Abstract
Inflammation has an important predictive role for long-term health. This is also true when looking at the specific population of trauma survivors with PTSD. There are emerging findings showing that PTSD is related to reduced somatic health as well as evidence linking inflammation with disease outcomes in this group, such as heart diseases and early mortality, regardless of age, gender or conventional risk factors. The effectiveness of cognitive behavioral therapy for PTSD symptom severity has been demonstrated by several previous studies. In contrast, literature is scarce, yet, whether inflammation improves over the course of treatment for PTSD. Therefore, the aim of this study was to investigate whether not only PTSD symptoms, but also inflammation changes over the course of psychological treatment. Twenty-nine PTSD patients were followed while attending an outpatient clinic receiving cognitive behavioral therapy. Inflammation, determined by the C-reactive protein (CRP) assessed via the dried blood spot (DBS) technique, and symptom severity, surveyed by the PTSD Checklist for DSM-5 (PCL) were measured at 5 points before trauma-focused therapy, as well as after four, eight and twelve weeks of intervention; furthermore, a 10-month follow-up assessment was conducted. Results revealed significant improvements of PTSD symptom severity during investigation, but no significant changes in the inflammatory biomarker (CRP). Results in terms of improvement in PTSD symptom severity are in line with prior findings. The results obtained for inflammation may suggest that risk factors for somatic health consequences in PTSD patients remain despite successful psychological treatment. Further longitudinal studies are needed to fully understand the relationship between inflammation and therapeutic outcome and to develop interventions normalizing inflammation in PTSD patients.
Keywords: Posttraumatic stress disorder, Trauma, Inflammation, CRP, Inflammatory biomarker, Psychological treatment, CBT
Highlights
-
•
PTSD symptoms decrease significantly in the course of cognitive behavioral therapy (CBT).
-
•
Inflammation (CRP) do not change significantly during CBT in PTSD patients.
-
•
Further longitudinal research regarding inflammatory biomarkers in PTSD during psychological treatment is needed.
1. Introduction
Posttraumatic stress disorder (PTSD) is defined as a disorder that can develop subsequent to one or more extremely stressful events. It does not only have direct consequences for quality of life and well-being of affected patients, but it can lead to a significant risk for severe somatic health consequences as well as an increased risk of death (O'Donovan et al., 2015; Smaardijk et al., 2020; Boscarino, 2008). These somatic conditions associated with PTSD range from so-called medically unexplained symptoms and somatic complaints, such as fibromyalgia or irritable bowel syndrome, to severe life-threatening diseases, such as cardiovascular disease and autoimmune disorders. It has been suggested that a possible mechanism linking PTSD with these conditions are dysregulated stress systems, ultimately leading to disinhibition of inflammatory processes (O'Donovan et al., 2015; Boscarino, 2008; Neigh and Ali, 2016; Pace and Heim, 2011; Rohleder and Karl, 2006). Inflammation is a process that is affected by stress systems and health behaviors and has profound effects on long-term health (Couzin-Frankel, 2010; Rohleder, 2019). Accordingly, inflammatory processes have been found to be dysregulated in PTSD: A meta-analysis from Passos et al. (2015) including 20 studies reported significantly increased plasma concentrations of inflammatory molecules such as IL-6 and IL-1beta in PTSD patients independent of comorbid depression. These results were confirmed in a meta-analysis by Renna et al. (2018) that included 41 studies with several anxiety disorders (23 on PTSD), which revealed significantly higher plasma pro-inflammatory molecules, including IL-6, TNF-alpha, CRP, and others, specifically in PTSD. Focusing on gene expression and the inflammatory transcriptome, Breen et al. (2015) reported a consistent upregulation of inflammatory processes in PTSD in five whole blood transcriptome studies that included more than 300 patients with different trauma types. While these reviews reveal strong evidence for inflammatory up-regulation being cross-sectionally related to PTSD, studies addressing longitudinal associations of inflammation with PTSD are largely lacking.
Trauma-focused therapy can reduce the hallmark symptoms of PTSD (intrusions, avoidance, hyperarousal) (Ehring et al., 2014), and is therefore an excellent intervention to investigate whether improvements in psychological symptoms are also predictive of or associated with improvements in pathways leading to disease, i.e., the inflammatory system. Given the role of inflammation for PTSD-related diseases and reductions in quality of life, knowledge about the impact of PTSD interventions on these systems is of critical importance. However, few studies to date have addressed this important question. Toft et al. (2018) investigated markers of low-grade inflammation in PTSD patients during a 12-week psychiatric inpatient treatment of PTSD patients with a combination of psychological treatment and psychoeducation. During therapy, a reduction of mental stress was found over time. In addition, they found an increasing inflammatory response over 12 weeks. The authors suggest these results could be explained by the intensive treatment reexperience of previous trauma through talk therapy or by exposure therapy or a disruption in their hypothalamic-pituitary-adrenal axis vs. immune system feedback. In contrast, Gill, Saligan, Lee, Rotolo and Szanton (Gill et al., 2013) demonstrated that women who had recovered from PTSD showed significantly lower concentrations of inflammatory biomarkers such as CRP compared to women with a current PTSD diagnosis. Himmerich et al. (Himmerich and Zimmermann, 2016) investigated circulating inflammatory markers in PTSD patients before and after therapy in two different treatment groups: The first group received eye movement desensitization and reprocessing therapy (EMDR) for six weeks. The second group was assigned to an outpatient clinical management group without EMDR and an undefined number of therapy sessions. Results showed no significant differences between the two groups but an increase of TNF-alpha, while sTNF-R p55 and sTNF-T p75 decreased significantly in both groups. Furthermore, another study examined men with a past history of PTSD compared to non-PTSD controls (Kawamura et al., 2001). Results showed that individuals who had remitted from PTSD had significant lower values for IFN-γ as well as IL-4 than controls and additionally suffered from long-lasting global immunosuppression.
Taken together, it is apparent that the few long-term studies that have addressed the relationship between inflammation and treatment success do not show consistent results. For a number of reasons, it is relevant to investigate whether alterations in inflammatory processes normalize with successful treatment. First, this will inform theoretical models on the role of inflammation as a state vs. trait marker in PTSD. Second, if current evidence-based treatments for PTSD are not successful in reducing inflammatory alterations, this may call for additional interventions using different mechanisms of change to specifically target this. In this study, we therefore aimed to contribute to this question by following PTSD patients in the course of therapy and measuring inflammatory biomarker (CRP) in addition to therapy success. We expected an improvement in subjective symptom severity as well as a change in inflammation values during therapy.
2. Material and methods
Ethical statement
The study design was approved by the Ethics Committee of the Friedrich-Alexander-University Erlangen-Nürnberg (FAU, ID 224_19) and additionally confirmed by the Ethics Committee of the Ludwig-Maximilians-Universität München and conducted in concordance with the Declaration of Helsinki. Written informed consent was obtained from all participants.
2.1. Participants
Participants were n = 29 trauma-exposed persons (4 male) with PTSD (age: M = 36.30, SD = 13.39). At time of enrollment, patients were receiving treatment in the outpatient treatment center of LMU Munich (Department of Psychology). The center provides evidence-based cognitive behavior therapy for trauma-related disorders. We asked the patients at the first or following appointment if they would like to participate and - after agreement - provided further information about the study.
Inclusion criteria were an age between 18 and 65 years as well as a diagnosis of PTSD according to DSM-5 and Clinician Administered PTSD Scale (see point 2.4). Exclusion criteria were acute psychosis, acute suicidality, current contact to the offender related to the traumatic event, intake of beta-blockers, glucocorticoids, or benzodiazepines, and endocrine or cardiovascular diseases.
Ten patients met the criteria of a comorbid affective disorder, three patients for anxiety disorder and two patients for personality disorder. Twelve patients received psychotropic drugs (antidepressants: n = 9, antipsychotics: n = 2, hypnotics: n = 1), whereby the doses did not change during the survey period. Additionally, illness duration in terms of PTSD was M = 3.39 (SD = 5.03) years. Eight patients reported sexual assault as traumatic experience. Two each described serious (non-traffic) accidents, domestic violence, violent attacks, the death of a loved one, or psychological abuse. One person each stated live-threatening illness and a traffic accidence.
2.2. Procedure
Patients underwent psychometric assessment, history taking, case formulation and treatment planning, as well as first therapeutic interventions, which did not directly correspond to trauma-specific treatment, during the first 5–7 appointments, which is a standard procedure for outpatient psychological treatment in Germany. Then, at the beginning of psychological treatment or later a project team member showed the patient how to take a set of Dried Blood Spots. Importantly, the first set of blood spots was always taken before any trauma-focused intervention started. After this, psychological treatment was performed or continued as usual. Dried Blood Spot samples were then taken from four further measurement points. These were repeated monthly for a duration of 12–20 weeks. In order to continue monitoring the effects, a further measurement was taken after about 10 months. Patients additionally completed a questionnaire (PTSD Checklist for DSM-5, see 2.4) about symptom improvement at each blood spot assessment.
2.2.1. Psychological treatment
Participants received an evidence-based trauma-focused CBT treatment for at least 12 weeks, with precise treatment components and duration being determined individually for each patient. Interventions included psychoeducation, complemented by mindfulness or relaxation methods, and skills training, if needed. The main focus of treatment was then on the application of trauma-focused interventions, e.g. EMDR, prolonged exposure, or imagery rescripting. Appointments took place once or twice weekly for 50 min per session. Standardization and quality of treatment was controlled by weekly team meetings and internal and external supervision of the whole therapeutic team.
2.3. Measures
All PTSD patients completed the Clinician Administered PTSD Scale (CAPS), the gold standard for measuring PTSD. The CAPS consists of a list of traumatic events, which the participants can use to assess the most serious events in terms of frequency and intensity of symptoms on a four-point scale. CAPS is a structured interview to measure the frequency and intensity based on the DSM-5 criteria (Weathers et al., 2018). Several studies have shown that CAPS is a reliable and valid instrument. It consists of 30 self-report items and is used to either make a current diagnosis of PTSD, a lifetime diagnosis or assess PTSD symptoms over the past week.
In addition, patients completed the PTSD Checklist for DSM-5 (PCL) for all 5 measuring points. PCL is one of the most frequently used self-evaluation instruments for the diagnosis of PTSD. The DSM-5 version was developed by Weathers, Litz, Keane, Palmieri, Marx & Schnurr (Weathers et al., 2013) and published by Krüger-Gottschalk et al. (2017) as an authorized German version. It can be used to screen for PTSD, to make a preliminary PTSD diagnosis, or to assess the severity of symptoms over time. In this case, it was used to monitor the current severity of PTSD during therapy.
Peripheral inflammation was assessed using the Dried Blood Spot (DBS) method (McDade, 2014; Danese et al., 2011). This method is well-suited for a less-invasive assessment of CRP levels and successfully established in our laboratory [e.g., 22,23]. Each sample was taken by first spraying an alcoholic skin antiseptic (e.g. Kodan Tinktur forte, Schülke, Germany) on one of the fingertips of the participant and wiping it off with a sterile cellulose swab. A disposable lancet was then applied to the disinfected fingertip and released. The first drop of blood was wiped off with a cellulose swab and discarded, then four separate drops of approximately 50 μl capillary blood were applied to four marked sectors of filter paper (Whatman 903, GE Healthcare Life Sciences, Germany). The filter paper was then dried for at least 8 h at room temperature and stored in an airtight envelope with desiccant. Within 36 h after blood collection, envelopes were transferred to storage at −30 °C. After completion of the study, CRP concentrations were determined as follows: 3.5 mm cores were punched out and eluted overnight in phosphate buffered saline containing 0.1% Tween 20 solution in a sealed polystyrene microplate at 4C. The following morning, microplates were shaken at 300 rpm for 1h, and then centrifuged at 2.000 g for 10 min. Subsequently, the eluate was subjected to a commercially available highly sensitivity enzyme immunoassay (IBL International, Hamburg, Germany) for determination of CRP concentrations. This assay has a detection limit of 0.225 μg/mL. Intra and inter-assay coefficients were below 10% in our laboratory. CRP concentrations in DBS show high correlations with plasma CRP levels (McDade et al., 2004).
2.4. Statistics
All statistical analyses were performed using the Statistical Package for the Social Science Version 28 (SPSS Institute, Chicago, Illinois). When logarithmic transformations were necessary, variables were transformed by applying the formula xln = ln(x+1). Data was expressed as means ± SEM, and p < .050 was set as the criterion for significance. T-Tests were performed for PCL and CRP to investigate differences between the first and the last measurements. For preliminary analyses of the influence of treatment on CRP and PCL over a maximum of five repeated measures for each patient, repeated measures ANOVA was used to examine the relationship between time and outcome measure for each variable and to explore any significant interaction. However, the majority of patients could not complete all five measurement time points. Because the linear model assumes that errors are independent (Field, 2013), and to manage these missing data points, we therefore used hierarchical linear modeling (HLM) with the random effects approach (Bryk and Raudenbush, 1992). Time-dependent differences in inflammation and the subjective symptom severity, as well as the relationship among CRP and current subjective symptom severity (PCL) were analyzed using two-level hierarchical linear modeling (HLM). Therefore, we have as level 1 variable five different points of measurements (Repeated CRP and PCL values, max = 5), which are nested in PTSD patients (level 2 variable). The two levels of the model were estimated using a random effect for intercept (to capture CRP and PCL upon beginning of treatment) and repeated time points of measurements (to capture the linear slope). In the within-person (Level 1) models, PCL and log-transformed CRP were estimated as a function of time since beginning of the treatment (coded in 5 points of measurement). To avoid multicollinearity and because the predictors do not have a meaningful zero point, we centered the level 1 variables (PCL and CRP) at mean. For estimating parameters, we used maximum likelihood (ML). For polynomials models, we interpreted the model that contains the linear trend, because this was the best fit of our model. For investigating if CRP or PCL are able to predict outcome, we used HLM with CRP and PCL values as level 1 variable, which are nested in symptom severity at the beginning of therapy investigated by CAPS (level 2 variable).
3. Results
3.1. Description of the sample
The group consisted of 29 patients (25 female, 4 male) with an average CAPS value of M = 43.52 (SD = 2.79). Average CRP level at beginning of the study was M = 3.06 mg/l (SD = 0.66) and average PCL-Score was M = 35.44 (SD = 2.59). After outlier analysis, three patients were excluded because of CRP values > 12.5 mg/l (Britting et al., 2021; Becker et al., 2021) resulting in a final sample size of n = 27 for the analyses. T-tests showed no differences between the beginning of therapy (T1) or after follow-up (T5) regarding CRP, t (14) = −0.64, p = .532, d = −0.17. Significant differences were found for PCL between first measurement (T1) and follow-up (T5), t (19) = 8.74, p = <.001, d = 0.88.
3.2. Subjective symptom severity
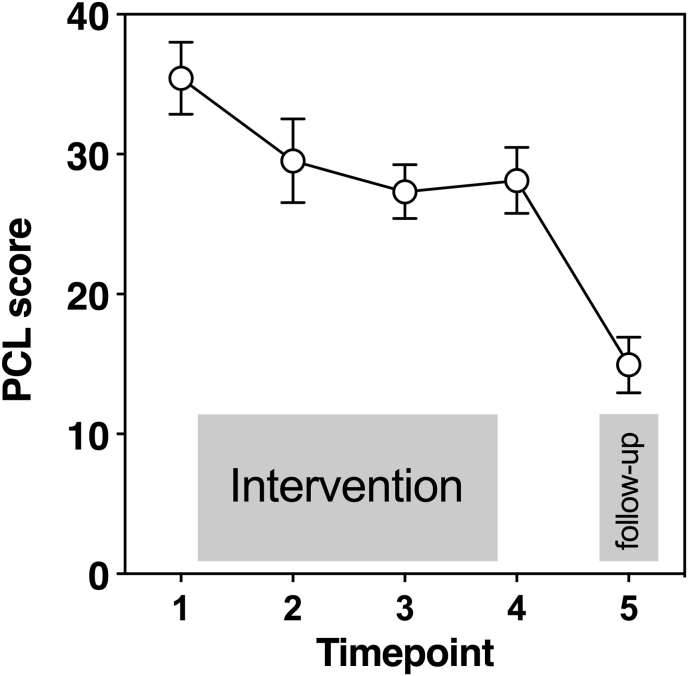
We found a significant variance of the random intercept, Var(u0j) = 230.81; SEM = 0.94, p = .011. This suggests that PCL at baseline varied significantly across participants. In addition, the slopes did not vary significantly across participants, Var(u1j) = 5.90; SEM = 4.15, p = .155. This could be explained by the change of subjective symptom variety over time, which did not vary significantly across individuals. The covariance between the slopes and intercepts, Cov(u0j,u1j) = −0.98 SEM = 0.07, p = <.001, suggests that while the intercept increased, the slope decreased. This trend reflects the reduction of subjective symptom severity in the course of therapy. Intraclass coefficient (ICC) was calculated (ICC = 0.251) revealing that about 25% of the overall variance can be explained by the person and 75% by time (indicating significant time effects). HLM revealed across all participants and all sampling times a mean PCL of β00 = 12.742 (standard error of the mean [SEM] = 2.67), which is significantly different from zero (t (62,91) = 4.78, p = <.001). The mean slope of time, which estimates the relation between time and PCL values showed a significant result (β10 = −5.042 (t (68,69) = −7.43, p =<.001). With every increase of the predictor ‘‘time’’ by 1 unit, the mean PCL level (intercept) decreases by 5.042 units (see Fig. 1). PCL Score at T1 did not significantly predict CRP after treatment (F (1, 12) = 1.65, p = .223). Analyses did not show different results when excluding (a) men or (b) participants with comorbid affective disorders.
Fig. 1.
Raw data of PTSD Checklist for DSM-5 (PCL) values at five different time points before (point 1), during psychological treatment (point 2-3), after (point 4) and follow-up (5). Figure shows mean and SEM.
3.3. Inflammation
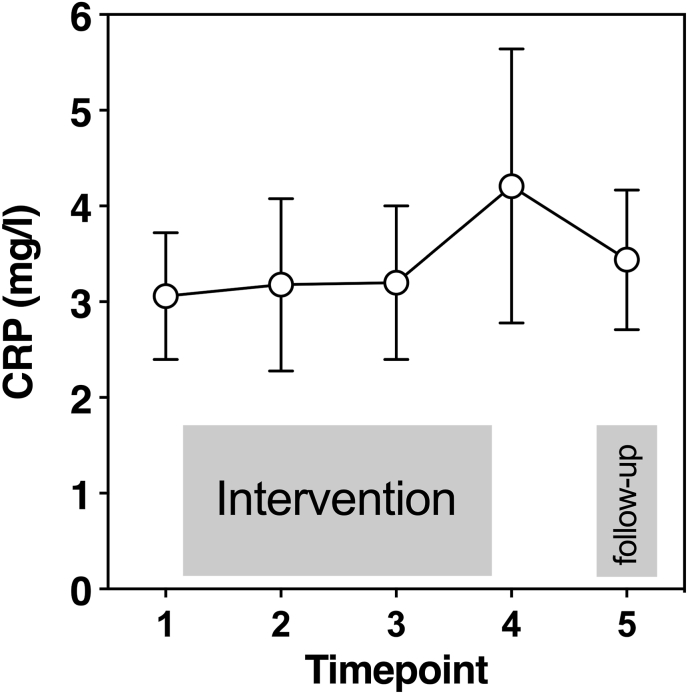
For CRP values, the variance of the random intercepts for CRP were significantly different, Var(u0j) = 0.07; SEM = 0.03, p. = 0.010, suggesting that CRP at baseline varied significantly across individuals. The slopes did not vary across participants, Var(u1j) = 0.002; SEM = 0.001, p = .204, which suggests that the change of CRP over time did not vary significantly across participants. We found a significant result for covariances between the slopes and intercepts, Cov(u0j,u1j) = −0.51, SEM = 0.24, p = .035. About 11.70% of the variance of the first level can be statistically explained with adding the factor “time” (ICC = 0.680). For log-transformed and mean-centered CRP the mean was β00 = −2.445 (SEM = 0.56). However, we found no significant differences regarding the relation between CRP and time (t (57.40) = −0.44, p = .663, see Fig. 2). Interestingly, the random intercept model revealed that CRP before therapy significantly predicted CRP values after follow-up (T5) (F (1, 12) = 19.45, p < .001). Similarly, as with PCL analyses, no different results were found when males or participants with comorbid affective disorders were excluded.
Fig. 2.
Raw data of C-reactive protein (CRP) (mg/l) values at five different time points before (point 1), during psychological treatment (point 2–3), after (point 4) and follow-up (5). Figure shows mean and SEM.
4. Discussion
In the present study, we investigated an inflammatory biomarker (CRP) in the course of psychological treatment by collecting five different sets of Dried Blood Spots with the first assessment taking place before any trauma-specific intervention had started. While there are many studies on the change of core symptoms of PTSD such as avoidance or intrusions during psychological treatment, only few studies to date have looked at the link between biomarkers, especially inflammation, and PTSD. When looking at PTSD symptoms, our results are in line with prior results indicating a significant reduction of subjective symptom severity (PCL) during psychological treatment (Ehring et al., 2014; Bradley et al., 2005). However, we found no significant changes in CRP concentrations, neither between the first and the last measuring point, nor when including all assessments in the analyses. Furthermore, CRP values at baseline were not able to predict psychotherapy outcome.
As detailed in the introduction, the literature on longitudinal changes of inflammation in trauma survivors with PTSD is still scarce and inconsistent (Sumner et al., 2020). Our findings regarding a lack of change in inflammatory markers in the course of treatment for PTSD is in line with some earlier studies, including Toft et al. (2018), who similarly found a subjectively reported symptom reduction during 12-week psychiatric inpatient treatment, but still higher IL-6 and TNF-alpha values after treatment in individuals suffering from PTSD. Despite methodological differences such as treatment in an inpatient ward vs. outpatient treatment center or the additional use of anti-inflammatory drugs in the earlier studies, results nevertheless converge in suggesting that treatment for PTSD does not lead to changes in the activation in the immune system. In contrast, some earlier studies had found a reduction of interleukins or CRP during the course of treatment (Tucker et al., 2004; Spitzer et al., 2014). As highlighted by Toft et al., one potentially relevant difference was these studies did not include trauma-focused interventions, whereas both our current study as well as the study by Toft et al. did include exposing patients to their trauma memories, as recommended as a first-line intervention for PTSD. Similarly, Himmerich et al. (Himmerich and Zimmermann, 2016) argued that increased TNF-alpha levels found in their study may be a side effect of exposure-based interventions used in their population, which may have induced a “fight or flight” response of the immune system. Another potential mechanism contributing to continuously increased levels of immune activation may be the fact that PTSD patients have an altered hypothalamic-pituitary-adrenal axis (HPA) with adapted cortisol release caused by chronic stress and inflammation (Pace and Heim, 2011; Rohleder and Karl, 2006; Gill et al., 2010). It has been suggested that this may lead to an impaired feedback regulation in the immune activation in the long run and contribute to stress-related pathology (Raison and Miller, 2003). Interestingly, a number of prior studies have found that after successful treatment, residual symptoms in the hyperarousal cluster of PTSD, for example insomnia, were the most common remaining PTSD symptoms after treatment (Kovacevic et al., 2022; Schnurr and Lunney, 2019). As PTSD patients are often predisposed to overreact to subsequent stressors, thereby making them more vulnerable to events (Kendall-Tackett, 2000), it may be important to test the hypothesis of whether these residual symptoms, which frequently remain after successful therapy, are associated with increases in the inflammatory system or with changes in the sympathetic nervous system or HPA axis.
On the other hand, some other earlier studies are in contrast to the current findings. For example, Gill et al. (2013) investigated women with current and past PTSD as well as a healthy non-traumatized control group. Interestingly, the authors found higher CRP concentrations in patients with current PTSD compared to controls as well as patients who had recovered from PTSD. Worth mentioning, Gill et al. only investigated female patients at the age of 18–45 years in their study, which could be a first methodological reason for their results not being generalizable to other clinical populations. More importantly, however, Gill et al. used a cross-sectional approach where participants in the remitted group had already recovered from PTSD some time ago, whereas the patients in our study were followed in the course of therapy beginning in its very early phase of recovering from PTSD symptoms. Moreover, the differences may be explained by the fact that our observation interval of ten months was too short. In order to resolve current inconsistencies in the literature, it appears necessary for future studies to include larger follow-up intervals in order to compare short-vs. long-term effects of recovering from PTSD on inflammatory processes. This would also allow testing hypotheses regarding changes in inflammatory biomarkers due to acute trauma-focused therapy, as mentioned in the previous paragraph.
We additionally found that CRP did not significantly predict outcome of psychological treatment, which is in line with a study by Maples-Keller et al. (2022) who investigated the relationship between CRP and response to psychological treatment. This is in contrast with a study by Eraly et al. (2014), who investigated 2978 participants before and after war zoned-deployed, reporting highly significant results for baseline CRP concentration as overall predictor of postemployment PTSD symptoms. Considering this, and our result that baseline CRP can predict outcome CRP, but no relationship between reported PTSD symptoms and CRP, it could be hypothesized that the inflammatory changes found in PTSD are not a consequence of the disorder, but a possibly persistent risk factor.
The following limitations should be considered when interpreting our results. First, the series of CRP measurements included only five different points in time, with the last follow-up being conducted at 10 months after beginning of treatment. Future studies should include more fine-grained analyses during the course of treatment as well as longer follow-up intervals. We are also limited with the uneven distribution of gender. Moreover, it would be interesting to investigate additional parameters for inflammation in order to draw a more comprehensive picture of the role of cytokines in the central nervous system. Our sample size was relatively small and consisted mostly of female subjects. It is possible that we could have found more subtle relationships and more generalizable results with a larger sample size and a more balanced gender distribution. As prior studies report trends of increased gene activity related to inflammation and/or cellular stress response in women with PTSD, but not in men, it would be interesting to investigate the moderation of sex (Neylan et al., 2011). Furthermore, the current study was carried out in patients receiving routine evidence-based treatment, which increases external validity and generalization of the study findings. On the other hand, this also carried the limitation that the treatment was not entirely standardized, as would be the case in randomized controlled efficacy trials. Thus, patients were in different phases of treatment for PTSD at different times. In order to better understand changes in inflammatory markers during the course of therapy, studies using more standardized treatments are needed in addition to studies investigating routine treatment. In addition, several patients received medication and were diagnosed with comorbidities. Future studies should try to select patients without medication. Finally, due to the lack of a control group we were not able to compare changes in inflammatory markers during treatment with its natural course. Future research should therefore ideally include a condition with PTSD patients not receiving psychological treatment (e.g., currently on the waiting list) as well as non-PTSD control groups assessed over time.
In summary, our findings indicate that routine psychological treatment in patients with current PTSD does not lead to changes regarding inflammatory CRP even in the presence of significant symptom reduction. Accordingly, we suggest that psychotherapy may not result in a change of inflammatory CRP in a 10-month follow-up period, which could indicate a risk factor for somatic health consequences. Employing a broader range of methodological approaches in future research will help to investigate the relationship between therapy and biomarkers in order to identify possible protective and risk factors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Kristin von Majewski is supported by a “Bavarian Equal Opportunities Sponsorship – Realisierung von Chancengleichheit von Frauen in Forschung und Lehre (FFL) – Realization Equal Opportunities for Women in Research and Teaching”.
Data availability
The data that has been used is confidential.
References
- Becker L., Dupke A., Rohleder N. Associations between C-reactive protein levels, exercise addiction, and athlete burnout in endurance athletes. Front. Psychol. 2021;12 doi: 10.3389/fpsyg.2021.615715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscarino J.A. A prospective study of PTSD and early-age heart disease mortality among Vietnam veterans: implications for surveillance and prevention. Psychosom. Med. 2008;70:668–676. doi: 10.1097/PSY.0b013e31817bccaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley R., Greene J., Russ E., Dutra L., Westen D. A multidimensional meta-analysis of psychotherapy for PTSD. Am. J. Psychiatr. 2005;162:214–227. doi: 10.1176/appi.ajp.162.2.214. [DOI] [PubMed] [Google Scholar]
- Breen M.S., Maihofer A.X., Glatt S.J., Tylee D.S., Chandler S.D., Tsuang M.T., et al. Gene networks specific for innate immunity define post-traumatic stress disorder. Mol. Psychiatr. 2015;20:1538–1545. doi: 10.1038/mp.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britting S., Kob R., Sieber C.C., Rohleder N., Freiberger E., Becker L. Physiological stress in safer cycling in older age (SiFAr-stress): effect of a multicomponent exercise intervention—a study protocol for a randomized controlled trial. Trials. 2021;22:552. doi: 10.1186/s13063-021-05481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryk A.S., Raudenbush S.W. Hierarchical linear models: applications and data analysis methods. Advanced qualitative techniques in the social sciences. 1992;1:265. https://psycnet.apa.org/fulltext/1992-97494-000.pdf Available: [Google Scholar]
- Couzin-Frankel J. Inflammation bares a dark side. Science. 2010;330:1621. doi: 10.1126/science.330.6011.1621. [DOI] [PubMed] [Google Scholar]
- Danese A., Caspi A., Williams B., Ambler A., Sugden K., Mika J., et al. Biological embedding of stress through inflammation processes in childhood. Mol. Psychiatr. 2011;16:244–246. doi: 10.1038/mp.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehring T., Welboren R., Morina N., Wicherts J.M., Freitag J., Emmelkamp P.M.G. Meta-analysis of psychological treatments for posttraumatic stress disorder in adult survivors of childhood abuse. Clin. Psychol. Rev. 2014;34:645–657. doi: 10.1016/j.cpr.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Eraly S.A., Nievergelt C.M., Maihofer A.X., Barkauskas D.A., Biswas N., Agorastos A., et al. Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatr. 2014;71:423–431. doi: 10.1001/jamapsychiatry.2013.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A. fourth ed. SAGE Publications; London, England: 2013. Discovering Statistics Using IBM SPSS Statistics. [Google Scholar]
- Gill J., Luckenbaugh D., Charney D., Vythilingam M. Sustained elevation of serum interleukin-6 and relative insensitivity to hydrocortisone differentiates posttraumatic stress disorder with and without depression. Biol. Psychiatr. 2010;68:999–1006. doi: 10.1016/j.biopsych.2010.07.033. [DOI] [PubMed] [Google Scholar]
- Gill J.M., Saligan L., Lee H., Rotolo S., Szanton S. Women in recovery from PTSD have similar inflammation and quality of life as non-traumatized controls. J. Psychosom. Res. 2013;74:301–306. doi: 10.1016/j.jpsychores.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Himmerich Willmund, Zimmermann . Psychiatria; 2016. Serum Concentrations of TNF-A and its Soluble Receptors during Psychotherapy in German Soldiers Suffering from Combat-Related PTSD.https://hrcak.srce.hr/file/259945 Available: [PubMed] [Google Scholar]
- Kawamura N., Kim Y., Asukai N. Suppression of cellular immunity in men with a past history of posttraumatic stress disorder. Am. J. Psychiatr. 2001;158:484–486. doi: 10.1176/appi.ajp.158.3.484. [DOI] [PubMed] [Google Scholar]
- Kendall-Tackett K.A. Physiological correlates of childhood abuse: chronic hyperarousal in PTSD, depression, and irritable bowel syndrome. Child Abuse Negl. 2000;24:799–810. doi: 10.1016/s0145-2134(00)00136-8. [DOI] [PubMed] [Google Scholar]
- Kovacevic M., Bravo K., Held P. Posttraumatic stress disorder and depression residual symptoms among veterans and service members who completed a 3-week cognitive processing therapy-based intensive treatment program. Psychol Trauma. 2022 doi: 10.1037/tra0001197. [DOI] [PubMed] [Google Scholar]
- Krüger-Gottschalk A., Knaevelsrud C., Rau H., Dyer A., Schäfer I., Schellong J., et al. The German version of the Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): psychometric properties and diagnostic utility. BMC Psychiatr. 2017;17:379. doi: 10.1186/s12888-017-1541-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maples-Keller J.L., Yasinski C., Stojek M., Ravi M., Watkins L.E., Patton S.C., et al. The relations between C-reactive protein and trauma exposure, PTSD and depression symptoms, and PTSD psychotherapy treatment response in treatment seeking veterans and service members. Brain Behav. Immun. 2022;101:84–92. doi: 10.1016/j.bbi.2021.12.025. [DOI] [PubMed] [Google Scholar]
- McDade T.W. Development and validation of assay protocols for use with dried blood spot samples. Am. J. Hum. Biol. 2014;26:1–9. doi: 10.1002/ajhb.22463. [DOI] [PubMed] [Google Scholar]
- McDade T.W., Burhop J., Dohnal J. High-sensitivity enzyme immunoassay for C-reactive protein in dried blood spots. Clin. Chem. 2004;50:652–654. doi: 10.1373/clinchem.2003.029488. [DOI] [PubMed] [Google Scholar]
- Neigh G.N., Ali F.F. Co-morbidity of PTSD and immune system dysfunction: opportunities for treatment. Curr. Opin. Pharmacol. 2016;29:104–110. doi: 10.1016/j.coph.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neylan T.C., Sun B., Rempel H., Ross J., Lenoci M., O'Donovan A., et al. Suppressed monocyte gene expression profile in men versus women with PTSD. Brain Behav. Immun. 2011;25:524–531. doi: 10.1016/j.bbi.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan A., Cohen B.E., Seal K.H., Bertenthal D., Margaretten M., Nishimi K., et al. Elevated risk for autoimmune disorders in Iraq and Afghanistan veterans with posttraumatic stress disorder. Biol. Psychiatr. 2015;77:365–374. doi: 10.1016/j.biopsych.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace T.W.W., Heim C.M. A short review on the psychoneuroimmunology of posttraumatic stress disorder: from risk factors to medical comorbidities. Brain Behav. Immun. 2011;25:6–13. doi: 10.1016/j.bbi.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Passos I.C., Vasconcelos-Moreno M.P., Costa L.G., Kunz M., Brietzke E., Quevedo J., et al. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatr. 2015;2:1002–1012. doi: 10.1016/S2215-0366(15)00309-0. [DOI] [PubMed] [Google Scholar]
- Raison C.L., Miller A.H. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am. J. Psychiatr. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- Renna M.E., O'Toole M.S., Spaeth P.E., Lekander M., Mennin D.S. The association between anxiety, traumatic stress, and obsessive-compulsive disorders and chronic inflammation: a systematic review and meta-analysis. Depress. Anxiety. 2018;35:1081–1094. doi: 10.1002/da.22790. [DOI] [PubMed] [Google Scholar]
- Rohleder . Psychoneuroendocrinology; 2019. Stress and Inflammation–The Need to Address the Gap in the Transition between Acute and Chronic Stress Effects.https://www.sciencedirect.com/science/article/pii/S0306453018306954 Available: [DOI] [PubMed] [Google Scholar]
- Rohleder N., Karl A. Role of endocrine and inflammatory alterations in comorbid somatic diseases of post-traumatic stress disorder. Minerva Endocrinol. 2006;31:273–288. [PubMed] [Google Scholar]
- Schnurr P.P., Lunney C.A. Residual symptoms following prolonged exposure and present-centered therapy for PTSD in female veterans and soldiers. Depress. Anxiety. 2019;36:162–169. doi: 10.1002/da.22871. [DOI] [PubMed] [Google Scholar]
- Smaardijk V.R., Maas A.H.E.M., Lodder P., Kop W.J., Mommersteeg P.M.C. Sex and gender-stratified risks of psychological factors for adverse clinical outcomes in patients with ischemic heart disease: a systematic review and meta-analysis. Int. J. Cardiol. 2020;302:21–29. doi: 10.1016/j.ijcard.2019.12.014. [DOI] [PubMed] [Google Scholar]
- Spitzer C., Wibisono D., Terfehr K., Löwe B., Otte C., Wingenfeld K. C-reactive protein, pre- and postdexamethasone cortisol levels in post-traumatic stress disorder. Nord. J. Psychiatr. 2014;68:296–299. doi: 10.3109/08039488.2013.844271. [DOI] [PubMed] [Google Scholar]
- Sumner J.A., Nishimi K.M., Koenen K.C., Roberts A.L., Kubzansky L.D. Posttraumatic stress disorder and inflammation: untangling issues of bidirectionality. Biol. Psychiatr. 2020;87:885–897. doi: 10.1016/j.biopsych.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft H., Bramness J.G., Lien L., Abebe D.S., Wampold B.E., Tilden T., et al. PTSD patients show increasing cytokine levels during treatment despite reduced psychological distress. Neuropsychiatric Dis. Treat. 2018;14:2367–2378. doi: 10.2147/NDT.S173659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker P., Ruwe W.D., Masters B., Parker D.E., Hossain A., Trautman R.P., et al. Neuroimmune and cortisol changes in selective serotonin reuptake inhibitor and placebo treatment of chronic posttraumatic stress disorder. Biol. Psychiatr. 2004;56:121–128. doi: 10.1016/j.biopsych.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Weathers F.W., Litz B.T., Keane T.M., Palmieri P.A., Marx B.P., Schnurr P.P. 2013. The PTSD Checklist for DSM-5 (PCL-5) [Google Scholar]
- Weathers F.W., Bovin M.J., Lee D.J., Sloan D.M., Schnurr P.P., Kaloupek D.G., et al. The Clinician-Administered PTSD Scale for DSM–5 (CAPS-5): development and initial psychometric evaluation in military veterans. Psychol. Assess. 2018;30:383–395. doi: 10.1037/pas0000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.