Abstract
Bovine viral diarrhea virus (BVDV) and bovine herpes virus-1 (BHV-1) have been known as the major pathogens with significant economic consequences on the cattle industry worldwide, including in Iran. In this cross-sectional study, the seroprevalences of BVDV and BHV-1 and their associated risk factors were investigated in dairy cattle farms of Fars province, Iran, where with 0.4 million head of cattle, mainly crossbred, is ranked the first in cow population in southern Iran. A total number of 420 cattle in 18 herds were randomly selected from three geographical regions (140 samples from each of the north, central, and south regions) and their serum samples were analyzed to detect antibodies to these viruses using a commercially available enzyme-linked immunosorbent assay kit. Chi-square test and logistic regression analyses were employed to investigate associations between risk factors and the seroprevalence of viruses. The overall seroprevalence rates for BVDV and BHV-1 were estimated at 91.67% and 77.87% at the herd level and 55.48% and 39.76% at the animal level, respectively. The only significant factor associated with the seroprevalence of both viruses at the herd level was found to be the literacy level of farmers (P<0.05). The significant risk factors for both viruses in cattle were identified as geographical region, age, and breeding method by the univariate analysis (P<0.05), while gender and farming system were determined as risk factors only for BHV-1 (P<0.001). A significant relationship of concurrent infection with BVDV and BHV-1 (φ=0.268, P<0.001) was recorded, and 28.6% of sera had antibodies to both viruses. The results of the present study demonstrated that BVDV and BHV-1 had a wide distribution in dairy cattle herds in southern Iran and highlighted the need for intensive surveillance and control strategies to reduce the risk of the spread of these viruses.
Keywords: BVDV, BHV-1, Risk factors, Southern Iran
1. Introduction
Herd health, in particular infectious diseases, has been shown to be of obvious critical importance in cattle herd efficiency ( 1 ). Bovine respiratory disease (BRD) constitutes one of the key health issues and is responsible for major economic losses in cattle in all parts of the world. Two of the most important viruses contributing to BRD include bovine herpes virus type 1 (BHV-1) and bovine viral diarrhea virus (BVDV). Both viruses are immunosuppressive and establish an environment that is favorable for the colonization and replication of pathogenic bacteria, resulting in a series of disease syndromes, especially BRD ( 2 ) and reproductive disorders ( 3 ).
Bovine viral diarrhea virus is a single-stranded RNA virus that belongs to the Pestivirus of Flaviviridae family and produces a wide range of detrimental effects, including respiratory disease, impaired reproductive performance, gastroenteric disorders, and a fatal form known as a mucosal disease ( 4 ). For BVDV, cytopathogenic (CP) and non-cytopathogenic (NCP) biotypes are defined. The CP biotype is usually encountered in association with clinical cases of mucosal disease and NCP strains are more prevalent and important in persistently infected (PI) animals, which generally remain lifelong virus carriers and shed the virus in most body excretions and secretions, while they remain seronegative ( 5 ). Decreased fertility and milk production, respiratory symptoms, reproductive and immunological dysfunctions, impaired herd performance, and the dreaded state of PI in calves are the substantial economic losses from BVDV ( 6 ).
Bovine herpes virus type 1 is a double-stranded DNA virus that belongs to Varicellovirus, a member of the Herpesviridae family, and consists of three subtypes. The clinical manifestation of BHV-1 infection, including respiratory syndromes, genital infections, and neurological disorders, is dependent on the nature of various subtypes. The transmission of BHV-1 occurs by contact with mucosal droplets from infected cattle. The virus can remain latent in some parts of the nervous system ganglia after the initial infection and shed following reactivation ( 7 ). The infection can cause severe economic losses due to a decrease in weight loss and milk production and restrictions in the international livestock trade ( 8 ).
While culture and virus isolation from clinical specimens remains the “gold standard” diagnostic technique, other methods, including virus-neutralization test and enzyme-linked immunosorbent assay (ELISA), are used to detect antibodies against BVDV and BHV-1. Enzyme-linked immunosorbent assay is a quick, simple, and economical method with good sensitivity, specificity, and repeatability employed in most seroprevalence studies, including the reports reviewed in the present study. Seroprevalence studies have already demonstrated a wide seropositivity distribution of 28.6-77.9% for BVDV and 10.7-72.2% for BHV-1 in different regions of Iran, while there is no documented report for the prevalence of these viruses in Fars province, Iran ( 9 - 13 ). Fars province, with 0.4 million head of cattle, mainly crossbred, ranks the first in cow population in southern Iran and plays an important role in the supply of milk and meat in the country ( 14 ). Due to the significant importance of BVDV and BHV-1, the present study aimed at estimating their seroprevalence and identifying potential risk factors in dairy cattle herds of Fars province.
2. Materials and Methods
2.1. Study Area
This study was carried out in Fars province, southern Iran. The 29 counties in the study area were classified based on geographical region to north, central, and south, and Eqlid, Marvdasht, and Lar counties were selected from each region, respectively. The dairy cattle herd and individual cattle were considered the target population and sampling unit, respectively. For this cross-sectional study, in each of these areas, three industrial dairy farms (large farms where cattle were raised according to modern procedures and had a capacity of more than 20 heads) and three traditional dairy farms (small farms where cattle were raised under householder management and had a capacity of fewer than 20 heads) were randomly selected. Sampling was performed twice: once in August as the warm time and the other time in March as the cold time in 2017. Some data were collected by asking some close-ended questions concerning farmer literacy level, management practices, and location and structure of the herd. These data were obtained by interviewing the person in charge of the herd and were used in the analysis of risk factors associated with the herd-level prevalence.
2.2. Sample Size
The sample size was calculated for at least 384 cases using the following formula ( 15 ):
Where: n = sample size, d = precision of 0.05 at 95% confidence level, and Pexp = expected prevalence. Pexp of 50% was used since the two diseases have rarely been studied in southern Iran.
In each of the 36 selected dairy farms, 6 cows, 3 bulls, and 3 calves under 6 months old were randomly selected for sampling. If there were not enough calves or bulls on the farm, they were replaced by cows. In total, 432 samples were collected from apparently healthy cattle; however, 12 samples were randomly omitted due to the lack of a test kit. Finally, considering approximately 10% higher than the calculated number, 420 samples (140 samples from each region) were included in this study. Cattle were generally not vaccinated against BVDV and BHV-1 in the study area.
2.3. Sample Collection and Detection of Antibodies
The blood samples were collected from the jugular vein (5 ml) into sterile vacuum tubes (VACUETTE®, Greiner Bio-One GmbH, Kremsmünster, Austria). All samples were immediately transported to the diagnostic laboratory. The samples were placed in a centrifuge, which was set at 3,000 rpm and 10 min, and the harvested serum was frozen at -18°C in a microtube until analysis.
An ELISA kit (Lot No: RES18D10, Bio-X Diagnostics, Rochefort, Belgium) was employed for screening antibodies to BVDV and BHV-1. According to the procedure provided in the kit, briefly, the test sera were diluted, added to the microplate, and coated by antigen. The plate was washed after incubation at 21°C for 1 h and the conjugate (peroxidase-labeled anti-bovine immunoglobulin G 1 monoclonal antibody) was added to the wells. After reincubation at 21°C for 1 h, the wells were washed,, and the reaction was revealed by adding the chromogen (tetramethylbenzidine). Finally, the reaction was stopped after 10 min by a stop solution (1 M phosphoric acid), and plates were read at 450 nm fairly soon after adding the stopping solution.
Corrected optical density (ODcorr) was calculated by subtracting optical density values in wells coated with BVDV and BHV-1 antigens or positive control from the OD values of corresponding wells containing the control antigen (ODtest antigen or positive control − ODcontrol = ODcorr). The percent positivity (PP) values were calculated as follows:
The sample was considered positive for BVDV or BHV-1 when PP was greater than 20% or 30%, respectively. Farms with at least two seropositive animals were considered positive herds.
2.4. Statistical Analysis
All statistical analyses were carried out in SPSS software for windows 16.0.0 (SPSS Inc. 2007). In the first step, serostatuses of BVDV and BHV-1 in the herd and individual animals were described. The herd-level variables, including geographical region, farming type, cattle purchasing, history of abortion, proper ventilation, distance to the main road, manure gathering intervals, and farmer literacy, were analyzed using the Chi-square test. The association of geographical region, animal factors (age and gender), and farm factors (farming type and breeding method) with BVDV and BHV-1 serostatuses of individual animals were examined using the Chi-square test, and the associated variables with a univariate p-value of < 0.05 were offered to the logistic regression test. The strength of the association between outcome and variables was assessed using odds ratios and a 95% confidence interval (CI). The simultaneous presence of BVDV and BHV-1 antibodies was assessed using Phi and Cramer's V test.
3. Results
Seroprevalence rates of BVDV and BHV-1 in the herd-level were estimated at 91.67% (95% CI: 78.18-97.13%) and 77.78% (95% CI: 61.92-88.29%), respectively. It was revealed the region and season did not significantly affect the herd seroprevalence of studied viruses (Figure 1). The results of univariable analysis for BVDV and BHV-1 seropositivity at the herd level are summarized in table 1. The association of geographical regions, farming type, adding cows to the farm, a history of abortion, proper ventilation, distance to road, and manure gathering interval was not significant with BVDV and BHV-1 seropositivity. The only significant factor associated with the seroprevalence of both viruses at the herd level was the literacy level of farmers (P<0.05).
Figure 1.
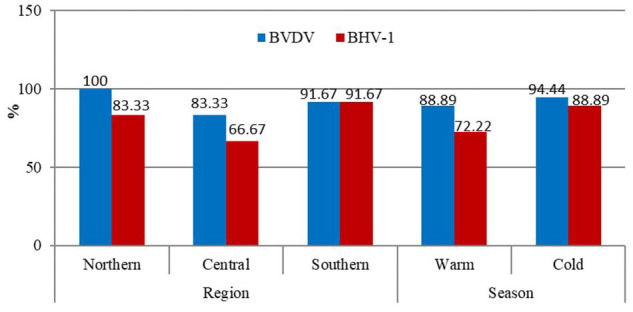
Percentage of seropositive farms for bovine viral diarrhea virus and bovine herpesvirus type 1in different regions and seasons
Table 1.
Univariable analysis of associated variables in the warm season for bovine viral diarrhea virus and bovine herpes virus-1 seropositivity at the herd level
| Viruses | BVDV | BHV-1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | Number | Seroprevalence (%) | 95% CI for seroprevalence | χ2* | P-value | Number | Seroprevalence (%) | 95% CI for seroprevalence | χ2 | P- value | ||||
| Tested | Positive | Upper | Lower | Tested | Positive | Upper | Lower | |||||||
| Geographic region | 1.125 | 0.570 | 2.215 | 0.330 | ||||||||||
| Central | 6 | 5 | 83.33 | 43.65 | 96.99 | 6 | 3 | 50.00 | 18.76 | 81.24 | ||||
| Northern | 6 | 6 | 100.00 | 60.97 | 100.00 | 6 | 5 | 83.33 | 43.65 | 96.99 | ||||
| Southern | 6 | 5 | 83.33 | 43.65 | 96.99 | 6 | 5 | 83.33 | 43.65 | 96.99 | ||||
| Farming type | 0.00 | 1.00 | 0.277 | 0.599 | ||||||||||
| Industrial | 9 | 8 | 88.89 | 56.50 | 98.01 | 9 | 6 | 66.67 | 35.42 | 87.94 | ||||
| Traditional | 9 | 8 | 88.89 | 56.50 | 98.01 | 9 | 7 | 77.78 | 45.26 | 93.68 | ||||
| Cattle purchasing | 0.281 | 0.596 | 0.554 | 0.457 | ||||||||||
| Yes | 2 | 2 | 100.00 | 34.24 | 100.00 | 2 | 1 | 50.00 | 9.45 | 90.55 | ||||
| No | 16 | 14 | 87.50 | 63.98 | 96.50 | 16 | 12 | 75.00 | 50.50 | 89.82 | ||||
| History of abortion | 0.117 | 0.732 | 0.004 | 0.952 | ||||||||||
| Yes | 11 | 10 | 90.91 | 62.27 | 98.38 | 11 | 8 | 72.73 | 43.44 | 90.26 | ||||
| No | 7 | 6 | 85.71 | 48.68 | 97.43 | 7 | 5 | 71.43 | 35.89 | 91.78 | ||||
| Proper ventilation | 0.281 | 0.596 | ||||||||||||
| Yes | 16 | 14 | 87.50 | 63.98 | 96.50 | 16 | 11 | 68.75 | 44.40 | 85.84 | 0.865 | 0.352 | ||
| No | 2 | 2 | 100.00 | 34.24 | 100.00 | 2 | 2 | 100.0 | 34.24 | 100.00 | ||||
| Distance to road | 2.250 | 0.134 | ||||||||||||
| Near | 9 | 9 | 100.00 | 70.09 | 100.00 | 9 | 6 | 66.67 | 35.42 | 87.94 | ||||
| Far | 9 | 7 | 77.78 | 45.26 | 93.68 | 9 | 7 | 77.78 | 45.26 | 93.68 | ||||
| Manure gathering interval | 1.125 | 0.289 | 0.138 | 0.710 | ||||||||||
| Daily | 6 | 6 | 100.00 | 60.97 | 100.00 | 6 | 4 | 66.67 | 30.00 | 90.32 | ||||
| > Daily | 12 | 10 | 83.33 | 55.19 | 95.30 | 12 | 9 | 75.00 | 46.77 | 91.11 | ||||
| Farmer literacy | 4.500 | 0.034 | 6.785 | 0.009 | ||||||||||
| ≤ Diploma | 12 | 12 | 100.00 | 75.75 | 100.00 | 12 | 11 | 91.67 | 64.62 | 98.51 | ||||
| > Diploma | 6 | 4 | 66.67 | 30.00 | 90.32 | 6 | 2 | 33.33 | 9.68 | 70.00 | ||||
BVDV: Bovine viral diarrhea virus; BHV-1: Bovine herpes virus-1: CI: Confidence interval
*Chi-square
Out of 420 animals, 233 (55.48%; 95% CI: 50.70-60.16%) and 167 (39.76%; 95% CI: 35.19-44.51%) cases were positive serologically for BVDV and BHV-1, respectively. Table 2 presents the results of Chi-square tests at the animal level. Geographical region, age, and breeding method significantly affected the BVDV serostatus (P<0.05). There was a statistically significant variation (P<0.05) in seroprevalence of BHV-1 in region, gender, age, farming system, and breeding type categories. Season did not significantly affect the seroprevalence of BVDV and BHV-1 at the animal level; therefore, this variable was not used in the multivariable logistic regression analysis. Furthermore, gender and farming system were omitted in the logistic regression analysis of serostatus of BVDV due to no significant association. In logistic regression analysis, all factors were still significantly related to the seroprevalence of BVDV and BHV-1 at the animal level, except gender for the status of BHV-1 (Table 3). The results showed that 28.6% of sera had antibodies to both viruses and 33.3% of sera were free from the antibodies. A significant association of concurrent infection with BVDV and BHV-1 was recorded (φ=0.268, P<0.001).
Table 2.
Univariable analysis of associated variables for bovine viral diarrhea virus and bovine herpes virus-1 seropositivity at the animal level
| Viruses | BVDV | BHV-1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | Number | Seroprevalence (%) | 95% CI for seroprevalence | χ2* | P- value | Number | Seroprevalence (%) | 95% CI for seroprevalence | χ2 | P- value | ||||
| Tested | Positive | Upper | Lower | Tested | Positive | Upper | Lower | |||||||
| Season | 1.63 | 0.202 | 1.68 | 0.195 | ||||||||||
| Warm | 210 | 110 | 52.38 | 45.64 | 59.03 | 210 | 77 | 36.67 | 30.45 | 43.37 | ||||
| Cold | 210 | 123 | 58.57 | 51.81 | 65.02 | 210 | 90 | 42.86 | 40.50 | 53.88 | ||||
| Geographic region | 6.03 | 0.049 | 49.28 | 0.000 | ||||||||||
| Central | 140 | 82 | 58.57 | 50.29 | 66.39 | 140 | 33 | 23.57 | 17.30 | 31.25 | ||||
| Northern | 140 | 85 | 60.71 | 52.44 | 68.41 | 140 | 46 | 32.86 | 25.63 | 41.01 | ||||
| Southern | 140 | 66 | 47.14 | 39.06 | 55.37 | 140 | 88 | 62.85 | 54.61 | 70.42 | ||||
| Gender | 0.51 | 0.476 | 10.02 | 0.002 | ||||||||||
| Male | 38 | 19 | 50.00 | 34.85 | 65.15 | 38 | 6 | 15.79 | 7.44 | 30.42 | ||||
| Female | 382 | 214 | 56.02 | 51.01 | 60.91 | 382 | 161 | 42.15 | 37.30 | 47.16 | ||||
| Age | 12.93 | 0.000 | 27.92 | 0.000 | ||||||||||
| Calf | 80 | 30 | 37.50 | 27.96 | 48.45 | 80 | 11 | 13.75 | 7.85 | 22.97 | ||||
| Adult | 340 | 203 | 59.71 | 54.42 | 64.79 | 340 | 156 | 45.88 | 40.66 | 51.19 | ||||
| Farming type | 1.68 | 0.194 | 10.56 | 0.001 | ||||||||||
| Industrial | 212 | 111 | 52.36 | 45.65 | 58.98 | 212 | 68 | 32.08 | 26.16 | 38.63 | ||||
| Traditional | 208 | 122 | 58.65 | 51.86 | 65.13 | 208 | 99 | 47.60 | 40.92 | 54.37 | ||||
| Breeding type | 9.47 | 0.002 | 14.30 | 0.000 | ||||||||||
| Artificial | 219 | 143 | 65.30 | 58.78 | 71.29 | 219 | 84 | 38.36 | 32.17 | 44.65 | ||||
| Mating | 113 | 54 | 47.79 | 38.81 | 56.92 | 113 | 68 | 60.18 | 50.96 | 68.73 | ||||
BVDV: Bovine viral diarrhea virus; BHV-1: Bovine herpes virus-1; CI: Confidence interval
*Chi-square
Table 3.
Logistic regression analysis of associated factors for bovine viral diarrhea virus and bovine herpes virus-1 seropositivity at the animal level
| Viruses | BVDV | BHV-1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Factor | Category | Odds ratio | 95% CI | P- value | Odds ratio | 95% CI | P- value | ||
| Upper | Lower | Upper | Lower | ||||||
| Region | Central | 1.56 | 0.99 | 2.54 | 0.056 | 1 | |||
| Northern | 1.73 | 1.08 | 2.78 | 0.023 | 1.23 | 0.69 | 2.18 | 0.479 | |
| Southern | 1 | 3.96 | 2.25 | 6.95 | 0.000 | ||||
| Gender | Male | 1 | |||||||
| Female | 1.31 | 0.45 | 3.78 | 0.620 | |||||
| Age | Calf | 1 | 1 | ||||||
| Adult | 2.47 | 1.50 | 4.08 | 0.000 | 3.16 | 1.47 | 6.78 | 0.003 | |
| Farming type | Industrial | 1 | |||||||
| Traditional | 1.92 | 1.25 | 2.95 | 0.003 | |||||
| Breeding type | Artificial | 2.06 | 1.30 | 3.26 | 0.002 | 1 | |||
| Mating | 1 | 2.429 | 1.53 | 3.87 | 0.000 | ||||
BVDV: Bovine viral diarrhea virus; BHV-1: Bovine herpes virus-1; CI: Confidence interval
4. Discussion
In this study, the seroprevalence rates of BVDV were estimated at 91.67% and 55.67% in cattle and herds, respectively. As vaccination against BVDV was not practiced in the herds in Fars province, it can be concluded that these found antibodies might have originated from the exposure of the animals to the virus, which could be the reason for high seroprevalence ( 16 ). The high herd-level BVDV serostatus was in agreement with other reports from Zanjan (90%) and Isfahan (90% and 100%) provinces in Iran ( 9 , 11 ). Worldwide seroprevalence of BVDV has been reported from 70% to 100% at the herd level ( 17 ). The herd seroprevalences of BVDV were reported at 95.6% and 80.0% in the Jimma town of Ethiopia and Selangor in Malaysia, respectively ( 18 , 19 ). The herd-level seroprevalence of BVDV was estimated at 100% in 161 Irish beef herds ( 1 ). Bovine viral diarrhea virus antibody was investigated serologically in bulk tank milk samples from dairy cattle herds in eastern China and northeast Thailand; accordingly, it was reported that 77.8% and 62.5% of herds were positive, respectively ( 20 , 21 ).
Globally, scarce studies have been conducted on risk factor analysis for BVDV seroprevalence at the herd level. Inconsistent with the results of the present study that did not identify animal purchasing as a risk factor for BVDV disease, those of a study in the State of Paraíba, Northeastern Brazil, showed that in addition to animal purchasing, other variables, such as the number of calves aged ≤ 12 months, rental of pasture, and presence of veterinary assistance, could be risk factors for BVDV seroprevalence in the farm ( 16 ).
In Iran, reports on animal-level seroprevalence of BVDV are considerable in comparison to herd-level reports. The animal-level seroprevalence of BVDV reported in the current study was in agreement with a previous report from Khorasan province (55.3%) and higher than those from Zanjan, Ahwaz, Chaharmahal Bakhtiari, and Esfahan provinces, Iran (28.6%, 28.5%, 50.7%, and 52.8% respectively) ( 9 , 13 , 22 ). The prevalence of seropositive animals was estimated at 66.0%, 73.3%, 74%, and 89% in Arak, Kerman, Semnan, and Sistan-Baluchestan provinces, Iran, respectively ( 10 , 12 , 13 ). The regional differences in the rate of seroprevalence can be explained by the size of the herd, compactness of cattle, transfer of animals between farms, and presence of PI animals ( 23 - 25 ).
In an analysis of variables, BVDV infection showed no significant association with season, gender, and farming system, which was not in agreement with the findings of the study conducted in Zanjan ( 9 ). Risk factors analysis revealed that other variables, including region, age, and breeding method, were significantly associated with BVDV seroprevalence. The risk for BVDV infection in dairy herds in the northern region of Iran increased significantly (P<0.05) by 1.73 times, compared to the southern region. The variation of seroprevalence in different regions can be attributable to the differences in herd characteristics (e.g., cattle density, herd size, housing systems, and biosecurity) and environmental conditions ( 19 , 26 ). It has been reported that BVDV infection risk was higher in adults (OD=2.47, P<0.001), which was consistent with the results of other studies ( 9 , 27 ). Longer exposure time to the virus during the animals’ life increases the chance of exposure to the virus in the older animals. Specific anti-BVDV antibodies are produced after infection and remain in serum forever; in this regard, seroprevalence reflects previous BVDV infection at any time of life ( 23 ). Another risk factor associated with BVDV seroprevalence was the breeding method; regarding this, higher seroprevalence was observed in cattle breeding by artificial insemination (AI) than in those by natural breeding (OD=2.06, P<0.01). There is a considerable amount of information about the risk of BVDV transmission through contaminated semen used for AI ( 28 ). Needle and palpation (if the same pair of gloves are worn for all exams) have also been shown to transmit BVDV when using AI ( 6 ). In contrast, the findings of other studies in Brazil and Ethiopia indicated no association between BVDV seropositivity and the breeding method ( 16 , 19 ).
The overall herd-level of BHV-1 seroprevalence in the present study was lower than those reported in Isfahan, Arak, Hamedan, and Zanjan provinces of Iran (100%, 91.6%, 82.3%, and 80%, respectively) ( 9 - 11 , 29 ). The variation in BHV-1 seroprevalence at the herd level was also observed in different countries, as it was reported at 90%, 83.2%, and 58.33% in Ireland, England, and Algeria, respectively. While in the present study, only the literacy level of farmer significantly affected BHV-1 seroprevalence at the herd level (χ2=6.785, P<0.01), in other studies, region, season, herd size, the introduction of a new animal to the herd, distance to neighboring farms, and hygiene were suggested as the main risk factors for BHV-1 infection ( 1 , 30 , 31 ).
In the current study, the animal-level prevalence of the BHV-1 (39.76%) was considered high, compared with the reports from Zanjan, Khorasan, Semnan, and Kerman provinces, Iran (11.4%, 21.7%, 25.6%, and 30.39%, respectively) ( 9 , 12 , 13 ). In other regions of Iran, the rates were reported at 72%, 58.74%, 57.7%, and 48.69% for Isfahan, Hamedan, Chaharmahal Bakhtiari, and Khouzestan provinces, respectively (13, 29, 32, 33). Differences in various studies may be due to discrepancies in the herd size, farming practices, and microclimate of the area ( 24 ).
Analysis of risk factors revealed a higher seroprevalence in the southern part than the central part of the Fars province (OD=3.96, P<0.001). The probable reason for this difference has been attributed to variation in climate, attitude, herd size, and management system ( 29 ). In the current study, a greater risk for BHV-1 infection was found in females than in males (χ2=10.02, P<0.01), which was inconsistent with the report from Zanjan province. On other hand, the findings of some studies have reported no significant difference between gender groups. These results highlight the importance of performing more studies to find the actual relationship between gender and infection with these viruses ( 9 , 33 ). In agreement with a recent report ( 11 ), the risk of BHV-1 seropositivity status was significantly higher in adults than in calves (OD=3.16, P<0.01). This difference may be explained by the fading of maternal antibodies, a history of a previous infection, and the presence of accumulative antibodies in adults.
The results of some studies have reported that the traditional system was associated with lower BHV-1 seropositivity ( 9 , 33 ). On the contrary, our results showed a higher risk in the traditional husbandry system (OD=1.92, P<0.01). This effect can result from housing characteristics (e.g., poor housing and ventilation and high animal density) and management practices (e.g., uncontrolled animal movement and poor hygiene) in dairy farms of Fars province under the traditional husbandry system. The results of the binary logistic regression in a previous study showed that the use of a bull instead of artificial insemination was a risk factor for BHV-1, which was in agreement with our finding ( 34 ). Cattle may be infected by BHV-1 directly from secretions (respiratory, eye, reproductive) or via semen from infected bulls and contaminated equipment ( 35 ); in this regard, the risk of infection increases through contact with infected individuals ( 31 ). A significant relationship of simultaneous infection with BVDV and BHV-1 was confirmed in previous studies, which was in line with the findings of the present study ( 9 , 11 , 32 , 36 ). The same risk factors or immunosuppressive effect of both viruses was mentioned to be involved in this relationship ( 10 ). It was indicated that rising in abortion and infertility problems in dairy cattle could result from the interaction between BVDV and BHV-1 ( 37 ).
In the light of our findings, it can be concluded that BVDV and BHV-1 infections were widespread in dairy herds and unvaccinated cattle in southern Iran. The awareness of farmers of identified risk factors is important to prevent infections and related economic losses. Further pieces of research are recommended to identify the biotypes and genotypes of viruses circulating and the establishment of surveillance and control programs in the region and country. Implementation of biosecurity strategies and detection of PI animals at the early stages of infection have to be considered essential for any control program.
Authors' Contribution
Study concept and design: M. H. and M. B.
Acquisition of data: M. H. and M. M.
Analysis and interpretation of data: M. H.
Drafting of the manuscript: M. H.
Critical revision of the manuscript for important intellectual content: M. B.
Statistical analysis: M. H.
Administrative, technical, and material support: M. M.
Ethics
All experimental procedures were carried out according to the approved protocol by Razi Vaccine and Serum Research Institute.
Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgment
This study was financially supported by Razi Vaccine and Serum Research Institute. The authors are gratefully thankful to the veterinary general office of Fars province for helping during the sampling procedure and Dr M. H. Hosseini for technical assistance during laboratory procedures.
References
- 1.Barrett D, Parr M, Fagan J, Johnson A, Tratalos J, et al. Prevalence of Bovine Viral Diarrhoea Virus (BVDV), Bovine Herpes Virus 1 (BHV 1), Leptospirosis and Neosporosis, and associated risk factors in 161 Irish beef herds. BMC Vet Res. 2018;14(1):8. doi: 10.1186/s12917-017-1324-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Urban-Chmiel R, Grooms DL. Prevention and Control of Bovine Respiratory Disease. J Livest Sci. 2012;3:27–36. [Google Scholar]
- 3.Newcomer BW, Givens D. Diagnosis and Control of Viral Diseases of Reproductive Importance: Infectious Bovine Rhinotracheitis and Bovine Viral Diarrhea. Vet Clin North Am Food Anim Pract. 2016;32(2):425–41. doi: 10.1016/j.cvfa.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Tautz N, Tews BA, Meyers G. The Molecular Biology of Pestiviruses. Adv Virus Res. 2015;93:47–160. doi: 10.1016/bs.aivir.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Lanyon SR, Hill FI, Reichel MP, Brownlie J. Bovine viral diarrhoea: pathogenesis and diagnosis. Vet J. 2014;199 (2):201–9. doi: 10.1016/j.tvjl.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 6.Khodakaram-Tafti A, Farjanikish GH. Persistent bovine viral diarrhea virus (BVDV) infection in cattle herds. Iran J Vet Res. 2017;18 (3):154–63. [PMC free article] [PubMed] [Google Scholar]
- 7.Biswas S, Bandyopadhyay S, Dimri U, Patra PH. Bovine herpesvirus-1 (BHV-1) - a re-emerging concern in livestock: a revisit to its biology, epidemiology, diagnosis, and prophylaxis. Vet Q. 2013;33(2):68–81. doi: 10.1080/01652176.2013.799301. [DOI] [PubMed] [Google Scholar]
- 8.Nandi S, Kumar M, Manohar M, Chauhan RS. Bovine herpese virus infections in cattle. Anim Health Res Rev. 2009;10(1):85–98. doi: 10.1017/S1466252309990028. [DOI] [PubMed] [Google Scholar]
- 9.Erfani AM, Bakhshesh M, Fallah MH, Hashemi M. Seroprevalence and risk factors associated with bovine viral diarrhea virus and bovine herpes virus-1 in Zanjan Province, Iran. Trop Anim Health Prod. 2019;51(2):313–9. doi: 10.1007/s11250-018-1687-3. [DOI] [PubMed] [Google Scholar]
- 10.Ghaemmaghami S, Ahmadi M, Deniko M, Mokhberosafa L, Bakhshesh M. Serological study of BVDV and BHV-1 infections in industrial dairy herds of Arak, Iran. Iran J Vet Sci Tech . 2013;5(2):53–61. [Google Scholar]
- 11.Noaman V, Nabinejad AR. Seroprevalence and risk factors assessment of the three main infectious agents associated with abortion in dairy cattle in Isfahan province, Iran. Trop Anim Health Prod. 2020:2001–9. doi: 10.1007/s11250-020-02207-8. [DOI] [PubMed] [Google Scholar]
- 12.Sakhaee E, Khalili M, Kazeminia S. Serological study of bovine viral respiratory diseases in dairy herds in Kerman province, Iran. Iran J Vet Res. 2009;10(1):49–53. [Google Scholar]
- 13.Nikbakht G, Tabatabaei S, Lotfollahzadeh S, Nayeri Fasaei B, Bahonar A, et al. Seroprevalence of bovine viral diarrhoea virus, bovine herpesvirus 1 and bovine leukaemia virus in Iranian cattle and associations among studied agents. J Appl Anim Res. 2015;43:22–5. [Google Scholar]
- 14. Agriculture Statistics of Iran. The yearbook of agriculture statistics of Iran, 2018. In: technology Bosai, editor. Tehran, Iran : The ministry of Jihad-E-agriculture; 2019. The yearbook of agriculture statistics of Iran, 2018. [Google Scholar]
- 15.Thrusfield M. Veterinary epidemiology. 3rd ed. Oxgord: Blackwell Science Ltd; 2007. [Google Scholar]
- 16.Fernandes LG, Nogueira AH, De Stefano E, Pituco EM, Ribeiro CP, et al. Herd-level prevalence and risk factors for bovine viral diarrhea virus infection in cattle in the State of Paraiba, Northeastern Brazil. Trop Anim Health Prod. 2016;48 (1):157–65. doi: 10.1007/s11250-015-0937-x. [DOI] [PubMed] [Google Scholar]
- 17.Houe H. Risk assessment. In: S.M. G, J.F. R, editors. Bovine viral diarrhea virus: diagnosis, management, and control. Iowa: Blackwell Publishing; 2008. pp. 35–64. [Google Scholar]
- 18.Daves L, Yimer N, Arshad SS. Seroprevalence of Bovine Viral Diarrhea Virus Infection and Associated Risk Factors in Cattle in Selangor, Malaysia. Vet Med Open J. 2016;1(1):22–8. [Google Scholar]
- 19.Tadesse T, Deneke Y, Deresa B. Seroprevalence of bovine viral diarrhea virus and its potential risk factors in dairy cattle of jimma town, southwestern Ethiopia. J Dairy Vet Anim Res. 2019;8(1):11–7. [Google Scholar]
- 20.Nilnont T, Aiumlamai S, Kanistanont K, Inchaisri C, Kampa J. Bovine viral diarrhea virus (BVDV) infection in dairy cattle herds in northeast Thailand. Trop Anim Health Prod. 2016;48(6):1201–8. doi: 10.1007/s11250-016-1075-9. [DOI] [PubMed] [Google Scholar]
- 21.Hou P, Zhao G, Wang H, He H. Prevalence of bovine viral diarrhea virus in dairy cattle herds in eastern China. Trop Anim Health Prod. 2019;51(4):791–8. doi: 10.1007/s11250-018-1751-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haji Hajikolaei MR, Seyfi Abad Shapouri MR. Serological study of bovine diarrhea virus infection of cattle in Ahvaz. J Vet Res. 2007;62:21–6. [Google Scholar]
- 23.Houe H. Epidemiology of bovine viral diarrhea virus. Vet Clin North Am Food Anim Pract. 1995;11(3):521–47. doi: 10.1016/s0749-0720(15)30465-5. [DOI] [PubMed] [Google Scholar]
- 24.Almeida LL, Miranda IC, Hein HE, Neto WS, Costa EF, et al. Herd-level risk factors for bovine viral diarrhea virus infection in dairy herds from Southern Brazil. Res Vet Sci. 2013;95(3):901–7. doi: 10.1016/j.rvsc.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Van Campen H. Epidemiology and control of BVD in the U.S. Vet Microbiol. 2010;142 (1-2):94–8. doi: 10.1016/j.vetmic.2009.09.049. [DOI] [PubMed] [Google Scholar]
- 26.Uddin MA, Ahasan A, Islam K, Islam MZ, Mahmood A, et al. Seroprevalence of bovine viral diarrhea virus in crossbred dairy cattle in Bangladesh. Vet World. 2017;10(8):906–13. doi: 10.14202/vetworld.2017.906-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aragaw K, Sibhat B, Ayelet G, Skjerve E, Gebremedhin EZ, et al. Seroprevalence and factors associated with bovine viral diarrhea virus (BVDV) infection in dairy cattle in three milksheds in Ethiopia. Trop Anim Health Prod. 2018;50(8):1821–7. doi: 10.1007/s11250-018-1624-5. [DOI] [PubMed] [Google Scholar]
- 28.Meyling A, Houe H, Jensen AM. Epidemiology of bovine virus diarrhoea virus. Rev Sci Tech. 1990;9(1):75–93. doi: 10.20506/rst.9.1.489. [DOI] [PubMed] [Google Scholar]
- 29.Bahari A, Gharekhani J, Zandieh M, Sadeghi-Nasab A, Akbarein H, et al. Serological study of bovine herpes virus type 1 in dairy herds of Hamedan province, Iran. Vet Res Forum. 2013;4(2):111–14. [PMC free article] [PubMed] [Google Scholar]
- 30.Kaddour A, Bouyoucef A, Fernandez G, Prieto A, Geda F, et al. Bovine herpesvirus 1 in the northeast of Algiers, Algeria: Seroprevalence and associated risk factors in dairy herd. J Adv Vet Anim Res. 2019;6(1):60–5. doi: 10.5455/javar.2019.f312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Schaik G, Dijkhuizen AA, Huirne RB, Schukken YH, Nielen M, et al. Risk factors for existence of Bovine Herpes Virus 1 antibodies on nonvaccinating Dutch dairy farms. Prev Vet Med. 1998;34(2-3):125–36. doi: 10.1016/s0167-5877(97)00085-8. [DOI] [PubMed] [Google Scholar]
- 32.Shirvani E, Lotfi M, Kamalzadeh M, Noaman V, Bahriari M, et al. Seroepidemiological study of bovine respiratory viruses (BRSV, BoHV-1, PI-3V, BVDV, and BAV-3) in dairy cattle in central region of Iran (Esfahan province) Trop Anim Health Prod. 2012;44(1):191–5. doi: 10.1007/s11250-011-9908-z. [DOI] [PubMed] [Google Scholar]
- 33.Adeli E, Pourmahdi Borujeni M, Haji Hajikolaei MR, Seifi Abad Shapouri MR. Bovine Herpesvirus-1 in Khouzestan province in Iran: seroprevalence and risk factors. Iran j rumin health res. 2017;2(1):47–56. [Google Scholar]
- 34.Quevedo DC, Benavides BB, Guillermo C, Carlos H. Seroprevalence and risk factors associated to BHV-1 and DVBV in dairy herds in Pasto, Colombia, in 2011. Rev Lasallista Investig. 2011;8(2):61–8. [Google Scholar]
- 35.Muylkens B, Thiry J, Kirten P, Schynts F, Thiry E. Bovine herpesvirus 1 infection and infectious bovine rhinotracheitis. Vet Res. 2007;38(2):181–209. doi: 10.1051/vetres:2006059. [DOI] [PubMed] [Google Scholar]
- 36.Aktas MS, Celik H. Seroprevalence of Bovine Viral Diarrhoea Virus, Bovine Herpesvirus Type 1 and Bovine Herpesvirus Type 4 infections in cattle in Ağrı Province, Eastern Anatolia Region, Turkey. Turkish J Vet Anim Sci. 2021;45:955–63. [Google Scholar]
- 37.Aslan ME, Azkur AK, Gazyagci S. Epidemiology and genetic characterization of BVDV, BHV-1, BHV-4, BHV-5 and Brucella spp. infections in cattle in Turkey. J Vet Med Sci. 2015;77(11):1371–77. doi: 10.1292/jvms.14-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]


