Abstract
Background: Triglyceride (TG) deposit cardiomyovasculopathy (TGCV) is a novel cardiovascular disorder and was recently encoded as an orphan disease in Europe (ORPHA code: 565612). Defective lipolysis results in TG accumulation in the myocardium and coronary arteries in TGCV. The myocardial washout rate (WR) of iodine-123-β-methyl iodophenyl-pentadecanoic acid (BMIPP) is an essential indicator to evaluate myocardial lipolysis in vivo. TGCV is classified into primary and idiopathic type with and without PNPLA2 mutation, respectively. Here, we present the clinical correlation perspectives of TGCV patients in Chiba, Japan, to increase the awareness of this orphan disease and facilitate its diagnosis.
Methods: We enrolled 234 patients who underwent BMIPP scintigraphy between September 2015 and July 2019. The diagnosis of TGCV was made based on the criteria we reported previously. Blood smear tests were performed for TGCV classification. The distributions of TGCV in each comorbidity were investigated.
Results: In total, 104 patients were diagnosed with definitive idiopathic TGCV (I-TGCV). They had various comorbid conditions, including heart failure with reduced ejection fraction and multivessel coronary artery disease requiring revascularization. Moreover, the serum TG levels in I-TGCV patients were not high, and there was no correlation between serum TG level and BMIPP WR (n=205, p-value=0.31), supporting the pathophysiological hypothesis of TGCV.
Conclusion: I-TGCV patients showed multiple coexistence of coronary artery disease, heart failure of unknown etiology, or diabetes mellitus. For patients with such clinical characteristics, BMIPP scintigraphy and calculation of WR should be considered proactively for the diagnosis of TGCV.
Keywords: Clinical characteristics, Comorbidity, Diagnosis, Iodine-123-β-methyl iodophenyl-pentadecanoic acid (BMIPP), Triglyceride deposit cardiomyovasculopathy (TGCV), Washout rate
Ten years have passed since the first discovery of triglyceride (TG) deposit cardiomyovasculopathy (TGCV) in a patient waiting to undergo heart transplant in Japan (1, 2). TGCV is a rare and intractable cardiovascular disorder that presents with severe heart failure, coronary artery disease, and arrhythmia (1–5). The pathophysiology of TGCV is characterized by excessive accumulation of TG in cardiomyocytes (6) and vascular smooth muscle cells (7, 8) due to defective intracellular lipolysis. Neither serum TG levels, body mass index, nor fat distribution is a diagnostic indicator of TGCV (3).
Diagnosing TGCV is challenging because the diagnosis is often obscure, and patients are instead treated symptomatically for coronary artery disease, heart failure, or cardiomyopathy in daily clinical practice (3). The potential number of TGCV patients in Japan was estimated to be 40,000–50,000 (3). Since the discovery of TGCV in 2008, the Japan TGCV study group has identified more than 200 patients with TGCV, and the diagnosis of TGCV is gradually being accepted. However, there is a huge discrepancy between the number of those diagnosed and the number of potential cases, and we are now tackling the issue of un-diagnosed or delayed-diagnosed situations (3).
Long-chain fatty acids (LCFAs) are the main energy source for the heart (9). LCFAs are taken up by cardiomyocytes, β-oxidized, and used for generating energy while some of them are stored in the cytoplasm in the form of TG, which is a compound of three fatty acids and a glycerol. In normal condition, stored TGs are hydrolyzed by enzymes such as intracellular lipases to release LCFAs. These processes are impaired in TGCV patients (10, 11).
A useful tool for diagnosing TGCV is myocardial fatty acid scintigraphy (12). TGCV patients tend to present a markedly decreased washout rate (WR) of iodine-123-β-methyl iodophenyl-pentadecanoic acid (BMIPP) in myocardial single photon emission computed tomography (SPECT) (3–6). A BMIPP molecule is a branched LCFA analog for a palmitate, which reflects the kinetics of fatty acids (13), and therefore directly represents TGCV pathophysiology. Hence, markedly reduced BMIPP WR has been a major diagnostic criterion for TGCV (4).
However, performing additional delayed imaging to calculate WR is burdensome in many institutions. Furthermore, it is not easy to identify patients who should undergo cardiac scintigraphy because TGCV presents a wide variety of clinical phenotypes. Selecting suitable cases for BMIPP scintigraphy and determining the characteristics of TGCV clusters remain issues of importance.
We have been trying to identify patients with TGCV and have diagnosed about 100 cases in Chiba University Hospital and its affiliated institutions. This study attempted to summarize the clinical correlation perspectives of these patients.
Materials and methods
Selection of participants and analysis
From September 2015 to July 2019, consecutive patients who underwent BMIPP scintigraphy were reviewed and diagnosed via the TGCV definitive clinical diagnostic criteria (4). For the classification of TGCV, blood smear tests were performed and vacuolar degeneration of peripheral blood polymorphonuclear leukocytes was examined. BMIPP scintigraphy has been proactively performed for patients who have either of the following: repeated percutaneous coronary intervention or coronary artery bypass grafting, early-onset ischemic heart disease, non-specific chest pain, unexplained impaired cardiac function or left ventricular asynergy, coronary diffuse narrowing or coronary spasm on coronary angiography, or diabetic complications. Among them, diagnoses of acute coronary syndrome, takotsubo cardiomyopathy, CD36 deficiency, mitochondrial cardiomyopathy, cardiac sarcoidosis, or chronic thromboembolic pulmonary hypertension were excluded from the analyses. Clinical characteristics were summarized in groups of TGCV, non-TGCV and the total.
All participants were divided into groups based on the presence of comorbidities: coronary diffuse narrowing, heart failure, and diabetes mellitus. The numbers and the ratios of confirmed TGCV patients were then calculated for each group. In addition, the serum TG levels of the TGCV and non-TGCV groups were compared and the correlation between BMIPP WRs and serum TG levels was assessed. Written informed consent was obtained from all the participants for analysis. This study was approved by the Research Ethics Committee of Chiba University Graduate School of Medicine (#2954).
BMIPP scintigraphy
Patients with markedly reduced BMIPP WRs in 123I-BMIPP/thallium-201 (201TI) dual-isotope SPECT were followed up with 123I-BMIPP single-isotope SPECT later. Following the protocol suggested by the Japanese Society of Nuclear Cardiology, after fasting for 12 hours or more, 111 MBq of 123I-BMIPP (Cardiodine; Nihon Medi-Physics Co. Ltd., Tokyo, Japan) was intravenously injected at rest. Early images were obtained after 20 minutes and delayed images were obtained after 180 to 210 minutes. WRs were calculated using the myocardial scintigraphy analysis software Heart Risk View-S (Nihon Medi-Physics) based on the data of SPECT short-axis images.
The SPECT system used was GE Infinia Hawkeye 4 (GE Healthcare Japan, Tokyo, Japan), equipped with an extended low-energy general-purpose (ELEGP) collimator. Collection was performed in a 64 × 64 matrix and by 180° step and shoot mode with a 6° sampling angle and 60 sec/view. The pixel size was 5.89 mm and slice width was 5.89 mm. The energy windows for 123I were 159 keV ± 10% (main) and 130 keV ± 10% (sub). The energy windows for 201TI were 70 keV ± 15% (main) and 54 keV ± 9% (sub). Reconstruction was performed with scatter corrections using filtered back-projection (with the ramp filter) and the 10th-order Butterworth filter of 0.4 cycle/cm cutoff frequency.
Statistical analyses
Continuous values were reported as mean ± standard deviation. Demographic and clinical characteristics were compared between TGCV and non-TGCV using Welch's t tests for continuous variables and chi-square tests for binary variables. Multiple comparison was performed using Benjamini-Hochberg procedure with false discovery rate threshold of 0.05. Welch's t test was used for comparing serum TG levels between TGCV and non-TGCV patients. Correlation between the BMIPP WRs and serum TG levels was assessed with the test of no correlation followed by the calculation of Pearson's correlation coefficients. Data was analyzed using R version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/).
Results
Data on 234 cases were analyzed. The clinical diagnoses are listed below (some cases had more than 1 comorbidity): there were 87, 40, 18, 13, and 8 cases of myocardial infarction, stable angina, coronary spastic angina, chronic total occlusion of coronary artery, and silent myocardial ischemia, respectively. Additionally, we observed 47 cases of heart failure and/or impaired cardiac function of unknown etiology. Moreover, there were 24, 14, 13, 11, and 5 cases of critical arrhythmia, cardiac arrest, valvular heart diseases, dilated cardiomyopathy, and hypertrophic cardiomyopathy, respectively. Further, 3 and 15cases of myocarditis and/or pericarditis and aortic and/or peripheral artery diseases were respectively diagnosed.
The clinical characteristics of the TGCV group, non-TGCV group and the total are shown in Table 1. Among them, 104 (44%) were diagnosed with definitive TGCV, and they all had the two major items of the diagnostic criteria. All cases were clinically diagnosed with I-TGCV because of the lack of typical Jordans' anomaly (large vacuolar degeneration in most peripheral blood polymorphonuclear leukocytes), which is essential for primary TGCV (14). The BMIPP WR of the TGCV patient group was −1.37 ± 10.6%. The TGCV group had higher rate of coronary artery disease (TGCV: 78 (75%) cases, non-TGCV: 68 (52%) cases, FDR adjusted p-value = 0.004). Although there were no significant differences in clinical characteristics except for coronary artery disease between the TGCV and non-TGCV groups, the TGCV group tended to have slightly lower left ventricular ejection fraction and higher rate of diabetes mellitus.
Table 1. Clinical characteristics of the study population.
| Characteristic | Total (N=234) | TGCV (n=104) | non-TGCV (n=130) | p-value/FDR adjusted p-value | |
|---|---|---|---|---|---|
|
|
Age (years) |
63.3 ± 13.7 |
64.6 ± 12.9 |
62.3 ± 14.3 |
0.21/0.62 |
|
|
Sex (male) |
173 (74%) |
77 (74%) |
96 (74%) |
0.97/0.97 |
|
|
Height (cm) |
163 ± 9.3 |
163 ± 9.8 |
163 ± 9.0 |
0.95/0.97 |
|
|
Body weight (kg) |
62.0 ± 14.5 |
61.7 ± 14.8 |
62.3 ± 14.3 |
0.79/0.95 |
|
|
Body mass index (kg/m2) |
23.1 ± 4.3 |
23.0 ± 4.2 |
23.2 ± 4.4 |
0.74/0.95 |
| Coronary artery disease | Total 146 (62%) | Total 78 (75%) | Total 68 (52%) | 0.0004/0.004 | |
|
|
|||||
| Heart | Coronary artery disease (details) | 1-vessel 28 (12%) | 1-vessel 14 (13%) | 1-vessel 14 (11%) | |
| 2-vessel 37 (16%) | 2-vessel 20 (19%) | 2-vessel 17 (13%) | |||
| 3-vessel 54 (23%) | 3-vessel 31 (30%) | 3-vessel 23 (18%) | |||
| LMT 15 (6%) | LMT 8 (8%) | LMT 7 (5%) | |||
| CSA 18 (8%) | CSA 10 (10%) | CSA 8 (6%) | |||
|
| |||||
| Heart failure or cardiac dysfunction of unknown etiology |
47 (20%) |
22 (21%) |
25(20%) |
0.72/0.95 |
|
| Post-resuscitation |
14 (6%) |
8 (8%) |
6 (5%) |
0.32/0.78 |
|
| LVEF (%) | 41.1 ± 18.7 | 38.1 ± 18.0 | 43.6 ± 18.9 | 0.026/0.15 | |
|
| |||||
| Hypertension |
97 (41%) |
45 (43%) |
52 (40%) |
0.61/0.95 |
|
| Comorbidity | Diabetes mellitus |
113 (48%) |
58 (56%) |
55 (42%) |
0.041/0.16 |
| Dyslipidemia |
101 (43%) |
43 (41%) |
58 (45%) |
0.62/0.95 |
|
Continuous values were reported as mean ± standard deviation. Demographic and clinical characteristics were compared between TGCV and non-TGCV using Welch's t tests for continuous variables and chi-square tests for binary variables. Multiple comparison was performed using Benjamini-Hochberg procedure with false discovery rate threshold of 0.05.
CSA: Coronary spastic angina, FDR: False discovery rate, LMT: Left main trunk, LVEF: Left ventricular ejection fraction
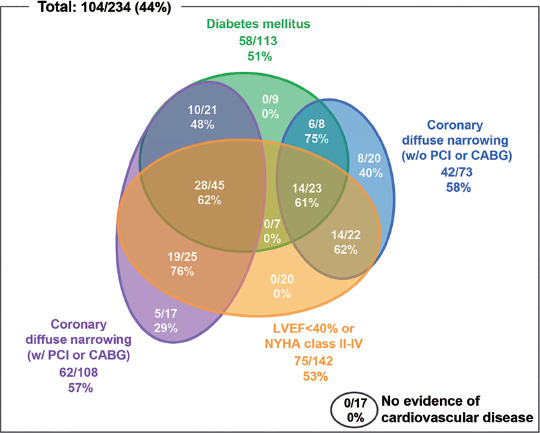
The patients had various comorbid conditions such as coronary artery disease, heart failure, and diabetes mellitus (Figure 1). Few TGCV patients had only one of these comorbidities. The group with the highest number of TGCV patients had 28 (62%) patients who have coronary diffuse narrowing with revascularization, heart failure, and diabetes mellitus, but the TGCV rate was not the highest. On the other hand, the group who have coronary diffuse narrowing with revascularization and heart failure alone showed the highest TGCV rate of 76% (19 patients).
Figure 1.
Correlation between TGCV diagnosis and presence of comorbidities.
Here, we present the comorbidities of the 104 TGCV cases out of 234 examined cases in our hospital. The presence of diabetes, coronary diffuse narrowing with or without revascularization, and left ventricular dysfunction or heart failure was analyzed. The numbers are listed for each group with the denominator as the total cases with that particular comorbidity and the numerator as total TGCV patients in that group, along with percentage.
CABG: Coronary artery bypass grafting, LVEF: Left ventricular ejection fraction, NYHA: New York Heart Association, PCI: Percutaneous coronary intervention
Among those who did not take lipid-lowering drugs, there was no difference in the serum TG level between TGCV patients and non-TGCV patients (TGCV: n=44, 127 ± 84.6 mg/dL, non-TGCV: n=66, 133 ± 70.7 mg/dL, p-value=0.73). Furthermore, the BMIPP WRs and serum TG levels were not found to be correlated (n=205, p-value=0.31).
Discussion
Clinical characteristics of TGCV
There are only a few clinical reports on TGCV. This study is the first report about the clinical characteristics of I-TGCV, summarizing 104 patients (about 40%) out of over 220 cases currently diagnosed in Japan. Many of the definitive I-TGCV patients presented with diverse comorbidities. TGCV cases accounted for over 40% in all the study population, which were enrolled mainly because of intractable or early-onset coronary artery disease, or heart failure of unknown etiology.
There was no difference between the serum TG levels of TGCV patients and those of non-TGCV patients; serum TG did not affect the intracellular TG accumulation in TGCV patients directly. This result is consistent with the pathophysiological hypothesis that the TG accumulation in the myocardial cytoplasm is due to intracellular lipase dysfunction. Furthermore, no correlation existed between BMIPP WRs and serum TG levels. This result suggests that BMIPP scintigraphy reflects the dynamics of fatty acids in cardiomyocytes independent from blood TG level, and especially reflects the accumulation of TG in TGCV patients.
One-half of the TGCV patients had diabetes mellitus as comorbidity, and the other half did not. It is known that TG and glucose are essential energy sources for the normal heart (15); it is of interest to investigate a possible association between the defective intracellular lipolysis observed in TGCV and abnormal cellular glucose utilization underlying diabetes mellitus.
BMIPP WR for the diagnosis of TGCV
We first reported the defective BMIPP WR in human (6) and murine genetic adipose triglyceride lipase deficiency (16), indicating the association between the molecular defect and BMIPP WR. Figure 2 shows the WRs of this study along with related previous reports using SPECT. Values of BMIPP WR were reported to be 33.6 ± 4.3% (n=8) without time-decay correction (17) and 19.4 ± 3.2% (n=10) with time-decay correction (18) in normal subjects; thus, the reference ranges of BMIPP WR are predictively calculated 22.8 to 44.4% without time-decay correction and 11.8 to 27.0% with time-decay correction, respectively (95% prediction interval).
Figure 2.
Range of 123I-BMIPP WR.
Markers show the mean values and bars show the standard deviations.
AP: Angina pectoris, BMIPP: Iodine-123-β-methyl iodophenyl-pentadecanoic acid, DC: Decay correction, DM: Diabetes mellitus, eAP: Effort angina pectoris, HD: Hemodialysis
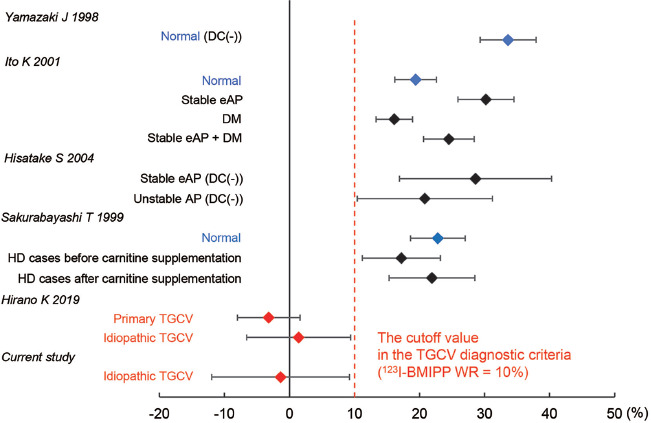
It has been reported that WR increases in ischemic myocardium and in effort angina (18), while it mildly decreases in diabetes mellitus (18), stable effort angina, and unstable angina (19). In addition, it has been reported that patients with chronic kidney disease on hemodialysis had a mild decrease in WR associated with secondary carnitine insufficiency (20). On the other hand, our data from the international registry of TGCV and related disorders demonstrated that the WR significantly reduced with primary TGCV −3.2 ± 4.8% and idiopathic 1.4 ± 8.0% (5). The patients with definitive diagnoses of TGCV in this study also had very low WRs (−1.37 ± 10.6%). Whereas, to date, there is no report of a marked decrease of WRs to less than 10% except in TGCV patients. Based on the above, the TGCV cutoff value of BMIPP WR<10% was considered appropriate.
Limitation
Chiba University Hospital is the only hospital certified to perform heart transplantation in the Chiba prefecture, where approximately 6.3 million people are living; hence, many patients with severe cardiovascular diseases in the prefecture and its surrounding areas are referred to this hospital. Therefore, patients with TGCV may be many in the current study population. To determine the prevalence and the number of TGCV patients in Japan, the Japan TGCV study group is preparing to launch the new TGCV registry as a governmental rare disease project.
Conclusion
TGCV is present in many patients with histories of coronary artery disease or heart failure of unknown etiology. TGCV is not correlated with serum TG level; therefore, a diagnosis of TGCV should be considered even in patients with normal serum TG levels. BMIPP WR is essential for the TGCV diagnosis; therefore, it is important to conduct BMIPP scintigraphy on suspected patients.
Author contribution
HM designed the research concept, and collected, analyzed, and interpreted data. HM and KHi wrote the manuscript. TI analyzed BMIPP SPECT and contributed to the discussion. MO examined blood smear test and contributed to the discussion. KHo provided scientific suggestions for writing the manuscript. YK contributed to the discussion. KHi is the principal investigator for the Japan TGCV study group.
Sources of funding
This study was partially supported by research grants on rare and intractable diseases from the Japan Agency for Medical Research and Development (AMED).
Conflicts of interest
KHi received research funds from Nihon Medi-Physics Co. Ltd. YK received remuneration for attending meetings (presentations) from Abbott Medical Japan, Daiichi Sankyo, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim and scholarship funds from Abbott Medical Japan, Daiichi Sankyo.
References
- 1.Hirano K, Ikeda Y, Zaima N, Sakata Y, Matsumiya G. Triglyceride deposit cardiomyovasculopathy. N Engl J Med 2008; 359: 2396–8. [DOI] [PubMed] [Google Scholar]
- 2.Hirano K. A novel clinical entity: triglyceride deposit cardiomyovasculopathy. J Atheroscler Thromb 2009; 16: 702–5. [DOI] [PubMed] [Google Scholar]
- 3.Hirano K. Triglyceride deposit cardiomyovasculopathy. Nihon Naika Gakkai Zasshi 2017; 106: 2385–90. (Article in Japanese) [Google Scholar]
- 4.Miyauchi H, Hashimoto C, Ikeda Y, Li M, Nakano Y, Kozawa J, et al. Diagnostic criteria and severity score for triglyceride deposit cardiomyovasculopathy. Ann Nucl Cardiol 2018; 4: 94–100. [Google Scholar]
- 5.Li M, Hirano K, Ikeda Y, Higashi M, Hashimoto C, Zhang B, et al. Triglyceride deposit cardiomyovasculopathy: a rare cardiovascular disorder. Orphanet J Rare Dis 2019; 14: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirano K, Ikeda Y, Sugimura K, Sakata Y. Cardiomyocyte steatosis and defective washout of iodine-123-β-methyl iodophenyl-pentadecanoic acid in genetic deficiency of adipose triglyceride lipase. Eur Heart J 2015; 36: 580. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda Y, Hirano K, Fukushima N, Sawa Y. A novel type of human spontaneous coronary atherosclerosis with triglyceride deposition. Eur Heart J 2014; 35: 875. [DOI] [PubMed] [Google Scholar]
- 8.Ikeda Y, Zaima N, Hirano K, Mano M, Kobayashi K, Yamada S, et al. Coronary triglyceride deposition in contemporary advanced diabetics. Pathol Int 2014; 64: 325–35. [DOI] [PubMed] [Google Scholar]
- 9.Bing RJ, Siegel A, Ungar I, Gilbert M. Metabolism of the human heart. II. Studies on fat, ketone and amino acid metabolism. Am J Med 1954; 16: 504–15. [DOI] [PubMed] [Google Scholar]
- 10.Hirano K, Tanaka T, Ikeda Y, Yamaguchi S, Zaima N, Kobayashi K, et al. Genetic mutations in adipose triglyceride lipase and myocardial up-regulation of peroxisome proliferated activated receptor- γ in patients with triglyceride deposit cardiomyovasculopathy. Biochem Biophys Res Commun 2014; 443: 574–9. [DOI] [PubMed] [Google Scholar]
- 11.Takagi A, Ikeda Y, Kobayashi K, Kobayashi K, Ikeda Y, Kozawa J, et al. Newly developed selective immunoinactivation assay revealed reduction in adipose triglyceride lipase activity in peripheral leucocytes from patients with idiopathic triglyceride deposit cardiomyovasculopathy. Biochem Biophys Res Commun 2018; 495: 646–51. [DOI] [PubMed] [Google Scholar]
- 12.Higashi M, Y Ikeda Y, Miyauchi H, Zaima N, Suzuki A, Li M, et al. Imaging modalities for triglyceride deposit cardiomyovasculopathy. Ann Nucl Cardiol 2017; 3: 94–102. [Google Scholar]
- 13.Knapp FF Jr, Ambrose KR, Goodman MM. New radioiodinated methyl-branched fatty acids for cardiac studies. Eur J Nucl Med 1986; 12 Suppl: S39–S44. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka M, Ikeda Y, Li M, Zaima N, Kawahara Y, Watanabe K, et al. A historical case of primary triglyceride deposit cardiomyovasculopathy. Pathol Int 2020; 70: 58–61. [DOI] [PubMed] [Google Scholar]
- 15.Neubauer S. The failing heart—an engine out of fuel. N Engl J Med 2007; 356: 1140–51. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki A, Yamaguchi S, Li M, Hara Y, Miyauchi H, Ikeda Y, et al. Tricarpin rescues myocardial abnormality in a mouse model of triglyceride deposit cardiomyovasculopathy. J Oleo Sci 2018; 8: 983–9. [DOI] [PubMed] [Google Scholar]
- 17.Yamazaki J. Clinical significance of 123I-BMIPP myocardial SPECT. Kaku Igaku 1998; 35: 367–73. (Article in Japanese) [PubMed] [Google Scholar]
- 18.Ito K, Sugihara H, Tanabe T, Yuba T, Doue T, Adachi Y, et al. Assessment of myocardial fatty acid metabolism in patients with angina pectoris and diabetes mellitus using 123I-BMIPP myocardial scintigraphy. Kaku Igaku 2001; 38: 699–705. (Article in Japanese) [PubMed] [Google Scholar]
- 19.Hisatake S, Yamashina S, Yamazaki J. Comparison between unstable angina pectoris and stable effort angina pectoris by using 123IBMIPP and 201Tl myocardial SPECT. Kaku Igaku 2004; 41: 9–16. (Article in Japanese) [PubMed] [Google Scholar]
- 20.Sakurabayashi T, Takaesu Y, Haginoshita S, Takeda T, Aoike I, Miyazaki S, et al. Improvement of myocardial fatty acid metabolism through L-Carnitine administration to chronic hemodialysis patients. Am J Nephrol 1999; 19: 480–4. [DOI] [PubMed] [Google Scholar]


