Abstract
OBJECTIVES
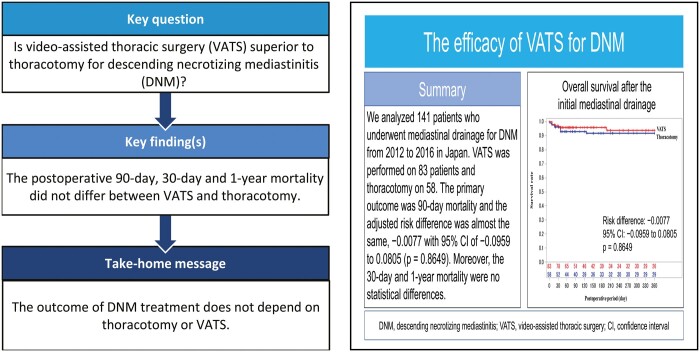
Thoracotomy is a reliable approach for descending necrotizing mediastinitis (DNM), and the use of video-assisted thoracic surgery (VATS), a minimally invasive procedure, has been increasing. However, which approach is more effective for DNM treatment is controversial.
METHODS
We analysed patients who underwent mediastinal drainage via VATS or thoracotomy, using a database with DNM from 2012 to 2016 in Japan, which was constructed by the Japanese Association for Chest Surgery and the Japan Broncho-esophagological Society. The primary outcome was 90-day mortality, and the adjusted risk difference between the VATS and thoracotomy groups using a regression model, which incorporated the propensity score, was estimated.
RESULTS
VATS was performed on 83 patients and thoracotomy on 58 patients. Patients with a poor performance status commonly underwent VATS. Meanwhile, patients with infection extending to both the anterior and posterior lower mediastinum frequently underwent thoracotomy. Although the postoperative 90-day mortality was different between the VATS and thoracotomy groups (4.8% vs 8.6%), the adjusted risk difference was almost the same, −0.0077 with 95% confidence interval of −0.0959 to 0.0805 (P = 0.8649). Moreover, we could not find any clinical and statistical differences between the 2 groups in terms of postoperative 30-day and 1-year mortality. Although patients who underwent VATS had higher postoperative complication (53.0% vs 24.1%) and reoperation (37.9% vs 15.5%) rates than those who underwent thoracotomy, the complications were not serious and most could be treated with reoperation and intensive care.
CONCLUSIONS
The outcome of DNM treatment does not depend on thoracotomy or VATS.
Keywords: Descending necrotizing mediastinitis, Mediastinal drainage, Thoracotomy, Video-assisted thoracic surgery
Descending necrotizing mediastinitis (DNM) is a serious infection that spreads from the neck, oral cavity and pharynx to the mediastinum.
INTRODUCTION
Descending necrotizing mediastinitis (DNM) is a serious infection that spreads from the neck, oral cavity and pharynx to the mediastinum. DNM spreads rapidly. Furthermore, it can lead to sepsis, which is fatal if immediate and aggressive treatment, particularly sufficient neck and mediastinum drainage, is delayed [1, 2]. DNM is a rare disease, and only ∼100 patients undergo mediastinal drainage for DNM annually in Japan [3]. There are various reports about the methods of mediastinal drainage [4–8]. However, several surgeons prefer thoracotomy, which is a reliable drainage method [9–12]. In recent years, there are concerns about the invasiveness of this procedure among patients with severe general condition. The number of patients who underwent video-assisted thoracic surgery (VATS), a minimally invasive approach, has increased [3, 13–15]. However, the efficacy of mediastinal drainage via VATS compared with thoracotomy remains questionable, and which approach is more effective for DNM treatment is controversial.
Therefore, further studies to examine the type of approach that is better for patients with DNM are desired. However, this has been challenging because DNM is a rare disease. Thus far, we have collected data and constructed a database with >200 DNM cases for the last 5 years in the joint study of the Japanese Association for Chest Surgery and the Japan Broncho-esophagological Society. A previous study reported the characteristics, pathophysiological examination results, and prognosis of patients with DNM [16]. The present study aimed to assess the usefulness of thoracotomy and VATS in mediastinal drainage among patients with DNM.
PATIENTS AND METHODS
Ethical statement
The protocol was in accordance with the principles of the Declaration of Helsinki, and the study was approved by the Clinical Research Area Ethics Committee of Kobe University Graduate School of Medicine (# B210202). The need for informed consent was waived owing to the nature of the study.
Study design
Similar to the joint retrospective study of Japanese Association for Chest Surgery and Japan Broncho-esophagological Society, clinical information including patient characteristics, treatments, and outcomes was collected. In total, 225 patients with DNM who were treated from January 2012 to December 2016 at 131 institutions in Japan were included, and a DNM database was constructed (Supplementary Material, Appendix S1) [16]. Using the collected clinical information, the efficacy of VATS and thoracotomy was retrospectively assessed.
Patient population
Using data collected from the database, we analysed patients with DNM who underwent transthoracic mediastinal drainage via VATS or thoracotomy. Patients who underwent mediastinal drainage using other approaches, such as subcutaneous, transcervical and subxiphoid, and those with missing data were excluded from the study.
Diagnosis and classification of DNM
The definition of DNM was established using the Esterera’s diagnostic criteria, which were as follows: (i) clinical evidence of severe oropharyngeal infection, (ii) characteristic roentgenographic features of mediastinitis, (iii) presence of necrotizing mediastinal infection during surgery and (iv) establishment of the association between oropharyngeal or cervical infection and the development of necrotizing mediastinal infection [16, 17]. The extent of mediastinal infection based on the classification proposed by Endo is as follows: type I, infection localized to the upper mediastinum above the carina; type IIA, infection extending to the anterior lower mediastinum; type IIB, infection extending to both the anterior and posterior lower mediastinum; and type IIC (a new classification), infection extending to the posterior lower mediastinum [16, 18].
Study outcomes
The primary outcome was 90-day mortality, and the secondary outcomes were 30-day mortality, 1-year mortality and reoperation rate. The other outcomes were surgery-related morbidity, surgical duration, volume of blood loss, indwelling time of mediastinal drainage, need for mechanical ventilation after surgery and length of hospital stay. Surgical mortality was defined as death from any cause after the initial surgery.
Postoperative complications were classified into 4 categories: pulmonary (prolonged air leakage, bronchopleural fistula, pneumonia, interstitial pneumonia, atelectasis and respiratory insufficiency), cardiovascular (cardiac infarction, heart failure, cerebral Haemorrhage, cerebral stroke, arrhythmia and pulmonary embolism), infectious (wound infection) and others. Prolonged air leakage was defined as postoperative air leakage that persisted for >7 days or that required additional postoperative procedure, such as surgery and adhesion therapy, within 7 days. Pneumonia was defined as the development of infection and lung consolidation on chest radiography or computed tomography scan. In terms of comorbidity, interstitial pneumonia was defined as the presence of characteristic features such as honeycombing and reticular pattern on chest computed tomography scan. If considered a postoperative complication, interstitial pneumonia was defined as acute exacerbation. Respiratory insufficiency was defined as the need for reintubation, tracheostomy or ventilatory assistance >48 h after surgery. Arrhythmia was defined as heartbeat irregularities that developed after surgery and required treatment.
VATS was defined as all intrathoracic procedures during surgery performed under monitor viewing and wound measuring ≤8 cm without rib dissection. The selection of either VATS or thoracotomy was based on the surgeon’s discretion at each institution.
Statistical analysis
Categorical variables, such as clinical characteristics and postoperative outcomes, were expressed as frequency and proportion and were compared using the Fisher’s exact test. Continuous variables were presented as mean and range and were compared using the Wilcoxon’s rank-sum test.
The primary outcome and the dichotomous secondary end points were expressed as proportion. To adjust the measured confounders, after the propensity scores of each participant were calculated based on age, sex, comorbidity, Eastern Cooperative Oncology Group performance status (ECOG PS) score and level of mediastinal extent, the adjusted risk difference was estimated by applying the generalized linear model for the binary outcome with the identity link function and robust standard error, in which the treatment approach and the logit-transformed propensity score were included as covariates. The unadjusted and adjusted risk differences between the VATS and thoracotomy groups and the 95% confidence intervals of 90-day mortality, 30-day mortality, 1-year mortality and reoperation rate were examined. The risk differences in postoperative complications were assessed using the same method. P-values were calculated with the Wald statistics. The unadjusted and adjusted Kaplan–Meier plots of 1-year survival were drawn. The confounding variables of the plot were adjusted using the Cox regression model with the logit transformation of the propensity score. Statistical analyses were performed using SAS 9.4 (SAS Institute, Inc., Cary, NC, USA).
RESULTS
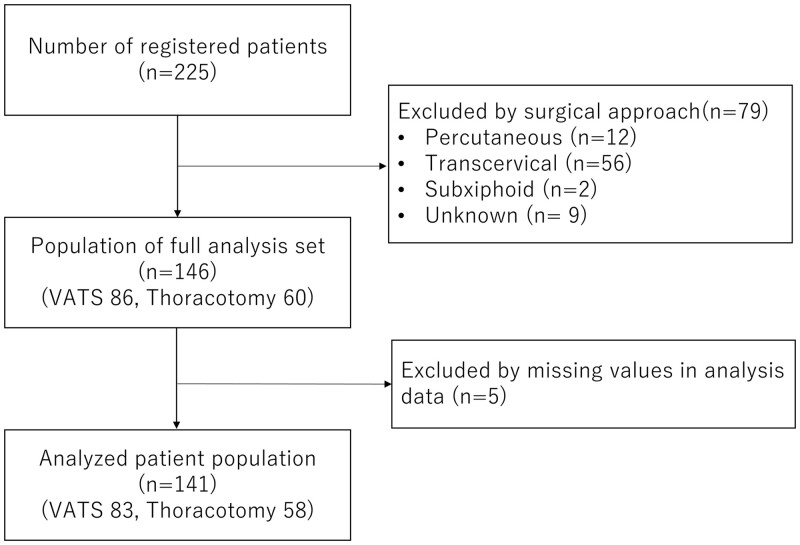
Figure 1 shows the flowchart of patient inclusion. VATS was performed on 83 patients and thoracotomy on 58. Table 1 depicts the clinical characteristics of patients with DNM who underwent transthoracic mediastinal drainage. There was no clinical and statistical difference between the VATS and thoracotomy groups in terms of age, sex, body mass index and symptoms at diagnosis. In terms of preoperative comorbidity, patients with hypertension commonly underwent thoracotomy. Meanwhile, patients with poor ECOG PS scores were more likely to undergo VATS.
Figure 1:
Flowchart of patient inclusion in this study.
Table 1:
Characteristics of the patients
| Factors | All patients | VATS | Thoracotomy | P-Value | |
|---|---|---|---|---|---|
| n = 141 | n = 83 | n = 58 | |||
| Age | Mean (range) | 62.6 (19–93) | 62.4 (25–86) | 62.8 (19–93) | 0.8471 |
| Sex | Male | 56 (39.7) | 34 (41.0) | 22 (37.9) | 0.7304 |
| Female | 85 (60.3) | 49 (59.0) | 36 (62.1) | ||
| BMI | Mean (range) | 22.5 (13.7–22) | 22.2 (13.7–38) | 22.9 (14.1–33.1) | 0.1608 |
| Comorbidity | Diabetes mellitus | 40 (28.4) | 24 (28.9) | 16 (27.6) | 1 |
| Malignancy | 4 (2.8) | 2 (2.4) | 2 (3.4) | 1 | |
| Autoimmune disease | 3 (2.1) | 2 (2.4) | 1 (1.7) | 1 | |
| Steroid use | 6 (4.3) | 3 (3.6) | 3 (5.2) | 0.6900 | |
| Ischaemic cardiac disease | 5 (3.5) | 4 (4.8) | 1 (1.7) | 0.6487 | |
| Cerebrovascular disease | 5 (3.5) | 4 (4.8) | 1 (1.7) | 0.6487 | |
| Renal failure | 10 (7.1) | 4 (4.8) | 6 (10.3) | 0.3177 | |
| Hypertension | 23 (16.3) | 8 (9.6) | 15 (25.9) | 0.0190 | |
| Others | 32 (22.7) | 16 (19.3) | 16 (27.6) | 0.3077 | |
| ECOG PS score | 0 | 67 (47.5) | 32 (38.6) | 35 (60.3) | 0.0167 |
| 1 | 27 (19.1) | 21 (25.3) | 6 (10.3) | ||
| 2 | 14 (9.9) | 8 (9.6) | 6 (10.3) | ||
| 3 | 17 (12.1) | 14 (16.9) | 3 (5.2) | ||
| 4 | 16 (11.3) | 8 (9.6) | 8 (13.8) | ||
| Symptoms | Fever | 104 (73.8) | 63 (75.9) | 41 (70.7) | 0.5609 |
| Pain | 107 (75.9) | 65 (78.3) | 42 (72.4) | 0.4313 | |
| Palpitation | 9 (6.4) | 7 (8.4) | 2 (3.4) | 0.3077 | |
| Redness | 52 (36.9) | 35 (42.2) | 17 (29.3) | 0.1560 | |
| Swelling | 90 (63.8) | 58 (69.9) | 32 (55.2) | 0.0787 | |
| Subcutaneous emphysema | 10 (7.1) | 7 (8.4) | 3 (5.2) | 0.5252 | |
| Dysphagia | 52 (36.9) | 32 (38.6) | 20 (34.5) | 0.7233 | |
| Dyspnoea | 58 (41.1) | 33 (39.8) | 25 (43.1) | 0.7300 | |
| Impaired consciousness | 10 (7.1) | 6 (7.2) | 4 (6.9) | 1 | |
| Sepsis | 27 (19.1) | 17 (20.5) | 10 (17.2) | 0.6700 | |
| Others | 16 (11.3) | 8 (9.6) | 8 (13.8) | 0.5906 | |
Values for categorical variables were presented as n (%) and were assessed using the Fisher’s exact test. Variables for continuous variables were expressed as mean and range and were examined using the Wilcoxon rank-sum test.
BMI: body mass index; ECOG PS: Eastern Cooperative Oncology Group performance status; VATS: video-assisted thoracic surgery.
Table 2 shows the extent and details of mediastinitis. Regarding the type of mediastinal extent, patients with type IIB and pleural effusion were more likely to undergo mediastinal drainage under thoracotomy.
Table 2:
Extent level and cause of mediastinitis
| Extent level and cause | All patients | VATS | Thoracotomy | P-Value | |
|---|---|---|---|---|---|
| n = 141 | n = 83 | n = 58 | |||
| Level of mediastinal extent | Above the carina (type I) | 47 (33.3) | 32 (38.6) | 15 (25.9) | 0.1797 |
| Anterior lower mediastinum (type IIA) | 16 (11.3) | 8 (9.6) | 8 (13.8) | ||
| Anterior and posterior lower mediastinum (type IIB) | 51 (36.2) | 25 (30.1) | 26 (44.8) | ||
| Posterior lower mediastinum (type IIC) | 27 (19.1) | 18 (21.7) | 9 (15.5) | ||
| Type of origin | Traumatic | 3 (2.1) | 2 (2.4) | 1 (1.7) | 0.7428 |
| Medical procedure related | 5 (3.5) | 2 (2.4) | 3 (5.2) | ||
| Infection | 133 (94.3) | 79 (95.2) | 54 (93.1) | ||
| Source of infection | Odontogenic | 24 (17.0) | 14 (16.9) | 10 (17.2) | 0.3229 |
| Oral | 5 (3.5) | 4 (4.8) | 1 (1.7) | ||
| Pharyngeal | 71 (50.4) | 43 (51.8) | 28 (48.3) | ||
| Cervical | 33 (23.4) | 16 (19.3) | 17 (29.3) | ||
| Oesophageal | 1 (0.7) | 1 (1.7) | |||
| Others | 7 (5.0) | 6 (7.2) | 1 (1.7) | ||
| Pleural effusion | Without effusion | 33 (23.4) | 24 (28.9) | 9 (15.5) | 0.0451 |
| Right | 28 (19.9) | 20 (24.1) | 8 (13.8) | ||
| Left | 9 (6.4) | 5 (6.0) | 4 (6.9) | ||
| Bilateral | 71 (50.4) | 34 (41.0) | 37 (63.8) | ||
| Empyema | Without empyema | 94 (66.7) | 58 (69.9) | 36 (62.1) | 0.2618 |
| Right | 19 (13.5) | 13 (15.7) | 6 (10.3) | ||
| Left | 11 (7.8) | 5 (6.0) | 6 (10.3) | ||
| Bilateral | 17 (12.1) | 7 (8.4) | 10 (17.2) | ||
Values were presented as n (%) and assessed using the Fisher’s exact test.
VATS: video-assisted thoracic surgery.
Table 3 shows the intraoperative and postoperative outcomes based on the thoracic approach. Patients who underwent thoracotomy had a significantly longer surgical duration than those who underwent VATS. Patients who underwent thoracotomy were more likely to require mechanical ventilation after surgery than those who underwent VATS. Patients who underwent thoracotomy had a higher mean volume of blood loss and longer indwelling time of mediastinal drainage, duration of mechanical ventilation and length of hospital stay than patients who underwent VATS. However, the results did not significantly differ.
Table 3:
Postoperative outcomes
| Outcomes | All patients | VATS | Thoracotomy | P-Value | |
|---|---|---|---|---|---|
| n = 141 | n = 83 | n = 58 | |||
| Operative time (min) | Mean (range) | 223.9 (22–667) | 206.6 (22–199) | 248.9 (79–667) | 0.0479 |
| Volume of blood loss (mL) | Mean (range) | 231.4 (0–110) | 186.3 (0–1100) | 296 (0–2560) | 0.2421 |
| Length of hospital stay (days) | Mean (range) | 57.1 (0–237) | 54.8 (10–223) | 60.2 (0–237) | 0.5188 |
| Indwelling time of mediastinal drainage (days) | Mean (range) | 21.6 (1–80) | 19.3 (3–59) | 25 (1–80) | 0.1188 |
| Mechanical ventilation | No | 29 (20.6) | 22 (26.5) | 7 (12.1) | 0.0555 |
| Yes | 112 (79.4) | 61 (73.5) | 51 (87.9) | ||
| Duration of mechanical ventilation (days) | Mean (range) | 18.7 (1–218) | 14.7 (1–58) | 23.6 (1–218) | 0.3245 |
Values for categorical variables were presented as n (%) and assessed with the Fisher’s exact test. Values for continuous variables were expressed as mean and range and evaluated with the Wilcoxon rank-sum test.
VATS: video-assisted thoracic surgery.
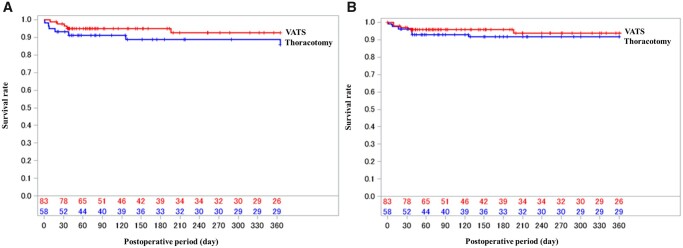
Figure 2 shows the distribution of the logit-transformed propensity scores of preoperative characteristics between the VATS and thoracotomy groups. The distributions differed between the 2 groups. Hence, the confounders should be adjusted. The 90-day mortality rates of the VATS and thoracotomy groups were 4.8% and 8.6%, respectively, and the risk difference between the 2 groups was −3.8%. The adjusted risk difference became close to zero. Regarding secondary outcomes, the 30-day mortality, 1-year mortality and reoperation rates did not differ between the thoracotomy and VATS groups (Table 4). Figure 3 shows the Kaplan–Meier curves of overall survival after the initial surgery. Patients who underwent VATS had a high incidence of all complications and cardiovascular complications. Meanwhile, infectious complications were more common in patients who underwent thoracotomy. However, there were no significant differences between the VATS and thoracotomy groups in terms of complication rates (Table 5). Supplementary Material, Table S1 presents detailed data on postoperative complications.
Figure 2:
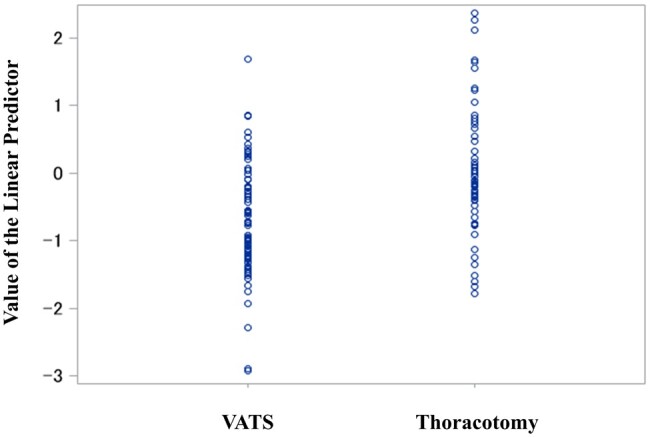
Logit-transformed propensity score comprised the preoperative characteristics of the VATS and thoracotomy groups. The distributions differed significantly between the VATS (mean: −0.7, SD: 0.81) and thoracotomy (mean: 0, SD: 0.95) groups (P < 0.0001). VATS: video-assisted thoracic surgery; SD: standard deviation.
Table 4:
Postoperative mortality and reoperation rates
| Outcomes | VATS (n = 83) | Thoracotomy (n = 58) | Unadjusted |
Adjusted |
||||
|---|---|---|---|---|---|---|---|---|
| RD | 95% CI | P-Value | RD | 95% CI | P-Value | |||
| 90-Day mortality | 4 (4.8) | 5 (8.6) | −0.038 | −0.1237 to 0.0477 | 0.3845 | −0.0077 | −0.095 to 0.0805 | 0.8649 |
| 30-Day mortality | 2 (2.4) | 4 (6.9) | −0.0449 | −0.118 to 0.2289 | 0.2289 | −0.0277 | −0.11 to 0.0545 | 0.5088 |
| 1-Year mortality | 5 (6.0) | 7 (12.1) | −0.0604 | −0.1587 to 0.0378 | 0.2278 | −0.0415 | −0.1517 to 0.0687 | 0.4604 |
| Reoperation rate | 20 (24.1) | 9 (15.5) | 0.0858 | −0.0452 to 0.2167 | 0.1991 | 0.066 | −0.0664 to 0.1985 | 0.3286 |
Values were presented as n (%). The unadjusted RD is the crude value. The adjusted RD including group and logit-transformed propensity scores was examined using the regression formula.
CI: confidence interval; RD: risk difference; VATS: video-assisted thoracic surgery.
Figure 3:
Kaplan−Meier curves of the overall survival after the initial mediastinal drainage. No significant differences were found between the VATS and thoracotomy groups based on the unadjusted (A) and estimated survival time analysed using the Cox regression model, which included approach and propensity scores as covariates (B). VATS: video-assisted thoracic surgery.
Table 5:
Postoperative complications
| Complications | VATS (n = 83) | Thoracotomy (n = 58) | Unadjusted |
Adjusted |
||||
|---|---|---|---|---|---|---|---|---|
| RD | 95% CI | P-Value | RD | 95% CI | P-Value | |||
| All | 44 (53.0) | 22 (37.9) | 0.1508 | −0.0139 to 0.3155 | 0.0727 | 0.164 | −0.0146 to 0.3426 | 0.0719 |
| Lung diseases | 26 (31.3) | 12 (20.7) | 0.1064 | −0.038 to 0.2507 | 0.1486 | 0.1343 | −0.0335 to 0.3022 | 0.1167 |
| CVD | 11 (13.3) | 3 (5.2) | 0.0808 | −0.0118 to 0.1734 | 0.0871 | 0.0791 | −0.0124 to 0.1706 | 0.0901 |
| Infection | 2 (2.4) | 5 (8.6) | −0.0621 | −0.1415 to 0.0173 | 0.1253 | −0.0636 | −0.1373 to 0.0102 | 0.0911 |
Values were presented as n (%). The unadjusted RD is the crude value. The adjusted RD including group and logit-transformed propensity scores was assessed using the regression formula.
CI: confidence interval; CVD: cardiovascular diseases; RD: risk difference; VATS: video-assisted thoracic surgery.
DISCUSSION
The mortality rate of DNM is >40% until the 1980s [2, 17]. In 1994, Marty-Ane et al. showed the importance of thoracotomy for mediastinal drainage, which is a good approach to all compartments of the mediastinum, thoracic cavity and pericardial region. Furthermore, aggressive mediastinal drainage under thoracotomy is recommended regardless of mediastinitis extent. The study assessed the outcomes of only 6 patients. However, the mortality rate was <20%, which was better than that of previous reports [9]. Thereafter, some case series had similar results about thoracotomy at various institutions in the 2000s. Currently, mediastinal drainage for DNM under thoracotomy is widely recognized as an effective approach [2, 10, 11, 18, 19]. Around 2010, progress in diagnostic imaging, antibiotic treatment and understanding of DNM led to early diagnosis and appropriate treatment, and the mortality rates became <10% in recent reports [6, 12, 15, 20]. Simultaneously, the number of institutions performing VATS, which is a minimally invasive procedure, has increased [3, 14–16]. Previously, the subxiphoid, parasternal and transcervical approaches were used because of concerns about the invasiveness of thoracotomy [5, 14, 18]. Compared with these approaches, VATS is more advantageous because it could be used to approach the entire mediastinum similar to thoracotomy. However, no study has compared VATS and thoracotomy. Hence, whether VATS can facilitate the sufficient removal of necrotic material and opening of the mediastinum from the top of the upper mediastinum to the diaphragm compared with thoracotomy remains controversial.
In this large-scale study of DNM, there was an interesting bias in the characteristics of patients who underwent VATS and thoracotomy. No differences in sex, age and comorbidity were found. However, patients with poor ECOG PS were more likely to undergo VATS. In the classification of DNM extent, thoracotomy was often performed for type IIB, which is the most severe extent of mediastinitis. These results indicated that surgeons recognize that VATS is minimally invasive but lesser effective in mediastinal drainage than thoracotomy because choosing between VATS and thoracotomy is at the discretion of the surgeon. Compared with the thoracotomy group, the VATS group had a significantly shorter operative time and a lower proportion of patients requiring postoperative mechanical ventilation. Therefore, VATS can be useful as it is minimally invasive.
In the current study, the primary outcome was postoperative 90-day mortality because the average length of hospital stay is ∼60 days, and most patients were not followed up for >1 year as DNM is a non-cancerous disease. The VATS group had better 30-day, 90-day and 1-year mortality rates than the thoracotomy group. However, the results did not significantly differ. Based on these results, the invasiveness of thoracotomy is not extremely significant to affect the prognosis of DNM compared with VATS. By contrast, the VATS group had higher rates of reoperation and cardiovascular and pulmonary complications than the thoracotomy groups. Debridement of necrotic tissue was not completely performed in VATS compared with thoracotomy. Prolonged mild mediastinitis could have been caused by incomplete drainage under VATS, which could have resulted in a higher rate of cardiopulmonary complications in patients with VATS compared with thoracotomy. In general, reoperation is often considered if mediastinitis does not improve even after the initial surgery and if various symptoms such as fever, cough and circulation insufficiency are prolonged. Moreover, previous studies have shown that delay or incomplete mediastinal drainage for DNM leads to cardiopulmonary complications [20, 21]. However, the VATS group had better outcomes such as length of hospital stay, dwelling time of mediastinal drainage, need for mechanical ventilation and duration of mechanical ventilation than the thoracotomy. That is, the incidence rate of VATS-related complications is high. However, they were not serious, and most could be treated with reoperation and intensive care. Patients with a poor PS who undergo VATS are more likely to benefit from a shorter operative time. In addition, since VATS could preserve the respiratory muscles, it is sufficiently advantageous for the treatment of DNM. However, treatment is based on sufficient drainage and necrotic tissue removal. Therefore, surgeons should consider conversion to thoracotomy if sufficient drainage cannot be achieved during VATS.
In this study, 90-day mortality was the primary end point and 1-year mortality was investigated as a secondary end point. Moreover, this study revealed similar 90-day and 1-year mortality rates. Since limited reports on transthoracic mediastinal drainage for DNM are available, we could not compare long-term outcomes of our study with other studies. However, limited late mortality in the present study suggested that DNM treatments have improved in the recent years.
Limitations
This study had several limitations. First, it was a retrospective database study. Although a prospective randomized study is ideal, it is extremely challenging to perform because of the small number of cases, urgency of treatment, and different patient characteristics. Second, selection bias for the surgical procedure could have existed because VATS or thoracotomy is chosen based on the surgeon’s discretion. However, we could not avoid the bias leading to unmeasured confounders in our analysis, which is a limitation of this database study. Third, detailed preoperative status of the patient, including PS, symptoms and comorbidities, was collected. With respect to the extent of mediastinal infection, we used the classification proposed by Endo. However, we could not collect additional information, such as severity of necrosis, the duration since onset, detailed anatomic mediastinal infected compartments and years of experience of the attending surgeon, since our database did not include this information. Fourth, the definition of VATS in this study was wound measuring ≤8 cm, corresponding to the domestic definition. However, most institutions performed VATS with a few ports of ≤2 cm for DNM treatment.
CONCLUSION
In conclusion, no clinical and statistical differences in prognosis were found between the VATS and thoracotomy groups. VATS is a useful minimally invasive procedure. However, DNM progresses rapidly, and inflammation and abscess often extend beyond the region identified on preoperative imaging. Therefore, thoracotomy should be considered if sufficient drainage cannot be performed under VATS.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to thank all data managers and hospitals for participating in this DNM database project and for their great efforts in entering the data.
Conflict of interest: none declared.
Glossary
ABBREVIATIONS
- DNM
Descending necrotizing mediastinitis
- ECOG PS
Eastern Cooperative Oncology Group performance status
- VATS
Video-assisted thoracic surgery
Contributor Information
Yugo Tanaka, Division of Thoracic Surgery, Kobe University Graduate School of Medicine, Hyogo, Japan.
Yoshimasa Maniwa, Division of Thoracic Surgery, Kobe University Graduate School of Medicine, Hyogo, Japan.
Kenji Sugio, Department of Thoracic and Breast Surgery, Oita University, Oita, Japan.
Tatsuro Okamoto, Department of Thoracic Oncology, National Hospital Organization Kyushu Cancer Center, Fukuoka, Japan.
Ken-Ichi Nibu, Department of Otolaryngology-Head and Neck Surgery, Kobe University Graduate School of Medicine, Hyogo, Japan.
Takashi Omori, Department of Clinical Biostatistics, Graduate School of Medicine, Kyoto University, Kyoto, Japan.
Shunsuke Endo, Department of Thoracic Surgery, Jichi Medical University, Tochigi, Japan.
Hiroyuki Kuwano, Fukuoka City Hospital, Fukuoka, Japan.
Masayuki Chida, Department of General Thoracic Surgery, Dokkyo Medical University School of Medicine, Shimotsuga, Japan.
Yasushi Toh, National Hospital Organization Kyushu Cancer Center, Fukuoka, Japan.
Morihito Okada, Department of Surgical Oncology, Hiroshima University, Hiroshima, Japan.
Akihiro Shiotani, Department of Otolaryngology, National Defense Medical College, Saitama, Japan.
Ichiro Yoshino, Department of General Thoracic Surgery, Chiba University Graduate School of Medicine, Chiba, Japan.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
DATA AVAILABILITY
Data are available on request.
Author contributions
Yugo Tanaka: Conceptualization; Data curation; Investigation; Methodology; Resources; Validation; Visualization; Writing—original draft. Yoshimasa Maniwa: Conceptualization; Data curation; Investigation; Project administration; Supervision; Writing—review & editing. Kenji Sugio: Conceptualization; Data curation; Supervision; Writing—review & editing. Tatsuro Okamoto: Conceptualization; Data curation; Writing—review & editing. Ken-Ichi Nibu: Data curation; Writing—review & editing. Takashi Omori: Formal analysis; Investigation; Methodology; Project administration; Validation; Writing—original draft. Shunsuke Endo: Data curation; Writing—review & editing. Hiroyuki Kuwano: Project administration; Supervision; Writing—review & editing. Masayuki Chida: Project administration; Supervision; Writing—review & editing. Yasushi Toh: Project administration; Supervision; Writing—review & editing. Morihito Okada: Conceptualization; Data curation; Project administration; Writing—review & editing. Akihiro Shiotani: Project administration; Supervision; Writing—review & editing. Ichiro Yoshino: Project administration; Resources; Supervision; Writing—review & editing.
Reviewer information
Interdisciplinary CardioVascular and Thoracic Surgery thanks the anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
- 1. Pearse HE. Mediastinitis following cervical suppuration. Ann Surg 1938;108:588–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wheatley MJ, Stirling MC, Kirsh MM, Gago O, Orringer MB.. Descending necrotizing mediastinitis: transcervical drainage is not enough. Ann Thorac Surg 1990;49:780–4. [DOI] [PubMed] [Google Scholar]
- 3. Shimizu H, Okada M, Toh Y, Doki Y, Endo S. et al. ; Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery. Thoracic and cardiovascular surgeries in Japan during 2018: annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2021;69:179–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ris HB, Banic A, Furrer M, Caversaccio M, Cerny A, Zbären P.. Descending necrotizing mediastinitis: surgical treatment via clamshell approach. Ann Thorac Surg 1996;62:1650–4. [DOI] [PubMed] [Google Scholar]
- 5. Tanaka Y, Maniwa Y, Yoshimura M, Okita Y.. Successful treatment of descending necrotizing mediastinitis. Gen Thorac Cardiovasc Surg 2007;55:366–9. [DOI] [PubMed] [Google Scholar]
- 6. Kocher GJ, Hoksch B, Caversaccio M, Wiegand J, Schmid RA.. Diffuse descending necrotizing mediastinitis: surgical therapy and outcome in a single-centre series. Eur J Cardiothorac Surg 2012;42:e66–e72. [DOI] [PubMed] [Google Scholar]
- 7. Casanova J, Bastos P, Barreiros F, Gomes MR.. Descending necrotising mediastinitis—successful treatment using a radical approach. Eur J Cardiothorac Surg 1997;12:494–6. [DOI] [PubMed] [Google Scholar]
- 8. Sancho LM, Minamoto H, Fernandez A, Sennes LU, Jatene FB.. Descending necrotizing mediastinitis: a retrospective surgical experience. Eur J Cardiothorac Surg 1999;16:200–5. [DOI] [PubMed] [Google Scholar]
- 9. Marty-Ane CH, Alauzen M, Alric P, Serres-Cousine O, Mary H.. Descending necrotizing mediastinitis. Advantage of mediastinal drainage with thoracotomy. J Thorac Cardiovasc Surg 1994;107:55–61. [PubMed] [Google Scholar]
- 10. Marty-Ané CH, Berthet JP, Alric P, Pegis JD, Rouvière P, Mary H.. Management of descending necrotizing mediastinitis: an aggressive treatment for an aggressive disease. Ann Thorac Surg 1999;68:212–7. [DOI] [PubMed] [Google Scholar]
- 11. Iwata T, Sekine Y, Shibuya K, Yasufuku K, Iyoda A, Iizasa T. et al. Early open thoracotomy and mediastinopleural irrigation for severe descending necrotizing mediastinitis. Eur J Cardiothorac Surg 2005;28:384–8. [DOI] [PubMed] [Google Scholar]
- 12. Wakahara T, Tanaka Y, Maniwa Y, Nishio W, Yoshimura M.. Successful management of descending necrotizing mediastinitis. Asian Cardiovasc Thorac Ann 2011;19:228–31. [DOI] [PubMed] [Google Scholar]
- 13. Roberts JR, Smythe WR, Weber RW, Lanutti M, Rosengard BR, Kaiser LR.. Thoracoscopic management of descending necrotizing mediastinitis. Chest 1997;112:850–4. [DOI] [PubMed] [Google Scholar]
- 14. Chen KC, Chen JS, Kuo SW, Huang PM, Hsu HH, Lee JM. et al. Descending necrotizing mediastinitis: a 10-year surgical experience in a single institution. J Thorac Cardiovasc Surg 2008;136:191–8. [DOI] [PubMed] [Google Scholar]
- 15. Sumi Y. Descending necrotizing mediastinitis: 5 years of published data in Japan. Acute Med Surg 2015;2:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sugio K, Okamoto T, Maniwa Y, Toh Y, Okada M, Yamashita T. et al. Descending necrotizing mediastinitis and the proposal of a new classification. JTCVS Open 2021;8:633–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Estrera AS, Landay MJ, Grisham JM, Sinn DP, Platt MR.. Descending necrotizing mediastinitis. Surg Gynecol Obstet 1983;157:545–52. [PubMed] [Google Scholar]
- 18. Endo S, Murayama F, Hasegawa T, Yamamoto S, Yamaguchi T, Sohara Y. et al. Guideline of surgical management based on diffusion of descending necrotizing mediastinitis. Jpn J Thorac Caridovasc Surg 1999;47:14–9. [DOI] [PubMed] [Google Scholar]
- 19. Lavini C, Natali P, Morandi U, Dallari S, Bergamini G.. Descending necrotizing mediastinitis. Diagnosis and surgical treatment. J Cardiovasc Surg (Torino) 2003;44:655–60. [PubMed] [Google Scholar]
- 20. Palma DM, Giuliano S, Cracchiolo AN, Falcone M, Ceccarelli G, Tetamo R. et al. Clinical features and outcome of patients with descending necrotizing mediastinitis: prospective analysis of 34 cases. Infection 2016;44:77–84. [DOI] [PubMed] [Google Scholar]
- 21. Makeieff M, Gresillon N, Berthet JP, Garrel R, Crampette L, Marty-Ane C. et al. Management of descending necrotizing mediastinitis. Laryngoscope 2004;114:772–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on request.
Author contributions
Yugo Tanaka: Conceptualization; Data curation; Investigation; Methodology; Resources; Validation; Visualization; Writing—original draft. Yoshimasa Maniwa: Conceptualization; Data curation; Investigation; Project administration; Supervision; Writing—review & editing. Kenji Sugio: Conceptualization; Data curation; Supervision; Writing—review & editing. Tatsuro Okamoto: Conceptualization; Data curation; Writing—review & editing. Ken-Ichi Nibu: Data curation; Writing—review & editing. Takashi Omori: Formal analysis; Investigation; Methodology; Project administration; Validation; Writing—original draft. Shunsuke Endo: Data curation; Writing—review & editing. Hiroyuki Kuwano: Project administration; Supervision; Writing—review & editing. Masayuki Chida: Project administration; Supervision; Writing—review & editing. Yasushi Toh: Project administration; Supervision; Writing—review & editing. Morihito Okada: Conceptualization; Data curation; Project administration; Writing—review & editing. Akihiro Shiotani: Project administration; Supervision; Writing—review & editing. Ichiro Yoshino: Project administration; Resources; Supervision; Writing—review & editing.
Reviewer information
Interdisciplinary CardioVascular and Thoracic Surgery thanks the anonymous reviewer(s) for their contribution to the peer review process of this article.