Review Highlights
-
•
Graphene oxide/Metal organic framework based composite gave the highest NRFX adsorption capacities (Qmax) of 1114.8 mg/g.
-
•
The NRFX adsorptive uptake was most described by Langmuir isotherm and pseudo-second order models.
-
•
Knowledge gaps in column studies and used adsorbent disposal method were seen for norfloxacin removal.
Keywords: Norfloxacin, Adsorption, Ecotoxicology, Water pollution, Desorption, Kinetics
Method name: A review of the adsorption method for norfloxacin reduction from aqueous media
Abstract
Norfloxacin (NRFX) is one of a class of antibiotics known as broad-spectrum fluoroquinolone antibiotic that is frequently used to treat infectious disorders in both animals and humans. NRFX is considered an emergent pharmaceutical contaminate. This review's objective is to evaluate empirical data on NRFX's removal from aqueous medium. The environmental danger of NRFX in the aquatic environment was validated by an initial ecotoxicological study. Graphene oxide/Metal Organic Framework (MOF) based composite, followed by Magnesium oxide/Chitosan/Graphene oxide composite gave the highest NRFX adsorption capacities (Qmax) of 1114.8 and 1000 mg/g, respectively. The main adsorption mechanisms for NRFX uptake include electrostatic interactions, H-bonds, π–π interactions, electron donor-acceptor interactions, hydrophobic interactions, and pore diffusion. The adsorptive uptake of NRFX were most suitably described by Langmuir isotherm and pseudo-second order implying adsorbate-to-adsorbent electron transfer on a monolayer surface. The thermodynamics of NRFX uptake is heavily dependent on the makeup of the adsorbent, and the selection of the eluent for desorption from the solid phase is equally important. There were detected knowledge gaps in column studies and adsorbent disposal method. There's great interest in scale-up and industrial applications of research results that will aid in management of water resources for sustainability.
Graphical abstract
Specifications table
| Subject area: | Environmental Science |
| More specific subject area: | Adsorption |
| Name of the reviewed methodology: | A review of the adsorption method for norfloxacin reduction from aqueous media |
| Keywords: | Norfloxacin; Adsorption; Ecotoxicology; Water pollution; Desorption; Kinetics |
| Resource availability: | Not applicable |
| Review question: | What was the maximum adsorption capacity (Qe) obtained for norfloxacin uptake? What adsorbent gave the maximum Qe for norfloxacin? Is the adsorption of norfloxacin a physicaly or chemically controlled process? |
Method details
Introduction
A new class of contaminants, known as emerging contaminants (ECs), have been discovered in wastewater. These include pharmaceutical compounds, illicit drugs, surfactants, etc. [1,2]. Antibiotics are the most significant and widely detected ECs because they are the third most widely used drugs in contemporary medicine, reportedly, above six percent of general prescription drugs [3]. They are commonly used to treat certain diseases in humans, chickens, cattle, fish, swine, etc. Indiscriminate discharge of these drugs has increased their presence in the environment, with a consequential impact on aquatic life, triggering changes in their growth and survival [4,5]. In addition, they also aid bacterial resistance, posing serious human health and environmental concerns [2,6].
Norfloxacin (NRFX) is a class of fluoroquinolone antibiotic commonly employed to combat both Gram-positive and Gram-negative bacteria [2,7,8]. NRFX has been broadly used as human medication (typically for the treatment of prostatitis and urinary tract infections) and veterinary medicine [9]. The antibiotics are located in sewage, animal husbandry, and hospital wastewater treatment plants [9]. Indiscriminate discharge of NRFX has led to its detection in sediments and rivers [10]. A recent work by Feng, Wang [11] reported the presence of NRFX in sewage treatment plant (STP) effluents and surface water from various parts of the world. Their report [11] revealed that the range of NRFX concentrations in the surface water is 33–1150 ng/L, while that of STP effluents is 9.4–170 ng/L.
Since the majority of conventional wastewater treatment facilities are not equipped to remove antibiotics, hence post-treatment effluent can release a sizable proportion of these NRFX-contaminated effluents into water bodies or the environment [5]. As a result, the removal of NRFX from aqueous solutions has gained the attention of researchers, particularly those in the field of the aquatic environment.
Several wastewater treatment methods have been investigated for the degradation/removal of NRFX from wastewater such as adsorption [1,4,6,7,9,[12], [13], [14], [15], [16], [17], [18]], membrane filtration [19], adsorption-photocatalytic [20], coagulation [21], electrocoagulation [22], [23], [24], photo-Fenton [25], electro-Fenton [26], photocatalyst [27], sonocatalytic [28], etc. The membrane separation method is costly and susceptible to fouling [18]. Photocatalytic and catalytic methods degrade antibiotics by breaking them down into small molecules. The high cost of catalysts limits their applications in large-scale operations and the identification of the noxiousness of the catalytic by-products is challenging [9]. Amongst these alternatives, adsorption is the most attractive technique in terms of economic viability [3,5]. Other advantages of adsorption over other alternatives in the removal of antibiotics from aqueous solutions include operational simplicity, high removal efficiency, applicability in a large sorbate concentration range, instrumentation cost benefit, etc., [4,13].
These promising attributes of adsorption towards the removal of antibiotics, in particular NRFX, from aqueous media, make it necessary to present a structured and comprehensive review of the recent research output in this area. The authors observed via painstaking research that despite years of studies in this area, there hasn't been a systematic review specifically focused on the adsorption of NRFX from aquatic solutions. NRFX was selected as the target pollutant for this study because of its wide usage in human and veterinary medicines making it highly detectable in the environment.
Hence, the focus of the present review is to comprehensively present, discuss, and summarize the research findings related to the adsorption of NRFX from an aqueous solution. The adsorbent materials with their respective adsorption capacities and the adsorption types, kinetics, isotherms, mechanisms, as well as thermodynamic equilibrium were elaborately analyzed. More so, the desorption/reusability capabilities of the individual adsorbents for NRFX sorption were discussed in detail. The review equally provided insight into main empirical research outcomes, identify research gaps and establish future research perspectives in the NRFX adsorption research arena. The papers published in the last decade were central to the review and the papers were all selected from the pool of reputable indexed journals.
Ecotoxicology of NRFX
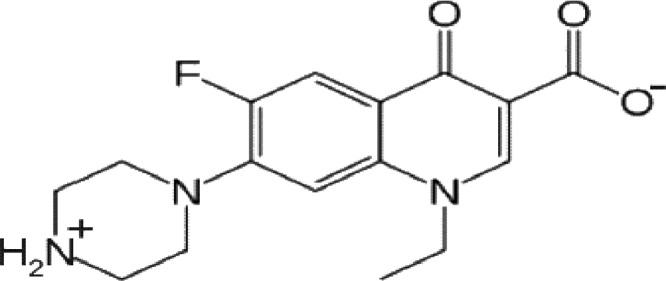
When the concentration of NRFX in the aquatic environment grows beyond the allowable limit, it becomes an environmental challenge and a threat to aquatic life. This section briefly provides insight into the adverse impacts of the NRFX contamination of the aquatic environment. This provides legitimate justification for the imperativeness of NRFX removal from aqueous media. The properties and the chemical structure of NRFX are presented in Table 1 and Fig. 1 respectively. The changes in the growth and survival of aquatic habitats triggered by sole NRFX accumulation or its combination with other pollution agents have been highlighted.
Table 1.
| Property | NRFX |
|---|---|
| Appearance CAS ID | crystalline powder (white to light-yellow) 70,458–96–7 |
| IUPAC Name | 1-Ethyl-6-fluoro-4-oxo-7-piperazin-1-ylquinoline-3-carboxylic acid |
| Chemical Formula | |
| Composition (%) | C (60.18), H (5.68), F (5.95), N (13.16), O (15.03) |
| Molecular weight (g/mol) | 319.33 |
| Exact molar mass (g/mol) | 319.13321961 |
| Polar surface area (Å2) | 72.9 |
| Water Solubility (at 25 °C, pH = 7) | 400 mg/L |
| λmax/nm | 278 |
| pKa (Dual) | 6.22 and 8.51 |
| Heavy atom count | 23 |
| Molecular dimensions, size (Å) | L12.2 Å×W8.2 Å×H7.0 Å |
| 1.03 | |
| H2 bond count (Donor) | 2 |
| H2 bond count (Acceptor) | 7 |
| Melting point | 220 – 221 °C |
Fig. 1.
Chemical structure of NRFX (Adopted from [2]).
The effect of NRFX contamination was investigated via co-culturing of Scenedesmus quadricauda-Chlorella vulgaris (SQCV) and Scenedesmus obliquus-Chlorella vulgaris (SOCV) in a short term and SOCV in a long term [29]. Their findings showed that NRFX impacted the interaction of species and reduced the stability over time of plankton communities, signifying that antibiotics such as NRFX have broader effects than currently understood. Wan, Long [30] demonstrated the impact of NRFX contamination on the aquatic food chain. Dual food chains, that is, Chlorella vulgaris-D. magna-larval and Scenedesmus quadricauda-D. magna-larval damselfly was used to study the impact of the presence (at different concentrations) of NRFX on trophic cascades. An increase in the concentration of NRFX triggered a reduction in the swimming capability of the species. Exposure to NRFX changed the trophic cascades' power and direction, and it revealed the specific variations of the species. Another study was conducted to determine the toxicity of NRFX towards Lactuca sativa via the estimation of the EC50 (5 days), that is, the effective concentration that would give a root elongation inhibition of 50% in 5 days [30]. As the concentration of NRFX increases, there is a reduction in the seeds’ ability to elongate their roots, hence, the samples' toxicity is raised. At up to 200 mg L − 1 NRFX concentration, the samples did not show toxicity, but from 350 mg L − 1 concentration, toxicity was demonstrated. Without a supporting electrolyte, the EC50 (5 days) for an NRFX-contaminated solution was recorded as 336 mg L − 1. Corresponding results were obtained in a related study by Pan, Yan [31] using Daphnia magna. On the hand, studies showed that Norfloxacin nicotinate is immunotoxic and is toxic and hazardous to the formative stages of the life of zebrafish [32]. These reports demonstrate the imperativeness of studies directed towards the effective removal of NRFX from aqueous media.
Overview of NRFX removal methods
This section presents an overview of the different NRFX removal methods. This helps to provide insight into the capabilities and the advantages of adsorption over the other NRFX removal methods, further lending credibility to why the adsorption method was considered for this review. From the literature, the methods that have been investigated for the removal of NRFX from aqueous solutions can be grouped into three, viz, physical, chemical, and biological methods.
The physical methods comprise of microfiltration [34], ultrafiltration [35], and nanofiltration [19,36] membranes, reverse osmosis [36], and adsorption [6,9,12,14]. In the membrane filtration techniques, semipermeable materials are used to remove a pollutant from the contaminated effluent [38]. Nanofiltration and reverse osmosis have demonstrated the most separation capabilities, delivering a > 99% NRFX removal at a pH of 8 [36]. Similarly, exactly 99.5% of NRFX were successfully removed from NRFX-contaminated pharmaceutical wastewater at a pH of 6.5 [19]. However, the membrane filtration technique is constrained by high installation and operating costs, and fouling susceptibility [37], [38], [39].
The chemical methods that have been reported include the coagulation-flocculation (C-F) [21], electrocoagulation (EC) [22], ion exchange [40], and advanced oxidation processes (AOPs) [[41], [42], [43], [44]]. AOPs are unconventional techniques for repollutants degradation, they benefit from the high oxidation capability of hydroxyl radical (•OH), azide radical (•N3), and sulfate radicals (SO4•−) produced during the treatment process [38,45] to give inert end products. The efficacy of the oxidant determines how well AOPs perform [45]. AOPs investigated for NRFX removal include photocatalysis [27,[46], [47], [48]], photolysis [49,50], chlorine processes [51], [52], [53], ozonolysis [54], [55], [56], sonolysis [57], sonocatalysis [28,58], Fenton (sono-Fenton, electro-Fenton, and photo-Fenton, etc.) [25,[59], [60], [61], [62]]. The degradation of NRFX photolysis enhanced by microwave has been demonstrated by Liao, Sharma [49]. Within five minutes of the MW/UV reaction, approximately 75% of NRFX was removed. The NRFX degradation was chiefly initiated by ·OH-photosensitization stages and a second-order reaction kinetics was followed. Novel FeVO4/Fe2TiO5-type composite photocatalyst was hydrothermally synthesized for NRFX degradation [46]. The novel photocatalyst irradiated with visible light exhibited enhanced photocatalytic activity and impressive stability during the treatment process. A study by Zhao, Wang [61] demonstrated a photo-Fenton NRFX degradation process via the immobilization of MIL-101(Fe)-NH2 on Al2O3. The prepared photo-Fenton catalyst delivered a > 90% NRFX removal efficiency which was attributed to its outstanding water and chemical stability.
In a coagulation-flocculation process, anionic polyacrylamide and polyaluminium chloride were used as coagulants according to He, Zhang [21]. The process could only deliver an NRFX removal efficiency of about 42%. The process was reported to be predominantly controlled by sweep flocculation, surface charge neutralization, and adsorption. In a similar study, the performance of electrocoagulation in NRFX degradation was demonstrated using six aluminum electrodes arranged in parallel [22]. The process variables were electrocoagulation reaction time, solution pH, and operating voltage. At optimal conditions, a 98.4% NRFX removal was achieved. Nonetheless, most of these chemical methods have some identified shortfalls. For instance, the AOPs are associated with high cost of chemical reagents, energy, and operation, it can also lead to the generation of unknown side products [63]. The ion exchange method would require a buffer, the acidity of the solution is raised, it also require high operating cost [37]. The coagulation-flocculation method is constrained by high effluent sludge volume and possibility of use of environmentally unsafe chemicals as coagulants [63,64]. The electrocoagulation is associated with high electricity and electrodes costs, passivation of the sacrificial electrodes, and the need for further treatment to eliminate the raised metal concentration brought about by the dissolution of the metal electrodes used [65], [66], [67], [68].
In biological methods, cultures of microorganisms are used to decompose contaminants [37]. Biological wastewater treatment is grouped into aerobic and anaerobic bioprocesses. Nitrogenous and organic components in the effluent can serve as nutrients for enhanced microbial proliferation in anaerobic or aerobic environments [37]. The performance of aerobic and anaerobic degradation techniques was reported by de Souza Santos, Teixeira [69]. The study revealed that NRFX degradation efficiency of 12% and 18% were obtained for activated sludge and anaerobic bio-digesters, respectively. The efficacy of microbial fuel cells for NRFX removal from aqueous media has also been investigated by Ondon and co-workers [70]. The NRFX removal efficiency of 65.5% was reported. As reported, raising the concentration of NRFX (up to 128 mg/L) had no substantial effect on its degradation efficiency. In an enzyme-based-activated sludge for NRFX degradation study, a degradation efficiency of 82.18% was obtained after 25 min, pH 5.0, and enzyme concentration of 1.5 × 10−9 mol L − 1 [71]. In another study, a high NRFX degradation efficiency via a bio-aerated filter incorporated with zeolite filler was demonstrated [72]. The degradation efficiency of NRFX was > 99.9%. The shortcomings of biological methods include the reality of requiring a longer time to complete the process, slow process kinetics, complexibility of the microbiological process, and some compounds are not biodegradable or poor in biodegradability [37,63].
The adsorption method of pollutants removal is one of the most widely researched areas [73,74] due to its numerous advantages over other alternative methods including, equipment simplicity and ease of operation, low operating cost, high pollutant removal efficiency, ease of process combination with other methods, etc. [37,63,75,76]. It also has the advantage of the use of widely available waste materials and biomass which are considered worthless as adsorbents in the adsorption process [38]. Numerous adsorbent materials have been investigated for adsorptive removal of NRFX from aqueous media, they include carbon-based adsorbents [3,16,77,78], polymers [79], [80], [81], clays [10,82], composites [2,83,84], nano-materials [6,9,13,18], magnetic materials [85,86], etc. It is on the premise of the wide availability of research on NRFX adsorption that this review was conceived.
Adsorbent performance for NRFX
This section discusses the performance of various adsorbents for NFRX sequestration, as well as the various factors affecting their performance. Studies on NRFX adsorption have shown the use of removal efficiency (RE%) and maximum adsorption capacity (Qmax) as indexes for the representation of the adsorbent's performance. For two reasons, this study utilized only the maximum adsorption capacity as its index. Firstly, while all studies on NRFX adsorption reported the strength of the adsorbent based on its adsorption capacity, not all studies reported the same for the removal efficiency. Secondly, previous studies have shown that the choice of operational parameters greatly affects the RE% [87,88]. For most of the studies performed, the Qmax of the adsorbents was obtained through Langmuir isotherm modeling. However, some exceptions exist, such as experiments [89,90], and other isotherm modeling such as Sips [5,17], and Dubinin-Astakhov [91]. This implies that the adsorption of NRFX mostly occurred on the monolayer surface of the adsorbent. Table 2 summarizes the findings of various researchers on the uptake of NRFX using the adsorption technique. From the table, it can be observed that the adsorbents with the highest Qmax value for NRFX uptake are Graphene oxide/Metal Organic Framework (MOF) based composites, followed by Magnesium oxide/Chitosan/Graphene oxide composites, with NRFX adsorption capacities of 1114.8 mg/g [83], and 1000 mg/g [92], respectively. These adsorbents fall into the class of composite-based adsorbents as buttressed by Ighalo, Omoarukhe [93]. Apart from having high adsorption capacity, composite-based adsorbents were also observed to be the most frequently used adsorbents for NRFX uptake. The perceived high performance of composite-based adsorbents could be due to the contribution of the individual chemical constituents inherent in them [94].
Table 2.
Adsorbent performance for NRFX adsorption from aqueous media.
| Adsorbent | (mg/g) | pH | Temp. (ºC) | SSA (m2/g) | Average pore volume (cm3/g) | Method of determination | Refs. |
|---|---|---|---|---|---|---|---|
| Graphene oxide/MOF based composite | 1114.8 | – | – | 49.95 | – | Langmuir | [83] |
| Magnesium oxide/Chitosan/Graphene oxide | 1000.0 | 7.0 | – | 294.0 | – | Langmuir | [92] |
| N-doped activated carbon from shaddock peel | 798.40 | – | 45 | 2481 | – | Langmuir | [102] |
| Trapa natans husk based AC | 638.60 | 7.5 | – | 1274 | 1.087 | Langmuir | [107] |
| Chitosan-hydroxyapatite nano-adsorbent | 625.00 | 7.0 | 45 | – | – | Langmuir | [6] |
| Pine needle based Fe/Cu oxides composite | 609.86 | 40 | 27.80 | 0.093 | Langmuir | [108] | |
| Magnetic halloysite nanotubes/molecularly imprinted polymer composite | 512.82 | – | 25 | – | – | Langmuir | [109] |
| Lotus stalk-based activated carbon | 456.18 | 5.5 | – | 1289 | – | Langmuir | [110] |
| Fe-Cu oxide nanocomposite from Loquat leaf extract | 453.70 | – | 40 | 13.38 | 0.055 | Langmuir | [103] |
| Loofah based activated carbon | 450.45 | 7.0 | 25 | 736.8 | – | Experiments | [89] |
| Pre-treated barley straw | 441.00 | 6.96 | 50 | – | – | Experiments | [90] |
| Chromium based sulphonated MOF | 408.20 | 7.0 | 30 | 1760 | – | Langmuir | [99] |
| Pre-treated barley straw | 396.00 | 7.0 | 25 | 1314 | – | Experiments | [111] |
| Graphene oxide | 374.90 | 7.0 | 31 | 67.00 | – | Sips | [17] |
| Chromium based metal-organic framework (MOF) | 360.00 | – | 30 | – | – | Experiments | [99] |
| Raw barley straw | 349.00 | 6.96 | 25 | – | – | Experiments | [90] |
| Polydopamine microspheres | 346.00 | 6.6 | 45 | – | – | Langmuir | [80] |
| Iron-trimesic metal-organic framework | 344.80 | 7.0 | 25 | 563.6 | – | Experiments | [112] |
| Cattail fibres based activated carbon | 310.70 | – | – | 907.0 | 1.00 | Langmuir | [113] |
| Magnetic biochar derived from furfural residue | 299.60 | 4.0 | – | 463.0 | 0.524 | Langmuir | [114] |
| Amine-functionalized magnetic bamboo-based AC | 293.20 | – | – | 923.2 | 0.51 | Langmuir | [33] |
| Luffa sponge biochar | 286.00 | – | 15 | 822.3 | – | Langmuir | [3] |
| Animal hairs based activated carbon | 285.40 | – | – | 514.0 | 0.243 | Langmuir | [113] |
| Layered chalcogenide | 271.10 | 4.0 | 40 | – | – | Langmuir | [115] |
| Raw montmorillonite | 255.44 | 5.0 | 25 | – | – | Langmuir | [116] |
| Zr-based metal organic framework composite | 253.16 | – | – | – | – | Langmuir | [117] |
| 3D peanut shell/graphene oxide | 228.83 | 6.2 | – | 64.24 | 0.108 | Experiments | [118] |
| Amino-functionalized Zr-based MOF | 222.50 | – | 45 | 713.2 | – | Langmuir | [9] |
| Surface-imprinted polymer microsphere | 220.30 | – | 25 | – | – | Langmuir | [119] |
| Salix mongolica based magnetic biochar | 215.80 | – | 35 | 1348 | 0.59 | Langmuir | [120] |
| Hypercross linked resin MN-202 | 196.10 | 7.0 | 45 | 1155 | – | Langmuir | [121] |
| Alkali-modified fly ash | 189.40 | 7.0 | 25 | 31.27 | 0.0773 | Langmuir | [122] |
| Macroporous resin XAD-4 | 178.60 | 7.0 | 15 | 880.0 | – | Langmuir | [121] |
| Magnetic bamboo-based AC | 173.30 | – | – | 861.6 | 0.45 | Langmuir | [33] |
| Montmorillonite | 169.22 | 7.0 | 25 | 65.50 | – | Langmuir | [2] |
| Aminated polystyrene resin MN-150 | 161.30 | 7.0 | 45 | 815.3 | – | Langmuir | [121] |
| Albizia lebbeck seed pods based activated carbon | 153.99 | – | 50 | 1824 | – | Langmuir | [123] |
| Iron-based mesoporous material (Fe-MCM-41) | 134.26 | – | – | 894.1 | – | Langmuir | [101] |
| Acid-modified fly ash | 129.70 | 7.0 | 25 | 18.37 | 0.0403 | Langmuir | [122] |
| Urea N-doped cocoa shell biochar | 120.30 | – | 25 | 8.450 | 0.016 | Langmuir | [98] |
| Granular AC | 112.86 | 7.0 | 25 | 1200 | 1.31 | Langmuir | [124] |
| Nitrogen rich-modified Calotropis gigantean fiber | 105.80 | 8.0 | – | – | – | Langmuir | [125] |
| Sulphuric acid activated termite feces | 104.40 | – | 55 | 81.30 | – | Sips | [5] |
| Diammonium hydrogen orthophosphate activated Pomelo peel based AC | 101.35 | – | 30 | 83.79 | 0.08 | Langmuir | [126] |
| Mg/Fe layered bimetallic hydroxides | 97.070 | 7.0 | 25 | – | – | Experiments | [127] |
| Thermally treated montmorillonite | 95.790 | 7.0 | 25 | 1.200 | – | Langmuir | [2] |
| Chromium based aminated MOF | 92.500 | – | 30 | – | – | Experiments | [99] |
| Carboxylated multi-walled carbon nanotubes | 89.300 | 7.0 | 45 | 160.0 | – | Langmuir | [121] |
| Copper loaded SBA-15 type mesoporous silica | 87.850 | – | 35 | – | – | Langmuir | [128] |
| Acid activated, thermally treated montmorillonite | 86.211 | 7.0 | 25 | 131.6 | – | Langmuir | [2] |
| Magnetic pomelo peel-based biochar | 85.080 | 45 | 20.73 | 0.110 | Langmuir | [104] | |
| Spent coffee ground biochar | 69.800 | 7.0 | – | 46.32 | – | Langmuir | [129] |
| Magnetic porous carbon from Fe3+-modified MOF-5 | 63.918 | 7.0 | 25 | 104.0 | 0.24 | Dubinin-Astakhov | [91] |
| Molecularly imprinted polymer | 62.500 | – | – | 7.950 | 0.34 | Langmuir | [81] |
| MCM-41type mesoporous silica | 61.400 | 7.0 | – | – | – | Experiments | [130] |
| Fly ash | 54.690 | 7.0 | 25 | 6.120 | 0.0233 | Langmuir | [122] |
| 2D copper-porphyrin metal-organic framework | 50.000 | 5.0 | – | 342.7 | 0.256 | Experiments | [131] |
| Iron loaded SBA-15 type mesoporous silica | 46.720 | – | 15 | – | – | Langmuir | [128] |
| Pomelo peel-based biochar | 45.270 | 45 | 1.706 | 0.003 | Langmuir | [104] | |
| Molecularly non-imprinted polymer | 44.600 | – | – | 5.850 | 0.29 | Langmuir | [81] |
| Magnetic molecularly imprinted polymer composite | 42.340 | – | 35 | – | – | Experiments | [132] |
| Sawdust biochar/Al2(SO4)3-modified thermal kaolin | 42.287 | – | – | 44.00 | 0.233 | Langmuir | [1] |
| Iron/Zinc/H3PO4 – modified sludge biochar | 39.300 | – | – | 39.10 | 0.129 | Langmuir | [133] |
| Phosphoric acid activated Pomelo peel based AC | 38.340 | – | 30 | 626.9 | 0.35 | Langmuir | [126] |
| Coffee husk waste | 33.560 | – | – | 1.217 | – | Langmuir | [16] |
| Iron-doped activated alumina | 32.580 | 6.5 | – | 140.6 | – | Langmuir | [110] |
| Montmorillonite/wheat straw biochar composite | 25.530 | 7.0 | 25 | 112.6 | 0.604 | Langmuir | [100] |
| Reduced graphene oxide/ magnetite composite | 23.260 | 6.2 | 25 | – | – | Langmuir | [134] |
| Rice husk waste | 20.120 | – | – | 0.902 | – | Langmuir | [16] |
| Magnetic molecularly imprinted polymer | 14.260 | – | 25 | 88.90 | 0.15 | Langmuir | [85] |
| Iron ore waste | 14.160 | – | 35 | 24.60 | – | Langmuir | [15] |
| Nickel-Aluminium layered double hydroxide | 12.990 | 7.0 | 25 | 60.83 | 0.099 | Langmuir | [34] |
| Urea N-doped biochar | 11.480 | 5.0 | 25 | 225.9 | 0.24 | Experiments | [135] |
| Wheat straw biochar | 10.580 | 7.0 | 25 | 20.10 | 0.138 | Langmuir | [100] |
| Magnesium-aluminum layered double hydroxides | 9.0660 | – | – | 37.30 | – | Experiments | [136] |
| Pomelo peel-based biochar | 7.4300 | 4.5 | 20 | – | – | Experiments | [137] |
| Corn stalks based activated magnetic biochar | 7.2469 | – | – | 760.7 | 0.3698 | Langmuir | [78] |
| Magnetic potato stems and leaves biochar-based manganese oxide composite | 6.9400 | 3.0 | 35 | 252.0 | 0.16 | Langmuir | [138] |
| Willow branches activated magnetic biochar | 6.2587 | – | – | 856.8 | 0.4007 | Langmuir | [78] |
| Pyrite-reed straw biochar composite | 5.9425 | 7.0 | 35 | 54.53 | 0.0532 | Langmuir | [139] |
| Potato stems and leaves biochar | 5.8200 | 3.0 | 53 | 99.43 | 0.078 | Langmuir | [138] |
| Natural attapulgite – potato stem hybrid biochar | 5.2400 | 6.1 | 35 | 90.40 | 0.1225 | Langmuir | [10] |
| Hematite-reed straw biochar composite | 4.4699 | 7.0 | 35 | 134.9 | 0.0857 | Langmuir | [139] |
| Reed stalks based activated magnetic biochar | 3.5139 | – | – | 777.5 | 0.3294 | Langmuir | [78] |
| Montmorillonite | 2.5530 | 6.5 | 25 | 148.3 | 0.202 | Experiments | [100] |
| Molecularly imprinted silica polymer | 2.2280 | – | 25 | 344.0 | – | Experiments | [140] |
| Carbonized Moringa oleifera pod husks | 2.0330 | – | 25 | 235.8 | – | Langmuir | [141] |
| Mesoporous silica MCM-41 | 1.7400 | 7.0 | 25 | 918.4 | 0.752 | Langmuir | [142] |
| Chemically treated Moringa oleifera pod husks | 1.5170 | – | 25 | 182.4 | – | Langmuir | [141] |
| Polystyrene microplastics | 0.9240 | – | 35 | – | − | Langmuir | [7] |
| Polybutylene succinate | 0.8340 | – | 35 | – | – | Langmuir | [7] |
| Polyethylene microplastics | 0.5140 | – | 35 | – | – | Langmuir | [7] |
The adsorbent's attributes or properties are one very important factor that affects its Qmax potential. These attributes can be divided into two types: physical and chemical properties. Examples of the adsorbent's physical attributes include its specific surface area, pore volume, and pore size [95], [96], [97]. To drive home this point, Zanli, Tang [98] varied the reaction conditions for the synthesis of cocoa shell-based biochar, which also caused a variation in the biochar's SSA, average pore diameter, and pore volume. The variation in these attributes was also observed to contribute to the variation in the uptake ability of NRFX. The chemical attributes of the adsorbent are another factor that affects its performance. A study by Guo, Kang [99] showed that the addition of an amine functional group to a chromium-based MOF decreased its adsorption capacity for NRFX from 360 mg/g to 92.5 mg/g, while the addition of a sulphonic acid functional group improved its adsorption capacity to 400.9 mg/g. pH is another sensitive parameter that affects the performance of the adsorbent . The pH at which maximum adsorption of NRFX was obtained varied from 3.0 to 8.0, with most of it falling within the range of 6–7, as shown in Table 2. Norfloxacin has two proton-binding sites, the piperazinyl and carboxyl groups, with acid dissociation constants (pKa) values of 6.22 and 8.51, respectively [100]. The implication of this is that NRFX exists in its cationic state at pH less than 6.22 and in its anionic state at pH greater than 8.51. Therefore, for optimum sorption, an adsorbent with a negatively charged surface is required for the uptake of NRFX at low pH, and a positively charged adsorbent surface is required for NRFX uptake at high pH due to electrostatic attraction. According to Fang, Miao [85], in the pH range of 6.2–8.5, where NRFX exists in its zwitterionic state, hydrogen bonds and van der Waals forces, rather than electrostatic forces, would dominate its adherence to the active sites of the adsorbent.
Research conducted by Feng, Liu [3] obtained a declining trend in NRFX adsorption capacity with an increase in temperature from 20 °C to 40 °C using biochar derived from Luffa sponge. Similar observations were also made by Chen, Li [101] using iron-based mesoporous material (Fe-MCM-41) as an adsorbent. Niu, Liu [102] obtained an opposite result as increase in temperature from 25 ℃ to 45 ℃, improved the adsorption capacity from 746 mg/g to 798 mg/g. Similar observations have also been obtained by several studies [103,104]. This disparity in Qmax value with respect to the change in temperature is hinged on the reaction temperature type. If the NRFX adsorption process is exothermic, decreasing the temperature of such a process is expected to increase the Qmax value [105]. A similar trend would also come into play by increasing the temperature of an endothermic reaction [106]. All things being equal, increasing the temperature of an exothermic adsorption reaction or decreasing the temperature of an endothermic adsorption reaction would lead to a decrease in Qmax. Other process parameters which can affect the adsorbent performance for NRFX uptake include adsorption duration, adsorbent dosage, NRFX concentration, ionic strength of the NRFX solution, and the presence of other chemical species in the solution matrix.
Adsorption mechanism
Understanding how well an adsorbent can remove a certain pollutant depends largely on the adsorption mechanism. This section discusses the adsorption mechanisms behind the interactions between NRFX and various adsorbents. Before focusing on particular adsorption mechanisms, it is important to analyze both empirical findings and analytical characterizations [143]. This is crucial since there are several processes involved in the adsorption of NRFX. The solution pH, the pH of the point of zero charge (pHpzc), the adsorbate pKa, isotherm and kinetic studies, thermodynamic studies, and desorption studies are all important variables in establishing the mechanism of adsorption. The pHpzc is the pH of the solution when the amount of positive charges on the adsorbent surface equals the amount of negative charges. When the pH< pHpzc, the adsorbent surface is protonated and positively charged, but when the pH> pHpzc, the surface is deprotonated and negatively charged [144]. NRFX has two proton-binding sites (carboxyl and piperazinyl groups) with respective pKa values of 6.22 and 8.51 [10]. When the pKa is less than 6.22, NRFX exists as NRFX+ (cationic form). It exists as NRFX± (zwitterionic form) when the pKa is between 6.22 and 8.51, and as NRFX− (anionic form) when the pKa is greater than 8.51 [104].
The mechanisms of NRFX adsorption onto various adsorbents are comprehensively summarized in Table 3, along with the optimum pH and pHpzc. According to the table, the main adsorption mechanisms for NRFX uptake include electrostatic interactions, H-bonds, π–π interactions, electron donor-acceptor interactions, hydrophobic interactions, and pore diffusion. Van der Waals forces, cation exchange, and surface complexation are a few additional often observed interactions. An electron donor-acceptor (EDA) interaction is typically used to describe the adsorption mechanism of ionic organic molecules on carbonaceous sorbents [114]. π–π EDA interaction is a specific and non-covalent bond that widely exists between electron-rich and electron-poor compounds [91]. NRFX is a strong acceptor compound due to the presence of a fluorine group in its chemical structure, which has a high tendency to attract electrons [145,146]. Other π interactions reported include, π–π stacking interactions [80,147,148] and n–π interactions [128].
Table 3.
Adsorption mechanisms for NRFX removal (NRFX pKa1 = 6.22 and pKa2 = 8.51).
| Adsorbent | Optimum pH | pHpzc | Adsorption mechanism | Ref. |
|---|---|---|---|---|
| Montmorillonite-biochar | 11 | 6.9 | Electrostatic interactions, H-bonds, pore-filling | [100] |
| Urea N-doped biochar | 5.0 | 4.0 | H-bonds, pore-filling, π–π EDA | [135] |
| N-doped/activated cocoa shell biochar | 5.0 | 4.1 | Pore-filling, H-bonds, π–π EDA, ion exchange, electrostatic attractions | [98] |
| UIO-66-NH2 nanomaterials | 8.0 | – | Electrostatic interactions, π–π interactions, H-bonds | [9] |
| Ni-Al LDH | 7.0 | 7.3 | Electrostatic interactions, anion exchange, hydrophobic interactions | [34] |
| Silica | 8.2 | 9.0 | Electrostatic interactions | [154] |
| Ferrihydrite nanoparticles | 5.5 | – | Electrostatic interactions, complexation | [155] |
| Bentonite-sludge biochar composite | 6.0 | – | H-bonds, complexation, precipitation, Electrostatic interactions, π–π interactions | [8] |
| Pretreated barley straw | 6.7 | 4.1 | Electrostatic attractions, H-bonds, π–π interactions, π–π EDA | [111] |
| Polydopamine microspheres | 7.0 | 6.3 | H-bonds, electrostatic interactions, π–π interactions | [80] |
| Microplastics | 5.0 | – | π–π conjugation, H-bonds, ion exchange, electrostatic interactions | [7] |
| Dissolved organic matter | 7.0 | – | π–π interactions, electrostatic interactions, H-bonds, van der Waals forces | [4] |
| (Fe/Zn + H3PO4)-modified sludge biochar | 7.0 | 2.2 | H-bonds, π–π interactions, electrostatic interactions, complexation | [133] |
| N rich—Calotropis gigantean fiber | 8.0 | – | Hydrophobic interactions, electrostatic interactions, H-bonds, π–π stacking interactions | [125] |
| Loofah AC | – | – | Physisorption, π–π interactions | [89] |
| Magnetic biochar from furfural residue | 4.0 | 3.4 | Electrostatic attractions, π–π EDA, van der Waals forces | [138] |
| Microplastics | 5.0 | 4.5 | Electrostatic interactions, π–π interactions, H-bonds, polar-polar interactions, van der Waals forces | [12] |
| Rice husk (Oryza sativa) | 6.2 | 5.2 | Electrostatic interactions, H-bonds | [16] |
| Coffee husk (Coffea arabica) | 6.2 | 4.1 | Electrostatic interactions, H-bonds | [16] |
| Montmorillonite | 4.5 | – | Complexation | [157] |
| Functionalized CNTs | – | – | π–π EDA, H-bonds | [145] |
| Iron ore waste | 7.0 | 8.5 | Cation exchange | [15] |
| Magnetic porous carbon-based sorbent | 7.0 | 7.6 | Electrostatic interactions, hydrophobic interactions, H-bonds, π–π EDA | [91] |
| Hematite/biochar composite | 4.0 | 7.7 | π–π EDA, H-bonds, ion exchange, complexation | [139] |
| Pyrite/biochar composite | 10 | 4.0 | π–π EDA, H-bonds, ion exchange, complexation | [139] |
| rGO/magnetic composites | 6.2 | – | π–π interactions, electrostatic repulsion | [134] |
| Potato stem/clay biochar composite | 11 | 7.8 | Electrostatic interactions, hydrophobic interactions, H-bonds, π–π EDA | [10] |
| Layered chalcogenide | 4.0 | – | Electrostatic attractions, π–π stacking interactions | [115] |
| Mg/Fe layered biometallic hydroxide | 6.0 | – | Coordination bonds, H-bonds | [127] |
| Activated magnetic biochar | 7.0 | – | π–π interactions, electrostatic force, pore fillings | [78] |
| Amine-functionalized magnetic bamoboo-based AC | 6.0 | 8.2 | π–π EDA, electrostatic interactions, hydrophobic interactions, H-bonds | [33] |
| KOH-modified biochar | 7.0 | – | Electrostatic interactions, π–π EDA, H-bonds | [158] |
| Chitosan/γ-Fe2O3 composites | 7.0 | – | H-bonds | [86] |
| Iron-doped activated alumina | 6.5 | 6.5 | Cation bridging, surface complexation | [110] |
| Lotus stalk-based AC | 5.5 | 5.4 | Hydrophobic interactions, cation exchange, π–π EDA | [110] |
| Carboxylated WMCNTs | 5.4 | 3.5 | Electrostatic interactions, H-bonds | [121] |
| Macroporous resin | 5.4 | – | Hydrophobic effect | [121] |
| Hypercrosslinked resin aminated polystyrene resin | 5.4 | 4.8 | Micropore fillings, electrostatic interactions, H-bonds, π–π EDA, hydrophobic effect | [121] |
| Fe-MCM-41 | 3.0 | 3.3 | Hydrophobic effect, surface complexation, π–π interactions, electrostatic interactions | [101] |
| Pretreated barley straw | 7.0 | – | π–π EDA, H-bonds, electrostatic interactions, hydrophobic interactions | [90] |
| Pomelo peel-based biochar | 3.0 | 11 | Cation exchange, H-bonds | [104] |
| γ-Fe2O3/biochar | 5.0 | 5.9 | Cation exchange, H-bonds, hydrophobic interactions | [104] |
| H2SO4-treated termite feces | 8.0 | 1.7 | π–π interactions, H-bonds, electrostatic interactions | [5] |
| Fe/Cu oxides nanocomposites | 7.0 | 3.7 | Electrostatic interactions, surface complexation, H-bonds, π–π EDA | [103] |
| Shaddock peel-derived N-doped AC | 7.0 | 8.0 | π–π EDA, hydrophobic effect, H-bonds, electrostatic interactions, Lewis acid-base effect | [102] |
| Carbon/montmorillonite hybrid | 7.0 | 2.0 | H-bonds, electrostatic interactions, π–π EDA | [2] |
| MCM-41 mesoporous silica | 7.0 | – | π–π interactions, hydrophobic interactions | [130] |
| Graphene oxide | 7.0 | 3.9 | Van der Waals force, π–π interactions, hydrogen bond donor and acceptor | [17] |
| Fe/mesoporous silica | 7.0 | 3.6 | Electrostatic interactions, n- π EDA, H-bonds, surface complexation | [128] |
| Cu/mesoporous silica | 7.0 | 5.1 | Electrostatic interactions, n- π EDA, H-bonds, surface complexation | [128] |
| Polydopamine microspheres | 6.0 | 6.3 | Electrostatic interactions, H-bonds, π–π stacking interactions | [80] |
| 3D pretreated peanut shell/graphene oxide | 6.2 | – | Electrostatic interactions, H-bonds, π–π conjugation, hydrophobic effect | [118] |
| Pomelo peel-derived AC/phosphoric acid | 7.0 | 6.3 | Electrostatic H-bonds, hydrophobic interactions | [126] |
| Luffa sponge biochar | 6.0 | 1.8 | Cation exchange, electrostatic interactions | [3] |
| Temperature-switchable graphene oxide | 7.0 | – | π–π EDA, H-bonds | [159] |
| MOF/graphene oxide composite | 7.0 | – | Electrostatic interactions, π–π interactions, H-bonds | [83] |
| Carbon/montmorillonite hybrids | 5.0 | – | π–π EDA, electrostatic interactions | [116] |
| Magnetic biochar from salix mongolica | 9.0 | 5.8 | π–π dispersion, hydrophobic interactions, H-bonds, electrostatic interactions | [120] |
| Hydroxyapatite/polymeric nanocomposite | 7.0 | 7.2 | Physisorption | [6] |
| Iron-trimesic MOFs | 7.0 | 3.6 | Coordination bonds, pore filling, π–π stacking interactions | [112] |
| Cassava dreg biochar | 7.0 | – | H-bonds, π–π interactions | [160] |
| Fe/Cu oxides composite particles | 8.1 | 4.0 | h-bonds, π–π stacking interactions, surface complexation, hydrophobic interactions | [148] |
| Humic acid | 3.0 | – | Cation exchange | [161] |
| MgO/chitosan/graphene oxide | 7.0 | 5.5 | Electrostatic interactions, π–π EDA | [92] |
| MIL-101(Cr)-SO3H | 7.0 | – | Electrostatic interactions | [99] |
| UiO-66-NH2@TpTGcl | 8.0 | – | Electrostatic interactions | [117] |
| ZIF-8 | 6.0 | – | Electrostatic interactions, π–π stacking interactions | [147] |
| Mg-Al-CO3-LDH | 8.7 | 8.7 | Electrostatic attraction | [136] |
| Mg-Al-Cl-LDH | 8.0 | 9.5 | Electrostatic attraction, anion exchange | [136] |
The protonation and deprotonation of NRFX pHpzc can promote electrostatic interaction, which is the interaction of molecules with opposing charges, resulting in electrostatic attraction or repulsion between the adsorbent surface and the adsorbate [149]. H-bond and van der Waals forces are relatively weak interactions that arises as a result of intermolecular dipole-dipole forces [149,150]. Numerous moieties found in the NRFX molecules engage with the adsorbent's surface functional groups to create H-bonds [100]. Hydrophobic interactions are non-covalent forces in which nonpolar species have the tendency to cluster in solution [150]. The formation of hydrophobic interactions is facilitated by the zwitterionic form of NRFX [9]. Pore filling entails the adsorption of contaminants onto meso‑ and microporous adsorbents (like biochar) [151]. The various interactions operate at different pH of the solution. For example, in the adsorption of NRFX onto the surface of montmorillonite/wheat straw-derived biochar composite [100], electrostatic attraction between negatively charged NRFX and the biochar surface dominated the mechanism at pH <5, while H-bond between NRFX molecules and oxygen-containing groups, as well as pore fillings, were the dominant mechanisms at pH >5.
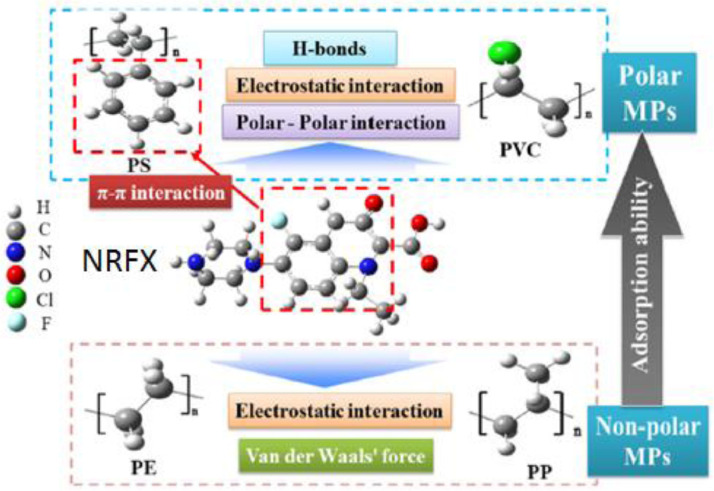
The adsorption mechanism can also be ascertained using analytical methods, such as FTIR, XPS, and XRD, as they reveal the functional groups present in the system and the positions of their interactions [152,153]. Zhang, Ni [12] used a combination of isotherm and kinetic studies, as well as various spectroscopic analyses, to determine the general adsorption mechanisms in the sorption of NRFX from simulated natural water and real surface water onto four different types of microplastics (polyethylene (PE), polystyrene (PS), polypropylene (PP), and polyvinyl chloride (PVC)) (Fig. 2). Several elements, such as H, C, N, O, Cl, and F, were detected on the microplastics using the XPS analysis, while FTIR revealed the presence of peaks assigned to hydroxyl and H-bonds. As depicted in Fig. 2, the microplastics can generally adsorb NRFX by a variety of mechanisms, including electrostatic, H-bond, van der Waals, π–π, and polar-polar interactions.
Fig. 2.
Schematic representation of NRFX adsorption on different microplastics (PP, PVC, PE, and PS). Reprinted from Zhang, Ni [12] with permission from Elsevier.
Isotherm and kinetic modeling
Isotherm studies
The knowledge of adsorption equilibrium is the most significant information required for effective interpretation of any adsorption process [34,181]. Analysis of adsorption is very essential for the general enhancement of process mechanism pathways and useful design of adsorption systems [159,133]. Adsorption isotherm study of any pollutant is carried out at constant temperature levels [182].
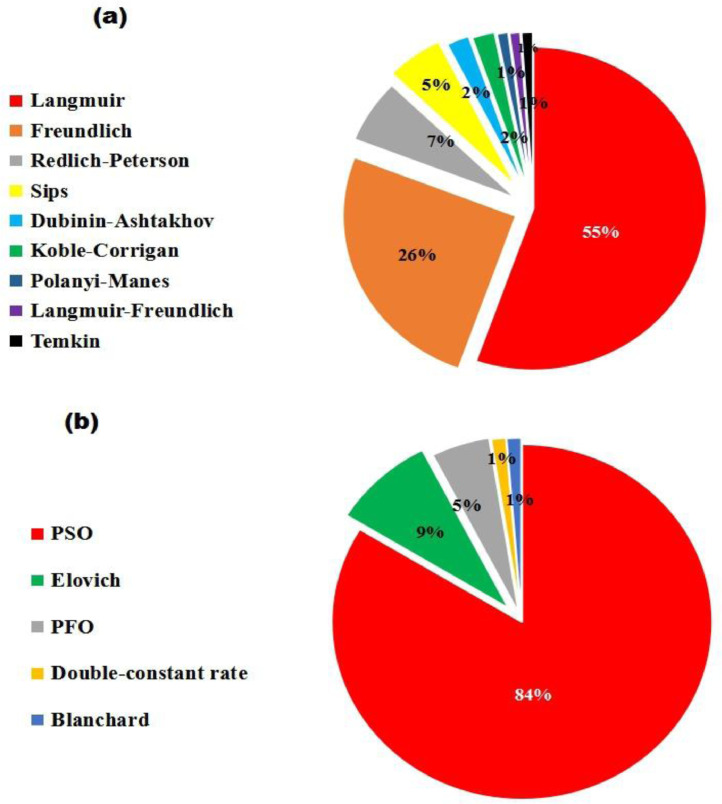
According to the information in Table 4 and Fig. 3(a), it is discernible that the adsorptive uptake of NRFX by various adsorbents were most suitably described by Langmuir isotherm (57%), followed by Freundlich isotherm (26%). This implies that NRFX adsorption is majorly monolayer in nature, where active sites exhibit uniform affinity for NRFX molecules [1,142]. However, NRFX uptake can involve multilayer adsorption occurring on heterogeneous surfaces, which form the main assumptions of Freundlich isotherm observed in 26% of the reviewed works (see Fig. 3 (b)). It is interesting to highlight that significant type of adsorbents involved in Freundlich's multilayer adsorption are thermal derivatives of biobased materials such as biochar [133,135,160], and activated carbon [113,126]. This could be attributed to the fact that biochar and activated carbon are characterized by irregular lamellar structure, which enhances its heterogeneous surface to promote multilayer adsorption of NRFX [3,114,141]. Aside Langmuir and Freundlich isotherm, a few other equilibrium models which effectively described NRFX adsorption include Redlich-Peterson [16,80,108,183], Sips [5,14,104], Dubinin-Astakhov [91,111], Koble-Corrigan [102,120], Polanyi-Manes [157], and Temkin [134].
Table 4.
Isotherm and kinetic parameters.
| Adsorbent | Isotherm |
Kinetics |
Ref. | ||||
|---|---|---|---|---|---|---|---|
| Best Fit | Nature | R2 | Best Fit | Nature | R2 | ||
| Octahedral UIO-66-NH2 nanomaterials | Langmuir | Nonlinear | 0.9930 | PSO | Linear | 0.999 | [9] |
| Tailored N-doped biochar from corn stalk | Freundlich | Nonlinear | 0.9780 | Elovich | Nonlinear | 0.999 | [135] |
| Biochar corn stalk | Langmuir | Nonlinear | 0.9950 | – | – | – | [135] |
| CoFe2O4@TiO2-MMIP | Langmuir | Nonlinear | 0.9945 | PSO | Nonlinear | 0.9940 | [15] |
| N-doped biochar derived from cocoa shell | Langmuir | Nonlinear | 0.9990 | Elovich | Nonlinear | 0.9990 | [98] |
| montmorillonite-biochar composite | Langmuir | Nonlinear | 0.9950 | – | – | – | [28] |
| animal hairs-based activated carbon | Freundlich | Nonlinear | 0.9967 | PSO | Nonlinear | 0.9999 | [113] |
| Polydopamine microspheres | Redlich-Peterson | Nonlinear | 0.9990 | PSO | Nonlinear | 0.984 | [80] |
| H3PO4 pretreated Raw barley straw | Dubinin-Astakhov | Nonlinear | 0.980 | PSO | Linear | 0.990 | [111] |
| Bentonite enhanced sludge biochar composite | Sips | Nonlinear | 0.9763 | – | – | – | [8] |
| ferrihydrite nanoparticles | Langmuir | Nonlinear | 0.9970 | PSO | Nonlinear | 0.978 | [155] |
| Biochar derived from spent coffee ground | Langmuir | Nonlinear | 0.9740 | [129] | |||
| Ceramic membrane | Langmuir | Nonlinear | 0.9750 | PSO | Nonlinear | 0.997 | [35] |
| Imprinted polymer microsphere (PMS@MIPs) | Freundlich | Linear | 0.9950 | PSO | Linear | 0.9999 | [112] |
| Non-imprinted polymer microsphere (PMS@NIPs) | Freundlich | Linear | 0.9960 | PSO | Linear | 0.9999 | [119] |
| Loofah activated carbon | Langmuir | Nonlinear | 0.981 | PSO | Linear | 0.9820 | [72] |
| Monomer molecularly imprinted sol-gel polymers from magnetic halloysite nanotubes | Freundlich | Nonlinear | 0.9904 | PSO | Nonlinear | 0.9987 | [109] |
| Nitrogen-rich Calotropis gigantea fiber | Freundlich | Nonlinear | 0.9891 | PSO | – | 0.9993 | [125] |
| modified sludge biochar | Freundlich | Nonlinear | 0.9970 | Elovich | Nonlinear | 0.9660 | [133] |
| Microplastics | Langmuir | Nonlinear | 0.9970 | PSO | Linear | 0.9990 | [7] |
| modified meta kaolin | Langmuir | Linear | 0.9790 | PSO | Nonlinear | 0.9960 | [1] |
| iron ore waste | Langmuir | Nonlinear | 0.9990 | PSO | Nonlinear | 0.9990 | [15] |
| mesoporous silica | Langmuir | Nonlinear | 0.9883 | – | – | – | [15] |
| carbon nanotubes | Polanyi–Manes | Nonlinear | 1.0000 | – | – | – | [157] |
| rice (Oryza sativa) husk wastes | Redlich-Peterson | Nonlinear | 0.9950 | PSO | Nonlinear | 1.0000 | [16] |
| coffee (Coffea arabica) husk wastes | Redlich-Peterson | Nonlinear | 0.9960 | PSO | Nonlinear | 1.0000 | [16] |
| Microplastics | Langmuir | Nonlinear | 0.9830 | PSO | Nonlinear | 0.9920 | [12] |
| Magnetic biochar | Freundlich | Nonlinear | 0.9970 | PSO | Linear | 0.982 | [114] |
| bamboo-based magnetic activated carbon | Langmuir | Nonlinear | 0.9990 | – | – | – | [34] |
| Plant based biochar | Langmuir | Nonlinear | 0.9982 | PSO | Nonlinear | 0.9731 | [78] |
| Mg/Fe bimetallic hydroxide | Freundlich | Linear | 0.9990 | PSO | Nonlinear | 0.9990 | [127] |
| Chalcogenide | Langmuir | Linear | 0.999 | – | – | – | [138] |
| graphene oxide/magnetite composites | Temkin | Nonlinear | 0.999 | PSO | Nonlinear | 0.9999 | [134] |
| clay-biochar composite | Langmuir | Nonlinear | 0.97 | PSO | Linear | 0.99 | [10] |
| iron ore – biochar composites | Freundlich | Nonlinear | 0.9913 | PSO | Linear | 0.9995 | [139] |
| MOF-derived carbon-based sorbent | Dubinin-Astakhov | Nonlinear | 0.948 | – | – | – | [55] |
| MOF@iCOF composite | Langmuir | Linear | 0.999 | PSO | Linear | 0.996 | [117] |
| iron and nitrogen co-doped biochar | Langmuir | Linear | 0.9986 | PSO | Linear | 0.9895 | [184] |
| ZIF-8 metal-organic framework | Freundlich | Nonlinear | 0.981 | PSO | Nonlinear | 0.999 | [147] |
| pretreated barley straw | Langmuir-Freundlich | Nonlinear | 0.955 | PSO | Linear | 0.999 | [90] |
| Fe-MCM-41 molecular sieves | Freundlich | Nonlinear | 0.9906 | PSO | Nonlinear | 0.9990 | [101] |
| Mg–Al layered double hydroxides | Freundlich | Linear | 0.9952 | PSO | Nonlinear | 0.9999 | [136] |
| porous resins | Langmuir | Nonlinear | 0.999 | PSO | Nonlinear | 0.99 | [139] |
| carbon nanotube | Langmuir | Nonlinear | 0.999 | PSO | Nonlinear | 0.99 | [139] |
| lotus stalk-based activated carbon | Langmuir | Nonlinear | 0.9996 | PSO | Nonlinear | 0.9990 | [110] |
| iron-doped activated alumina | Freundlich | Nonlinear | 0.9931 | PSO | Nonlinear | 0.9990 | [110] |
| Chitosan | Langmuir | Nonlinear | 0.997 | PFO | Nonlinear | 0.916 | [36] |
| chitosan/Fe2O3 composites | Langmuir | Nonlinear | 0.993 | PSO | Nonlinear | 0.878 | [36] |
| KOH modified biochar | Langmuir | NS | 0.9300 | PSO | 0.9700 | [158] | |
| Pomelo Peel Activated Carbon | Freundlich | Nonlinear | 0.9970 | PSO | Nonlinear | 0.9900 | [126] |
| Carbon/montmorillonite | Langmuir | Nonlinear | 0.99 | – | – | – | [2] |
| mesoporous metallic silicon | Langmuir | Nonlinear | 0.998 | PSO | Linear | 0.999 | [128] |
| magnetic biochar | Koble-Corrigan | Nonlinear | 0.999 | Elovich | Nonlinear | 0.990 | [20] |
| luffa sponge biochar | Freundlich | Nonlinear | 0.9995 | PSO | NS | 0.9998 | [3] |
| Graphene Oxide | Langmuir | Nonlinear | 0.996 | PSO | Nonlinear | 0.972 | [159] |
| MOF@GO composite | Langmuir | Linear | 0.99929 | PSO | Linear | 0.937 | [83] |
| montmorillonite | Langmuir | Nonlinear | 0.98 | – | – | – | [116] |
| carbon/montmorillonite hybrids | Langmuir | Nonlinear | 0.99 | – | – | – | [116] |
| fly ash | Langmuir | Nonlinear | 0.982 | Elovich | Nonlinear | 0.997 | [122] |
| Acid-modified fly ash | Langmuir | Nonlinear | 0.998 | Elovich | Nonlinear | 0.995 | [122] |
| Alkali-modified fly ash | Langmuir | Nonlinear | 0.955 | PSO | Nonlinear | 0.992 | [122] |
| Molecularly imprinted polymer | Freundlich | Linear | 0.9970 | PSO | Linear | 0.9829 | [81] |
| magnetic molecularly imprinted polymers | Freundlich | Linear | 0.976 | PFO | Linear | 0.9607 | [132] |
| MOFs | Langmuir | Nonlinear | 0.9994 | PSO | NS | 0.9999 | [99] |
| Cassava Dreg Biochar | Freundlich | Linear | 0.9980 | PSO | Nonlinear | 1.000 | [160] |
| Fe/Cu oxides composite | Redlich-Peterson | Nonlinear | 0.9980 | Double-constant rate | Nonlinear | 0.999 | [108] |
| microporous activated carbon | Langmuir | Linear | 0.9999 | PSO | Linear | 0.9975 | [123] |
| iron-trimesic metal-organic frameworks (Fe-BTC) | Langmuir | Nonlinear | 0.9950 | PSO | Nonlinear | 0.9990 | [112] |
| Chitosan-based sorbent | Langmuir | Linear | 0.997 | PSO | Linear | 0.9950 | [92] |
| chitosan-hydroxyapatite | Langmuir | Nonlinear | 0.99 | PSO | Linear | 0.99 | [6] |
| hexagonal mesoporous silica | Langmuir | NS | 0.9950 | PSO | NS | – | [130] |
| copper-porphyrin MOF | Langmuir | Linear | 0.9990 | PSO | Linear | 0.9990 | [131] |
| Humic acid | Freundlich | Nonlinear | 0.985 | – | – | – | [161] |
| Nano Alumina | Langmuir | Linear | 0.979 | PFO | Linear | 0.993 | [13] |
| Carbonized Moringa pod husks | Freundlich | Linear | 0.995 | Blanchard | Linear | 0.989 | [141] |
| Ammonium chloride-treated | Langmuir | Linear | 0.991 | Blanchard | Linear | 0.988 | [141] |
| Amino-functionalized Zr-based MOF | Langmuir | Nonlinear | 0.993 | PFO | Linear | 0.999 | [9] |
| Graphene oxide | Sips | Nonlinear | 0.997 | PSO | – | – | [17] |
| KOH-modified biochar | Langmuir | Nonlinear | 0.93 | PSO | Linear | 0.970 | [158] |
| Polydopamine microspheres | Redlich-Peterson | Nonlinear | 0.999 | PSO | Nonlinear | 0.984 | [80] |
| peanut shell-supported graphene oxide (PPS/GO) | Freundlich | Nonlinear | 0.9611 | PSO | Linear | 0.9999 | [118] |
| BC | – | – | – | PSO | Nonlinear | 0.980 | [137] |
| BC | Sips | Nonlinear | 0.9955 | PSO | Nonlinear | 0.9938 | [54] |
| γ-Fe2O3@BC | Sips | Nonlinear | 0.9986 | PSO | Nonlinear | 0.9742 | [54] |
| Acid activated termite feces | Sips | Nonlinear | 0.9976 | PSO | Nonlinear | 0.9941 | [5] |
| Modified Activated Carbon | Langmuir | Nonlinear | 0.9656 | PSO | Nonlinear | 0.9540 | [146] |
| N-doped activated carbon | Koble-Corrigan | Nonlinear | 0.9996 | PSO | Nonlinear | 0.9996 | [102] |
| BC | Langmuir | NS | 0.9939 | PSO | Nonlinear | 0.9978 | [8] |
| Magnetic biochar-based Manganese oxide | Langmuir | NS | 0.9694 | PSO | Nonlinear | 0.9999 | [8] |
| Modified TiO2 | Langmuir | Nonlinear | 0.9990 | PSO | Nonlinear | 0.9980 | [46] |
| Activated Carbon | Langmuir | Nonlinear | 0.9937 | PSO | Nonlinear | 0.9993 | [107] |
| Magnetic MIP | Langmuir | Nonlinear | 0.9910 | PSO | Linear | 0.9960 | [185] |
| Granular activated carbon | Freundlich | Nonlinear | 0.9890 | PSO | Nonlinear | 1.0000 | [124] |
| Activated carbon from agro waste | Redlich-Peterson | Nonlinear | 0.9930 | Elovich | Nonlinear | 0.9980 | [183] |
Fig. 3.
Isotherm and kinetic adsorptive uptake of NFRX by various adsorbents distribution.
Given that Langmuir and Freundlich isotherms were the dominant models that best described NRFX uptake, it is imperative to briefly elucidate these models and their associated parameters. The nonlinear form of Langmuir isotherm is given my Eq. (1).
| (1) |
Where is equilibrium NRFX concentration (mg/L), is equilibrium adsorption capacity (mg/g), is the Langmuir constant (L/mg), is the theoretical maximum monolayer coverage (mg/g). Linearising Eq. (1) will give Eq. (2).
| (2) |
Plot of vs will give and as slope and intercept, respectively.
Freundlich has the nonlinear form given in Eq. (3).
| (3) |
Where represents the Freundlich constant, represents the heterogeneity factor. and are obtained as slope and intercept from plotting vs of the linear form given in Eq. (4).
| (4) |
Adsorption kinetics
Adsorption kinetics is traditionally illustrated by sorption vs. time plot [181]. This plot determines the initiation of all kinetic investigations because its shape and outlook indicates the fundamental kinetics of any process [146,8]. Furthermore, sorption kinetics provide prerequisite reaction parameters and facilitates in estimating thermodynamic data required for design and ultimate scale-up of lab-model experiments [112]. From the data collected for kinetics in Table 4 and the associated pie chart in Fig. 3(b), it is apparent that the adsorption of NRFX is best described by pseudo-second order (PSO) model, while a few works (16%) reported the superiority of other models [36,98,133,135,13,132,141,183].
Pseudo-second order (PSO) model assumes a second order sorption rate with regards to available active sites [181] . The linear and nonlinear forms of PSO are given in Eqs. (5) and (6), respectively.
| (5) |
| (6) |
Where k is the kinetic rate constant, and is the adsorption capacity at time, t. A plot of vs. t produces a straight line for PSO conforming kinetics [9,111,119].
The acceptability of PSO equation in accurate modeling of adsorption processes has been challenged by two major drawbacks: the first is over-fitting anomalies associated with the model [182], the other is unreliable prediction of the linear form of PSO owing to regression errors [188]. However, the work of Tran et al. [182] demonstrated that PSO model, regardless of the form, performed satisfactorily better than other models in adsorption kinetics of antibiotics similar to NRFX. In view of the fact that PSO was superior in the prediction of NRFX adsorption kinetics, it follows that the fundamental assumptions of the model such as a chemisorption process which features adsorbate-to-adsorbent electron transfer, dominates the chelation process [181].
Thermodynamic studies
Thermodynamic studies provide data relating to temperature changes in a process. This information is exemplified by the values of thermodynamic parameters such as Gibbs free energy (ΔGo), change in enthalpy (ΔHo), and entropy (ΔSo) [186]. These parameters portray the favor-ability, spontaneity, energy delivery (exothermic) or utilization (endothermic), and degree of randomness of any process under investigation [8]. The thermodynamic parameters are estimated from Van't Hoff model, and the free energy equation given in Eqs. (7) and (8), respectively.
| (7) |
| (8) |
Where K is the equilibrium constant, R is the universal gas constant and T is the temperature of the system in kelvin. The thermodynamic studies summarized in Table 5 reveals that NRFX adsorption onto biochars [78,103], activated carbon [123,141], clay-biochar composite [10,8], metal organic frameworks [83,147], modified biosorbents [157], and polymers [7,80], were majorly favorable as illustrated by decrease in ΔGo values occasioned by temperature increment. Furthermore, the negative ΔGo observed here indicates that NRFX uptake using these adsorbents was spontaneous at all temperature levels. This spontaneous and favorable outlook could be as a result of multi adsorption sites provided by oxygen loaded functional groups suitable for NRFX uptake [78,103]. A significantly different trend was observed for ferrous sorbents [54,139], where NRFX uptake was highlighted to be unfavorable even though the process maybe spontaneous. This implies NRFX uptake by magnetic adsorbents would be triggered by an external influence [54,186]. Non-spontaneity was observed by the use of synthetic polymers (PS, PBS, PE) indicating that NRFX adsorption requires energy to promote the process [7,80].
Table 5.
Thermodynamic parameters.
| Adsorbent | Thermodynamic parameters |
Ref. | |||
|---|---|---|---|---|---|
| Temp (K) | Δ | Δ | Δ | ||
| CoFe2O4@TiO2-MMIP | 323 | –2.28 | 11.35 | 40 | [85] |
| Bentonite enhanced sludge biochar composite | 313 | − 9.33 | 6.03 | 49.06 | [8] |
| Loofah activated carbon | 298 | − 5.97 | – | – | [72] |
| modified sludge biochar | 308 | −7.23 | 3.84 | 0.0359 | [133] |
| Potential of polystyrene (PS) | 288 | 9.005 | 10.341 | 4.8 | [7] |
| Polybutylene succinate (PBS) | 298 | 9.945 | 3.541 | −21.3 | [7] |
| Polyethylene (PE) | 288 | 12.799 | 30.312 | 62.0 | [7] |
| iron ore waste | 308.15 | −33.01 | −1.09 | 103.59 | [15] |
| rice (Oryza sativa) husk wastes | 338 | −48.07 | 41.55 | 263.50 | [16] |
| coffee (Coffea arabica) husk wastes | 338 | −45.33 | 4.858 | 148.40 | [16] |
| Plant based biochar | 308.15 | − 4.5909 | 32.194 | 0.1196 | [78] |
| Mg/Fe bimetallic hydroxide | 298 | − 5.5400 | −41.41 | 0.12 | [127] |
| graphene oxide/magnetite composites | 308 | −20.49 | −19.17 | 4.30 | [134] |
| clay-biochar composite | 308.15 | −1.924 | 22.50 | 0.079 | [10] |
| Biochar | 288.15 | 2.886 | 28.94 | 0.091 | [10] |
| pyrite-biochar composites | 288.15 | −20.5437 | −31.9914 | −39.6 | [139] |
| hematite-biochar composites | 308.15 | −17.2404 | 49.2746 | 216.8 | [139] |
| ZIF-8 metal-organic framework | 313 | −6.27 | 5.68 | 39.00 | [147] |
| Fe-MCM-41 molecular sieves | 308 | −5.09 | 13.92 | 61.73 | [101] |
| Mg–Al layered double hydroxides (CO3−MA21) | 318 | −17.61 | −3.03 | 45.87 | [136] |
| Mg–Al layered double hydroxides (Cl-MA21) | 318 | −20.50 | −27.65 | −22.21 | [136] |
| KOH modified biochar | 308 | −15.54 | 6.13 | 70 | [158] |
| mesoporous metallic silicon (Cu/SBA-15) | 308 | −2.60 | 10.28 | 41.82 | [128] |
| mesoporous metallic silicon (Fe/SBA-15) | 288 | −2.39 | −7.27 | −16.96 | [128] |
| magnetic biochar | 308 | –3.79 | 38.41 | 136.94 | [20] |
| luffa sponge biochar | 308 | –30.55 | –8.6 | –126 | [159] |
| MOF@GO composite | 310 | –13,324.33 | –0.000011 | –306.735 | [83] |
| fly ash | 288 | –20.857 | –35.541 | –51 | [122] |
| Acid-modified fly ash | 288 | –20.594 | –27.372 | –24 | [122] |
| Alkali-modified fly ash | 288 | –21.573 | –30.804 | –32 | [122] |
| Cassava Dreg Biochar | 308 | –78.60 | 326.40 | 1320 | [160] |
| Fe/Cu oxides composite | 313 | –4.46 | 1.47 | 18.94 | [108] |
| microporous activated carbon | 303 | –12.604 | –30.362 | –58.53 | [123] |
| iron-trimesic metal-organic frameworks (Fe-BTC) | 318 | −12.935 | 5.903 | 59.24 | [112] |
| Carbonize Moringa pod husks | 328 | −32.091 | −23.919 | 24.443 | [141] |
| Ammonium chloride-treated | 328 | −31.377 | −13.203 | 5.662 | [141] |
| Amino-functionalized Zr-based MOF | 318 | −8.76 | 8.401 | 54.014 | [9] |
| Graphene oxide | 304.15 | −7.784 | −13.141 | −18 | [17] |
| KOH-modified biochar | 308 | −21.06 | 31.26 | 17.00 | [158] |
| Polydopamine microspheres | 318 | –17.4 | 7.30 | 77.6 | [80] |
| BC | 318.15 | –1.9386 | –10.5651 | 39.3865 | [104] |
| γ-Fe2O3@BC | 318.15 | 2.5136 | 6.9232 | 29.5070 | [104] |
| Fe/Cu oxides composite | 313 | –4.32 | 2.72 | 22.5 | [120] |
| Acid activated termite feces | 328 | –3.54 | 62.62 | 198.7 | [5] |
| N-doped activated carbon | 318 | –37.83 | 34.73 | 230 | [102] |
| BC | 308 | − 18.54 | 16.97 | 115.3 | [8] |
| Magnetic BC/MnO2 composite | 308 | − 24.61 | 18.12 | 138.9 | [8] |
Enthalpy calculations specifies the nature and type of the adsorption process. Among all the adsorbents listed in Table 5 metallic compounds mainly exhibited exothermic (Ho<0) nature, unlike other sorbants [8,101,169] where endothermic (Ho > 0) nature dominated the norfloxcin adsorption process. One probable justification for this endothermicity in terms of adsorption enthalpy is the inevitable loss of hydration layer from NRFX molecule at the point of adsorption [103,158]. This dehydration phenomenon utilizes more energy than exothermicity of NRFX molecules attached to the surface [158]. Similarly, NRFX uptake was observed to be predominantly physical type, with very few reports indicating chemisorption [160]. According to Chen et al., [187], the heat given off during physisorption process is identical in scale as the heat of condensation (Heat = 2.1 - 20.9 kJ/mol; Energy = −20 to 0 kJ/mol), while chemisorption energy and heat demands are found within the range of −80 to −400 kJ/mol and 80 - 200 kJ/mol, respectively. Given that physical adsorption dominates, it implies that the driving force is electrostatic (Van der Waals’ force) interaction, where NRFX molecules are not restricted to a specific location on the adsorbent surface [7,8].
Entropy changes (ΔSo) signifies the degree of disorderliness (randomness) and also depicts the capacity of any adsorbent to remove the targeted adsorbate [186]. The entropy sign convention gives a hint of either increased randomness (ΔSo > 0) or reduced randomness (ΔSo < 0) of an adsorption process. From the change in entropy values outlined in Table 5, most adsorbents displayed strong affinity for NRFX adsorbate as illustrated by the positive entropy values. This phenomenon is attributed to the formation of well structured active sites following special functionalization procedures which aided efficient sequestration of NRFX on the surface of the adsorbent [112,160]. A few reports of reduced randomness were indicated by application of biochar [3,91], activated carbon [123], ferrors sorbents [139], MOF [83], metallic compounds [59], and synthetic polymer [7]. This reduced randomness results from NRFX adsorption difficulties, which implies that the adsorbate molecules even after the adsorption process may likely remain in the bulk effluent. This drawback could be eliminated either by improving the adsorbent preparation step to produce better structured adsorbents, or ensuring that no other effluent is competing with the adsorbate of interest for occupation of active sites.
Generally, from analysis of results obtained from temperature effects, it is evident that NRFX adsorption is a physisorption process that is significantly spontaneous with increased surface randomness characterized by either exothermic (for non-metallic adsorbents) or endothermic nature (for metallic adsorbents).
Competitive adsorption
Since most wastewater contains some salts, the presence of these salts or co-ions in the solution may have an effect on the adsorption process. A competitive adsorption study is used to determine the ability of an adsorbent to selectively adsorb an adsorbate in the presence of other species vying for the available active sites on the surface of the adsorbent [162,163]. Determining the suitability of an adsorbent for practical wastewater treatment requires such a study [164].
The results of various experiments investigating the affinity of various adsorbents towards NRFX and other contaminants are shown in Table 6. In most of the studies, it can be seen that the presence of these other species led to a reduction in the absorption of NRFX. But some of them increased the removal impact [9,104,159], while others had no effect [139,165]. In the adsorption of NRFX using pomelo peel-based biochar and γ-Fe2O3-loaded biochar (γ-Fe2O3/BC) [104], it was discovered that the presence of Na+ reduced the adsorption of NRFX by the biochar adsorbent, and the reduction was stronger as the concentration of the Na+ increases (from 0.02 to 0.10 mol/L). This is due to the fact that Na+ may engage in cation exchange and rival NRFX for active binding sites on the biochar surface. A weaker H-bond will result between the biochar surface and NRFX as a result of the additional Na+ partially replacing the protons of the biochar surface. In contrast, adding Na+ increased the adsorption of NRFX by γ-Fe2O3/BC. This is because the ionic form of NRFX in water will be reduced by the additional NaCl, a process known as “salt-out-effect”, and will precipitate into the molecular form of NRFX. The γ-Fe2O3/BC is then coupled with the NRFX of the molecular state via hydrophobic action to increase its adsorption capacity.
Table 6.
Summary of competitive adsorption NRFX.
| Adsorbent | Competing species | Maximum change with competing species | Ref. |
|---|---|---|---|
| Nanocomposite optosensor | Ciprofloxacin | No change | [165] |
| Magnetic molecularly imprinted polymer | K+, Na+ | Little influence | [85] |
| Magnetic molecularly imprinted polymer | Cu2+ | 41.5% reduction | [85] |
| Magnetic molecularly imprinted polymer | Fe2+ | 84.3%reduction | [85] |
| Magnetic molecularly imprinted polymer | Mn+, Al3+, Ca2+, Mg2+ | Decrease | [85] |
| N-doped/activated cocoa shell biochar | Na+, Ca2+ | Decrease | [98] |
| UIO-66-NH2 nanomaterials | NaCl, CaCl2 | Increase | [9] |
| Spent coffee ground-derived biochar | Na+, Ca2+ | Decrease | [129] |
| Bentonite/sludge biochar composite | Na+ | Increase | [8] |
| Polydopamine microspheres | CaCl2, NaCl | Decrease | [80] |
| Polystyrene microplastic | NaCl | 76.8% decrease | [7] |
| Polybutylene succinate microplastic | NaCl | 84.7% decrease | [7] |
| Polyethylene microplastic | NaCl | 90.9% decrease | [7] |
| Dissolved organic matter | Na+ | Decrease | [4] |
| Surface molecularly imprinted polymer | Enrofloxacin, ciprofloxacin, sulfonamide | Increase | [119] |
| Magnetic biochar from furfural residue | Chloramphenicol, ciprofloxacin, tetracycline | Decrease | [114] |
| Microplastics | Humic acid | 75.3% decrease | [12] |
| Iron ore waste | Na+ | 25.4% decrease | [15] |
| Iron ore waste | Ca+ | 45.3% decrease | [15] |
| Iron ore waste | Mg2+ | 61% decrease | [15] |
| Hematite/biochar composite | Ca2+ | Increased at low concentration of Ca2+ but decreased at high concentration | [139] |
| Pyrite/biochar composite | Ca2+ | Negligible effect | [139] |
| Amine-functionalized magnetic bamboo-based AC | NaCl | Slight increase | [34] |
| Fe-MCM-41 | Na+, Ca2+ | Decrease | [155] |
| Pomelo peel-based biochar | Na+ | Decrease | [104] |
| γ-Fe2O3/biochar | Na+ | Increase | [104] |
| Fe/Cu oxides nanocomposites | NaCl, CaCl2 | Decrease | [103] |
| Magnetic biochar-based/manganese oxide composite | CaCl2 | Slight decrease | [138] |
| Zinc oxide/zinc sulfide composite | Fe2+ | 10% increase | [20] |
| Zinc oxide/zinc sulfide composite | Na+, Cu2+, Zn2+ | Na+= Slight (2%) decrease, Cu2+ = 99% decrease, Zn2+ = 62% decrease | [20] |
| Zinc oxide/zinc sulfide composite | CO32− | Slight increase | [20] |
| Zinc oxide/zinc sulfide composite | SO42−, NO3−, Cl−, C6H5O73− | Decrease | [20] |
| Polydopamine microspheres | NaCl, CaCl2 | Decrease | [80] |
| 3D pretreated peanut shell/graphene oxide | CaCl2 | 56% decrease | [118] |
| 3D pretreated peanut shell/graphene oxide | NaCl | 16.4% decrease | [118] |
| Pomelo peel-derived AC/phosphoric acid | Na+ | Slight decrease | [126] |
| Pomelo peel-derived AC/phosphoric acid | Mg2+, NO−, SO42+ | Sharp decrease | [126] |
| Temperature-switchable graphene oxide | Humic acid | Increase | [159] |
| Magnetic biochar from salix mongolica | NaCl, CaCl2 | Decrease | [120] |
| Fe/Cu oxides composite particles | CaCl2 | 33% decrease | [148] |
| Fe/Cu oxides composite particles | NaCl | 9% decrease | [148] |
Liu, He [20] investigated the effect of competing ions for NRFX binding on the surface of a carbon-based composite photocatalytic material of zinc oxide and zinc sulfide (ZnO/ZnS@BC) using several cations (SO42+, NO3−, Cl−, CO32−, C6H5O73−). The result revealed that NRFX degraded at varying rates in different ion solutions. For the cations, Fe2+ slightly enhanced the removal effect, while Na+ had almost no effect (only decreased by 2%). However, the removal effectiveness was significantly inhibited by Cu2+ and Zn2+ to the extent of 99% and 62%, respectively. This is due to the fact that NRFX caused the formation of new, structurally stable, and difficult-to-decompose clathrate in the presence of the cations. On the other hand, all the anions (with the exception of CO32−) hindered the removal effect, with NO3− showing the highest rate of inhibition (34%). CO32− slightly promoted the removal of NRFX by ameliorating the pH of the solution [20].
Column adsorption
The adsorption of NRFX can be carried out in either a batch or continuous system. The latter is usually carried out with the aid of a fixed bed column [166,167]. Fixed-bed adsorption is more advantageous than batch adsorption as it is more effective and efficient in treating large quantities of polluted solutions, thus making the transition from laboratory to industrial treatment easier [168]. The pH of the environment, the height of the adsorbent bed, the NRFX flow rate in the column, the concentration of NRFX in the solution, the adsorbent quantity, and the number of regeneration cycles of the adsorbent bed all influence NRFX adsorption in a column. The summary of studies done by various authors for fixed bed adsorption of NRFX and the results obtained is given in Table 7.
Table 7.
Summary of column adsorption for NRFX adsorption from aqueous media.
| Adsorbent | Best-fit model | Conditions |
Adsorption Capacity (mg/g) | Rate constant (l/min.mg) | Refs. | ||
|---|---|---|---|---|---|---|---|
| Bed height (cm) | Vol. flow rate (mL/min) | Inlet Conc. (mg/L) | |||||
| Phoenix dactylifera L. based activated carbon | Thomas and Yoon-Nelson | 25 | 0.5 | 150 | 1.992 | [169] | |
| Amine-functionalized magnetic bamboo-based AC | – | 60 | 0.5 | 300 | 315.7 | – | [33] |
| Magnetic bamboo-based AC | – | 60 | 0.5 | 300 | 193.4 | – | [33] |
| Granular AC | – | 25 | 1.5 | 225 | – | – | [170] |
Darweesh and Ahmed [169] studied the uptake of NRFX onto granular activated carbon (GAC) in a continuous packed bed column by varying the process parameters in a bid to obtain the optimum conditions for NRFX uptake. The volumetric flow rate was adjusted from 0.5 to 1.5 mL/min, the bed height was adjusted from 15 to 25 cm, and the initial concentration was adjusted from 75 to 250 mg/L. Results showed that the higher the flow rate, the lesser the interaction between GAC and NRFX, which would lead to a lower uptake capacity. The increase in bed height translates to more contact and diffusion time between NRFX and the pores of GAC. An increase in inlet concentration was noted to affect the uptake capacity the most in relation with other process variables, as an increase in NRFX concentration decreases the contact time between NRFX and GAC and also ensures improved filling of the adsorption site of the activated carbon [169]. The potential of using amine-functionalized magnetic bamboo-based activated carbon and non-functionalized magnetic bamboo-based activated carbon as adsorbents for NRFX uptake under similar conditions was explored by Peng, Hu [34]. The study revealed that since NRFX is a π-electron donor, the presence of the amine functional group, which is a π-electron acceptor on the surface of the adsorbent, contributed to the higher adsorption of the functionalized adsorbent [34].
Desorption and regeneration
A desorption/reusability study is usually carried out to determine how many times an adsorbent can be recycled and reused before its efficacy is jeopardized [143,171]. An adsorbent can be recovered by using an eluent to remove the NRFX from its surface. The percentage difference between the regenerated adsorbents' and the initial adsorbent's adsorption capacity is known as the adsorbent recovery [172,173]. This is especially significant because it reduces the cost of the process. Adsorption is typically more cost-effective if the adsorbent can be collected and recycled. The summary of several studies on the desorption and reusability of different adsorbents used for the adsorption of NRFX is shown in Table 8. It was observed that after 5 cycles, most of the adsorbents still had the ability to adsorb more than 75% of the initial concentration of NRFX. This shows the high recyclability of such adsorbents. The highest recovery of 97.10% after four regeneration cycles was obtained by Qin, Zhao [140] with the use of molecularly imprinted silica polymer and methanol/ethanol mixture as the eluent. For this to occur, different solvents have been utilized as eluents, with methanol and ethanol being the most commonly used. Yi, Liang [125] studied the desorption potential of nitrogen-rich Calotropis gigantean fiber using four different eluents. The eluents and their percentage desorption include aqueous sodium hydroxide (83%), hydrochloric acid (55%), sodium chloride (17%), and methanol (5%). This shows the efficiency of sodium hydroxide for the recyclability of NRFX-saturated adsorbents. The efficacy of sodium hydroxide as an eluent was also made in a study that reviewed that desorption/reusability of another antibiotics – levofloxacin [143]. In another study, Khan, Shaheen [83] observed that after three cycles of regeneration, graphene oxide/MOF based composite adsorbent gave a removal efficiency of 88.41%.
Table 8.
Desorption and reusability of various adsorbents for NRFX adsorption from aqueous media.
| Adsorbent | Elution agent | % qm after 1st cycle | Number of cycles (n) | % qm retained after n cycles | Refs. |
|---|---|---|---|---|---|
| Magnetic halloysite nanotubes/molecularly imprinted polymer composite | – | 90.30 | 7 | 85.60 | [109] |
| Molecularly imprinted polymer | HCOOH/CH3OH (1:1) | 90.00 | 6 | ˃ 85.00 | [81] |
| Amino-functionalized Zr-based MOF | 0.1 mol/L NaOH | ˃ 95.00 | 5 | ˃ 80.00 | [9] |
| Nitrogen-rich Calotropis gigantean fiber | 0.1 mol/L NaOH | – | 5 | ˃ 75.00 | [125] |
| 3D peanut shell/graphene oxide | C2H5OH(aq) | 89.73* | 5 | 77.52* | [118] |
| Natural attapulgite-potato stem hybrid biochar | CH3OH | 84.20 | 5 | 82.00 | [10] |
| Sawdust biochar/Al2(SO4)3-modified thermal kaolin | CH3OH/H2O | – | 5 | 80.20 | [1] |
| Amine-functionalized magnetic bamboo-based AC | C2H5OH(aq) | – | 5 | 80.00 | [34] |
| Molecularly imprinted silica polymer | CH3OH/ CH3COOH (9:1 v/v) | – | 4 | 97.10 | [140] |
| Magnesium oxide/Chitosan/Graphene oxide | CH3OH | – | 4 | ˃ 80.00 | [92] |
| Surface-imprinted polymer microsphere | CH3OH/ CH3COOH | 83.20* | 4 | 77.48* | [119] |
| Magnetic molecularly imprinted polymer composite | CH3OH/ CH3COOH | 90.00 | 4 | 75.50 | [132] |
| Graphene oxide/MOF based composite | C2H5OH/HCl (10:1) | 93.61 | 3 | 88.40 | [83] |
| Layered chalcogenide | 1.0 M KCl | 95.00 | 3 | ˃ 90.00 | [115] |
| Magnetic potato stems and leaves biochar-based manganese oxide composite | – | 98.00 | 3 | 95.00 | [138] |
= mg/g.
Computer simulation of NRFX removal
Many approaches for molecular modeling of adsorption processes have been developed, including the force field method, quantum chemistry method, Monte Carlo method, and molecular dynamics method [174]. Molecular simulation employs a computer to construct molecular modeling at the atom level, which can then simulate the physical, chemical, and biological properties of molecular systems. Several studies have investigated various aspects of NRFX adsorption from aqueous solutions using a range of molecular modeling methodologies and computational tools. Li and Fang [174] utilised a computational chemistry tool called Hyperchem 8.0 for simulating molecule conformation and binding, and molecular mechanics optimiser was used to find the best molecular conformation. The total energies of the molecules were estimated after simulating the molecular conformations of the molecular imprinted particles (MIPs) with NRFX. The binding dynamics of NRFX to MIPs were investigated using molecular modeling and confirmed by binding studies. The energy changes of the molecules throughout the MIPs' binding process with NOR were computed, and the results were utilised to forecast the binding efficiency towards these molecules. There were two types of NRFX binding sites discovered on the molecular imprinted particles (MIPs). The first included hydrogen bonds between the oxygen atoms of MIPs and the carbonyl group of NRFX, while the second involved hydrogen bonds between the oxygen atoms of MIPs and the hydroxyl group of NRFX. Furthermore, the molecules' energies change was 1.69×106 J/mol. The results showed that the MIPs had good NRFX selectivity.
Dan, Li [118] investigated the adsorption of NRFX onto a 3D peanut shell/graphene oxide aerogel using DFT. Various interactions were involved in the adsorption of NRFX by 3D PPS/GO aerogel, including electrostatic attraction, H-bonding, π-π conjugation, and hydrophobic effect. Zhang, Cheng [175] used DFT calculations to determine the adsorption mechanism between sesame straw biochar (935 m2g−1) and NRFX; computational simulations revealed that interactions between the biochar and NRFX were electrostatic interaction, hydrogen bond, and - interaction, all of which were conducive to chemical adsorption. The energy of adsorption for NRFX with biochar in water was 0.3977 Hartree. The higher the energy of adsorption, the more stable the adsorption arrangement between adsorbate and adsorbent. Yadav, Goel [176] also performed DFT calculations to determine the thermodynamic feasibility of the adsorption process and the nature of interactions of NRFX with the graphene oxide; the negative values of adsorption energy suggest the process's thermodynamic feasibility.
Additionally, computational investigations for NRFX uptake onto graphene oxides [177], graphene oxide/chitosan composites [178], and NRFX molecularly imprinted polymers [179] have been described.
Knowledge gaps and visions for the future
On the basis of this study of the literature, various knowledge gaps are highlighted below. Despite the recent publication of a great quantity of material on the absorption of NRFX via adsorption, numerous unresolved issues still exist and warrant further study. It is not possible to scale up a process for industrial use without investigations on column studies. Because fixed-bed adsorption is more effective and efficient at handling large amounts of contaminated solutions, it is preferable to batch adsorption. For column studies, just three researches were observed. Rarely is the waste management research on utilized adsorbents that will be discharged back into the environment highlighted in literature. It is advised that researchers consider alternatives to the usual combustion method for disposing of these adsorbents. Numerous academics have also noted a similar dearth of disposal studies [38,39,180]. For financial considerations and potential future applications of the described adsorbents, the budget of NFRX removal from pharmaceutical/hospital effluents is crucial.
Conclusion
This review's objective is to evaluate empirical data on NRFX's removal from aqueous medium. The environmental danger of IBP in the aquatic environment was validated by an initial ecotoxicological study. It is on the premise of the wide availability of research on NRFX adsorption that this review was conceived. Composite based adsorbents gave the highest NRFX adsorption capacities (Qmax) value for NRFX uptake, with Graphene oxide/ MOF giving 1114.8 mg/g. The type of adsorbent and the reactivity of the solution affect the adsorption process. The main adsorption mechanisms for NRFX uptake include electrostatic interactions, H-bonds, π–π interactions, electron donor-acceptor interactions, hydrophobic interactions, and pore diffusion. Langmuir isotherm and pseudo-second order kinetics models were superior in the prediction of NRFX which features adsorbate-to-adsorbent electron transfer on monolayer surface. The thermodynamics of NRFX adsorption is heavily dependent on the makeup of the adsorbent, and the selection of the eluent for desorption from the solid phase is equally important. The paper also examined several computational techniques that have been used by other studies to remove NRFX. There were detected knowledge gaps in adsorbent disposal and column investigations. Every research project will eventually be scaled up for commercial environmental protection applications.
Ethics statements
Not applicable.
CRediT authorship contribution statement
Paschal Enyinnaya Ohale: Conceptualization, Writing – original draft, Writing – review & editing, Validation, Visualization. Chinenye Adaobi Igwegbe: Conceptualization, Supervision, Data curation, Writing – original draft, Writing – review & editing, Visualization. Kingsley O. Iwuozor: Writing – original draft, Writing – review & editing, Validation, Visualization. Ebuka Chizitere Emenike: Writing – original draft, Writing – review & editing, Validation, Visualization. Christopher Chiedozie Obi: Writing – original draft, Writing – review & editing, Validation, Visualization. Andrzej Białowiec: Supervision, Writing – review & editing, Validation, Visualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Contributor Information
Chinenye Adaobi Igwegbe, Email: ca.igwegbe@unizik.edu.ng, chinenyeigwegbe@upwr.edu.pl.
Kingsley O. Iwuozor, Email: kingsleyiwuozor5@gmail.com.
Data Availability
No data was used for the research described in the article.
References
- 1.Yang Y., et al. Efficient with low-cost removal and adsorption mechanisms of norfloxacin, ciprofloxacin and ofloxacin on modified thermal kaolin: experimental and theoretical studies. J. Hazard. Mater. 2022;430 doi: 10.1016/j.jhazmat.2022.128500. [DOI] [PubMed] [Google Scholar]
- 2.Zelaya Soulé M.E., et al. Carbon/montmorillonite hybrids with different activation methods: adsorption of norfloxacin. Adsorption. 2019;25(7):1361–1373. [Google Scholar]
- 3.Feng Y., et al. Norfloxacin removal from aqueous solution using biochar derived from luffa sponge. J. Water Supply: Res. Technol.-AQUA. 2018;67(8):703–714. [Google Scholar]
- 4.Wang Y., et al. Effects of dissolved organic matter on the adsorption of norfloxacin on a sandy soil (fraction) from the Yellow River of Northern China. Sci. Total Environ. 2022;848 doi: 10.1016/j.scitotenv.2022.157495. [DOI] [PubMed] [Google Scholar]
- 5.Chahm T., et al. Use of chemically activated termite feces a low-cost adsorbent for the adsorption of norfloxacin from aqueous solution. Water Sci. Technol. 2019;79(2):291–301. doi: 10.2166/wst.2019.052. [DOI] [PubMed] [Google Scholar]
- 6.Nayak A., Bhushan B., Kotnala S. Fabrication of chitosan-hydroxyapatite nano-adsorbent for removal of norfloxacin from water: isotherm and kinetic studies. Mater. Today: Proc. 2022;61:143–149. [Google Scholar]
- 7.Sun M., et al. Adsorption behaviors and mechanisms of antibiotic norfloxacin on degradable and nondegradable microplastics. Sci. Total Environ. 2022;807 doi: 10.1016/j.scitotenv.2021.151042. [DOI] [PubMed] [Google Scholar]
- 8.Cao X., et al. Co-adsorption capabilities and mechanisms of bentonite enhanced sludge biochar for de-risking norfloxacin and Cu2+ contaminated water. Chemosphere. 2022;299 doi: 10.1016/j.chemosphere.2022.134414. [DOI] [PubMed] [Google Scholar]
- 9.Fang X., et al. High-efficiency adsorption of norfloxacin using octahedral UIO-66-NH2 nanomaterials: dynamics, thermodynamics, and mechanisms. Appl. Surf. Sci. 2020;518 [Google Scholar]
- 10.Li Y., et al. Removal of Norfloxacin from aqueous solution by clay-biochar composite prepared from potato stem and natural attapulgite. Colloids Surf. A. 2017;514:126–136. [Google Scholar]
- 11.Feng M., et al. Metal-mediated oxidation of fluoroquinolone antibiotics in water: a review on kinetics, transformation products, and toxicity assessment. J. Hazard. Mater. 2018;344:1136–1154. doi: 10.1016/j.jhazmat.2017.08.067. [DOI] [PubMed] [Google Scholar]
- 12.Zhang, Y., et al., Mechanistic insight into different adsorption of norfloxacin on microplastics in simulated natural water and real surface water. Environ. Pollut., 2021. 284: p. 117537. [DOI] [PubMed]
- 13.Parashar A., Sikarwar S., Jain R. Studies on adsorption kinetics of norfloxacin using nano alumina in aqueous medium. Anal. Chem. Lett. 2020;10(2):227–239. [Google Scholar]
- 14.Lebron Y.A.R., et al. Statistical physics modeling and optimization of norfloxacin adsorption onto graphene oxide. Colloids Surf. A. 2020;606 [Google Scholar]
- 15.Fang N., et al. Toward broader applications of iron ore waste in pollution control: adsorption of norfloxacin. J. Hazard. Mater. 2021;418 doi: 10.1016/j.jhazmat.2021.126273. [DOI] [PubMed] [Google Scholar]
- 16.Paredes-Laverde M., Silva-Agredo J., Torres-Palma R.A. Removal of norfloxacin in deionized, municipal water and urine using rice (Oryza sativa) and coffee (Coffea arabica) husk wastes as natural adsorbents. J. Environ. Manage. 2018;213:98–108. doi: 10.1016/j.jenvman.2018.02.047. [DOI] [PubMed] [Google Scholar]
- 17.Moreira V.R., et al. Graphene oxide in the remediation of norfloxacin from aqueous matrix: simultaneous adsorption and degradation process. Environ. Sci. Pollut. Res. Int. 2020;27(27):34513–34528. doi: 10.1007/s11356-020-09656-6. [DOI] [PubMed] [Google Scholar]
- 18.Azhar M.R., et al. Adsorptive removal of antibiotic sulfonamide by UiO-66 and ZIF-67 for wastewater treatment. J. Colloid Interface Sci. 2017;500:88–95. doi: 10.1016/j.jcis.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 19.de Souza D.I., et al. Nanofiltration for the removal of norfloxacin from pharmaceutical effluent. J. Environ. Chem. Eng. 2018;6(5):6147–6153. [Google Scholar]
- 20.Liu W., et al. Synergistic adsorption-photocatalytic degradation effect and norfloxacin mechanism of ZnO/ZnS@ BC under UV-light irradiation. Sci. Rep. 2020;10(1):1–12. doi: 10.1038/s41598-020-68517-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He J., et al. Understanding and characteristics of coagulation removal of composite pollution of microplastic and norfloxacin during water treatment. Sci. Total Environ. 2022;831 doi: 10.1016/j.scitotenv.2022.154826. [DOI] [PubMed] [Google Scholar]
- 22.Balarak D., et al. Survey electrocoagulation process in removal of norfloxacin antibiotic from aqueous solutions. J. Pharmaceutical Res. Int. 2020;32(3):53–60. [Google Scholar]
- 23.Baran W., et al. Removal of veterinary antibiotics from wastewater by electrocoagulation. Chemosphere. 2018;194:381–389. doi: 10.1016/j.chemosphere.2017.11.165. [DOI] [PubMed] [Google Scholar]
- 24.Bajpai M., et al. A review on electrocoagulation process for the removal of emerging contaminants: theory, fundamentals, and applications. Environ. Sci. Pollut. Res. Int. 2022;29:1–30. doi: 10.1007/s11356-021-18348-8. [DOI] [PubMed] [Google Scholar]
- 25.Tian Y., et al. 0D/3D coupling of g-C3N4 QDs/hierarchical macro-mesoporous CuO-SiO2 for high-efficiency norfloxacin removal in photo-Fenton-like processes. J. Hazard. Mater. 2021;419 doi: 10.1016/j.jhazmat.2021.126359. [DOI] [PubMed] [Google Scholar]
- 26.Yu D., et al. Mineralization of norfloxacin in a CoFe–LDH/CF cathode-based heterogeneous electro-fenton system: preparation parameter optimization of the cathode and conversion mechanisms of H2O2 to· OH. Chem. Eng. J. 2021;417 [Google Scholar]
- 27.Prabavathi S.L., et al. Construction of heterostructure CoWO4/g-C3N4 nanocomposite as an efficient visible-light photocatalyst for norfloxacin degradation. J. Ind. Eng. Chem. 2019;80:558–567. [Google Scholar]
- 28.Zhang H., et al. Preparation of Ce4+-doped BaZrO3 by hydrothermal method and application in dual-frequent sonocatalytic degradation of norfloxacin in aqueous solution. Ultrason. Sonochem. 2018;42:356–367. doi: 10.1016/j.ultsonch.2017.11.043. [DOI] [PubMed] [Google Scholar]
- 29.Pan Y., et al. Norfloxacin pollution alters species composition and stability of plankton communities. J. Hazard. Mater. 2020;385 doi: 10.1016/j.jhazmat.2019.121625. [DOI] [PubMed] [Google Scholar]
- 30.Wan L., et al. Effect of norfloxacin on algae–cladoceran grazer–larval damselfly food chains: algal morphology-mediated trophic cascades. Chemosphere. 2020;256 doi: 10.1016/j.chemosphere.2020.127166. [DOI] [PubMed] [Google Scholar]
- 31.Pan Y., et al. Lethal/sublethal responses of Daphnia magna to acute norfloxacin contamination and changes in phytoplankton-zooplankton interactions induced by this antibiotic. Sci. Rep. 2017;7(1):1–10. doi: 10.1038/srep40385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang X., et al. Effects of norfloxacin nicotinate on the early life stage of zebrafish (Danio rerio): developmental toxicity, oxidative stress and immunotoxicity. Fish Shellfish Immunol. 2020;96:262–269. doi: 10.1016/j.fsi.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Peng X., et al. Amine-functionalized magnetic bamboo-based activated carbon adsorptive removal of ciprofloxacin and norfloxacin: a batch and fixed-bed column study. Bioresour. Technol. 2018;249:924–934. doi: 10.1016/j.biortech.2017.10.095. [DOI] [PubMed] [Google Scholar]
- 34.Sharma V., et al. Integrated adsorption-membrane filtration process for antibiotic removal from aqueous solution. Powder Technol. 2017;321:259–269. [Google Scholar]
- 35.Wu H., et al. Retentions of bisphenol A and norfloxacin by three different ultrafiltration membranes in regard to drinking water treatment. Chem. Eng. J. 2016;294:410–416. [Google Scholar]
- 36.Dolar D., et al. Photolysis of enrofloxacin and removal of its photodegradation products from water by reverse osmosis and nanofiltration membranes. Sep. Purif. Technol. 2013;115:1–8. [Google Scholar]
- 37.Oba S.N., et al. Removal of ibuprofen from aqueous media by adsorption: a comprehensive review. Sci. Total Environ. 2021;780 doi: 10.1016/j.scitotenv.2021.146608. [DOI] [PubMed] [Google Scholar]
- 38.Igwegbe C.A., et al. Adsorption of ciprofloxacin from water: a comprehensive review. J. Ind. Eng. Chem. 2021;93:57–77. [Google Scholar]
- 39.Lippi M., et al. State of art of landfill leachate treatment: literature review and critical evaluation. Ciência e Natura. 2018:e78. [Google Scholar]
- 40.Mora-Gómez J., et al. Electrochemical degradation of norfloxacin using BDD and new Sb-doped SnO2 ceramic anodes in an electrochemical reactor in the presence and absence of a cation-exchange membrane. Sep. Purif. Technol. 2019;208:68–75. [Google Scholar]
- 41.Shankaraiah G., et al. Degradation of antibiotic norfloxacin in aqueous solution using advanced oxidation processes (AOPs)—a comparative study. Desalination Water Treat. 2016;57(57):27804–27815. [Google Scholar]
- 42.Bartolomeu M., et al. Wastewater chemical contaminants: remediation by advanced oxidation processes. Photochem. Photobiol. Sci. 2018;17(11):1573–1598. doi: 10.1039/c8pp00249e. [DOI] [PubMed] [Google Scholar]
- 43.Serna-Galvis E.A., et al. Degradation of seventeen contaminants of emerging concern in municipal wastewater effluents by sonochemical advanced oxidation processes. Water Res. 2019;154:349–360. doi: 10.1016/j.watres.2019.01.045. [DOI] [PubMed] [Google Scholar]
- 44.Matavos-Aramyan S., Moussavi M. Advances in Fenton and Fenton based oxidation processes for industrial effluent contaminants control-a review. Int. J. Environ. Sci. Nat. Resources. 2017;2(4):1–18. [Google Scholar]
- 45.Klavarioti M.D., et al. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 2009;35(2):402–417. doi: 10.1016/j.envint.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 46.Li J., et al. Fabrication of FeVO4/Fe2TiO5 composite catalyst and photocatalytic removal of norfloxacin. Chem. Eng. J. 2016;298:300–308. [Google Scholar]
- 47.García-Muñoz P., et al. Evaluation of photoassisted treatments for norfloxacin removal in water using mesoporous Fe2O3-TiO2 materials. J. Environ. Manage. 2019;238:243–250. doi: 10.1016/j.jenvman.2019.02.109. [DOI] [PubMed] [Google Scholar]
- 48.Ahmadi S., et al. Sono electro-chemical synthesis of LaFeO3 nanoparticles for the removal of fluoride: optimization and modeling using RSM, ANN and GA tools. J. Environ. Chem. Eng. 2021;9(4) [Google Scholar]
- 49.Liao W., et al. Microwave-enhanced photolysis of norfloxacin: kinetics, matrix effects, and degradation pathways. Int. J. Environ. Res. Public Health. 2017;14(12):1564. doi: 10.3390/ijerph14121564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Souza Santos L.V., Meireles A.M., Lange L.C. Degradation of antibiotics norfloxacin by Fenton, UV and UV/H2O2. J. Environ. Manage. 2015;154:8–12. doi: 10.1016/j.jenvman.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 51.Lu J., et al. Kinetics, structural effects and transformation pathways for norfloxacin oxidation using the UV/chlorine process. J. Water Process. Eng. 2021;44 [Google Scholar]
- 52.Su R., et al. Chlorine dioxide radicals triggered by chlorite under visible-light irradiation for enhanced degradation and detoxification of norfloxacin antibiotic: radical mechanism and toxicity evaluation. Chem. Eng. J. 2021;414 [Google Scholar]
- 53.Carrillo-Abad J., et al. Effect of the CuO addition on a Sb-doped SnO2 ceramic electrode applied to the removal of Norfloxacin in chloride media by electro-oxidation. Chemosphere. 2020;249 doi: 10.1016/j.chemosphere.2020.126178. [DOI] [PubMed] [Google Scholar]
- 54.Wang H., et al. pH-dependent norfloxacin degradation by E+-ozonation: radical reactivities and intermediates identification. J. Clean. Prod. 2020;265 [Google Scholar]
- 55.Chen W., et al. Synthesis of MnOx/SBA-15 for Norfloxacin degradation by catalytic ozonation. Sep. Purif. Technol. 2017;173:99–104. [Google Scholar]
- 56.Chen H., et al. Catalytic ozonation of norfloxacin using Co3O4/C composite derived from ZIF-67 as catalyst. Chemosphere. 2021;265 doi: 10.1016/j.chemosphere.2020.129047. [DOI] [PubMed] [Google Scholar]
- 57.Ma X., et al. Ultrasound-enhanced nanosized zero-valent copper activation of hydrogen peroxide for the degradation of norfloxacin. Ultrason. Sonochem. 2018;40:763–772. doi: 10.1016/j.ultsonch.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 58.Hu S.-.B., et al. Aqueous norfloxacin sonocatalytic degradation with multilayer flower-like ZnO in the presence of peroxydisulfate. Ultrason. Sonochem. 2017;38:446–454. doi: 10.1016/j.ultsonch.2017.03.044. [DOI] [PubMed] [Google Scholar]
- 59.Özcan A., Özcan A.A., Demirci Y. Evaluation of mineralization kinetics and pathway of norfloxacin removal from water by electro-Fenton treatment. Chem. Eng. J. 2016;304:518–526. [Google Scholar]
- 60.Wang G., et al. Removal of norfloxacin by surface Fenton system (MnFe2O4/H2O2): kinetics, mechanism and degradation pathway. Chem. Eng. J. 2018;351:747–755. [Google Scholar]
- 61.Zhao Q., Wang C.-.C., Wang P. Effective norfloxacin elimination via photo-Fenton process over the MIL-101 (Fe)-NH2 immobilized on α-Al2O3 shee. Chin. Chem. Lett. 2022;33(11):4828–4833. [Google Scholar]
- 62.Wang Y., et al. Bio-Electron-Fenton (BEF) process driven by sediment microbial fuel cells (SMFCs) for antibiotics desorption and degradation. Biosens. Bioelectron. 2019;136:8–15. doi: 10.1016/j.bios.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 63.Crini G., Lichtfouse E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019;17(1):145–155. [Google Scholar]
- 64.Nwabanne J.T., Obi C.C. Coagulation-flocculation performance of snail shell biomass in abattoir wastewater treatment. J. Chem. Technol. Metallurgy. 2019;54(6) [Google Scholar]
- 65.Nwabanne J.T., Obi C.C. Abattoir wastewater treatment by electrocoagulation using iron electrodes. Chem. Sin. 2017;8(2):254–260. [Google Scholar]
- 66.Nwabanne J., Obi C.C. Electrocoagulation treatment of abattoirwastewater using aluminium electrode pairs. NSChE J. 2018;33(1):65. [Google Scholar]
- 67.Obi C.C., et al. Multi-characteristic optimization and modeling analysis of electrocoagulation treatment of abattoir wastewater using iron electrode pairs. J. Water Process. Eng. 2022;49 [Google Scholar]
- 68.Igwegbe C.A., Onukwuli O.D., Onyechi P.C. Optimal Route for Turbidity removal from Aquaculture Wastewater by Electrocoagulation-flocculation process. J. Eng. Appl. Sci. 2019;15(1):99–108. [Google Scholar]
- 69.de Souza Santos L.V., et al. Evaluation of the aerobic and anaerobic biodegradability of the antibiotic norfloxacin. Water Sci. Technol. 2014;70(2):265–271. doi: 10.2166/wst.2014.214. [DOI] [PubMed] [Google Scholar]
- 70.Ondon B.S., et al. Simultaneous removal and high tolerance of norfloxacin with electricity generation in microbial fuel cell and its antibiotic resistance genes quantification. Bioresour. Technol. 2020;304 doi: 10.1016/j.biortech.2020.122984. [DOI] [PubMed] [Google Scholar]
- 71.Zhao R., et al. Efficient enzymatic degradation used as pre-stage treatment for norfloxacin removal by activated sludge. Bioprocess. Biosyst. Eng. 2017;40(8):1261–1270. doi: 10.1007/s00449-017-1786-y. [DOI] [PubMed] [Google Scholar]
- 72.Wang G., et al. Efficient removal of norfloxacin by biological aerated filters: effect of zeolite modification and analysis of microbial communities. J. Water Process. Eng. 2021;40 [Google Scholar]
- 73.Iheanacho O.C., et al. Packed bed column adsorption of phenol onto corn cob activated carbon: linear and nonlinear kinetics modeling. S. Afr. J. Chem. Eng. 2021;36:80–93. [Google Scholar]
- 74.Nwabanne J.T., et al. Linear and nonlinear kinetics analysis and adsorption characteristics of packed bed column for phenol removal using rice husk-activated carbon. Appl. Water Sci. 2022;12(5):1–16. [Google Scholar]
- 75.Igwegbe C., et al. Studies on adsorption characteristics of corn cobs activated carbon for the removal of oil and grease from oil refinery desalter effluent in a downflow fixed bed adsorption equipment. Eur. J. Sustainable Dev. Res. 2020;5(1) [Google Scholar]
- 76.Igwegbe C.A., et al. Equilibrium and kinetics analysis on vat yellow 4 uptake from aqueous environment by modified rubber seed shells: nonlinear modelling. J. Mater. Environ. Sci. 2020;11(9):1424–1444. [Google Scholar]
- 77.Qin T., et al. A novel biochar derived from cauliflower (Brassica oleracea L.) roots could remove norfloxacin and chlortetracycline efficiently. Water Sci. Technol. 2017;76(12):3307–3318. doi: 10.2166/wst.2017.494. [DOI] [PubMed] [Google Scholar]
- 78.Wang B., et al. Preparation of biochar by simultaneous carbonization, magnetization and activation for norfloxacin removal in water. Bioresour. Technol. 2017;233:159–165. doi: 10.1016/j.biortech.2017.02.103. [DOI] [PubMed] [Google Scholar]
- 79.Luo Y., et al. CuNiN@ C coupled with peroxymonosulfate as efficient catalytic system for the removal of norfloxacin by adsorption and catalysis. Sep. Purif. Technol. 2020;252 [Google Scholar]
- 80.Wan Y., et al. Optimization adsorption of norfloxacin onto polydopamine microspheres from aqueous solution: kinetic, equilibrium and adsorption mechanism studies. Sci. Total Environ. 2018;639:428–437. doi: 10.1016/j.scitotenv.2018.05.171. [DOI] [PubMed] [Google Scholar]
- 81.Tegegne B., et al. Molecularly imprinted polymer for adsorption of venlafaxine, albendazole, ciprofloxacin and norfloxacin in aqueous environment. Sep. Sci. Technol. 2021;56(13):2217–2231. [Google Scholar]
- 82.Jing F., et al. Mechanistic insight into adsorptive removal of ionic NOR and nonionic DEP organic contaminates by clay-biochar composites. Environ. Pollut. 2022;310 doi: 10.1016/j.envpol.2022.119881. [DOI] [PubMed] [Google Scholar]
- 83.Khan N.A., et al. Efficient removal of norfloxacin by MOF@ GO composite: isothermal, kinetic, statistical, and mechanistic study. Toxin Rev. 2021;40(4):915–927. [Google Scholar]
- 84.Zhu F., et al. Degradation mechanism of norfloxacin in water using persulfate activated by BC@ nZVI/Ni. Chem. Eng. J. 2020;389 [Google Scholar]
- 85.Fang L., et al. Efficient removal of norfloxacin in water using magnetic molecularly imprinted polymer. Chemosphere. 2021;262 doi: 10.1016/j.chemosphere.2020.128032. [DOI] [PubMed] [Google Scholar]
- 86.Wu X., et al. Recognizing removal of norfloxacin by novel magnetic molecular imprinted chitosan/γ-Fe2O3 composites: selective adsorption mechanisms, practical application and regeneration. Sep. Purif. Technol. 2016;165:92–100. [Google Scholar]
- 87.Iwuozor K.O., et al. Aqueous phase adsorption of aromatic organoarsenic compounds: a review. J. Water Process. Eng. 2022;49 [Google Scholar]
- 88.Ighalo J.O., et al. Utilisation of adsorption as a resource recovery technique for lithium in geothermal water. J. Mol. Liq. 2022 [Google Scholar]
- 89.Wang Z., et al. Loofah activated carbon with hierarchical structures for high-efficiency adsorption of multi-level antibiotic pollutants. Appl. Surf. Sci. 2021;550 [Google Scholar]
- 90.Yan B., Niu C.H., Wang J. Kinetics, electron-donor-acceptor interactions, and site energy distribution analyses of norfloxacin adsorption on pretreated barley straw. Chem. Eng. J. 2017;330:1211–1221. doi: 10.1021/acs.est.7b00327. [DOI] [PubMed] [Google Scholar]
- 91.Chen D., et al. MOF-derived magnetic porous carbon-based sorbent: synthesis, characterization, and adsorption behavior of organic micropollutants. Adv. Powder Technol. 2017;28(7):1769–1779. [Google Scholar]
- 92.Nazraz M., Yamini Y., Asiabi H. Chitosan-based sorbent for efficient removal and extraction of ciprofloxacin and norfloxacin from aqueous solutions. Microchim. Acta. 2019;186(7):1–9. doi: 10.1007/s00604-019-3563-x. [DOI] [PubMed] [Google Scholar]
- 93.Ighalo J.O., et al. Cost of adsorbent preparation and usage in wastewater treatment: a review. Cleaner Chem. Eng. 2022;3 [Google Scholar]
- 94.Iwuozor K.O., et al. An empirical literature analysis of adsorbent performance for methylene blue uptake from aqueous media. J. Environ. Chem. Eng. 2021;9(4) [Google Scholar]
- 95.Ighalo J.O., et al. Verification of pore size effect on aqueous-phase adsorption kinetics: a case study of methylene blue. Colloids Surf. A. 2021;626 [Google Scholar]
- 96.Ighalo J.O., et al. A review of pine-based adsorbents for the adsorption of dyes. Biomass-Derived Mater. Environ. Appl. 2022:319–332. [Google Scholar]
- 97.Iwuozor K.O., et al. Do adsorbent pore size and specific surface area affect the kinetics of methyl orange aqueous phase adsorption? J. Chem. Lett. 2021;4:11. [Google Scholar]
- 98.Zanli B.L.G.L., Tang W., Chen J. N-doped and activated porous biochar derived from cocoa shell for removing norfloxacin from aqueous solution: performance assessment and mechanism insight. Environ. Res. 2022;214(3) doi: 10.1016/j.envres.2022.113951. [DOI] [PubMed] [Google Scholar]
- 99.Guo X., et al. Exploration of functional MOFs for efficient removal of fluoroquinolone antibiotics from water. Microporous Mesoporous Mater. 2019;286:84–91. [Google Scholar]
- 100.Zhang J., et al. Effects of pH, dissolved humic acid and Cu2+ on the adsorption of norfloxacin on montmorillonite-biochar composite derived from wheat straw. Biochem. Eng. J. 2018;130:104–112. [Google Scholar]
- 101.Chen W., et al. Efficient adsorption of Norfloxacin by Fe-MCM-41 molecular sieves: kinetic, isotherm and thermodynamic studies. Chem. Eng. J. 2015;281:397–403. [Google Scholar]
- 102.Niu X., et al. High specific surface area N-doped activated carbon from hydrothermal carbonization of shaddock peel for the removal of norfloxacin from aqueous solution. Water Sci. Technol. 2022;85(10):2964–2979. doi: 10.2166/wst.2022.163. [DOI] [PubMed] [Google Scholar]
- 103.Liu Q., et al. Green synthesis of stable Fe, Cu oxide nanocomposites from loquat leaf extracts for removal of Norfloxacin and Ciprofloxacin. Water Sci. Technol. 2020;81(4):694–708. doi: 10.2166/wst.2020.152. [DOI] [PubMed] [Google Scholar]
- 104.Wang J., et al. Adsorption characteristics and mechanism of norfloxacin in water by γ-Fe2O3@ BC. Water Sci. Technol. 2020;82(2):242–254. doi: 10.2166/wst.2020.078. [DOI] [PubMed] [Google Scholar]
- 105.Iwuozor K.O., et al. A review on the mitigation of heavy metals from aqueous solution using sugarcane bagasse. SugarTech. 2021;23:19. [Google Scholar]
- 106.Ighalo J.O., et al. Adsorption of persistent organic pollutants (POPs) from the aqueous environment by nano-adsorbents: a review. Environ. Res. 2022;212 doi: 10.1016/j.envres.2022.113123. [DOI] [PubMed] [Google Scholar]
- 107.Xie H., et al. Sorption of norfloxacin from aqueous solutions by activated carbon developed from Trapa natans husk. Sci. China Chem. 2011;54(5):835–843. [Google Scholar]
- 108.Ma P., et al. Green synthesis of Fe/Cu oxides composite particles stabilized by pine needle extract and investigation of their adsorption activity for norfloxacin and ofloxacin. J. Dispers. Sci. Technol. 2021;42(9):1350–1367. [Google Scholar]
- 109.Li G., et al. Bifunctional monomer molecularly imprinted sol-gel polymers based on the surface of magnetic halloysite nanotubes as an effective extraction approach for norfloxacin. Appl. Clay Sci. 2018;162:409–417. [Google Scholar]
- 110.Liu W., et al. Sorption of norfloxacin by lotus stalk-based activated carbon and iron-doped activated alumina: mechanisms, isotherms and kinetics. Chem. Eng. J. 2011;171(2):431–438. [Google Scholar]
- 111.Yan B., Niu C.H. Adsorption behavior of norfloxacin and site energy distribution based on the Dubinin-Astakhov isotherm. Sci. Total Environ. 2018;631:1525–1533. doi: 10.1016/j.scitotenv.2018.03.119. [DOI] [PubMed] [Google Scholar]
- 112.Zhou Y., et al. Simultaneous removal of tetracycline and norfloxacin from water by iron-trimesic metal-organic frameworks. J. Environ. Chem. Eng. 2022;10(3) [Google Scholar]
- 113.Liu H., et al. Evaluation of animal hairs-based activated carbon for sorption of norfloxacin and acetaminophen by comparing with cattail fiber-based activated carbon. J. Anal. Appl. Pyrolysis. 2013;101:156–165. [Google Scholar]
- 114.Li C., et al. Synergistic effects of anionic surfactants on adsorption of norfloxacin by magnetic biochar derived from furfural residue. Environ. Pollut. 2019;254 doi: 10.1016/j.envpol.2019.113005. [DOI] [PubMed] [Google Scholar]
- 115.Li J.-.R., et al. Can adsorbent of layered chalcogenide be regenerated? A case study of norfloxacin adsorbed by layered chalcogenide in water. Colloids Surf. A. 2018;537:287–293. [Google Scholar]
- 116.Zelaya Soulé M.E., et al. Norfloxacin adsorption on montmorillonite and carbon/montmorillonite hybrids: pH effects on the adsorption mechanism, and column assays. J. Environ. Sci. Health, Part A. 2020;56(1):113–122. doi: 10.1080/10934529.2020.1842042. [DOI] [PubMed] [Google Scholar]
- 117.Xu Y., et al. Crystal engineering of novel MOF@ iCOF composite as a multifunction platform for targeted monitoring and capturing of norfloxacin. Chem. Eng. J. 2022;435 [Google Scholar]
- 118.Dan H., et al. Mechanism of sonication time on structure and adsorption properties of 3D peanut shell/graphene oxide aerogel. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139983. [DOI] [PubMed] [Google Scholar]
- 119.Hu Y., et al. Surface-imprinted polymer microsphere based on self-stabilized precipitation polymerization for selective removal of norfloxacin. Appl. Surf. Sci. 2022;574 [Google Scholar]
- 120.Liu P., et al. Preparation of magnetic biochar obtained from one-step pyrolysis of Salix mongolica and investigation into adsorption behavior of sulfadimidine sodium and norfloxacin in aqueous solution. J. Dispers. Sci. Technol. 2020;41(2):214–226. [Google Scholar]
- 121.Yang W., et al. Adsorption behavior and mechanisms of norfloxacin onto porous resins and carbon nanotube. Chem. Eng. J. 2012;179:112–118. [Google Scholar]
- 122.Chen J., et al. Comparison of adsorption characteristics of acid-base modified fly ash to norfloxacin. Spectrosc. Lett. 2020;53(6):416–429. [Google Scholar]
- 123.Ahmed M.J., Theydan S.K. Fluoroquinolones antibiotics adsorption onto microporous activated carbon from lignocellulosic biomass by microwave pyrolysis. J. Taiwan Inst. Chem. Eng. 2014;45(1):219–226. [Google Scholar]
- 124.Liu P., et al. Sorption of sulfadiazine, norfloxacin, metronidazole, and tetracycline by granular activated carbon: kinetics, mechanisms, and isotherms. Water Air Soil Pollut. 2017;228(4):1–14. [Google Scholar]
- 125.Yi L., et al. Rapid nitrogen-rich modification of Calotropis gigantea fiber for highly efficient removal of fluoroquinolone antibiotics. J. Mol. Liq. 2018;256:408–415. [Google Scholar]
- 126.Prarat, P., et al., Removal of norfloxacin antibiotic from aqueous solution by activated carbon prepared from pomelo peel biomass waste by chemical activation. Environ. Asia, 2020. 13.
- 127.Qiu Z., et al. Regenerable Mg/Fe bimetallic hydroxide for remarkable removal of low-concentration norfloxacin from aqueous solution. Colloids Surf. A. 2022;644 [Google Scholar]
- 128.Che G., et al. Unraveling influence of metal species on norfloxacin removal by mesoporous metallic silicon adsorbent. Environ. Sci. Pollut. Res. Int. 2020;27(28):35638–35649. doi: 10.1007/s11356-020-09829-3. [DOI] [PubMed] [Google Scholar]
- 129.Nguyen V.-.T., et al. Adsorption of norfloxacin from aqueous solution on biochar derived from spent coffee ground: master variables and response surface method optimized adsorption process. Chemosphere. 2022;288 doi: 10.1016/j.chemosphere.2021.132577. [DOI] [PubMed] [Google Scholar]
- 130.Otalvaro J.O., Avena M., Brigante M. Adsorption of norfloxacin on a hexagonal mesoporous silica: isotherms, kinetics and adsorbent reuse. Adsorption. 2019;25(7):1375–1385. [Google Scholar]
- 131.Zhao S., et al. Microwave-assisted hydrothermal assembly of 2D copper-porphyrin metal-organic frameworks for the removal of dyes and antibiotics from water. Environ. Sci. Pollut. Res. Int. 2020;27(31):39186–39197. doi: 10.1007/s11356-020-09865-z. [DOI] [PubMed] [Google Scholar]
- 132.Gaho M.M., et al. Synthesis of novel magnetic molecularly imprinted polymers by solid-phase extraction method for removal of norfloxacin. Chin. J. Anal. Chem. 2022;50(6) [Google Scholar]
- 133.Ma Y., et al. Iron/zinc and phosphoric acid modified sludge biochar as an efficient adsorbent for fluoroquinolones antibiotics removal. Ecotoxicol. Environ. Saf. 2020;196 doi: 10.1016/j.ecoenv.2020.110550. [DOI] [PubMed] [Google Scholar]
- 134.Tang Y., et al. Synthesis of reduced graphene oxide/magnetite composites and investigation of their adsorption performance of fluoroquinolone antibiotics. Colloids Surf. A. 2013;424:74–80. [Google Scholar]
- 135.Wu J., et al. Norfloxacin adsorption and subsequent degradation on ball-milling tailored N-doped biochar. Chemosphere. 2022 doi: 10.1016/j.chemosphere.2022.135264. [DOI] [PubMed] [Google Scholar]
- 136.Sui M., et al. Adsorption of norfloxacin in aqueous solution by Mg–Al layered double hydroxides with variable metal composition and interlayer anions. Chem. Eng. J. 2012;210:451–460. [Google Scholar]
- 137.Zhang M., et al. Study on optimal adsorption conditions of norfloxacin in water based on response surface methodology. Water Supply. 2022;22(4):3661–3672. [Google Scholar]
- 138.Li R., et al. Magnetic biochar-based manganese oxide composite for enhanced fluoroquinolone antibiotic removal from water. Environ. Sci. Pollut. Res. Int. 2018;25(31):31136–31148. doi: 10.1007/s11356-018-3064-1. [DOI] [PubMed] [Google Scholar]
- 139.Yang X., et al. Mechanistic insights into removal of norfloxacin from water using different natural iron ore–biochar composites: more rich free radicals derived from natural pyrite-biochar composites than hematite-biochar composites. Appl. Catal. B. 2019;255 [Google Scholar]
- 140.Qin D., et al. Selective extraction and detection of norfloxacin from marine sediment and seawater samples using molecularly imprinted silica sorbents coupled with HPLC. Mar. Pollut. Bull. 2020;150 doi: 10.1016/j.marpolbul.2019.110677. [DOI] [PubMed] [Google Scholar]
- 141.Wuana R.A., Sha'Ato R., Iorhen S. Preparation, characterization, and evaluation of Moringa oleifera pod husk adsorbents for aqueous phase removal of norfloxacin. Desalination Water Treat. 2016;57(25):11904–11916. [Google Scholar]
- 142.Liang Z., et al. Adsorption of quinolone antibiotics in spherical mesoporous silica: effects of the retained template and its alkyl chain length. J. Hazard. Mater. 2016;305:8–14. doi: 10.1016/j.jhazmat.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 143.Iwuozor K.O., et al. Mitigation of levofloxacin from aqueous media by adsorption: a review. Sustain. Water Resour. Manag. 2021;7(6):1–18. [Google Scholar]
- 144.Emenike E.C., et al. Recent advances in nano-adsorbents for the sequestration of copper from water. J. Water Process. Eng. 2022;47 [Google Scholar]
- 145.Peng H., et al. Adsorption of ofloxacin and norfloxacin on carbon nanotubes: hydrophobicity-and structure-controlled process. J. Hazard. Mater. 2012;233:89–96. doi: 10.1016/j.jhazmat.2012.06.058. [DOI] [PubMed] [Google Scholar]
- 146.Li X., et al. Modified activated carbon fiber felt for the electrosorption of norfloxacin in aqueous solution. Sustainability. 2020;12(10):3986. [Google Scholar]
- 147.Zhou L., et al. Simultaneous removal of mixed contaminants, copper and norfloxacin, from aqueous solution by ZIF-8. Chem. Eng. J. 2019;362:628–637. [Google Scholar]
- 148.Igwegbe C.A., et al. Electrocoagulation-flocculation of aquaculture effluent using hybrid iron and aluminium electrodes: a comparative study. Chem. Eng. J. Adv. 2021;6 [Google Scholar]
- 149.Barquilha C.E., Braga M.C. Adsorption of organic and inorganic pollutants onto biochars: challenges, operating conditions, and mechanisms. Bioresour. Technol. Rep. 2021;15 [Google Scholar]
- 150.Roy K., et al. Understanding the Basics of QSAR For Applications in Pharmaceutical Sciences and Risk Assessment. 1st edn. Academic; London: 2015. Background of QSAR and historical developments. [Google Scholar]
- 151.Ambaye T., et al. Mechanisms and adsorption capacities of biochar for the removal of organic and inorganic pollutants from industrial wastewater. Int. J. Environ. Sci. Technol. 2021;18(10):3273–3294. [Google Scholar]
- 152.Onu C.E., et al. Decolourization of bromocresol green dye solution by acid functionalized rice husk: artificial intelligence modeling, GA optimization, and adsorption studies. J. Hazard Mater. Adv. 2023;9:1–14. [Google Scholar]
- 153.Emenike E.C., et al. Efficient recycling of disposable face masks via co-carbonization with waste biomass: a pathway to a cleaner environment. Clean. Environ. Syst. 2022 [Google Scholar]
- 154.Lorphensri O., et al. Sorption of acetaminophen, 17α-ethynyl estradiol, nalidixic acid, and norfloxacin to silica, alumina, and a hydrophobic medium. Water Res. 2006;40(7):1481–1491. doi: 10.1016/j.watres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 155.Chen Y., Li Z. Interaction of norfloxacin and hexavalent chromium with ferrihydrite nanoparticles: synergistic adsorption and antagonistic aggregation behavior. Chemosphere. 2022;299 doi: 10.1016/j.chemosphere.2022.134386. [DOI] [PubMed] [Google Scholar]
- 156.Pei Z.-.G., et al. Insight to ternary complexes of co-adsorption of norfloxacin and Cu (II) onto montmorillonite at different pH using EXAFS. J. Hazard. Mater. 2011;186(1):842–848. doi: 10.1016/j.jhazmat.2010.11.076. [DOI] [PubMed] [Google Scholar]
- 157.Luo J., et al. Sorption of norfloxacin, sulfamerazine and oxytetracycline by KOH-modified biochar under single and ternary systems. Bioresour. Technol. 2018;263:385–392. doi: 10.1016/j.biortech.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 158.Yao N., et al. Norfloxacin and bisphenol-A removal using temperature-switchable graphene oxide. ACS Appl. Mater. Interfaces. 2018;10(34):29083–29091. doi: 10.1021/acsami.8b07233. [DOI] [PubMed] [Google Scholar]
- 159.Feng D., et al. Adsorption characteristics of norfloxacin by biochar prepared by cassava dreg: kinetics, isotherms, and thermodynamic analysis. Bioresources. 2015;10(4):6751–6768. [Google Scholar]
- 160.Zhao L., et al. Sorption of copper and norfloxacin onto humic acid: effects of pH, ionic strength, and foreign ions. Environ. Sci. Pollut. Res. Int. 2019;26(11):10685–10694. doi: 10.1007/s11356-019-04515-5. [DOI] [PubMed] [Google Scholar]
- 161.Emenike E.C., et al. Delonix regia biochar potential in removing phenol from industrial wastewater. Bioresour. Technol. Rep. 2022 [Google Scholar]
- 162.Ogunlalu O., et al. Trends in the mitigation of heavy metal ions from aqueous solutions using unmodified and chemically-modified agricultural waste adsorbents. Curr. Res. Green Sustainable Chem. 2021;4 [Google Scholar]
- 163.Emenike E.C., Iwuozor K.O., Anidiobi S.U. Heavy metal pollution in aquaculture: sources, impacts and mitigation techniques. Biol. Trace Elem. Res. 2021;200:1–17. doi: 10.1007/s12011-021-03037-x. [DOI] [PubMed] [Google Scholar]
- 164.Bunkoed O., Donkhampa P., Nurerk P. A nanocomposite optosensor of hydroxyapatite and graphene quantum dots embedded within highly specific polymer for norfloxacin detection. Microchem. J. 2020;158 [Google Scholar]
- 165.Aibinu A.M., et al. Green synthesis of CuO nanocomposite from watermelon (Citrullus lanatus) rind for the treatment of aquaculture effluent. Reg. Stud. Marine Sci. 2022;52 [Google Scholar]
- 166.Iwuozor K.O., et al. Removal of pollutants from aqueous media using cow dung-based adsorbents. Curr. Res. Green Sustainable Chem. 2022;5 [Google Scholar]
- 167.Iwuozor K.O., et al. Adsorption of organophosphate pesticides from aqueous solution: a review of recent advances. Int. J. Environ. Sci. Technol. 2022 In press. [Google Scholar]
- 168.Darweesh T.M., Ahmed M.J. Adsorption of ciprofloxacin and norfloxacin from aqueous solution onto granular activated carbon in fixed bed column. Ecotoxicol. Environ. Saf. 2017;138:139–145. doi: 10.1016/j.ecoenv.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 169.Hu Q., et al. Prediction of breakthrough curves in a fixed-bed column based on normalized Gudermannian and error functions. J. Mol. Liq. 2021;323 [Google Scholar]
- 170.Ighalo J.O., et al. Utilization of avocado (Persea americana) adsorbents for the elimination of pollutants from water: a review. Biomass-Der. Mater. Environ. Appl. 2022:333–348. [Google Scholar]
- 171.Firozjaee T.T., et al. The removal of diazinon from aqueous solution by chitosan/carbon nanotube adsorbent. Desalin. Water Treat. 2017;79:291–300. [Google Scholar]
- 172.Ighalo J., et al. Recent advances in hydrochar application for the adsorptive removal of wastewater pollutants. Chem. Eng. Res. Des. 2022;184:38. [Google Scholar]
- 173.Li S., Fang L. Advanced Materials Research. 2014. Modeling of the adsorption mechanisms and selectivity of the molecular imprinted particles for norfloxacin. Trans Tech Publ. [Google Scholar]
- 174.Zhang Y., Cheng L., Ji Y. A novel amorphous porous biochar for adsorption of antibiotics: adsorption mechanism analysis via experiment coupled with theoretical calculations. Chem. Eng. Res. Des. 2022;186:362–373. [Google Scholar]
- 175.Yadav S., et al. Removal of fluoroquinolone from aqueous solution using graphene oxide: experimental and computational elucidation. Environ. Sci. Pollut. Res. Int. 2018;25(3):2942–2957. doi: 10.1007/s11356-017-0596-8. [DOI] [PubMed] [Google Scholar]
- 176.Zhang X., et al. Interactions between antibiotics and graphene-based materials in water: a comparative experimental and theoretical investigation. ACS Appl. Mater. Interfaces. 2016;8(36):24273–24280. doi: 10.1021/acsami.6b09377. [DOI] [PubMed] [Google Scholar]
- 177.Guo R., Lin K., Ai Y. Insights into the interface mechanism of three typical antibiotics onto the graphene oxide/chitosan composite: an experimental and theoretical investigation. Chin. J. Chem. Phys. 2021 [Google Scholar]
- 178.Liang D., et al. Design, preparation and adsorption performances of norfloxacin molecularly imprinted polymers. J. Mol. Graphics Modell. 2022;114 doi: 10.1016/j.jmgm.2022.108197. [DOI] [PubMed] [Google Scholar]
- 179.Ighalo J.O., et al. Mitigation of metronidazole (flagyl) pollution in aqueous media by adsorption: a review. Environ. Technol. Rev. 2020;9(1):137–148. [Google Scholar]
- 180.Ohale P.E., et al. Adsorptive kinetics, isotherm and thermodynamic analysis of fishpond effluent coagulation using chitin derived coagulant from waste Brachyura shell. Chem. Eng. J. Adv. 2020;4 [Google Scholar]
- 181.Tran H.N., et al. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: a critical review. Water Res. 2017;120:88–116. doi: 10.1016/j.watres.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 182.Bednárek J., et al. Revelation of high-adsorption-performance activated carbon for removal of fluoroquinolone antibiotics from water. Biomass Convers. Biorefin. 2022:1–15. [Google Scholar]
- 183.Xi M., et al. Enhanced norfloxacin degradation by iron and nitrogen co-doped biochar: revealing the radical and nonradical co-dominant mechanism of persulfate activation. Chem. Eng. J. 2021;420 [Google Scholar]
- 184.Huang W., et al. Preparation and characterization of novel thermosensitive magnetic molecularly imprinted polymers for selective recognition of norfloxacin. J. Polym. Res. 2016;23(5):1–11. [Google Scholar]
- 185.Ohale P.E., et al. A comparative optimization and modeling of ammonia–nitrogen adsorption from abattoir wastewater using a novel iron-functionalized crab shell. Appl. Water Sci. 2022;12(8):1–27. [Google Scholar]
- 186.Chen S., et al. Study on the adsorption of dyestuffs with different properties by sludge-rice husk biochar: adsorption capacity, isotherm, kinetic, thermodynamics and mechanism. J. Mol. Liq. 2019;285:62–74. [Google Scholar]
- 187.Aniagor C., Menkiti M. Kinetics and mechanistic description of adsorptive uptake of crystal violet dye by lignified elephant grass complexed isolate. J. Environ. Chem. Eng. 2018;6(2):2105–2118. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.