Abstract
The role of collagen XII in regulating injury repair and reestablishment of corneal function is unknown. This manuscript aims to investigate the role(s) of collagen XII in the repair of incisional and debridement injuries in an adult mouse model. Two different types of injury in wild type and Col12a1−/− corneas were created to investigate the effects of collagen XII -in wound repair and scar formation- by using clinical photographs, immunohistology, second harmonic generation imaging and electron microscopy. Results showed that collagen XII is a regulator of wound closure after incisional injuries. Absence of collagen XII retarded wound closure and the wound healing process. These findings show that collagen XII regulates fibrillogenesis, CD68 cell lineage infiltration, and myofibroblast survival following injury. In vitro studies suggest that collagen XII regulates deposition of an early and provisional matrix by interacting with two proteins regulating early matrix deposition: fibronectin and LTBP1(latent transforming growth factor β binding protein 1). In conclusion, collagen XII regulates tissue repair in corneal incisional wounds. Understanding the function of collagen XII during wound healing has significant translational value.
Keywords: regeneration, stroma, fibroblasts, wound, collagen XII
Introduction
Collagen XII is a member of the (Fibril Associated Collagens with Interrupted Triple helices) FACIT family that are expressed in a wide range of connective tissues including the corneal stroma and Descemet’s membrane.(Hemmavanh et al., 2013; Kabosova et al., 2007; Sun et al., 2020; Wessel et al., 1997) Collagen XII is a homotrimer that binds to the surface of collagen I fibrils via short collagenous domains on its C terminus.(Gordon et al., 1987; Keene et al., 1991; Koch et al., 1995) Non-collagenous domains on its N terminus are known to bind several extracellular matrix components including decorin, fibromodulin, cartilage oligomeric matrix protein and tenascin X in the interfibrillar space.(Agarwal et al., 2012; Font et al., 1996; Veit et al., 2006)
Collagen XII is a regulator of extracellular matrix and cell function. In humans, collagen XII deficiency causes distal myopathy and a myopathic form of Ehlers-Danlos syndrome.(Hicks et al., 2014; Mohassel et al., 2019; Zou et al., 2014) Collagen XII knockout mice (Col12a1−/−) display muscle weakness, reduced grip strength, bone fragility, and kyphosis.(Izu et al., 2011) In skin, collagen XII acts as a stress absorber that maintains collagen suprastructure.(Schonborn et al., 2020) In the cornea, collagen XII regulates stromal structure and function.(Sun et al., 2020) Col12a1−/− corneas are thicker with increased number of keratocytes. Fibril density is increased and the stromal lamellae are layered in a disorganized fashion.(Sun et al., 2020) From a functional perspective, we recently demonstrated that collagen XII is a significant regulator of stromal TGF-β storage and release from the extracellular matrix.(Sun et al., 2022) Previous studies suggest that collagen XII is a regulator of wound healing and scar formation.(Schonborn et al., 2020) Collagen XII is found in fibrotic lung tissue, in skin scars and in corneal scars. (Bergmeier et al., 2018; Massoudi et al., 2012; Tzortzaki et al., 2003) In bleomycin induced pulmonary fibrosis models in mice, collagen XII expression is markedly upregulated between 4- and 8-weeks post insult.(Tzortzaki et al., 2003) In skin injuries, collagen XII is deposited in the wound edge and later throughout the entire wound bed. In Col12a1−/− mice, granulation tissue contraction and retraction in skin wounds are delayed compared to wild type mice, leading to delayed closure of wounds.(Schonborn et al., 2020) Interestingly, wound healing in mice models overexpressing collagen XII is also abnormal with larger amounts of granulation tissue and macrophage infiltration.(Schonborn et al., 2020) Collagen XII was overexpressed in human corneal scars obtained from five patients with injuries due to trauma or infections.(Massoudi et al., 2012) In mouse corneas, collagen XII was overexpressed in scars after full thickness injury models.(Massoudi et al., 2012) Abnormal deposition of collagen XII has also been found in human corneas with pseudophakic and aphakic bullous keratopathy in areas where fibrosis is present.(Ljubimov et al., 1996)
Multiple extracellular matrix components and cells are relevant in the different stages of wound healing. In the early phase of injury, fibronectin is essential to form a provisional matrix where fibroblasts migrate and initiate collagen fibrillogenesis. Fibronectin forms a framework for collagen deposition and growth factors entrapment including transforming growth factor β (TGF-β). LTBP1 is a major regulator of TGF-β1 activity by regulating its entrapment and release within the matrix. Once secreted out of cells, TGF-β storage and activation are regulated by the extracellular matrix where LTBP-1 is crosslinked to the matrix to fibronectin and fibrillin most likely by a transglutaminase.(Annes et al., 2003; Rifkin et al., 2022; Rifkin, 2005) LTBP-1 plays a critical role in modulating the function of TGF-β by controlling its storage and mechanical release from the extracellular matrix.(Annes et al., 2003; Holm et al., 2011; Rifkin et al., 2018; Robertson et al., 2015; Robertson and Rifkin, 2016) Besides, myofibroblasts cells known to regulate matrix contraction and scar formation, macrophages are known to play regulatory roles in wound healing and fibrosis.(Wynn and Vannella, 2016) We used CD68 -monocyte lineage marker- to identify monocytes, macrophages and resident dendritic cells.(Wynn and Vannella, 2016)
Appropriate and timely wound closure is of utmost importance in achieving successful restoration of function after accidental or surgical injury. Delayed healing or poor wound maturation increase the risk of infection and permanent tissue dysfunction that in the cornea translates to loss of transparency and changes in shape with subsequent loss of vision. In this study we aim to investigate the effects of collagen XII on the healing response following two different types of corneal injuries aiming to recreate perforating and penetrating corneal injuries.
Methods
1.1. Description of stromal scar formation by stromal burr injury
All experiments conformed to the use of Laboratory Animals and ARVO statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committee of the University of South Florida College of Medicine. Adult, 60-days-old male C57BL/6 mice (Jackson Labs, Maine, USA), and 60-days-old collagen XII knockout mice(Sun et al., 2020) (Dr Manuel Koch, Cologne, Germany) were anesthetized by intraperitoneal injection of ketamine (50 mg/kg) and xylazine (10 mg/kg). Male mice were used for this study since trauma is significantly more common in males.(Akgun et al., 2022; Vlasov et al., 2017) Once the mouse was under general anesthesia, 0.1 ml of 0.5% proparacaine hydrochloride and atropine 1% was added to the ocular surface. A single dose of proparacaine was used since it is known to affect wound healing if used twice a day for 3 days.(Zhang et al., 2022) A subcutaneous injection of slow-release buprenorphine was given for analgesia. All injuries were performed on the left cornea. Under microscope visualization the corneal epithelium was removed with an AlgerBrush II diamond burr (The Alger Company, Lago Vista, TX). Stromal burr injury was then performed with the AlgerBrush II diamond burr passed 5 times along the horizontal axis of the eye from limbus to limbus.(Cogswell et al., 2021) Immediately after the procedure drops of moxifloxacin and artificial tear ointment were applied to the ocular surface.
1.2. Evaluation of wound closure after full-thickness keratotomy
Adult, eight 60-days-old male C57BL/6 mice, and eight 60-days-old collagen XII knockout mice were anesthetized, and subcutaneous analgesia was administered as described above. Once the mouse was under general anesthesia, 0.1 ml of 0.5% proparacaine hydrochloride and atropine 1% was added to the ocular surface. Under microscope visualization a full-thickness corneal incision, 1 mm length, was made centrally in the cornea with a 15-degree blade. Complete separation of the corneal stromal wound edges in all cases was noted after this injury. The stromal matrix was labeled with 5([4,6-dichlorotriazin-2yl]-amino)fluorescein (DTAF) in some corneas to demarcate area of tissue repair. Immediately after the procedure drops of moxifloxacin and artificial tear ointment were applied to the ocular surface.
1.3. Scar density grading and wound size measurement
Photographs were obtained using a standardized protocol at 1 week and 3 weeks after stromal burr injury.(Cogswell et al., 2021) Photographs were performed using the same standardized protocol 10 days after full-thickness keratotomy wounding. Our preliminary data and pilot studies showed that a 1 mm corneal incision closes within 7–10 days. Two light sources were positioned to avoid reflections. Two masked examiners classified the severity of scars, based on printed standardized photographs. Each photograph was evaluated twice in a randomized, masked manner by both observers within a one-week interval.(Cogswell et al., 2021) Depending on the severity of opacification and the area of involvement, scars were classified on a scale from 0 to 4 based on a modified Fantes haze scale, with 4 being the most severe, see Table 1 for a description of modified Fantes haze scale.(Fantes et al., 1990)
| Grade | Description |
|---|---|
| 0 | Clear, no opacity seen |
| 1 | Trace diffuse opacity or small (<0.5 mm2) focal opacity of moderate density. Able to visualize all iris and lens details |
| 2 | Mild diffuse opacity or medium-sized (<1 mm2) focal dense opacity. Able to visualize iris and lens but unable to visualize iris details in area of opacity. |
| 3 | Moderate diffuse opacity or large-sized (>1 mm2) focal dense opacity. Able to visualize part of iris and lens. |
| 4 | Dense diffuse opacity. No anterior chamber view, unable to visualize iris or lens. |
1.4. Immunofluorescence microscopy
Corneal tissue was evaluated using a standardized immunostaining protocol 7 – 10 days after injury.(Espana et al., 2015; Hemmavanh et al., 2013) After euthanasia, the injured left cornea from each mouse was embedded and frozen in OCT medium on dry ice (Sakura Finetek, Torrance, CA). Cross sections of 6 μm thickness were cut using an NX 50 cryostat. Sections from burr injured corneas were blocked in 10% donkey serum (Sigma-Aldrich, St. Louis, MO) and then incubated overnight at 4°C in anti-fibronectin (Abcam, Cambridge, MA, catalog # ab2413) and anti-α-Smooth Muscle Actin (α-SMA), (Abcam, Cambridge, MA, catalog #ab7817). Sections from keratotomy wounded corneas were blocked in 10% donkey serum (Sigma-Aldrich, St. Louis, MO) and then incubated overnight at 4°C in anti-collagen XII (kind gift of Dr Manuel Koch, Hamburg), anti-CD68 (BioLegend, San Diego, CA, catalog #137001), anti-LTBP1 (a generous gift from Dr Carl-Henrik Heldin, Uppsala University, Uppsala, Sweden) and anti-α-SMA. The same procedure was repeated for the right corneas of each mouse which were used as controls. Mouse fibroblasts were cultured as previously described and were fixed and stained using similar protocols.(Sun et al., 2021) Glass slides were incubated overnight at 4°C in above antibodies: anti-collagen XII, anti-LTBP1 and anti-fibronectin.
1.5. Second harmonic generation microscopy
Corneal tissue, 7– 10 days after creating a full-thickness keratotomy wound, was evaluated using standardized protocol.(Cogswell et al., 2021; Sun et al., 2022; Sun et al., 2021) Following euthanasia the injured left cornea from each mouse was embedded and frozen in OCT medium (Sakura Finetek, Torrance, CA). Cross sections, 15 μm thick, were cut using an NX 50 cryostat and each section was incubated overnight at 4°C in anti-LTBP1. Each section was imaged using an Olympus MPE-RS microscope using a 25× (0.95 NA) water-immersion objective (Olympus). Propidium iodine (Thermo Fisher Scientific, Waltham, MA) in a 1:100 concentration was added to the Optisol solution at the time of imaging. Two-photon second harmonic generation (SHG) signals were created using a mode-locked titanium:sapphire laser at 960 nm. The SHG forward-scattered signals passing through the corneal sections were collected using a 0.8 NA condenser lens with a narrow band-pass filter (465–485 nm). All samples were scanned using a 2-μm z-axis step size from the back to the front of the section. The two-photon excited fluorescent signal from propidium iodine was captured with a band pass filter (575–630 nm)
1.6. Transmission electron microscopy (TEM)
Ten days after full-thickness keratotomy wounds were created, the tissue was evaluated using a standardized protocol.(Hemmavanh et al., 2013) After euthanasia the injured left cornea from each mouse was infiltrated and embedded in a mixture of Embed 812, nadic methyl anhydride, dodecenyl succinic anhydride, and DMP-30 (Electron Microscopy Sciences, Hatfield, PA). Thin sections (~80 nm) were cut with a Leica ultramicrotome and poststained with 2% aqueous uranyl acetate and 1% phosphotungstic acid, pH 3.2. The sections were examined at 80 kV with a JEOL 1400 transmission electron microscope equipped with a Gatan Ultrascan US1000 2K digital camera.
1.7. RNA isolation and quantification of mRNA
Whole corneas were dissected from a group of adult mice that underwent eye injury as described above, 3 weeks after injury. One group had a burr injury. Another group had a full thickness keratotomy. The tissue from the full thickness keratotomy was separated into 2 regions: area of injury (I) where the wound was created and remote area (R) where no injury existed. Isolated corneal tissues were incubated with Dispase II (Roche, Indianapolis, IN) to remove the cornea epithelium. The stroma of cornea was cut into small pieces and total RNA was extracted using QIAzol Lysis Reagent (Qiagen, Venlo, Limburg) and RNeasy MinElute Cleanup Kit (Qiagen, Venlo, Limburg). Reverse transcription and quantitative real-time PCR analysis was performed as described before.(Sun et al., 2020b) Basically, 4 ng total RNA per well was subjected to reverse transcription by using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Waltham, MA) and the real-time PCR was performed with PowerTrack™ SYBR Green Master Mix (Applied Biosystems, Waltham, MA) on a StepOnePlus Real Time PCR system (Applied Biosystems, Waltham, MA) following the manufacturer’s protocol. Comparative CT method with Actb as a reference gene was used for quantification. The fast-cycling parameters include an initial denaturation step at 95 °C for 5 min, followed by 40 cycles of 5 s at 95 °C, 30 s at 60°C. A melting curve was performed at the end of the RT-qPCR run with 15 seconds at 95°C and then 15 seconds each at 0.3°C increments between 60°C and 95°C. The following primer sequences were used: Col12a1 FW: CCCTACAACAGATGGGCCTAC, Col12a1 RV:TCTTCTCCCCTGGCTTTGTA; Actb FW: AGATGACCCAGATCATGTTTGAGA, Actb RV: CACAGCCTGGATGGCTACGT. Each sample was run in duplicate PCR reaction, statistical analysis was performed from 3–6 corneas of different mice at each time point.
1.8. Corneal fibroblasts expansion on plastic dishes
Corneal fibroblasts were harvested from adult, 60-days-old, male C57BL/6 and Col12a1−/− mice using an isolation technique previously described.(Sun et al., 2021) To simulate the study of a provisional matrix in vitro, keratocytes were seeded onto cell culture chamber slides treated for cell culture (Nunc™ Lab-Tek™ II Chamber Slide™ System, Thermo Fisher Scientific, Waltham, MA, USA). Corneal fibroblasts were expanded on 5% FBS in DMEM (Sigma Aldrich, St Louis, MO) and supplemented with ascorbic acid (Sigma Aldrich, St Louis, MO). Medium was then exchanged every 2 days and kept in a 37°C incubator.
1.9. Statistical analysis
GraphPad Prism version 9.1.2 (San Diego, CA, USA) was used for all statistical analyses and all data are shown as means ± standard deviation. Statistical significance between two conditions was evaluated by non-parametric tests, Mann Whitney and Wilcoxon. Values with P < 0.05 were considered statistically significant, (*P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.001).
Results
2.1. Collagen XII expression is upregulated during normal wound repair following corneal injury
To evaluate whether collagen XII is upregulated during wound healing in adult corneas, stromal expression of collagen XII after injury -was studied by fluorescence microscopy and quantified by mRNA Col12a1 expression- 3 weeks after burr injury and incisional stromal keratotomy. Following stromal burr injury, expression of collagen XII was documented in the anterior corneal stroma, Fig 1A. Area of burr injury is shown by uptake of DTAF, Fig 1B. Quantification of Col12a1 mRNA transcription showed upregulation of collagen XII in the cornea with burr injury compared to control eye, Fig 1C. In a full-thickness keratotomy model, expression of collagen XII was observed throughout newly regenerated extracellular matrix, Fig 1D. Area of formed new matrix is localized between DTAF marking done at time of surgery, Fig 1E. Quantification of Col12a1 mRNA transcription showed expression of collagen XII in the injured area compared to the remote area (uninjured), Fig 1F.
Figure 1: Expression of collagen XII following trauma three weeks after injury in two different types of injury.
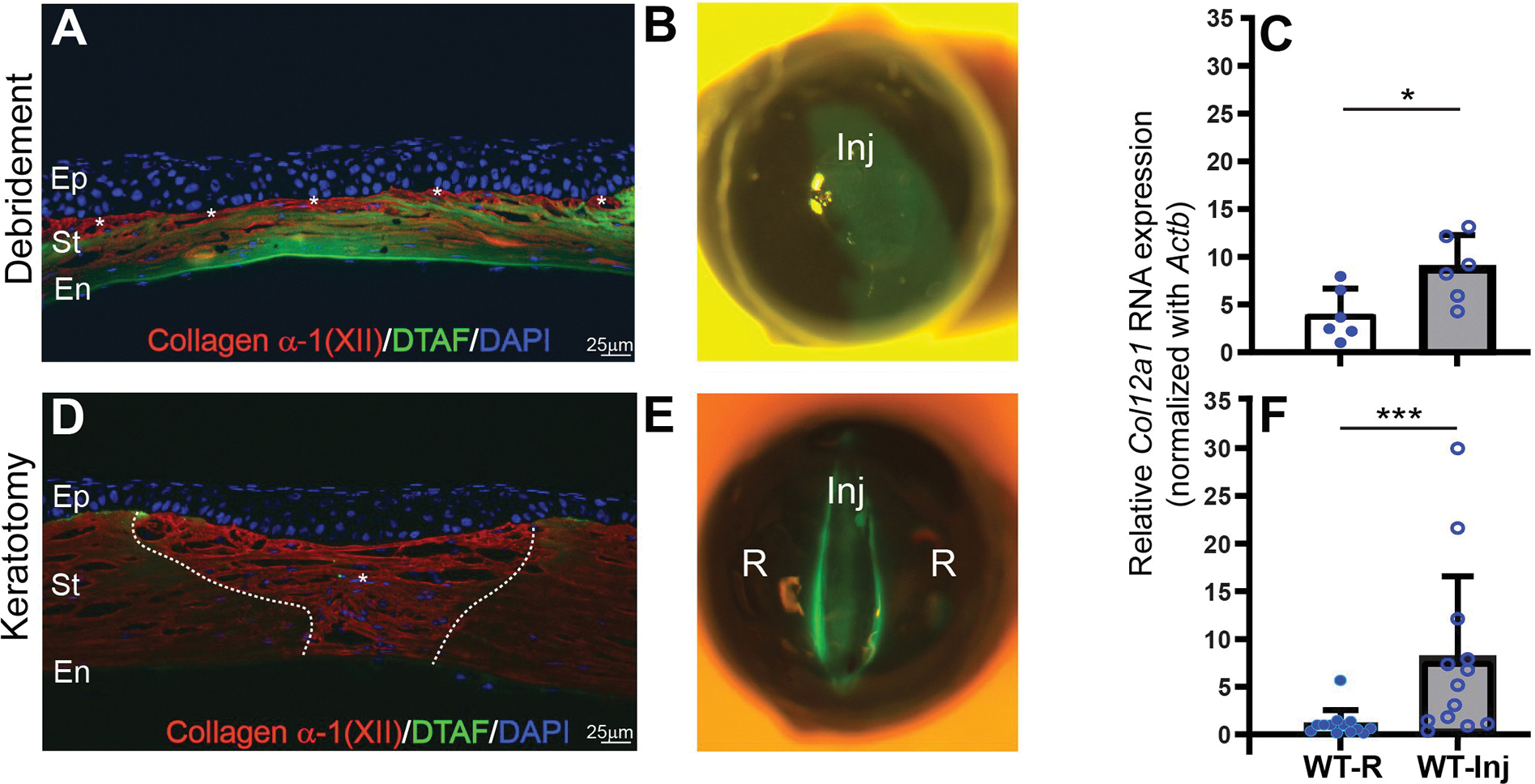
(A) A model of deep burr injury in the stroma with loss of stromal tissue shows expression of collagen XII in the surface of the cornea, white asterisks. (B) Slit-lamp microphotograph demonstrates area of burr injury demarcated by DTAF. (C) Quantitative PCR quantification shows statistically significant expression of Col12a1 mRNA transcripts compared to control contralateral uninjured eye; Wilcoxon paired test. (D) A lineal full thickness keratotomy injury with expression of collagen XII in the newly regenerated wound. White asterisk shows newly regenerated tissue expressing collagen XII. (E) Slit-lamp microphotograph demonstrates area of full thickness keratotomy injury demarcated by DTAF. New tissue regeneration and remodeling is located within DTAF area. (F) Quantitative PCR quantification shows statistically significant expression of Col12a1 mRNA transcripts compared to control uninjured area of the same eye. Wilcoxon paired test. DTAF: (5-(4,6-dichlorotriazinyl) aminofluorescein). Inj: area of injury, R: area outside injury. *P < 0.05; ***P < 0.005. Bar 25μm.
2.2. Collagen XII deficiency does not affect scar density after deep corneal debridement
No difference was found in average Fantes scar score following stromal burr injury in Col12a1−/− mice (average score of 1.26) compared to wild type (average score of 1.30) at 3 weeks. See Supplemental figure 1.
2.3. Collagen XII regulates wound closure after full-thickness keratotomy
Based on our preliminary data and pilot studies, a 1 mm corneal incision closes within 10 days. To assess the role of collagen XII in regulating early phases of wound healing, we studied corneal immature wounds -no older than 10 days after a full thickness keratotomy-, in wild type and Col12a1−/− mice. Slit-lamp examination showed corneas in wild type mice with a formed anterior chamber, epithelialized corneal surface, and a well-developed scar, Fig. 2A. In contrast, the process of wound closure in the Col12a1−/− was delayed and an immature thin wound with the presence of descemetocele (tissue bulging) was seen, Fig 2B. A statistically significant difference in scar density after full thickness keratotomy was found. Col12a1−/− corneas had an average Fantes scar score of 2.71 compared to 1.71 for wild type mice (p<0.01). A statistically significant difference in wound diameter as measured using ImageJ software (version 1.53c NIH, USA) was found as well between wild type mice (120 microns ± 50) and Col12a1−/− corneas (210 microns ± 83) (p=0.04).
Figure 2: Collagen XII regulates wound closure after corneal injury -1 week- after penetrating keratotomy.
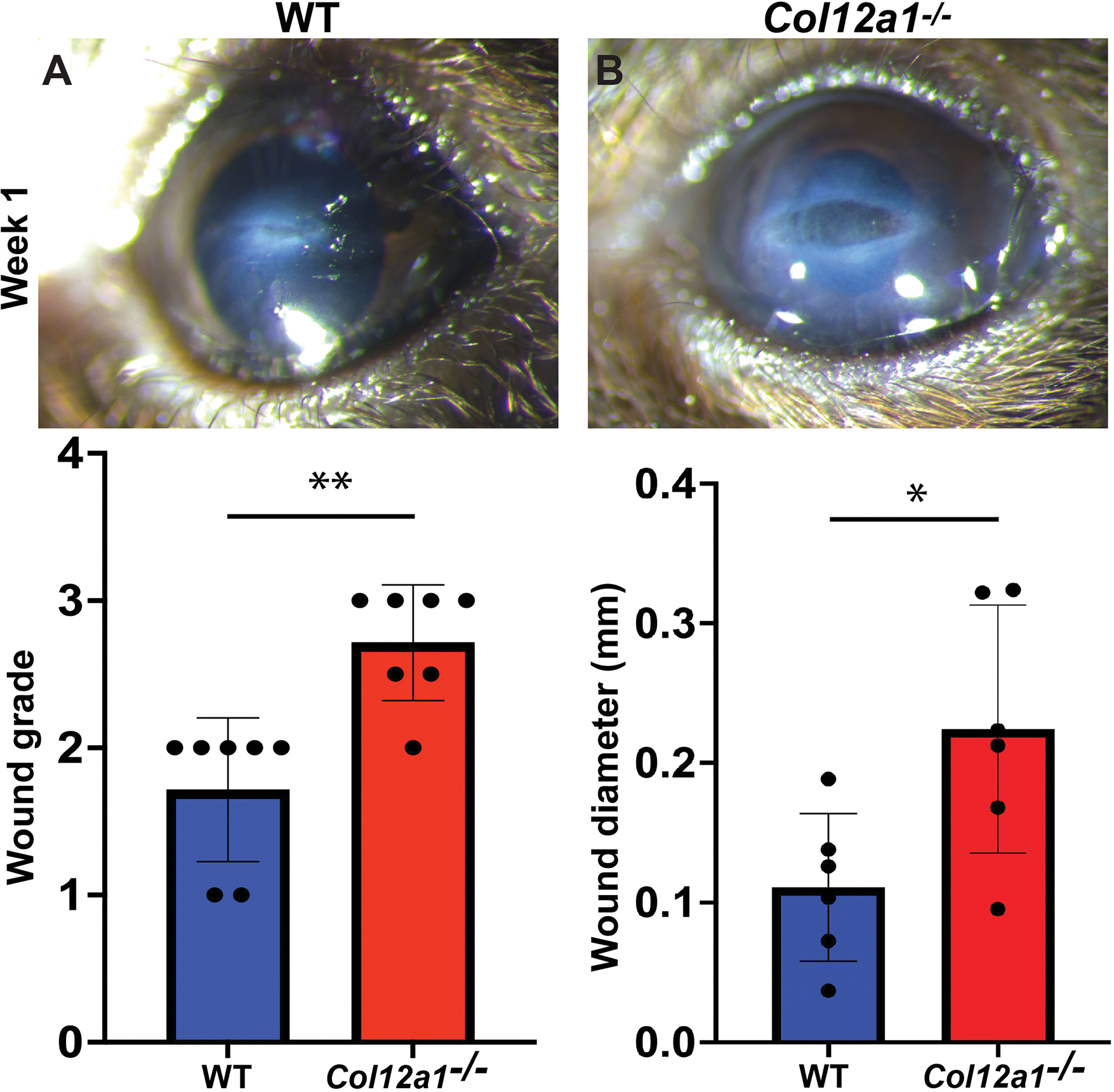
Progressive closure of a 1 mm penetrating full thickness cut (keratotomy) of the cornea. (A) Representative photograph at 1 week in a WT cornea. (B) Representative photographs show delayed wound closure in the absence of collagen XII. (C) Denser scar formation in the absence of collagen XII. (D) Larger immature wound developing in the absence of collagen XII. Two tailed Mann Whitney test, *P < 0.05; **P < 0.01.
When specific expression of collagen XII in the forming extracellular matrix closing the wound was examined, 10 days after injury, we found collagen XII expression in regenerated bridging extracellular matrix connecting the two wound edges in wild type injury model, (Figs 3A and 3B). In contrast, no collagen XII staining was observed in Col12a1−/− corneas where a much larger gap in tissue was evident, (Figs 3C and 3D).
Figure 3: Collagen XII regulates tissue repair during early stages of repair (day 10) and is present in the provisional matrix that bridges both ends of the wound.
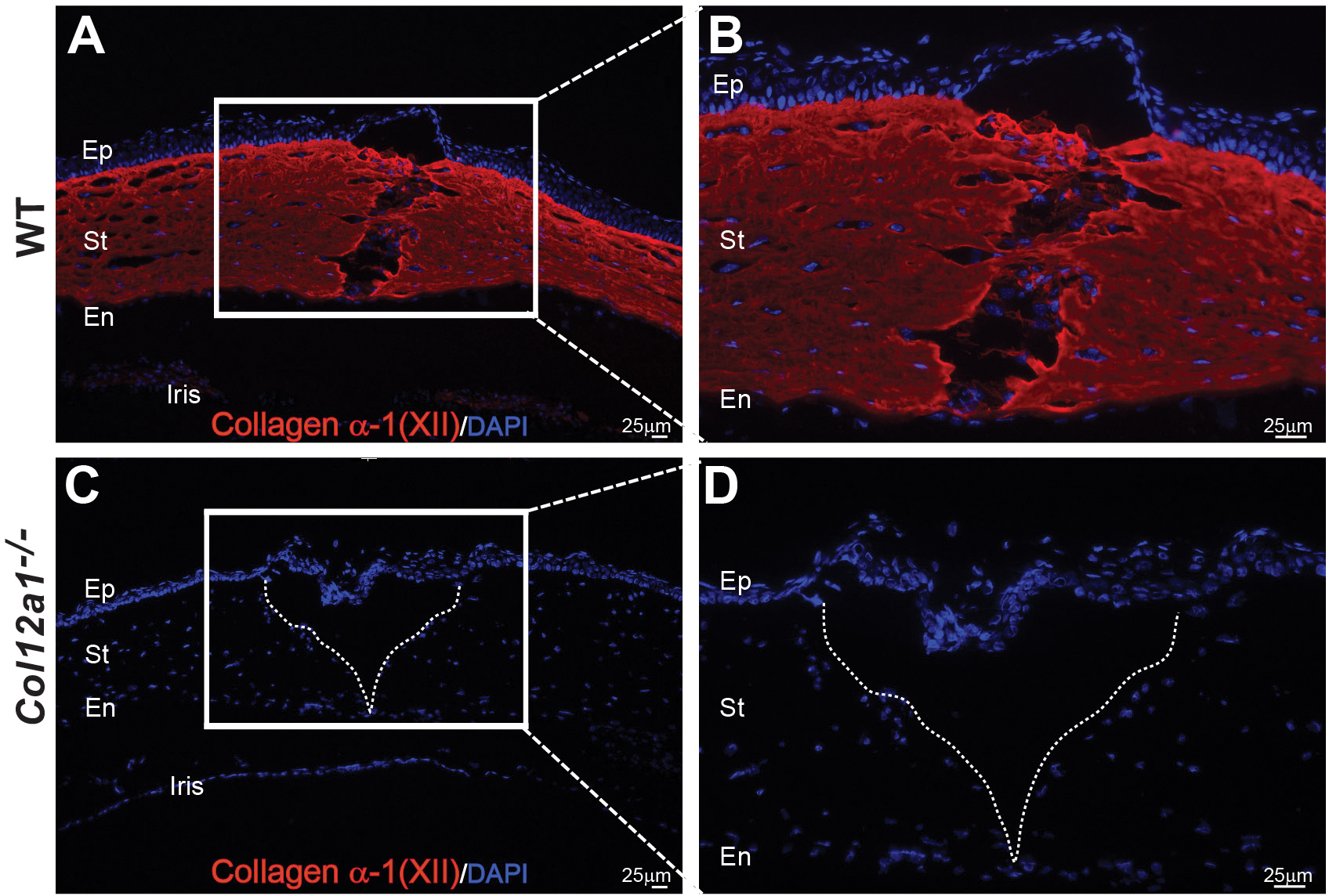
(A) Collagen XII expression is noted in the regenerated tissue bridging ends of the keratotomy injury during early tissue repair. (B) Higher magnification image shows collagen XII expression in regenerated matrix where migrating fibroblasts are secreting collagen XII-note collagen XII around cells. (C) Control, Col12a1−/− mouse cornea show epithelialized larger wound and no expression of collagen XII. (D) A poorly healed wound in higher magnification- note no stromal cells in area. Bar 25 μm.
2.4. Collagen XII down-regulates wound maturation as demonstrated by myofibroblast and CD68 + cells infiltration in the wound
We rarely identified CD68 positive cells or α-SMA expression at the wound in wild type corneas, Fig 4A and B, respectively. Following full thickness keratotomy, Col12a1−/− corneas displayed increased CD68 positive cells and increased α-SMA signal, (Fig 4D). The number of CD68 positive cells at the wound site was quantified at 20X magnification field. Collagen XII knockout mice displayed a higher mean number of CD68 positive cells (16.78 cells/20X field) compared to wild type mice (11.2 cells/20X field), but the difference was not statistically significant (unpaired t-test, p=0.085).
Figure 4: Collagen XII regulates infiltration of CD68 lineage cells and survival of myofibroblasts in maturing wound.
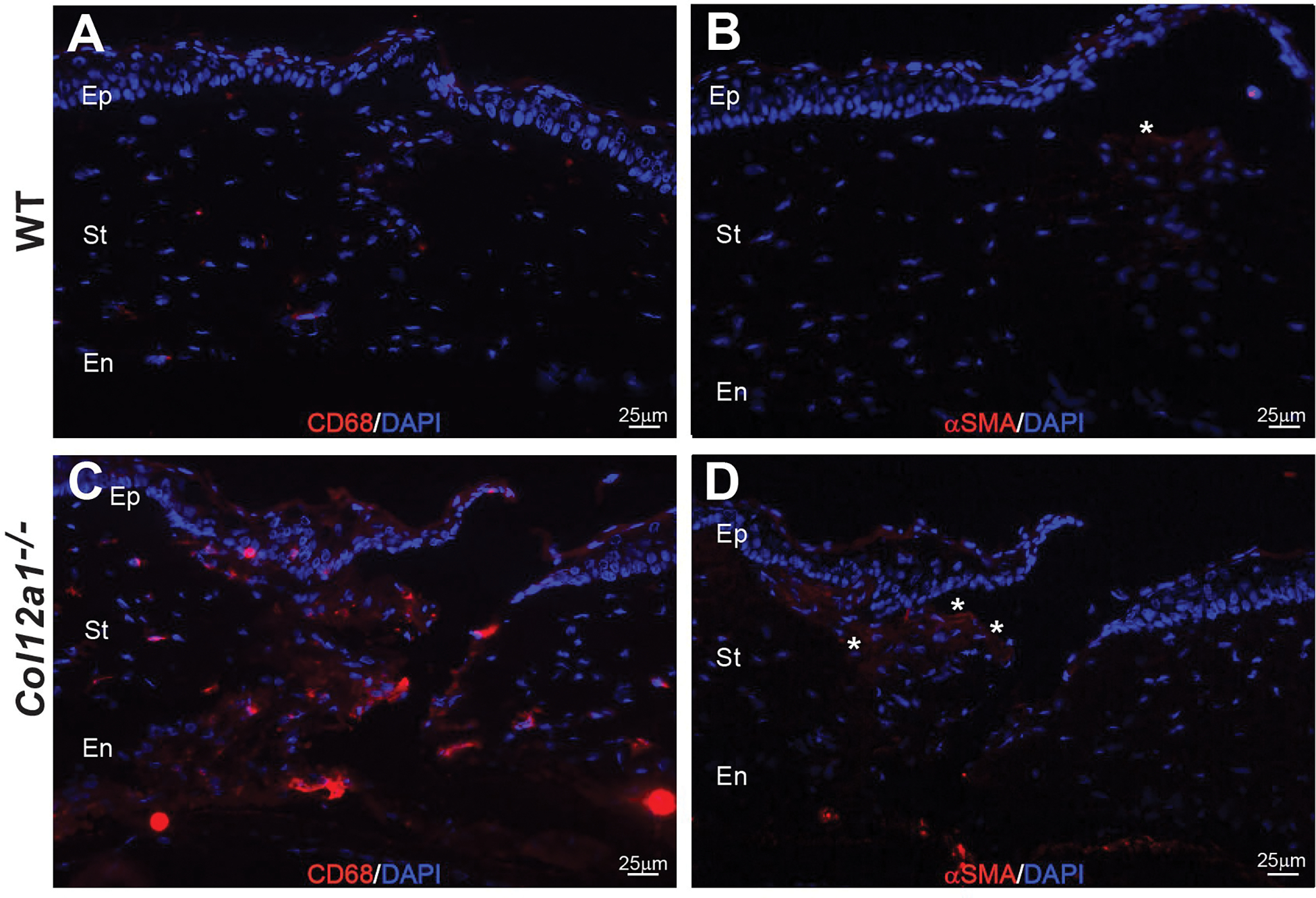
(A) Increased infiltration of CD68 lineage cells (red) in the wound is minimal in the WT mouse. (C) Increased infiltration of CD68 lineage cells in the wound in the absence of collagen XII is evident. (B) Minimal presence of myofibroblasts (red expression and shown by asterisk) in the maturing wound of an injured WT cornea. (D) Increased expression of α-smooth muscle actin cells (red expression) in the wound deficient in collagen XII, shown by asterisks. Bar 25 μm.
2.5. Collagen XII regulates wound organization and maturation following full thickness Keratotomy
Second harmonic generated imaging was performed on full-thickness keratotomy wounds from both wild type and Col12a1−/− corneas to evaluate matrix collagen fibrillogenesis in the remodeling area closing the wound. Collagen fibrils were noted partially filing the injury area in wild type mice but not in Col12a1−/− corneas. LTBP-1 staining was added to assist in identifying non-collagenous components of the extracellular matrix in the forming provisional matrix, Fig. 5A. Col12a1−/− corneas had greater areas of unfilled matrix in the wounds compared to wild type corneas. Keratotomy wounds in the Col12a1−/− corneas displayed increased LTBP-1 expression and contained more cells compared to wild type mice in the uninjured area, Fig. 5B. Microphotographs from transmission electron microscopy confirmed these findings. Wild type wounds appeared to exist in a more advanced stage of repair and were closer to restoring the regularly arranged collagen network seen in healthy, uninjured controls, Figs 6A and B. Increased cellularity in keratotomy wounds from Col12a1−/− mice compared to wild type mice was evident, with larger activated cells being the predominant type found in wounds of Col12a1−/− mice, Fig. 6C. Collagen XII deficient wounds had noticeably less matrix material compared to wild type wounds and contained ill-defined extracellular matrix material within disorganized collagen networks, Fig. 6D.
Figure 5: Collagen XII regulates wound maturation and deposition of a provisional matrix during tissue repair.
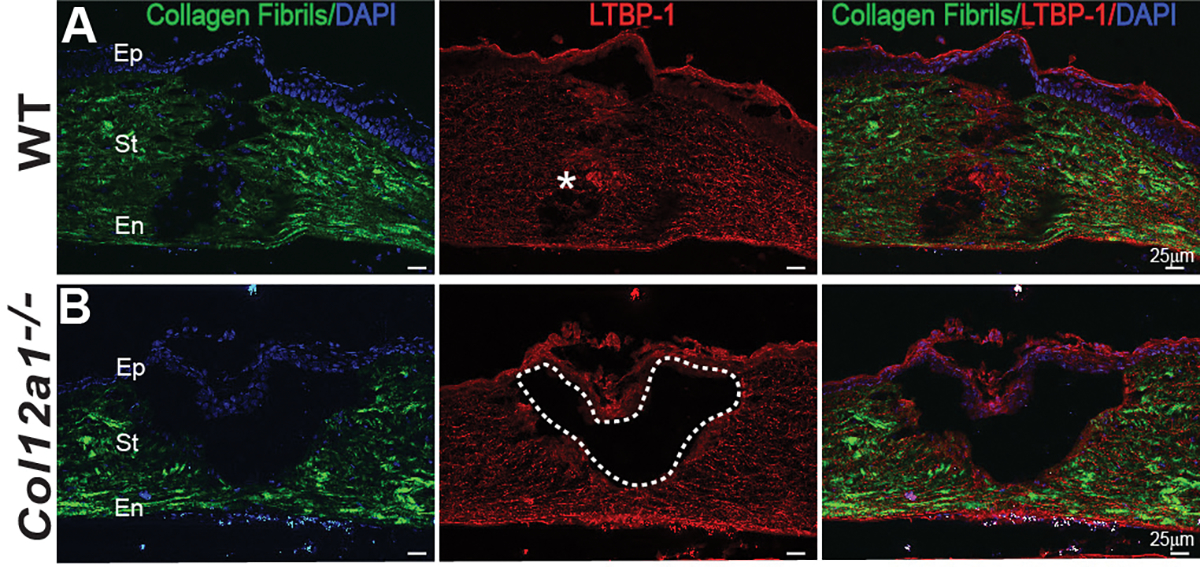
(A) Histology sections showed regenerated collagen in the injury area in WT cornea, shown in green by SHG imaging. LTBP-1 expression is noted, marked by asterisk, filling in the wound where fibrillogenesis is occurring. Areas of colocalization of LTBP-1 expression and fibrillogenesis are noted in the maturing wound. (B) Immature open wound with no regenerated collagen in the wound in the absence of collagen XII. There is no LTBP-1 expression in the open wound in the collagen XII deficient cornea. Dashed line demarcates area of open wound. Bar 25 μm.
Figure 6: Transmission electron microscopy microphotographs demonstrating the role of collagen XII in regulating fibrillogenesis and wound maturation 7 – 10 days pot injury.
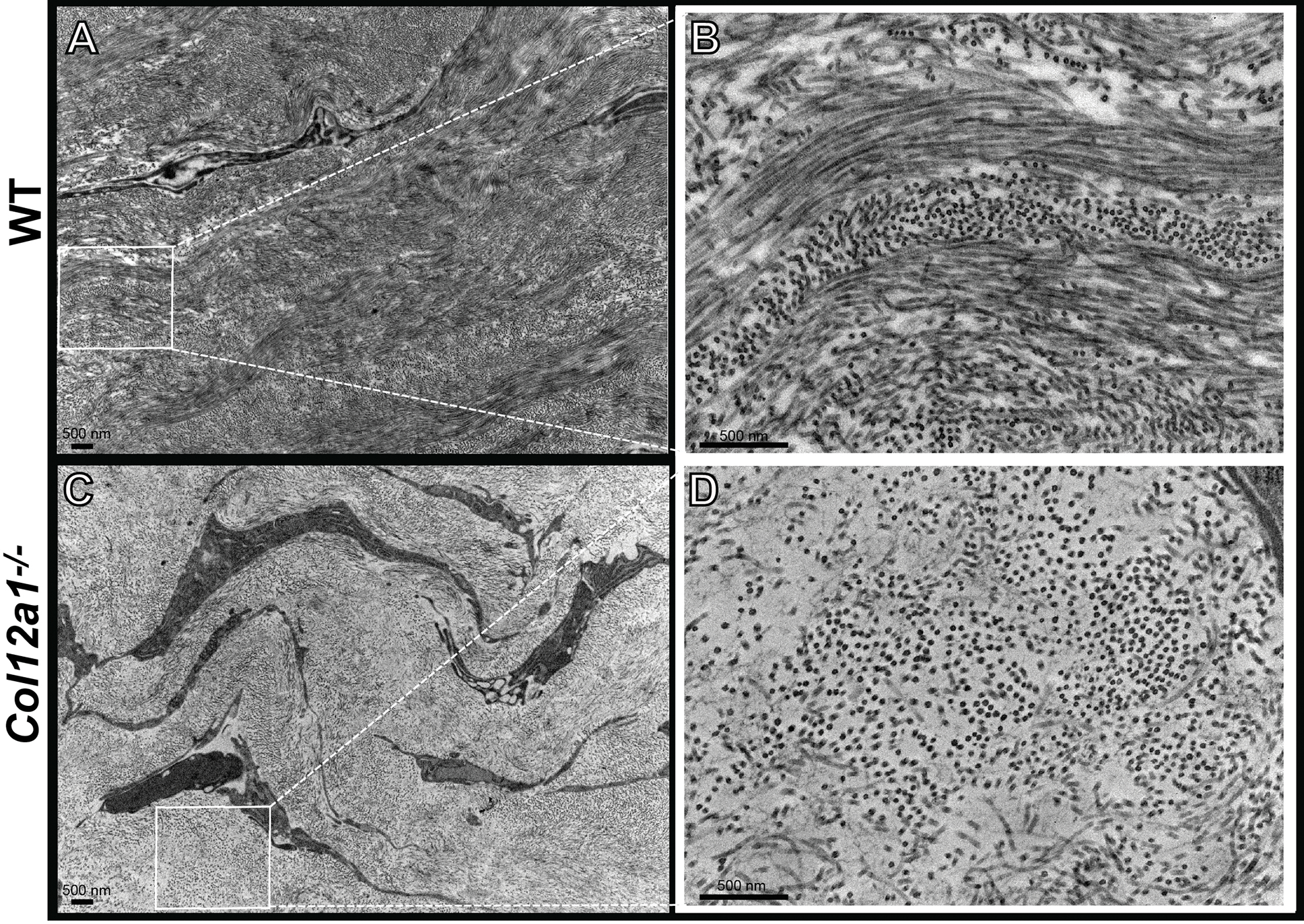
(A) Activated fibroblasts in the WT corneal matrix show significant fibrillogenesis and activated fibroblasts in the maturing wound. A1: Normal alignment of fibrils and lamellae in WT corneas. (B) Larger and apparently activated fibroblasts in the collagen XII deficient matrix in a regenerated matrix with decreased fibrillogenesis in the maturing wound. B1: Higher magnification confirms poor fibrillogenesis and poor fibril organization in a regenerated matrix without collagen XII. Bar 500 nm.
2.6. Collagen XII colocalizes with fibronectin and LTBP1 in vitro
To explore a mechanism that explains how collagen XII can regulate formation of new matrix, we investigated whether collagen XII interacts with matrix components involved in the establishment of a late provisional matrix during wound healing.(Barker and Engler, 2017) We studied fibronectin, main component of provisional matrix, and LTBP-1 because this protein is known to regulate the storage and release of TGF-β1 from matrix. We found using double staining techniques that collagen XII colocalizes with both fibronectin and LTBP-1. Collagen XII was localized intracellularly and in the extracellular space surrounding cells. Colocalization with fibronectin was evident in the extracellular space around fibroblasts, Fig. 7A. Similarly, colocalization with LTBP-1 was found in the extracellular space around fibroblasts as well as intracellularly, Fig. 7B.
Figure 7: Collagen XII colocalizes with provisional matrix components, fibronectin and LTBP1 in vitro, suggesting regulation of temporary matrix formation and organization by collagen XII.
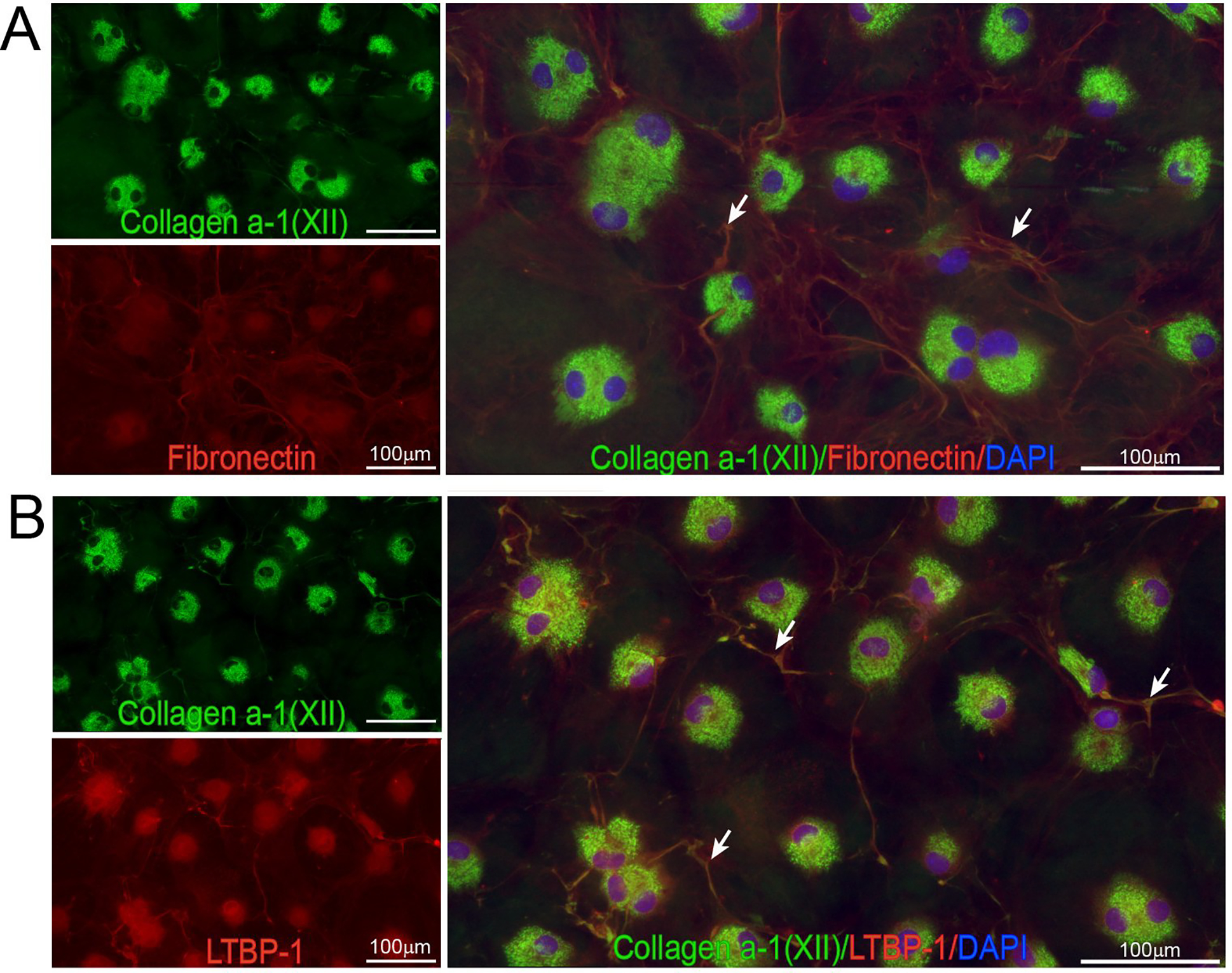
(A) Colocalization of collagen XII and fibronectin was noted during in vitro expansion of corneal derived fibroblasts suggestive of interactions between collagen XII and fibronectin. (B) Colocalization of collagen XII and LTBP-1 in the matrix formed in vitro during expansion of corneal derived fibroblasts suggestive of interactions between collagen XII and LTBP-1. Bar 100 μm.
Discussion
The unique components of the corneal extracellular matrix form a complex tissue array that provides structure, resists mechanical stress and strain, sequesters-growth factors and cytokines, and gives mechanical and chemical feedback to resident cells during homeostasis and after injury.(Bonnans et al., 2014; Espana and Birk, 2020; Hynes, 2009) Understanding the roles of collagen XII in regulating corneal wound healing will open new venues to modulate scar formation, and reduce visual morbidity following injury. In this manuscript we show that collagen XII plays a significant role in corneal wound healing by regulating wound closure and maturation.
How Collagen XII, a structural collagen family member, regulates tissue structure and cell function during tissue repair is a fundamental question. We hypothesize that although collagen XII is classified as a collagen, its unique structure and large size with different splicing variants likely provides it with functional in addition to structural properties. The largest variant, 300 kDa, contain different domains (18 FN3, fibronectin like 3 domains, 4 VWA, Von Willenbrand factor type A domains, 1 thrombospondin domain, and 2 collagenous domains), See Fig. 8. All these domains can hypothetically interact with different cytokines and other matrix components to regulate cytokine and proteases storage, release, and activation from the matrix during development and if the process of tissue repair is activated.(Hynes, 2009)
Figure 8: Collagen XII structure.
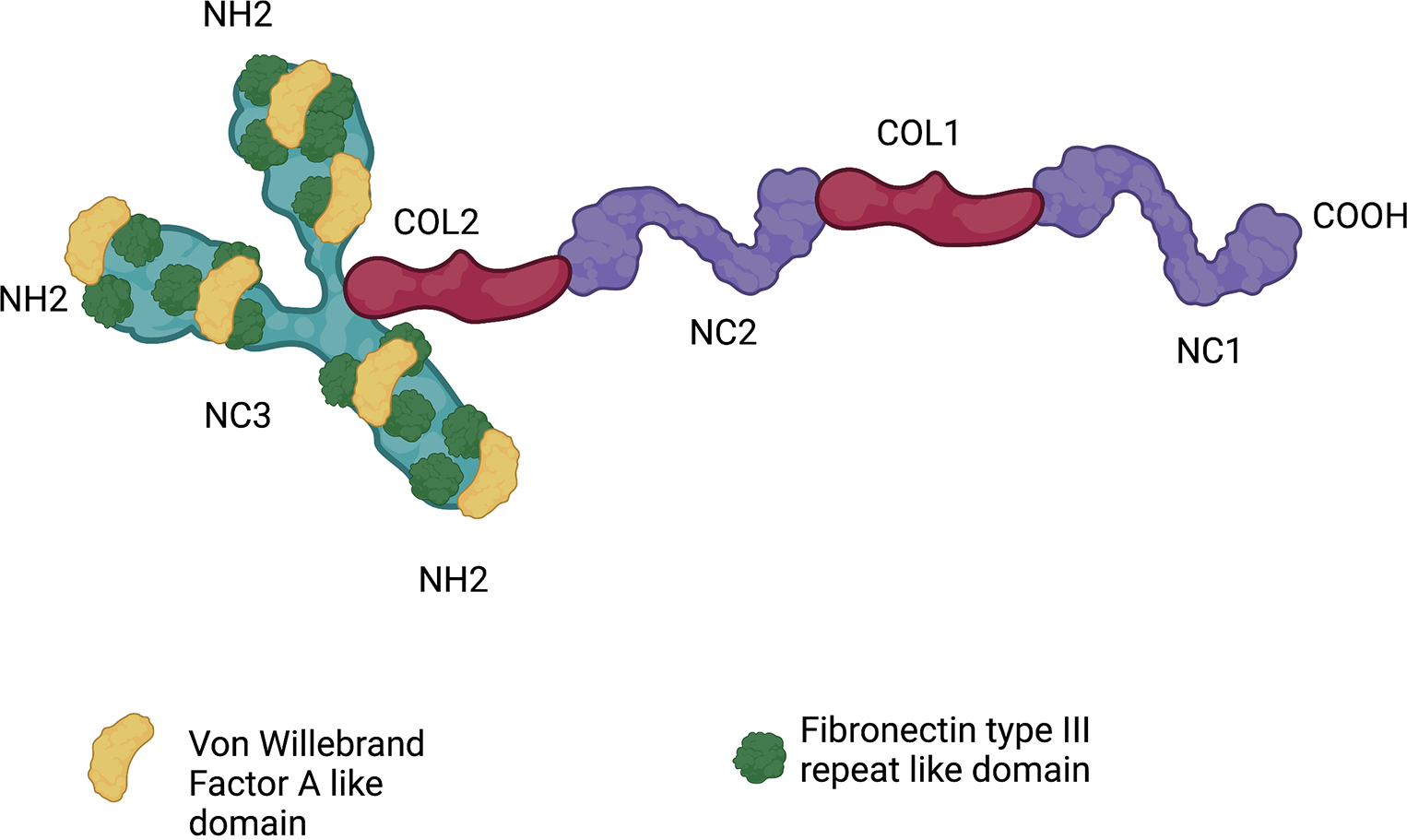
Homotrimeric molecule composed of two collagenous domains (COL1–COL2) and three noncollagenous domains (NC1 to NC3)
The data in this study strongly support the hypothesis that collagen XII acts as a regulator of wound closure and scar maturation: 1- we identified collagen XII expression in repaired tissue and scars following stromal burr injury and full-thickness keratotomy; 2- collagen XII is present in the new provisional matrix that bridges the two ends of the wound during tissue repair; 3- incomplete wound closure following full-thickness keratotomy in the absence of collagen XII suggests a role for this protein in wound contraction and consolidation of wound/scar mechanical and tensile properties. One of the mechanisms that could explain delayed wound closure and scar maturation is dysregulation of TGF-β storage and activation by collagen XII. We have recently demonstrated that collagen XII regulates TGF-β storage and release by corneal keratocytes and fibroblasts in vitro and in vivo, with higher levels of active TGF-β present in the stroma in the absence of collagen XII.(Sun et al., 2022) Similarly, in a skin punch injury model, increased tissue levels of active TGF-β1 in Col12a1−/− mice were detected compared to wild type controls.(Schonborn et al., 2020) Despite increased active TGF-β1 in Col12a1−/− group, delayed skin granulation as well as poor tissue contraction and retraction with delayed closure of wounds were noted in the absence of collagen XII.(Schonborn et al., 2020) However, the exact mechanism(s) by which collagen XII regulates TGF-β activity remains elusive.
We explored if collagen XII influences wound healing by regulating fibroblast activity, macrophage infiltration, fibrillogenesis, tissue mechanics or all these factors together. To investigate the function of collagen XII in regulating those cells populations known to modulate wound healing, we looked at the presence of activated fibroblasts and cells of monocyte lineage (expression of CD68 includes resident dendritic cells and circulating macrophages) in the wound. We found that collagen XII influences both, CD68+ infiltration as well as the activation of fibroblasts. Infiltration by CD68+ cells and expansion of fibroblasts and acquisition of a myofibroblast phenotype are prominent features of the proliferative phase of wound healing. Compared to wounds in wild type corneas, keratotomy wounds in Col12a1−/− had increased number of CD68+ and cells expressing α-SMA. We selected 1 week post injury, time point, to evaluate CD68+ and α-SMA expression and this needs to be remembered when elucidating our findings. These data are supported by the increase of activated fibroblasts in Col12a−/− observed in the TEM microphotographs where increased numbers of activated fibroblasts are present. Our finding aligns with previously published results from studies in skin injury, where increased macrophage and activated fibroblast numbers in the wound do not translate to increased wound contracture or faster wound healing. Evaluation of naïve contralateral eyes showed normal stromal cell numbers and morphology. mRNA analysis showed no significant difference in the expression of different matrix components (collagens V, XI, XII, and XIV) and the remote area of uninjured area.
Why is wound closure delayed if more macrophages and activated fibroblasts/myofibroblasts - known to upregulate wound closure- are present in the wounds of Col12a−/− corneas, a matrix without collagen XII? Our data show that collagen XII regulates fibrillogenesis and matrix organization during wound maturation and scar consolidation. It may also synchronize micro-mechanical tissue interactions. Images generated by SHG microscopy and TEM microphotographs of maturing keratotomy wounds, in a collagen XII deficient matrix during the formation of a provisional matrix, each displayed decreased collagen fibrillogenesis with ill-defined extracellular matrix and disorganization of collagen networks when compared to wild type corneas. Thus, we hypothesize that an abnormal matrix and tissue with dysfunctional mechanical properties develop in early stages of wound repair in the absence of collagen XII. We have recently shown that collagen XII is a regulator of stromal stiffness.(Nair et al., 2022) This dysfunctional matrix will create a vicious cycle affecting fibroblast activation and myofibroblast survival. An immature and dysfunctional matrix will promote fibroblast activation and will inhibit myofibroblast apoptosis. Together, these findings suggest that collagen XII reexpression during tissue repair is essential to reestablish the properties of matrix and tissue function. Collagen XII expression affects wound healing from early stages when repairing cells are secreting a provisional matrix.
To further elucidate the mechanisms behind the observed delay in wound healing and contracture, we performed in vitro experiments designed to detect the interaction of two key provisional matrix components, fibronectin and LTBP-1, with collagen XII. The expression of collagen XII in vivo, 10 days post injury as in Figure 3, as well as our colocalization in vitro studies show that collagen XII interacts with fibronectin and LTBP-1 and suggest that collagen XII plays a role in regulating key extracellular matrix components as early as during the provisional matrix formation phase. Interestingly, all three matrix components studied in this work are regulators of TGF-β function. These results suggest that the reason for impaired wound contraction is not due to a cellular defect. Rather these impairments are more likely due to defects in extracellular matrix composition, functionality, signaling and assembling in the absence of collagen XII. We are continuing with additional studies to determine the role of collagen XII in regulating myofibroblast conversion, survival and contraction.
The regulation of TGF-β activity by collagen XII may be exerted through multiple pathways. These can include the regulation of tissue mechanics which can potentially facilitate or disturb the activation of TGF-β1, binding of collagen XII to other matrix components (our preliminary data show that collagen XII binds LTBP1 and fibronectin), and regulation of MMPs or other proteolytic enzymes by collagen XII.(Nair et al., 2022) We are currently working on an inducible mouse model where we can manipulate the expression of collagen XII during different stages of wound closure and scar maturation. We plan to explore the possibility that down-regulating collagen XII at different stages of wound repair is beneficial to reestablish matrix properties and tissue function. Manipulating collagen XII expression during later stages of wound healing and scar maturation by gene therapy could hypothetically downregulate matrix mechanics and scar density. We have recently shown that collagen XII is a regulator of corneal matrix stiffness, and it is well established that myofibroblasts survival is sensitive to tissue mechanics.(Nair et al., 2022; Pakshir et al., 2020)
In conclusion, collagen XII regulates wound and scar maturation. Wound closure is delayed and activated fibroblasts and myofibroblasts are more commonly present in wounds when collagen XII is not expressed. Our findings suggest that collagen XII is necessary to consolidate matrix regeneration and formation of scar tissue. Manipulation of collagen XII expression at different stages of wound repair could be employed for therapeutic proposes to modify the process or healing and minimize the adverse consequences of scarring.
Supplementary Material
Highlights:
Collagen XII is essential to attain wound closure and scar maturation.
Incisional injuries heal poorly and closure is delayed in the absence of collagen XII.
Collagen XII is deposited early during wound closure and interacts with other important components of the provisional matrix to regulate wound closure.
Acknowledgments
This study was supported by NIH/NEI grants EY029395 (EME) and EY034114 (EME).
Footnotes
Proprietary Interests: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal P, Zwolanek D, Keene DR, Schulz JN, Blumbach K, Heinegard D, Zaucke F, Paulsson M, Krieg T, Koch M, Eckes B, 2012. Collagen XII and XIV, new partners of cartilage oligomeric matrix protein in the skin extracellular matrix suprastructure. J Biol Chem 287, 22549–22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akgun Z, Palamar M, Egrilmez S, Yagci A, Selver OB, 2022. Clinical Characteristics and Severity Distribution of Tertiary Eye Center Attendance by Ocular Chemical Injury Patients. Eye Contact Lens 48, 295–299. [DOI] [PubMed] [Google Scholar]
- Annes JP, Munger JS, Rifkin DB, 2003. Making sense of latent TGFbeta activation. J Cell Sci 116, 217–224. [DOI] [PubMed] [Google Scholar]
- Barker TH, Engler AJ, 2017. The provisional matrix: setting the stage for tissue repair outcomes. Matrix Biol 60–61, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmeier V, Etich J, Pitzler L, Frie C, Koch M, Fischer M, Rappl G, Abken H, Tomasek JJ, Brachvogel B, 2018. Identification of a myofibroblast-specific expression signature in skin wounds. Matrix Biol 65, 59–74. [DOI] [PubMed] [Google Scholar]
- Bonnans C, Chou J, Werb Z, 2014. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 15, 786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogswell D, Sun M, Greenberg E, Margo CE, Espana EM, 2021. Creation and grading of experimental corneal scars in mice models. Ocul Surf 19, 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana EM, Birk DE, 2020. Composition, structure and function of the corneal stroma. Exp Eye Res, 108137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana EM, Sun M, Birk DE, 2015. Existence of Corneal Endothelial Slow-Cycling Cells. Invest Ophthalmol Vis Sci 56, 3827–3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantes FE, Hanna KD, Waring GO 3rd, Pouliquen Y, Thompson KP, Savoldelli M, 1990. Wound healing after excimer laser keratomileusis (photorefractive keratectomy) in monkeys. Arch Ophthalmol 108, 665–675. [DOI] [PubMed] [Google Scholar]
- Font B, Eichenberger D, Rosenberg LM, van der Rest M, 1996. Characterization of the interactions of type XII collagen with two small proteoglycans from fetal bovine tendon, decorin and fibromodulin. Matrix Biol 15, 341–348. [DOI] [PubMed] [Google Scholar]
- Gordon MK, Gerecke DR, Olsen BR, 1987. Type XII collagen: distinct extracellular matrix component discovered by cDNA cloning. Proc Natl Acad Sci U S A 84, 6040–6044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmavanh C, Koch M, Birk DE, Espana EM, 2013. Abnormal corneal endothelial maturation in collagen XII and XIV null mice. Invest Ophthalmol Vis Sci 54, 3297–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks D, Farsani GT, Laval S, Collins J, Sarkozy A, Martoni E, Shah A, Zou Y, Koch M, Bonnemann CG, Roberts M, Lochmuller H, Bushby K, Straub V, 2014. Mutations in the collagen XII gene define a new form of extracellular matrix-related myopathy. Hum Mol Genet 23, 2353–2363. [DOI] [PubMed] [Google Scholar]
- Holm TM, Habashi JP, Doyle JJ, Bedja D, Chen Y, van Erp C, Lindsay ME, Kim D, Schoenhoff F, Cohn RD, Loeys BL, Thomas CJ, Patnaik S, Marugan JJ, Judge DP, Dietz HC, 2011. Noncanonical TGFbeta signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science 332, 358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO, 2009. The extracellular matrix: not just pretty fibrils. Science 326, 1216–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izu Y, Sun M, Zwolanek D, Veit G, Williams V, Cha B, Jepsen KJ, Koch M, Birk DE, 2011. Type XII collagen regulates osteoblast polarity and communication during bone formation. J Cell Biol 193, 1115–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabosova A, Azar DT, Bannikov GA, Campbell KP, Durbeej M, Ghohestani RF, Jones JC, Kenney MC, Koch M, Ninomiya Y, Patton BL, Paulsson M, Sado Y, Sage EH, Sasaki T, Sorokin LM, Steiner-Champliaud MF, Sun TT, Sundarraj N, Timpl R, Virtanen I, Ljubimov AV, 2007. Compositional differences between infant and adult human corneal basement membranes. Invest Ophthalmol Vis Sci 48, 4989–4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene DR, Lunstrum GP, Morris NP, Stoddard DW, Burgeson RE, 1991. Two type XII-like collagens localize to the surface of banded collagen fibrils. J Cell Biol 113, 971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Bohrmann B, Matthison M, Hagios C, Trueb B, Chiquet M, 1995. Large and small splice variants of collagen XII: differential expression and ligand binding. J Cell Biol 130, 1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljubimov AV, Burgeson RE, Butkowski RJ, Couchman JR, Wu RR, Ninomiya Y, Sado Y, Maguen E, Nesburn AB, Kenney MC, 1996. Extracellular matrix alterations in human corneas with bullous keratopathy. Invest Ophthalmol Vis Sci 37, 997–1007. [PubMed] [Google Scholar]
- Massoudi D, Malecaze F, Soler V, Butterworth J, Erraud A, Fournie P, Koch M, Galiacy SD, 2012. NC1 long and NC3 short splice variants of type XII collagen are overexpressed during corneal scarring. Invest Ophthalmol Vis Sci 53, 7246–7256. [DOI] [PubMed] [Google Scholar]
- Mohassel P, Liewluck T, Hu Y, Ezzo D, Ogata T, Saade D, Neuhaus S, Bolduc V, Zou Y, Donkervoort S, Medne L, Sumner CJ, Dyck PJB, Wierenga KJ, Tennekoon G, Finkel RS, Chen J, Winder TL, Staff NP, Foley AR, Koch M, Bonnemann CG, 2019. Dominant collagen XII mutations cause a distal myopathy. Ann Clin Transl Neurol 6, 1980–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Ambekar YS, Zevallos-Delgado C, Mekonnen T, Sun M, Zvietcovich F, Singh M, Aglyamov S, Koch M, Scarcelli G, Espana EM, Larin KV, 2022. Multiple Optical Elastography Techniques Reveal the Regulation of Corneal Stiffness by Collagen XII. Invest Ophthalmol Vis Sci 63, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakshir P, Noskovicova N, Lodyga M, Son DO, Schuster R, Goodwin A, Karvonen H, Hinz B, 2020. The myofibroblast at a glance. J Cell Sci 133. [DOI] [PubMed] [Google Scholar]
- Rifkin D, Sachan N, Singh K, Sauber E, Tellides G, Ramirez F, 2022. The role of LTBPs in TGF beta signaling. Dev Dyn 251, 95–104. [DOI] [PubMed] [Google Scholar]
- Rifkin DB, 2005. Latent transforming growth factor-beta (TGF-beta) binding proteins: orchestrators of TGF-beta availability. J Biol Chem 280, 7409–7412. [DOI] [PubMed] [Google Scholar]
- Rifkin DB, Rifkin WJ, Zilberberg L, 2018. LTBPs in biology and medicine: LTBP diseases. Matrix Biol 71–72, 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson IB, Horiguchi M, Zilberberg L, Dabovic B, Hadjiolova K, Rifkin DB, 2015. Latent TGF-beta-binding proteins. Matrix Biol 47, 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson IB, Rifkin DB, 2016. Regulation of the Bioavailability of TGF-beta and TGF-beta-Related Proteins. Cold Spring Harb Perspect Biol 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonborn K, Willenborg S, Schulz JN, Imhof T, Eming SA, Quondamatteo F, Brinckmann J, Niehoff A, Paulsson M, Koch M, Eckes B, Krieg T, 2020. Role of collagen XII in skin homeostasis and repair. Matrix Biol 94, 57–76. [DOI] [PubMed] [Google Scholar]
- Sun M, Koudouna E, Cogswell D, Avila MY, Koch M, Espana EM, 2022. Collagen XII Regulates Corneal Stromal Structure by Modulating Transforming Growth Factor-beta Activity. Am J Pathol 192, 308–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Zafrullah N, Adams S, Devaux F, Avila MY, Ziebarth N, Margo CE, Koch M, Espana EM, 2021. Collagen XIV Is an Intrinsic Regulator of Corneal Stromal Structure and Function. Am J Pathol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Zafrullah N, Devaux F, Hemmavanh C, Adams S, Ziebarth NM, Koch M, Birk DE, Espana EM, 2020. Collagen XII Is a Regulator of Corneal Stroma Structure and Function. Invest Ophthalmol Vis Sci 61, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzortzaki EG, Tischfield JA, Sahota A, Siafakas NM, Gordon MK, Gerecke DR, 2003. Expression of FACIT collagens XII and XIV during bleomycin-induced pulmonary fibrosis in mice. Anat Rec A Discov Mol Cell Evol Biol 275, 1073–1080. [DOI] [PubMed] [Google Scholar]
- Veit G, Hansen U, Keene DR, Bruckner P, Chiquet-Ehrismann R, Chiquet M, Koch M, 2006. Collagen XII interacts with avian tenascin-X through its NC3 domain. J Biol Chem 281, 27461–27470. [DOI] [PubMed] [Google Scholar]
- Vlasov A, Ryan DS, Ludlow S, Coggin A, Weichel ED, Stutzman RD, Bower KS, Colyer MH, 2017. Corneal and Corneoscleral Injury in Combat Ocular Trauma from Operations Iraqi Freedom and Enduring Freedom. Mil Med 182, 114–119. [DOI] [PubMed] [Google Scholar]
- Wessel H, Anderson S, Fite D, Halvas E, Hempel J, SundarRaj N, 1997. Type XII collagen contributes to diversities in human corneal and limbal extracellular matrices. Invest Ophthalmol Vis Sci 38, 2408–2422. [PubMed] [Google Scholar]
- Wynn TA, Vannella KM, 2016. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 44, 450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang E, Gupta S, Olson E, Sinha PR, Hesemann NP, Fraunfelder FW, Mohan RR, 2022. Effects of Regular/Dilute Proparacaine Anesthetic Eye Drops in Combination with Ophthalmic Antibiotics on Corneal Wound Healing. J Ocul Pharmacol Ther 38, 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Zwolanek D, Izu Y, Gandhy S, Schreiber G, Brockmann K, Devoto M, Tian Z, Hu Y, Veit G, Meier M, Stetefeld J, Hicks D, Straub V, Voermans NC, Birk DE, Barton ER, Koch M, Bonnemann CG, 2014. Recessive and dominant mutations in COL12A1 cause a novel EDS/myopathy overlap syndrome in humans and mice. Hum Mol Genet 23, 2339–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


