Abstract
Although mRNA vaccines for COVID-19 are highly beneficial and are recommended for patients with kidney disease, adverse reactions in some patients after vaccination have been problematic. Various vasculitis and renal disorders have been reported after vaccination; however, a causal relationship has not yet been identified. In this report, we describe a case of rapidly progressive glomerulonephritis that developed after SARS-CoV-2 vaccination, in which both anti-glomerular basement membrane (anti-GBM) and myeloperoxidase antineutrophil cytoplasmic antibodies (MPO-ANCA) were present. The patient’s renal biopsy showed that of the 48 glomeruli in total, four showed global sclerosis and none showed segmental sclerosis. The biopsy showed 11 cellular glomerular crescents and 5 fibrocellular glomerular crescents. Renal function improved with steroids, rituximab, and plasma exchange. Approximately 9 months later, MPO-ANCA was again elevated, and the pulmonary lesions worsened, again requiring multidisciplinary treatment. This case suggests that caution should be exercised in the development of double-positive disease after vaccination, and that long-term observation may be necessary because of the possibility of relapse.
Keywords: SARS-CoV-2 vaccination, MPO-ANCA, Anti-GBM disease
Introduction
Anti-glomerular basement membrane (GBM) disease and ANCA-associated vasculitis (AAV) are rare conditions. However, the co-presentation of both anti-GBM and ANCA antibodies in individual patients is well-recognized and occurs at a much higher frequency than would be expected by chance alone. This “double-positive” phenomenon was first reported within a few years of the first description of ANCA in the 1980s. Various studies have reported that between 21 and 47% of patients with anti-GBM disease are double-positive for ANCA [1].
In patients with severe COVID-19, multi-organ failure reportedly progresses due to endothelial cell damage, increased thrombotic inflammation, and cytokine overproduction. The kidney has also been recognized as a major target organ, and acute kidney injury (AKI) due to COVID-19 is of great interest [2].mRNA vaccines for COVID-19 have been developed and applied at unprecedented rates. Vaccination is highly beneficial and is recommended for patients with renal disease, including those on maintenance dialysis [3]. However, the adverse reactions observed in some patients after vaccination pose a serious problem. Various vasculitis and renal disorders have been reported after vaccination, but no causal relationship has been identified [4]. In this report, we describe a case of rapidly progressive glomerulonephritis with both anti-GBM antibodies and myeloperoxidase antineutrophil cytoplasmic antibodies (MPO-ANCA) that developed after SARS-CoV-2 vaccination, in which renal function improved with steroids, rituximab, and plasma exchange. The patient had re-elevation of MPO-ANCA and exacerbation of pulmonary lesions after approximately 9 months.
Case report
A 74-year-old woman was admitted to our hospital with persistent fever and malaise after receiving her second COVID-19 vaccination (Pfizer-BioNTech). The patient’s symptoms started two days after vaccination. The patient’s medical history included nontuberculous mycobacterial (NTM) infection and sinusitis. She had smoked 20 cigarettes/day for 30 years from the age of 20–50. Prior to the current episode, the patient was followed up every 3 months for chronic kidney disease. Additionally, her sCre was stable at around 0.85–0.88 mg/dL, and both her urine protein and occult blood were continuously negative.
At the previous hospital, laboratory findings were as follows: serum creatinine, 1.28 mg/dL, C-reactive protein (CRP), 20.3 mg/dL, and urinary RBCs, 10–19 RBC/HPF. These findings showed decreased renal function with an abnormal urine analysis and a high inflammatory response. Anti-GBM antibody and MPO-ANCA were both positive (19.7 (reference range 0.0–2.99) IU/mL and 30.7 (reference range 0.0–3.4) IU/mL, respectively). The patient was then transferred to our department for further evaluation and treatment (Day 1). On admission, the patient had no physical symptoms other than fever and malaise. The patient’s body temperature was 37.5 °C, pulse rate was 88 beats/min, blood pressure was 130/76 mmHg, and oxygen saturation was 97% while breathing ambient air. The patient’s weight was 55.8 kg, height was 157.7 cm, and body mass index was 22.44 kg/m2.
On auscultation, the lungs were clear, and heart sounds were normal. No neurological findings or rashes were observed on physical examination. A chest radiograph obtained upon admission showed no findings suggestive of pneumonia or pleural effusion. Computed tomography (CT) of the chest, abdomen, and pelvis performed after admission showed small nodules and cord shadows in the middle lobe of the right lung, consistent with a history of NTM infection and slightly swollen bilateral kidneys. Laboratory data are presented in Table 1. The Birmingham vasculitis activity score (BVAS) version 3 [5] was used to evaluate each organ system. The patient’s scores were as follows: General 0, Cutaneous 0, Mucous membranes/eyes 0, ENT 0, Chest 0 (persistent nodules and pleural effusion), Cardiovascular 0, Abdominal 0, Renal 12 (new onset of hematuria, creatinine 1.41–2.82 mg/dL, and 30% rise in serum creatinine), and Nervous system 0. The patient’s total score was 12.
Table 1.
Laboratory findings during the disease course
| Day 1 | Day 21 | Day 102 | Day 288 | Day 511 | |
|---|---|---|---|---|---|
| Blood test | |||||
| Albumin (g/dL) | 2.0 | 4.0 | 3.4 | 3.7 | 4.1 |
| Aspartate aminotransferase (IU/L) | 33 | 11 | 22 | 13 | 14 |
| Alanine transaminase (IU/L) | 21 | 17 | 38 | 11 | 10 |
| Urea nitrogen (mg/dL) | 16.1 | 20.9 | 27.9 | 14.5 | 21.0 |
| Creatinine (mg/dL) | 1.52 | 1.24 | 1.30 | 1.34 | 1.41 |
| Urine acid (mg/dL) | 5.3 | 3.4 | 5.8 | 5.9 | 6.1 |
| Sodium (mEq/L) | 144 | 137 | 142 | 142 | 145 |
| Potassium (mEq/L) | 3.8 | 4.1 | 4.1 | 3.7 | 3.7 |
| Chloride (mEq/L) | 107 | 103 | 107 | 108 | 109 |
| Corrected calcium (mEq/L) | 10.4 | 9.4 | 9.6 | 9.7 | 9.8 |
| C-reactive protein (mg/dL) | 26.06 | 0.14 | 0.03 | 0.27 | 0.56 |
| Hemoglobin A1c (%) | 5.9 | 5.4 | |||
| IgG (mg/dL) | 1892.0 | 272.0 | 687.0 | ||
| IgA (mg/dL) | 296.0 | 92.0 | 195.0 | ||
| IgM (mg/dL) | 89.0 | 72.0 | 57.0 | ||
| IgG4 (mg/dL) | 209 | 55 | |||
| Complement C3 (mg/dL) | 108.0 | 35.0 | 95.0 | ||
| Complement C4 (mg/dL) | 20.9 | 9.8 | 25.4 | ||
| Rheumatoid factor (IU/mL) | 35.6 | 48.0 | |||
| Anti-nuclear antibody titer | Negative | Negative | |||
| PR3-ANCA (IU/mL) | Negative | Negative | Negative | ||
| MPO-ANCA (IU/mL) | 19.7 | 2.4 | < 0.5 | 10.0 | 3.6 |
| Anti-GBM antibody (EU) | 22.7 | 1.0 | 0.7 | < 1.5 | < 1.5 |
| ASO (IU/mL) | 14 | 53 | |||
| ASK (IU/mL) | < 40 | < 40 | |||
| White blood cell (/μL) | 11,570 | 8520 | 5190 | 9850 | 19,020 |
| Hemoglobin (g/dl) | 8.6 | 8.3 | 9.2 | 11.1 | 12.7 |
| Platelet (× 103/μL) | 518 | 147 | 209 | 22.1 | 23.3 |
| Spot urine urinalysis | |||||
| Specific gravity | 1.005 | 1.011 | 1.013 | 1.007 | 1.016 |
| Blood | 3+ | 3+ | 1+ | 3+ | 2+ |
| Protein | (−) | 1 + | (−) | (−) | (−)〜(±) |
| RBCs (/HPF) | 20–29 | 50–99 | 5–9 | 50–99 | 1–4 |
| Protein (g/gCre) | 0.19 | 1.15 | 0.26 | 0.12 | 0.18 |
Shown are blood and urine data at admission (day 1), 3 weeks after admission (day 21), during remission (day 102), at relapse (day 288), and at time of writing (day 511)
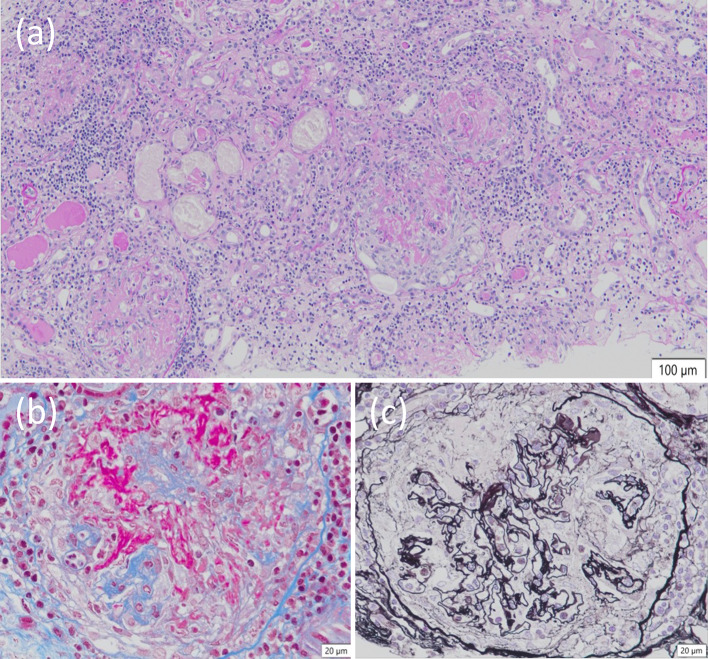
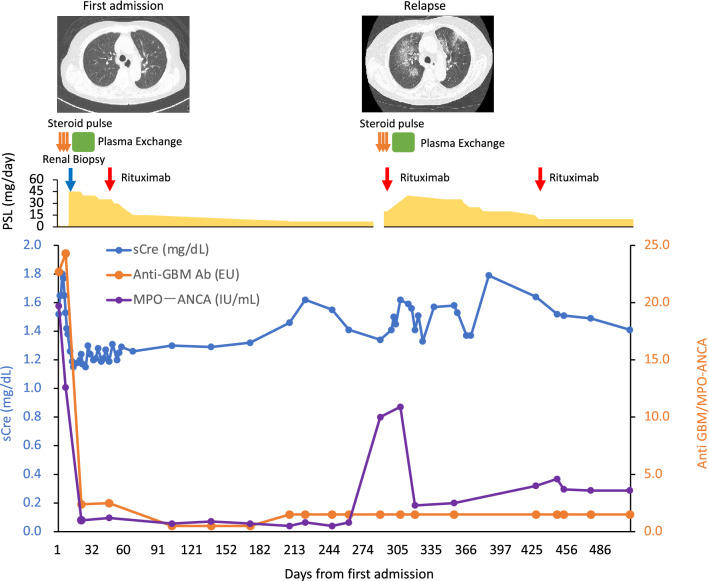
A renal biopsy was performed. Of the 48 glomeruli in total, 4 showed global sclerosis, and none showed segmental sclerosis. There were 11 cellular glomerular crescents and 5 fibrocellular glomerular crescents. Cellular crescent formation with Bowman's cyst rupture and infiltration of cellular components were conspicuous, and fibrin spreading from the basement membrane rupture area to Bowman's cyst was also observed. No basement membrane thickening, doubling, or spike formation was observed. No increase was observed in the mesangium. A kidney biopsy revealed crescentic necrotizing glomerulonephritis with moderate interstitial inflammation under light microscopy (Fig. 1A–C). Immunofluorescence analysis confirmed pauci-immune glomerulonephritis (data not shown). When considered in conjunction with the immunostaining findings, the diagnosis of diffuse crescentic necrotizing glomerulonephritis, the pauci-immune type, was determined to be reasonable. The clinical diagnosis was ANCA-associated nephritis (microscopic polyangiitis), with a positive MPO-ANCA/anti-GBM antibody. The patient was started on intravenous methylprednisolone (1000 mg/day) for three days and oral prednisolone (PSL) 0.8 mg/kg daily after that. The patient underwent plasma exchange therapy. Initially, the patient received fresh-frozen plasma; however, midway through treatment, the patient developed a skin rash. Chlorpheniramine maleate and hydrocortisone were used, but there was no improvement. Therefore, the modality was changed to selective plasma exchange after the third session. (Total plasma exchange was performed on days 7 and 8. Selective plasma exchange was performed on days 11, 13, 15, 18, and 20). As shown in Fig. 2, the serum creatinine levels improved from 1.80 to 1.24 mg/dL, the inflammatory response subsided after plasma exchange, and both anti-GBM antibody and MPO-ANCA turned seronegative on day 21. We gradually reduced the PSL dose and administered 500 mg rituximab on day 50. The patient was discharged on day 58. The patient's BVAS score improved to 0 at the outpatient clinic three months after admission (day 102). The patient's PSL was gradually tapered down to a dose of 7.5 mg/day.
Fig. 1.
Light microscopy findings of kidney biopsy. a Necrotizing glomerulonephritis with moderate interstitial inflammation (Periodic acid–Schiff stain, original magnification × 100). b Glomerular basement membrane breaks and fibrin deposition (Masson stain, original magnification × 400). c The collapse of the glomerular tuft and disruption of Bowman capsule (periodic acid-methenamine-silver original magnification × 400)
Fig. 2.
Clinical course. Days since initial admission, serum creatinine and ANCA, and anti-GBM antibodies are shown. Treatment during the course of the disease and CT findings of the chest are shown in the upper panel. sCre serum creatinine, PSL prednisolone
On the 288th day, the patient’s blood sample was positive for MPO-ANCA (10.0 IU/mL) and negative for anti-GBM antibody. This patient was originally scheduled to receive a second dose of rituximab 6 months after the initial dose but had to postpone it due to an ophthalmology surgery for cataracts in both eyes. As a result, 8 months after the first dose of rituximab, MPO-ANCA was again positive, and the patient was considered to have relapsed. There were no significant episodes of vasculitis leading to relapse in this case. The third dose of the COVID-19 vaccine was not administered as the patient did not wish to receive it. This patient also did not have an episode of COVID-19.
At the time of relapse, the patient had occult blood in the urine, mild urinary protein levels, and an elevated inflammatory response. The patient was diagnosed with recurrent vasculitis and readmitted to the hospital. Crackles were heard in the bilateral lower lung fields. Oxygenation was at SpO2 95% (room air). Chest CT ground-glass opacity and reticular shadows were present mainly around broncho-vascular bundles in the upper lobes of both lungs, and ground-glass opacity also appeared in the middle lobe of the right lung. The findings were consistent with alveolar hemorrhage (Fig. 2). We treated the patient with PSL 1000 mg/day for three days as remission induction therapy, followed by maintenance therapy with PSL 40 mg/day. Since the serum creatinine was also elevated (from 1.34 to 1.62 mg/dL), which was complicated by alveolar hemorrhage, at the time of relapse, the patient was treated with SePE therapy (seven times) in addition to rituximab 500 mg to control the vasculitis caused by ANCA (Table 1, Fig. 2). SePE was administered seven times. Pneumonia improved on chest CT on day 307. On the first admission, CD19 and CD20 were 11.7% and 13.9%, respectively, before the first dose of rituximab. After administration of rituximab, they were 2.1% and 0.1% respectively. CD19 was monitored during outpatient visits and remained at 0.0–0.1%. It slightly increased to 0.3% at relapse and decreased to 0% after the second dose of rituximab. The PSL dose was gradually decreased, and the patient was discharged on day 328. At the time of writing (day 511), the patient was attending the outpatient clinic, and her renal function and urinary findings were stable (Table 1).
Discussion
As vaccination programs are being rolled out globally, many COVID-19 vaccine-related adverse effects have recently been reported, but the causal association between COVID-19 vaccination and de novo autoimmune-related diseases remains unclear. Several cases of new-onset autoimmune-related diseases have been reported after COVID-19 vaccination [6]. Previous studies have revealed that SARS-CoV-2 infection can trigger an autoimmune reaction [7]. Wu et al. reported that among 48 cases of de novo nephrotic syndrome following COVID-19 vaccination, minimal change disease was the most common histopathological diagnosis, followed by IgA nephropathy and vasculitis [4]. Jeffs et al. noted increased ANCA production following viral RNA influenza and rabies vaccine administration. They also demonstrated that this unusual response is linked to vaccines containing viral RNA [8]. Taeschler et al. reported that severely ill COVID-19 patients had a high prevalence of ANCA during acute illness, which was associated with high SARS-CoV-2-specific antibody titers in COVID-19 patients and individuals who received the COVID-19 vaccine [9].
We have reviewed several case reports of single-positive AAV and anti-GBM disease after COVID-19 vaccination [10–27]. To the best of our knowledge, only two cases of anti-GBM antibody and ANCA seropositivity have been reported (Table 2) [16, 27]. In this study, we report the first case of double-positivity for ANCA and anti-GBM antibodies after COVID-19 vaccination, followed by temporary remission by apheresis or other means, which relapsed during the course.
Table 2.
Existing reports of ANCA-associated vasculitis after SARS-CoV-2 vaccination, anti-GBM disease, or double-positive [10–27]
| No | Author | Sex | Age | Complications | Diagnosis | Days | Vaccine type | Vaccine doses | Anti-GBM | ANCA |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Tan | F | 60 | IgAN | Anti-GBM | 1 | Pfizer | 2 | Y | N |
| 2 | Sacker | F | N/A | None | Anti-GBM | 14 | Moderna | 2 | Y | N |
| 3 | Nagai | F | 70 | None | Anti-GBM | 9 | N/A | 2 | Y | N |
| 4 | Shakoor | F | 78 | DM, HTN, Af | AAV | 6 | Pfizer | 2 | N | Y |
| 5 | Dube | F | 29 | Cystic lung disease | AAV | 16 | Pfizer | 2 | N | Y |
| 6 | Sekar | M | 52 | HTN | AAV | 14 | Moderna | 2 | N | Y |
| 7 | Andergg | M | 81 | N/A | AAV | 22 | Moderna | 2 | N | Y |
| 8 | Baier | F | 57 | None | AAV | 5 | Pfizer | 2 | N | Y |
| 9 | Okuda | F | 37 | Graves’ disease | AAV | 12 | Pfizer | 1 | N | Y |
| 10 | Hakroush | F | 79 | HTN, degenerative disk disease | AAV | 14 | Pfizer | 2 | N | Y |
| 11 | Villa M | M | 63 | N/A | AAV | 7 | AstraZeneca | 1 | N | Y |
| 12 | Feghali | M | 58 | N/A | AAV | 4 | Moderna | 2 | N | Y |
| 13 | Felzer | F | 60 | N/A | AAV | 1 | Moderna | 1 | N | Y |
| 14 | Garcia | F | 78 | Odontogenic maxillary sinusitis | AAV | 14 | Sinovac | 1 | N | Y |
| 15 | Ibrahim | F | 79 | Asthma, gastroesophageal reflux disease | AAV | 7 | Moderna | 2 | N | Y |
| 16 | Prabhahar | M | 51 | N/A | AAV | 15 | AstraZeneca | 1 | N | Y |
| 17 | Prema | M | 45 | N/A | AAV | 12 | Bharat | 1 | N | Y |
| 18 | Gupta | M | 23 | Fragile X syndrome, interstitial lung disease | AAV | 14 | Moderna | 2 | Y | Y |
| 19 | Prema | M | 58 | N/A | AAV | 14 | Bharat | 2 | Y | Y |
Double-positive patients have a greater risk of relapse than patients with single-positive anti-GBM disease [28]. The significance of positive anti-GBM antibodies in ANCA-predisposed rapidly progressive glomerulonephritis (RPGN)s continues to be debated. Bosch et al. proposed the theory that ANCA damages GBM by a histotoxic immune response, and that the damaged GBM exposes new antigenic sites and produces anti-GBM antibodies [29]. While ANCA reappears at a high rate of 25–81% after treatment, anti-GBM antibodies disappear 12–18 months after treatment, and recurrence is reported to be very rare [30]. In a rat model of AAV, Kanzaki et al. found that additional administration of anti-GBM antibodies increased albuminuria, enhanced cytokine signaling in glomeruli, and exacerbated crescent formation [31]. Therefore, anti-GBM antibodies may be pathogenic in their own right, even in (ANCA-predominant) double-positive disease. This suggests that, in the presence of anti-GBM antibodies, even if the histopathological diagnosis is AAV, the pathogenic capacity of anti-GBM antibodies should be considered when choosing the treatment for anti-GBM disease.
Cases have also been reported in which plasma exchange was necessary after SARS-CoV-2 vaccination. Anderegg et al. reported an 81-year-old man with AKI after Moderna vaccine administration, proteinuria in non-nephrotic areas, and elevated proteinase 3 (PR3) ANCA titer. The patient had severe nephrotic syndrome [10]. The patient presented with severe pauci-immune crescentic nephritis and was treated with high-dose glucocorticoids, cyclophosphamide, and plasmapheresis [10]. Prema et al. reported a case of remission after steroid pulse therapy, cyclophosphamide, and plasma exchange in a patient double-positive for anti-GBM antibodies and ANCA after vaccination. No long-term follow-up was performed [27]. Plasma exchange therapy has been known to be effective for removing anti-GBM antibodies since the 1980s [32]. Cui et al. showed that plasma exchange therapy is also effective in double-positive cases, suggesting that treatment according to anti-GBM disease status improves outcomes [33]. As noted above, patients diagnosed with anti-GBM disease after administration of the SARS-CoV-2 vaccine have been reported, all of whom were treated with steroids, cyclophosphamide, and plasma apheresis [19, 21, 24]. In the present case, simple plasma exchange using fresh-frozen plasma (FFP) was initially performed because there was concern about the appearance of alveolar hemorrhage. However, due to allergy problems associated with the use of FFP, the patient was switched to SePE. The only randomized controlled trial on the efficacy of plasma exchange therapy for anti-GBM disease was reported in the 1980s by Johnson et al. in which 17 patients were treated. In this randomized controlled trial, patients treated with CS + CY + plasma exchange had significantly faster anti-GBM antibody-negative results and significantly lower serum Cre levels at the end of treatment than those not treated with plasma exchange therapy [32]. Recently, selective plasma exchange has been shown to remove anti-GBM antibodies in the IgG region [34], but has the problem of simultaneously removing coagulation factors. In our case, rituximab was administered as maintenance therapy in addition to steroids and plasma exchange for post-vaccine double-positive disease.
In summary, we report a case of anti-GBM antibody- and ANCA-positive AAV that developed after SARS-CoV-2 vaccination. The patient was followed up for 1 year. The patient was once remitted with multidisciplinary treatment including plasma exchange but relapsed and had to be re-treated. We consider this case valuable because it is the first case in the English literature to report a relapse with long-term follow-up, in addition to the extremely rare occurrence of both anti-GBM antibody and ANCA positivity after SARS-CoV-2 vaccination. Although the direct relationship between vaccination and vasculitis is not clear, it was considered necessary to note that long-term observation may be required, as in this case.
Author contributions
KT, TN, and DK performed renal biopsies and treated the patient during hospitalization; AS, AH, MS, and TH treated the patient during relapse. KT and DK prepared the manuscript. All the authors have read and approved the final manuscript.
Funding
This work was partly supported by Grants-in-Aid for Research from the National Center for Global Health and Medicine (21A2002).
Data availabilty
All data generated or analyzed during this study are included in this published article.
Declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee at which the studies were conducted and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from the patient included in the study. This article does not contain any studies with animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Segelmark M, Hellmark T. Anti-glomerular basement membrane disease: an update on subgroups, pathogenesis and therapies. Nephrol Dialysis Transpl. 2019;34(11):1826–1832. doi: 10.1093/ndt/gfy327. [DOI] [PubMed] [Google Scholar]
- 2.Katagiri D. For safe and adequate blood purification therapy in severe COVID-19—what we have learned so far. Glob Health Med. 2022;4(2):94–100. doi: 10.35772/ghm.2022.01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katagiri D, Kikuchi K. The impact and treatment of COVID-19 in hemodialysis patients. J Clin Med. 2023;12(3):838. doi: 10.3390/jcm12030838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu HHL, Kalra PA, Chinnadurai R. New-onset and relapsed kidney histopathology following COVID-19 vaccination: a systematic review. Vaccines (Basel) 2021;9(11):1252. doi: 10.3390/vaccines9111252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mukhtyar C, Lee R, Brown D, Carruthers D, Dasgupta B, Dubey S, et al. Modification and validation of the Birmingham Vasculitis Activity Score (version 3) Ann Rheum Dis. 2009;68(12):1827–1832. doi: 10.1136/ard.2008.101279. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Xu Z, Wang P, Li XM, Shuai ZW, Ye DQ, et al. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology. 2022;165(4):386–401. doi: 10.1111/imm.13443. [DOI] [PubMed] [Google Scholar]
- 7.Ehrenfeld M, Tincani A, Andreoli L, Cattalini M, Greenbaum A, Kanduc D, et al. COVID-19 and autoimmunity. Autoimmun Rev. 2020;19(8):102597. doi: 10.1016/j.autrev.2020.102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeffs LS, Nitschke J, Tervaert JW, Peh CA, Hurtado PR. Viral RNA in the influenza vaccine may have contributed to the development of ANCA-associated vasculitis in a patient following immunisation. Clin Rheumatol. 2016;35(4):943–951. doi: 10.1007/s10067-015-3073-0. [DOI] [PubMed] [Google Scholar]
- 9.Taeschler P, Cervia C, Zurbuchen Y, Hasler S, Pou C, Tan Z, et al. Autoantibodies in COVID-19 correlate with antiviral humoral responses and distinct immune signatures. Allergy. 2022;77(8):2415–2430. doi: 10.1111/all.15302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderegg MA, Liu M, Saganas C, Montani M, Vogt B, Huynh-Do U, et al. De novo vasculitis after mRNA-1273 (Moderna) vaccination. Kidney Int. 2021;100(2):474–476. doi: 10.1016/j.kint.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baier E, Olgemöller U, Biggemann L, Buck C, Tampe B. Dual-positive MPO- and PR3-ANCA-associated vasculitis following SARS-CoV-2 mRNA booster vaccination: a case report and systematic review. Vaccines (Basel) 2022;10(5):653. doi: 10.3390/vaccines10050653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dube GK, Benvenuto LJ, Batal I. Antineutrophil cytoplasmic autoantibody-associated glomerulonephritis following the Pfizer-BioNTech COVID-19 vaccine. Kidney Int Rep. 2021;6(12):3087–3089. doi: 10.1016/j.ekir.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feghali EJ, Zafar M, Abid S, Santoriello D, Mehta S. De-novo antineutrophil cytoplasmic antibody-associated vasculitis following the mRNA-1273 (Moderna) vaccine for COVID-19. Cureus. 2021;13(11):e19616. doi: 10.7759/cureus.19616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felzer JR, Fogwe DT, Samrah S, Michet CJ, Jr, Specks U, Baqir M, et al. Association of COVID-19 antigenicity with the development of antineutrophilic cytoplasmic antibody vasculitis. Respirol Case Rep. 2022;10(1):e0894. doi: 10.1002/rcr2.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia DS, Martins C, da Fonseca EO, de Carvalho VCP, de Rezende RPV. Clinical Images: severe proteinase 3 antineutrophil cytoplasmic antibody glomerulonephritis temporally associated with Sinovac Biotech's inactivated SARS-CoV-2 vaccine. ACR Open Rheumatol. 2022;4(4):277–278. doi: 10.1002/acr2.11397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta RK, Ellis BK. Concurrent antiglomerular basement membrane nephritis and antineutrophil cytoplasmic autoantibody-mediated glomerulonephritis after second dose of SARS-CoV-2 mRNA vaccination. Kidney Int Rep. 2022;7(1):127–128. doi: 10.1016/j.ekir.2021.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hakroush S, Tampe B. Case report: ANCA-associated vasculitis presenting with rhabdomyolysis and pauci-immune crescentic glomerulonephritis after Pfizer-BioNTech COVID-19 mRNA vaccination. Front Immunol. 2021;12:762006. doi: 10.3389/fimmu.2021.762006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibrahim H, Alkhatib A, Meysami A. Eosinophilic granulomatosis with polyangiitis diagnosed in an elderly female after the second dose of mRNA vaccine against COVID-19. Cureus. 2022;14(1):e21176. doi: 10.7759/cureus.21176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagai K, Iwase M, Ueda A. A case of anti-GBM nephritis following centipede bites and COVID-19 vaccination. CEN Case Rep. 2022;11(2):166–170. doi: 10.1007/s13730-021-00646-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okuda S, Hirooka Y, Sugiyama M. Propylthiouracil-induced antineutrophil cytoplasmic antibody-associated vasculitis after COVID-19 vaccination. Vaccines (Basel). 2021;9(8):842. doi: 10.3390/vaccines9080842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sacker A, Kung V, Andeen N. Anti-GBM nephritis with mesangial IgA deposits after SARS-CoV-2 mRNA vaccination. Kidney Int. 2021;100(2):471–472. doi: 10.1016/j.kint.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sekar A, Campbell R, Tabbara J, Rastogi P. ANCA glomerulonephritis after the Moderna COVID-19 vaccination. Kidney Int. 2021;100(2):473–474. doi: 10.1016/j.kint.2021.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shakoor MT, Birkenbach MP, Lynch M. ANCA-associated vasculitis following Pfizer-BioNTech COVID-19 Vaccine. Am J Kidney Dis. 2021;78(4):611–613. doi: 10.1053/j.ajkd.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan HZ, Tan RY, Choo JCJ, Lim CC, Tan CS, Loh AHL, et al. Is COVID-19 vaccination unmasking glomerulonephritis? Kidney Int. 2021;100(2):469–471. doi: 10.1016/j.kint.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villa M, Díaz-Crespo F, Pérez de José A, Verdalles Ú, Verde E, Almeida Ruiz F, et al. A case of ANCA-associated vasculitis after AZD1222 (Oxford-AstraZeneca) SARS-CoV-2 vaccination: casualty or causality? Kidney Int. 2021;100(4):937–938. doi: 10.1016/j.kint.2021.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prabhahar A, Naidu G, Chauhan P, Sekar A, Sharma A, Sharma A, et al. ANCA-associated vasculitis following ChAdOx1 nCoV19 vaccination: case-based review. Rheumatol Int. 2022;42(4):749–758. doi: 10.1007/s00296-021-05069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prema J, Muthukumaran A, Haridas N, Fernando E, Seshadri J, Kurien AA. Two cases of double-positive antineutrophil cytoplasmic autoantibody and antiglomerular basement membrane disease after BBV152/covaxin vaccination. Kidney Int Rep. 2021;6(12):3090–3091. doi: 10.1016/j.ekir.2021.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McAdoo SP, Tanna A, Hrušková Z, Holm L, Weiner M, Arulkumaran N, et al. Patients double-seropositive for ANCA and anti-GBM antibodies have varied renal survival, frequency of relapse, and outcomes compared to single-seropositive patients. Kidney Int. 2017;92(3):693–702. doi: 10.1016/j.kint.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosch X, Mirapeix E, Font J, Borrellas X, Rodríguez R, López-Soto A, et al. Prognostic implication of anti-neutrophil cytoplasmic autoantibodies with myeloperoxidase specificity in anti-glomerular basement membrane disease. Clin Nephrol. 1991;36(3):107–113. [PubMed] [Google Scholar]
- 30.Rutgers A, Heeringa P, Damoiseaux JG, Tervaert JW. ANCA and anti-GBM antibodies in diagnosis and follow-up of vasculitic disease. Eur J Intern Med. 2003;14(5):287–295. doi: 10.1016/S0953-6205(03)00097-9. [DOI] [PubMed] [Google Scholar]
- 31.Kanzaki G, Nagasaka S, Higo S, Kajimoto Y, Kanemitsu T, Aoki M, et al. Impact of anti-glomerular basement membrane antibodies and glomerular neutrophil activation on glomerulonephritis in experimental myeloperoxidase-antineutrophil cytoplasmic antibody vasculitis. Nephrol Dialysis Transpl. 2016;31(4):574–585. doi: 10.1093/ndt/gfv384. [DOI] [PubMed] [Google Scholar]
- 32.Johnson JP, Moore J, Jr, Austin HA, 3rd, Balow JE, Antonovych TT, Wilson CB. Therapy of anti-glomerular basement membrane antibody disease: analysis of prognostic significance of clinical, pathologic and treatment factors. Medicine. 1985;64(4):219–227. doi: 10.1097/00005792-198507000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Cui Z, Zhao J, Jia XY, Zhu SN, Jin QZ, Cheng XY, et al. Anti-glomerular basement membrane disease: outcomes of different therapeutic regimens in a large single-center Chinese cohort study. Medicine. 2011;90(5):303–311. doi: 10.1097/MD.0b013e31822f6f68. [DOI] [PubMed] [Google Scholar]
- 34.Yamatani S, Shirai A, Hirohata Y, Kumamoto N, Kosaka K, Kikuta J, et al. Treatment of rapidly progressive anti-GBM antibody-type glomerulonephritis with combination therapy involving simple plasma exchange and selective plasma exchange: a case report. Nihon Toseki Igakkai Zasshi. 2018;51(9):557–563. doi: 10.4009/jsdt.51.557. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.