Abstract
Ocular infection is a significant public health issue in many countries and is a leading cause of morbidity and blindness worldwide. The current study aimed to determine the prevalence of bacterial isolates, and their role in patients with external ocular infections, according to age, sex, and residence in rural and urban areas. In addition, investigate the antibiotic sensitivity and resistance patterns commonly used for treating patients suffering from external ocular infections in Babylon Governorate, Iraq. Two hundred patients with clinical suspicion of external ocular infections, ranging in age from 20 to 68 years old and from both sexes, participated in this study. The eye swabs were collected and cultured to distinguish between bacterial and viral isolates. About 105 isolates (52.5%) were found to be positive bacterial cultures and taken as a study sample, whereas 95 (47.5%) negative bacterial cultures were excluded. The results revealed that ocular infections most frequently occur in the age groups between 20-49years, and most patients were males 65 (61.9%). The most common eye infection was conjunctivitis, which affected 45.7% of patients. This was followed by blepharitis, which affected 21.9% of patients; blepharoconjunctivitis, which affected 14.3% of patients; dacryocystitis, which affected 12.4% of patients; and keratitis, which affected 5.7% of patients. Also, the results indicated that the most common bacteria implicated in ocular infections are Staphylococcus aureus (37.1%), followed by Coagulase negative Staphylococci (CoNS) (26.7%), Haemophilusinfluenzae (21.9%), Streptococcuspneumoniae (6.7%), Klebsiella pneumonia (3.8%), Streptococcus pyogenes (1.9%), Pseudomonas aeruginosa (1.9%). Furthermore, some antibiotics were tested for these pathogenic bacterial isolates to show their effects against these bacteria. It was found that most bacterial isolates were resistant to Ampicillin, Penicillin, and Tetracycline, whereas they were susceptible to Ciprofloxacin, Gentamycinand Chloramphenicol.
Keywords: Conjunctivitis, Blepharitis, Bacteria, Multidrug resistance
1. Introduction
Ocular infection is a significant public health issue in many countries and is a leading cause of morbidity and blindness worldwide ( 1 ). The most prevalent pathogens that cause external ocular infections are bacteria. Conjunctivitis, blepharitis, periorbital cellulitis, dacryocystitis, and orbital are the most prevalent external ocular infections ( 2 ). Infections like these are linked to higher rates of morbidity and blindness worldwide ( 3 ).
Gram-positive commonly cause ocular infections, and Gram-negative bacteria such as S. aureus, St. pneumoniae, Haemophilus influenza, Pseudomonas aeruginosa, Bacillus, Enterobacteriaceae, Morraxellaspp., and Neisseria gonorrhoeae are among bacteria that cause ocular infections ( 4 ). Significant contributors to the development of ocular infections include "the virulence of the pathogen, inadequate personal cleanliness, poor living conditions, a compromised immune system, surgery, trauma, chronic nasolacrimal duct obstruction, and systemic disorders” ( 5 ). In hospitals, the rise of resistant bacterial strains to widely used antibiotics is a global problem ( 2 ). Furthermore, bacterial agents that cause ocular illnesses and their sensitivity to different antibiotics differ according to geographic location and from one hospital to another ( 6 ).
The current study aimed to determine the prevalence of bacterial isolates, and their role in patients with external ocular infections, according to age, sex, and residence in rural and urban areas. In addition, investigate the antibiotic sensitivity and resistance patterns commonly used for treating patients suffering from external ocular infections in Babylon Governorate, Iraq.
2. Materials and Methods
2.1. Study Populations and Specimen Collection
All 200 patients with clinical signs and symptoms of external ocular infections of age groups ranging from 20 to 68 years old and from both sexes were attending the Ophthalmic Unit of HillaTeaching Hospital and other primary health centers from the interval beginning of September (2020) to the end of February (2021) in Babylon Governorate. The clinical features of ocular infection cases and other information were recorded, including patient age, sex, and residence. Also, the patients should not have antibiotics to avoid false negative results.
The eye swabs were obtained from patients and put into a sterilized swab tube that contained specific transport media and kept in an ice box—then transferred to the laboratory within two hours from collecting.
2.2. Cultivation and Identification of Bacteria
MacConkey's agar, Blood agar, and Chocolate agar (heated blood agar) were inoculated with the laboratory's collected clinical sample of eye swabs, then incubated at 37°C for 24 hours. A single colony of pure bacterial isolates were identified using morphological characteristics such as the following assays: Gram's stain and biochemical tests, including “Oxidase, Catalase, Coagulase, Optochin and Bacitracin tests, Lysine decarboxylation, Indole production, Motility, Urease, Carbohydrate fermentation, Citrate utilization, Gas production, and H2S production,". In addition, satellitism test was done to identify Haemophilusinfluenzae isolates ( 7 ).
2.3. Antibiotic Susceptibility Test
It was done for all identified bacterial isolates on Muller-Hinton agar using the disc diffusion method suggested by the Clinical and Laboratory Standard Institute (CLSI) 2014 guideline ( 8 ). The inoculum was prepared by adding growth from (3–5) isolated colonies of the test organism to 5 ml of nutrient broth. The culture was incubated at 37°C for 2 hours to produce a moderate turbidity bacterial suspension. An inoculum was obtained from the standardized culture using a sterile swab. The inoculum was then streaked onto the surface of the Muller-Hinton agar plate, and antibiotic discs were equally dispersed throughout the agar's surface using sterile forceps. After that, the plates were incubated at 37°C for 24 hours, and the zone inhibition diameters were determined.
3. Results
In the present study, 200 good clinical samples of eye swabs were collected from patients (113 male and 87 female) suffering from external ocular infections, ranging in age from 20 to 68 years old. Of them, 105 isolates (52.5%) were found to be positive bacterial cultures, and 95 isolates (47.5%) had negative bacterial cultures (Table 1).
Table 1.
Distribution of eye swabs based on the bacterial culture results
| Bacterial culture results | No. of eye swabs (%) | Gender | |
|---|---|---|---|
| Male | Female | ||
| Positive bacterial culture | 105 (52.5) | 65 | 40 |
| Negative bacterial culture | 95 (47.5) | 48 | 47 |
| Total | 200 (100%) | 113 | 87 |
The negative bacterial isolates 95 were excluded, whereas all 105 positive bacterial isolates from patients (65 male and 40 female) suffering from ocular infections were taken as a study sample (Table 2).
Table 2.
Distribution of adult patients with ocular infections based on their age groups and gender
| Age groups in years | No. of patients (%) | Gender | |
|---|---|---|---|
| Male | Female | ||
| 20 – 29 | 25 (23.8%) | 15 | 10 |
| 30 – 39 | 30 (28.6%) | 19 | 11 |
| 40 – 49 | 33 (31.4%) | 21 | 12 |
| 50 – 59 | 10 (9.5%) | 6 | 4 |
| ≥ 60 | 7 (6.7%) | 4 | 3 |
| Total | 105(100%) | 65 (61.9%) | 40 (38.1%) |
The results in table 2 reveal that ocular infections occur in patients of all age groups, but it most frequently occurs in the age groups between 20-49 years.
Also, it is clear that ocular infections occur in males and females, and most patients were males 65 (61.9%). Male predominance was observed in patients aged 20-49 years, while there was no difference in other age groups.
Furthermore, the high prevalence of ocular infections in a rural areas (74.3%) than in urban areas (25.7%) (Figure 1).
Figure 1.
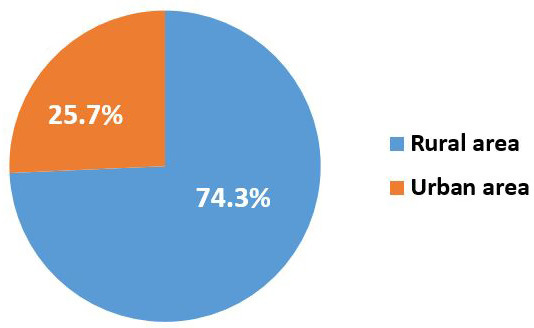
The prevalence of ocular diseases in rural and urban areas
Among the 105 positive bacterial isolates obtained from individuals with ocular infections, conjunctivitis was found to be the most prevalent eye infection, accounting for 45.7%. This was followed by blepharitis (21.9%), blepharoconjunctivitis (14.3%), dacryocystitis (12.4%), and keratitis (5.7%) (Table 3).
Table 3.
Frequency of ocular infections types among patients in relation to the identified clinical features
| Ocular infections types | No. of cases | Percentage |
|---|---|---|
| Conjunctivitis | 48 | 45.7% |
| Blepharitis | 23 | 21.9% |
| Blepharoconjunctivitis15 | 14.3% | |
| Dacryocystitis13 | 12.4% | |
| Keratitis | 6 | 5.7% |
| Total | 105 | 100% |
Out of 105 bacterial isolates, 17 (35.4%) were confirmed to be Haemophilusinfluenzae, followed by Staphylococcus aureus 16 (33.3%), Coagulase negative Staphylococci (CoNS) 11 (22.9%), Streptococcus pneumoniae 2 (4.2%), Klebsiella pneumoniae 1 (2.1%), and Streptococcus pyogenes 1 (2.1%). (2.1 percent). From the cases of blepharitis and blepharoconjunctivitis, Staphylococcus aureus was the most common bacteria, accounting for 8 (34.8%) and 8 (53.3%), respectively. In addition, Coagulase-negative Staphylococci (CoNS) 8 (61.5 %) were identified as the most prevalent bacteria associated with dacryocystitis, followed by Staphylococcus aureus 4 (30.8%). Furthermore, the most common bacteria associated with dacryocystitis were Coagulase-negative Staphylococci (CoNS) (n=8, 61.5 %), followed by Staphylococcus aureus (n=4, 30.8 %). While Staphylococcus aureus was the most common bacteria isolated from keratitis cases, accounting for 3 (50%), it was followed by Pseudomonas aeruginosa (n=2, 33.3 %) and 1 (16.7%) Streptococcus pneumoniae (Table 4).
Table 4.
Frequency of pathogenic bacteria in patients with various clinical features of ocular infections types
| Pathogenic bacteria | Ocular infections types | |||||
|---|---|---|---|---|---|---|
| Conjunctivitis | Blepharitis | Blepharoconjunctivitis | Dacryocystitis | Keratitis | Total (%) | |
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | ||
| S. aureus | 16 (33.3) | 8 (34.8) | 8 (53.3) | 4 (30.8) | 3 (50) | 39 (37.1) |
| CoNS | 11 (22.9) | 6 (26.1) | 3 (20) | 8 (61.5) | 0 | 28 (26.7) |
| H. influenzae | 17 (35.4) | 4 (17.4) | 2 (13.3) | 0 | 0 | 23 (21.9) |
| S. pneumoniae | 2 (4.2) | 2 (8.7) | 1 (6.7) | 1 (7.7) | 1 (16.7) | 7 (6.7) |
| K. pneumoniae | 1 (2.1) | 2 (8.7) | 1 (6.7) | 0 | 0 | 4 (3.8) |
| St. pyogenes | 1 (2.1) | 1 (4.3) | 0 | 0 | 0 | 2 ( 1.9) |
| P. aeruginosa | 0 | 0 | 0 | 0 | 2 (33.3) | 2 (1.9) |
| Total | 48 (45.7) | 23 (21.9) | 15 (14.3) | 13 (12.4) | 6 (5.7) | 105 (100) |
The results tabulated in table 4 revealed that Staphylococcus aureus is the most prevalent bacteria leading to external ocular infections being responsible for (37.1%) of infections, followed by Coagulase negative Staphylococci (CoNS) (26.7%), Haemophilusinfluenzae (21.9%), Streptococcus pneumoniae (6.7%), Klebsiella pneumonia (3.8%), Streptococcus pyogenes (1.9%), and Pseudomonas aeruginosa (1.9%) (Figure 2).
Figure 2.
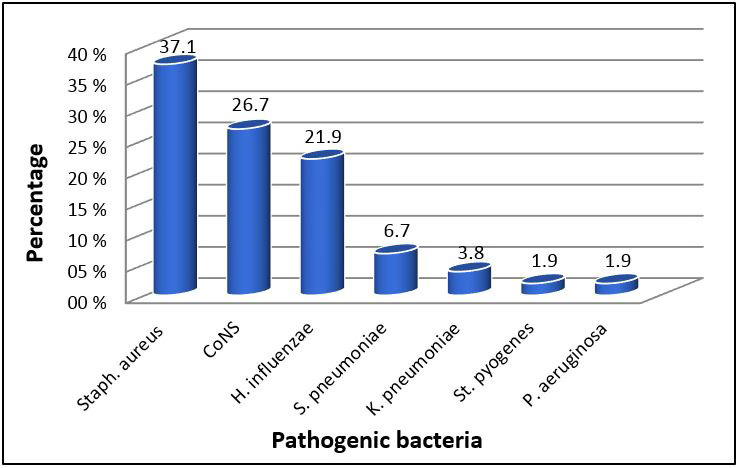
Frequency of pathogenic bacteria among patients with external ocular infections
Moreover, certain antibiotics were tested for pathogenic bacteria isolated from patients with ocular infections to show their effects against these bacteria (Table 5).
Table 5.
Antibiotic susceptibility of pathogenic bacteria isolated from adult patients with external ocular Infections
| Bacterial isolates | No. of isolates | Antibiotic susceptibility pattern | AMP | C | CIP | CN | P | TE |
|---|---|---|---|---|---|---|---|---|
| S. aureus | 39 | S% | 2 (5.1) | 35 (89.7) | 37 (94.9) | 39 (100) | 1 (2.6) | 10 (25.6) |
| R% | 37 (94.9) | 4 (10.3) | 2 (5.1) | 0 | 38 (97.4) | 29 (74.4) | ||
| CoNS | 28 | S% | 3 (10.7) | 23 (82.1) | 25 (89.3) | 26 (92.9) | 2 (7.1) | 8 (28.6) |
| R% | 25 (89.3) | 5 (17.9) | 3 (10.7) | 2 (7.1) | 26 (92.9) | 20 (71.4) | ||
| H.influenzae | 23 | S% | 14 (60.9) | 18 (78.3) | 22 (95.7) | 19 (82.6) | 1 (4.3) | 4 (17.4) |
| R% | 9 (39.1) | 5 (21.7) | 1 (4.4) | 4 (17.4) | 22 (95.7) | 19 (82.6) | ||
| S.pneumoniae | 7 | S% | 4 (57.1) | 5 (71.4) | 6 (85.7) | 5 (71.4) | 4 (57.1) | 5 (71.4) |
| R% | 3 (42.9) | 2 (28.6) | 1 (14.3) | 2 (28.6) | 3 (42.9) | 2 (28.6) | ||
| K. pneumoniae | 4 | S% | 0 | 2 (50) | 4 (100) | 4 (100) | 0 | 1 (25) |
| R% | 4 (100) | 2 (50) | 0 | 0 | 4 (100) | 3 (75) | ||
| S. pyogenes | 2 | S% | 1 (50) | 2 (100) | 2 (100) | 2 (100) | 1 (50) | 1 (50) |
| R% | 1 (50) | 0 | 0 | 0 | 1 (50) | 1 (50) | ||
| P. aeruginosa | 2 | S% | 1(50) | 1 (50) | 2 (100) | 2 (100) | 0 | 1 (50) |
| R% | 1(50) | 1 (50) | 0 | 0 | 2 (100) | 1 (50) |
S: Susceptible; R: Resistance; AMP: Ampicillin (10 μg); C: Chloramphenicol (30 μg); CIP: ciprofloxacin (5 μg); CN: Gentamicin (10 μg); P: Penicillin (10 μg); TE: Tetracycline (30 μg)
4. Discussion
Ocular bacterial infections are a significant public health problem in developing countries and are strongly linked to antibiotic resistance ( 9 ). Exogenous bacterial pathogens are the most common cause of ocular infections. They can, however, penetrate the eye and cause infection in some conditions.
In the current study, the total prevalence of bacterial isolates associated with external ocular infection was (52.5%). Our result agrees with the previous studies reported in Iran and Gondar at a prevalence rate of about (52.4% and 54.2%) respectively ( 10 , 11 ). However, this study's results are lower than those of other previous studies reported in Babylon, Jimma, and India at a prevalence rate of about (92.1% and 74.7%) respectively ( 12 , 13 ). On the other hand, the results of this study are higher than those of a study carried out in Bangalore (34.5%) ( 14 ).
This study showed that ocular infections most frequently occur in ages (20-49). This result corresponded to the result conducted in Yemen, which found that human eye infection can affect people of all age groups, and the infection is more common in the age groups (21-30) ( 15 ). The results also showed that ocular infection had affected both sexes, but most patients were males 65 (61.9%) because of their outside activities. These results corresponded to those reported in Ethiopia ( 16 ), which indicated that external ocular infections were more common in male than female patients.
On the other hand, the high prevalence of ocular infections in a rural areas (74.3%) than in urban areas (25.7%). The difference in distribution is probably due to poor hygiene and the fact that many rural communities use river waters for swimming, making them a source of eye infection. External ocular infections were more common in farmers and individuals without work ( 15 ).
Additionally, the results revealed that conjunctivitis is the most common external ocular infection, accounting for 45.7%, followed by blepharitis (21.9%) and blepharoconjunctivitis (14.3%), dacryocystitis (12.4%), and keratitis (5.7%). These findings are consistent with those reported in Ethiopia ( 1 ), which stated that conjunctivitis is the most prevalent external ocular infection (40.5%), followed by blepharitis (22.3%), blepharoconjunctivitis (12.6%), dacryocystitis (10.2%) while keratitisis the least common (4.2%). In contrast, the findings of this study disagree with the findings of another study carried out in India ( 17 ), which found that dacryocystitis is the most predominant external ocular infection than other infections.
Furthermore, the results of our study showed that the most prevalent bacteria leading to external ocular infections is Staphylococcus aureus being responsible for (37.1%) of infections, followed by Coagulase negative Staphylococci (CoNS) (26.7%), Haemophilusinfluenzae (21.9%), Streptococcus pneumoniae (6.7%), Klebsiella pneumonia (3.8%), Streptococcus pyogenes (1.9%), and Pseudomonas aeruginosa (1.9%). These results agreed with the other previous studies ( 18 ), which stated that Gram-positive cocci were more common as the leading cause of bacterial ocular infections. On the other hand, previous studies reported in Jimma ( 19 ) and India ( 20 ) revealed that Staphylococcus aureus was the most predominant pathogen identified from ocular infections.
The present study's data showed that the followed bacteria associated with ocular infections is Coagulase-negative Staphylococci (CoNS). This result is supported by the previous study obtained by Musa, Nazeerullah ( 21 ). In the current study, H. influenzae was the most common isolate (21.9%) among Gram-negative bacteria associated with ocular infections. This result contrasts with Getahun, Gelaw ( 22 ), who found that K. Pneumonia was the most common Gram-negative bacteria leading to associate ocular infections. However, another study in Saudi Arabia showed that P.aeruginosa was the most prevalent bacteria associated with ocular infections ( 23 ). Also, in the current study, the predominant bacteria isolated from dacryocystitis cases were Staphylococci (CoNS). This result corresponds to the result reported in Iran ( 24 ). In Iraq, Al-Buhilal, Rhumaid ( 25 ) showed that the non-typeable H.influenzae was a significant bacterium in eye specimens of patients suffering from conjunctivitis.
Also, the in vitro effects of some antibiotics for the bacterial isolates of the present study were investigated. It was shown that the antibiotic sensitivity in bacterial ocular infections was varied, with increased resistance to most antibiotics. The ciprofloxacin was highly effective against all bacteria isolates from adult patients with ocular infections, followed by chloramphenicol and gentamicin. These results parallel previous studies conducted in different locations ( 26 , 27 ).
According to table 5, it was found that all isolates of S. aureus were highly resistant to ampicillin (94.9%) and penicillin (97.4%). Additionally, an antibiotic susceptibility pattern of S. pneumoniae and S. pyogenes isolates showed that (85.7% and 100%) sensitivity pattern to ciprofloxacin, (57.1% and 50%) sensitivity pattern to ampicillin, and penicillin. Other studies in Ethiopia reported the same pattern as our finding ( 16 ). S. aureus isolates appeared to have the highest resistance rate to penicillin and ampicillin, and this might be related to the synthesis of beta-lactamase enzymes and changes in the penicillin-binding proteins ( 28 ). All isolates of CoNS in the current study were resistant to penicillin, ampicillin, and tetracycline at a rate of (92.9%, 89.3%, and 71.4%), respectively. Besides, the results also showed that H. influenzae and P. aeruginosa isolates were highly sensitive to ciprofloxacin (95.7% and 100%), gentamycin (82.6% and100%), and to a lesser degree to chloramphenicol (78.3% and 50%). Previous studies carried out in southwest Ethiopia showed similar results ( 29 ).
Moreover, K. Pneumonia isolates were susceptible (100%) to ciprofloxacin and Gentamycin but showed highly resistant (100%) to ampicillin and penicillin, and some isolates showed resistance to a lesser degree to tetracycline (75%). Similar results were reported in Northwest Ethiopia ( 29 ).
Antimicrobial resistance may be raised probably due to ineffective dosing regimens, self-medications, inappropriate use of antibiotics without a prescription, prolonged therapy duration, and lack of personal hygiene. However, regular face washing can help prevent bacteria-caused external eye infections; thus, health education is essential.
The bacterial infection is higher than other infections. External ocular infections most frequently occur in the age groups between (20-49) years and infect both males and females, but male predominance was observed in this age group. The predominant bacteria implicated in external ocular infections are Staphylococcus aureus, coagulase-negative Staphylococci (CoNS), Haemophilus influenza, Streptococcus pneumoniae, Klebsiella pneumonia, Streptococcuspyogenes, and Pseudomonasaeruginosa. Most bacterial isolates were showed resistance to Ampicillin, Penicillin, and Tetracycline, whereas susceptible to Ciprofloxacin, Gentamycin, and Chloramphenicol.
Authors' Contribution
All the authors contributed significantly to the study design, the study sample collection, laboratory testing, data analysis and interpretation, the creation of a draft, manuscript revisions, as well as final approval of the version that has been published.
Ethics
The study protocol was approved by the medical ethics board of the Middle Technical University, Baghdad, Iraq. The study included only adults and written informed consents were provided by all the subjects participated in the study.
Conflict of Interest
The authors declare that they have no conflict of interest.
Grant Support
Self-funded work.
References
- 1.Aklilu A, Bitew A, Dessie W, Hailu E, Asamene N, Mamuye Y. Prevalence and drug susceptibility pattern of bacterial pathogens from ocular infection in St. Paul’s Hospital Millennium Medical College, Ethiopia. J Bacteriol Mycol. 2018;5(8):1085. [Google Scholar]
- 2.Teweldemedhin M, Gebreyesus H, Atsbaha AH, Asgedom SW, Saravanan M. Bacterial profile of ocular infections: a systematic review. BMC Ophthalmol. 2017;17(1):1–9. doi: 10.1186/s12886-017-0612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aweke T, Dibaba G, Ashenafi K, Kebede M. Bacterial pathogens of exterior ocular infections and their antibiotic vulnerability pattern in southern Ethiopia. African J Immunol Res. 2014;1(1):019–25. [Google Scholar]
- 4.Miller JM, Binnicker MJ, Campbell S, Carroll KC, Chapin KC, Gilligan PH, et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2018 update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin Infect Dis. 2018;67(6):e1–e94. doi: 10.1093/cid/ciy381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sreekumar H, Manikandan P, Aiswariya P, Narendran V, Gomathi P, Priya R, et al. Prevalence and antimicrobial susceptibility pattern of staphylococci and streptococci causing ocular infections from a tertiary eye care hospital. South India Biomed Res. 2016;27(3):780–6. [Google Scholar]
- 6.Ayehubizu Z, Mulu W, Biadglegne F. Common bacterial causes of external ocular infections, associated risk factors and antibiotic resistance among patients at ophthalmology unit of Felege Hiwot Referral Hospital, Northwest Ethiopia: a cross-sectional study. J Ophthalmic Inflamm Infect. 2021;11(1):1–10. doi: 10.1186/s12348-021-00238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacFaddin J. Biochemical tests for identification of medical bacteria, williams and wilkins. Philadelphia, PA. 2000;113 [Google Scholar]
- 8.Watts A, Ke D, Wang Q, Pillay A, Nicholson-Weller A, Lee JC. Staphylococcus aureus strains that express serotype 5 or serotype 8 capsular polysaccharides differ in virulence. Infect Immun. 2005;73(6):3502–11. doi: 10.1128/IAI.73.6.3502-3511.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haun N, Hooper-Lane C, Safdar N. Healthcare personnel attire and devices as fomites: a systematic review. Infect Control Hosp Epidemiol. 2016;37(11):1367–73. doi: 10.1017/ice.2016.192. [DOI] [PubMed] [Google Scholar]
- 10.Anagaw B, Biadglegne F, Belyhun Y, Mulu A. Bacteriology of ocular infections and antibiotic susceptibility pattern in Gondar University Hospital, north west Ethiopia. Ethiop Med J. 2011;49(2):117–23. [PubMed] [Google Scholar]
- 11.Ansari MR, Madani H, Ghaderi E. Conjunctival bacterial flora and antibiotic resistance pattern in patients undergoing cataract surgery. Pak J Med Sci. 2008;24(4):581–5. [Google Scholar]
- 12.Hamid B, Hadi Bacterial Conjunctivitis In Patients With Graves' Disease And Its Association With Il-17 Level In Babylon Province, Iraq. Asian J Microbiol Biotech Env Sc. 2018;20:104–9. [Google Scholar]
- 13.Tesfaye T, Beyene G, Gelaw Y, Bekele S, Saravanan M. Bacterial profile and antimicrobial susceptibility pattern of external ocular infections in Jimma University specialized hospital, Southwest Ethiopia. Am J Infect Dis. 2013;1(1):13–20. [Google Scholar]
- 14.Bharathi MJ, Ramakrishnan R, Shivakumar C, Meenakshi R, Lionalraj D. Etiology and antibacterial susceptibility pattern of community-acquired bacterial ocular infections in a tertiary eye care hospital in south India. Indian J Ophthalmol. 2010;58(6):497. doi: 10.4103/0301-4738.71678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdel-Sater M, Al-Zubeiry A, Badah K. Microbiota associated with human eye infections in Taїz city, Yemen. J Basic Appl Mycol. 2012;3:1–11. [Google Scholar]
- 16.Shiferaw B, Gelaw B, Assefa A, Assefa Y, Addis Z. Bacterial isolates and their antimicrobial susceptibility pattern among patients with external ocular infections at Borumeda hospital, Northeast Ethiopia. BMC ophthalmol. 2015;15(1):1–8. doi: 10.1186/s12886-015-0078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar A, Pandya S, Kavathia G, Antala S, Madan M, Javdekar T. Microbial keratitis in Gujarat, Western India: findings from 200 cases. Pan Afr Med. 2011;10 [PMC free article] [PubMed] [Google Scholar]
- 18.Organization WH. Universal eye health: a global action plan 2014–2019 [Google Scholar]
- 19.Chaudhary M, Bhattarai A, Adhikari S, Bhatta D. Bacteriology and antimicrobial susceptibility of adult chronic dacryocystitis. Nepal J Ophthalmol. 2010;2(2):105–13. doi: 10.3126/nepjoph.v2i2.3716. [DOI] [PubMed] [Google Scholar]
- 20.Biradar S, Chandrashekhar D, Gangane R, Chandrakanth C, Biradar K, VinodKumar C. Spectrum of microbial keratitis and antimicrobial susceptibility at tertiary care teaching hospital in north Karnataka. Int J Pharm Biomed Res. 2012;3(2):117–20. [Google Scholar]
- 21.Musa AA, Nazeerullah R, Sarite SR. Bacterial profile and antimicrobial susceptibility pattern of anterior blepharitis in Misurata region, Libya. Dent Medical Res. 2014;2(1):8. [Google Scholar]
- 22.Getahun E, Gelaw B, Assefa A, Assefa Y, Amsalu A. Bacterial pathogens associated with external ocular infections alongside eminent proportion of multidrug resistant isolates at the University of Gondar Hospital, northwest Ethiopia. BMC Ophthalmol. 2017;17(1):1–10. doi: 10.1186/s12886-017-0548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed E. Bacterial spectrum of external ocular infections: prevalence and associated in vitro antimicrobial susceptibility and resistance in a tertiary care hospital. J Adv Res. 2018;6(1):869–78. [Google Scholar]
- 24.Eshraghi B, Abdi P, Akbari M, Fard MA. Microbiologic spectrum of acute and chronic dacryocystitis. Int J Ophthalmol. 2014;7(5):864. doi: 10.3980/j.issn.2222-3959.2014.05.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Buhilal JAM, Rhumaid AK, Al-Tabtabai AMH, AL-Rubaey NKF. Molecular detection of Haemophilus influenzae isolated from eye swabs of patients with conjunctivitis in Hilla Province, Iraq. J appl biol. 2022;10(2):1–7. [Google Scholar]
- 26.Namitha B, Mahalakshmi RA, Auchat R. Aerobic Bacteriological Profile in Cases of Ocular Infections in a Tertiary Care Hospital (Navodaya Medical College and Research Centre, Raichur) J Dent Med Sci. 2014;13(11):14–21. [Google Scholar]
- 27.Mshangila B, Paddy M, Kajumbula H, Ateenyi-Agaba C, Kahwa B, Seni J. External ocular surface bacterial isolates and their antimicrobial susceptibility patterns among pre-operative cataract patients at Mulago National Hospital in Kampala, Uganda. BMC ophthalmol. 2013;13(1):1–6. doi: 10.1186/1471-2415-13-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deyno S, Fekadu S, Astatkie A. Resistance of Staphylococcus aureus to antimicrobial agents in Ethiopia: a meta-analysis. Antimicrobial Resistance & Infection Control. 2017;6(1):1–15. doi: 10.1186/s13756-017-0243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diriba K, Kassa T, Alemu Y, Bekele S. In vitro biofilm formation and antibiotic susceptibility patterns of bacteria from suspected external eye infected patients attending ophthalmology clinic, Southwest Ethiopia. Int J Microbiol. 2020;2020 doi: 10.1155/2020/8472395. [DOI] [PMC free article] [PubMed] [Google Scholar]


