collaboration between the behavior circuit and ovarian activity, which is under the tight control of the hypothalamic-pituitary–gonadal (HPG) axis. Notably, there are two other types of female mating strategies in mammals that do not require simultaneous ovulation, including mating-induced ovulation (e.g. rabbits and cats) and ovulation-decoupled mating (e.g. humans) (Bakker and Baum, 2000; Brenner and West, 1975). In this review, we will focus on the peri-ovulation mating in rodents, summarizing our current understanding regarding the neural circuits controlling various phases of female sexual behaviors and their interaction with the HPG axis.
The appetitive phase of female sexual behaviors
Female sexual behaviors comprise three phases: appetitive, action (consummatory), and refractory Figure 1) (Beach, 1976; Everitt, 1995; Gutierrez-Castellanos et al., 2022; Micevych and Meisel, 2017). The appetitive phase can be further divided into detection, approach, and investigation (Wei et al., 2021). During detection, the female identifies the unique sensory cues emitted by a distant male. During approach, the female moves towards the male to establish physical contact. During investigation, the female closely explores the male to determine its identity and perhaps acquire certain social and health-related information. The latency to approach and the time spent investigating could reflect the female’s interest in the male and its readiness to engage in sexual behaviors, i.e. sexual motivation. While in the wild, the appetitive phase is likely essential for successful reproduction as copulation can only occur when two animals are physically close, this phase is minimal in the standard laboratory mating assays. Indeed, when the male and female are placed in a shoe-box-sized cage, their chance of encountering is high. Nevertheless, the approach frequency and investigation duration still reflect females’ sexual interest in males (Yin et al., 2022).
Figure 1.
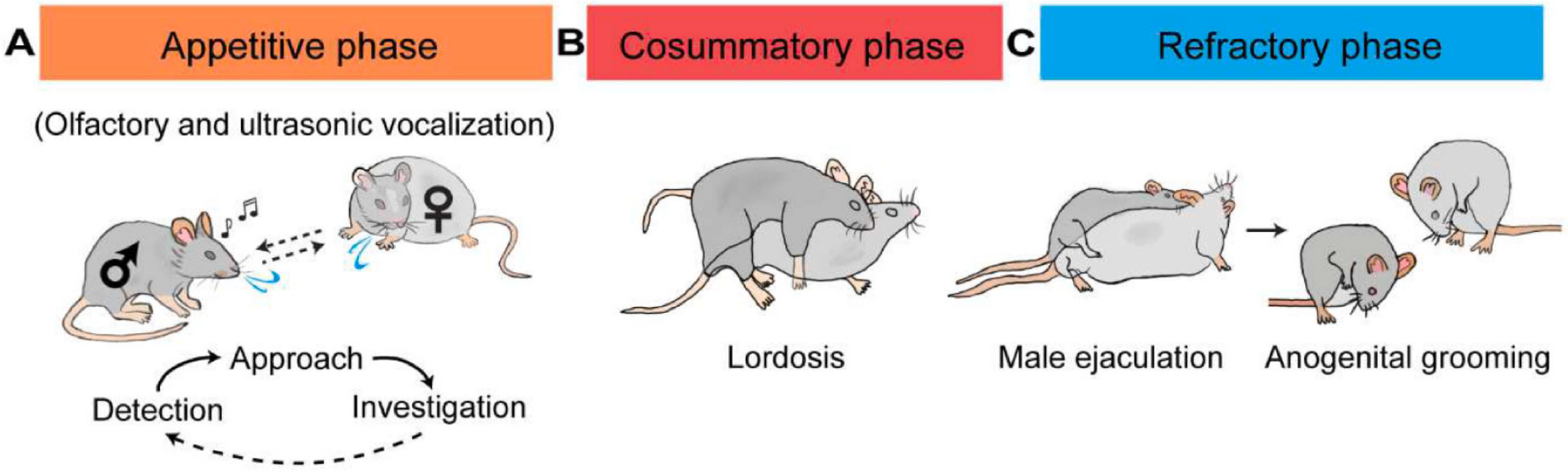
Three phases of female sexual behaviors in rodents. (A) The appetitive phase includes detection, approach, and investigation. These three actions occur in loops until the consummatory phase begins. (B) During the consummatory phase, the male initiates mounting, positioning itself on top of the female while holding the female’s flanks with its front paws. If the female is receptive, it stays stationary with its back arching downward, a posture known as lordosis. (C) After the male ejaculates, both males and females show reduced sexual interest and spend most time grooming and licking themselves. Dark gray: male; light gray: female.
Several behavioral paradigms were designed to specifically determine the role of brain regions in detection, approach, and investigation. In the habituation-dishabituation test, the females are presented with urine from a conspecific 3–4 times, followed by urine from a different conspecific (Figure 2A)(Baum and Keverne, 2002). As the female becomes increasingly familiar with the same urine, it decreases investigation time. When unfamiliar urine is presented, the investigation time increases. This assay is robust and straightforward. Importantly, it can reveal deficits in detection and discrimination independent of sexual interest. Social preference test is commonly used for determining changes in sexual interest (Figure 2B, C) (Keller et al., 2008). Intact male odor, e.g. urine, and castrated male odor (or female odor) are each placed at one end of a Y maze or a three-compartment arena, and females are allowed to explore the maze freely. The relative time the female spent on investigating each odor is then measured to determine its sexual interest. Females with higher sexual interest is expected to prefer the intact male cue over castrated or female cues. A more sophisticated nose entry test is also used to assess interest/motivation towards the male cues (Figure 2D) (Demir et al., 2020). In the assay, females are placed in a chamber with two ports that contain different social cues. The frequency of port entry reflects the female’s interest in a cue. Thus, females with high sexual interests are expected to poke the male cue port more frequently. However, the social preference test and nose port test could not distinguish a deficit in detection vs. interest as they are both manifested as a reduced number of poking. Thus, they should be used in combination with the habituation-dishabituation test to determine the exact cause of a deficit (Keller et al., 2008). Lastly, paced mating test (Figure 2E) is often used to assess female sexual motivation during copulation, which is typically dominated by the males (Johansen et al., 2008; Paredes and Vazquez, 1999). In this assay, the test chamber is divided by a partition, and the female can enter or exit the side where the male is confined. The frequency and duration of visits are used to measure the motivational state of the female. Females with high sexual interests are expected to visit the male chamber more frequently.
Figure 2.
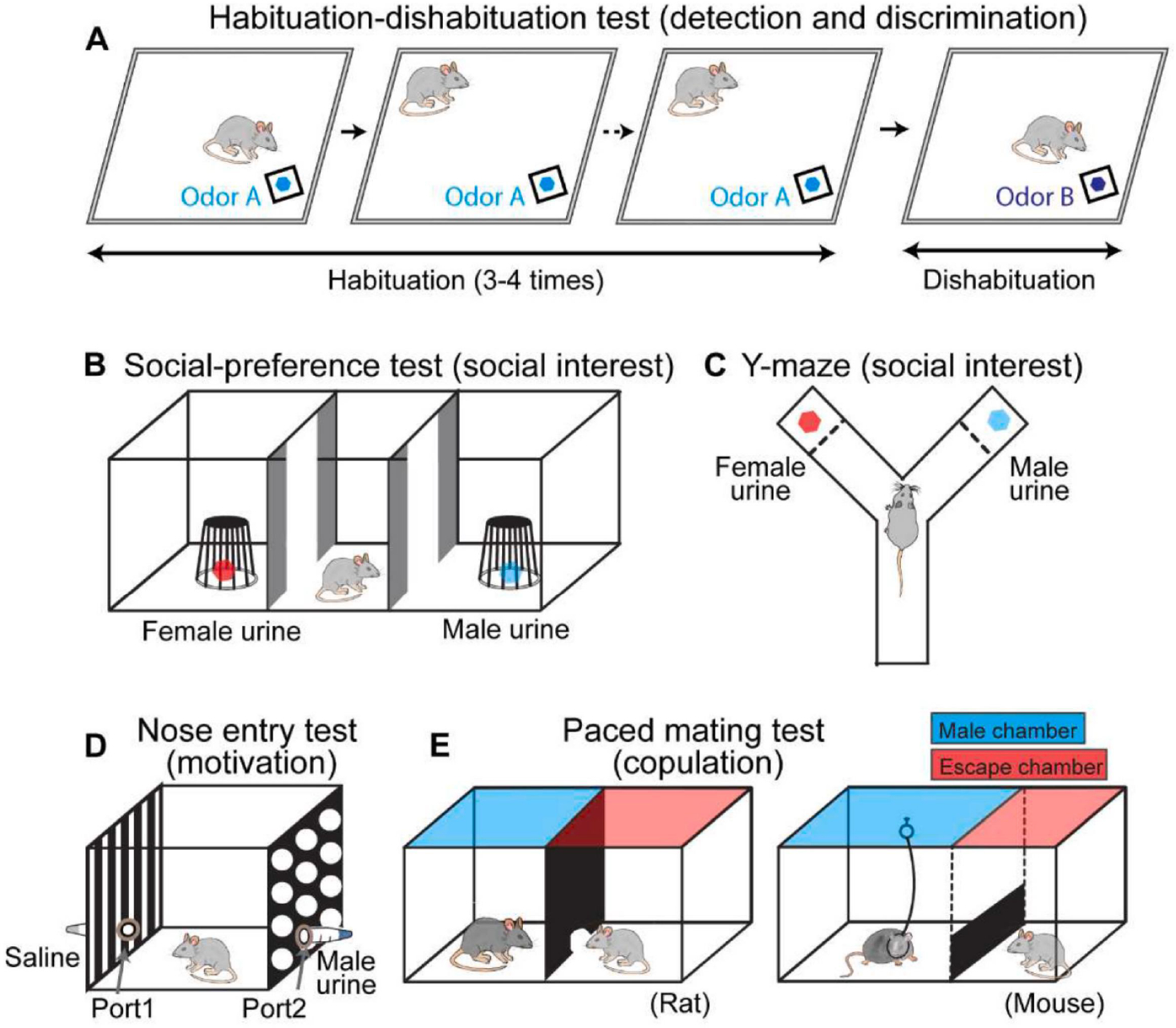
Behavioral paradigms to assess social odor detection and sexual interest. (A) Habituation-dishabituation test to measure social odor detection and discrimination. The test animals are presented with urine from a conspecific 3–4 times, followed by urine from a different conspecific. (B, C) Three-chamber (B) and Y-Maze (C) social preference tests to assess animals’ social interest. Each end chamber/arm contains one type of odor source or a conspecific. (D) Nose entry test to examine females’ interest in a specific odor. Each tube attached to the nose port contains an odor source, e.g., urine. (E) Paced mating test to measure females’ sexual motivation. (Left) For rats, the test arena is divided into a ‘male’ chamber and an ‘escape’ chamber by a barrier with a small hole that females, but not males, can fit through. (Right) For mice, the male is tethered to the “male” chamber while the female could easily jump through the barrier to enter the escape the “male chamber”.
Neural substrates essential for the appetitive phase of female sexual behaviors
Rodents are nocturnal and rely heavily on olfaction. Thus, volatiles emitted by distant males are essential for detection, although other sensory modalities, such as ultrasound vocalization, likely also contribute (Asaba et al., 2014). The main olfactory system is responsible for sensing and processing volatiles (Figure 3). Airborne chemicals first bind to the olfactory receptors in the olfactory sensory neurons (OSNs) of the main olfactory epithelium (MOE), then the information passes to the main olfactory bulb (MOB) and reaches multiple brain regions, including the anterior olfactory nucleus (AON), olfactory tubercle (OT), piriform cortex (Pir), cortical amygdala (anterior and posterolateral part; CoAa and CoApl, respectively), and entorhinal cortex (EC) (Spehr et al., 2006). Genetic ablation or ZnSO4-induced MOE lesion severely impaired females’ performance in the habitation-dishabituation test as evidenced by the low investigation time to both familiar and unfamiliar urine samples (Keller et al., 2006a; Ma et al., 2002).
Figure 3.
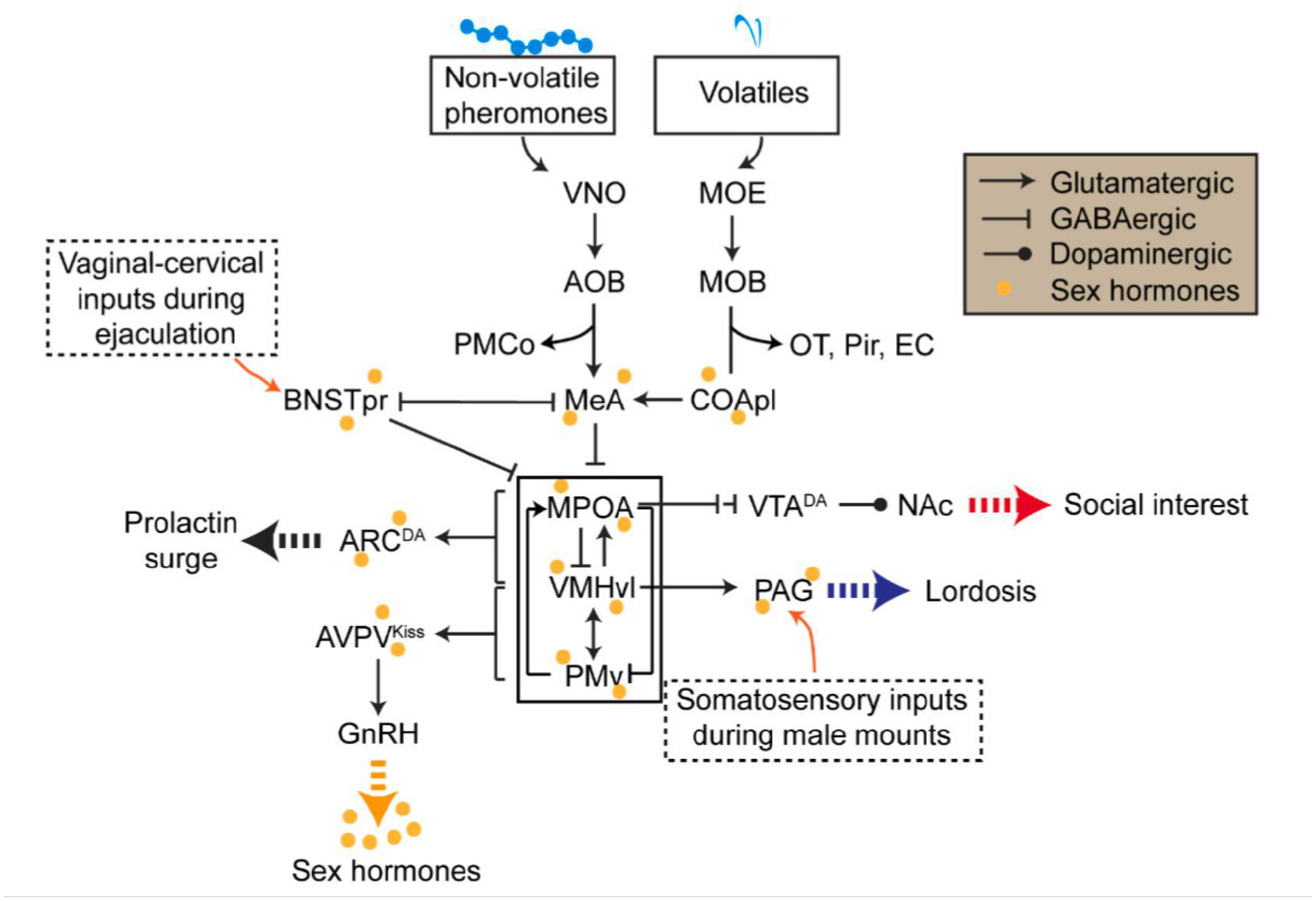
A hypothesized model describing the neural circuits underlying various aspects of female sexual behaviors. During the appetitive phase of female sexual behaviors, male-related volatiles are detected by the MOE-MOB-CoApl circuit and converge with the non-volatile cues detected via VNO-AOB at the MeA. These chemosensory cues then evoke dopamine release at the NAc through the MPOA-VTA-NAc pathway to mediate the female’s high interest in the male. During the consummatory phase, male mounting associated somatosensory inputs activate the PAG to evoke the lordosis reflex. PAG is gated by the VMHvl, which is under the strong influence of sex hormones and male chemosensory inputs. PMv facilitates lordosis and MPOA inhibits lordosis by providing excitatory and inhibitory inputs to the VMHvl, respectively. When the male ejaculates, responses of the VMHvl and MPOA trigger prolactin surges essential for uterus development via ARCDA cells. Meanwhile, ejaculation causes a strong activation of BNSTpr cells, inhibiting the VMHvl and reducing the sexual interest. The mating circuit also interacts with the HPG axis extensively. VMHvl and PMv provide excitatory inputs to AVPVKiss cells to facilitate GnRH surge during proestrus. In turn, sex hormones released from the HPG axis modulate the overall state of the mating circuit. MOE: main olfactory epithelium; MOB: main olfactory bulb; OT: olfactory tubercle; Pir: piriform cortex; EC: entorhinal cortex; CoApl: posterolateral part of cortical amygdala; VNO: vomeronasal organ; AOB: accessory olfactory bulb; MeA: medial amygdala; PMCo: posteromedial cortical amygdala; BNSTpr: principal nucleus of bed nucleus of the stria terminalis; MPOA: medial preoptic area; VMHvl: ventrolateral part of the ventromedial hypothalamus; PMv: ventral part of premammillary nucleus; VTADA: ventral tegmental area dopaminergic cells; NAc: nucleus accumbens; PAG: periaqueductal gray; ARCDA: arcuate nucleus dopaminergic cells; AVPVKiss: anteroventral periventricular nucleus kisspeptin cells; GnRH: gonadotropin-releasing hormone.
In contrast to the MOE ablation, lesioning the vomeronasal organ (VNO), the peripheral organ to sense non-volatile pheromones, did not affect the detection or discrimination of conspecific volatiles. However, VNO lesioned animals (VNOx) showed a generally low level of investigation of non-volatiles in urine or soiled bedding and no preference for non-volatiles from intact male urine over castrated male urine (Keller et al., 2006b). Thus, non-volatile pheromones, detected by the VNO, are essential for sustaining the interest of the female upon contact. VNO sends the pheromone signal to the accessory olfactory bulb (AOB), which in turn passes the information to the medial amygdala (MeA), posteromedial cortical amygdala (PMCo), and the principal nucleus of bed nucleus of the stria terminalis (BNSTpr)(Spehr et al., 2006). As key relays of the accessory olfactory pathways, both AOB and MeA are essential for females’ interest in male pheromones. For example, Darcin is a male-specific major urinary protein (MUP) that can strongly induce female’s interest, revealed by poking in the nose entry test (Figure 2D)(Demir et al., 2020). When AOB was suppressed optogenetically, Darcin-induced interest in female mice was blocked entirely. Furthermore, activating Darcin-responsive MeA cells increased female’s port entry while inhibiting MeA abolished female’s attraction to Darcin, suggesting the AOB-MeA pathway is essential for mediating female’s innate interest in male pheromones (Demir et al., 2020). In addition to non-volatile cues, volatile cues converge onto the MeA through CoApl (Martinez-Marcos, 2009). Lesion of MeA, especially its posterior subdivision (MeAp), also blocked the preference of female mice for male volatiles without affecting her ability to detect and discriminate the male odor from castrated male odor in the habituation-dishabituation test (DiBenedictis et al., 2012). Thus, the innate preference for male cues, regardless of volatiles or non-volatiles, requires intact MeA in females.
From the MeA, the converged male chemosensory cues are sent to the medial hypothalamus (MH), either directly or via the BNSTpr (Spehr et al., 2006) (Figure 3). However, BNSTpr-routed information appears to be non-essential as lesioning the area does not affect female’s preference for the male urine in a two-choice investigation test in hamsters (Martinez and Petrulis, 2011). It is worth noting that BNSTpr function in processing social cues is sexually dimorphic. For example, aromatase-expressing cells in BNSTpr in male mice respond differentially to male and female cues, whereas the same cells in females do not respond to intruder of either sex (Bayless et al., 2019).
At the MH, the medial preoptic area (MPOA), the ventrolateral part of the ventromedial hypothalamus (VMHvl), and the ventral part of premammillary nucleus (PMv) are the main targets of MeAp and BNSTpr (Figure 3). Chemogenetic inhibition of VMHvl cells expressing Cckar (VMHvlCckar) abolished the preference of estrous females in males, whereas activating the cells enhanced the preference for males in diestrous females (Knoedler et al., 2022; Yin et al., 2022). MPOA is also essential for female sexual interest. Deficits in MPOA caused by lesion, optogenetic inactivation, or estrogen receptor alpha (Esr1) knockdown all diminished female’s preference for intact male odor over castrated male or female odor (Guarraci and Clark, 2006; Martinez and Petrulis, 2013; McHenry et al., 2017; Spiteri et al., 2012a; Xiao et al., 2005). Additionally, in paced mating test, the MPOA lesion in rats consistently reduced the female’s voluntary interaction with the males (Guarraci et al., 2004; Xiao et al., 2005; Yang and Clements, 2000). Given the strong excitatory projections of PMv cells to both MPOA and VMHvl and the high responsivity of PMv cells to conspecific olfactory cues, PMv likely also play a positive role in sexual interest in females, although experimental evidence is still lacking (Canteras et al., 1992; Motta et al., 2013; Soden et al., 2016).
From the MH, the projection to the ventral tegmental area (VTA) is likely essential for driving the high interest towards the males (Figure 3). Optogenetic activation of the projection from MPOA neurotensin cells to VTA enhanced the investigation of males in female mice (McHenry et al., 2017). VTA contains abundant dopamine neurons, which are likely disinhibited by MPOA GABAergic inputs to VTA GABAergic cells (Fang et al., 2018). Indeed, Optogenetic activation of MPOA neurotensin cell projection to VTA caused dopamine release in the nucleus accumbens (NAc), the primary target of VTA (McHenry et al., 2017). The VTA-NAc dopamine release is causally linked to social interest. Optogenetic activation of VTA dopamine cells or their terminals in the NAc strongly increased social interest in mice, whereas inhibiting VTA dopamine cells reduced social interest (Dai et al., 2022; Gunaydin et al., 2014).
Altogether, current knowledge suggests a vital role of the main olfactory system in detecting and locating male cues. Once the females and males are in close contact, non-volatile pheromones induce dopamine release in the NAc through the VNO-AOB-MeA-(MPOA, VMHvl, PMv)-VTA-NAc circuit to maintain the high interest towards the males (Figure 3). In addition, male volatiles could also contribute to the sustained interest by activating MeA through CoApl. Importantly, the same male cue does not evoke the same level of interest from the female every time. Rather, the behavior output is strongly modulated by the female’s internal state, which is determined collectively by sex hormone level, experience, age, metabolic state and health condition etc. Sex hormones, which fluctuate across the estrus cycle, are particularly important for modulating females’ interest in males. Indeed, the entire circuit mentioned above expresses abundant sex hormone receptors (Mitra et al., 2003). As a result, the circuit’s response and hence the female’s interest in the male wanes and waxes across the estrous cycle (Gutierrez-Castellanos et al., 2022) (Figure 3).
The action phase of female sexual behaviors
The action phase of female sexual behaviors is remarkably simple in its motor pattern: the female stays stationary with her back arching downward, a posture known as lordosis (Pfaff et al., 2008). This posture exposes the vagina to facilitate penile insertion. Lordosis quotient is the percentage of trials the female expresses lordosis when being mounted by a male. It is a common measure of the sexual receptivity of a female. As males’ mounting success tightly links to females’ behavior, the likelihood of males advancing sexual behaviors, e.g. the ratio between mount and mount attempt and that between intromission and mount, could also be used as a readout of female’s receptivity level (Yin et al., 2022). In mice, when the female’s receptivity is low, instead of lordotic, it could actively reject the male’s mounting attempt by kicking, pushing, or flight. Females may also be semi-receptive: while they do not actively reject the male, they wiggle and twist when being mounted (Yin et al., 2022). Manual flank-perineum stimulation (palpation) that mimics male action during mounting is sufficient to elicit lordosis in rats, suggesting the reflexive nature of lordosis (Kow et al., 1979). Notably, the tendency of females to show lordosis is strongly modulated by sex hormones (Jennings and de Lecea, 2020; Komisaruk, 1974; Micevych and Meisel, 2017; Pfaff, 1973), the presence of specific male pheromones (Haga et al., 2010), and vaginal-cervical stimulation (VCS) (Rodriguez-Sierra et al., 1975).
The neural substrates for lordosis
The VMH, especially the ventrolateral part where sex steroid receptors are densely expressed, is perhaps the best characterized brain area for regulating female sexual behaviors (Jennings and de Lecea, 2020; Kow and Pfaff, 1998; Lenschow and Lima, 2020; Micevych and Meisel, 2017; Pfaff et al., 2008). To date, VMHvl remains the only brain region that could rapidly increase female sexual receptivity upon artificial activation. D.W. Pfaff and colleagues first showed that prolonged electric stimulation of the VMH region could facilitate the lordosis response to manual somatosensory stimulation or male mounting in female rats (Pfaff and Sakuma, 1979). Conversely, lesions in the VMH suppressed the lordosis reflex in rats (Pfaff and Sakuma, 1979). Subsequent experiments showed that sex hormone implants in the VMH could facilitate lordosis (Rubin and Barfield, 1980, 1983a, b), while shRNA-mediated Esr1 knockdown had the opposite effect (Musatov et al., 2006). More recent studies focusing on molecularly defined VMHvl populations showed that VMHvl cells express progesterone receptor (VMHvlPR) is necessary for female sexual receptivity in mice (Inoue et al., 2019; Yang et al., 2013). However, activating VMHvlPR cells failed to facilitate lordosis (Inoue et al., 2019). This surprising negative result likely reflects the functional heterogeneity of the VMHvlPR cells. Our study revealed that VMHvlEsr1 cells in female mice, which overlap nearly 100% with VMHvlPR cells, contain two subdivisions: a medial subdivision activated during aggression and a lateral subdivision activated during female sexual behaviors (Hashikawa et al., 2017). These subdivisions show differential projection patterns and transcriptomic profiles, although they are both enriched of Esr1 and PR (Hashikawa et al., 2017; Kim et al., 2019; Knoedler et al., 2022). Liu et. al. recently found that VMHvl cells expressing Npy2r, a medial subdivision-biased gene, can bi-directionally modulate female mouse aggression, whereas Nyp2r−Esr1+ cells can modulate female sexual behaviors (Liu et al., 2022). Studies from our group and Knoedler et. al. further demonstrated that VMHvl cells expressing Cckar, a lateral subdivision-biased gene, are necessary for female sexual receptivity but not female aggression in mice (Knoedler et al., 2022; Yin et al., 2022). Furthermore, we found that chemogenetic and optogenetic activation of VMHvlCckar cells is sufficient to promote female sexual receptivity even in ovariectomized (OVX) female mice (Figure 4) (Yin et al., 2022). Thus, VMHvlCckar cell activity can bypass the sex hormone requirement to powerfully control female sexual receptivity. In other words, sex hormone is likely required because it plays an import role in activating VMHvlCckar cells. Artificial activation of VMHvlCckar cells fulfills this requirement and eliminates the need of sex hormones. Indeed, virtually all VMHvlCckar cells in female mice express Esr1 and VMHvlCckar cells show a high level of plasticity over estrus cycle (Yin et al., 2022). Specifically, in vivo activity, intrinsic excitability, and synaptic transmission of VMHvlCckar cells are high during estrus and low during diestrus (Yin et al., 2022). However, it is important to note that VMHvlCckar activity does not increase during acute expression of lordosis (Yin et al., 2022). When VMHvlCckar activity in female mice was artificially increased, it facilitated female receptivity but did not induce lordosis posture in the absence of male mounting attempt, suggesting that VMHvl does not control moment-to-moment lordosis reflex (Yin et al., 2022).
Figure 4.
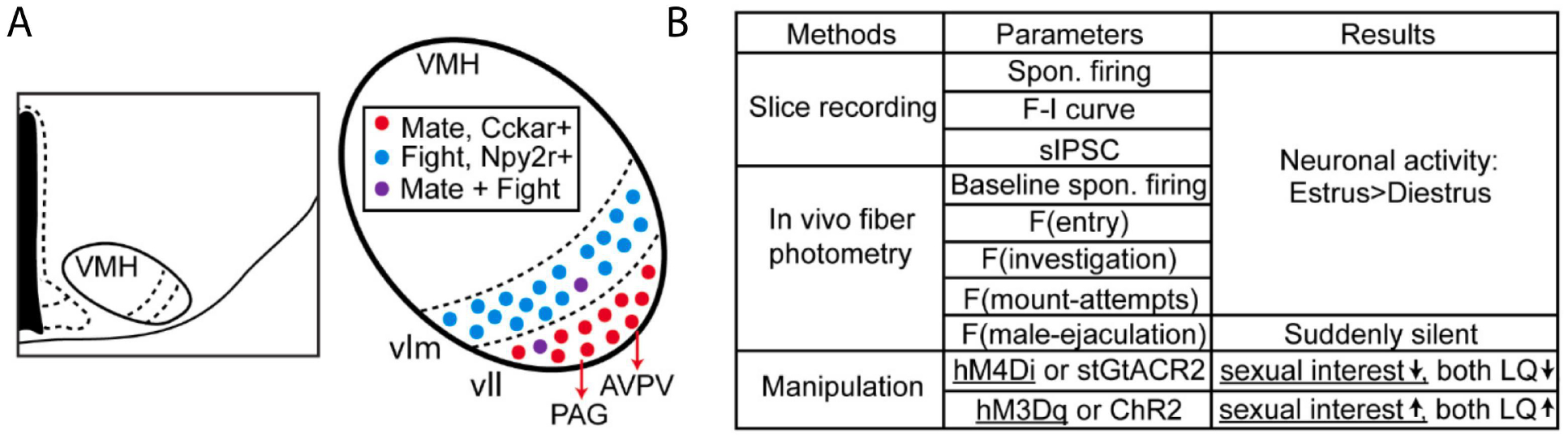
The role of VMHvllCckar cells in female sexual behaviors. (A) Diagram showing the location of VMH in the hypothalamus and its functionally and molecularly distinct subdivisions. Mating-related VMHvll cells may project to PAG and AVPV to control female lordosis and the HPG axis, respectively. (B) A summary of the in vivo and in vitro activity patterns of VMHvllCckar cells during estrus and diestrus and the behavior changes induced by VMHvllCckar manipulation (Yin et al., 2022). LQ: Lordosis quotient.
As mentioned earlier, VMHvl receives substantial inputs from MeA and BNSTpr, two key regions for processing male non-volatile and volatile cues (Figure 3). These chemosensory inputs are important for the full expression of lordosis, as any deficit along the VNO-AOB (or MOE-MOB) -MeA pathway impairs female sexual receptivity. For example, ablation of VNO or knocking out Trpc2, an ion channel essential for signal transduction of VNO cells, consistently reduced lordosis quotient in mice, rats, and hamsters (Curtis et al., 2001; Hellier et al., 2018; Keller et al., 2006a; Kimchi et al., 2007; Mackay-Sim and Rose, 1986; Oboti et al., 2014; Rajendren et al., 1990). Knocking out cyclic nucleotide-gated channel subunit alpha 2 (cnga2), an essential ion channel for OSNs in the MOE, impaired female sexual receptivity in mice (Mandiyan et al., 2005). Optogenetic silencing AOB neurons projecting to the MeA reduced the expression of lordosis in sexually experienced estrous female mice (McCarthy et al., 2017). Lesion, chemogenetic inhibition, and optogenetic inactivation of the MeA cells, especially GABAergic cells, all reduced lordosis (DiBenedictis et al., 2012; Johnson et al., 2021; McCarthy et al., 2017; Rajendren and Moss, 1993). Inhibiting MeA cells also suppressed the lordosis-promoting effect of Exocrine gland-secreting peptide 1 (ESP1), a male mouse pheromone (Ishii et al., 2017). In contrast to the critical role of MeA in promoting female receptivity, the role of BNSTpr appears to be very limited. Lesion, chemogenetic inactivation, or ablation of BNSTpr cells failed to impair female lordosis in mice and hamsters (Bayless et al., 2019; Ishii et al., 2017; Martinez and Petrulis, 2011), although a recent study showed that BNSTpr cells expressing st18 are active during lordosis in mice (Zhou et al., 2023).
Why is chemosensory input important for lordosis? It is not a direct trigger of the lordosis reflex as females do not show lordosis upon sniffing the males or male urine, nor is it necessary, as manual cutaneous stimulation could induce lordosis in the absence of any chemosensory cues. We consider the primary role of male pheromones is to boost the overall activity level of VMHvl cells. When the male is around, VMHvl cells in female mice not only acutely increase activity during each episode of close contact but also show a sustained baseline activity increase (Hashikawa et al., 2017; Nomoto and Lima, 2015; Yin et al., 2022). Both of these activity changes are likely mainly driven by chemosensory inputs as VMHvl cells respond strongly to male urine and minimally to auditory cues, e.g. ultrasound vocalization and all our recordings were performed under infrared light, allowing minimal visual inputs (Hashikawa et al., 2017; Yin et al., 2022). While the acute response during close social contact is likely mediated by fast synaptic transmission, we speculate that the sustained baseline activity change is mediated by some slow-acting molecules, such as neuropeptides or sex hormones. Consistent with this hypothesis, gonadotropin-releasing hormone (GnRH) gradually increased in the basal medial hypothalamus in females over the course of mating (Lin and Ramirez, 1991), and VNO lesion blunted such increase (Rajendren et al., 1990). The GnRH release is functionally relevant as supplementing GnRH to VNO-ablated females restored the female’s sexual receptivity in various rodent species (Keller et al., 2006b; Mackay-Sim and Rose, 1986; Rajendren et al., 1990). These results suggest a model that during male-female interactions, male pheromones activate the accessory olfactory system through VNO to increase GnRH release and potentially other related hormones, boosting VMHvlCckar cell activity and sexual receptivity (Figure 3). The pheromone-induced activity boost is likely sex hormone state dependent as we observed a higher elevation in baseline activity of VMHvlCckar cells in estrous than diestrous female mice after male presentation (Yin et al., 2022). Beyond the HPG axis, pheromone inputs may also induce neuromodulator release, such as dopamine, norepinephrine and serotonin to influence activities of cells related to lordosis (Gunaydin et al., 2014; Li et al., 2016; Vathy and Etgen, 1989).
Other VMHvl-connected medial hypothalamic regions, including PMv and MPOA, also play roles in lordosis. PMv plays a positive role as bilateral lesions of the PMv in female mice significantly reduced lordosis (Ross et al., 2018). Interestingly, MPOA is found consistently to suppress instead of promoting female receptivity. MPOA lesion or site-specific Esr1 knockdown increased lordosis quotient while electric stimulation of MPOA had the opposite effect (Hoshina et al., 1994; Malsbury et al., 1980; Powers and Valenstein, 1972; Spiteri et al., 2012b; Xiao et al., 2005). MPOA cells expressing μ-opioid receptor (MOR) could be particularly relevant for suppressing lordosis as MOR activation and internalization in the MPOA closely tracks the female receptivity level during the estrus cycle (Micevych and Sinchak, 2013). MPOA cells provide strong GABAergic inputs to the VMHvl, which could be an important pathway for suppressing female receptivity (Karigo et al., 2021). Whether the sexual motivation-promoting and lordosis-suppressing cells in the MPOA are distinct, as suggested by P.E. Micevych and R.L. Meisel, or identical, remains to be addressed in future studies (Micevych and Meisel, 2017).
The periaqueductal gray (PAG) has been proposed as an important downstream region of the VMHvl that controls hindbrain premotor neurons to drive lordosis (Shelley et al., 2006). Lesioning PAG reduced lordosis, while its electric stimulation increased the output of premotor neurons relevant to lordosis and facilitated the behavior in rats (Cottingham et al., 1987; Sakuma and Pfaff, 1979a, b). Anterograde tracing revealed moderate projection from VMHvlCckar to ventrolateral PAG in female mice (Yin et al., 2022), whereas polysynaptic retrograde tracing revealed indirect projection from PAG to lumbar epaxial muscles controlling lordosis (Daniels et al., 1999). Furthermore, electrophysiological recording from PAG cells in female rats revealed that approximately 40% of PAG cells were excited by cutaneous stimuli similar to those experienced in male mounting, and one-third of PAG cells were excited by VMH inputs after estrogen supplement (Sakuma and Pfaff, 1980). Thus, the somatosensory and VMHvl inputs appear to converge onto PAG cells that control the lordosis-producing muscles. Consistent with this idea, Vaughn et. al. recently characterized the molecular profiles of PAG cells relevant for various instinctive behaviors in mice, including female sexual behaviors, using single nucleus RNA sequencing (snRNA-seq) and Multiplexed Error-Robust Fluorescence in Situ Hybridization (MERFISH)(Vaughn et al., 2022). They found that female sexual behaviors mainly activate lateral and ventral part of the caudal PAG, which interestingly is the part preferentially targeted by VMHvlEsr1 and VMHVlCckar cells (Falkner et al., 2020; Hashikawa et al., 2017; Yin et al., 2022). Notably, many of the female mating-activated PAG cells express Esr1, suggesting that they are likely modulated by sex hormones (Vaughn et al., 2022). The lordosis-activated cells in the PAG appears to be largely glutamatergic and project to medullary reticular formation (MRF), a brainstem region required for lordosis in female rats (Yamada and Kawata, 2014).
Taken together, we propose a simple circuit driving lordosis reflex (Figure 5). During male mounting, the somatosensory inputs activate PAG cells which then indirectly activate lumbar epaxial muscles. VMHvl inputs to the PAG act as a permissive gate. It does not control the moment-to-moment activation of PAG cells but determines whether PAG is close enough to the activation threshold to release the motor response upon receiving the somatosensory inputs. VMHvl output level is determined mainly by the sex hormone environment that varies across the estrous cycle. During male-female interactions, male pheromones may enhance the ongoing VMHvl activity not only by increasing synaptic inputs to the cells but also by boosting the HPG axis and other neuromodulatory systems through the accessory olfactory pathway involving VNO, AOB, and MeA.
Figure 5.
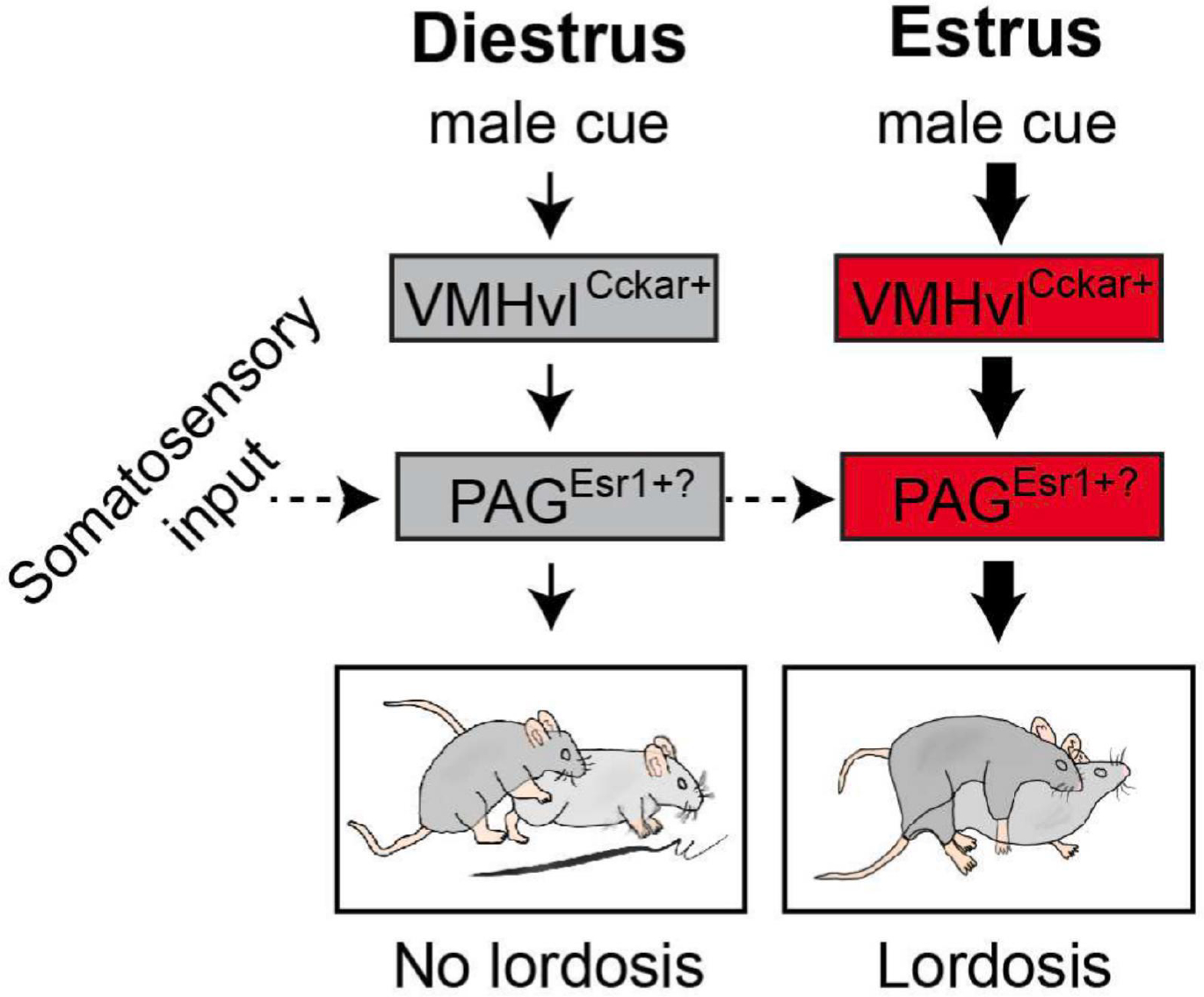
A model of the lordosis reflex circuit. During diestrus, VMHvl is in a low activity state and consequently PAG is far from its activation threshold. When the somatosensory input related to male mounting reaches PAG, it fails to push the PAG pass its activation threshold to drive lordosis. In contrast, during estrus, VMHvl Cckar cells are in a high activity state, moving PAG closer to its activation threshold. Therefore, the same male mounting-associated somatosensory input now can drive the PAG pass the threshold to induce lordosis. PAG cells relevant for female sexual behaviors could be Esr1+
Neural mechanisms underlying behavior and neuroendocrine changes during the post-ejaculation refractory phase
After the male ejaculates, once or several times depending on the species, the intense interest of the male and female towards each other vanishes (Figure 6). Each animal grooms and licks itself repeatedly and occasionally investigates the other. In the hours to days afterward, the animals remain sexually unmotivated towards the same or a new mate, a phenomenon known as post-ejaculation refraction (Bermant, 1961; McGill, 1963; Seizert, 2018; Yang and Clemens, 1996; Yin et al., 2022; Zhang et al., 2021). Our recent study found that while estrous female mice strongly preferred males over females, they overwhelmingly preferred females over males after being ejaculated (Yin et al., 2022). In mice, females have approximately 75% probability of becoming pregnant after male ejaculation (See https://www.criver.com/products-services/research-models-services/animal-models/timed-pregnant-mice-and-rats?region=3616). Thus, the sharp decrease in females’ interest in males is likely an energy-saving strategy to prevent females from engaging in unnecessary copulation.
Figure 6.
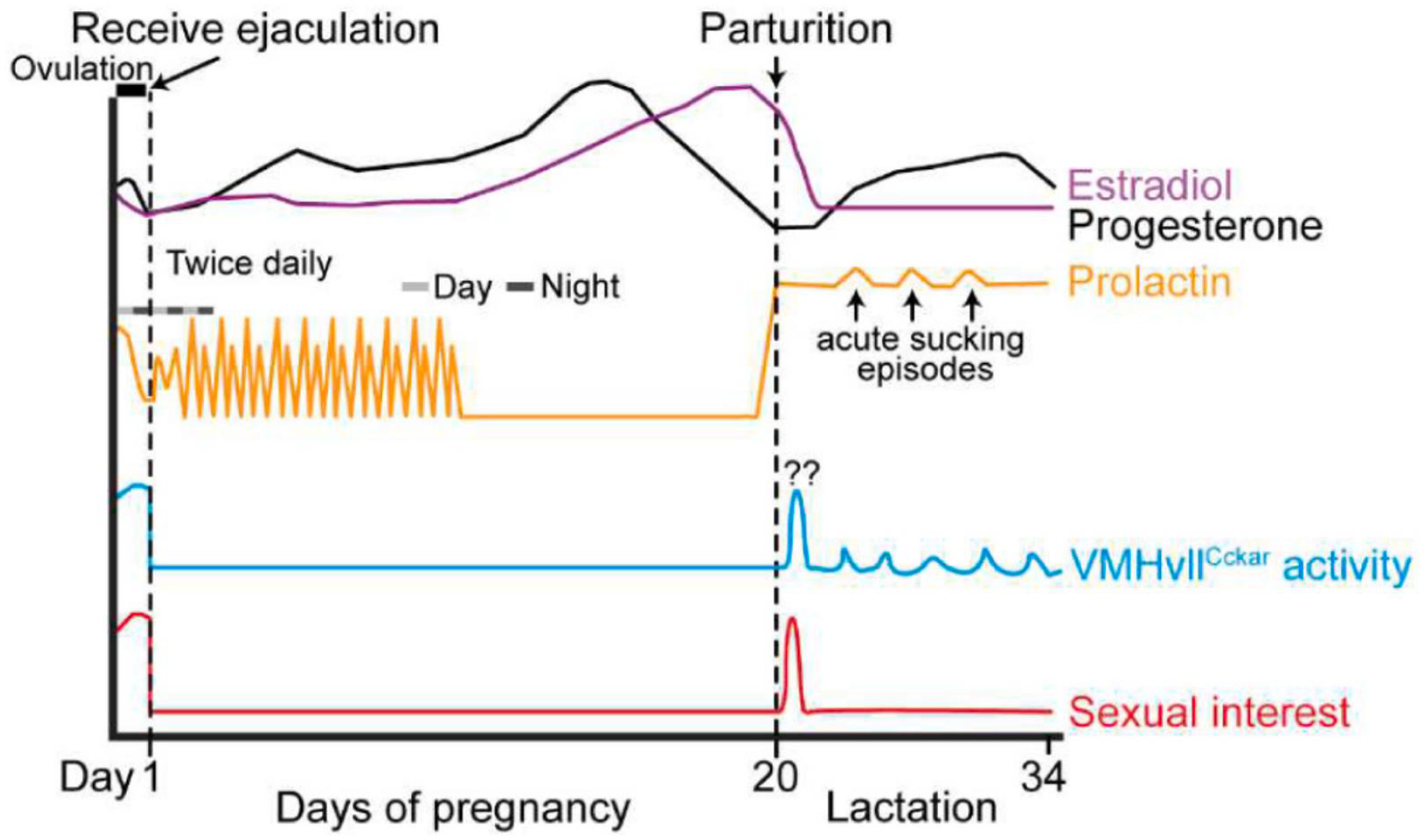
Schematic illustration of the hormonal, neural, and behavioral changes during pregnancy and lactation in rodents. In rodents, after the female receives ejaculation, serum progesterone (black) increases with the progress of pregnancy, reaches its peak value at gestation day 16, and rapidly decreases before parturition. Serum estradiol (purple) increases from mid-pregnancy to term and declines rapidly before parturition. Prolactin (orange) transiently increases right after ejaculation and then surges twice daily for the first half of pregnancy. Prolactin surges again the night before parturition and maintains a high level during lactation. The activity of VMHvllCckar cells is highly correlated with female sexual receptivity: both are high during estrus and low during pregnancy and lactation. During postpartum day 1, female sexual receptivity is high, a phenomenon known as postpartum heat, and VMHvllCckar cell activity is possibly also high, although no recording data is currently available. Light gray bar: daytime; dark gray bar: nighttime.
What are the neural mechanisms that cause sexual interest to plummet during the post-ejaculation period? Answers to this question remain poorly understood. The sharp reduction in sexual interest is likely caused by reduced activity of brain regions essential for sexual interest, such as MeA, MPOA, VMHvl, PMv, VTA, and NAc. Consistent with this hypothesis, we observed sustained suppression of VMHvlCckar activity immediately after ejaculation and throughout pregnancy in female mice (Yin et al., 2022) (Figure 6). Whether a similar suppression occurs in other brain regions in the mating circuit remains to be investigated. The suppression of the female reproduction circuit could be due to centrally acting mechanisms or through circulating hormones that are triggered centrally. In both humans and rodents, prolactin, a hormone secreted from the anterior pituitary gland, increases rapidly and transiently after ejaculation/orgasm and thus has been considered a prime suspect in causing sexual refraction (Exton et al., 1999; Exton et al., 2000; Kruger et al., 2002; Voogt et al., 2001). Consistent with this hypothesis, transient or chronic prolactin elevation decreased sexual receptivity in rats and humans (Dudley et al., 1982). However, a recent study found that blocking post-ejaculation prolactin surge or inducing its occurrence did not alter the post-ejaculation refractory period in male mice, shedding doubts on prolactin’s role in sexual refraction in males (Valente et al., 2021). In addition to prolactin, some other neurochemical events associated with ejaculation, e.g. serotonin increase in the lateral hypothalamus and dopamine decrease in NAc and MPOA, have been suggested to mediate male sexual refraction in mice and rats (Lorrain et al., 1997; Lorrain et al., 1999; Zhang et al., 2021). However, whether changes in prolactin, serotonin or dopamine contribute to female sexual refraction remains to be investigated.
Regardless of which neurochemical mediating the post-ejaculation sexual refraction, brain regions activated during male ejaculation are likely essential in triggering the post-ejaculation behavior switch. Several brain regions, including MPOA, VMHvl, BNSTpr, MeA, and arcuate nucleus (ARC), have been found to express a higher level of c-Fos in female rats that were ejaculated than those that were only mounted (Polston and Erskine, 1995; Voogt et al., 2001). The ejaculation-induced c-Fos increase in BNSTpr is particularly intriguing as BNSTpr has been found to play no role in the appetitive and action phases of female sexual behaviors so far despite its strong connection with other sexual behavior-related regions and its strong activation after mating (Bayless et al., 2019; Ishii et al., 2017; Martinez and Petrulis, 2011). BNSTpr provides dense GABAergic projection to both VMHvl and PMv, and thus is well positioned to suppress these key mating-related regions after ejaculation (Dong and Swanson, 2004; Ortiz-Juza et al., 2021) (Figure 3). Consistent with this hypothesis, Zhou et. al. recently found that estrogen receptor 2 (Esr2)-expressing cells in the BNSTpr are highly activated during male mouse ejaculation and become hyperexcited for days afterwards (Zhou et al., 2023). Inhibiting BNSTpr Esr2 cells led to fast recovery from the sexual refraction in both males and females. Additionally, exocrine gland-secreting peptide 22 (ESP22), a female receptivity-suppressing lacrimal protein from juvenile mice, increased c-Fos expression in the BNST (Osakada et al., 2018). When BNST GABAergic cells or their projections to the VMHvl were pharmacogenetically inhibited, ESP22-induced increase in sexual rejection was blocked, suggesting that ESP22 promotes sexual rejection via activation of BNSTpr cells (Osakada et al., 2018). Thus, BNSTpr is a key region in suppressing female receptivity under various contexts.
In addition to the behavior change, a series of well-coordinated hormone events occur during pregnancy, starting from repeated twice-daily surges of prolactin (Exton et al., 1999; Grattan et al., 2008; Voogt et al., 2001) (Figure 6). In rodents, prolactin surges rescue the short-lived corpus luteum after ovulation to sustain its progesterone secretion, which is necessary for uterine endometrial development and implantation of the blastocyst (Gunnet and Freeman, 1983). The exact mechanism that triggers the twice-daily prolactin surges during early pregnancy remains elusive, but several lines of evidence suggest that the surges are likely triggered in the brain rather than from reproductive organs. First, fertilization is not required to initiate the prolactin surge. Mating with sterile males could similarly trigger prolactin and subsequent progesterone increase (Yang et al., 2009). Second, sex hormones released from ovary is not required for the initiation of prolactin surge. In OVX rats, prolactin surge can be induced by brief cervical stimulation, although sex hormone supplement enhances the magnitude of the surge (Freeman and Sterman, 1978; Gorospe and Freeman, 1981). Third, early studies suggest that MPOA and VMH are critical for the prolactin surge. In rats, lesions in the VMH and surrounding areas blocked the surges, whereas MPOA lesions caused repetitive nocturnal prolactin surges without mating (Freeman and Banks, 1980; Gunnet and Freeman, 1985; Gunnet et al., 1981). Conversely, electric stimulation of the VMH region induced prolactin release (Beach et al., 1978; Quinn and Everett, 1967). These results suggest that MPOA normally suppresses while VMH promotes prolactin surge. A recent tracing study in mice revealed that both MPOA and VMH, as well as their downstream regions, such as the anteroventral periventricular nucleus (AVPV) and tuberal nucleus (TU), project heavily to ARC dopaminergic (ARCDA) neurons (Esteves et al., 2019). The ARCDA neurons hold a prominent role in controlling prolactin secretion. These cells exert tonic inhibition on the anterior pituitary lactotroph cells through dopamine D2 receptors, and prolactin release occurs when ARCDA-D2 inhibition reduces (Fitzgerald and Dinan, 2008). Thus, we speculate that activity changes in the MPOA and VMHvl during and after ejaculation alter the activity of ARCDA cells, which ultimately leads to prolactin surges (Figure 3). Future in vivo recording and functional manipulation studies will help test this hypothesis.
Interaction between the female sexual behavior circuit and the HPG axis
In mice and rats, the expression of female sexual behavior is highly coordinated with ovarian activity, which is cyclic and lasts approximately 4–5 days per cycle (McLean et al., 2012). Each cycle contains four stages: proestrus, estrus, metestrus, and diestrus (Figure 7). In rodents, proestrus corresponds to the follicular phase of the menstrual cycle in humans (Hawkins and Matzuk, 2008). During this phase, several follicles (average 8) grow and mature and eventually release oocytes into the oviduct, i.e. ovulation, marking the beginning of estrus (Nakamura and Husbandry, 1957). Metestrus corresponds to the early luteal phase in humans when the leftover follicles develop into corpus luteum and release progesterone to prepare the uterus for egg implantation. If conception does not occur, the animals enter diestrus, corresponding to the late luteal phase in humans, when corpus luteum degrades and progesterone level declines (McLean et al., 2012).
Figure 7.
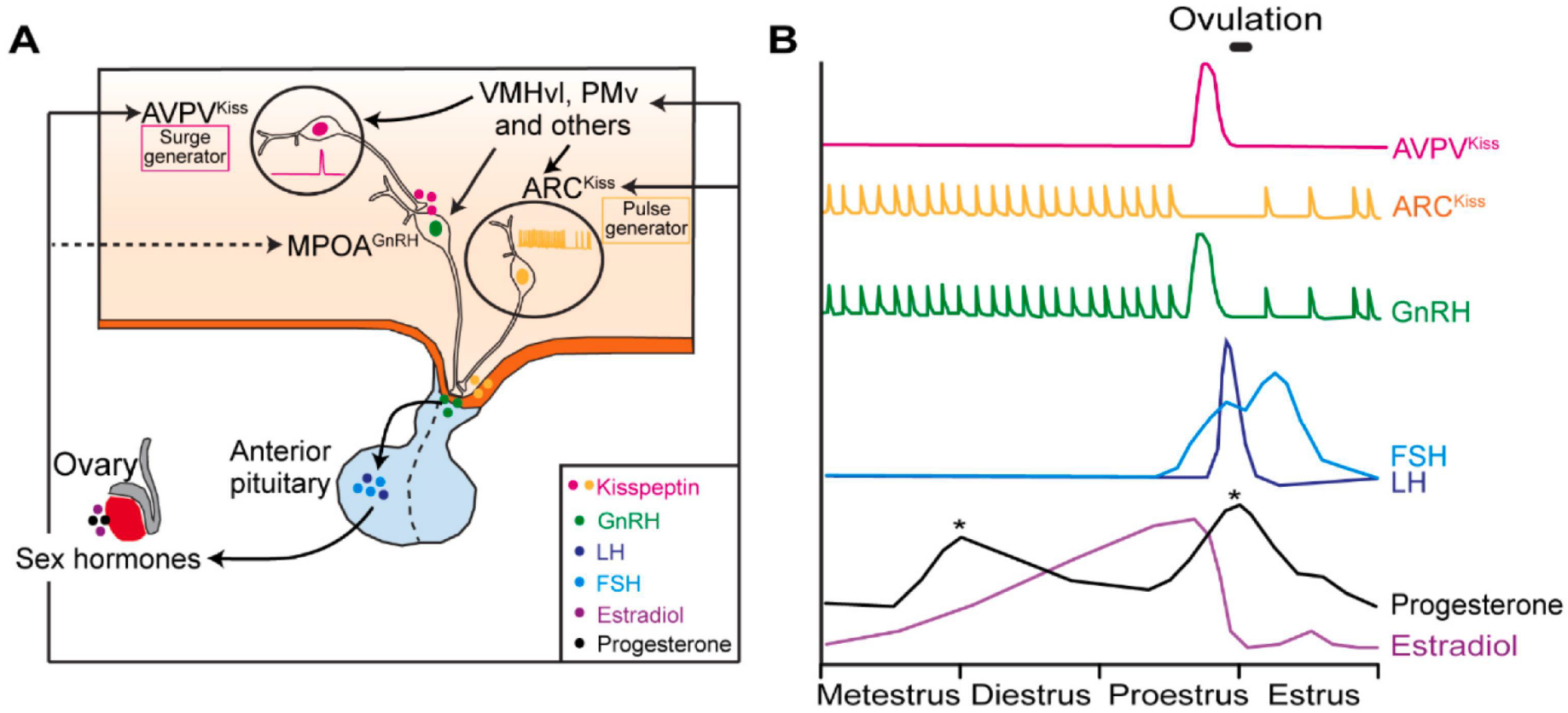
Neural control of the HPG axis (A) A model showing the hypothalamic regions/populations controlling the cascade of hormone release along the HPG axis. Specifically, ARCKiss controls the pulsatile release of GnRH and AVPVKiss controls GnRH surge. VMHvl and PMv facilitate GnRH surge through their inputs to ARCKiss, AVPVKiss and GnRH cells. GnRH stimulates the biosynthesis and the release of LH and FSH from the anterior pituitary gland, which promotes the release of sex hormones (estradiol and progesterone) from ovaries. (B) Schematics showing the putative activity patterns of AVPVKiss and ARCKiss cells (Herbison, 2020) and the release patterns of GnRH, LH, FSH, progesterone, and estradiol across the estrus cycle. ARCKiss: arcuate nucleus kisspeptin cells; AVPVKiss: anteroventral periventricular nucleus kisspeptin cells; GnRH, gonadotropin-releasing hormone; FSH, folliclestimulating hormone; LH, luteinizing hormone.
The ovarian cycle is under the control of a cascade of hormone events, namely the HPG axis, and GnRH lies at the head of this cascade (Figure 7). GnRH is released in pulses by GnRH cells in the medial preoptic nucleus approximately once per hour during metestrus, diestrus, and early proestrus. During estrus, GnRH pulse frequency decreases to approximately 0.2 pulse/hour (Herbison, 2018, 2020). In addition to the pulsed release, during late proestrus, a surge of GnRH occurs, which leads to the bulk release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the anterior pituitary gland. LH and FSH facilitate the maturation of follicles, ovulation, and formation of corpus luteum, whereas follicles and corpus luteum, in turn, release sex hormones, including estrogen and progesterone, to modulate GnRH release (Herbison, 2018, 2020). Across the estrus cycle, the estrogen level is the lowest during estrus, begins to rise by late metestrus and throughout diestrus, reaches peak concentration during mid-proestrus, and then drops rapidly, reaching basal value during early estrus (Levine, 2015) (Figure 7). Progesterone shows two peaks during the estrous cycle: the first peak occurs during late-proestrus and the second peak occurs during metestrus due to activation of the newly formed corpus luteum (Levine, 2015) (Figure 7).
Importantly, due to a lack of Esr1, which is necessary for estrogen to exert feedback on GnRH secretion, GnRH cells are believed to be not directly controlled by sex hormones (Herbison, 1998). Instead, sex hormones modulate GnRH cell activity by influencing the inputs to GnRH cells from brain regions sensitive to sex hormones. Kisspeptin inputs to the GnRH cells represent a vital driving force for GnRH release. Kisspeptin is expressed mainly in two clusters of cells, one in the ARC and the other in the AVPV. ARC kisspeptin (ARCKiss) cells are considered the GnRH pulse generator as they show intermittent bursts that correlate perfectly with pulsatile LH secretion (Clarkson et al., 2017; McQuillan et al., 2019).
In contrast, AVPV kisspeptin (AVPVKiss) cells are considered the GnRH surge generator (Clarkson et al., 2008; Herbison, 2020; Smith et al., 2006) (Figure 7). AVPVKiss cells are enriched of Esr1 and thus are well positioned to directly sense estrogen rise during proestrus, a crucial trigger for GnRH surge, and transmit the information to GnRH cells (Christian and Moenter, 2010; Clarkson et al., 2008; Plant, 2012; Smith et al., 2006). Those two GnRH generators also receive inputs from other hypothalamic structures. The VMHvl represents one of the estrogen-sensitive inputs to the AVPV (Figure 3). During estrus in female mice, VMHvlCckar cells increased excitability (Yin et al., 2022) and the density of axon terminals from VMHvlPR cells to the AVPV increased drastically (Inoue et al., 2019). In OVX female mice, estrogen supplement nearly tripled the post-synaptic response of AVPV cells to VMHvlPR input, although, in this study, kisspeptin cells were not explicitly labeled (Inoue et al., 2019). PMv is another region in the mating circuit that could relay estrogen information to AVPVKiss cells (Figure 3). Channelrhodopsin-assisted circuit mapping showed that PMv PACAP cells make monosynaptic connections onto both AVPVKiss and ARCKiss cells in female mice (Ross et al., 2018). Deleting PACAP in PMv cells severely disrupted the estrus cycle (Ross et al., 2018). In female rats, lesions of the PMv decreased LH increase and reduced AVPV and GnRH neuron activation during preovulatory GnRH surge (Donato et al., 2009). It is worth noting that AVPVKiss neurons not only receive but also send information to regions in the mating circuit. Hellier et al. recently showed a projection from AVPVKiss cells to the VMHvl in female mice, which can modulate lordosis bi-directionally, highlighting the complex crosstalk between the HPG axis and the mating circuit (Hellier et al., 2018). In addition to modulating the HPG axis through AVPVKiss cells, tracing studies in mice also revealed direct projections from the VMHvl and PMv to GnRH cells (Boehm et al., 2005; Yoon et al., 2005). Thus, the mating circuit could serve as “estrogen sensors” and facilitate preovulatory GnRH surge by enhancing excitatory inputs to GnRH cells, directly or indirectly, to ensure the synchronization between sexual interest/receptivity and ovulation.
Pheromones modulate the HPG axis through regions in the female sexual behavior circuit
The HPG axis is also influenced by external cues. The presence of a male can hasten HPG activation to get the females physically ready for reproduction. In sheep and goats, non-cycling females can enter estrus and ovulate by the sudden introduction of a new ram, a phenomenon known as the “Ram effect” or “Male effect” (Shelton, 1960). In mice, exposure to males can synchronize the estrous cycle of a group of females, known as the “Whitten effect” (Whitten, 1956) or promote the onset of the estrus cycle in prepubertal females, known as the “Vandenbergh effect” (Vandenbergh, 1967). Exposing recently mated female mice to unfamiliar males during a three- to four-day critical period for embryo implantation causes a high rate of pregnancy failure, known as the “Bruce effect” (Bruce, 1959). All phenomena mentioned above are mediated by male chemosensory cues, as exposing the females to male urine or fleece has similar impacts on the estrus cycle and ovulation (Dominic, 1965; Gelez and Fabre-Nys, 2004; Marsden and Bronson, 1964; Vandenbergh, 1969). Non-volatiles detected by VNO are essential for the Bruce effect, whereas volatiles are mainly responsible for the Whitten effect and Ram effect (Gangrade and Dominic, 1984; Gelez and Fabre-Nys, 2004; Hattori et al., 2017; Kelliher et al., 2006; Ma et al., 2002; Oboti et al., 2014).
How do the male chemosensory cues influence the HPG axis? Limited studies suggest that kisspeptin cells are involved. Kisspeptin cells showed increased c-Fos expression after male pheromone exposure (Bakker et al., 2010; Fabre-Nys et al., 2015). When the kisspeptin receptor was blocked, male cues could no longer induce GnRH surge in anestrous ewes (De Bond et al., 2013). We speculate that male pheromone signals are relayed through AOB and MeA to reach VMHvl, PMv, and then kisspeptin cells (Figure 3). PMv could be a particularly important region given that it is highly responsive to male pheromones in females and projects directly to kisspeptin as well as GnRH cells (Leshan et al., 2009; Ross et al., 2018). Consistent with this model, unilateral VNO lesions combined with contralateral PMv lesions blocked male cue-induced LH surge in female rats (Beltramino and Taleisnik, 1985).
Altogether, current results support an intimate relationship between the female mating circuit and the HPG axis (Figure 3). On the one hand, the HPG axis determines the sex hormone level and, consequently, the active window for the female mating circuit. On the other hand, the mating circuit is an integral part of the sex hormone feedback loop that modulates the HPG axis. They are highly interdependent: the mating circuit cannot be activated without an appropriate sex hormone environment; conversely, the HPG axis cannot cycle properly without inputs from the sexual behavior circuit. Mate-specific chemosensory cues can travel through critical regions in the sexual behavior circuit to activate the HPG axis, which in turn mobilizes the sexual behavior circuit through sex hormone release. Ultimately, this process allows the females to get ready to reproduce when the opportunity arises.
Concluding remarks
Here, we summarized our current understanding regarding the neural circuits controlling the three phases of female sexual behaviors: appetitive, action, and refractory phases. The medial hypothalamus, including MPOA, VMHvl, and PMv, is at the core of the neural circuit for each phase (Figure 3). During the appetitive phase, the main olfactory pathway first detects distal male volatiles. Upon reaching the target, non-volatile pheromones channel through the accessory olfactory pathway to activate the MPOA, evoking dopamine release from the VTA in the NAc to maintain social interest toward the male. During the action phase, when the VMHvl is sufficiently activated by sex hormones and male pheromones, it permits the PAG to drive the lordosis reflex in response to appropriate somatosensory inputs. During the refractory phase, VMHvl activity shuts down, possibly due to strong activation of BNSTpr and/or actions of specific neuropeptides. While much progress has been made on the neural circuits of female sexual behaviors, many questions remain. For example, what are the molecular identities of MPOA cells promoting sexual interest vs. inhibiting lordosis? Are they the same cells? What is the molecular identity of PAG cells downstream of VMHvlCckar cells? Are the same PAG cells receiving somatosensory inputs during mounting, driving lordosis, and integrating inputs from VMHvl? Or are there additional microcircuit components? The neural circuit controlling the sudden loss of sexual interest after male ejaculation just starts to emerge. Does BNSTpr exert its function in sexual refraction through the VMHvl? How does ejaculation trigger the action of neuroendocrine systems (prolactin, 5-HT, et al.) during early pregnancy? Is the VMHvl or MPOA projection to ARCDA cells a relevant pathway? Future studies combining in vivo recording and pathway-specific manipulations will help answer these questions.
The sexual behavior circuit is tightly linked to the HPG axis owing to the high dependence of female sexual behaviors and their associated outcomes, e.g. pregnancy, on sex hormones. The sexual behavior circuit can influence the HPG axis through direct synaptic inputs, whereas the HPG axis controls the release of sex hormones to determine the state (e.g. activatable or not) of the sexual behavior circuit. Despite their close relationship, most studies have examined the sexual behavior circuit and HPG axis separately. A better understanding of their interaction will be essential to gain a complete view of female reproduction control. Many fundamental questions remain to be addressed. For example, what are the major relays in the sexual behavior circuit that modulate the HPG axis? How are the external pheromone cues and internal ovarian cues integrated? What are the key regions that pass pheromone information to the HPG axis? Is there any plasticity in the system? Does mating experience change the type of cues that activate the HPG axis? In the era of cross-field research, the investigation of neural and endocrine control of female reproduction shall converge.
Highlights.
Female sexual behaviors comprise appetitive, consummatory, and refractory phases.
The medial hypothalamus plays a central role in the neural circuits mediating all phases of female sexual behaviors.
The state of the sexual behavior circuits is under strong modulation of sex hormones.
The sexual behavior circuit is an integral part of the HPG axis feedback loop and a key mediator allowing external cues to influence the HPG axis.
Acknowledgement
We thank the following funding sources: NIH grants R01MH101377, R01MH124927, R01HD092596, U19NS107616 and U01NS113358 (D.L.); the Vulnerable Brain Project (D.L.); and Mathers Foundation (D.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asaba A, Hattori T, Mogi K, and Kikusui T (2014). Sexual attractiveness of male chemicals and vocalizations in mice. Front Neurosci 8, 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker J, and Baum MJ (2000). Neuroendocrine regulation of GnRH release in induced ovulators. Front Neuroendocrinol 21, 220–262. [DOI] [PubMed] [Google Scholar]
- Bakker J, Pierman S, and González-Martínez D (2010). Effects of aromatase mutation (ArKO) on the sexual differentiation of kisspeptin neuronal numbers and their activation by same versus opposite sex urinary pheromones. Hormones behavior 57, 390–395. [DOI] [PubMed] [Google Scholar]
- Baum MJ, and Keverne EB (2002). Sex difference in attraction thresholds for volatile odors from male and estrous female mouse urine. Horm Behav 41, 213–219. [DOI] [PubMed] [Google Scholar]
- Bayless DW, Yang T, Mason MM, Susanto AA, Lobdell A, and Shah NM (2019). Limbic neurons shape sex recognition and social behavior in sexually naive males. Cell 176, 1190–1205. e1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach FA (1976). Sexual attractivity, proceptivity, and receptivity in female mammals. Horm Behav 7, 105–138. [DOI] [PubMed] [Google Scholar]
- Beach JE, Tyrey L, and Everett JW (1978). Prolactin secretion preceding delayed pseudopregnancy in rats after electrical stimulation of the hypothalamus. Endocrinology 103, 2247–2251. [DOI] [PubMed] [Google Scholar]
- Beltramino C, and Taleisnik S (1985). Ventral premammillary nuclei mediate pheromonal-induced LH release stimuli in the rat. Neuroendocrinology 41, 119–124. [DOI] [PubMed] [Google Scholar]
- Bermant G (1961). Response Latencies of Female Rats during Sexual Intercourse. Science 133, 1771–1773. [DOI] [PubMed] [Google Scholar]
- Boehm U, Zou Z, and Buck LB (2005). Feedback loops link odor and pheromone signaling with reproduction. Cell 123, 683–695. [DOI] [PubMed] [Google Scholar]
- Brenner RM, and West NB (1975). Hormonal regulation of the reproductive tract in female mammals. Annu Rev Physiol 37, 273–302. [DOI] [PubMed] [Google Scholar]
- Bruce HM (1959). An exteroceptive block to pregnancy in the mouse. Nature 184, 105. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, and Swanson LW (1992). Projections of the ventral premammillary nucleus. J Comp Neurol 324, 195–212. [DOI] [PubMed] [Google Scholar]
- Christian CA, and Moenter SM (2010). The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocr Rev 31, 544–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, de Tassigny X.d.A., Moreno AS, Colledge WH, and Herbison AE (2008). Kisspeptin–GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. Journal of Neuroscience 28, 8691–8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, Han SY, Piet R, McLennan T, Kane GM, Ng J, Porteous RW, Kim JS, Colledge WH, Iremonger KJ, et al. (2017). Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci U S A 114, E10216–E10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottingham SL, Femano PA, and Pfaff DW (1987). Electrical stimulation of the midbrain central gray facilitates reticulospinal activation of axial muscle EMG. Exp Neurol 97, 704–724. [DOI] [PubMed] [Google Scholar]
- Curtis JT, Liu Y, and Wang Z (2001). Lesions of the vomeronasal organ disrupt mating-induced pair bonding in female prairie voles (Microtus ochrogaster). Brain Res 901, 167–174. [DOI] [PubMed] [Google Scholar]
- Dai B, Sun F, Tong X, Ding Y, Kuang A, Osakada T, Li Y, and Lin D (2022). Responses and functions of dopamine in nucleus accumbens core during social behaviors. Cell Rep 40, 111246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels D, Miselis RR, and Flanagan-Cato LM (1999). Central neuronal circuit innervating the lordosis-producing muscles defined by transneuronal transport of pseudorabies virus. J Neurosci 19, 2823–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bond J-AP, Li Q, Millar RP, Clarke IJ, and Smith JT (2013). Kisspeptin signaling is required for the luteinizing hormone response in anestrous ewes following the introduction of males. PloS one 8, e57972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir E, Li K, Bobrowski-Khoury N, Sanders JI, Beynon RJ, Hurst JL, Kepecs A, and Axel R (2020). The pheromone darcin drives a circuit for innate and reinforced behaviours. Nature 578, 137–141. [DOI] [PubMed] [Google Scholar]
- DiBenedictis BT, Ingraham KL, Baum MJ, and Cherry JA (2012). Disruption of urinary odor preference and lordosis behavior in female mice given lesions of the medial amygdala. Physiol Behav 105, 554–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominic C (1965). The origin of the pheromones causing pregnancy block in mice. Reproduction 10, 469–472. [DOI] [PubMed] [Google Scholar]
- Donato J Jr., Silva RJ, Sita LV, Lee S, Lee C, Lacchini S, Bittencourt JC, Franci CR, Canteras NS, and Elias CF (2009). The ventral premammillary nucleus links fasting-induced changes in leptin levels and coordinated luteinizing hormone secretion. J Neurosci 29, 5240–5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, and Swanson LW (2004). Projections from bed nuclei of the stria terminalis, posterior division: implications for cerebral hemisphere regulation of defensive and reproductive behaviors. J Comp Neurol 471, 396–433. [DOI] [PubMed] [Google Scholar]
- Dudley CA, Jamison TS, and Moss RL (1982). Inhibition of lordosis behavior in the female rat by intraventricular infusion of prolactin and by chronic hyperprolactinemia. Endocrinology 110, 677–679. [DOI] [PubMed] [Google Scholar]
- Esteves FF, Matias D, Mendes AR, Lacoste B, and Lima SQ (2019). Sexually dimorphic neuronal inputs to the neuroendocrine dopaminergic system governing prolactin release. J Neuroendocrinol 31, e12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ (1995). Neuroendocrine mechanisms underlying appetitive and consummatory elements of masculine sexual behavior. The pharmacology of sexual function dysfunction, 15–31. [Google Scholar]
- Exton MS, Bindert A, Kruger T, Scheller F, Hartmann U, and Schedlowski M (1999). Cardiovascular and endocrine alterations after masturbation-induced orgasm in women. Psychosom Med 61, 280–289. [DOI] [PubMed] [Google Scholar]
- Exton NG, Truong TC, Exton MS, Wingenfeld SA, Leygraf N, Saller B, Hartmann U, and Schedlowski M (2000). Neuroendocrine response to film-induced sexual arousal in men and women. Psychoneuroendocrinology 25, 187–199. [DOI] [PubMed] [Google Scholar]
- Fabre-Nys C, Kendrick KM, and Scaramuzzi RJ (2015). The “ram effect”: new insights into neural modulation of the gonadotropic axis by male odors and socio-sexual interactions. Frontiers in neuroscience 9, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner AL, Wei D, Song A, Watsek LW, Chen I, Chen P, Feng JE, and Lin D (2020). Hierarchical Representations of Aggression in a Hypothalamic-Midbrain Circuit. Neuron 106, 637–648 e636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang YY, Yamaguchi T, Song SC, Tritsch NX, and Lin D (2018). A Hypothalamic Midbrain Pathway Essential for Driving Maternal Behaviors. Neuron 98, 192–207 e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald P, and Dinan TG (2008). Prolactin and dopamine: what is the connection? A review article. Journal of Psychopharmacology 22, 12–19. [DOI] [PubMed] [Google Scholar]
- Freeman ME, and Banks JA (1980). Hypothalamic sites which control the surges of prolactin secretion induced by cervical stimulation. Endocrinology 106, 668–673. [DOI] [PubMed] [Google Scholar]
- Freeman ME, and Sterman JR (1978). Ovarian steroid modulation of prolactin surges in cervically stimulated ovariectomized rats. Endocrinology 102, 1915–1920. [DOI] [PubMed] [Google Scholar]
- Gangrade BK, and Dominic CJ (1984). Studies of the male-originating pheromones involved in the Whitten effect and Bruce effect in mice. Biol Reprod 31, 89–96. [DOI] [PubMed] [Google Scholar]
- Gelez H, and Fabre-Nys C (2004). The “male effect” in sheep and goats: a review of the respective roles of the two olfactory systems. Horm Behav 46, 257–271. [DOI] [PubMed] [Google Scholar]
- Gorospe WC, and Freeman ME (1981). An ovarian role in prolonging and terminating the two surges of prolactin in pseudopregnant rats. Endocrinology 108, 1293–1298. [DOI] [PubMed] [Google Scholar]
- Grattan DR, Steyn FJ, Kokay IC, Anderson GM, and Bunn SJ (2008). Pregnancy-induced adaptation in the neuroendocrine control of prolactin secretion. J Neuroendocrinol 20, 497–507. [DOI] [PubMed] [Google Scholar]
- Guarraci FA, and Clark AS (2006). Ibotenic acid lesions of the medial preoptic area disrupt the expression of partner preference in sexually receptive female rats. Brain Res 1076, 163–170. [DOI] [PubMed] [Google Scholar]
- Guarraci FA, Megroz AB, and Clark AS (2004). Paced mating behavior in the female rat following lesions of three regions responsive to vaginocervical stimulation. Brain Res 999, 40–52. [DOI] [PubMed] [Google Scholar]
- Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, et al. (2014). Natural neural projection dynamics underlying social behavior. Cell 157, 1535–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnet JW, and Freeman ME (1983). The Mating-induced Release of Prolactin: A Unique Neuroendrocine Response. Endocrine Reviews 4, 44–61. [DOI] [PubMed] [Google Scholar]
- Gunnet JW, and Freeman ME (1985). The interaction of the medial preoptic area and the dorsomedial-ventromedial nuclei of the hypothalamus in the regulation of the mating-induced release of prolactin. Neuroendocrinology 40, 232–237. [DOI] [PubMed] [Google Scholar]
- Gunnet JW, Mick C, and Freeman ME (1981). The role of the dorsomedial-ventromedial area of the hypothalamus in the control of prolactin secretion induced by cervical stimulation. Endocrinology 109, 1846–1850. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Castellanos N, Husain BFA, Dias IC, and Lima SQ (2022). Neural and behavioral plasticity across the female reproductive cycle. Trends Endocrinol Metab. [DOI] [PubMed] [Google Scholar]
- Haga S, Hattori T, Sato T, Sato K, Matsuda S, Kobayakawa R, Sakano H, Yoshihara Y, Kikusui T, and Touhara K (2010). The male mouse pheromone ESP1 enhances female sexual receptive behaviour through a specific vomeronasal receptor. Nature 466, 118–122. [DOI] [PubMed] [Google Scholar]
- Hashikawa K, Hashikawa Y, Tremblay R, Zhang J, Feng JE, Sabol A, Piper WT, Lee H, Rudy B, and Lin D (2017). Esr1(+) cells in the ventromedial hypothalamus control female aggression. Nat Neurosci 20, 1580–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T, Osakada T, Masaoka T, Ooyama R, Horio N, Mogi K, Nagasawa M, Haga-Yamanaka S, Touhara K, and Kikusui T (2017). Exocrine Gland-Secreting Peptide 1 Is a Key Chemosensory Signal Responsible for the Bruce Effect in Mice. Curr Biol 27, 3197–3201 e3193. [DOI] [PubMed] [Google Scholar]
- Hawkins SM, and Matzuk MM (2008). The menstrual cycle: basic biology. Ann N Y Acad Sci 1135, 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellier V, Brock O, Candlish M, Desroziers E, Aoki M, Mayer C, Piet R, Herbison A, Colledge WH, and Prévot V (2018). Female sexual behavior in mice is controlled by kisspeptin neurons. Nature communications 9, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE (1998). Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocrine reviews 19, 302–330. [DOI] [PubMed] [Google Scholar]
- Herbison AE (2018). The Gonadotropin-Releasing Hormone Pulse Generator. Endocrinology 159, 3723–3736. [DOI] [PubMed] [Google Scholar]
- Herbison AE (2020). A simple model of estrous cycle negative and positive feedback regulation of GnRH secretion. Front Neuroendocrinol 57, 100837. [DOI] [PubMed] [Google Scholar]
- Hoshina Y, Takeo T, Nakano K, Sato T, and Sakuma Y (1994). Axon-sparing lesion of the preoptic area enhances receptivity and diminishes proceptivity among components of female rat sexual behavior. Behavioural brain research 61, 197–204. [DOI] [PubMed] [Google Scholar]
- Inoue S (2022). Neural basis for estrous cycle-dependent control of female behaviors. Neurosci Res 176, 1–8. [DOI] [PubMed] [Google Scholar]
- Inoue S, Yang R, Tantry A, Davis CH, Yang T, Knoedler JR, Wei Y, Adams EL, Thombare S, Golf SR, et al. (2019). Periodic Remodeling in a Neural Circuit Governs Timing of Female Sexual Behavior. Cell 179, 1393–1408 e1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii KK, Osakada T, Mori H, Miyasaka N, Yoshihara Y, Miyamichi K, and Touhara K (2017). A Labeled-Line Neural Circuit for Pheromone-Mediated Sexual Behaviors in Mice. Neuron 95, 123–137 e128. [DOI] [PubMed] [Google Scholar]
- Jennings KJ, and de Lecea L (2020). Neural and Hormonal Control of Sexual Behavior. Endocrinology 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JA, Clemens LG, and Nunez AA (2008). Characterization of copulatory behavior in female mice: evidence for paced mating. Physiol Behav 95, 425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CS, Hong W, and Micevych PE (2021). Posterodorsal Medial Amygdala Regulation of Female Social Behavior: GABA versus Glutamate Projections. J Neurosci 41, 8790–8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karigo T, Kennedy A, Yang B, Liu M, Tai D, Wahle IA, and Anderson DJ (2021). Distinct hypothalamic control of same- and opposite-sex mounting behaviour in mice. Nature 589, 258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Baum MJ, and Bakker J (2008). Olfactory control of sex-recognition and sexual behavior in mice. In Chemical Signals in Vertebrates 11 (Springer; ), pp. 241–250. [Google Scholar]
- Keller M, Douhard Q, Baum MJ, and Bakker J (2006a). Destruction of the main olfactory epithelium reduces female sexual behavior and olfactory investigation in female mice. Chem Senses 31, 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Pierman S, Douhard Q, Baum MJ, and Bakker J (2006b). The vomeronasal organ is required for the expression of lordosis behaviour, but not sex discrimination in female mice. European Journal of Neuroscience 23, 521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher KR, Spehr M, Li XH, Zufall F, and Leinders-Zufall T (2006). Pheromonal recognition memory induced by TRPC2-independent vomeronasal sensing. Eur J Neurosci 23, 3385–3390. [DOI] [PubMed] [Google Scholar]
- Kim D-W, Yao Z, Graybuck LT, Kim TK, Nguyen TN, Smith KA, Fong O, Yi L, Koulena N, Pierson N, et al. (2019). Multimodal Analysis of Cell Types in a Hypothalamic Node Controlling Social Behavior. Cell 179, 713–728.e717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi T, Xu J, and Dulac C (2007). A functional circuit underlying male sexual behaviour in the female mouse brain. Nature 448, 1009–1014. [DOI] [PubMed] [Google Scholar]
- Knoedler JR, Inoue S, Bayless DW, Yang T, Tantry A, Davis C. h., Leung NY, Parthasarathy S, Wang G, Alvarado M, et al. (2022). A functional cellular framework for sex and estrous cycle-dependent gene expression and behavior. Cell Online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komisaruk BR (1974). Neural and hormonal interactions in the reproductive behavior of female rats. Adv Behav Biol 11, 97–129. [DOI] [PubMed] [Google Scholar]
- Kow LM, Montgomery MO, and Pfaff DW (1979). Triggering of lordosis reflex in female rats with somatosensory stimulation: quantitative determination of stimulus parameters. J Neurophysiol 42, 195–202. [DOI] [PubMed] [Google Scholar]
- Kow LM, and Pfaff DW (1998). Mapping of neural and signal transduction pathways for lordosis in the search for estrogen actions on the central nervous system. Behav Brain Res 92, 169–180. [DOI] [PubMed] [Google Scholar]
- Kruger TH, Haake P, Hartmann U, Schedlowski M, and Exton MS (2002). Orgasm-induced prolactin secretion: feedback control of sexual drive? Neurosci Biobehav Rev 26, 31–44. [DOI] [PubMed] [Google Scholar]
- Lenschow C, and Lima SQ (2020). In the mood for sex: neural circuits for reproduction. Curr Opin Neurobiol 60, 155–168. [DOI] [PubMed] [Google Scholar]
- Leshan RL, Louis GW, Jo YH, Rhodes CJ, Munzberg H, and Myers MG Jr. (2009). Direct innervation of GnRH neurons by metabolic- and sexual odorant-sensing leptin receptor neurons in the hypothalamic ventral premammillary nucleus. J Neurosci 29, 3138–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JE (2015). Neuroendocrine Control of the Ovarian Cycle of the Rat. Knobil and Neill’s Physiology of Reproduction (Fourth Edition), 1199–1257. [Google Scholar]
- Li Y, Zhong W, Wang D, Feng Q, Liu Z, Zhou J, Jia C, Hu F, Zeng J, Guo Q, et al. (2016). Serotonin neurons in the dorsal raphe nucleus encode reward signals. Nat Commun 7, 10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WW, and Ramirez VD (1991). Effect of mating behavior on luteinizing hormone-releasing hormone release in female rabbits as monitored with push-pull cannulae. Neuroendocrinology 53, 229–235. [DOI] [PubMed] [Google Scholar]
- Liu M, Kim D-W, Zeng H, and Anderson D (2022). Make war not love: The neural substrate underlying a state-dependent switch in female social behavior. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain DS, Matuszewich L, Friedman RD, and Hull EM (1997). Extracellular serotonin in the lateral hypothalamic area is increased during the postejaculatory interval and impairs copulation in male rats. J Neurosci 17, 9361–9366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain DS, Riolo JV, Matuszewich L, and Hull EM (1999). Lateral hypothalamic serotonin inhibits nucleus accumbens dopamine: implications for sexual satiety. J Neurosci 19, 7648–7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Allen ND, Van Bergen YC, Jones CM, Baum MJ, Keverne EB, and Brennan PA (2002). Selective ablation of olfactory receptor neurons without functional impairment of vomeronasal receptor neurons in OMP‐ntr transgenic mice. European Journal of Neuroscience 16, 2317–2323. [DOI] [PubMed] [Google Scholar]
- Mackay-Sim A, and Rose JD (1986). Removal of the vomeronasal organ impairs lordosis in female hamsters: effect is reversed by luteinising hormone-releasing hormone. Neuroendocrinology 42, 489–493. [DOI] [PubMed] [Google Scholar]
- Malsbury CW, Pfaff DW, and Malsbury AM (1980). Suppression of sexual receptivity in the female hamster: neuroanatomical projections from preoptic and anterior hypothalamic electrode sites. Brain Research 181, 267–284. [DOI] [PubMed] [Google Scholar]
- Mandiyan VS, Coats JK, and Shah NM (2005). Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nature neuroscience 8, 1660–1662. [DOI] [PubMed] [Google Scholar]
- Marsden HM, and Bronson FH (1964). Estrous Synchrony in Mice: Alteration by Exposure to Male Urine. Science 144, 1469. [DOI] [PubMed] [Google Scholar]
- Martinez-Marcos A (2009). On the organization of olfactory and vomeronasal cortices. Prog Neurobiol 87, 21–30. [DOI] [PubMed] [Google Scholar]
- Martinez LA, and Petrulis A (2011). The bed nucleus of the stria terminalis is critical for sexual solicitation, but not for opposite-sex odor preference, in female Syrian hamsters. Hormones behavior 60, 651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LA, and Petrulis A (2013). The medial preoptic area is necessary for sexual odor preference, but not sexual solicitation, in female Syrian hamsters. Hormones behavior 63, 606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy EA, Maqsudlu A, Bass M, Georghiou S, Cherry JA, and Baum MJ (2017). DREADD-induced silencing of the medial amygdala reduces the preference for male pheromones and the expression of lordosis in estrous female mice. Eur J Neurosci 46, 2035–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill TE (1963). Sexual Behavior of the Mouse after Long-Term and Short-Term Postejaculatory Recovery Periods. J Genet Psychol 103, 53–57. [DOI] [PubMed] [Google Scholar]
- McHenry JA, Otis JM, Rossi MA, Robinson JE, Kosyk O, Miller NW, McElligott ZA, Budygin EA, Rubinow DR, and Stuber GD (2017). Hormonal gain control of a medial preoptic area social reward circuit. Nat Neurosci 20, 449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean AC, Valenzuela N, Fai S, and Bennett SA (2012). Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J Vis Exp, e4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuillan HJ, Han SY, Cheong I, and Herbison AE (2019). GnRH Pulse Generator Activity Across the Estrous Cycle of Female Mice. Endocrinology 160, 1480–1491. [DOI] [PubMed] [Google Scholar]
- Micevych P, and Sinchak K (2013). Temporal and concentration-dependent effects of oestradiol on neural pathways mediating sexual receptivity. J Neuroendocrinol 25, 1012–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych PE, and Meisel RL (2017). Integrating Neural Circuits Controlling Female Sexual Behavior. Front Syst Neurosci 11, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, and Schaeffer JM (2003). Immunolocalization of estrogen receptor β in the mouse brain: comparison with estrogen receptor α. Endocrinology 144, 2055–2067. [DOI] [PubMed] [Google Scholar]
- Motta SC, Guimarães CC, Furigo IC, Sukikara MH, Baldo MV, Lonstein JS, and Canteras NS (2013). Ventral premammillary nucleus as a critical sensory relay to the maternal aggression network. Proceedings of the National Academy of Sciences 110, 14438–14443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Kaplitt MG, and Ogawa S (2006). RNAi-mediated silencing of estrogen receptor {alpha} in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proc Natl Acad Sci U S A 103, 10456–10460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, and Husbandry A (1957). Cytological studies on abnormal ova in mature ovaries of mice observed at different phases of oestrous cycle. Journal of the Faculty of Fisheries 1, 343–351. [Google Scholar]
- Nomoto K, and Lima SQ (2015). Enhanced male-evoked responses in the ventromedial hypothalamus of sexually receptive female mice. Curr Biol 25, 589–594. [DOI] [PubMed] [Google Scholar]
- Oboti L, Pérez-Gómez A, Keller M, Jacobi E, Birnbaumer L, Leinders-Zufall T, Zufall F, and Chamero P (2014). A wide range of pheromone-stimulated sexual and reproductive behaviors in female mice depend on G protein Gαo. BMC biology 12, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Juza MM, Alghorazi RA, and Rodriguez-Romaguera J (2021). Cell-type diversity in the bed nucleus of the stria terminalis to regulate motivated behaviors. Behav Brain Res 411, 113401. [DOI] [PubMed] [Google Scholar]
- Osakada T, Ishii KK, Mori H, Eguchi R, Ferrero DM, Yoshihara Y, Liberles SD, Miyamichi K, and Touhara K (2018). Sexual rejection via a vomeronasal receptor-triggered limbic circuit. Nat Commun 9, 4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes RG, and Vazquez B (1999). What do female rats like about sex? Paced mating. Behavioural brain research 105, 117–127. [DOI] [PubMed] [Google Scholar]
- Pfaff DW (1973). Luteinizing hormone-releasing factor potentiates lordosis behavior in hypophysectomized ovariectomized female rats. Science 182, 1148–1149. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Kow LM, Loose MD, and Flanagan-Cato LM (2008). Reverse engineering the lordosis behavior circuit. Horm Behav 54, 347–354. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, and Sakuma Y (1979). Deficit in the lordosis reflex of female rats caused by lesions in the ventromedial nucleus of the hypothalamus. J Physiol 288, 203–210. [PMC free article] [PubMed] [Google Scholar]
- Plant TM (2012). A comparison of the neuroendocrine mechanisms underlying the initiation of the preovulatory LH surge in the human, Old World monkey and rodent. Front Neuroendocrinol 33, 160–168. [DOI] [PubMed] [Google Scholar]
- Polston EK, and Erskine MS (1995). Patterns of induction of the immediate-early genes c-fos and egr-1 in the female rat brain following differential amounts of mating stimulation. Neuroendocrinology 62, 370–384. [DOI] [PubMed] [Google Scholar]
- Powers B, and Valenstein ES (1972). Sexual receptivity: facilitation by medial preoptic lesions in female rats. Science 175, 1003–1005. [DOI] [PubMed] [Google Scholar]
- Quinn DL, and Everett JW (1967). Delayed pseudopregnancy induced by selective hypothalamic stimulation. Endocrinology 80, 155–162. [DOI] [PubMed] [Google Scholar]
- Rajendren G, Dudley CA, and Moss RL (1990). Role of the vomeronasal organ in the male-induced enhancement of sexual receptivity in female rats. Neuroendocrinology 52, 368–372. [DOI] [PubMed] [Google Scholar]
- Rajendren G, and Moss RL (1993). The role of the medial nucleus of amygdala in the mating-induced enhancement of lordosis in female rats: the interaction with luteinizing hormone-releasing hormone neuronal system. Brain Res 617, 81–86. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sierra JF, Crowley WR, and Komisaruk BR (1975). Vaginal stimulation in rats induces prolonged lordosis responsiveness and sexual receptivity. J Comp Physiol Psychol 89, 79–85. [DOI] [PubMed] [Google Scholar]
- Ross RA, Leon S, Madara JC, Schafer D, Fergani C, Maguire CA, Verstegen AM, Brengle E, Kong D, Herbison AE, et al. (2018). PACAP neurons in the ventral premammillary nucleus regulate reproductive function in the female mouse. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BS, and Barfield RJ (1980). Priming of estrous responsiveness by implants of 17 beta-estradiol in the ventromedial hypothalamic nucleus of female rats. Endocrinology 106, 504–509. [DOI] [PubMed] [Google Scholar]
- Rubin BS, and Barfield RJ (1983a). Induction of estrous behavior in ovariectomized rats by sequential replacement of estrogen and progesterone to the ventromedial hypothalamus. Neuroendocrinology 37, 218–224. [DOI] [PubMed] [Google Scholar]
- Rubin BS, and Barfield RJ (1983b). Progesterone in the ventromedial hypothalamus facilitates estrous behavior in ovariectomized, estrogen-primed rats. Endocrinology 113, 797–804. [DOI] [PubMed] [Google Scholar]
- Sakuma Y, and Pfaff DW (1979a). Facilitation of female reproductive behavior from mesensephalic central gray in the rat. Am J Physiol 237, R278–284. [DOI] [PubMed] [Google Scholar]
- Sakuma Y, and Pfaff DW (1979b). Mesencephalic mechanisms for integration of female reproductive behavior in the rat. Am J Physiol 237, R285–290. [DOI] [PubMed] [Google Scholar]
- Sakuma Y, and Pfaff DW (1980). Covergent effects of lordosis-relevant somatosensory and hypothalamic influences on central gray cells in the rat mesencephalon. Exp Neurol 70, 269–281. [DOI] [PubMed] [Google Scholar]
- Seizert CA (2018). The neurobiology of the male sexual refractory period. Neurosci Biobehav Rev 92, 350–377. [DOI] [PubMed] [Google Scholar]
- Shelley DN, Choleris E, Kavaliers M, and Pfaff DW (2006). Mechanisms underlying sexual and affiliative behaviors of mice: relation to generalized CNS arousal. Soc Cogn Affect Neurosci 1, 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton M (1960). Influence of the presence of a male goat on the initiation of estrous cycling and ovulation of Angora does. Journal of Animal Science 19, 368–375. [Google Scholar]
- Smith JT, Popa SM, Clifton DK, Hoffman GE, and Steiner RA (2006). Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. Journal of Neuroscience 26, 6687–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soden ME, Miller SM, Burgeno LM, Phillips PE, Hnasko TS, and Zweifel LS (2016). Genetic isolation of hypothalamic neurons that regulate context-specific male social behavior. Cell reports 16, 304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spehr M, Spehr J, Ukhanov K, Kelliher K, Leinders-Zufall T, and Zufall F (2006). Parallel processing of social signals by the mammalian main and accessory olfactory systems. Cell Mol Life Sci 63, 1476–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiteri T, Ogawa S, Musatov S, Pfaff DW, and Agmo A (2012a). The role of the estrogen receptor alpha in the medial preoptic area in sexual incentive motivation, proceptivity and receptivity, anxiety, and wheel running in female rats. Behav Brain Res 230, 11–20. [DOI] [PubMed] [Google Scholar]
- Spiteri T, Ogawa S, Musatov S, Pfaff DW, and Ågmo A (2012b). The role of the estrogen receptor α in the medial preoptic area in sexual incentive motivation, proceptivity and receptivity, anxiety, and wheel running in female rats. Behavioural brain research 230, 11–20. [DOI] [PubMed] [Google Scholar]
- Valente S, Marques T, and Lima SQ (2021). No evidence for prolactin’s involvement in the post-ejaculatory refractory period. Commun Biol 4, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbergh JG (1967). Effect of the presence of a male on the sexual maturation of female mice. Endocrinology 81, 345–349. [DOI] [PubMed] [Google Scholar]
- Vandenbergh JG (1969). Male odor accelerates female sexual maturation in mice. Endocrinology 84, 658–660. [DOI] [PubMed] [Google Scholar]
- Vathy I, and Etgen AM (1989). Hormonal activation of female sexual behavior is accompanied by hypothalamic norepinephrine release. Journal of neuroendocrinology 1, 383–388. [DOI] [PubMed] [Google Scholar]
- Vaughn E, Eichhorn S, Jung W, Zhuang X, and Dulac C (2022). Three-dimensional Interrogation of Cell Types and Instinctive Behavior in the Periaqueductal Gray. bioRxiv, 2022.2006.2027.497769. [Google Scholar]
- Voogt JL, Lee Y, Yang S, and Arbogast L (2001). Regulation of prolactin secretion during pregnancy and lactation. Prog Brain Res 133, 173–185. [DOI] [PubMed] [Google Scholar]
- Wang H, and Dey SK (2006). Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet 7, 185–199. [DOI] [PubMed] [Google Scholar]
- Wei D, Talwar V, and Lin D (2021). Neural circuits of social behaviors: Innate yet flexible. Neuron 109, 1600–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitten WK (1956). Modification of the oestrous cycle of the mouse by external stimuli associated with the male. J Endocrinol 13, 399–404. [DOI] [PubMed] [Google Scholar]
- Xiao K, Kondo Y, and Sakuma Y (2005). Differential regulation of female rat olfactory preference and copulatory pacing by the lateral septum and medial preoptic area. Neuroendocrinology 81, 56–62. [DOI] [PubMed] [Google Scholar]
- Yamada S, and Kawata M (2014). Identification of neural cells activated by mating stimulus in the periaqueductal gray in female rats. Front Neurosci 8, 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CF, Chiang MC, Gray DC, Prabhakaran M, Alvarado M, Juntti SA, Unger EK, Wells JA, and Shah NM (2013). Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell 153, 896–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JJ, Larsen CM, Grattan DR, and Erskine MS (2009). Mating-induced neuroendocrine responses during pseudopregnancy in the female mouse. J Neuroendocrinol 21, 30–39. [DOI] [PubMed] [Google Scholar]
- Yang LY, and Clemens LG (1996). Relation of intromissions to the female’s postejaculatory refractory period in rats. Physiol Behav 60, 1505–1511. [DOI] [PubMed] [Google Scholar]
- Yang LY, and Clements LG (2000). MPOA lesions affect female pacing of copulation in rats. Behav Neurosci 114, 1191–1202. [PubMed] [Google Scholar]
- Yin L, Hashikawa K, Hashikawa Y, Osakada T, Lischinsky JE, Diaz V, and Lin D (2022). VMHvll(Cckar) cells dynamically control female sexual behaviors over the reproductive cycle. Neuron 110, 3000–3017 e3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H, Enquist LW, and Dulac C (2005). Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell 123, 669–682. [DOI] [PubMed] [Google Scholar]
- Zhang SX, Lutas A, Yang S, Diaz A, Fluhr H, Nagel G, Gao S, and Andermann ML (2021). Hypothalamic dopamine neurons motivate mating through persistent cAMP signalling. Nature 597, 245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Li A, Mi X, Li Y, Ding Z, An M, Chen Y, Li W, Tao X, Chen X, et al. (2023). Hyperexcited limbic neurons represent sexual satiety and reduce mating motivation. Science, eabl4038. [DOI] [PubMed] [Google Scholar]


