Abstract
Theranostics describes the coupling of a diagnostic biomarker and a therapeutic agent (i.e., a theranostic pair) that have a common target in tumor cells or their microenvironment. The term is increasingly associated with in vivo nuclear medicine oncologic applications that couple diagnostic imaging by means of gamma radiation with concomitant localized high-energy particulate radiation to a tissue expressing the common target. Several theranostic pairs have been translated into clinical practice in the United States and are poised to become a mainstay of cancer treatment. The purposes of this article are to review experience with theranostics for solid-organ malignancies and to address the practical integration into care pathways of β-emitting therapies that include somatostatin analogue radioligands for neuroendocrine tumors, PSMA-directed therapy for prostate cancer, and 131I-MIBG therapy for tumors of neural crest origin. Toxicities related to theranostics administration and indications for cessation of therapy in patients who experience adverse events are also discussed. A multidisciplinary team-based approach for identifying patients most likely to respond to these agents, determining the optimal time for therapy delivery, and managing patient care throughout the therapeutic course is critical to the success of a radiotheranostic program.
Keywords: nuclear therapy, radioligand therapy, theranostics
Theranostics, a term believed to have first been used in 1998, describes the coupling of a diagnostic biomarker and a therapeutic agent that share a specific target in either tumor cells or their microenvironment [1, 2]. The term broadly refers to any combination of diagnostic and therapeutic modalities. Potential treatment strategies in theranostics include nucleic acid delivery, chemotherapy, thermal ablation, and radiation therapy. Theranostics entails the use of a spectrum of imaging probes, including MRI contrast agents and PET and SPECT agents [3]. The term is increasingly associated with in vivo nuclear medicine oncologic applications, which are the focus of this article.
Radioactive iodine therapy for thyroid disease, first administered in the 1940s, is a paradigm of a theranostic treatment strategy [4]. Unlike the radioiodine used to detect functioning thyroid tissue solely for diagnostic purposes, 131I is a dual γ- and β-emitting radionuclide administered orally in the form of sodium iodide to treat well-differentiated thyroid cancers (papillary and follicular), hyperthyroidism, and nontoxic nodular goiter. Determination of the administered therapeutic activity depends on many factors, including pretreatment radioiodine uptake measurements. Radioiodine treatment exemplifies the critical concept of theranostic treatment strategy by coupling diagnostic imaging through gamma radiation with the high linear energy transfer of concomitantly released β-radiation. Diagnostic and therapeutic radiopharmaceuticals with a common target are referred to as theranostic pairs.
In association with advances in PET/CT and PET/MRI technology, several theranostic pairs have been translated into clinical practice in the United States (Fig. 1). At a patient-specific level, the likelihood of treatment success and prognostic information can be evaluated before and during therapy; some patients can avoid unnecessary treatments and their associated side effects.
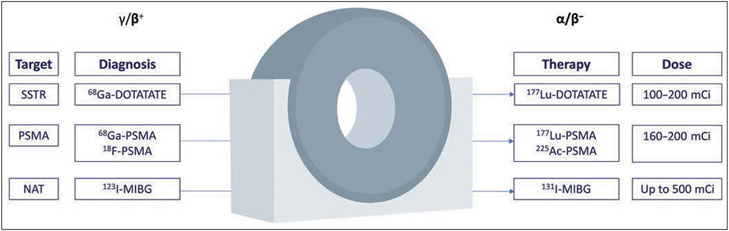
Fig. 1—
Schematic shows overview of frequently used theranostic pairs in nuclear radiology. Specific target is used for diagnostic PET examination performed to evaluate presence and magnitude of uptake by tumor. This assessment guides therapy and dose selection (100 mCi = 3700 MBq). SSTR = somatostatin receptor, NAT= noradrenaline transporter.
This article reviews experience with theranostics for solid-organ malignancies. We address the integration into patient care pathways of currently available β-emitting radiopharmaceutical therapies, including somatostatin analogue (SSA) radioligands, PSMA-directed theranostics, and 131I-labeled MIBG therapy. We also briefly comment on α-particle therapy and various promising future radioligands. Emerging theranostic agents for the treatment of hematologic malignancies are not discussed [5].
Beta-Emitting Therapies
Somatostatin Analogue Radioligands
Experimental advances in peptide receptor radiotherapy (PRRT), coupled with the emergence of PET, facilitated the imaging diagnosis of neuroendocrine tumors (NETs) and potential theranostic targets. PET with a 68Ga-labeled somatostatin receptor (SSTR) has superseded octreotide scintigraphy in many clinical situations. The American College of Radiology now considers use of SSTR PET appropriate for initial staging after a histologic diagnosis of NET, localization of NETs with an unknown primary site, staging of NETs before surgery, and evaluation of a mass suggestive of an NET but inaccessible for biopsy. The use of 68Ga-labeled SSTR PET to select patients for whom SSTR-targeted PRRT is suitable is also considered appropriate [6].
Large multicenter clinical trials have shown the efficacy of PRRT for treatment of advanced NETs after disease progression with long-acting SSA therapy (Fig. 2). Interim analysis in a phase 3 trial of 177Lu-DOTATATE for midgut NETs (Neuroendocrine Tumors Therapy 1 [NETTER-1] trial) showed that treatment with PRRT resulted in longer progression-free survival than did therapy with long-acting repeatable SSAs. Clinically significant myelosuppression was observed in fewer than 10% of patients [7]. At the final prespecified analysis of overall survival, 177Lu-DOTATATE did not significantly improve overall survival compared with long-acting octreotide. Median overall survival was 48 months in the 177Lu-DOTATATE group and 36.3 months in the octreotide group. However, 11.7-month improvement in median overall survival was considered clinically significant [8]. A total of 3% (3/111) of patients treated with 177Lu-DOTATATE had grade 3 or worse adverse events; 2% (2/111) had myelodysplastic syndrome [9].
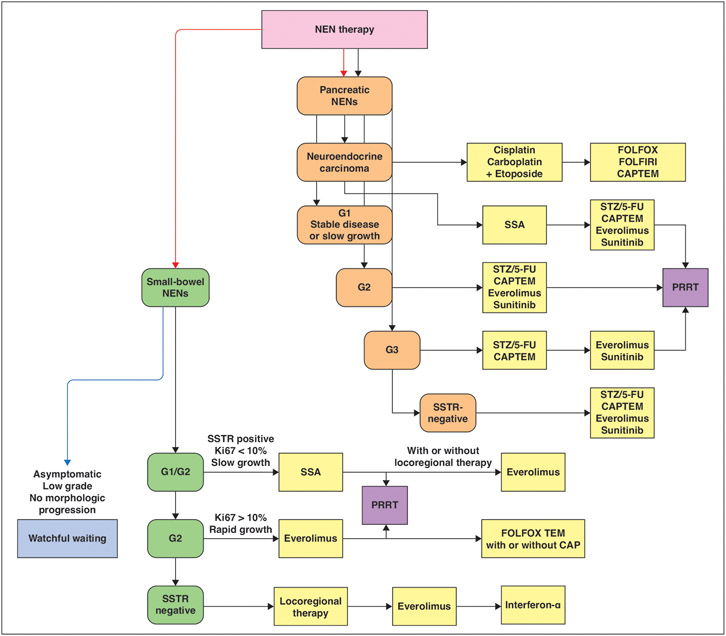
Fig. 2—
Schematic shows potential time points for peptide receptor radionuclide therapy (PRRT) therapy in management of neuroendocrine neoplasms (NENs) according to European Society for Medical Oncology clinical practice guidelines [25]. PRRT can be considered after initial medical management in patients with somatostatin receptor (SSTR)-positive small-bowel and pancreatic NENs. Specifically, PRRT can be considered for grade 2 (G2) small-bowel NENs after progression with serotonin analogue (SSA) therapy and is considered equivalent alternative to everolimus. PRRT can be considered for grade 1 (G1) and potentially grade 2 pancreatic NENs if progression occurs with SSA therapy and is considered equivalent to other available agents. FOLFOX = leucovorin calcium (folinic acid), fluorouracil, and oxaliplatin, FOLFIRI = leucovorin calcium (folinic acid), fluorouracil, and irinotecan hydrochloride, CAPTEM = capecitabine and temozolomide, STZ = streptozotocin, 5-FU = 5-fluorouracil, Ki67 = nuclear protein Ki67.
Potential adverse events (AE) encountered during PRRT can be classified as early or late. Early AEs include nausea and emesis, predominantly caused by the amino acid solutions infused for renal radioprotection during therapy. The reported incidences of nausea and emesis were 59% and 52%, respectively, in the NETTER-1 trial [7, 10]. Renal radioprotection may alternatively be performed with compounded amino acid solutions that contain only positively charged L-lysine and L-arginine and markedly reduce gastrointestinal side effects compared with the side effects of higher-osmolality solutions [11]. Hormonal crises, such as carcinoid and hypertensive crises, are other rare early AEs [12, 13]. Patients with hepatic metastases or with preexisting heart disease are at risk of carcinoid crisis.
Patients should be assessed for pretreatment electrolyte disturbances or dehydration, which should be corrected before PRRT is initiated. Additional pretreatment with steroids, 5-hydroxytryptamine 3 receptor antagonists, or a bolus dose of octreotide for 1–2 hours should be considered before PRRT in high-risk patients. Practitioners should be familiar with the clinical manifestations and treatment of acute carcinoid crisis. Patients with pheochromocytoma or paragangliomas are at increased risk of hypertensive crises. Many centers administer combined α- and β-adrenergic blockade 2 weeks before therapy [14].
Hematotoxicity is the most common delayed AE of PRRT [15] and encompasses a broad spectrum of severities and durations. Although as many as 11% of patients develop grade 3 or 4 leukopenia or thrombocytopenia, only 2% have persistent hematotoxicity [15-17]. Greater administered activity of PRRT and pretreatment cytopenia are positively associated with the occurrence of hematotoxicity [16]. Additional risk factors include older age, increased marrow uptake on pretreatment imaging, and other myelotoxic therapies [18]. Table 1 summarizes the common indications for cessation of 177Lu-DOTATATE therapy after the development of toxicity.
TABLE 1:
Indications for Cessation of 177Lu-DOTATATE or 177Lu-Vipivotide Tetraxetan Therapy After Toxicity Develops
| Therapy and Toxicity | Indication for Cessation |
|---|---|
| l77Lu- DOTATATEa | |
| Thrombocytopenia | Recurrent grade 2, 3, or 4 AE after withholding of agent until complete or partial resolution (grade 0 or 1 AE) and restarting at a reduced dose Permanently discontinue if grade 2 or greater thrombocytopenia results in a delay of 16 wk or more |
| Anemia and neutropenia | Recurrent grade 3 or 4 AE after withholding of agent until complete or partial resolution (grade 0, 1, or 2 AE) and restarting at a reduced dose Permanently discontinue if grade 3 or greater anemia or neutropenia results in a delay of 16 wk or more |
| Renal toxicity | Recurrent renal toxicity after withholding of agent until complete resolution or return to baseline and restarting at a reduced dose Permanently discontinue if renal toxicity results in a delay 16 wk or more |
| Hepatotoxicity | Recurrent hepatotoxicity after withholding of agent until complete resolution or return to baseline and restarting at a reduced dose Permanently discontinue if hepatotoxicity results in a delay 16 wk or more |
| Any other possibly related CTCAE grade 3 or grade 4 AE | Recurrent grade 3 AE after dose reduction after withholding of agent until complete or partial resolution (grade 0, 1, or 2 AE) and restarting at a reduced dose Permanently discontinue for grade 3 or greater AE that results in a delay 16 wk or more |
| l77Lu–vipivotide tetraxetanb | |
| Myelosuppression | Recurrent grade 3 or greater AE after one dose reduction |
| Renal toxicity | Grade 3 or greater AE Recurrent renal toxicity after one dose reduction |
| Xerostomia | Grade 3 or greater AE |
| Gastrointestinal toxicity | Grade 3 or greater AE (not amenable to medical intervention) Recurrent grade 3 or greater AE after one dose reduction |
| Transaminitis | Five times upper limit of normal, in the absence of hepatic metastases |
| Nonhematologic toxicity | Any unacceptable toxicity Any serious AE that delays therapy more than 4 wk Any grade 3 or 4 AE or persistent intolerable grade 2 AE after one dose reduction |
Initial imaging with radiolabeled SSAs is crucial for identifying suitable candidates for PRRT. The SUVmax of the tumor during SSTR PET/CT correlates with radioligand uptake in PRRT [19]. The Krenning score developed for octreotide imaging may be used for the evaluation of SSTR PET/CT [20]. In 2018, a standardized tool (SSTR Reporting and Data System [SSTR-RADS] version 1.0) was proposed for the interpretation and reporting of SSTR PET/CT [21]. The aims of SSTR-RADS are to improve categorization of the likelihood that findings represent NETs and to identify patients who will respond to PRRT [21]. SSTR-RADS scores of 1 and 2 correspond to benign and likely benign lesions. SSTR-RADS scores of 4 and 5 correspond to lesions in sites typical for NETs that have intense uptake and that either lack (score 4) or have concordant (score 5) anatomic imaging findings typical of NETs. PRRT should be considered for lesions with an SSTR-RADS score of 4 or 5 but not for lesions with a score of 1 or 2. Lesions with an SSTR-RADS score of 3 either suggest NET but have insufficient uptake to merit PRRT or have sufficient uptake but have imaging features in-consistent with an NET such that tissue sampling is advised before potential treatment.
Uptake is quantified on a 3-point Likert scale. Level 1 uptake is less than that of blood pool; level 2 uptake is above that of blood pool but less than that of physiologic liver; and level 3 uptake is above that of physiologic liver. To our knowledge, no current evidence supports routine imaging during delivery of PRRT [15]. However, some centers use planar scintigraphy, SPECT, or SPECT/CT after delivery of the therapeutic dose of 177Lu-DOTATATE for dosimetry and dose adjustment. Repeat SSTR PET may be useful in patients with disease that has progressed but who previously benefited from PRRT to guide a decision for possible retreatment [15]. Some evidence shows prognostic utility of performing dual-radiotracer PET with both radiolabeled SSAs and FDG before the initiation of PRRT [22, 23]. A systematic review and meta-analysis showed that disease control rates after PRRT were lower for patients with FDG-positive tumors [24]. Furthermore, patients with FDG-positive tumors at baseline were at higher risk of disease progression and death during PRRT. Dual-tracer PET could thus facilitate a personalized treatment strategy by assisting in disease prognostication, identifying patients likely to benefit from PRRT or systemic chemotherapy, and providing a baseline for surveillance of disease activity [24].
The timing of initiation of PRRT is critical in the management of NETs. Given the absence of randomized data, the sequence in which treatments are administered, particularly to patients with disease that progresses with first-line SSAs, is typically individualized on the basis of the WHO grade of disease, primary disease site, rate of progression, disease burden, dominant organ of metastasis, and the patient’s symptoms, functional performance, and underlying comorbidities [25-27]. Multiple clinical trials—including the Prospective, Randomized, Controlled, Open-Label, Multicenter Phase III Study to Evaluate Efficacy and Safety of Peptide Receptor Radionuclide Therapy (PRRT) With 177Lu-Edotreotide Compared to Targeted Molecular Therapy With Everolimus in Patients (COMPETE) [28]; Prospective, Randomized, Controlled, Open-Label, Multicenter Trial to Evaluate Efficacy, Safety, and Patient-Reported Outcomes of Peptide Receptor Radionuclide Therapy (PRRT) with Lutetium (177Lu) Edotreotide Compared to Best Standard of Care in Patients (COMPOSE) [29]; and Testing Lutetium Lu 177 Dotatate in Patients With Somatostatin Receptor Positive Advanced Bronchial Neuroendocrine Tumors (NCT 04665739)—are ongoing and will yield randomized prospective data on the safety and efficacy of PRRT compared with either everolimus or other standard-of-care interventions.
In addition to the established and emerging roles of PRRT in managing NETs, SSTR-targeted radionuclide therapy may have a role in the treatment of patients with radioiodine-refractory differentiated thyroid cancer (DTC) and metastatic medullary thyroid cancer (MTC). The current standard-of-care treatment of most patients with DTC comprises surgery and radioiodine therapy. However, among patients who receive this combination, recurrence rates range from 2% to 14%, and recurrent cancers can be resistant to radioiodine therapy [30, 31]. By contrast, MTC is inherently insensitive to treatment with radioiodine therapy [32]. PRRT could be a therapeutic option for patients with DTC or MTC whose tumor is found to express SSTR subtypes on scintigraphy or PET. Maghsoomi et al. [33] conducted a systematic review of SSTR-targeted PRRT with 90Y, 111In, or 177Lu in patients with DTC or MTC. The biochemical and objective response rates were 35.3% and 10.5% among patients with DTC and 37.2% and 10.6% among patients with MTC. The time to death after PRRT ranged from 1 to 63 months among patients with DTC and from 1 to 26.8 months among patients with MTC.
PSMA Radioligands for Prostate Cancer
PSMA is expressed in high levels on the surface of prostate cancer cells, including both the primary site and metastases, and remains highly expressed after multiple lines of therapy [34]. Experience with 177Lu-PSMA therapy in the treatment of metastatic castration-resistant prostate cancer (mCRPC) is rapidly expanding since the FDA approval in March 2022 of 177Lu–vipivotide tetraxetan (also known as 177Lu-PSMA-617) to treat patients with PSMA-positive mCRPC who have been previously treated with an androgen receptor pathway inhibitor and taxane-based chemotherapy (Figs. 3 and 4) [35]. Like 177Lu-DOTATATE therapy, PSMA PET is needed to confirm treatment eligibility. Although PSMA PET is highly sensitive and specific, physiologic and pathologic causes aside from prostate cancer can exhibit uptake. PSMA Reporting and Data System (PSMA-RADS) version 1.0 was introduced in 2018 to address this uncertainty [36, 37].
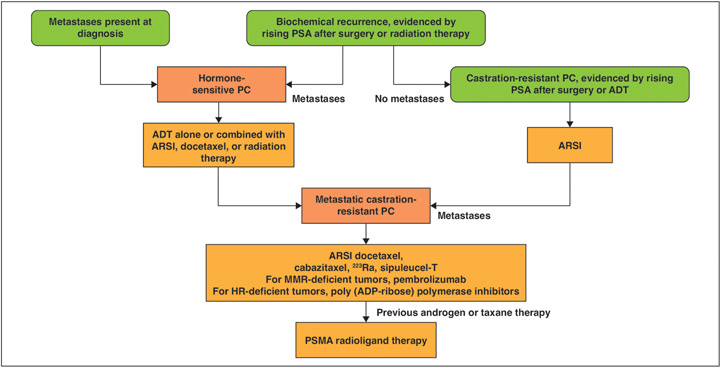
Fig. 3—
Schematic shows current therapeutic options for patients with advanced prostate cancer, indicating potential role of PSMA radioligand therapy. PC = prostate cancer, ADT = androgen deprivation therapy, ARSI = androgen receptor signaling inhibitor, MMR = mismatch repair, HR = homologous recombination, ADP = adenosine diphosphate. (Information derived from [72].)
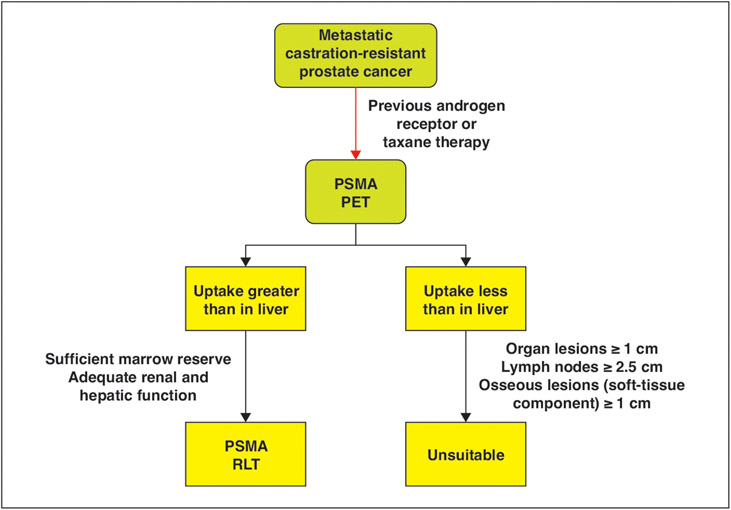
Fig. 4—
Schematic shows overview of 177Lu-labeled PSMA ligands. Eligible patients include those with metastatic castration-resistant prostate cancer previously treated with androgen receptor inhibitors or taxane-based therapy. Patients subsequently undergo PSMA PET. After multidisciplinary discussion, PSMA radioligand therapy (RLT) should be considered in patients with evidence of PSMA uptake greater than liver uptake, sufficient marrow reserve, and adequate renal and hepatic function. PSMA RLT should not be considered in patients with measurable disease (including organ lesions measuring ≥ 1 cm, lymph nodes measuring ≥ 2.5 cm, and bony lesions measuring ≥ 1 cm) that lack PSMA uptake greater than that of liver.
The VISION trial examined the role of 177Lu–vipivotide tetraxetan therapy in patients with mCRPC [38]. Patients in the trial had been treated previously with at least one androgen-receptor pathway inhibitor and one or two taxane regimens. All patients had increased PSMA uptake greater than liver uptake in at least one lesion and no PSMA-negative lesions. Patients were randomized in a 2:1 ratio to undergo standard-of-care therapy combined with 177Lu–vipivotide tetraxetan therapy or to undergo standard-of-care therapy alone. Patients who had undergone chemotherapy, immunotherapy, 223Ra therapy, or therapy with investigational drugs were excluded. The addition of 177Lu–vipivotide tetraxetan therapy to standard-of-care therapy lengthened imaging-based progression-free survival (8.7 vs 3.4. months) and overall survival (15.3 vs 11.3 months). The TheraP trial was a randomized phase 2 trial that compared 177Lu–vipivotide tetraxetan and cabazitaxel in the care of patients with mCRPC [39]. Two hundred eligible patients were identified with PSMA PET/CT; 98 patients were randomized to undergo 177Lu–vipivotide tetraxetan treatment, and 101 patients were randomized to undergo cabazitaxel treatment. A greater PSA response and fewer serious AEs occurred in the 177Lu–vipivotide tetraxetan group.
The European Association of Nuclear Medicine recommends that PSMA-targeted therapy be considered in patients with mCRPC who do not respond to or are ineligible for all alternative treatment options and who have uptake greater than liver uptake on pretherapy PSMA PET [40]. In its announcement approving 177Lu–vipivotide tetraxetan, the FDA also indicated that patients should be selected for therapy on the basis of evidence of increased uptake on 68Ga-PSMA-11 PET, as defined in the VISION trial protocol [38], and at least one tumor lesion exhibiting uptake greater than liver uptake. Patient selection should also consider the presence of PSMA-negative lesions [35]. Eligible patients should have sufficient bone marrow reserve and adequate renal and hepatic function and should discontinue myelosuppressive therapy [41]. The FDA label indicates that 177Lu–vipivotide tetraxetan be administered in six doses at 6-week intervals [35], according to the schedule in the VISION study. The indications for dose delays, dose reductions, and therapy cessation after development of toxicity are summarized in Table 1.
Therapy With 131I-MIBG
MIBG is a structural norepinephrine analogue taken up by the noradrenaline transporter. Tumors of neural crest origin, such as neuroblastoma, pheochromocytoma, carcinoid tumors, and MTC, express noradrenaline transporter, which in turn takes up MIBG. Decaying 131I releases ionizing radiation, presumed to be the agent’s mechanism of therapeutic effect. Iodine-131-labeled MIBG therapy for NETs has been used since the 1980s [42, 43]. In 2018, after a phase 2 multicenter clinical trial showed promising results, the FDA approved 131I-MIBG therapy for use in recurrent or unresectable malignant pheochromocytoma or paraganglioma [44, 45]. The study showed a 50% or greater reduction in antihypertensive medication in 25% of participants and overall tumor response (based on RECIST 1.0) in 22% of participants; 53% of these patients had a response duration of at least 6 months.
In advance of therapy, results of a pretreatment imaging study should ensure adequate uptake of radiolabeled MIBG. Because free 131I can accumulate and irradiate the thyroid gland, thyroid blockade is instituted typically 1–3 days before therapy and continued for 7–14 days after treatment. More aggressive thyroid blockade can be considered in patients with neuroblastoma, including combination therapy with thyroxine, methimazole, and potassium iodide [46].
Contraindications to 131I therapy include pregnancy, inability to cease breastfeeding, renal failure requiring short-term dialysis, and life expectancy less than 3 months (except for palliation in patients with refractory bone pain). The most critical toxicity related to 131I-MIBG is dose-dependent hematotoxicity, which occurs within weeks of administration but can persist for months. Relative contraindications to 131I therapy include a WBC count less than 3000/μL or a platelet count less than 100 ×103/μL. Acute nonhematologic toxicities include sialadenitis, nausea, and anorexia. These toxicities usually occur within days of therapy and are typically mild and self-limited. Hypothyroidism is in general a late-onset toxicity that can occur despite appropriate thyroid blockade. Severe nonhematologic toxicities are rare but can be fatal. They include acute respiratory distress syndrome, bronchiolitis obliterans, and pulmonary embolism [46].
Although 131I-MIBG can be used to treat certain carcinoid tumors, these tumors have lower uptake of 131I-MIBG than of therapeutic SSAs such as 177Lu-DOTATATE. Iodine-131-labeled MIBG in carcinoid tumors therefore is reserved for patients who have contraindications to SSA radioligands, such as impaired renal function.
During administration of 131I-MIBG, vital sign monitoring, particularly blood pressure monitoring, is essential. After administration, vital signs should be assessed at least twice daily but more frequently in patients with catecholamine-secreting tumors. An acute hypertensive crisis can occur during infusion, and short-acting α- or β-blockers should be readily available for management. Many classes of drugs are known to interact with 131I-MIBG uptake and should theoretically be stopped before MIBG infusion. However, patients with catecholamine-secreting tumors often undergo α- or β-blockade before radioligand therapy (RLT), and withdrawing these medications could precipitate a hypertensive crisis. The European Association of Nuclear Medicine therefore recommends that despite the potential effects on therapeutic MIBG uptake, patients should not stop these medications before therapy [46].
Targeted Alpha Therapy With 225Ac-PSMA Radioligands
Despite the success of 177Lu-PSMA in the management of mCRPC, disease progresses in a significant proportion of patients, even after an initial response [47]. Targeted alpha therapy (TAT) directed at PSMA has therefore gained interest for patients who do not have a sustained response to β-emitting therapy or whose condition is unsuitable for beta therapy at the outset. A small number of α-emitting radionuclides are suitable for clinical translation; 225Ac is the most extensively studied. However, prospective comparative data on 225Ac-PSMA activity are lacking, and safety experience to date with 225Ac-PSMA therapy is limited [48]. At present, 225Ac-PSMA TAT has been offered as compassionate access salvage therapy; it has a unique toxicity profile that includes bone marrow suppression and grade 1 and grade 2 xerostomia in as many as 88% and 12% of patients; both toxicities can be permanent [49]. Experience with 225Ac-PSMA therapy has primarily been in patients in whom prior 177Lu-PSMA therapy has failed. At present it cannot be determined whether 225Ac-PSMA can be considered an alternative to 177Lu-PSMA therapy in patients with mCRPC. Moreover, the optimal timing of TAT as a sequential treatment after 177Lu-PSMA RLT is uncertain given insufficient understanding of the mechanisms of 177Lu-PSMA resistance and of the potential for reduced PSMA expression to negate the effects of TAT [48].
Emerging Radioligands for Solid-Organ Malignancies
PSMA-Targeted Therapies for Other Solid Cancers
PSMA is expressed not only on the surface of prostate cancer cells but also in the neovasculature of a variety of other tumors, including lung, breast, hepatocellular, and thyroid cancers. Given expanding evidence for the use of PSMA RLT and its favorable toxicity profile, the use of PSMA RLT for solid tumors other than prostate cancer will likely expand in the next decade. To date, case reports have described use of PSMA RLT in patients with salivary gland cancer, glioblastoma, thyroid cancer, and hepatocellular carcinoma [50]. Whereas PSMA expression in prostate cancer occurs in the tumor cells themselves, PSMA expression in other solid tumors typically occurs in the tumor neovasculature [51]. Theoretically, such expression may result in rapid RLT washout and reduced tumoricidal effects. Furthermore, the potential interaction with the tumor microenvironment could result in secondary immune responses [50]. Prospective clinical trials are needed to establish any potential role for PSMA RLT in other solid cancers.
Bombesin Analogues and Gastrin-Releasing Peptide Receptors
Gastrin-releasing peptide receptors (GRPRs) are peptides that elicit a multitude of effects on tissues via G protein–coupled receptor binding, including hormone release, smooth muscle contraction, and cell growth [52, 53]. GRPR is highly expressed in many cancers, such as breast, prostate, and lung cancers, and has comparatively little expression in physiologically normal tissues aside from the pancreas and gastrointestinal tract [54-56]. Because of these properties GRPR is an ideal theranostic candidate. Efforts to translate GRPR analogues into clinical practice have predominantly focused on prostate cancer given high expression in prostate cancer but minimal-to-no expression in normal prostate tissue or in benign prostatic hyperplasia [52, 53]. GRPR expression has also been found to be associated with the Gleason score of prostate cancer [57, 58].
GRPR agents can be broadly divided into agonists, antagonists, or a combination of the two. Agonists bind to the GRPR receptor and are internalized; this mechanism of action is preferred for the delivery of particulate radiation. However, clinical translation has been hindered by severe AEs reported during phase 1 clinical trials [52]. Subsequent efforts have focused on developing GRPR antagonists, which do not activate the GRPR receptor, to help reduce AEs [52]. Experience with such agents has been predominantly with the 68Ga-labeled RM-2 GRPR antagonist. The 68Ga-RM2 GRPR antagonist has been studied in healthy volunteers and in patients with breast cancer and prostate cancer [59-61]. In patients with breast cancer, 68Ga-RM2 uptake in the primary tumor was positively correlated with estrogen receptor status. In contrast, in patients with prostate cancer, uptake was significantly higher in the primary tumor site than in the surrounding normal tissue [60, 61].
For patients with mCRPC, 177Lu-RM2 has been developed as a theranostic agent. In 35 patients with mCRPC, 68Ga-RM-2 PET/CT results confirmed GRPR expression, and four of these patients proceeded to treatment with 177Lu-RM2 [62]. The dosimetry was calculated from SPECT/CT of the upper and lower abdomen 1, 24, 48, and 72 hours after injection. The tumors absorbed doses at a therapeutic level, and there was rapid clearance from GRPR-rich organs. A high absorbed dose to the pancreas may limit the maximum therapeutic dose that can be administered and poses a significant barrier to the future use of GRPR-targeted therapy [62, 63]. Furthermore, emerging evidence suggests that GRPR expression may be encountered more frequently in low-grade disease and less frequently in high-grade disease. Although the former relation is helpful for lesion detection, the latter relation potentially reduces the likely role of the agent in theranostic management [64].
Fibroblast Activation Protein Ligands
Fibroblast activation protein (FAP) is a type II membrane-bound glycoprotein enzyme with peptidase activity. FAP is highly expressed by cancer-associated fibroblasts. It has comparatively lower expression in normal tissues. Exceptions include the endometrium of premenopausal women, breast tissue, and areas of bony remodeling (including degenerative arthropathy) [65]. FAP-targeted PET has attracted considerable recent attention because of its broad range of clinical targets and high uptake-to-background ratio [66]. Gallium-68-labeled FAP inhibitor (FAPI) has been used to image many cancers and, among cancers, has had the highest uptake values in sarcoma; esophageal, breast, and lung cancers; and cholangiocarcinoma [66]. The high ratios of uptake to primary and metastatic disease sites and the improved contrast due to low background uptake have prompted interest in the use of FAPI-based PET tracers for tumor characterization and RLT [66-68]. In a series of nine patients with advanced-stage solid cancer who underwent 90Y-FAPI-46 RLT, three patients had radiographic disease control after therapy. Grade 3 or grade 4 hematotoxicity occurred in four patients, and no acute toxicities attributable to 90Y-FAPI-46 were found [69]. Further optimization of FAPI molecular radiotherapy is required, including matching the physical half-life of the isotope to the tumor retention time when accounting for rapid clearance of FAPI from tumors [67].
Practical Integration of a Theranostics Program
Increasing breadth of experience with a multitude of established radiopharmaceutical therapies coupled with the emergence of novel theranostic pairs is leading to a central role of such therapies in oncology and nuclear medicine. It is incumbent on nuclear medicine physicians in practice and in training to understand the practicalities of implementing a theranostics program, including patient-specific dosing, management of AEs, and indications for therapy cessation. To implement a theranostics program, key requirements in terms of infrastructure, personnel, training, and knowledge are needed to deliver safe and effective care (Table 2). Requirements include ease of access to SPECT/CT and PET/CT and access to inpatient services for the management of AEs. The multidisciplinary care team should have sufficient training and experience in dose prescription and radiation safety. Reflecting rapid advances in the field, continuing education of the nuclear medicine team, including nurses, technologists, physicists, and physicians, is critical, Furthermore, a sufficient number of treatments should be performed for the team to remain proficient at implementing care. Table 3 summarizes key prevention and management strategies for various radiotheranostic emergencies.
TABLE 2:
Key Requirements for Implementing a Radiotheranostics Program
| Category | Requirement |
|---|---|
| Infrastructure | Ease of access to PET/CT and SPECT/CT Inpatient facilities for management of adverse events Access to radiopharmaceuticals Appropriate radiopharmaceutical receiving area, hot laboratory, and radioactive waste storage Shielded infusion bays with resuscitation equipment Software for imaging archiving, interpretation, and dosimetry |
| Personnel | Authorized user nuclear medicine or nuclear radiology physician Certified nuclear technologists Radiation safety officer Reimbursement team Additional multidisciplinary team membersa |
| Training and knowledge | Sufficient number of therapies performed annually Extensive knowledge about therapy appropriateness, benefits, and adverse events Continuous medical education with a specific focus on radiopharmaceutical therapies |
Varies by institution but may include nuclear physicists, advanced nonphysician practitioners, and nuclear pharmacists.
TABLE 3:
Prevention and Management of Radiotheranostic Emergencies
| Theranostic Agent |
Emergency | Prevention | Management |
|---|---|---|---|
| 177Lu-DOTATATE | Hormonal crisis | Identify patients at high risk Correct any preexisting electrolyte or metabolic disturbances In patients with hormonally functional PPGL, pretreat with α-blockers (first choice) and avoid aggravating medications (e.g., sympathomimetics) |
Octreotide bolus or continuous infusion H1 receptor blockers, H2 receptor blockers Steroids Consider ICU transfer in the presence of hypotension [14] |
| 177Lu-DOTATATE or 177Lu-PSMA | Tumor lysis syndrome | Assess for preexisting factors (e.g., chronic renal or cardiac disease) Assess for electrolyte disturbance Consider prehydration for patients with elevated serum creatinine level and large tumor burden Consider allopurinol for patients with elevated uric acid [70] |
Supportive care including treating electrolyte disturbances Maximize length of administration (e.g., 4 h) [71] |
| 131I-MIBG | Catecholamine surge (unstable hypertension) | In patients with metabolically active catecholamine-secreting tumors, continue antihypertensives, including β-blockers, acknowledging that these may reduce uptake and storage of MIBG | Stop or slow the infusion α- or β-blockade [46] |
Note—PPGL = pheochromocytoma and paraganglioma.
A critical element of the success of a theranostics program is multidisciplinary input during initial consideration, therapy, and posttreatment follow-up. Medical oncologists, radiation oncologists, and nuclear medicine physicians should work collaboratively to identify not only patients whose condition is appropriate but also the appropriate time for treatment administration. Input should be sought from practitioners in additional medical subspecialties on a per-patient basis. Many centers have a standardized team-based framework for patient evaluation that includes advanced nonphysician practitioners and nuclear technologists with interest and expertise in theranostic approaches, who work under appropriate physician supervision [14]. Multidisciplinary tumor boards are an optimal platform for addressing complex cases and potential therapeutic challenges. Decisions should consider the early and delayed AEs of theranostic regimens, and efforts should be made to minimize potential toxicities before delivery.
Consensus Statements.
Radiotheranostics have rapidly expanded in clinical applications. Targeted radiopharmaceutical therapies for solid malignancies that have received FDA approval include 131I-MIBG therapy in 2018 and 77Lu-vipivotide tetraxetan therapy in 2022.
PRRT, with 177Lu-DOTATATE for example, is a therapeutic strategy in patients with advanced NETs who have experienced disease progression during long-acting SSA therapy and in patients with radioiodine-refractory DTC or metastatic MTC.
Nuclear medicine physicians responsible for the management of 177Lu-DOTATATE therapy should have expertise in interpretation of 68Ga-DOTATATE PET/CT examinations performed before initiation of PRRT and should be familiar with potential therapy-related toxicities and indications for therapy cessation.
The VISION trial showed longer imaging-based progression-free survival and overall survival among patients with mCRPC who underwent 177Lu–vipivotide tetraxetan therapy in combination with standard-of-care therapy than among those who underwent standard-of-care therapy alone. Managing nuclear medicine physicians should recognize indications for cessation of 177Lu–vipivotide tetraxetan therapy.
Critical factors for the success of a radiotheranostics program include ease of access to PET/CT and SPECT/CT, multidisciplinary team input, a sufficient case volume, and continuing education of treating health care practitioners.
Acknowledgments
Supported by a K award (K08CA249047) from the NIH (P Heidari).
Footnotes
The authors declare that there are no disclosures relevant to the subject matter of this article.
References
- 1.Gomes Marin JF, Nunes RF, Coutinho AM, et al. Theranostics in nuclear medicine: emerging and re-emerging integrated imaging and therapies in the era of precision oncology. RadioGraphics 2020; 40:1715–1740 [DOI] [PubMed] [Google Scholar]
- 2.Idée JM, Louguet S, Ballet S, Corot C. Theranostics and contrast-agents for medical imaging: a pharmaceutical company viewpoint. Quant Imaging Med Surg 2013; 3:292–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelkar SS, Reineke TM. Theranostics: combining imaging and therapy. Bioconjug Chem 2011; 22:1879–1903 [DOI] [PubMed] [Google Scholar]
- 4.Silberstein EB, Alavi A, Balon HR, et al. The SNMMI practice guideline for therapy of thyroid disease with 131I 3.0. J Nucl Med 2012; 53:1633–1651 [DOI] [PubMed] [Google Scholar]
- 5.Caers J, Duray E, Vrancken L, et al. Radiotheranostic agents in hematological malignancies. Front Immunol 2022; 13:911080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hope TA, Bergsland EK, Bozkurt MF, et al. Appropriate use criteria for somatostatin receptor PET imaging in neuroendocrine tumors. J Nucl Med 2018; 59:66–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strosberg J, El-Haddad G, Wolin E, et al. ; NETTER-1 Trial Investigators. Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med 2017; 376:125–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Virgolini I. Overall survival results from the NETTER-1 trial in neuroendocrine tumours: an important milestone. Lancet Oncol 2021; 22:1645–1646 [DOI] [PubMed] [Google Scholar]
- 9.Strosberg JR, Caplin ME, Kunz PL, et al. ; NETTER-1 investigators. 177Lu-DOTATATE plus long-acting octreotide versus highdose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol 2021; 22:1752–1763 [DOI] [PubMed] [Google Scholar]
- 10.Wu Q, Chen B, Yan G, Yang Z, Xiong L, He J. A systematic review and meta-analysis of gastrointestinal events associated with nonoperative therapies for neuroendocrine tumors. OncoTargets Ther 2018; 11:7655–7668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Toubah T, Sikaria D, Jesurajan J, et al. Comparison of nausea and vomiting associated with amino acid formulations coinfused with peptide receptor radionuclide therapy: commercial parenteral nutrition formulas versus compounded arginine/lysine. Pancreas 2021; 50:513–515 [DOI] [PubMed] [Google Scholar]
- 12.Kong G, Hicks RJ. Peptide receptor radiotherapy: current approaches and future directions. Curr Treat Options Oncol 2019; 20:77. [DOI] [PubMed] [Google Scholar]
- 13.de Keizer B, van Aken MO, Feelders RA, et al. Hormonal crises following receptor radionuclide therapy with the radiolabeled somatostatin analogue [177Lu-DOTA0,Tyr3]octreotate. Eur J Nucl Med Mol Imaging 2008; 35:749–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Olmo-García MI, Muros MA, López-de-la-Torre M, et al. Prevention and management of hormonal crisis during theragnosis with LU-DOTA-TATE in neuroendocrine tumors. a systematic review and approach proposal. J Clin Med 2020; 9:2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burkett BJ, Dundar A, Young JR, et al. How we do it: a multidisciplinary approach to 177Lu DOTATATE peptide receptor radionuclide therapy. Radiology 2021; 298:261–274 [DOI] [PubMed] [Google Scholar]
- 16.Sabet A, Ezziddin K, Pape UF, et al. Long-term hematotoxicity after peptide receptor radionuclide therapy with 177Lu-octreotate. J Nucl Med 2013; 54:1857–1861 [DOI] [PubMed] [Google Scholar]
- 17.Hörsch D, Ezziddin S, Haug A, et al. Effectiveness and side-effects of peptide receptor radionuclide therapy for neuroendocrine neoplasms in Germany: a multi-institutional registry study with prospective follow-up. Eur J Cancer 2016; 58:41–51 [DOI] [PubMed] [Google Scholar]
- 18.Bergsma H, Konijnenberg MW, Kam BLR, et al. Subacute haematotoxicity after PRRT with (177)Lu-DOTA-octreotate: prognostic factors, incidence and course. Eur J Nucl Med Mol Imaging 2016; 43:453–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kratochwil C, Stefanova M, Mavriopoulou E, et al. SUV of [68Ga]DOTATOC-PET/CT predicts response probability of PRRT in neuroendocrine tumors. Mol Imaging Biol 2015; 17:313–318 [DOI] [PubMed] [Google Scholar]
- 20.Hofman MS, Lau WFE, Hicks RJ. Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: clinical utility, normal patterns, pearls, and pitfalls in interpretation. RadioGraphics 2015; 35:500–516 [DOI] [PubMed] [Google Scholar]
- 21.Werner RA, Solnes LB, Javadi MS, et al. SSTR-RADS version 1.0 as a reporting system for SSTR PET imaging and selection of potential PRRT candidates: a proposed standardization framework. J Nucl Med 2018; 59:1085–1091 [DOI] [PubMed] [Google Scholar]
- 22.Basu S, Sirohi B, Shrikhande SV. Dual tracer imaging approach in assessing tumor biology and heterogeneity in neuroendocrine tumors: its correlation with tumor proliferation index and possible multifaceted implications for personalized clinical management decisions, with focus on PRRT. Eur J Nucl Med Mol Imaging 2014; 41:1492–1496 [DOI] [PubMed] [Google Scholar]
- 23.Zhang P, Yu J, Li J, et al. Clinical and prognostic value of PET/CT imaging with combination of 68Ga-DOTATATE and 18F-FDG in gastroenteropancreatic neuroendocrine neoplasms. Contrast Media Mol Imaging 2018; 2018:2340389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alevroudis E, Spei ME, Chatziioannou SN, et al. Clinical utility of 18F-FDG PET in neuroendocrine tumors prior to peptide receptor radionuclide therapy: a systematic review and meta-analysis. Cancers (Basel) 2021; 13:1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pavel M, Öberg K, Falconi M, et al. ; ESMO Guidelines Committee. Gastroenteropancreatic neuroendocrine neoplasms: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020; 31:844–860 [DOI] [PubMed] [Google Scholar]
- 26.Hope TA, Bodei L, Chan JA, et al. NANETS/SNMMI consensus statement on patient selection and appropriate use of 177Lu-DOTATATE peptide receptor radionuclide therapy. J Nucl Med 2020; 61:222–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah MH, Goldner WS, Benson AB, et al. Neuroendocrine and adrenal tumors, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2021; 19:839–868 [DOI] [PubMed] [Google Scholar]
- 28.Pavel ME, Rinke A, Baum RP. COMPETE trial: peptide receptor radionuclide therapy (PRRT) with 177Lu-edotreotide vs. everolimus in progressive GEP-NET. Ann Oncol 2018; 29(suppl 8):478 [Google Scholar]
- 29.Halfdanarson TR, Reidy DL, Vijayvergia N, et al. Pivotal phase III COMPOSE trial will compare 177Lu-edotreotide with best standard of care for well-differentiated aggressive grade 2 and grade 3 gastroenteropancreatic neuroendocrine tumors. J Clin Oncol 2022; 40(suppl):TPS514 [Google Scholar]
- 30.Tuttle RM, Tala H, Shah J, et al. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid 2010; 20:1341–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aashiq M, Silverman DA, Na’ara S, Takahashi H, Amit M. Radioiodine-refractory thyroid cancer: molecular basis of redifferentiation therapies, management, and novel therapies. Cancers (Basel) 2019; 11:E1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meijer JA, Bakker LE, Valk GD, et al. Radioactive iodine in the treatment of medullary thyroid carcinoma: a controlled multicenter study. Eur J Endocrinol 2013; 168:779–786 [DOI] [PubMed] [Google Scholar]
- 33.Maghsoomi Z, Emami Z, Malboosbaf R, Malek M, Khamseh ME. Efficacy and safety of peptide receptor radionuclide therapy in advanced radioiodine-refractory differentiated thyroid cancer and metastatic medullary thyroid cancer: a systematic review. BMC Cancer 2021; 21:579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fendler WP, Rahbar K, Herrmann K, Kratochwil C, Eiber M. 177Lu-PSMA radioligand therapy for prostate cancer. J Nucl Med 2017; 58:1196–1200 [DOI] [PubMed] [Google Scholar]
- 35.U.S. Food and Drug Administration website. FDA approves Pluvicto for metastatic castration-resistant prostate cancer. www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pluvicto-metastatic-castration-resistant-prostate-cancer. Published Mar 23, 2022. Accessed Nov 13, 2022
- 36.Kaewput C, Vinjamuri S. Update of PSMA Theranostics in prostate cancer: current applications and future trends. J Clin Med 2022; 11:2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rowe SP, Pienta KJ, Pomper MG, Gorin MA. PSMA-RADS version 1.0: a step towards standardizing the interpretation and reporting of PSMA-targeted PET Imaging studies. Eur Urol 2018; 73:485–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sartor O, de Bono J, Chi KN, et al. ; VISION Investigators. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med 2021; 385:1091–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hofman MS, Emmett L, Sandhu S, et al. ; TheraP Trial Investigators and the Australian and New Zealand Urogenital and Prostate Cancer Trials Group. [77Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet 2021; 397:797–804 [DOI] [PubMed] [Google Scholar]
- 40.Kratochwil C, Fendler WP, Eiber M, et al. EANM procedure guidelines for radionuclide therapy with 177Lu-labelled PSMA-ligands (177Lu-PSMA-RLT). Eur J Nucl Med Mol Imaging 2019; 46:2536–2544 [DOI] [PubMed] [Google Scholar]
- 41.Fendler WP, Kratochwil C, Ahmadzadehfar H, et al. 177Lu-PSMA-617 therapy, dosimetry and follow-up in patients with metastatic castration-resistant prostate cancer [in German]. Nuklearmedizin 2016; 55:123–128 [PubMed] [Google Scholar]
- 42.Sisson J, Shapiro B, Beierwaltes WH, et al. Treatment of malignant pheochromocytoma with a new radiopharmaceutical. Trans Assoc Am Physicians 1983; 96:209–217 [PubMed] [Google Scholar]
- 43.Treuner J, Klingebiel T, Feine U, et al. Clinical experiences in the treatment of neuroblastoma with 131I-metaiodobenzylguanidine. Pediatr Hematol Oncol 1986; 3:205–216 [DOI] [PubMed] [Google Scholar]
- 44.Pryma D, Chin B, Noto R, et al. Azedra (iobenguane I 131) in patients with malignant, recurrent and/or unresectable pheochromocytoma or paraganglioma (PPGL): updated efficacy and safety results from a multi-center, open-label, pivotal phase 2 study. J Clin Oncol 2018; 36(15 suppl):400 [Google Scholar]
- 45.Pryma DA, Chin BB, Noto RB, et al. Efficacy and safety of high-specific-activity 131I-MIBG therapy in patients with advanced pheochromocytoma or paraganglioma. J Nucl Med 2019; 60:623–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giammarile F, Chiti A, Lassmann M, Brans B, Flux G. EANM. EANM procedure guidelines for 131I-meta-iodobenzylguanidine (131I-mIBG) therapy. Eur J Nucl Med Mol Imaging 2008; 35:1039–1047 [DOI] [PubMed] [Google Scholar]
- 47.Sathekge MM, Bruchertseifer F, Vorster M, Morgenstern A, Lawal IO. Global experience with PSMA-based alpha therapy in prostate cancer. Eur J Nucl Med Mol Imaging 2021; 49:30–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dhiantravan N, Hofman MS, Ravi Kumar AS. Actinium-225 prostate-specific membrane antigen theranostics: will α beat β? Eur Urol 2021; 79:351–352 [DOI] [PubMed] [Google Scholar]
- 49.Feuerecker B, Tauber R, Knorr K, et al. Activity and adverse events of actinium-225-PSMA-617 in advanced metastatic castration-resistant prostate cancer after failure of lutetium-177-PSMA. Eur Urol 2021; 79:343–350 [DOI] [PubMed] [Google Scholar]
- 50.Uijen MJM, Derks YHW, Merkx RIJ, et al. PSMA radioligand therapy for solid tumors other than prostate cancer: background, opportunities, challenges, and first clinical reports. Eur J Nucl Med Mol Imaging 2021; 48:4350–4368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holzgreve A, Biczok A, Ruf VC, et al. PSMA expression in glioblastoma as a basis for theranostic approaches: a retrospective, correlational panel study including immunohistochemistry, clinical parameters and PET imaging. Front Oncol 2021; 11:646387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mansi R, Nock BA, Dalm SU, Busstra MB, van Weerden WM, Maina T. Radio-labeled bombesin analogs. Cancers (Basel) 2021; 13:5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baratto L, Jadvar H, Iagaru A. Prostate cancer theranostics targeting gastrin-releasing peptide receptors. Mol Imaging Biol 2018; 20:501–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morgat C, MacGrogan G, Brouste V, et al. Expression of gastrin-releasing peptide receptor in breast cancer and its association with pathologic, biologic, and clinical parameters: a study of 1,432 primary tumors. J Nucl Med 2017; 58:1401–1407 [DOI] [PubMed] [Google Scholar]
- 55.Mattei J, Achcar RD, Cano CH, et al. Gastrin-releasing peptide receptor expression in lung cancer. Arch Pathol Lab Med 2014; 138:98–104 [DOI] [PubMed] [Google Scholar]
- 56.Li X, Cai H, Wu X, Li L, Wu H, Tian R. New frontiers in molecular imaging using peptide-based radiopharmaceuticals for prostate cancer. Front Chem 2020; 8:583309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beer M, Montani M, Gerhardt J, et al. Profiling gastrin-releasing peptide receptor in prostate tissues: clinical implications and molecular correlates. Prostate 2012; 72:318–325 [DOI] [PubMed] [Google Scholar]
- 58.Nagasaki S, Nakamura Y, Maekawa T, et al. Immunohistochemical analysis of gastrin-releasing peptide receptor (GRPR) and possible regulation by estrogen receptor βcx in human prostate carcinoma. Neoplasma 2012; 59:224–232 [DOI] [PubMed] [Google Scholar]
- 59.Roivainen A, Kähkönen E, Luoto P, et al. Plasma pharmacokinetics, whole-body distribution, metabolism, and radiation dosimetry of 68Ga bombesin antagonist BAY 86-7548 in healthy men. J Nucl Med 2013; 54:867–872 [DOI] [PubMed] [Google Scholar]
- 60.Stoykow C, Erbes T, Maecke HR, et al. Gastrin-releasing peptide receptor imaging in breast cancer using the receptor antagonist (68)Ga-RM2 and PET. Theranostics 2016; 6:1641–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kähkönen E, Jambor I, Kemppainen J, et al. In vivo imaging of prostate cancer using [68Ga]-labeled bombesin analog BAY86-7548. Clin Cancer Res 2013; 19:5434–5443 [DOI] [PubMed] [Google Scholar]
- 62.Kurth J, Krause BJ, Schwarzenböck SM, Bergner C, Hakenberg OW, Heuschkel M. First-in-human dosimetry of gastrin-releasing peptide receptor antagonist [177Lu]Lu-RM2: a radiopharmaceutical for the treatment of metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging 2020; 47:123–135 [DOI] [PubMed] [Google Scholar]
- 63.Minamimoto R, Hancock S, Schneider B, et al. Pilot comparison of 68Ga-RM2 PET and 68Ga-PSMA-11 PET in patients with biochemically recurrent prostate cancer. J Nucl Med 2016; 57:557–562 [DOI] [PubMed] [Google Scholar]
- 64.Faviana P, Boldrini L, Erba PA, et al. Gastrin-releasing peptide receptor in low grade prostate cancer: can it be a better predictor than prostate-specific membrane antigen? Front Oncol 2021; 11:650249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kessler L, Ferdinandus J, Hirmas N, et al. Pitfalls and common findings in 68Ga-FAPI PET: a pictorial analysis. J Nucl Med 2022; 63:890–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kratochwil C, Flechsig P, Lindner T, et al. 68Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med 2019; 60:801–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Calais J. FAP: the next billion dollar nuclear theranostics target? J Nucl Med 2020; 61:163–165 [DOI] [PubMed] [Google Scholar]
- 68.Sollini M, Kirienko M, Gelardi F, Fiz F, Gozzi N, Chiti A. State-of-the-art of FAPI-PET imaging: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging 2021; 48:4396–4414 [DOI] [PubMed] [Google Scholar]
- 69.Ferdinandus J, Costa PF, Kessler L, et al. Initial clinical experience with 90Y-FAPI-46 radioligand therapy for advanced-stage solid tumors: a case series of 9 patients. J Nucl Med 2022; 63:727–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang K, Brenner W, Prasad V. Tumor lysis syndrome: a rare but serious complication of radioligand therapies. J Nucl Med 2019; 60:752–755 [DOI] [PubMed] [Google Scholar]
- 71.Makis W, McCann K, McEwan AJ. The challenges of treating paraganglioma patients with (177)Lu-DOTATATE PRRT: catecholamine crises, tumor lysis syndrome and the need for modification of treatment protocols. Nucl Med Mol Imaging 2015; 49:223–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mateo J, McKay R, Abida W, et al. Accelerating precision medicine in metastatic prostate cancer. Nat Can 2020; 1:1041–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.U.S. Food and Drug Administration website. Highlights of prescribing information. www.accessdata.fda.gov/drugsatfda_docs/label/2018/208700s000lbl.pdf. Revised Jan 2018. Accessed Oct 25, 2022 [Google Scholar]