Abstract
Most feed materials are predominantly complex and in insoluble forms as animals use them. The aim of digestion processes, therefore, is to sequentially modify the feed substances into simple and soluble forms that are appropriate for absorption and ingestion. This study aimed to investigate the possible effect of pomegranate peel aqueous extract on the histological structure of the small intestine of local male rabbits (Oryctolagus cuniculus). Twelve healthy adult male rabbits acquired from a local market in Iraq- Baghdad was used in this study. The animals were divided into two main groups, six animals in the control group, while the other six were considered as treated groups with pomegranate peel extract. The pomegranate extract was prepared by the maceration method. The experiment lasted for two months, from 1/10/2020 to 1/12/2020. The results showed that the tunica mucosa of the duodenum of the experimental group has huge overcrowded intestinal villi associated with hypertrophy of mucosal villi, in addition to a marked increase in the population of goblet cells and their secretory activities. The tunica submucosa was occupied by a thick layer of Brunner's glands, which were compound tubular mucous alveolar type and had clear cytoplasm. The jejunum of the experimental group showed that the mucosal layer was characterized by a marked increase of intestinal plica circularis, which is associated with the projection of pyramidal-shaped intestinal mucosa. The epithelial mucosa was (pseudostratified columnar epithelium) covered by a thick brush border of microvilli. The histological arrangement of various layers of the ileum was nearly analogous to that of the jejunum except for some distinctive differences. An evident aggregated lymphocytes forming Payers patches occupied the tunica submucosa in both the control and treated groups. The most historical parameters in all segments of the small intestine revealed a significant increase in experimental animals compared to control animals. In conclusion, the aqueous extract of pomegranate peels have apparent positive effects on the histological structure of the small intestine of rabbits improving, positively the digestive efficiency of animals and enhancing the efficiency of the immune system in the animal through an apparent increase in the numbers of goblet cell that plays importance for immunity of the body.
Keywords: Histology, Maceration method, Tunica submucosa, Brunner’s glands, Iraq
1. Introduction
Most feed materials are predominantly complex and insoluble as animals use them. The aim of digestion processes, therefore, is to sequentially modify the feed substances into simple and soluble forms that are appropriate for absorption and ingestion. The digestive canal can be defined as a tube that extends from the mouth cavity of the animal's body to its anal opening, constructed with dilations and constrictions throughout its dimension to form some portions, each with its particular assignment in the digestive process.
Pomegranate has a high nutritive value, health advantages, and antioxidants and, therefore, is considered a medical feed. This plant is frequently subjected to medical grassy among many infectious diseases such as influenza and respiratory infections. Peel or seeds are considered waste products; however, processing these materials transforms them into more valuable products for pharmaceutical, cosmetic and industrial issues ( 1 ). Peels, when dried, can be used for the treatment of diarrhea as well as therapy for urinary and respiratory diseases. In addition, peels can exert variable pharmacological activity, including antioxidant effect, antifertility effect, cytotoxic activity, hepatoprotective activity, and hypoglycemic activity ( 2 ). Several studies confirmed that pomegranate and derivatives have free radicals that play potential scavenger and antioxidant roles ( 3 ). In variances species of animals, there are several kinds of the gastrointestinal tract or digestive canal. These differences appeared palpably in the structural picture and functional role and commonly determine the style of animal feeding, but with all these diversities, a considerable resemblance is also present among species ( 4 ). The small intestine is the first site of the digestive system that deals with the secretion and absorption of fatty acids, amino acids, and carbohydrates.
Consequently, it might be essential in increasing the digestive rate and detraction digesta load. Histological and morphological modifications in the small intestine appear as a unique necessary specialization for the rapid breakdown of substances taken into the body as nourishment, food and drink, and absorption of its contents ( 5 ). Animal models are a good subject for several biomedical and experimental studies involving immunological and metabolic research, biochemical, physiological, and anatomical issues, as well as for overgrowth investigation and experimental transplantation. Rabbits are productive breeders, producing vast quantities of tasty meat for human consumption. The production rate of these animals is faster than that of pigs, goats, or sheep ( 6 ).
This study aimed to investigate the possible effect of pomegranate peel aqueous extract on the histological structure of the small intestine of local male rabbits (Oryctolagus cuniculus).
2. Materials and Methods
2.1. Animals of Study
This study was performed from October to December (2020) among 12 healthy local male rabbits 5-6 months age-old and 1200 g of weight. All rabbits were subjected to a preparation period for 3 weeks, during which they were examined clinically to ensure their health status, acclimated to a new place and fed a pellet with tap water for drinking. Randomly, rabbits were divided equally into two groups:
1. The 1st group was a control group that was fed a daily freely on a diet.
2. The 2nd group was an experimental group that daily fed on a diet plus oral administration of pomegranate peel extracts at a dose of 200 g.
2.2. Samples Collection
After scarifying the animal, the abdominal region was opened up; the small intestine was enucleated pleasantly, then washed with normal saline to expel any possible remains, and soon lay down in an adequate volume of fixative liquid as much as possible to avert post-mortem modification (in minimum the fluid volume ten times the size of sample). Through cut-off transversally, a squeaky lancet trimming was carried out for the duodenum, jejunum, and ileum. Then 10% neutral buffered formalin solution was employed in labeled containers to submerge the samples for 48 hours ( 7 ). Following the completion of the fixation process, the samples of the intestine were transferred on the histological slides for microscopic evaluations ( 8 ).
2.3. Statistical Analysis
Data were analyzed by SPSS software using the Chi-square (x2) test, and values, Mean±Standard error (M±SE), were considered significant at P<0.05.
3. Results
3.1. Duodenum
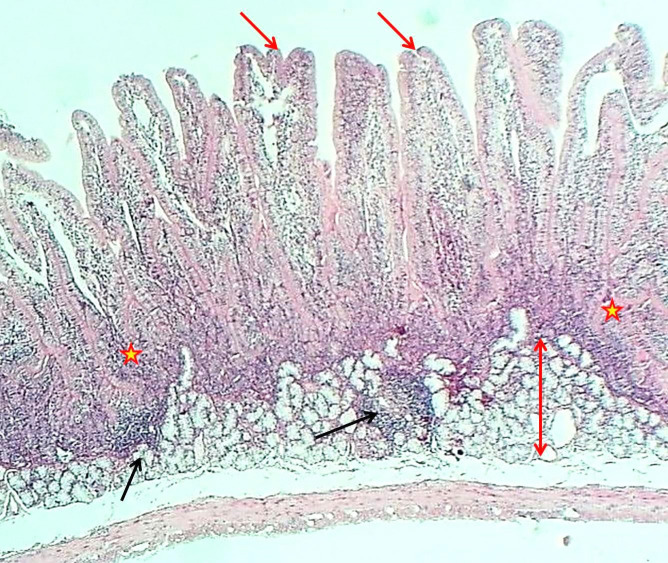
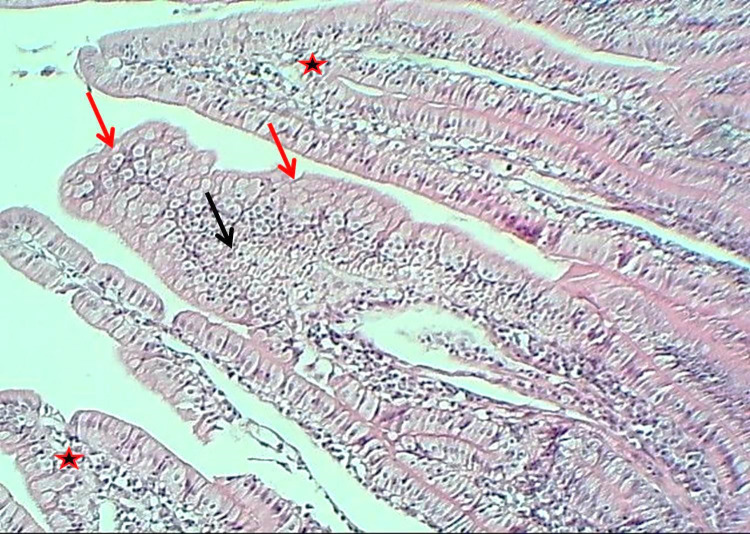
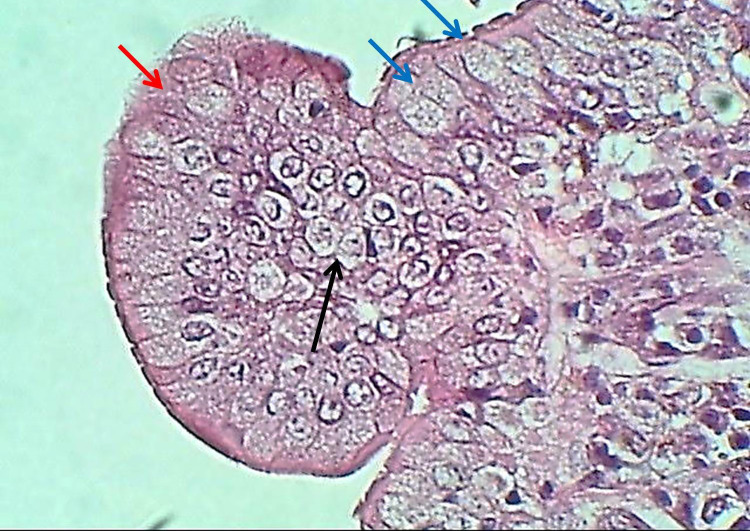
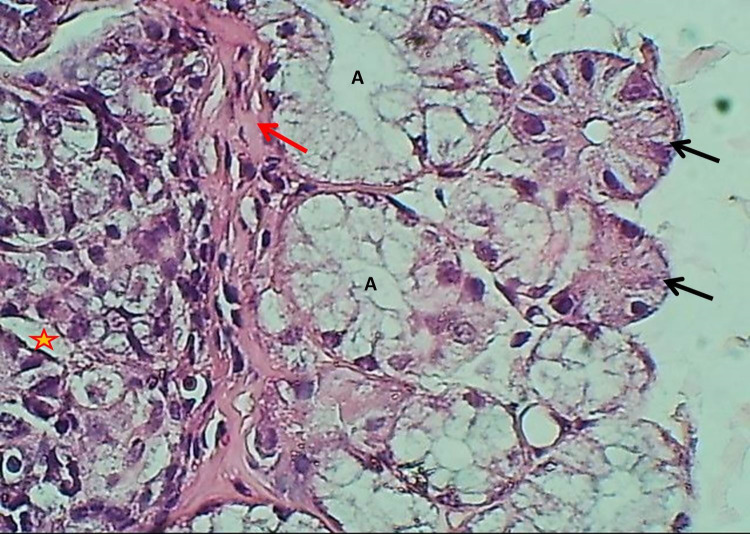
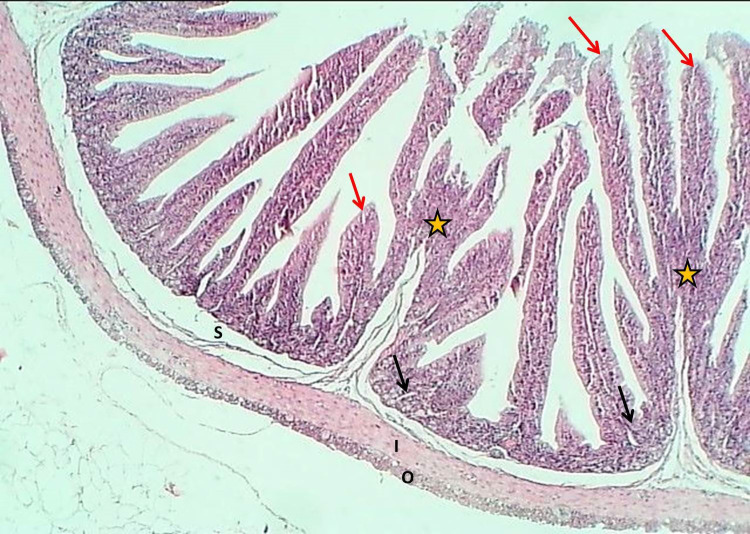
The duodenum of the experimental group showed that the tunica mucosa was revealed with huge overcrowded intestinal villi associated with hypertrophy of mucosal villi; most of these villi showed marked branching and fusion (Figures 1 and 2). The epithelial mucosa was buildup by simple columnar epithelium, which caused active hyperplasia and a marked increase in the population of goblet cells and their secretory activities (Figure 3). The loose connective tissue of lamina propria within epithelial crypts and tunica submucosa was characterized by marked aggregation of lymphocytic forming lymphocytic nodules (Figures 1 and 4). The submucosal Brunner's glands revealed two types of secretory units; mucous alveolar and serous acinar secretory units; the mucous type was predominant and revealed large alveoli that had wide lumen and foamy lightly stained cytoplasm meanwhile the second type was little revealed small size serous acinar contained granular eosinophilic cytoplasm (Figure 5). The historical results revealed a significant elevation in numbers and lengths of villi and most histological parameters of the duodenum in the treated group compared to the control one (Table 1).
Figure 1.
The histological section of the duodenum (Experimental group) shows hypertrophy and division of villi (Red arrows), thickening of epithelial crypts (asterisk) and a layer of Brunner's glands (Double head arrow) and lymphoid nodules (Black arrows), H&E stain, 40×
Figure 2.
The histological section of the duodenum (Experimental group) shows secretory activities (Red arrows), hyperplasia of epithelial cells (Black arrows), a fusion of villi (Red asterisk), H&E stain, 100×
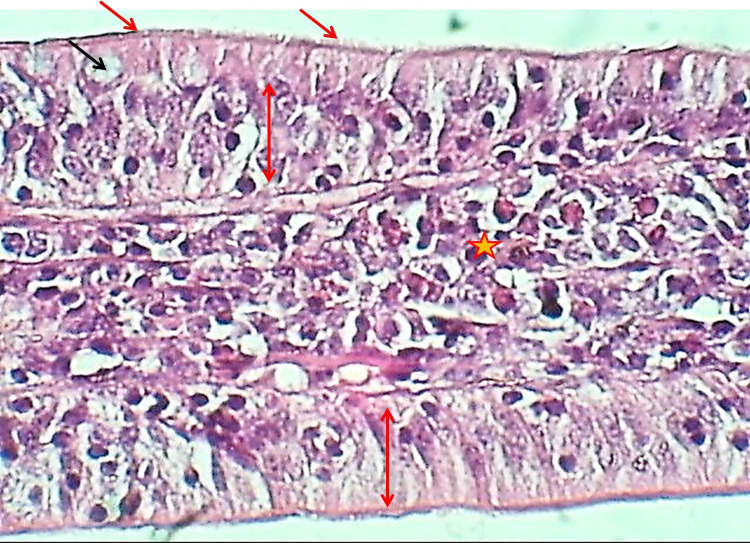
Figure 3.
The histological section of the villus of the duodenum (Experimental group) shows epithelial hyperplasia (Black arrow), increased goblet cells (Blue arrows), secretory activity (Red arrows), H&E stain, 400×
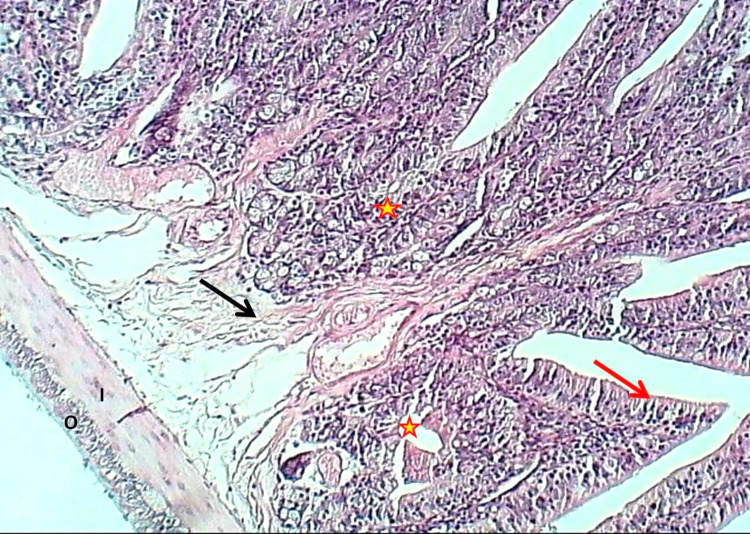
Figure 4.
Section of the duodenum (Experimental group) shows thickening of villi (arrows) and aggregation of lymphoid tissue with nodules formation (asterisk), H&E stain, 100×
Figure 5.
The histological section of the duodenum (Experimental group) shows muscularis mucosa (Red arrow), epithelial crypts (Asterisk), mucous alveolar (A), and serous acinus of Brunner's glands (Black arrows) H&E stain, 400×
Table 1.
The thickness of tunica mucosa, submucosa, muscularis, and some histological parameters of the duodenum in control and experimental groups
| Histological parameter | Control group (µm) | The experimental group (µm) | LSD |
|---|---|---|---|
| Tunica mucosa | 676.43±12.59 | 697.83±9.10 | 21.4 NS |
| Tunica submucosa | 183.06±8.19 | 259.22±9.36 | 76.16 * |
| Tunica muscularis | 72.66±2.26 | 68.56±3.48 | 4.1 NS |
| Height of villi | 476.40±11.21 | 513.22±7.90 | 36.82 * |
| Width of villi | 62.51±1.09 | 83.01±2.98 | 20.5 * |
| Depth of crypts | 155.88±7.38 | 177.13±12.65 | 21.25 * |
| Diameter of alveoli | 48.57±1.87 | 49.98±2.07 | 34.59 * |
| Epithelial height | 19.37±0.98 | 29.01±0.87 | 9.64 * |
| Numbers of goblet cells/villi | 5±1.01 | 9±1.91 | 4 * |
* Significance (*) at P<0.05
3.2. Jejunum
The jejunum of the experimental group showed that the tunica mucosa was characterized by a marked increase of intestinal plica circularis associated with projection of pyramidal-shaped intestinal mucosa and submucosal connective tissue showed 6-8 villi (Figures 6 and 7). The same epithelial mucosa (pseudostratified columnar epithelium) was covered by a thick brush border of microvilli (Figure 8).
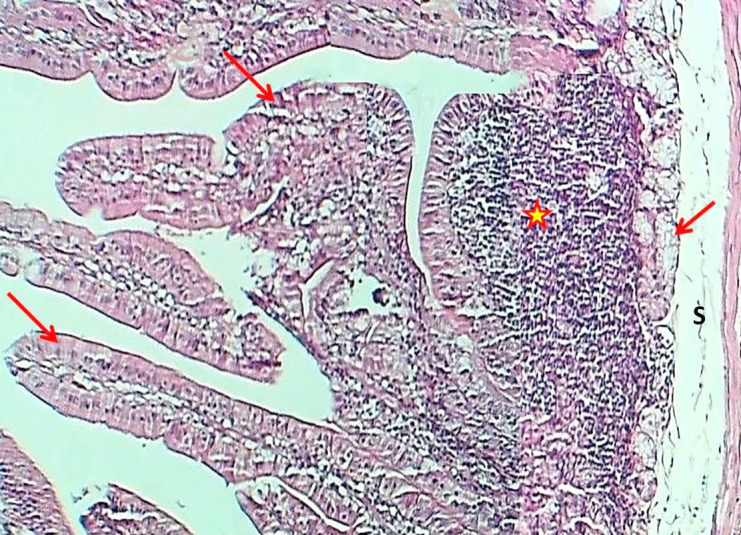
Figure 6.
The histological section of Jejunum (Experimental group) shows a marked increase in plica circularis (Asterisks) and thickness of villi (Red arrows), little epithelial crypts (Black arrows), thin submucosa (Asterisks), with thick inner smooth muscle fibers of tunica muscularis (I) and thin outer layer (O) H&E stain, 40×
Figure 7.
The histological section of Jejunum (Experimental group) shows marked thickened plica circularis with little epithelial crypts (Asterisks), stratified epithelial cells (Red arrow), submucosal loose connective tissue (Black arrow), thick inner smooth muscle fibers of tunica muscularis (I) and thin outer layer (O), H&E stain, 100×
Figure 8.
The histological section of jejunum villus (Experimental group) shows pseudostratified columnar epithelium (Red double head arrows) with thickened brush border microvilli of enterocytes (Red arrows), goblet cells (Black arrows) & thick, highly cellular lamina propria (Asterisk), H&E stain, 400×
The core of each villus formed by lamina propria was characterized by marked thickening associated with a thick layer of cellular loose connective tissue. The historical measurements revealed a significant increase in the numbers of goblet cells and all other parameters in the experimental group's jejunum compared to the treated group's animals (Table 2).
Table 2.
The thickness of tunica mucosa, submucosa, muscularis, and some histological parameters of jejunum in control and experimental groups
| Histological parameter | Control group (µm) | Experimental group (µm) | LSD |
|---|---|---|---|
| Tunica mucosa | 593.15±15.09 | 942.80±11.35 | 349.65 * |
| Tunica submucosa | 81.18±3.16 | 103.30±3.32 | 21.12 * |
| Tunica muscularis | 60.32±3.46 | 73.23±1.26 | 12.91 * |
| Height of villi | 394.44±7.48 | 487.14±8.10 | 83.7 * |
| Width of villi | 75.28±1.24 | 66.07±3.38 | 9.21 * |
| Depth of crypts | 78.61±1.37 | 129.28±4.98 | 51.08 * |
| Epithelial height | 21.86±1.90 | 29.69±2.35 | 7.83 * |
| Numbers of goblet cells/villi | 8±1.21 | 14±2.01 | 4 * |
* Significance (*) at P<0.05
3.3. Ileum
There were no significant differences between the results of ileum and jejunum in experimental animals treated with the extract. This similarity is represented by the remarkable augmentation of intestinal plica circularis, which seems to be 8-9 short villi projected in the mucosal and submucosal layer (Figures 9 and 10). Pseudostratified columnar epithelium covered by microvilli as brush border makes the mucosal epithelium and thick lamina propria in the core of villi. The historical findings showed a noticeable increase in villus height, crypt depth, and generality of studied parameters of ileum in dosed animals (Table 3).
Figure 9.
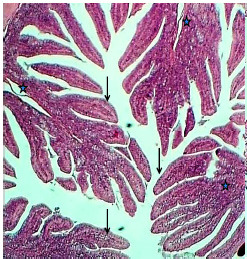
The histological section of the ileum (Experimental group) shows mucosal much of plica circularis (Asterisks) and numerous villi (Arrows), H&E stain, 40×
Figure 10.
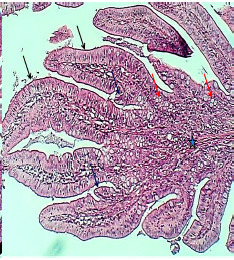
The histological section of the ileum villus (Experimental group) shows very thick villi (Black arrows), much more goblet cells (Red arrows), branching of villi (Black arrows), and thick lamina propria (Asterisk), H&E stain, 40×
Table 3.
The thickness of tunica mucosa, submucosa, muscularis, and some histological parameters of ileum in control and experimental groups
| Histological parameter | Control group (µm) | The experimental group (µm) | LSD |
|---|---|---|---|
| Tunica mucosa | 593.92±13.19 | 689.71±8.01 | 95.79* |
| Tunica submucosa | 82.18±3.61 | 201.69±8.92 | 119.51* |
| Tunica muscularis | 72.32±3.39 | 91.23±4.50 | 30.91* |
| Height of villi | 484.44±7.48 | 632.26±12.98 | 147.82* |
| Width of villi | 75.28±1.24 | 81.53±7.73 | 6.25 NS |
| Depth of crypts | 78.61±1.37 | 91.47±8.94 | 12.86* |
| Epithelial height | 21.86±1.90 | 34.11±3.58 | 12.25* |
| Numbers of goblet cells/villi | 9±2.07 | 14±1.06 | 5* |
* Significance (*) at P<0.05
4. Discussion
The positive effect of pomegranate peel extract may be represented in its ability to improve digestion in the dosed animals by increasing the number and length of villi in the studied parts of the intestine. Increased length of villi correlates to the health status of the digestive canal and high efficacy of nutrient absorption ( 9 ), whereas a decrease in the length of villi with deep crypts refers to the persistence of toxins or high regeneration of tissues ( 10 ). Although food activates the intestinal morphological development, low villi density in the intestine could reduce both the width and height of villi, resulting in decreasing digestion and absorption, leading to a severe impact on the body weight ( 11 , 12 ).
The significant improvement in the alveolar diameters of Brunner's glands in treated animals may reflect the good digestive effect of the aqueous extract of pomegranate peels on the intestinal lining.
Mohammad, Ali ( 13 ) mentioned that the submucosa layer appears as a thin layer of loose connective tissue below the mucosal layer, which in the duodenum is comprised of simple tubular glands named (Brunner's glands) which developed highly increased in density in the submucosa of the duodenum of rabbits and some others animals. Brunner's glands differ in secretory unit acini in rabbit serous and mucous acini were characterized by their relatively wide lumen, and their cells seemed pale. The duodenum is the position of most of the breakdown of the nutrients passing through it ( 14 ). Brunner's glands have an essential function represented by neutralizing chyme reaching the duodenum from the stomach, protecting the mucous membrane, and making the intestinal contents at the optimum pH for pancreatic enzyme work ( 15 ).
The significant increase in the number of goblet cells in the jejunum of rabbits treated with pomegranate peel gives a clear indication of the effectiveness of this extract in improving digestion by developing the intestinal lining to the high ability to enhance the immune system of the animal. This hypothesis becomes more acceptable with that detected by Birchenough, Johansson ( 16 ) as Goblet cells play an essential role in preserving intestinal homeostasis. Recent studies have shown that goblet cells can perform as antigen importers and may play a significant role in regulating innate immune function. Goblet cells aid maintains homeostasis by their function of providing bicarbonate for suitable mucin unfolding in the small intestine. Goblet cells may also form a line of protection in the intestinal mucosa and have a common secretive function.
The results mentioned above of animals dosed with pomegranate peel extract show an apparent positive effect on the histological structure of the jejunum through the development of the mucosal layer by increasing its thickness and number of intestinal plica circularis, which in turn leads to enhanced digestion and increasing the absorptive surface area of the small intestine. Bartholomew, Dahlin ( 17 ) reported that the small intestine presents an enormous absorptive surface, not only because of its great length but because the mucous membrane is thrown into crescentic circular folds or plica circularis. These are most prominent in the upper jejunum, which gradually becomes smaller towards the lower ileum, where they disappear. The absorptive surface is also increased by the intestinal villi, which are most developed in the jejunum, again becoming fewer and shorter towards the lower ileum. There is sizeable intestinal mucosa entirely composed of mucus-secreting or goblet cells. In the same context, Bello and Danmaigoro ( 18 ) mentioned that the inner surface of the small intestine is covered with finger-like projections referred to as villi, which increase the surface area available for the absorption of nutrients from the gut content. By increasing the surface area of the small intestine, villi increase the chance of a food particle encountering a digestive enzyme and being absorbed across the epithelium and into the bloodstream. The villous lamina propria is rich in capillaries and lymphatics that help transport nutrients absorbed by enterocytes across the luminal surface.
It could detect the functional status of the small intestine through critical parameters such as crypt depth and height of villi ( 19 ). In the absorptive epithelium, the villi and crypts play a very substantial role in the terminal stage of digestion and absorption of food ( 7 ). Any modifications in the morphology of the small intestine could affect the ability of metabolism and performance of nutrients ( 19 ). For example, the increase in the height of villi could result in an augment of the broad luminal villus absorptive area, which in turn leads to the pleasurable activity of the digestive enzyme and higher transportation of food at the villus surface ( 20 ). The evolution of the small intestine could be estimated by measuring the height of the villi, depth of the crypt, and surface area to detect the available zone for digestion and absorption ( 21 , 22 ).
A higher villus height to crypt depth ratio results in a decreased turnover of the intestinal mucosa. A slower turnover rate of the intestinal epithelium leads to a lower maintenance requirement and, finally, can result in a higher growth efficiency of the animal ( 23 ). Overall, it can be said that villus height, crypt depth, and the ratio of villus height to crypt depth reflect the small intestine morphology and absorption capacity ( 24 ). Therefore, an increase in villus height, villus height to crypt depth ratio, or decrease in the crypt depth is correlated with an improvement in the digestion and absorption of nutrients ( 25 - 27 ).
The aqueous extract of pomegranate peels has an apparent positive effect on the histological structure of the small intestine in rabbits; through the significant increase of most historical parameters, which is reflected positively in improving the digestive efficiency of the animal. On the other hand, this extract enhances the efficiency of the immune system in animals through an apparent increase in the number of goblet cells that play a critical immune role in the body.
Authors' Contribution
Study concept and design: A. S. A.
Acquisition of data: B. A. A.
Analysis and interpretation of data: A. S. A.
Drafting of the manuscript: B. A. A.
Critical revision of the manuscript for important intellectual content: A. S. A.
Statistical analysis: B. A. A.
Administrative, technical, and material support: B. A. A.
Ethics
This study was licensed by the Scientific Committee of the Department of Anatomy and Histology in the College of Veterinary Medicine (University of Baghdad, Baghdad, Iraq).
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Dhumal S, Karale A, Jadhav S, Kad V. Recent advances and the developments in the pomegranate processing and utilization: a review. J Agric Crop Sci. 2014;1(1):1–17. [Google Scholar]
- 2.Thring TS, Hili P, Naughton DP. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement Med Ther. 2009;9(1):1–11. doi: 10.1186/1472-6882-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenblat M, Hayek T, Aviram M. Anti-oxidative effects of pomegranate juice (PJ) consumption by diabetic patients on serum and on macrophages. Atherosclerosis. 2006;187(2):363–71. doi: 10.1016/j.atherosclerosis.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Damron WS. Introduction to animal science: Global, biological, social, and industry perspectives. 6th ed: Pearson. 2013. [Google Scholar]
- 5.Igwebuike U, Eze UU. Morphological characteristics of the small intestine of the African pied crow (Corvus albus) Anim Res Int. 2010;7(1):1116–20. [Google Scholar]
- 6.Foster HL, Small JD, Fox JG. The mouse in biomedical research: normative biology, immunology, and husbandry: Academic press. 2014 [Google Scholar]
- 7.Wang J, Peng K. Developmental morphology of the small intestine of African ostrich chicks. Poult Sci. 2008;87(12):2629–35. doi: 10.3382/ps.2008-00163. [DOI] [PubMed] [Google Scholar]
- 8.Gharban HA, Al-Shaeli SJ, Al-Fattli HH, Altaee MN. Molecular and histopathological confirmation of clinically diagnosed lumpy skin disease in cattle, Baghdad Province of Iraq. Vet World. 2019;12(11):1826. doi: 10.14202/vetworld.2019.1826-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia V, Catala-Gregori P, Hernandez F, Megias M, Madrid J. Effect of formic acid and plant extracts on growth, nutrient digestibility, intestine mucosa morphology, and meat yield of broilers. J Appl Poult Res. 2007;16(4):555–62. [Google Scholar]
- 10.Miles R, Butcher G, Henry P, Littell R. Effect of antibiotic growth promoters on broiler performance, intestinal growth parameters, and quantitative morphology. Poult Sci. 2006;85(3):476–85. doi: 10.1093/ps/85.3.476. [DOI] [PubMed] [Google Scholar]
- 11.Uni Z, Ganot S, Sklan D. Posthatch development of mucosal function in the broiler small intestine. Poult Sci. 1998;77(1):75–82. doi: 10.1093/ps/77.1.75. [DOI] [PubMed] [Google Scholar]
- 12.Yamauchi KE, Isshiki Y. Scanning electron microscopic observations on the intestinal villi in growing White Leghorn and broiler chickens from 1 to 30 days of age. Br Poult Sci. 1991;32(1):67–78. doi: 10.1080/00071669108417328. [DOI] [PubMed] [Google Scholar]
- 13.Mohammad H, Ali K, Al-Ali Z. Histomorphologal and histochemical structure in the duodenum of sheep (Ovis aries) and rabbit (Oryctolagus cuniculus)-a comparative study. Online J Anim Feed Res. 2020;10(6):251–8. [Google Scholar]
- 14.Elnasharty M, Abou-Ghanema I, Sayed-Ahmed A, Elnour AA. Mucosal-submucosal changes in rabbit duodenum during development. World Acad Eng Technol. 2013:7–14. [Google Scholar]
- 15.Ergun E, Ergun L, Ozen A, Kurum A, Bayraktaroglu AG. Histomorphology of the Brunner's glands in the Angora rabbit. J Anim Vet Adv. 2010;9(5):887–91. [Google Scholar]
- 16.Birchenough GM, Johansson ME, Gustafsson JK, Bergström JH, Hansson GC. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 2015;8(4):712–9. doi: 10.1038/mi.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartholomew LG, Dahlin DC, Waugh JM. Intestinal polyposis associated with mucocutaneous melanin pigmentation (Peutz-Jeghers syndrome): review of literature and report of six cases with special reference to pathologic findings. Gastroenterology. 1957;32(3):434–51. [PubMed] [Google Scholar]
- 18.Bello A, Danmaigoro A. Histomorphological observation of the small intestine of Red Sokoto Goat: a review. MOJ Anatomy & Physiology. 2019;6 [Google Scholar]
- 19.Laudadio V, Passantino L, Perillo A, Lopresti G, Passantino A, Khan R, et al. Productive performance and histological features of intestinal mucosa of broiler chickens fed different dietary protein levels. Poult Sci. 2012;91(1):265–70. doi: 10.3382/ps.2011-01675. [DOI] [PubMed] [Google Scholar]
- 20.Tufarelli V, Desantis S, Zizza S, Laudadio V. Performance, gut morphology and carcass characteristics of fattening rabbits as affected by particle size of pelleted diets. Arch Anim Nutr. 2010;64(5):373–82. doi: 10.1080/1745039X.2010.496945. [DOI] [PubMed] [Google Scholar]
- 21.Franco JRG, Murakami AE, Natali MRM, Garcia E, Furlan AC. Influence of delayed placement and dietary lysine levels on small intestine morphometrics and performance of broilers. Braz J Poult Sci. 2006;8(4):233–41. [Google Scholar]
- 22.Swatson HK, Gous R, Iji PA, Zarrinkalam R. Effect of dietary protein level, amino acid balance and feeding level on growth, gastrointestinal tract, and mucosal structure of the small intestine in broiler chickens. Anim Res. 2002;51(6):501–15. [Google Scholar]
- 23.Van Nevel CJ, Decuypere JA, Dierick NA, Molly K. Incorporation of galactomannans in the diet of newly weaned piglets: effect on bacteriological and some morphological characteristics of the small intestine. Arch Anim Nutr. 2005;59(2):123–38. doi: 10.1080/17450390512331387936. [DOI] [PubMed] [Google Scholar]
- 24.Montagne L, Pluske J, Hampson D. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim Feed Sci Technol. 2003;108(1-4):95–117. [Google Scholar]
- 25.Hou Y, Wang L, Ding B, Liu Y, Zhu H, Liu J, et al. Dietary α-ketoglutarate supplementation ameliorates intestinal injury in lipopolysaccharide-challenged piglets. Amino acids. 2010;39(2):555–64. doi: 10.1007/s00726-010-0473-y. [DOI] [PubMed] [Google Scholar]
- 26.Hou Y, Wang L, Yi D, Ding B, Yang Z, Li J, et al. N-acetylcysteine reduces inflammation in the small intestine by regulating redox, EGF and TLR4 signaling. Amino acids. 2013;45(3):513–22. doi: 10.1007/s00726-012-1295-x. [DOI] [PubMed] [Google Scholar]
- 27.Yao K, Yin Y, Li X, Xi P, Wang J, Lei J, et al. Alpha-ketoglutarate inhibits glutamine degradation and enhances protein synthesis in intestinal porcine epithelial cells. Amino acids. 2012;42(6):2491–500. doi: 10.1007/s00726-011-1060-6. [DOI] [PubMed] [Google Scholar]