Abstract
Background
The optimal time to intubate patients with SARS-CoV-2 pneumonia has not been adequately determined. While the use of non-invasive respiratory support before invasive mechanical ventilation might cause patient-self-induced lung injury and worsen the prognosis, non-invasive ventilation (NIV) is frequently used to avoid intubation of patients with acute respiratory failure (ARF). We hypothesized that delayed intubation is associated with a high risk of mortality in COVID-19 patients.
Methods
This is a secondary analysis of prospectively collected data from adult patients with ARF due to COVID-19 admitted to 73 intensive care units (ICUs) between February 2020 and March 2021.
Intubation was classified according to the timing of intubation. To assess the relationship between early versus late intubation and mortality, we excluded patients with ICU length of stay (LOS) < 7 days to avoid the immortal time bias and we did a propensity score and a cox regression analysis.
Results
We included 4,198 patients [median age, 63 (54‒71) years; 71% male; median SOFA (Sequential Organ Failure Assessment) score, 4 (3‒7); median APACHE (Acute Physiology and Chronic Health Evaluation) score, 13 (10‒18)], and median PaO2/FiO2 (arterial oxygen pressure/ inspired oxygen fraction), 131 (100‒190)]; intubation was considered very early in 2024 (48%) patients, early in 928 (22%), and late in 441 (10%). ICU mortality was 30% and median ICU stay was 14 (7‒28) days. Mortality was higher in the “late group” than in the “early group” (37 vs. 32%, p < 0.05). The implementation of an early intubation approach was found to be an independent protective risk factor for mortality (HR 0.6; 95%CI 0.5‒0.7).
Conclusions
Early intubation within the first 24 h of ICU admission in patients with COVID-19 pneumonia was found to be an independent protective risk factor of mortality.
Trial registration
The study was registered at Clinical-Trials.gov (NCT04948242) (01/07/2021).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12871-023-02081-5.
Keywords: SARS-COV2, COVID-19 pneumonia, Timing to intubation, Mechanical ventilation
Background
Patients with coronavirus disease 2019 (COVID-19), caused by the SARS-CoV-2 virus, can develop severe hypoxemia and acute respiratory distress syndrome (ARDS) [1]. Delayed intubation in ARDS patients has been reported to increase mortality, so ARDS guidelines recommend early recognition of acute respiratory failure (ARF) and intubation [2]. At the beginning of the pandemic when ARF was labelled as ARDS, the indication was to immediately intubate [3-7]. Subsequently, it was observed that these patients could tolerate even a severe degree of hypoxemia without or seldom respiratory symptoms. These issues, as well as the lack of ventilators and ICU rooms, resulted in patients being intubated later. So, it was observed that some of these patients never ended up intubated, and potential secondary effects associated with invasive mechanical ventilation (IMV), as ventilator-induced lung injury (VILI) and ventilator-associated pneumonia (VAP) were avoided [8, 9]. Published COVID-19 clinical practice guidelines recommended a trial of non-invasive respiratory support first [10].
Nowadays, whether to intubate early or late remains controversial. Several expert consensus recommend early intubation in patients with a severe presentation of COVID-19 pneumonia because a large proportion of these patients could potentially develop ARF requiring emergency orotracheal intubation, which is associated to a higher risk of viral transmission to healthcare workers [3-6]. Moreover, some documents also recommended early intubation to prevent patient-self-induced lung injury (P-SILI) [11, 12]. On the other hand, there is probably not enough evidence for P-SILI in NIV and obviously there are many uncertainties regarding the right strategy to recommend the use of an invasive or not mechanical ventilation in patients with COVID-19 pneumonia [13].
A recent meta-analysis including more than 8,000 critically ill patients with COVID-19 suggested that the timing of intubation may have no impact on mortality [14]. This study acknowledged the huge heterogeneity of the studies analysed, that reflects different clinical strategies regarding this topic and it would preclude definitive conclusions. We hypothesized that delayed intubation is associated with higher mortality in COVID-19. To test this hypothesis, we aimed to determine the association of intubation timing on ICU mortality in a large population of critically ill COVID-19 patients.
Methods
Design
This a secondary analysis of prospectively collected data from adult patient with ARF due to COVID-19 admitted to 73 intensive care units (ICUs) (71 in Spain, 1 in Andorra, and 1 in Ireland) between February 22, 2020 and March 11, 2021. Data were retrieved from the Spanish Society of Intensive Care Medicine and Coronary Units’ (SEMICYUC) registry of COVID-19 patients. The study was registered at Clinical-Trials.gov (NCT04948242) (01/07/2021).
We selected all consecutive patients ≥ 15 years old who met the criteria for COVID-19 pneumonia and ARDS according to the Berlin criteria [15]. We excluded patients with limitations on life support and those with missing data. Patients were followed up to ICU discharge or death.
Data collection
Patients’ demographic and clinical data were recorded on a case report form, anonymized, and sent to the coordinating centre, where all the information was entered in the COVID-19 registry, as reported elsewhere [16]. Demographic characteristics (age, sex, and body mass index), comorbidities, laboratory tests, microbiologic results, radiological findings, time to intubation, non-invasive respiratory support (oxygen, high-flow nasal cannula [HFNC], non-invasive ventilation [NIV], and IMV) at ICU admission and 24 h after admission, complications, organ-support measures, treatments administered, and outcomes were registered. Disease severity was evaluated 24h after ICU admission using the APACHE II score and SOFA score.
Clinical decisions (e.g., indicating intubation, respiratory support, and treatments) were not protocolized and were left to the discretion of attending physicians. COVID-19 diagnosis was confirmed by reverse transcription-polymerase chain reactions (rt-PCR) for SARS-CoV-2 and COVID-19 pneumonia was diagnosed through clinical signs of pneumonia with acute respiratory failure and lung infiltrates on chest imaging [17].
Outcome
The primary outcome was all-cause ICU mortality.
Secondary outcomes included hospital mortality, ICU and hospital LOS, and time under IMV.
Analysis plan
We classified intubation time as 1) very early: before or at ICU admission; 2) early: < 24 h after ICU admission, 3) late: > 24 h after ICU admission and 4) never intubated. We compared early versus late groups. We first displayed the clinical characteristics of the patients using the very early group as a reference group since these group patients showed clear signs of ARF and emergency orotracheal intubation. Therefore, our main analysis will focus on patients who were intubated within the first 24 h of ICU admission compared to those who were intubated after this time period.
Statistical analysis
No statistical sample size calculation was performed, and sample size was equal to the number of patients admitted to the participating ICUs during the study. Categorical variables were expressed as frequencies and percentages, and continuous variables were expressed as medians and first and third quartiles (Q1-Q3). To compare baseline characteristics between groups, we used chi-square or Fisher’s exact tests for categorical variables and the Mann-Whitney U for continuous variables. All tests were two-tailed, and significance was set at p < 0.05.
To investigate possible hospital-level or inter-hospital variation in ICU mortality (as random effects), we used multilevel logistic regression analysis with a conditional random intercept model [16], according to the total number of beds in each hospital (< 200, 200−500, or > 500). Regression coefficients were summarized as the variance with standard deviation and the interclass correlation coefficient (ICC).
All patients were classified as intubated within the first day of ICU stay or thereafter. The first time recorded for the purpose of the study was the day of ICU admission, but all patients were censored within the first 7 days after ICU admission and were discarded to avoid immortal time bias. Propensity score (PS) using the genetic matching algorithm was used to reduce selection bias and balance the covariance matrix of both groups. The match was 1:1 with replacement and ties, and no calibrators. The variables selected for inclusion in the matching model were the variables related to outcome. These variables included demographic characteristics and comorbidities, disease severity and laboratory data.
To investigate the association between baseline (ICU admission) variables and early or late intubation, a multivariate analysis (binary logistic regression) was performed. The multivariate model comprised factors of clinical interest and all significant covariates in the univariate analysis. The results are presented as odds ratios (OR) and 95% confidence intervals (CI).
Finally, to confirm the results, a survival analysis (Cox hazard regression) was performed to investigate whether survival time was related to covariates, and to estimate the effect size of intubation timing and ICU mortality in the PS matched cohort. The results are presented as hazard ratio (HR) and 95% confidence intervals (CI).
SPSS version 24 (IBM Corp. Armonk, NY, USA) and R software (cran.r-project.org) were used for the data analyses.
Results
Whole population
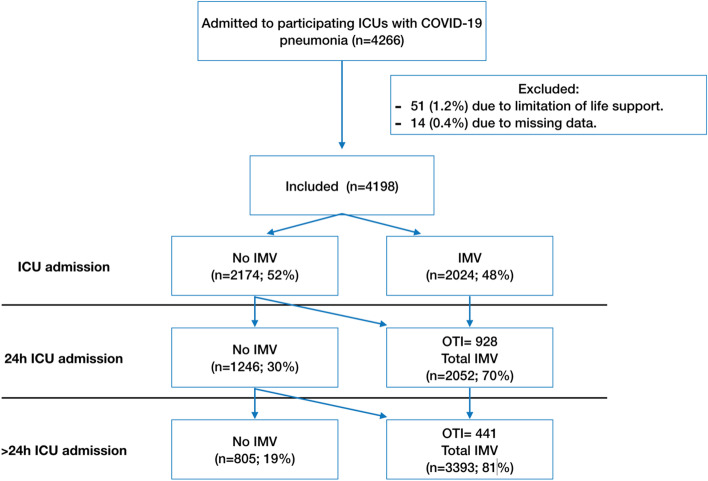
During the study period, 4,266 patients were admitted to the participating ICUs with COVID-19 pneumonia; 51 (1.2%) were excluded from the analyses due to end-of-life decisions during their ICU stay and 17 (0.4%) were excluded due to missing data (Fig. 1). Thus, we analyzed data from 4,198 patients [median age, 63 (54‒71) years; 71% men; median SOFA score, 4 (3‒7), median APACHE II score, 13 (10‒18); and median PaO2/FiO2 ratio at ICU admission, 131 (90‒190)] (Table 1).
Fig. 1.
Flowchart of the study. ICU: Intensive Care Unit, IMV= Invasive mechanical ventilation, OTI= Orothraqueal intubation
Table 1.
General characteristics of the population
| Variable | Entire population (N = 4198) | Very early group (N = 2024) | Early group (N = 928) | Late group (N = 441) | Never intubated (N = 805) |
|---|---|---|---|---|---|
| Demographics and severity of illness | |||||
| Male, n (%) | 2971 (71) | 1436 (71) | 670 (72) | 317 (72) | 548 (68) |
| Age, in years, median (p25-75) | 63 (54‒71) | 65 (57‒72) | 64 (56‒71) | 62 (50‒70) | 57 (48‒66) |
| SOFA, median (p25‒75) | 4 (3‒7) | 6 (4‒8) | 4 (3‒7) | 3 (2‒6) | 3 (2‒4) |
| APACHE II, median (p25‒75)a | 13 (10‒18) | 15 (11‒20) | 14 (10‒17) | 13 (10‒16) | 10 (7‒13) |
| Comorbidities | |||||
| Hypertension, n (%) | 1931 (46) | 980 (48) | 466 (50) | 175 (40) | 310 (39) |
| Obesity (> 30 kg/m2), n (%) | 1508 (36) | 739 (36) | 354 (38) | 150 (34) | 265 (33) |
| Diabetes, n (%) | 949 (23) | 458 (23) | 262 (28) | 85 (19) | 144 (18) |
| Chronic heart failure, n (%) | 139 (3) | 68 (3) | 32 (3) | 18 (4) | 21 (3) |
| Chronic lung disease, n (%) | 286 (7) | 162 (8) | 69 (7) | 24 (5) | 31 (4) |
| Asthma, n (%) | 262 (6) | 123 (6) | 60 (6) | 26 (6) | 53 (7) |
| Ischemic heart disease, n (%) | 256 (6) | 128 (6) | 64 (7) | 18 (4) | 37 (5) |
| Immunosuppression, n (%) | 222 (5) | 88 (4) | 60 (6) | 28 (6) | 46 (6) |
| Chronic kidney disease, n (%) | 210 (5) | 94 (5) | 58 (6) | 24 (5) | 34 (4) |
| Laboratory findings at admission | |||||
| Procalcitonin (ng/ml), median (p25–75) | 0.28 (0.11‒0.82) | 0.3 (0.1‒1) | 0.3 (0.1‒0.9) | 0.3 (0.1‒0.8) | 0.2 (0.1‒0.4) |
| C-reactive protein (mg/dL), median (p25–75) | 14.19 (7.9‒22.3) | 15 (9‒23) | 15 (9‒23) | 14 (7‒22) | 6 (11‒17) |
| White blood cells count (109/ml), median (p25–75) | 8.9 (6.3‒12.4) | 10 (7‒13) | 8.6 (6.3‒12) | 8.2 (5.8‒11.5) | 8.3 (5.0‒11.1) |
| LDH (U/L), median (p25–75) | 489 (368‒670) | 561 (434‒727) | 512 (405‒629) | 484 (384‒583) | 409 (326‒507) |
| D-dimer (ng/ml), median (p25–75) | 1000 (580.5‒2260) | 1899 (813‒5659) | 1128 (600‒2496) | 991 (595‒2437) | 837 (500‒1661) |
| Creatinine (mg/dL), median (p25–75) | 0.76 (0.53‒1.03) | 0.9 (0.7‒1.2) | 0.8 (0.7‒1.1) | 0.8 (0.7‒1.05) | 0.8 (0.6‒0.9) |
| Urea (mg/dL), median (p25–75) | 44 (32‒61) | 47 (35‒67) | 45 (32‒60) | 44 (33‒57) | 38 (29‒50) |
| Lactate (mmol/L), median (p25–75) | 1.5 (1.1‒2.1) | 1.1 (1.5‒2.1) | 1.5 (1.1‒2.1) | 1.6 (1.1‒2.3) | 1.4 (1‒2.1) |
| PaO2/FiO2 ratio, median (p25–75) | 131 (90‒190) | 155 (98‒215) | 100 (75‒148) | 116 (90‒152) | 142 (106‒182) |
| Complications and outcomes | |||||
| Acute renal failure, n (%) | 1123 (27) | 676 (33) | 283 (30) | 111 (25) | 53 (7) |
| Renal replacement therapy, n (%) | 383 (9) | 235 (12) | 105 (11) | 39 (9) | 4 (1) |
| Acute heart failure, n (%) | 376 (9) | 208 (10) | 104 (11) | 42 (9) | 22 (3) |
| ICU LOS (days), median (p25–75)b | 14 (7‒28)b | 19 (12‒34) | 19 (12‒34) | 25 (8‒42) | 3 (5‒8) |
| Hospital LOS (days), median (p25–75)b | 29 (18‒46)b | 37 (25‒54) | 35 (23‒53) | 41 (20‒46) | 15 (12‒21) |
| ICU mortality, n (%) | 1270 (30) | 787 (39) | 293 (32) | 163 (37) | 0 (0) |
| Hospital mortality, n (%) | 1327 (32) | 824 (41) | 309 (33) | 176 (40) | 0 (0) |
SOFA Sequential Organ Failure Assessment, APACHE Physiology and Chronic Health Evaluation, LOS Length of stay, LDH Lactate dehydrogenase, ICU Intensive Care Medicine, PaO2/FiO2 Arterial oxygen pressure/ inspired oxygen fraction
aMeasured after 24 h of admission
bCalculated on survivors
The conditional random intercept model found no effects on ICU mortality attributable to the centre (variance 0.39, SD 0.63; ICC 0.10) or to hospital size (variance 0.001, SD 0.04; ICC 0.0004). ICU mortality was 30% (n = 1260/4198) and hospital mortality was 32% (n = 1327/4198), with a median ICU length of stay (LOS) of 14 (7‒28) days and hospital LOS of 29 (18‒46) days. The complete characteristics of the whole population and groups are shown in Table 1.
At ICU admission, 2,024 (48%) patients were on IMV or immediately intubated (very early group). Additionally, 928 (22%) patients were intubated during the first 24 h after ICU admission (early group), 441 (10%) were intubated > 24 h after ICU admission (late group) and 805 (19%) were never intubated. Thus, a total of 3,393 (81%) received IMV during the ICU stay (Fig. 1). Very early intubation was more commonly performed in the first wave of the pandemic (56 vs 34%, p < 0.001) and patients never intubated were more common in the second wave (23 vs 17%, p < 0.001) (Table S1). Non-invasive respiratory support devices were used more often in the second wave (Table S2). Nevertheless, no significant differences in ICU mortality were observed between the first and second waves (31 vs 28%, p = 0.1).
Patients in the very early group were older and presented with a higher APACHE II and SOFA severity scores than the other groups. The crude ICU mortality in the very early group (39%) was higher than that observed in the early group (31%, p < 0.001) but similar to that of the late group (37%, p = 0.48) (Table 1).
Early versus late intubation comparison
Patients in the late group were younger (62 vs. 64, p = 0.01), with less severe disease (APACHE II: 13 vs. 14, p = 0.007, and SOFA: 3 vs. 4, p < 0.001), and had higher PaO2/FiO2 ratio on ICU admission (116 vs. 100, p < 0.001). However, the mortality in the late intubation group (37%) was higher than the observed ICU mortality in the early intubation group (32%, p = 0.05) and hospital mortality in the late group was higher than in the early group (40 vs 33% respectively, p = 0.03, Table 2). There was no statistical difference in ICU (19 vs 20, p = 0.2) and hospital LOS (37 vs 35, p = 0.7) or days of IMV (20 vs 19, p = 0.99).
Table 2.
Univariate analysis. Early vs. late intubation
| Variables | Early intubation (n = 928) | Late intubation (n = 441) | P values |
|---|---|---|---|
| General characteristics | |||
| Male, n (%) | 670 (73) | 317 (72) | 0.9 |
| Age (years), median (p25–75) | 64 (56‒71) | 62 (50‒73) | 0.01 |
| Comorbidities | |||
| Hypertension, n (%) | 466 (50) | 175 (40) | <0.001 |
| Obesity (> 30 kg/m2), n (%) | 354 (38) | 150 (34) | 0.1 |
| Diabetes, n (%) | 262 (28) | 85 (19) | <0.001 |
| Chronic lung disease, n (%) | 69 (7) | 24 (5) | 0.2 |
| Asthma, n (%) | 60 (6) | 26 (6) | 0.7 |
| Immunosuppression, n (%) | 60 (6) | 28 (6) | 0.9 |
| Chronic kidney disease, n (%) | 58 (6) | 24 (5) | 0.5 |
| Chronic heart failure, n (%) | 32 (3) | 18 (4) | 0.5 |
| Ischemic heart disease, n (%) | 64 (7) | 27 (6) | 0.6 |
| Severity of illness | |||
| SOFA, median (p25–75) | 4 (3‒7) | 3 (2‒6) | <0.001 |
| APACHE II, median (p25–75)a | 14 (10‒17) | 13 (10‒16) | 0.001 |
| PaO2/Fio2 ratio at admission, median (p25–75) | 100 (75‒148) | 116 (90‒152) | <0.001 |
| Shock at admission, n (%) | 281 (30) | 36 (8) | <0.001 |
| Laboratory variables | |||
| Procalcitonin (ng/ml), median (p25–75) | 0.3 (0.1‒0.9) | 0.3 (0.1‒0.8) | 0.06 |
| C-reactive protein (mg/dL), median (p25–75) | 15 (9‒23) | 14 (7‒22) | 0.6 |
| White blood cells count (109/ml), median (p25–75) | 8.6 (6.3‒12) | 8.2 (5.8‒11.5) | 0.04 |
| LDH (U/L), median (p25–75) | 512 (405‒629) | 484 (384‒583) | 0,002 |
| D-dimer (ng/ml), median (p25–75) | 1128 (600‒2496) | 991 (595‒2437) | 0.1 |
| Creatinine (mg/dL), median (p25–75) | 0.8 (0.7‒1.1) | 0.8 (0.7‒1.05) | 0.1 |
| Urea (mg/dL), median (p25–75) | 45 (32‒60) | 44 (33‒57) | 0.5 |
| Lactate (mmol/L), median (p25–75) | 1.5 (1.1‒2.1) | 1.6 (1.1‒2.3) | 0.2 |
| Outcomes | |||
| Days for IVM, median (p25–75)b | 19 (12‒34) | 20 (12‒34) | 0.99 |
| ICU mortality, n (%) | 293 (32) | 163 (37) | 0.05 |
| ICU LOS, median (p25–75)b | 20 (12‒35) | 19 (12‒34) | 0.2 |
| Hospital mortality, n (%) | 309 (33) | 176 (40) | 0.03 |
| Hospital LOS, median (p25–75)b | 35 (23‒53) | 37 (24‒54) | 0.7 |
SOFA Sequential Organ Failure Assessment, APACHE Physiology and Chronic Health Evaluation, LDH Lactate dehydrogenase, IMV Invasive mechanical ventilation, ICU Intensive care unit, PaO2/FiO2 Arterial oxygen pressure/ inspired oxygen fraction
aMeasured after 24 h of admission
bCalculated on survivors
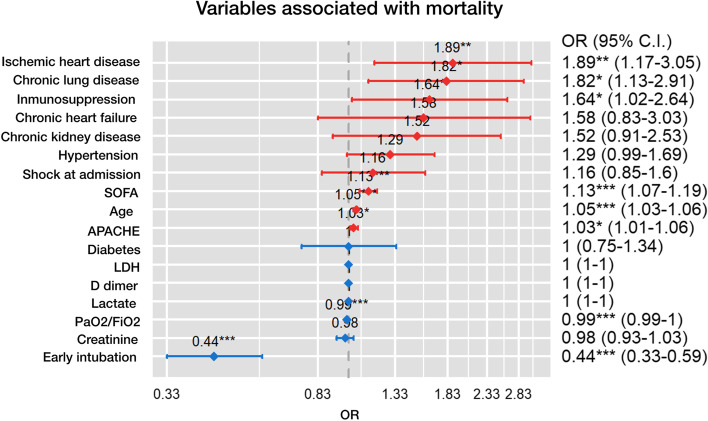
Age, severity of illness (APACHE II and SOFA), hypertension, chronic kidney disease, COPD, diabetes, immunosuppression, coronary disease, and chronic heart failure were more frequent in non-survivors (Table S3) and were included in the multivariate model. Early intubation was found as an independent protective risk factor of mortality OR 0.44 (95%CI 0.33–0.59) (Fig. 2, Table S4).
Fig. 2.
Variables associated with mortality. OR= Odds ratio, SOFA= Sequential Organ Failure Assessment, APACHE= Physiology and Chronic Health Evaluation, PaO2/FiO2 (arterial oxygen pressure/ inspired oxygen fraction), LDH: lactate dehydrogenase
To confirm these findings, propensity score (PS) matching was applied after excluding patients with less than 7 days of ICU LOS. 840 early patients and 371 late intubated patients were matched. The APACHE II score, SOFA score, age, presence of shock at ICU admission, diabetes, hypertension, LDH, PCT and PaO2/FiO2 ratio at ICU admission were the variables included in the PS model (Table S5). The summaries of balance for unmatched and matched data are shown in Table S6 and Fig. S1.
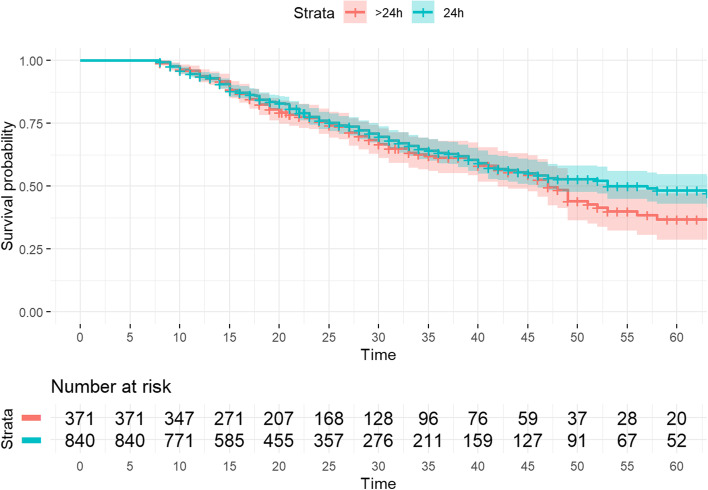
Finally, to determine the impact of early intubation on ICU mortality, a Cox regression analysis adjusted for potential confounding factors (Table S3) was performed in the matched cohort. The Cox model showed that the early intubation was significantly associated with a lower ICU mortality rate (HR 0.60; 95% CI 0.47-0.76) (Table 3, Fig. 3).
Table 3.
Mortality in the ICU: Cox regression
| Variables | HR | IC | P values |
|---|---|---|---|
| General characteristics | |||
| Age | 1.03 | 1.02‒1.05 | <0.001 |
| Comorbidities | |||
| Chronic lung disease | 1.3 | 0.9-1.8 | 0.2 |
| Chronic heart failure | 1.4 | 0.9-2.1 | 0.13 |
| Chronic kidney injury | 1.1 | 0.8‒1.8 | 0.5 |
| Diabetes | 1.1 | 0.9‒1.4 | 0.4 |
| Ischemic heart disease | 1.5 | 1.1‒2.1 | 0.01 |
| Hypertension | 1.1 | 0.9‒1.4 | 0.5 |
| Immunosuppression | 1.3 | 0.9‒1.8 | 0.1 |
| Severity of illness | |||
| APACHE II | 1 | 0-9‒1.03 | 0.3 |
| SOFA | 1.1 | 1.02‒1.1 | 0.004 |
| Shock at admission | 0.88 | 0.7–1.2 | 0.4 |
| PaO2/FiO2 ratio | 0.99 | 0.99–0.99 | <0.001 |
| Laboratory variables | |||
| LDH (U/L) | 1 | 0.99–1 | 0.3 |
| D-dimer (ng/ml) | 1 | 1–1 | 0.2 |
| Lactate (mmol/L) | 1 | 0.99–1.002 | 0.1 |
| Creatinine (mg/dL) | 1 | 0.9.1.1 | 0.8 |
| Intubation timing | |||
| Early intubation | 0.6 | 0.5‒0.8 | <0.001 |
APACHE Physiology and Chronic Health Evaluation, SOFA Sequential Organ Failure Assessment, PaO2/FiO2 Arterial oxygen pressure/ inspired oxygen fraction, LDH Lactate dehydrogenase
Fig. 3.
Cox regression ICU mortality
Discussion
The main finding of this research piece is that in critically ill patients with COVID-19 pneumonia who did not require intubation at admission to the ICU, intubation within the 24 h after ICU admission was found to be associated with a lower risk of mortality compared to those that were intubated out of the 24h window. Remarkably, we found higher mortality in the late intubation group compared to patients that were intubated within the first 24h even though those patients were younger, presented with less severe disease, and with a higher PaO2/FiO2 ratio at ICU admission.
The cut-off between early and late intubation remains controversial and somewhat arbitrary. Several studies using the cut-off 24-hours after ICU admission found no significant differences in ICU mortality [13, 18-21]. Studies using a 48-h cut-off have reported contradictory findings, with some observing higher in-hospital and out-of-hospital mortality rate in patients intubated after the cut-off [22] and others finding no significant differences [23, 24]. Both cut-off points were considered in the same sample in a prospective Spanish matched analysis study, finding increased mortality risk in both delayed intubation more than 24 and 48 h [25]. A retrospective observational study including only 40 patients with COVID-19 found lower mortality in patients intubated < 50 h after admission to ICU, but obviously the small sample could preclude conclusions about the timing of intubation [26]. Other prospective studies found an increase in mortality for each additional day between hospital admission and intubation [27, 28].
It might be more logical to establish the cut-off according to the onset of severe hypoxemic respiratory failure, although it will probably be coincidental to the ICU admission moment. Ranieri et al. [29] found no significant differences in mortality, ICU LOS, or days under IMV between patients intubated 24 h before or after the onset of ARDS diagnosed according to the Berlin criteria. However, another prospective study carried out in Spain, established the first 48 h after the start of any respiratory support as the cut-off point, and found a HR 2.2 for late intubation group [30].
When the decision to intubate is related with clear signs of ARF and late intubation is considered, early intubation is related with less mortality risk [31], as expected. In a study where patients were intubated if they developed hemodynamic instability, altered level of consciousness, or respiratory distress defined as the use of accessory respiratory muscles or inability to speak, Siempos et al. [32] found no significant differences in mortality, ICU LOS, or ventilator-free days. This study compared a group comprising non intubated patients together with those who were intubated after receiving non-invasive respiratory support for > 24 h to avoid intubation, versus the remaining intubated patients, probably because all the patients were intubated with clear signs of respiratory failure. A prospective observational study to assess the usefulness of a protocol in which patients were intubated for increased work of breathing after HFNC and awake prone manoeuvre found higher mortality in patients intubated > 48 h after ICU admission [33]. Finally, one of the latest meta-analyses published showed no statistical difference in terms of mortality between early and late intubation, however they analysed studies with different cut-off points and high heterogeneity, so the results must be interpreted with care [34]. The results obtained so far are contradictory, in part by the different cut-off points, wave patterns observed in the pandemic and intubation criteria in the studies analysed. More robust and protocolized studies, randomized, if possible, should be performed.
We established a 24 h after ICU admission cut-off point due to the characteristics of our database. Our findings were similar to those seen in patients with ARDS [35, 36] and some of the COVID-19 studies mentioned above, where delaying intubation increases mortality risk. This is supported by pathophysiology, as P-SILI is avoided when intubated due to decrease in respiratory drive and transpulmonary pressure [37]. It has been demonstrated that there are two phenotypes of COVID 19, type L with low elastance, low ventilation-perfusion ratio, low lung weight and low lung recruitment, and type H with high elastance, high lung weight and high recruitment. The second one could be the progression of the first one due to the illness evolution but also due to P-SILI [38].
Our study has several strengths. It is a multi-centre study conducted in 73 ICUs and including many critically ill patients. Moreover, data were collected over a 1-year period including different waves of the pandemic, thus avoiding possible biases related to variations in treatment arising from differences in the availability of resources and knowledge about the disease [16, 39, 40]. Finally, our robust statistical analysis adjusted for demographic variables, comorbidities, and severity scores and included propensity matched analysis.
Our study also has some limitations. The decision to intubate was not protocolized, and the reason for intubation was not recorded. Unfortunately, in order to obtain a large sample size with low missing data, we did not collect additional mechanical ventilation variables (e.g., driving pressure and mechanical power) [41]. Nevertheless, this epidemiological database compiled during the COVID-19 pandemic enables us to differentiate patients according to the timing of intubation in a real scenario. Our observational analysis carries the risk of selection bias, and other confounding factors cannot be excluded. For these reasons, despite the large number of critically ill patients included, our results may not be generalizable to other populations or scenarios.
Conclusions
n COVID-19 patients, the decision to intubate within the first 24 h of ICU admission was associated with a protective benefit. It showed a lower risk of mortality compared to performing intubation after the first 24-h window of ICU admission. Our data suggest that physicians would consider a 24-h window to perform an intubation on COVID-19 patients admitted to ICU. Based on the observational nature of the data, this information should be validated further in future studies.
Supplementary Information
Additional file 1: Table S1. OTI timing in different waves. Table S2. Oxygen-therapy at ICU admission differentiate by waves. Table S3. Univariate mortality analysis. Table S4. Mortality in the ICU: Binary logistic regression. Table S5. Early vs late group after excluding patients with < 7 days of ICU LOS. Table S6. Propensity score early and late groups. Figure S1. Propensity score early and late groups.
Acknowledgements
To John Giba for English manuscript edition.
To SEMICYUC COVID-19 working group investigators:
Andalucía:
UCI Hospital Universitario Virgen de Valme (Sevilla): Ana Loza; UCI Hospital Quirón (Huelva): Diego Matallana Zapata; UCI Hospital Universitario Puerto Real (Cádiz): Isabel Díaz Torres, Sonia Ibañez Cuadros, María Recuerda Nuñez, Maria Luz Carmona Pérez, Jorge Gómez Ramos, Alba Villares Casas; UCI Hospital Universitario Virgen de la Macarena (Sevilla): María Luisa Cantón, José Javier González Contreras, Helena Pérez Chomón, Nerissa Alvarez Chicote, Alberto Sousa González; UCI Hospital Universitario Reina Sofía (Córdoba): María De Alba Aparicio, Juan Carlos Pozo Laderas; UCI Hospital Universitario de Jerez (Jerez de la Frontera): Angel Estella, Sara moreno Cano. UCI Hospital Infanta Elena (Huelva): Diego Matallana Zapata
Aragón:
UCI Hospital Nuestra Señora de Gracia (Zaragoza): Ruth Jorge García; UCI Hospital Clínico Universitario Lozano Blesa (Zaragoza): Laura Sánchez Montori, Sandra Herrero García, Paula Abanses Moreno, Carlos Mayordomo García. UCI Hospital General San Jorge(Huesca): Tomás Mallor Bonet, Paula Omedas Bonafonte, Enric Franquesa Gonzalez, Nestor Bueno Vidales, Paula Ocabo Buil, Carlos Serón Arbeloa; UCI Hospital Universitario Miguel Servet(Zaragoza): Isabel Sancho, Pablo Guerrero Ibañez, Pablo Gutierrez, UCI Hospital Obispo Polanco (Teruel): María Concepción Valdovinos, Raquel Canto
Asturias:
UCI Hospital Universitario San Agustín (Avilés): Ana Luz Balán Mariño, María José Gutiérrez Fernández, Marta Martín Cuadrado, Belén García Arias; UCI Hospital Universitario Central de Asturias (Oviedo): Lorena Forcelledo Espina, Lucía Viña Soria, Lorena Martín Iglesias, Lucía López Amor, Elisabet Fernández Rey, Emilio García Prieto. UCI Hospital Cabueñes (Gijón): Débora Fernández Ruíz, Carla Martínez González
Baleares:
UCI hospital Universitario Son Llatzer (Palma de Mallorca): Lorenzo Socias, Marcio Borges-Sá, María Aranda Pérez, Antonia Socias. UCI Hospital Quirón Salud Palmaplanas (Palma de Mallorca): José Mª Bonell Goytisolo, Inmaculada Alcalde Mayayo, Carlos Corradini, Isabel Ceniceros, Edwin Rodríguez; UCI Hospital Universitario Son Espases (Palma de Mallorca): Jose Ignacio Ayestarán Rota, Mariana Andrea Novo Novo, Joaquim Colomina Climent, Albert Figueras Castilla, Tomàs Leal Rullan, Maria Magdalena Garcias Sastre; UCI Hospital Comarcal d’Inca(Inca): Rossana Pérez Senoff; UCI Hospital Mateu Orfila (Mao): Ramón Fernández-Cid Bouza
Canarias:
UCI Complejo Hospitalario Universitario Insular - Materno Infantil (Las Palmas de G.C): Juan Carlos Martín González, Carmen Pérez Ortiz, José Luciano Cabrera Santana, Juan José Cáceres Agra, Domingo González Romero, Ana Casamitjana Ortega; UCI Hospital General de la Palma (Tenerife): Luis Alberto Ramos Gómez, Carolina Montelongo Ojeda; UCI Hospital Universitario Dr. Negrín (Las Palmas de G.C): Jordi Solé-Violán.
Cataluña:
UCI Hospital Universitari de Tarragona Joan XXIII (Tarragona): Alejandro Rodríguez, María Bodí, Gerard Moreno, Sandra Trefler, Laura Claverias, Raquel Carbonell, Erika Esteve, Montserrat Olona, Xavier Teixidó. UCI Hospital Universitari Arnau de Vilanova (Lleida): Monserrat Vallverdú Vidal, Begoña Balsera Garrido. UCI Hospital Universitari Vall d’Hebron (Barcelona): Elisabeth Papiol Gallofré, Raquel Albertos Martell, Rosa Alcaráz Peñarrocha, Xavier Nuvials Casals, Ricard Ferrer Roca; UCI Hospital Verge de la Cinta (Tortosa): Eric Adrián Mayor Vázquez, Ferrán Roche Campo, Pablo Concha Martínez, Diego Franch Llasat;UCI Hospital del Mar (Barcelona): Joan Ramón Masclanz , Judith Marín-Corral, Purificación Pérez, Rosana Muñoz , Clara Vila; UCI Hospital Mutua de Terrasa (Terrasa): Francisco Javier González de Molina, Elisabeth Navas Moya, Josep Trenado; UCI Hospital Sant Joan (Reus): Imma Vallverdú, Eric Castañé; UCI Hospital Parc Tauli (Sabadell): Emili Díaz Santos , Gemma Goma, Edgar Moglia; UCI Hospital German Trias i Pujol (Badalona): Fernando Arméstar , Alba Herraiz, Regina Roig, Beatriz Catalán, Paula Lluís, Javier Marichalar
Cantabria
UCI Hospital Universitario Marqués de Valdecillas(Santander): Borja Suberviola
Castilla La Mancha:
UCI Hospital Universitario de Guadalajara (Guadalajara): Antonio Albaya Moreno, Carlos Marian Crespo. UCI Hospital Nuestra Señora del Prado (Toledo): Carmen Carolina Sena Pérez, Francisca Arbol Linde
Castilla y León:
UCI Hospital Virgen de la Concha (Zamora): Diana Monge Donaire, Vega Losada Martínez, Nuria Rodrigo Castroviejo, Gerardo Ferrigno, Reyes Beltrán, Carolina Sanmartino, Concepción Tarancón Maján, Alfredo Marcos Gutiérrez; UCI Complejo Asistencial de Segovia(Segovia): Virginia Hidalgo Valverde, Caridad Martín López; UCI Hospital universitario de Burgos (Burgos): Oihane Badallo, María del Valle Ortiz, Rebeca Vara Arlanzón, David Iglesias Posadilla; UCI Hospital Clínica de Salamanca (Salamanca): María Teresa Recio, Juan Carlos Ballesteros; UCI Complejo Asistencial Universitario de Palencia (Palencia):
Ceuta
UCI Hospital Universitario de Ceuta: Enrique Laza Laza
Extremadura
UCI Hospital San Pedro de Alcántara (Cáceres): Elena Gallego Curto, Mª Carmen Sánchez García; UCI Hospital de Mérida(Mérida): Miguel Díaz-Tavora, Rosa Mancha
Galicia:
UCI Hospital Montecelo (Pontevedra): Ana Ortega Montes, Isabel Gallego Barbachano, Eva Sanmartín Mantiñán. UCI CHUAC A Coruña (A Coruña): María Lourdes Cordero, Raquel María Rodríguez García, Jorge Gámez Zapata, María Gestal Vázquez. UCI Centro Hospital Universitario de Ferrol (Ferrol): María José Castro Orjales, María Isabel Álvarez Diéguez. UCI Hospitalario Clínico Universitario de Santiago (Santiago de Compostela): Carmen Rivero Velasco, Beatriz Lence Massa, Aitziber Santiago Langarica; REA CHUAC A Coruña (A Coruña): María Gestal Vázquez. UCI Hospital Lucus Augusti (Lugo): Ignacio Martínez Varela
Huelva:
UCI Hospital Infanta Elena (Huelva): Diego Matallana Zapata,
Madrid:
UCI IFEMA (Madrid): Alberto Hernández Tejedor; UCI Hospital Príncipe de Asturias (Madrid): Esther Mª López Ramos, Laura Alcázar Sánchez Elvira, Rocío Molina Montero, Mª Consuelo Pintado Delgado, María Trascasa Muñoz de la Peña, Yaiza Betania Ortiz de Zárate Ansotegui, Alejandra Acha Aranda, Juan Higuera Lucas; UCI Hospital de la Princesa (Madrid): Juan Antonio Sanchez Giralt, Marta Chicot Llano, Nuria Arevalillo Fernández, Marta Sánchez Galindo, Ricardo Andino Ruiz, Alfonso Canabal Berlanga; UCI Hospital Clinico San Carlos (Madrid): Miguel Sánchez, Mercedes Nieto; UCI Hospital HLA la Moncloa(Madrid):, Adoración Bueno Blázquez, Rosa María de la Casa, Fátima Martín, Samuel González López, Juan José Oñoro Cañaveral.
Murcia
UCI Hospital Morales Meseguer (Murcia): Elena Martínez Quintana, Bernardo Gil Rueda, Áurea Higon Cañigral, Laura López Gómez , Pablo Safwat Bayoumi Delis, Augusto Montenegro Muore, Ángel Andrés Agamez Luengas; UCI Hospital Clínico Universitario Virgen de la Arrixaca (Murcia): Enriqueta Andreu Soler, Ana Beatriz Pérez Pérez, José Higinio de Gea García, Rubén Jara Rubio, Silvia Sánchez Cámara, Alba Moreno Flores , José Moya Sánchez, Daniel Francisco Pérez Martínez,-Mª Desamparados del Rey Carrión; UCI Hospital Reina Sofía (Murcia): María José Rico Lledó, Juana María Serrano Navarro, Juan Francisco Martín Ruíz, Julián Triviño Hidalgo, África López Ferrer, Isabel Cremades Navalón; UCI Hospital Santa Lucía (Cartagena): Josefa Murcia Payá, JM Allegre Gallego ; UCI Hospital Rafael Méndez (Lorca): María del Carmen Lorente; UCI Hospital Universitario Mar Menor (San Javier):Marta Gonsalvez
Navarra
UCI Hospital Reina Sofía (Tudela): Ruth González Natera, Raquel Garrido López de Murillo, Tania Ojuel Gros, Raquel Flecha Viguera, Isabel López González; UCI Hospital García Orcoyen(Estella-Lizarra): Adriana García Herrera
País Vasco
UCI Hospital Universitario de Donostia (Donostia): Loreto Vidaur Tello, Maialen Aseguinolaza, Itziar Eguibar;
Sevilla:
UCI Hospital Universitario Virgen de la Macarena(Sevilla): María Luisa Cantón Bulnes, Jose Javier González Contreras, Helena Pérez Chomón, Nerissa Álvarez Chicote, Alberto Sousa González
Valencia:
UCI Hospital Universitario de La Ribera (Alzira): Asunción Marqués Parra, Sergio García Marti, Alberto Lorenzo Aguilar, Laura Bellver Bosch, Victor Gascón Sanchez, Sonia De la Guía Ortega. UCI Hospital Dr. Peset (Valencia): Martín Parejo Montell, Alberto Belenguer Muncharaz, Hector Hernández Garces, Victor Ramírez Montero, Mónica Crespo Gómez, Verónica Martí Algarra; UCI Hospital Universitari i Politècnic La Fe (Valencia): Susana Sancho Chinesta, Joaquin Arguedas Cervera, Faustino Álvarez Cebrian, Begoña Balerdi Pérez, Rosa Jannone Fores, Javier Botella de Maglia; UCI Hospital Clínico Universitario de Valencia (Valencia): Nieves Carbonell Monleón, Jose Ferreres Franco, Ainhoa Serrano Lazaro, Mar Juan Díaz, María Luisa Blasco Cortés; UCI Hospital Virgen de los Lirios de Alcoy (Alicante): Laura Fayos, Julia Giménez, Gaspar Soriano, Ricardo Navarro. UCI Hospital Arnau de Vilanova (Valencia): Sonia Mas, Elena Bisbal, Laura Albert, Johncard Romero, Juan Fernández Cabreara; UCI Hospital Comarcal de Vinarós (Vinarós): Andrea Ortíz
Principado de Andorra
ICU Hospital Nostra Senyora de Meritxell (Les Esclades): Antonio Margarit Ribas, Neus Guasch
Abbreviations
- ARDS
Acute respiratory distress syndrome
- IMV
Invasive mechanical ventilation
- VILI
Ventilator induced lung injury
- P-SILI
Patient-self-induced lung injury
- HFNC
High-flow nasal cannula
- NIV
Non-invasive ventilation
- APACHE
Acute Physiology and Chronic Health Evaluation
- SOFA
Sequential Organ Failure Assessment
- OR
Odds ratio
- CI
Confidence interval
- SD
Standard deviation
- ICC
Interclass correlation coefficient
- LOS
Length of stay
- LDH
Lactate dehydrogenase
- PaO2/FiO2
Arterial oxygen pressure/ inspired oxygen fraction
Authors’ contributions
SM, AR, LC. MM, MB, and ED had substantial contributions to conception and design of the study. SM, AR, ST, LC, MM, and LC had substantial contribution for data acquisition. SM, AR, LC, MM, MB, IML, JSV, JRM, FG, ED, EBA, RGN, AAM, MV, JCB, LS and SS had substantial contribution for analysis and interpretation of data for the study. SM, AR, IML, MM, MB, and ED drafting of the manuscript. JRM, FG, JSV, RGN, AAM, LS, SS EBA and JCB critically reviewed the draft manuscript. The corresponding author (SM) had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors approved the final version of the manuscript. The views expressed in this article are those of the authors and not necessarily those of the SEMICYUC.
Funding
This study was supported by the Spanish Intensive Care Society (SEMICYUC) and Ricardo Barri Casanovas Foundation. The study sponsors have no role in the study design, data collection, data analysis, data interpretation, or writing of the report. This study has been funded by Instituto de Salud Carlos III (ISCIII) through the project " FIS PI20/01674" and co-funded by the European Union.
Availability of data and materials
The anonymized database and the data dictionary that defines each field in the set, will be available to reviewers if they consider necessary prior confidentiality agreement. Information contact: Alejandro Rodríguez, Critical Care Department – Hospital Universitario de Tarragona Joan XXIII, Tarragona, Spain, whose mail is ahr1161@yahoo.es.
Declarations
Ethics approval and consent to participate
The study was approved by the institutional review board of reference of the Joan XXIII University Hospital and the Pere Virgili Research Institute (IRB# CEIM/066/2020) and each participating centre. It has also been performed in accordance with the Declaration of Helsinki. The ethics committee (the Joan XXIII University Hospital and the Pere Virgili Research Institute (IRB# CEIM/066/2020)) did not consider it necessary to sign informed consent given the pandemic status considerations and the epidemiological characteristics of the study.
Competing interests
The authors declare no competing interests.
Footnotes
The complete list of SEMICYUC COVID-19 working Group is shown in Acknowledgements.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sara Manrique and Laura Claverias are first authors.
Sara Manrique and Laura Claverias Claverias contributed equally to this work.
References
- 1.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papazian L, Aubron C, Brochard L, Chiche JD, Combes A, Dreyfuss D, Forel JM, Guérin C, Jaber S, Mekontso-Dessap A, Mercat A, Richard JC, Roux D, Vieillard-Baron A, Faure H. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care. 2019;9(1):69. doi: 10.1186/s13613-019-0540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brewster DJ, Chrimes N, Do TBT, Fraser K, Groombridge CJ, Higgs A, et al. Consensus statement: Safe Airway Society principles of airway management and tracheal intubation specific to the COVID-19 adult patient group. Med J Aust. 2020;212:472–81. doi: 10.5694/mja2.50598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown CA, Mosier JM, Carlson JN, Gibbs MA. Pragmatic recommendations for intubating critically ill patients with suspected COVID-19. J Am Coll Emerg Phys Open. 2020;1:80–4. doi: 10.1002/emp2.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuo M, Huang Y, Ma W, Xue Z, Zhang J, Gong Y, et al. Expert recommendations for tracheal intubation in critically iii patients with noval coronavirus disease 2019. Chin Med Sci J. 2020;35:105–9. doi: 10.24920/003724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook TM, El-Boghdadly K, McGuire B, McNarry AF, Patel A, Higgs A. Consensus guidelines for managing the airway in patients with COVID- 19: guidelines from the Difficult Airway Society, the Association of Anaesthetists the Intensive Care Society, the Faculty of Intensive Care Medicine and the Royal College of Anaesthetists. Anaesth Blackwell Publ. 2020;75:785–99. doi: 10.1111/anae.15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alhazzani W, Moller MH, Arabi YM et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed]
- 8.Kobayashi H, Uchino S, Takinami M, Uezono S. The impact of ventilator-associated events in critically ill subjects with prolonged mechanical ventilation. Respir Care. 2017 doi: 10.4187/respcare.05073. [DOI] [PubMed] [Google Scholar]
- 9.Finfer SR, Vincent J-L, Slutsky AS, Ranieri VM. Critical care medicine ventilator induced lung injury. N Engl J Med. 2013;369:2126–62. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 10.Attaway AH, Scheraga RG, Bhimraj A, Biehl M, Hatipoğlu U. Severe covid-19 pneumonia: pathogenesis and clinical management. BMJ. 2021;372:n436. doi: 10.1136/bmj.n436. [DOI] [PubMed] [Google Scholar]
- 11.Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020;323(22):2329–2330. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 12.Cruces P, Retamal J, Hurtado DE, et al. A physiological approach to understand the role of respiratory effort in the progression of lung injury in SARS-CoV-2 infection. Crit Care. 2020;24:494. doi: 10.1186/s13054-020-03197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tobin MJ, Laghi F, Jubran A. Caution about early intubation and mechanical ventilation in COVID-19. Ann Intensive Care. 2020;10:78. doi: 10.1186/s13613-020-00692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papoutsi E, Giannakoulis VG, Xourgia E, Routsi C, Kotanidou A, Siempos II. Effect of timing of intubation on clinical outcomes of critically ill patients with COVID-19: a systematic review and meta-analysis of non-randomized cohort studies. Crit Care. 2021;25(1):121. doi: 10.1186/s13054-021-03540-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Force ADT, Ranieri V, Rubenfeld G, Thompson B, Ferguson N, Caldwell E. Acute respiratory distress syndrome. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 16.Carbonell R, Urgeles S, Rodriguez A, et al. Mortality comparison between the first and second/ third waves among 3,795 critical COVID-19 patients with pneumonia admitted to the ICU: a multicentre retrospective cohort study. Lancet Reg Health Eur. 2021;11:100243. doi: 10.1016/j.lanepe.2021.100243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vidal-Cortés P, Díaz E, Agular E, et al. Recommendations for the management of critically ill patients with COVID-19 in Intensive Care Units. Med Intensiva (Engl Ed) 2022;46(2):81–89. doi: 10.1016/j.medine.2021.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez-Romieu AC, Adelman MW, Hockstein MA, et al. Timing of intubation and mortality among critically ill coronavirus disease 2019 patients: a single-center cohort study. Crit Care Med. 2020;48(11):e1045–e1053. doi: 10.1097/CCM.0000000000004600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mellado-Artigas R, Ferreyro BL, Angriman F, Hernández-Sanz M, Arruti E, Torres A, et al. High-flow nasal oxygen in patients with COVID-19-associated acute respiratory failure. Crit Care. 2021;25:58. doi: 10.1186/s13054-021-03469-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fayed M, Patel N, Yeldo N, Nowak K, Penning DH, Vasconcelos Torres F, Natour AK, Chhina A. Effect of intubation timing on the outcome of patients with severe respiratory distress secondary to COVID-19 pneumonia. Cureus. 2021;13(11):e19620. doi: 10.7759/cureus.19620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Tarbsheh A, Chong W, Oweis J, Saha B, Feustel P, Leamon A, Chopra A. Clinical outcomes of early versus late intubation in COVID-19 patients. Cureus. 2022;14(1):e21669. doi: 10.7759/cureus.21669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dupuis C, Bouadma L, de Montmollin E, et al. Association between early invasive mechanical ventilation and day-60 mortality in acute hypoxemic respiratory failure related to coronavirus disease-2019 pneumonia. Crit Care Explor. 2021;3(1):e0329. doi: 10.1097/CCE.0000000000000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matta A, Chaudhary S, Bryan Lo K, et al. Timing of intubation and its implications on outcomes in critically Ill patients with coronavirus disease 2019 infection. Crit Care. 2020;2:0262. doi: 10.1097/CCE.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chong WH, Saha BK, Murphy DJ, Chopra A. Comparison of clinical characteristics and outcomes of COVID-19 patients undergoing early versus late intubation from initial hospital admission: a systematic review and meta-analysis. Respir Investig. 2022;60(3):327–336. doi: 10.1016/j.resinv.2022.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riera J, Barbeta E, Tormos A, et al. Effects of intubation timing in patients with COVID-19 throughout the four waves of the pandemic: a matched analysis. Eur Respir J. 2022:2201426. 10.1183/13993003.01426-2022. Epub ahead of print. PMID: 36396142; PMCID: PMC9686319. [DOI] [PMC free article] [PubMed]
- 26.Zhang Q, Shen J, Chen L, et al. Timing of invasive mechanic ventilation in critically ill patients with coronavirus disease 2019. J Trauma Acute Care Surg. 2020;89(6):1092–1098. doi: 10.1097/TA.0000000000002939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hyman JB, Leibner ES, Tandon P, et al. Timing of intubation and in-hospital mortality in patients with coronavirus disease 2019. Crit Care Expl. 2020;2:e0254. doi: 10.1097/CCE.0000000000000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le Terrier C, Suh N, Wozniak H, et al. Delayed intubation is associated with mortality in patients with severe COVID-19: a single-centre observational study in Switzerland. Anaesth Crit Care Pain Med. 2022;41(4):101092. doi: 10.1016/j.accpm.2022.101092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez J, Benitez ID, De Gonzalez-Clavo I, et al. Impact of time to intubation on mortality and pulmonary sequelae in critically ill patients with COVID-19: a prospective cohort study. Crit Care. 2022;26:18. doi: 10.1186/s13054-021-03882-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto R, Kaito D, Homma K, et al. Early intubation and decreased in-hospital mortality in patients with coronavirus disease 2019. Crit Care. 2022;26(1):124. doi: 10.1186/s13054-022-03995-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siempos II, Xourgia E, Ntaidou TK, Zervakis D, Magira EE, Kotanidou A, et al. Effect of early vs delayed or no intubation on clinical outcomes of patients with COVID-19: an observational study. Front Med Front. 2020;7:614152. doi: 10.3389/fmed.2020.614152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vera M, Kattan E, Born P, et al. Intubation timing as determinant of outcome in patients with acute respiratory distress syndrome by SARS-CoV-2 infection. J Crit Care. 2021;65:164–169. doi: 10.1016/j.jcrc.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee HJ, Kim J, Choi M, Choi WI, Joh J, Park J, Kim J. Early intubation and clinical outcomes in patients with severe COVID-19: a systematic review and meta-analysis. Eur J Med Res. 2022;27(1):226. doi: 10.1186/s40001-022-00841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kangelaris KN, Ware LB, Wang CY, et al. Timing of intubation and clinical outcomes in adults with ARDS. Crit Care Med. 2016;44(1):120–129. doi: 10.1097/CCM.0000000000001359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bauer PR, Gajic O, Nanchal R, et al. Association between timing of intubation and outcome in critically ill patients: a secondary analysis of the ICON audit. J Crit Care. 2017;42:1–5. doi: 10.1016/j.jcrc.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 37.Brochard L, Slutsky A, Pesenti A. Mechanical Ventilation to Minimize Progression of Lung Injury in Acute Respiratory Failure. Am J Respir Crit Care Med. 2017;195(4):438–442. doi: 10.1164/rccm.201605-1081CP. [DOI] [PubMed] [Google Scholar]
- 38.Gattinoni L, Chiumello D, Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care. 2020;24:154. doi: 10.1186/s13054-020-02880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karagiannidis C, Windisch W, McAuley DF, et al. Major differences in ICU admissions during the first and second COVID-19 wave in German. Lancet Respir Med. 2021;9(5):e47–e48. doi: 10.1016/S2213-2600(21)00101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doidge JC, Gould DW, Fernando-vivas P, et al. Trends in intensive care for patients with COVID-19 in England, Wales, and Northern Ireland. Am J Respir Crit Care Med. 2021;203(5):565–574. doi: 10.1164/rccm.202008-3212OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.SerpaNeto A, et al. Mechanical power of ventilation is associated with mortality in critically ill patients: an analysis of patients in two observational cohorts. Intensive Care Med. 2018;44(11):1914–1922. doi: 10.1007/s00134-018-5375-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. OTI timing in different waves. Table S2. Oxygen-therapy at ICU admission differentiate by waves. Table S3. Univariate mortality analysis. Table S4. Mortality in the ICU: Binary logistic regression. Table S5. Early vs late group after excluding patients with < 7 days of ICU LOS. Table S6. Propensity score early and late groups. Figure S1. Propensity score early and late groups.
Data Availability Statement
The anonymized database and the data dictionary that defines each field in the set, will be available to reviewers if they consider necessary prior confidentiality agreement. Information contact: Alejandro Rodríguez, Critical Care Department – Hospital Universitario de Tarragona Joan XXIII, Tarragona, Spain, whose mail is ahr1161@yahoo.es.