Abstract
Since more than 100 years, the adsorption of the radioactive noble gas radon (222Rn) is performed on activated charcoal at cryogenic temperatures. There is little—if any—progress in the field of radon adsorption at ambient conditions to facilitate the development of simple and compact radon adsorption systems. We report here on the truly remarkable property of the synthetic silver-exchanged zeolites Ag-ETS-10 and Ag-ZSM-5 to strongly adsorb radon gas at room temperature. 222Rn breakthrough experiments in nitrogen carrier gas have shown that these materials exhibit radon adsorption coefficients exceeding 3000 m3/kg at 293 K, more than two orders of magnitude larger than any noble gas adsorbent known to date. Water vapor and carrier gas type were found to strongly influence radon adsorption, practically qualifying these silver exchanged materials as a new class of radon adsorbents. Our results demonstrate that Ag-ETS-10 and Ag-ZSM-5 are materials that show high affinity towards radon gas at ambient temperatures making them candidate materials for environmental and industrial 222Rn mitigation applications. Adsorption systems based on silver loaded zeolites have the potential to replace activated charcoal as material of choice in many radon related research areas by avoiding the necessity of cryogenic cooling.
Subject terms: Environmental impact, Nuclear chemistry, Risk factors, Chemical engineering, Porous materials, Environmental monitoring
Introduction
Radon (222Rn, T1/2 = 3.8235 days) is a radioactive noble gas omnipresent in the environment. Being part of the 238U/226Ra decay chain, it has been found to considerably contribute to the radiation dose to the public1. Exposure to 222Rn at home and workplace is closely monitored in risk areas with high radon concentration and specific guidelines exist worldwide to mitigate exposure2. There is a reviving interest in 226Ra as raw material in the production of medical isotopes for cancer therapy that will require the handling of radiologically significant quantities of radon gas3. 222Rn has also gained considerable attention in particle physics research, where it is considered as unwanted contaminant in low-background environments4. There has been extensive research on 222Rn removal since the discovery of its adsorption on activated charcoal by Rutherford in 19065 and numerous scientific publications exist on the adsorption properties of this material, including investigations on the influence of temperature, gas flow velocity, type of investigated charcoal etc.6–8. In the recent decades, additional materials have been proposed for 222Rn adsorption such as metal organic frameworks (MOFs)9,10, carbon molecular sieves (CMSs)7 and natural or synthetic zeolites11. However, most of these materials including activated charcoal were shown to exhibit adsorption coefficients below 10 m3/kg at room temperature and as of today, charcoal still remains the material of choice despite its limited radon retention characteristics at ambient temperatures12. Examples for radon reduction systems exist in underground laboratories, where 222Rn adsorption relies on cryogenic cooling of a charcoal bed involving large bed volumes13,14.
It is interesting to note that ongoing research at CEA, France, related to radioactive Xe capture for monitoring nuclear activities in the frame of the comprehensive nuclear-test-ban treaty as well as for reducing emissions from nuclear processing facilities, has considerably advanced towards the usage of synthetic zeolites for noble gas adsorption at room temperature. Xenon, being the homologue of radon, was found to be efficiently retained on materials such as Ag-ZSM-5 and Ag-ETS-10 and experimental evidence exists on the nature of strong adsorption sites majorly influencing the interaction of the noble gas with metallic silver nanoparticles15,16. Adsorption enthalpies for Xe on Ag-ZSM-5 were reported to be as high as 65 kJ/mol and remarkable findings have been reported for room temperature Xe capture and purification using adsorbent-supported silver nanoparticles16,17. Based on first test results18, there is strong evidence to assume that 222Rn will exhibit even stronger adsorption behavior towards these materials. While it was shown that radon has affinity to bind to metal surfaces19 and that silver in synthetic zeolites has a strong influence on the adsorption process20,21, an experimental determination of adsorption coefficients for 222Rn with a synthetic zeolite material containing silver has never been published in peer reviewed journals.
The ability to exchange a broad variety of cations into the framework of the Engelhard Titano-Silicate (ETS) and Zeolite Soconi Mobil (ZSM) structures has opened up a broad field of applications for these materials. Having a variety of different crystalline phases, synthetic zeolites such as ETS-4 or ZSM-5 have been investigated as catalytic reagents in the petroleum industry and separation chemistry22–25. Ag-ZSM-5 and Ag-ETS-10 are the silver exchanged variants of these materials known for exhibiting antibacterial properties26. Both forms are unusual in their ability to, upon heating, auto-reduce exchanged silver to nanospheres of dimensions of < 10 nm held on the zeolite surface at extraordinary large concentrations with essentially pure metallic character27. The usage of zeolites in the nuclear sector is especially interesting due to their radiation and fire resistance thanks to a rigid structure and inorganic nature. For example, the silver exchanged form of 13X zeolite (Ag-13X) has been studied as adsorbent for iodine28 and radon20 originating from processing of radioactive materials.
Since no publication exists on the interaction of Ag-ETS-10 and Ag-ZSM-5 with radon gas, a series of 222Rn adsorption experiments was performed at ambient temperature using these synthetic zeolites including Ag-13X and compared with two activated charcoals commonly encountered in radon adsorption.
Comparison of 222Rn adsorbents
For the experimental evaluation of 222Rn adsorption on the studied zeolite materials, a dedicated system was designed in order to ensure data reproducibility. It was noted in the early stages of this study that different weather conditions influenced the obtained results by changes in relative humidity of the laboratory. Only an improved leak-tight setup enabling in-situ thermal regeneration of the studied materials resulted in satisfactory data quality and reproducibility.
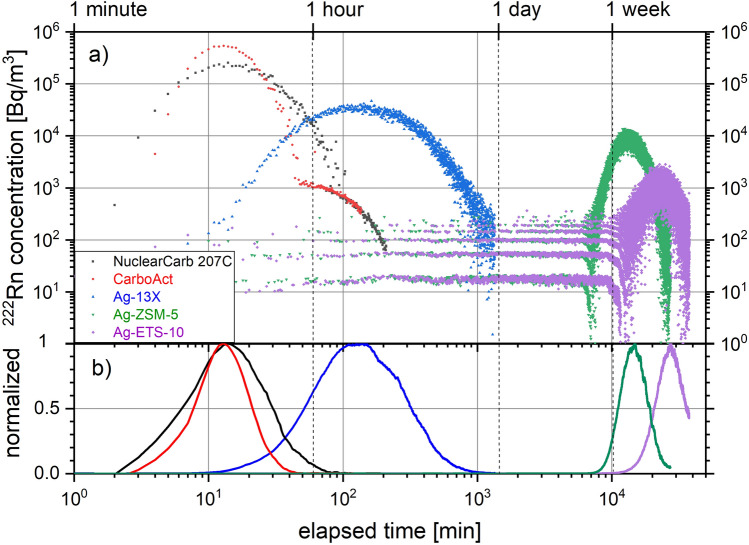
The experimentally measured breakthrough curves at ambient temperature on a 17 cm3 adsorption column for two activated carbons NuclearCarb 207C and CarboAct as well as three investigated silver-exchanged zeolites Ag-13X, Ag-ZSM-5 and Ag-ETS-10 are given in Fig. 1. While the breakthrough of 222Rn on both activated carbons occurred within several minutes after injection, its retention on Ag-ZSM-5 and on Ag-ETS-10 is considerably longer (note that both axes in Fig. 1 are given in logarithmic scale). For the last named material, measurable release of 222Rn was observed to begin 7 days after injection and a full breakthrough curve could not be obtained within 4 weeks. Only 1/32th of its initial activity could be detected in the ejected gas stream due to its radioactive decay along the adsorption bed. For Ag-13X we found a stronger retention of 222Rn compared to the activated carbons, although considerably less than for the other two zeolite structures. A summary of the mass of each adsorbent material, the injected and ejected 222Rn activity, the Rn retention time, the packing density and the measured adsorption coefficient K is given in Table 1. For both investigated charcoals, the measured adsorption coefficients for radon are of same magnitude as typically reported in literature7.
Figure 1.
(a) Measured breakthrough curves of 222Rn on NuclearCarb 207C, CarboAct, Ag-13X, Ag-ZSM-5 and Ag-ETS-10 for an adsorbent bed volume of 17 cm3; temperature 293 K; N2 carrier gas flow rate 2 NL/min; (b) normalized, decay-corrected and adjacent-averaged representation of data in (a).
Table 1.
Overview of experimental parameters, obtained retention times and adsorption coefficients for the five tested materials given in Fig. 1; for NuclearCarb 207C, CarboAct and Ag-13X the injected and released 222Rn activities do not significantly differ within their uncertainties (± 10% at a confidence level of 68%).
| Material | Packing density [kg/m3] | Mass | Mean retention time | Adsorption coefficient K | Injected 222Rn activity | Released 222Rn activity |
|---|---|---|---|---|---|---|
| [g] | [min] | [m3/kg] | [kBq] | [kBq] | ||
| NuclearCarb 207C | 450 | 7.65 | 21 | 5.6 | 11.7 | 12.4 |
| CarboAct | 250 | 4.20 | 15 | 7.2 | 16.3 | 16.0 |
| Ag-13X | 970 | 16.50 | 240 | 29 | 16.3 | 20.8 |
| Ag-ZSM-5 | 520 | 8.85 | 1.54·104 | 3500 | 1030 | 132 |
| Ag-ETS-10 | 950 | 16.30 | 2.76·104 | 3400 | 1070 | 34.2 |
Carrier gas influence
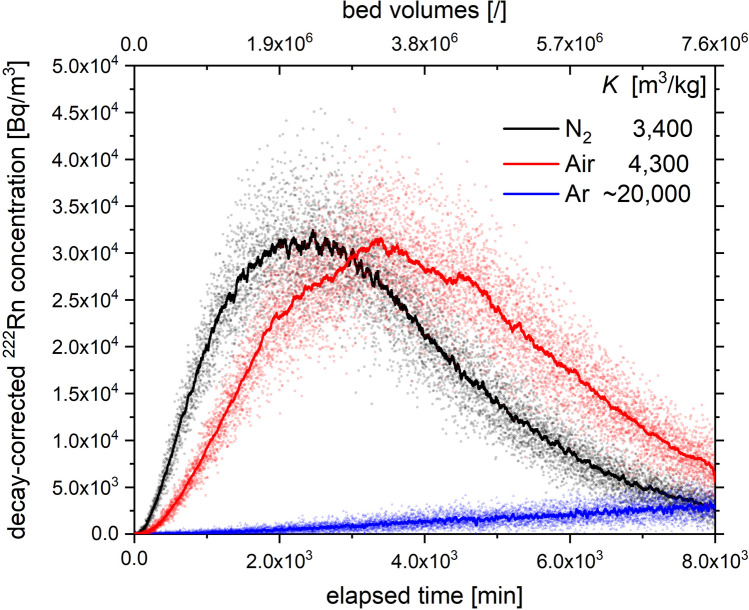
Additionally, the influence of the carrier gas on the breakthrough of 222Rn has been measured on Ag-ETS-10 using 3.0 g of adsorbent at a flow rate of 3 NL/min to enable shorter experimental durations compared to experiments shown in Fig. 1. The experimental breakthrough curves in N2, compressed air and Ar are shown in Fig. 2. The results indicate a similar retention behavior of 222Rn in N2 and dry air, indicating that trace constituents of air that are not scavenged on the 13X-APG drying column have negligible adsorption competition with radon. Experimental 222Rn adsorption coefficients K were found to be 3400 and 4300 m3/kg for N2 and dry air, respectively. For Ar as carrier gas, however, no complete breakthrough curve could be obtained. Substantially stronger 222Rn retention on the adsorption bed was observed when compared to N2/air data, practically hindering the acquisition of a meaningful elution curve under the investigated conditions due to significant 222Rn decay loss on the adsorbent. Computational analysis indicated the 222Rn adsorption coefficient in Ar to be approx. a factor six larger than that in N2 at 293 K.
Figure 2.
Decay-corrected experimental ambient temperature breakthrough curves of 222Rn with N5.0 N2, compressed air and bottled N4.8 Ar as carrier gases; Ag-ETS-10 adsorbent mass 3.0 g; volumetric flow rate 3 NL/min; injected 222Rn activity 485 kBq in each experiment.
Water competition
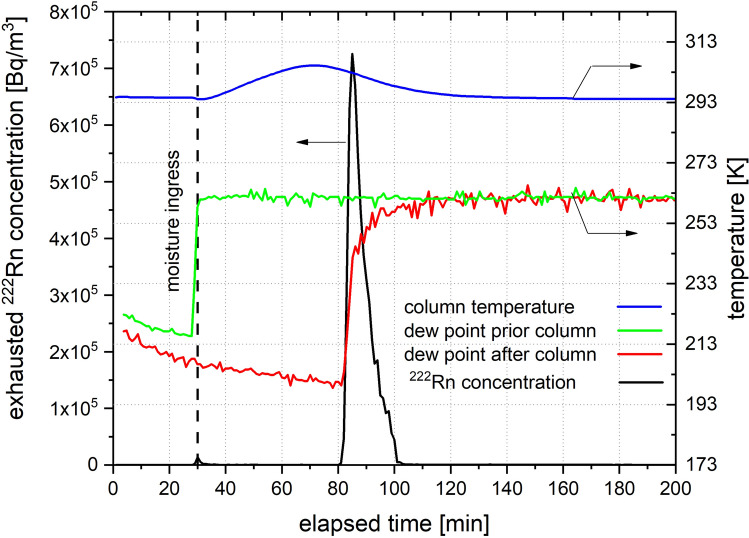
As described in the Materials and Methods section, all experiments were performed with pre-dried carrier gases to ensure low residual water content. The clear competitive effect of moisture towards 222Rn adsorption has been experimentally proven when laboratory air was allowed to pass over the adsorbent bed of Ag-ETS-10 loaded with 222Rn. Figure 3 gives the graphical representation of the 222Rn breakthrough that exactly coincides with the increase in dew point of the ejected air stream. A temperature increase of the adsorption bed is measured upon moisture entering the column indicating exothermic water adsorption. It can be seen that 222Rn leaves the adsorption bed as a sharply rising pulse, in contrary to the broad peak as observed in the previous experiments given in Figs. 1 and 2. This behavior can be explained by the competition of water molecules with 222Rn for the active adsorption sites on the adsorbent. Similar observations were made with Ag-ZSM-5. The subsequent thermal regeneration of the materials indicated that the desorption of 222Rn occurred quantitatively since no radon could be detected in the effluent gas stream. When saturated with water, Ag-ETS-10 and Ag-ZSM-5 showed negligible 222Rn retention compared to their regenerated form.
Figure 3.
Measured release of 222Rn from Ag-ETS-10 upon contact with laboratory air of 55% rel. humidity at 294 K; flow rate 2 NL/min; mass of adsorbent—16.3 g; injected 222Rn activity—11.7 kBq; 30 min post injection the 13X dehumidification column was disconnected and laboratory air was allowed to enter the adsorption bed.
Environmental radon capture
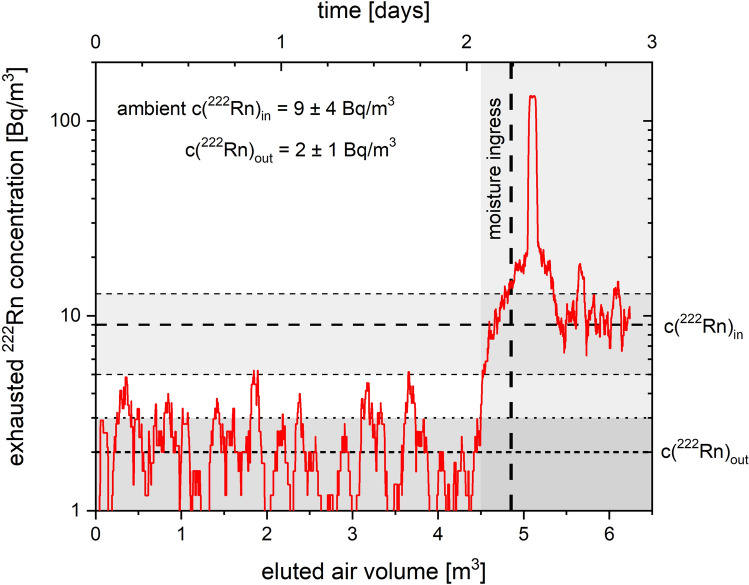
Laboratory air at 294 K with a 222Rn concentration of 9 ± 4 Bq/m3 and a relative humidity between 30 and 55% was pre-dried using 13X-4A and passed over a column containing 16.2 g of Ag-ETS-10. The measured 222Rn concentration in the exhausted air stream was 2 ± 1 Bq/m3, representing the lowest end in the measurement range of the employed radon detector. As seen in Fig. 4, these readings could be maintained for about 2 days, until a rise in radon concentration was noted and the pre-drying column subsequently removed similarly to the previous experiment represented in Fig. 3. A sharp increase in the measured 222Rn concentration was observed, indicating that 222Rn, which has accumulated on the Ag-ETS-10, was displaced by water molecules. Afterwards the measured radon concentration approached as would be expected for an adsorption bed that lost its radon retention property. Although suffering from in-leakage of moisture and radon into the adsorption bed and the employed radon detector operating in under-pressure, this experiment qualitatively shows the capability of the material to capture radon under environmental conditions.
Figure 4.
Measured 222Rn concentration in pre-dried laboratory air at 294 K after passing 16.2 g of Ag-ETS-10 at a flow rate of 1.5 NL/min; the 13X-4A dehumidification column was removed 2.25 d after start of the experiment; graph represents a 60 points adjacent average of collected data.
Discussion
Based on the measured breakthrough curves of 222Rn on the investigated materials, Ag-ETS-10 and Ag-ZSM-5 both showed remarkable retention properties towards radon at room temperature. For each of these two synthetic zeolite-type frameworks, substantial reduction of 222Rn in the effluent gas stream can be achieved. With 16.3 g of Ag-ETS-10 it was possible to retain 1 MBq of 222Rn in 2 NL/min N2 as carrier gas over a period of 1 week and more than 20 m3 (or 106 bed volumes) of N2 have passed over the column before a measurable increase in 222Rn concentration could be detected at the exhaust. At maximum breakthrough, the radon release from the Ag-ETS-10 adsorption bed was delayed for 17 days, corresponding to approximately 4.5 half-lifes of 222Rn. With N2 as carrier gas, Ag-ZSM-5 and Ag-ETS-10 exhibit radon adsorption coefficients K that are at least two orders of magnitude larger compared to NuclearCarb 207C, CarboAct and Ag-13X. To our knowledge, no measurement has yet been reported in scientific literature showing a material with radon adsorption coefficients exceeding 3000 m3/kg at room temperature. It is also shown for Ag-ETS-10 that experiments with dry air resulted in 20% higher K values, whereas with argon only an estimation could be obtained due to substantially stronger 222Rn retention.
Moisture control was found to be of crucial importance for the evaluation of the obtained results. The investigated silver exchanged zeolites showed to be very potent desiccants and care must be taken interpreting 222Rn breakthrough experiments with regard to possible interference with moisture co-adsorption. Careful in-situ regeneration of the material avoiding exposure to ambient humidity has proven to be of high importance for data reproducibility. The competitive behavior of water towards 222Rn adsorption is similar to observations made with Xe adsorption on Ag-ETS-1029, and our results give evidence that the displacement of radon occurs quantitatively from the adsorption bed.
Non-polar gas species as noble gases have increasing polarizability with increasing electron shell radius30. This accounts for the increasing affinity of noble gases to strongly polarizing adsorption sites in the order Ar < Kr < Xe < Rn. In analogy, a similar behavior can be expected for diatomic molecules, where N2 shows higher polarizability if compared to Ar and O231. Our experiments have shown that the choice of carrier gas had a significant influence on 222Rn adsorption since radon retention is majorly driven by competition with carrier gas molecules. A larger K value was measured for air (4300 kg/m3) than for pure N2 (3400 kg/m3), indicating that on macroscopic level, 21 vol.% of O2 in air induces a measurable difference in radon adsorption. For Ar, having a polarizability similar to that of O2, the radon adsorption coefficient on Ag-ETS-10 is approx. 6 times larger than for N2, comparable to the selectivity of N2 over Ar measured for this material32. It can be concluded that the physisorption of radon on the silver containing adsorption bed must be exceptionally strong when no carrier gas competition dominates. Changes in K values of this magnitude are not observed for activated charcoal at room temperature33, qualifying silver exchanged zeolites as a new class of radon adsorbents.
It is generally accepted in scientific literature that the interaction between xenon and the zeolite framework significantly enhances upon incorporation of silver34,35. It was speculated early that a substantial change in the electrostatic potential of the surface of the zeolite occurs upon loading with silver particles, leading to significantly enhanced noble gas affinity34. There is, however, no general consensus on what physico-chemical form of silver contributes to that effect. While some authors argue that adsorption in silver exchanged zeolites predominantly occurs via interaction with metallic silver nanopaticles16,17, others have recently reported evidence that the interaction is via Ag+ ions incorporated into the zeolite framework34–37. There is also no conclusive data to what extend the distribution and concentration of silver influence the adsorption properties towards noble gases. Since our results show that the obtained K values for Ag-ZSM-5 and Ag-ETS-10 do not significantly differ within their uncertainties, it appears that 222Rn retention does not primarily depend on the quantity of silver present when comparing Ag-exchanged zeolites to each other. This observation contradicts earlier findings made with Xe where adsorption capacity and the concentration of active adsorption sites was found to increase with larger silver loading29,38. While the ETS-10 framework is a titano-silicate with a wide pore structure able to exchange up to 30 wt% of silver, ZSM-5, being an alumino-silicate, has less cationic exchange sites to incorporate silver ions—only 10% by weight—and a smaller density. The latter material should thus have considerably less silver adsorption sites available for noble gases resulting in smaller K values—an observation that was not made in our experiments with radon. Moreover, Ag-13X, having similar Ag content and a density comparable to that of Ag-ETS-10, showed far inferior 222Rn retention properties giving rise to the conclusion that either the zeolite framework itself or in combination with the physical form, concentration and (size) distribution of silver defines the strength of noble gas interaction. It can be speculated that the procedure of preparing nano-dispersed silver onto the zeolite framework might have an influence on the noble gas adsorption behavior. Further investigations are necessary in order to fully understand the influence of material properties of silver exchanged zeolites towards noble gas adsorption.
The revolutionary nature of Ag-ETS-10 and Ag-ZSM-5 can be visualized by a following example of a Rn reduction system for air purification in laboratories requiring low background environments. For a certain radon inlet and outlet concentration and , respectively, the 222Rn reduction factor (RF) is given by Eq. (1):
| 1 |
where K is the adsorption coefficient, m the mass of the adsorbent, f the volumetric flow rate and the half-life of the respective Rn isotope. For a constant inflow of 222Rn on an adsorption bed, a significant accumulation of 222Rn occurs on the column upon reaching steady-state conditions where the decay rate of adsorbed 222Rn approaches its incoming flux. Assuming a throughput of f = 100 m3/h of air with a 222Rn activity concentration of = 20 Bq/m3, an adsorption bed of 20 cm diameter and 50 cm height (m = 15 kg) could delay the incoming 222Rn stream by 600 h or 25 days (K = 4000 m3/kg). This would correspond to a breakthrough 222Rn activity concentration of = 0.22 Bq/m3 in the effluent air stream of a single continuously working adsorption column at room temperature. While similar adsorption systems based on activated charcoal require several cubic meters of bed volume and cryogenic cooling, this example clearly shows the economic potential of silver exchanged zeolites in 222Rn related research and gas separation applications. As results in Fig. 4 indicate, radon can be then efficiently stripped in a limited volume by allowing moisture to pass over the adsorption bed. Smaller scale radon reduction systems could be employed to reduce indoor 222Rn levels in dwellings located in elevated radon risk areas.
In contrast to adsorption beds based on charcoal, silver exchanged zeolite beds require periodic thermal regeneration in order to restore their initial 222Rn retention performance. Although it has been reported earlier that ionizing radiation might be the cause for the deactivation of Ag-13X20, we have strong reason to believe that primarily gaseous water co-adsorption is responsible for this effect by blocking the active sites of the adsorbent. This is supported by the fact that Ag-ETS-10 practically loses its radon retention properties if exposed to humidity levels typically present in the environment. Therefore, it needs to be clearly stated that proper removal of water using known dehumidification processes to achieve dew points below 200 K is crucial for the efficient operation of silver exchanged zeolite radon adsorbents. The usage of commonly employed gas drying agents such as 13X or 4A zeolites have proven to be practical for this purpose. Additionally, based on the results presented in Fig. 4, it can be concluded that proper in-situ regeneration of the adsorption material in leak-proof equipment is advisable to avoid moisture interference since a significant drop in 222Rn retention might be expected upon moisture co-adsorption.
Apart from water vapor, recent investigations also indicate that organic species containing chlorine show a measurable poisoning effect on Ag-ZSM-5 with regard to Xe adsorption39. It can be concluded that proper protection of the adsorption bed from species attacking the active sorption sites will be required. Yet another peculiarity that comes with the usage of these materials is the economic value of silver, making Ag-exchanged zeolites significantly more costly than activated carbon (5000 EUR/kg as of 2022). Due to their cost, the durability towards long term usage and multiple regeneration cycles has to be experimentally proven. Although first promising experience in studies with xenon exists29, it is important to verify physical and chemical adsorbent stability in representative environmental conditions and to understand the behavior towards trace constituents of air typically present in much higher atmospheric concentrations than 222Rn.
Simply based on polarizability considerations, fluorinated greenhouse gases such as chlorofluorocarbons (CFCs) or SF6 should exhibit significantly stronger retention on Ag-ETS-10 and Ag-ZSM-5 if compared to charcoal. Based on results for radon interaction with polycrystalline metal surfaces19, ETS-10 and ZSM-5 frameworks containing stable metal nanoparticles other than silver should exhibit even larger affinity towards 222Rn than reported herein. The result of this study should motivate further detailed investigations of metal exchanged zeolites towards novel developments in radon and other gas separation applications.
In conclusion, our results demonstrate exceptionally strong interaction of radon with silver exchanged ETS-10 and ZSM-5 frameworks, analogous to observations published earlier on xenon17. We have shown that moisture and the polarizability of the carrier gas have a strong influence on the adsorption behavior of 222Rn, practically making these silver exchanged zeolites a new family of radon adsorbents. More compact and simple 222Rn adsorption systems can be designed using these zeolite materials, considerably reducing the impact of 222Rn originating from 226Ra emanations in environmental, scientific and industrial applications.
Materials and methods
Materials
Ag-ETS-10 (granular, binderless, 16 × 30 mesh, packing density 950 kg/m3) was prepared and provided by Extraordinary Adsorbents Inc. of Edmonton, Alberta, Canada. Ag-13X (spherical, + 20 mesh, packing density of 970 kg/m3) was obtained from Sigma-Aldrich, Belgium. Nuclearcarb 207C (granular, 6 × 12 mesh, packing density of 450 kg/m3) was obtained from Chemviron Carbon, United Kingdom, and CarboAct High Purity Carbon (fragmented, 0.01–0.4 cm, packing density of 250 kg/m3) was obtained from Carbo-act International, Netherlands. Ag-ZSM-5 (spherical, particle size 0.2–0.5 mm, packing density of 520 kg/m3) was kindly provided by CEA, France, and was synthetized by Ag exchange of Na-PZ-2/25 obtained from Zeolyst, USA, according to the description given in the thesis of Deliere18. The moisture adsorbent 13X-APG was obtained from UOP CH Sarl, Switzerland, as spherical 8 × 12 mesh beads.
222Rn was generated and supplied by degassing a PYLON RN-1025 flow-through 3.77 MBq 226Ra source (reference date: 25/04/2014) supplied by PYLON Electronics Inc., Canada. Nitrogen was supplied in N5.0 purity from an in-house LN2 supply tank with a nominal pressure of 8 bar and a dew point of 203 K. Compressed air was supplied from an in-house air compressing station at 6 bar with a dew point of 243 K. Technical grade N4.8 argon was supplied in bottles by Air Products NV, Belgium.
Methods
Synthesis of Ag-ETS-10
ETS-10 was synthesized by the suspension of particulates of anatase (TiO2) in a sodium silicate solution (~ 30% SiO2, 8.5% Na2O). After the addition of ETS-10 seeds and a fluoride mineralizer, the mixture was crystallized for 72 h at 473 K in a 3.8 L Parr stainless steel autoclave at autogenous pressure. After filtering, washing and drying, the crystalline product underwent granulation and was sieved into 16 × 30 mesh aggregates. The high purity, as synthesized, ETS-10 was loaded into a column and strip exchanged with an excess of silver nitrate in solution at 363 K for 36 h. The silver-exchanged ETS-10 (now Ag-ETS-10) was thoroughly washed with deionized water and dried at 473 K in a forced air oven.
Experimental Rn adsorption setup
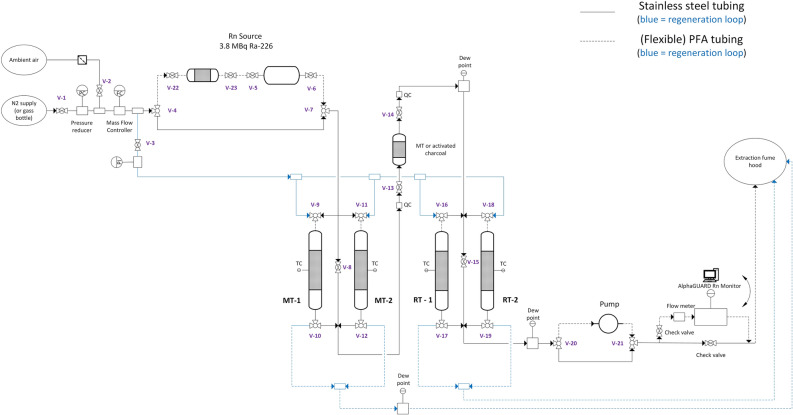
Dynamic 222Rn adsorption experiments were performed in a laboratory scale setup schematically depicted in Fig. 5. The trapping device consisted of two redundant moisture traps and two Rn adsorption columns, hygrometers for indication of the moisture content in the system, flow controllers (MFC and VA-flowmeter), a vacuum pump and a radon monitor. The system was built out of stainless steel fittings, valves and tubing (1/4″ OD) to assure leak tightness for minimal moisture ingress. A part of the system contained flexible perfluoroalkoxy alkane (PFA) tubing to ease operation.
Figure 5.
Schematic representation of the setup used for 222Rn adsorption experiments.
Supplied N2, air and Ar was reduced in pressure and fed into the experimental system as carrier gas. The flow of the carrier gas in the setup was controlled by a thermal mass flow controller (Red-y smart, Vögtlin Instruments GmbH, Switzerland). The setup was equipped with two redundant moisture traps (MT-1 and MT-2) filled with molecular sieve 13X-APG to reduce the dew point of the incoming gas below 200 K. Redundancy was provided for continuous operation in case of column saturation. Both columns have a volume of approx. 385 cm3 (Øin = 4.05 cm, L = 30 cm) and are made of stainless steel (SS 316). The radon adsorption was performed with one of the adsorption columns RT-1 and RT-2, both having a volume of 17 cm3 (Øin = 1.2 cm, L = 15 cm) and made of stainless steel (SS 316). All columns are designed and manufactured in-house. For all columns, a CF flange with a copper seal on top of the column is installed to ensure leak tightness. The seal is regularly exchanged and a leak test is performed after each column opening via pressurization. Two dew point transmitters (Easidew online, Michell Instruments, United Kingdom, range between 173 and 293 K) are installed in the setup to measure the dew point of the carrier gas at the exhaust of MT-1/2 and RT-1/2, respectively.
The setup enables regeneration of different sorbents in situ, i.e. while keeping the sorbent in the column. The heating of the stainless steel column was performed via an electrical heating wire connected to a temperature controller that regulates the temperature to a predetermined set-point via a thermocouple. The thermocouple was positioned on the outer wall of the column. The columns and heating wire are thermally insulated with glass fiber tape. The setup was equipped with a vacuum pressure transmitter (pressure range − 1 to 2.5 barg) for pressure monitoring and leak tightness validation of the setup and the columns in particular after opening and closing.
A (removable) adsorbent column (120 cm3) can be inserted into the experimental loop via quick-connects to enable the degassing of the 222Rn source. Once removed, the loop was closed via stainless steel tubing. The outgoing radon concentration was monitored by sampling a part of the exhaust stream with a AlphaGUARD Professional Radon Monitor DF2000, Bertin GmbH, Germany. It enables a continuous determination of the volumetric radon concentration with a measuring range from 2 Bq/m3 to 2 MBq/m3 with a data sampling frequency of 1 min. The monitor was equipped with a flow-regulated pump which can be adjusted from 0.05 L/min to 2 L/min, allowing sampling of the exhausted gas stream. For the experiments described herein, the device was operated in the “1 min, flow, radon” mode at a flow rate of 1 L/min.
Experiments for environmental radon adsorption were performed in a non-radioactive laboratory at room temperature by using a KNF Laboport N86 vacuum pump and a mass flow controller (Red-y smart, Vögtlin Instruments GmbH, Switzerland) in suction mode through one MT400-4 and one MT120-4 13X-4A moisture traps (both obtained from Agilent, USA) and a glass column (Øin = 1.2 cm, L = 15 cm manufactured at Glasatelier Saillard, Belgium) containing the tested adsorbent. All components were connected by flexible PFA tubing. The exhausted air stream was sampled using a newly purchased AlphaGUARD DF2000 radon monitor having a negligible intrinsic background count rate.
Breakthrough experiments
Breakthrough experiments were performed at laboratory room temperature, which was 293 ± 2 K. A stepwise procedure was applied. At first, the Pylon RN-1025 was degassed to remove accumulated 222Rn in equilibrium with 3.77 MBq 226Ra. In order to control the total 222Rn activity injected in each performed experiment, the degassing was performed with dried laboratory air for 1 h at a flow rate of 0.5 NL/min, where the flushed 222Rn was adsorbed on a 50 g NuclearCarb 207C column that was subsequently removed. After degassing, radon started accumulating again in the RN-1025 source at a rate of 473 Bq/min. In a next step, the accumulated radon was injected into the selected adsorbent column by flushing the radon source with the desired carrier gas (N2, Ar or air) and flow rate (0.5–5 NL/min) for 5 min. In order to reach the desired activity for injection, the time between the end of the degassing and the end of the injection was adjusted, being 25–35 min for experiments with the activated carbons and Ag-13X and 18–40 h for Ag-ZSM-5 and Ag-ETS-10, respectively. After injection, the radon source was closed and N2, Ar or air was fed to the column until all injected radon has passed through the column (complete breakthrough) or the experiment terminated preliminarily (incomplete breakthrough).
Material regeneration
Each tested adsorbent underwent a regeneration cycle via heat treatment under inert gas conditions prior to each experiment. In each case, N5.0 nitrogen was used as regeneration gas. The regeneration was performed for at least 15 h at 503 K for Ag-ETS-10, 523 K for Ag-13X and Ag-ZSM-5, while for both activated carbons 423 K was chosen. The moisture adsorbent 13X-APG was regenerated at 553 K. The regeneration of 13X-4A and Ag-ETS-10 for environmental 222Rn adsorption was performed in a muffle furnace (type AAF 11/7, Carbolite, UK) at 503 K overnight and each material was subsequently filled manually into the respective adsorption column.
Data treatment
For each performed experiment, the data acquisition system of the AlphaGUARD DF2000 radon monitor provided the volumetric 222Rn activity concentration of the ejected gas stream as function of experimental time. These data were first corrected for the intrinsic background of the device, which was periodically determined by flushing pure N2 through the system and ranged typically between 20 and 100 Bq/m3. Afterwards, decay correction was applied to the background corrected dataset to account for 222Rn decay since the start of each breakthrough experiment. For each experimental data set of N measurement points, the breakthrough curves were then smoothed via the adjacent averaging method over points. The adsorption coefficient K [m3/kg] for each tested adsorbent was calculated using the formula given as
where tr is the mean retention time in [h], m the mass of adsorbent in [kg] and f the volumetric flow rate in [m3/h]. The retention time tr is the time of the eluted decay-corrected radon activity to be 50% of the total injected radon activity. Fitting of the breakthrough curves and the subsequent estimation of tr was performed using software data analysis applying the chromatographic plate model function6 given as
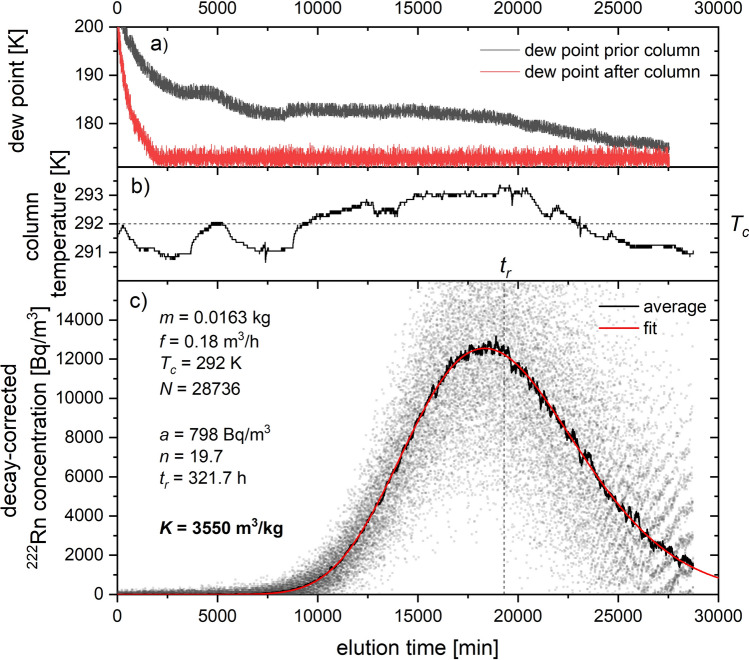
where a is the amplitude of the injected 222Rn spike and n the number of theoretical plates of the adsorbent column. The model also served as computational analysis tool to estimate tr for incomplete break-through curves by fitting the expected total ejected 222Rn activity to the activity initially injected into the column. An example of a fitting curve with the obtained plate model parameters is given in Fig. 6 together with data on temperature and dew point evolution for a typical breakthrough experiment.
Figure 6.
Experimental data for a breakthrough experiment with 16.3 g Ag-ETS-10 at a flow rate of 3 NL/min of N2 showing the evolution of (a) the dew point prior and after the adsorption column, (b) the column temperature and (c) the averaged decay-corrected 222Rn concentration with its plate model fit.
The uncertainties for the adsorbent mass m and volumetric flow rate f were given as 1% and 2%, respectively. Variations in column temperature during experiments induced the largest uncertainty in the determination of tr. The overall uncertainty associated to the measured adsorption coefficient K was estimated to be in the order of 10% at a confidence level of 68%.
Acknowledgements
The authors express their gratitude to Sylvain Topin and Gabriel Couchaux, both affiliated to CEA, France, for enabling the usage of Ag-ZSM-5 in the underlying investigation. Data published herein were presented at the 19th Radiochemical Conference (RadChem 2022) held in Mariánské Lázně, Czech Republic, on 15–20 May 2022.
Author contributions
S.H. wrote the main manuscript text; J.M., J.R. and C.G. were involved in the design of the experimental setup; synthesis of Ag-ETS-10 material was done by N.I., D.K. and S.K.; J.M. performed main experimental work with the aid of S.H., D.M. and H.S.; analysis of the results were performed by J.M. and S.H.; A.A. and T.C. enabled the described research activities; all authors reviewed the manuscript.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on request.
Competing interests
The co-authors Natalie Ireland, Daniel Kuznicki and Steven Kuznicki are co-owners of the company Extraordinary adsorbents Inc. commercially distributing the material Ag-ETS-10 studied herein. SCK CEN has a pending patent application with the number WO 2022/167669 with the inventors being Jasper Mermans, Dominic Maertens, Hanna Skliarova, Stephan Heinitz and Thomas Cardinaels. The authors Jos Rutten, Christophe Gueibe and Alexander Aerts do not have any competing interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Porstendorfer J. Properties and behavior of radon and thoron and their decay products in the air. J. Aerosol. Sci. 1994;25:219–263. doi: 10.1016/0021-8502(94)90077-9. [DOI] [Google Scholar]
- 2.Zeeb H. WHO Handbook on Indoor Radon: A Public Health Perspective. WHO Press; 2009. [PubMed] [Google Scholar]
- 3.Engle JW. The production of Ac-225. Curr. Radiopharm. 2018;11:173–179. doi: 10.2174/1874471011666180418141357. [DOI] [PubMed] [Google Scholar]
- 4.Udovicic V, et al. Radon problem in an underground low-level laboratory. Radiat. Meas. 2009;44:1009–1012. doi: 10.1016/j.radmeas.2009.10.038. [DOI] [Google Scholar]
- 5.Rutherford E. Absorption of the radio-active emanations by charcoal. Nature. 1906;74:634–634. doi: 10.1038/074634a0. [DOI] [Google Scholar]
- 6.Pushkin K, et al. Study of radon reduction in gases for rare event search experiments. Nucl. Instrum. Meth. A. 2018;903:267–276. doi: 10.1016/j.nima.2018.06.076. [DOI] [Google Scholar]
- 7.Busto J. Radon adsorption in nanoporous carbon materials. Aip. Conf. Proc. 2013;1549:112–115. doi: 10.1063/1.4818088. [DOI] [Google Scholar]
- 8.Gaul WC, Underhill DW. Dynamic adsorption of radon by activated carbon. Health Phys. 2005;88:371–378. doi: 10.1097/01.HP.0000152110.01409.3e. [DOI] [PubMed] [Google Scholar]
- 9.Zeng XF, Chen F, Cao DP. Screening metal-organic frameworks for capturing radioactive gas Rn in indoor air. J. Hazard. Mater. 2019;366:624–629. doi: 10.1016/j.jhazmat.2018.12.042. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, et al. Thermodynamics-kinetics-balanced metal-organic framework for in-depth radon removal under ambient conditions. J. Am. Chem. Soc. 2022;144:13634–13642. doi: 10.1021/jacs.2c04025. [DOI] [PubMed] [Google Scholar]
- 11.Bikit I, et al. Radon adsorption by zeolite. Radiat. Meas. 2015;72:70–74. doi: 10.1016/j.radmeas.2014.12.001. [DOI] [Google Scholar]
- 12.Pressyanov D. New generation of highly sensitive radon detectors based on activated carbon with compensated temperature dependence. Sci. Rep. 2022;12:8479. doi: 10.1038/s41598-022-12502-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodak R, et al. Characterization and long-term performance of the Radon Trapping Facility operating at the Modane Underground Laboratory. J. Phys. G Nucl. Partic. 2019 doi: 10.1088/1361-6471/ab368e. [DOI] [Google Scholar]
- 14.Fajt L, et al. Compact anti-radon facility. Aip. Conf. Proc. 2015 doi: 10.1063/1.4928008. [DOI] [Google Scholar]
- 15.Deliere L, et al. Role of silver nanoparticles in enhanced xenon adsorption using silver-loaded zeolites. J. Phys. Chem. C. 2014;118:25032–25040. doi: 10.1021/jp507590a. [DOI] [Google Scholar]
- 16.Kuznicki SM, et al. Xenon adsorption on modified ETS-10. J. Phys. Chem. C. 2007;111:1560–1562. doi: 10.1021/jp067630t. [DOI] [Google Scholar]
- 17.Deliere L, et al. Breakthrough in xenon capture and purification using adsorbent-supported silver nanoparticles. Chem.-Eur. .J. 2016;22:9660–9666. doi: 10.1002/chem.201601351. [DOI] [PubMed] [Google Scholar]
- 18.Deliere L. Adsorption et séparation des gaz rares sur des adsorbants dopés à l’argent. Université Claude Bernard; 2015. [Google Scholar]
- 19.Eichler R, Schadel M. Adsorption of radon on metal surfaces: A model study for chemical investigations of elements 112 and 114. J. Phys. Chem. B. 2002;106:5413–5420. doi: 10.1021/jp015553q. [DOI] [Google Scholar]
- 20.Hedstrom H, Foreman M, Ekberg C, Rameback H. Radon capture with silver exchanged zeolites. Radiochim. Acta. 2012;100:395–399. doi: 10.1524/ract.2012.1932. [DOI] [Google Scholar]
- 21.Tyndall DW. Aparatus and process for air cleaning. US Patent. 2008;7381244:B2. [Google Scholar]
- 22.Yu ZN, Sawada JA, An WZ, Kuznicki SM. PtZn-ETS-2: A novel catalyst for ethane dehydrogenation. Aiche J. 2015;61:4367–4376. doi: 10.1002/aic.15010. [DOI] [Google Scholar]
- 23.Grande CA, Lind A, Vistad O, Akporiaye D. Olefin-paraffin separation using calcium-ETS-4. Ind. Eng. Chem. Res. 2014;53:15522–15530. doi: 10.1021/ie5004703. [DOI] [Google Scholar]
- 24.Figueiredo BR, Portugal I, Rocha J, Silva CM. Fixed-bed removal of Cs+ from aqueous solutions by microporous silicate ETS-4: Measurement and modeling of loading-regeneration cycles. Chem. Eng. J. 2016;301:276–284. doi: 10.1016/j.cej.2016.04.112. [DOI] [Google Scholar]
- 25.Rahimi N, Karimzadeh R. Catalytic cracking of hydrocarbons over modified ZSM-5 zeolites to produce light olefins: A review. Appl Catal a-Gen. 2011;398:1–17. doi: 10.1016/j.apcata.2011.03.009. [DOI] [Google Scholar]
- 26.Sánchez MJ, Mauricio JE, Paredes AR, Gamero P, Cortés D. Antimicrobial properties of ZSM-5 type zeolite functionalized with silver. Mater. Lett. 2017;191:65–68. doi: 10.1016/j.matlet.2017.01.039. [DOI] [Google Scholar]
- 27.Anson A, Maham Y, Lin CCH, Kuznicki TM, Kuznicki SM. XPS characterization of silver exchanged ETS-10 and mordenite molecular sieves. J. Nanosci. Nanotechnol. 2009;9:3134–3137. doi: 10.1166/jnn.2009.044. [DOI] [PubMed] [Google Scholar]
- 28.Cheng QH, et al. Adsorption of gaseous radioactive iodine by Ag/13X zeolite at high temperatures. J. Radioanal. Nucl. Ch. 2015;303:1883–1889. doi: 10.1007/s10967-014-3736-3. [DOI] [Google Scholar]
- 29.Gueibe C, et al. Application of silver-exchanged zeolite for radioxenon mitigation at fission-based medical isotope production facilities. Process Saf. Environ. Prot. 2022;158:576–588. doi: 10.1016/j.psep.2021.12.031. [DOI] [Google Scholar]
- 30.Saha R, Jana G, Pan S, Merino G, Chattaraj PK. How far can one push the noble gases towards bonding? A personal account. Molecules. 2019;24:2933. doi: 10.3390/molecules24162933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller TM, Bederson B. Atomic and molecular polarizabilities-a review of recent advances. In: Bates DR, Bederson B, editors. Advances in Atomic and Molecular Physics. Academic Press; 1978. pp. 1–55. [Google Scholar]
- 32.Ansón A, et al. Adsorption of argon, oxygen, and nitrogen on silver exchanged ETS-10 molecular sieve. Micropor. Mesopor. Mat. 2008;109:577–580. doi: 10.1016/j.micromeso.2007.04.026. [DOI] [Google Scholar]
- 33.Underhill D. W. The adsorption of argon, krypton, and xenon on activated charcoal. Health Phys.71(2), 160–166. 10.1097/00004032-199608000-00006 (1996). [DOI] [PubMed]
- 34.Munakata K, Kanjo S, Yamatsuki S, Koga A, Ianovski D. Adsorption of noble gases on silver-mordenite. J. Nucl. Sci. Technol. 2003;40:695–697. doi: 10.3327/jnst.40.695. [DOI] [Google Scholar]
- 35.Coopersmith KJ, et al. Investigating the role of silver oxidation state on the thermodynamic interactions between xenon and silver-functionalized zeolites. J. Phys. Chem. C. 2023;127:1598–1606. doi: 10.1021/acs.jpcc.2c05783. [DOI] [Google Scholar]
- 36.Oda A, et al. Orbital trap of xenon: Driving force distinguishing between Xe and Kr found at a single Ag(I) site in MFI zeolite at room temperature. J. Phys. Chem. C. 2022;126:8312–8326. doi: 10.1021/acs.jpcc.2c01515. [DOI] [Google Scholar]
- 37.Torcivia MA, Demers SME, Broadwater KL, Hunter DB. Investigating the effects of Ag, Cu, and Pd functionalized chabazite on the adsorption affinities of noble gases Xe, Kr, and Ar. J. Phys. Chem. C. 2023;127:3800–3807. doi: 10.1021/acs.jpcc.2c08401. [DOI] [Google Scholar]
- 38.Daniel C, et al. Xenon capture on silver-loaded zeolites: Characterization of very strong adsorption sites. J. Phys. Chem. C. 2013;117:15122–15129. doi: 10.1021/jp403934r. [DOI] [Google Scholar]
- 39.Monpezat A, et al. Effect of chlorine-containing VOCs on silver migration and sintering in ZSM-5 used in a TSA process. Catalysts. 2019 doi: 10.3390/catal9080686. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on request.