Abstract
Background
Therapeutic trials in Alzheimer’s disease (AD) face many obstacles—particularly with regard to screening and recruitment.
Discussion
Decentralized clinical trials (DCTs) are being developed in other diseases and appear to be of value for overcoming these difficulties. The use of remote visits offers hope of broader recruitment and thus a reduction in inequalities due to age, geography, and ethnicity. Furthermore, it might be easier to involve primary care providers and caregivers in DCTs. However, further studies are needed to determine the feasibility of DCTs in AD.
Summary
A mixed-model DCT might constitute the first step towards completely remote trials in AD and should be assessed first.
Keywords: Therapeutic trials, Alzheimer’s disease, Digital tools, Decentralized trials
Background
According to Alzheimer Disease International (ADI), the number of people with dementia was 50 million in 2018 and will rise to 150 million in 2050 [1]—even though more recent data suggest that the incidence and prevalence of dementia are falling in western Europe [2]. Among people aged 60 or over, the estimated prevalence of mild cognitive impairment (which can convert to dementia) is between 16 and 20% [3]. Cognitive impairment is still challenging to prevent, diagnose, and care for, and so this major public health issue requires significant clinical research efforts.
On one hand, the global pandemic of coronavirus disease, since early 2019, exposed weaknesses in the clinical research system: ongoing trials halted their recruitment and study procedures, while new trials were left on hold [4]. On the other hand, this crisis period catalyzed the emergence of innovative clinical trials methodologies and tools [5, 6]. With the use of today’s digital health technologies (telemedicine, wearable devices, mobile apps, etc.), the decentralized clinical trial (DCT) might now be a valuable way of bypassing pinch points in clinical research [7]. In a DCT, participants do not have to visit a central investigating center (such as a hospital or research institute) on a regular basis. Study visits can be avoided though the use of digital tools for the remote collection and monitoring of the participants’ health data. DCTs can also involve decentralized trial networks that involve several investigating centers for the real-time sharing and analysis of data. Digital health technologies allow for the remote collection of information at every stage of the clinical trial and can improve recruitment and participation rates via a “patient-centric trial” approach [8].
Discussion
In the field of cognitive disorders, the clinical research issues are huge. ADI advises investing 1% of the societal cost of dementia in clinical research [1]. Alzheimer’s disease (AD) is a major health issue because of its current high prevalence and the poor therapeutic arsenal. However, the failure rate for therapeutic trials in AD is particularly high: 99.6% in 2014, according to Cummings et al. [9]. Nevertheless, in 2022, 143 drugs were being tested in 172 trials [10]. Only two molecules have given encouraging results since then [11, 12]. Several other challenges have been identified, such as slow recruitment and missing data [13]. We believe that new communication technologies and the development of telemedicine and connected health will improve randomized clinical trials at all steps.
Recruitment is often a slow process, with an estimated rate of 0.2 patients per site per month. Due to screening failures, it has been estimated that the screening phase should be from 1.8 to 3.8 times longer than the treatment phase (depending on the trial phase and the population’s cognitive status) [10, 13]. Myers et al. [14] suggested that virtual visits can eliminate geographical barriers and enable safe, efficient recruitment in clinical trials in Parkinson’s disease. Indeed, this suggestion has been made for all clinical trials [15] and could be applied to AD trials. Moreover, recruitment into AD trials has limitations related to ethnicity and age. Indeed, a recent cross-sectional study concluded that some ethnic groups, people with a lower educational level, and women were under-represented in AD trials [16]. DCTs might constitute a good way of reducing inequalities in research participation [17]. This is the view held by some of the speakers at the 2019 “Virtual Clinical Trials: Challenges and Opportunities” workshop hosted by the US National Academy of Sciences [18]. Furthermore, a qualitative study in UK highlighted the need of a caregiver in recruitment [19]. DCT may also be especially beneficial for study partners and caregivers who may have difficulty traveling to a trial site due to factors such as distance, cost, or mobility issues. Therefore, one of the major benefits of a virtual trial can be precisely to reduce the burden on participants and their caregivers.
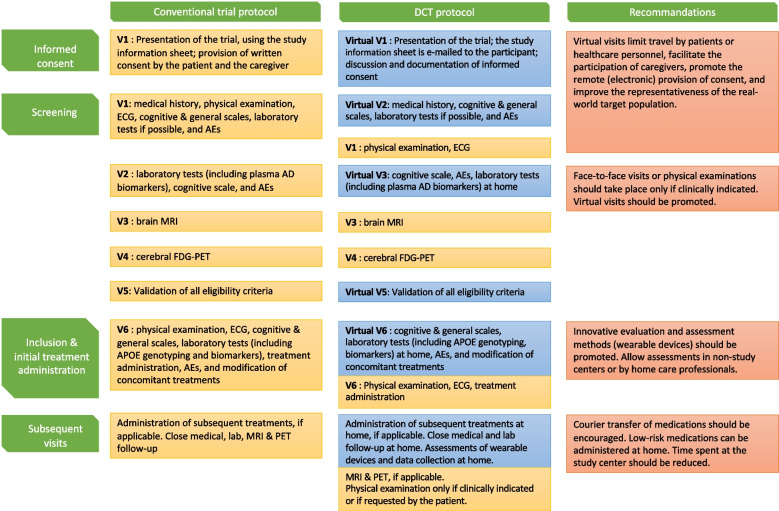
AD trials are also known for their high screening failure rate [13]. This failure necessitates a broad pre-screening process, depending on the setting and the recruitment pathway. In a study of four cohorts in Europe, the number needed to prescreen per amyloid-positive participant ranged from 6.9 to 88.5 [20]. The US National Institute on Aging has suggested a number of strategies for improving the trial recruitment of older adults with dementia. These recommendations include the development of community partnerships and the promotion of science to healthcare providers and caregivers [21]. In our opinion, remote screening and recruitment might also increase the screening rate and facilitate the involvement of primary care providers and centers (e.g., private-practice physicians, home care nurses, local hospitals, and nursing homes) in AD clinical trials. Digital health technologies (e.g., wearable devices) might also facilitate patient follow-up and safety monitoring during routine care [18]. As a result, the home administration of drugs (and especially low-risk drugs) would also become safer [7]. Through these advantages, DCTs might facilitate various aspects of patient recruitment and inclusion in the field of AD (Fig. 1). In addition to their value in the early phases of trials, digital tools might also enable greater standardization of tests and data collection and thus the composition of more uniform study subgroups.
Fig. 1.
Trials designs in Alzheimer's disease. A comparison of conventional and DCT designs in AD, with recommendations for optimizing research processes. AD, Alzheimer’s disease; AE, adverse event; APOE, apolipoprotein E; ECG, electrocardiogram; FDG-PET, fluorodeoxyglucose positron emission tomography; MRI, magnetic resonance imaging
However, DCTs have a number of limitations, especially when considering patients with cognitive impairment. Firstly, the data from a clinical examination cannot be collected remotely, and so a fully remote virtual clinical trial is probably not appropriate. On the same lines, remote cognitive assessments must be validated before they can be considered as judgment and inclusion criteria in a DCT. There are few data on this topic [22], but further dedicated studies are now warranted. This also raises questions about the administration and safety of the investigated therapies in general and parenteral treatments in particular. For example, it would be more difficult to monitor the occurrence of adverse events such as amyloid-related imaging abnormalities (which requires repeated brain MRI scans) in a virtual trial. Secondly, DCTs can represent new costs that might have not been considered previously by trial sponsors. Thirdly, and despite their theoretical utility in standardizing data collection, DCTs will lack the human factor to some extent—a factor that is important to cognitively impaired patients and their study partners and caregivers, whose role is required for key cognitive evaluation batteries. Moreover, the reliability and accuracy of remotely collected data can be questioned [23]. The implementation of virtual trials would thus require dedicated training of the healthcare personnel involved. Fourthly, more data are needed to confirm the quality and benefits of DCTs. Researchers are encouraged to publish their findings (including negative results and operational details) and thus guide the future development of DCTs [24]. The experiences of patients, their caregivers, and care providers must also be assessed, in order to identify potential levers for the virtual approach.
Summary
Some of the obstacles in AD trials might be resolved by the use of digital health tools, such as remote recruitment and assessment. Some types of health data can be collected more flexibly and safety via wearable devices. Digital health technology might help to make clinical research more inclusive and more representative. By facilitating the recruitment of people from diverse settings, we can also hope to increase study sample sizes and statistical power. However, measuring the quality of digital trials remains an issue. Since virtual trials have other limitations, a hybrid approach (i.e., a combination of virtual visits at home and face-to-face visits at investigating centers or other centers) might be a good compromise. DCTs emerge as useful innovative approaches for observational and interventional trials in AD. However, further research (including feasibility studies) is needed before decentralized components can be exported to randomized, controlled trials. Lastly, the use of digital health tools in observational studies might trigger a new era of development in this field.
Acknowledgements
Not applicable
Authors’ contributions
VL, as co-first author, designed the discussion, performed the literature search, and drafted the manuscript. WG, as co-first-author, designed the discussion, performed the literature search, and drafted the manuscript. AA designed the discussion and revised the manuscript. JN designed the discussion and revised the manuscript. ACB designed the discussion and revised the manuscript. DB designed the discussion, drafted the figure, and revised the manuscript. CD designed the discussion and revised the manuscript. BF designed the discussion and revised the manuscript. The authors read and approved the final manuscript.
Funding
We received no funding for this article.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Victoire Leroy and Wassim Gana contributed equally to this work and co-first authorship.
References
- 1.Alzheimer’s Disease International. World Alzheimer Report 2018 The state of the art of dementia research: New frontiers. September 2018.
- 2.Wu Y-T, Fratiglioni L, Matthews FE, et al. Dementia in western Europe: epidemiological evidence and implications for policy making. Lancet Neurol. 2016;15:116–124. doi: 10.1016/S1474-4422(15)00092-7. [DOI] [PubMed] [Google Scholar]
- 3.Roberts R, Knopman DS. Classification and epidemiology of MCI. Clin Geriatr Med 2013; 29: 10.1016/j.cger.2013.07.003. [DOI] [PMC free article] [PubMed]
- 4.Dorsey ER, Kluger B, Lipset CH. The new normal in clinical trials: decentralized studies. Ann Neurol. 2020;88:863–866. doi: 10.1002/ana.25892. [DOI] [PubMed] [Google Scholar]
- 5.Jimenez-Maggiora GA, Bruschi S, Qiu H, So J-S, Aisen PS. ATRI EDC: a novel cloud-native remote data capture system for large multicenter Alzheimer’s disease and Alzheimer’s disease-related dementias clinical trails. JAMIA Open. 2022;5(1):2022, 1–14. [DOI] [PMC free article] [PubMed]
- 6.Walter S, Clanton TB, Langford OG, et al. Recruitment into the Alzheimer Prevention Trials (APT) Webstudy for a Trial-Ready Cohort for Preclinical and Prodromal Alzheimer’s Disease (TRC-PAD) J Prev Alzheimers Dis. 2020;7:219–225. doi: 10.14283/jpad.2020.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doherty GJ, Goksu M, de Paula BHR. Rethinking cancer clinical trials for COVID-19 and beyond. Nat Cancer. 2020;1:568–572. doi: 10.1038/s43018-020-0083-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma NS. Patient centric approach for clinical trials: current trend and new opportunities. Perspect Clin Res. 2015;6:134–138. doi: 10.4103/2229-3485.159936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cummings JL, Morstorf T, Zhong K. Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther. 2014;6:37. doi: 10.1186/alzrt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cummings J, Lee G, Nahed P, et al. Alzheimer’s disease drug development pipeline: 2022. Alzheimers Dement (N Y) 2022;8:e12295. doi: 10.1002/trc2.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J Prev Alzheimers Dis. 2022;9(2):197–210. [DOI] [PubMed]
- 12.van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388:9–21. doi: 10.1056/NEJMoa2212948. [DOI] [PubMed] [Google Scholar]
- 13.Cummings J, Aisen P, Barton R, et al. Re-engineering Alzheimer clinical trials: global Alzheimer’s Platform network. J Prev Alzheimers Dis. 2016;3:114. doi: 10.14283/jpad.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myers TL, Augustine EF, Baloga E, et al. Recruitment for remote decentralized studies in Parkinson’s disease. J Parkinsons Dis. 2022;12:371–380. doi: 10.3233/JPD-212935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aiyegbusi OL, Davies EH, Myles P et al. Digitally enabled decentralised research: opportunities to improve the efficiency of clinical trials and observational studies. BMJ Evid Based Med. 2023;bmjebm-2023–112253. [DOI] [PMC free article] [PubMed]
- 16.Raman R, Quiroz YT, Langford O, et al. Disparities by race and ethnicity among adults recruited for a preclinical Alzheimer disease trial. JAMA Netw Open. 2021;4:e2114364. doi: 10.1001/jamanetworkopen.2021.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beauchet O, Galery K, Lafontaine C et al. Frailty, e-health and prevention of late-onset Alzheimer disease and related disorders: it is time to take action. Aging Clin Exp Res. 2022;34(5):1179–81. [DOI] [PMC free article] [PubMed]
- 18.National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Sciences Policy; Forum on Drug Discovery, Development, and Translation. Virtual Clinical Trials: Challenges and Opportunities: Proceedings of a Workshop. Washington (DC): National Academies Press (US), 2019. [PubMed]
- 19.Clement C, Selman LE, Kehoe PG, Howden B, Lane JA, Horwood J. Challenges to and facilitators of recruitment to an Alzheimer’s disease clinical trial: a qualitative interview study. J Alzheimers Dis. 2019;69:1067–1075. doi: 10.3233/JAD-190146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vermunt L, Muniz-Terrera G, Ter Meulen L, et al. Prescreening for European Prevention of Alzheimer Dementia (EPAD) trial-ready cohort: impact of AD risk factors and recruitment settings. Alzheimers Res Ther. 2020;12:8. doi: 10.1186/s13195-019-0576-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Strategy for Recruitment and Participation in Alzheimer’s and Related Dementias Clinical Research. National Institute on Aging https://www.nia.nih.gov/research/recruitment-strategy (26 April 2022, date last accessed). [DOI] [PMC free article] [PubMed]
- 22.Beishon LC, Elliott E, Hietamies TM et al. Diagnostic test accuracy of remote, multidomain cognitive assessment (telephone and video call) for dementia. Cochrane Database Syst Rev. 2022(4). [DOI] [PMC free article] [PubMed]
- 23.McDermott MM, Newman AB. Remote research and clinical trial integrity during and after the coronavirus pandemic. JAMA. 2021;325:1935–1936. doi: 10.1001/jama.2021.4609. [DOI] [PubMed] [Google Scholar]
- 24.Rogers A, De Paoli G, Subbarayan S, et al. A systematic review of methods used to conduct decentralised clinical trials. Br J Clin Pharmacol. 2022;88:2843–2862. doi: 10.1111/bcp.15205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.