Abstract
Triglyceride deposit cardiomyovasculopathy (TGCV) is a newly identified disease that was discovered in individuals who required cardiac transplantation in Japan in 2008. Defective intracellular lipolysis causes triglyceride (TG) accumulation in the myocardium and coronary artery vascular smooth muscle cells, which results in severe heart failure and coronary artery disease with poor prognosis. A known cause of TGCV is a genetic deficiency of adipose triglyceride lipase (ATGL), a rate-limiting enzyme in the intracellular hydrolysis of TG. TGCV is classified into primary TGCV with ATGL mutations and idiopathic TGCV without ATGL mutations. Since its discovery, the Japan TGCV Study Group has attempted to elucidate its pathophysiology, develop diagnostic procedures, and specific treatment. Myocardial scintigraphy with iodine-123-β-methyl iodophenyl-pentadecanoic acid (123I-BMIPP) is a unique imaging modality for evaluating myocardial lipolysis in vivo. The washout rate of 123I-BMIPP is an essential indicator for the diagnosis of TGCV. Along with our efforts to provide awareness of and insights into this disease concept, we found that the cumulative number of clinically diagnosed patients has reached >200 and the cases are distributed throughout Japan. In addition, we successfully completed three investigator-initiated clinical trials of a potential therapeutic agent (CNT-01) for TGCV, which was assigned by the Ministry of Health, Labour, and Welfare, Japan, under the SAKIGAKE Designation System in June 2020. Here, we provide the Diagnostic Criteria 2020 for TGCV in order to further promote this “rare and intractable disease” project.
Keywords: Adipose triglyceride lipase, BMIPP, Diagnostic criteria, Rare and intractable diseases, Tricaprin, Triglyceride deposit cardiomyovasculopathy
Triglyceride deposit cardiomyovasculopathy (TGCV) is a rare and intractable cardiovascular disorder that was first reported in Japanese patients with a genetic deficiency of adipose triglyceride lipase (ATGL) (1). Lack of ATGL activity results in defective hydrolysis of intracellular triglyceride (TG), thereby causing lipotoxicity and energy failure which lead to diffuse-narrowing, TG-deposit coronary atherosclerosis and cardiomyocyte steatosis (2, 3). TGCV is classified into two subtypes: primary and idiopathic TGCVs with and without genetic mutation of PNPLA2 encoding ATGL, respectively (4, 5). In both TGCV subtypes, patients have severe heart failure, arrhythmia, coronary artery disease resistant to standard therapies such as lipid-lowering drugs and/or percutaneous coronary intervention, and a poor prognosis (6, 7).
Long-chain fatty acids (LCFAs) are the major energy source of the normal heart (8). After LCFAs are taken up by cardiomyocytes, they are immediately incorporated into the cellular TG pool. Intracellular lipases such as ATGL hydrolyze TG to release LCFAs, which are oxidized in the mitochondria to produce adenosine triphosphate, the energy that generates heartbeats. Myocardial scintigraphy with iodine-123-β-methyl iodophenyl-pentadecanoic acid (123I-BMIPP), an LCFA analog, reflects the kinetics of LCFAs (9). 123I-BMIPP scintigraphy became available clinically in Japan in the 1990s. Pioneering works with canine model treated with Etomoxir by Nohara et al. showed washout of 123I-BMIPP may be increased in ischemic condition (10, 11). In contrast, we found the marked reduction of washout rate (WR) of 123I-BMIPP in human (3, 12) and murine with genetic deficiency of ATGL (13), indicating the association of molecular defects and 123I-BMIPP WR. Furthermore, we reported that the 123I-BMIPP WR was low in both primary and idiopathic TGCVs based on data from the first registry of TGCV and related disorders.
Basic and clinical research studies have been accelerated to develop effective diagnostic methods and treatments for TGCV as soon as possible. In 2020, we launched the TGCV Diagnostic Criteria Review Committee as a governmental project on rare and intractable diseases (Health, Labour and Welfare Sciences Research Grants), to collect and evaluate the latest information on TGCV. In this manuscript, we provide the Diagnostic Criteria 2020 for TGCV and the latest information on our activities to overcome the intractable disease.
The Diagnostic Criteria 2020 for TGCV and the classification algorithms for primary and idiopathic TGCVs
As shown in Table 1, the present criteria consist of three essential and three major items. The essential items signify the metabolic basis of TGCV, such as impaired LCFA metabolism or TG deposition in the myocardium. Major items signify clinical phenotypes, including reduced cardiac function, specific appearance of coronary arteries, or typical Jordans' anomaly (14). Both essential and major items with one or more positive indicate definite TGCV, and at least one positive essential item denotes probable TGCV. Supportive items include diabetes mellitus and hemodialysis, which are common features of TGCV (15), but their relationship or degree of contribution to the development of TGCV is unknown.
Table 1. Diagnostic Criteria 2020 for triglyceride deposit cardiomyovasculopathy.
| Items | Clinical findings |
|---|---|
| 1. Essential items | Impaired LCFA metabolism or TG deposition in myocardium |
| 1) Decreased washout rate (<10%) in myocardial 123I-BMIPP SPECT | |
| 2) Myocardial TG deposition by biopsy specimens (a) | |
| 3) Myocardial TG deposition by CT or MR spectroscopy | |
| 2. Major items | 1) Decreased left ventricular ejection fraction (<40%) |
| 2) Diffuse narrowing of coronary arteries documented by CAG and/or coronary CT angiography (b) | |
| 3) Typical Jordans' anomaly (apparent vacuoles >1 μm in size) of polymorphonuclear leucocytes in peripheral blood smear (c) |
Diagnosis
Definite TGCV: One or more essential items and one or more major items are met.
Probable TGCV: At least one essential item is met.
Supportive items (d)
Diabetes mellitus (e)
Hemodialysis
(a) For tissue TG contents examination, frozen sections with osmium fixation, but not paraffin sections, should be used for prevention of lipid elution
(b) The presence or absence of a significant stenosis is not considered
(c) For difficult cases, May-Giemsa staining slides of peripheral blood smear will be evaluated by the Japan TGCV Study Group
(d) These items are commonly present according to autopsy heart analysis and small-scale cohort analysis performed by the Japan TGCV Study Group but not proven to have diagnostic accuracy or causal relationship.
(e) According to the diagnostic criteria of diabetes mellitus by the Japan Diabetes Society.
If imaging protocols for 123I-BMIPP myocardial scintigraphy, cardiac CT and cardiac MR spectroscopy are needed, contact Japan TGCV Study Group (E-mail: inffo@tgcv.org)
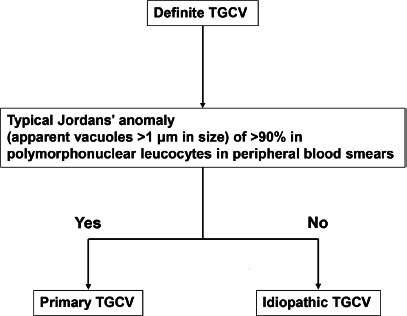
The classification algorithms for primary TGCV and idiopathic TGCV are described in Figure 1.
Abbreviations: TG: triglyceride, LCFA: long chain fatty acid, 123I-BMIPP: iodine-123-β-methyl iodophenyl-pentadecanoic acid, SPECT: single-photon emission computed tomography, CT: computed tomography, MR: magnetic resonance, CAG: coronary angiography, TGCV: triglyceride deposit cardiomyovasculopathy
The classification algorithms for primary and idiopathic TGCVs are also provided in the present diagnostic criteria (Figure 1). Definite TGCV is classified as either primary or idiopathic TGCV, according to the presence or absence of typical Jordans' anomaly (apparent vacuoles >1 μm in size) of >90% in polymorphonuclear leucocytes in peripheral blood smears.
Figure 1.
The classification algorithms for primary and idiopathic triglyceride deposit cardiomyovasculopathy (TGCV).
Definite TGCV is classified as either primary or idiopathic TGCV according to the presence or absence of typical Jordans' anomaly.
Differential diagnoses of TGCV
The following diseases should be differentiated from TGCV.
-
Cardiovascular diseases presenting with heart failure
Hypertrophic cardiomyopathy, dilated cardiomyopathy, dilated phase of hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, mitochondrial cardiomyopathy, alcoholic heart disease, metabolic myocardial disorders (e. g. Fabry disease, Pompe disease, Danon disease, mitochondrial diseases, CD36 deficiency, and cholesteryl ester storage disease), carnitine deficiency (drug-induced or dialysis-related), diabetic cardiomyopathy, and excess epicardial fat deposition
-
Other diseases presenting with Jordans' anomaly
Neutral lipid storage disease with ichthyosis, carnitine palmitoyltransferase deficiency
(Neutral lipid storage disease with myopathy is a clinical continuum of primary TGCV because the responsible gene is identical.)
Activities of the Japan TGCV Study Group inside and outside Japan
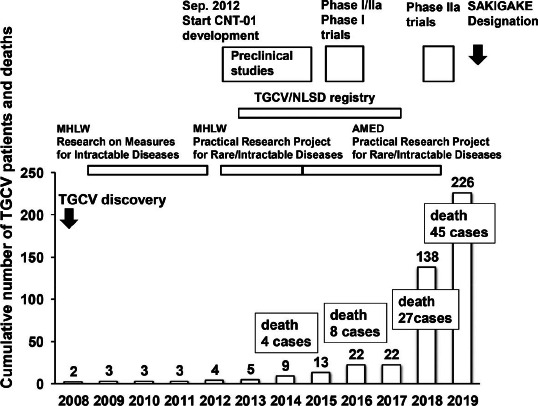
We organized the Japan TGCV Study Group in 2009 to save the lives of patients with TGCV. We created and published diagnostic criteria, a self-questionnaire to score disease symptom and activities of daily living for TGCV (16). On the last day of February (World Rare Disease Day) in 2013, we launched the International Registry for TGCV and related disorders, namely neutral lipid storage disease (NLSD) with myopathy (17, 18) and NLSD with ichthyosis (19, 20), in order to clarify the clinical profiles and natural history of TGCV. As analysis of the enrolled cases revealed that TGCV is intractable disease with cardiac involvement independent of NLSD (5), TGCV was encoded as a rare cardiovascular disorder in Orphanet, the largest rare disease network in Europe in 2019 (ORPHA code. 565612, https://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=EN&Expert=565612). In Japan, the number of diagnosed TGCV cases reached 226 in November 2019 (Figure 2). The member of our study group now deploys nationwide (please see the appendix).
Figure 2.
Cumulative number of patients with TGCV and activities of the Japan TGCV Study Group.
Abbreviations: AMED: Japan Agency for Medical Research and Development, MHLW: Ministry of Health, Labour, and Welfare, NLSD: neutral lipid storage disease, TGCV: triglyceride deposit cardiomyovasculopathy
Potential of a new therapeutic agent, CNT-01
Our previous experiments revealed that LCFAs induced TG accumulation in fibroblasts derived from patients with TGCV (21, 22). We developed a newnutritional therapeutic agent using medium-chain fatty acids (MCFAs), an alternative energy source to LCFA and glucose (23, 24), as we found that MCFAs do not induce TG deposition in ATGL-deficient fibroblasts (unpublished data). CNT-01, a capsule formulation of tricaprin (capric acid TG), was manufactured at the Pharmaceutical Department of Osaka University Hospital. After obtaining information that CNT-01 reduced myocardial lipid deposition, improved left ventricular function, and extended life expectancy in a mouse model of TGCV (13), we performed the following serial investigator-initiated clinical trials, 1) phase I/IIa trials for idiopathic TGCV (ClinicalTrials.gov Identifier: NCT02502578), 2) a phase I study in healthy subjects (UMIN 000022174), and 3) a multicenter, placebo-controlled, double-blind phase IIa study for idiopathic TGCV (UMIN000035403). These trials indicated that CNT-01 is safe and may improves TG metabolism in patients with TGCV (data not shown). As a result, CNT-01 was assigned as a therapeutic agent through “SAKIGAKE Designation System” of the Ministry of Health, Labour, and Welfare, which promotes research and development in Japan aiming at early practical application of innovative pharmaceutical products, medical devices, and regenerative medicines. The mission of our study group is to succeed another clinical trial for CNT-01 to be approved as the world's first drug for TGCV in collaboration with the TGCV patient group and pharmaceutical company.
Remaining issues for the future
More than 1,000 hospitals have a single-photon emission computed tomography (SPECT) apparatus in Japan. However, the number of hospitals where delay images and WR calculation are proactively made remains limited. As described earlier, 123I-BMIPP scintigraphy is an essential and important diagnostic nuclear imaging modality for TGCV that is used to evaluate myocardial lipolysis in vivo. Not only awareness of TGCV but also scientific investigation and discussions on 123I-BMIPP WR, including detailed kinetics of 123I-BMIPP, its molecular mechanism(s) and pathophysiological significance, are required.
It is an important future research focus to investigate underlying mechanisms of defective intracellular lipolysis in patients with idiopathic TGCV. We recently reported reduced ATGL activity in peripheral leucocytes in patients with idiopathic TGCV (25). The presence of ATGL protein in idiopathic TGCV (5) suggests that the mechanism of the decrease in ATGL activity may be due to the structural changes of ATGL, decrease in auxiliary factors, or increase in inhibitors, although no available data support this hypothesis.
Conclusion
We, the Japan TGCV Study Group, officially announce the Diagnostic Criteria 2020 for TGCV.
Acknowledgments
We thank Ms. Yumi Iriguchi and Ms. Mao Ishikawa for their secretarial assistance.
Author contribution
KK and KH wrote the manuscript. KK, YS, YI, HM, YN, KS, KN, JK, HH, TA, HY, and CH are members of the TGCV Diagnostic Criteria Review Committee. TI contributed to the discussion for the algorithm for Jordans' anomaly. KH is the principal investigator of the Japan TGCV Study Group.
Sources of funding
This study was partially supported by research grants for rare and intractable diseases from the Japan Agency for Medical Research and Development (AMED grant No. 17ek0109092h0003) and Health Labour Sciences Research Grant from the Ministry of Health, Labour, and Welfare (grant No. 20FC1008).
Conflicts of interest
KH received grants from Nihon Medi-Physics Co. Ltd. KN received grants from Nihon Medi-Physics Co. Ltd. HY received honoraria for speaking activities from Bayer, Kowa, and Takeda Co. Ltd.
Appendix
The members of the Japan TGCV Study Group are as follows:
Ken-ichi Hirano (Laboratory of Cardiovascular Disease, Novel, Non-invasive, and Nutritional Therapeutics and Triglyceride Research Center, Graduate School of Medicine, Osaka University), Tetsuya Amano (Department of Cardiology, Aichi Medical University), Toshihisa Anzai (Department of Cardiovascular Medicine, Hokkaido University Graduate School of Medicine), Yoshihiko Ikeda (Department of Pathology, National Cerebral and Cardiovascular Center), Hiroyasu Iso (Public Health Graduate School of Medicine, Osaka University), Tomomi Ide (Department of Cardiovascular Medicine, Faculty of Medical Sciences, Kyushu University), Tomonori Itoh (Division of Cardiology, Department of Internal Medicine, Iwate Medical University), Tohru Inaba (Department of Infection Control and Laboratory Medicine, Kyoto Prefectural University of Medicine), Takahiro Okumura (Department of Cardiology, Nagoya University Graduate School of Medicine), Kouji Kajinami (Department of Cardiology, Kanazawa Medical University), Junji Kozawa (Department of Metabolic Medicine, Graduate School of Medicine, Osaka University), Kunihisa Kobayashi (Department of Endocrinology and Diabetes Mellitus, Fukuoka University Chikushi Hospital), Yasuhiko Sakata (Department of Cardiovascular Medicine, Tohoku University Graduate School of Medicine), Kazunori Shimada (Department of Cardiovascular Biology and Medicine, Juntendo University Graduate School of Medicine), Koichiro Sugimura (Department of Cardiology, International University of Health and Welfare School of Medicine), Yasuyuki Nagasawa (Division of Kidney and Dialysis, Department of Internal Medicine, Hyogo College of Medicine), Hiroyuki Hao (Department of Pathology, Nihon University School of Medicine), Masahiro Higashi (Department of Radiology, National Hospital Organization Osaka National Hospital), Hideyuki Miyauchi (Department of Cardiovascular Medicine, Chiba University Graduate School of Medicine), Hiroshi Yoshida (Department of Laboratory Medicine, The Jikei University Kashiwa Hospital), Seiya Kato (Division of Pathology, Saiseikai Fukuoka General Hospital), Katsuhiro Kawaguchi (Department of Cardiology, Komaki City Hospital), Eiryu Sai (Department of Cardiovascular Biology and Medicine, Juntendo University Graduate School of Medicine), Tomohiro Sakamoto (Division of Cardiology, Cardiovascular Center, Saiseikai Kumamoto Hospital), Kazuhiro Shimizu (Department of Internal Medicine, Toho University Sakura Medical Center), Kohji Shirai (Mihama Hospital), Atsuko Takagi (Laboratory of Cardiovascular Disease, Novel, Non-invasive, and Nutritional Therapeutics and Triglyceride Research Center, Graduate School of Medicine, Osaka University), Kenichi Nakajima (Department of Functional Imaging and Artificial Intelligence, Kanazawa University), Tomoaki Nakata (Hakodate Goryoukaku Hospital), Daisaku Nakatani (Center for Global Health, Department of Medical Innovation, Osaka University Hospital), Yusuke Nakano (Department of Cardiology, Aichi Medical University), Hiroshi Nakamura (Kure Medical Center and Chugoku Cancer Center, National Hospital Organization), Chikako Hashimoto (Laboratory of Cardiovascular Disease, Novel, Non-invasive, and Nutritional Therapeutics and Triglyceride Research Center, Graduate School of Medicine, Osaka University), Akihiro Hayashida (Department of Cardiology, The Sakakibara Heart Institute of Okayama), Yuji Hisamatsu (Division of Cardiology, JCHO Shimonoseki Medical Center), Makito Hirano (Department of Neurology, Kindai University, Faculty of Medicine), Takayuki Honjo (Department of Cardiology, Kagawa University Hospital), Yoko Yasui (Faculty of Human Life Science, Osaka City University), Sohsuke Yamada(Department of Pathology and Laboratory Medicine, Kanazawa Medical University), Hidekatsu Yanai (Department of Internal Medicine, National Center for Global Health and Medicine Kohnodai Hospital)
References
- 1.Hirano K, Ikeda Y, Zaima N, Sakata Y, Matsumiya G. Triglyceride deposit cardiomyovasculopathy. N Engl J Med 2008; 359: 2396–8. [DOI] [PubMed] [Google Scholar]
- 2.Hirano K. A novel clinical entity: triglyceride deposit cardiomyovasculopathy. J Atheroscler Thromb 2009; 16: 702–5. [DOI] [PubMed] [Google Scholar]
- 3.Higashi M, Ikeda Y, Miyauchi H, Zaima N, Suzuki A, Li M, et al. Imaging modalities for triglyceride deposit cardiomyovasculopathy. Ann Nucl Cardiol 2017; 3: 94–102. [Google Scholar]
- 4.Ikeda Y, Zaima N, Hirano K, Mano M, Kobayashi K, Yamada S, et al. Coronary triglyceride deposition in contemporary advanced diabetics. Pathol Int 2014; 64: 325–35. [DOI] [PubMed] [Google Scholar]
- 5.Li M, Hirano K, Ikeda Y, Higashi M, Hashimoto C, Zhang B, et al. Triglyceride deposit cardiomyovasculopathy: a rare cardiovascular disorder. Orphanet J Rare Dis 2019; 14: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kozawa J, Higashi M, Shimomura I, Hirano K. Intractable coronary artery disease in a patient with type 2 diabetes presenting with triglyceride deposit cardiomyovasculopathy. Diabetes Care 2019; 42: 983–6. [DOI] [PubMed] [Google Scholar]
- 7.Nakano Y, Suzuki M, Hirano K, et al. Association of triglyceride deposit cardiomyovasculopathy with drug-eluting stent restenosis among patients with diabetes. JAMA Netw Open 2020; 3: e2012583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bing RJ, Siegel A, Ungar I, Gilbert M. Metabolism of the human heart. II. Studies on fat, ketone and amino acid metabolism. Am J Med 1954; 16: 504–15. [DOI] [PubMed] [Google Scholar]
- 9.Knapp FF Jr, Ambrose KR, Goodman MM. Newradioiodinated methyl-branched fatty acids for cardiac studies. Eur J Nucl Med 1986; 12 Suppl: S39–44. [DOI] [PubMed] [Google Scholar]
- 10.Hosokawa R, Nohara R, Fujibayashi Y, Okuda K, Ogino M, Hata T, et al. Metabolic fate of iodine-123-BMIPP in canine myocardium after administration of etomoxir. J Nucl Med 1996; 37: 1836–40. [PubMed] [Google Scholar]
- 11.Nohara R, Hosokawa R, Hirai T, Okuda K, Ogino M, Fujibayashi Y, et al. Basic kinetics of 15-(p-iodophenyl)-3-R, S-methylpentadecanoic acid (BMIPP) in canine myocardium. Int J Card Imaging 1999; 15: 11–20. [DOI] [PubMed] [Google Scholar]
- 12.Hirano K, Ikeda Y, Sugimura K, Sakata Y. Cardiomyocyte steatosis and defective washout of iodine-123-β-methyl iodophenyl-pentadecanoic acid in genetic deficiency of adipose triglyceride lipase. Eur Heart J 2015; 36: 580. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki A, Yamaguchi S, Li M, Hara Y, Miyauchi H, Ikeda Y, et al. Tricaprin rescues myocardial abnormality in a mouse model of triglyceride deposit cardiomyovasculopathy. J Oleo Sci 2018; 67: 983–9. [DOI] [PubMed] [Google Scholar]
- 14.Jordans GH. The familial occurrence of fat containing vacuoles in the leukocytes diagnosed in two brothers suffering from dystrophia musculorum progressiva (ERB.). Acta Med Scand 1953; 145: 419–23. [DOI] [PubMed] [Google Scholar]
- 15.Miyauchi H, Iimori T, Hoshi K, Ohyama M, Hirano K, Kobayashi Y. Correlation perspectives for the diagnosis of idiopathic triglyceride deposit cardiomyovasculopathy. Ann Nucl Cardiol 2020; 6: xx–xx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyauchi H, Hashimoto C, Ikeda Y, Nakano Y, Kozawa J, Sai E, et al. Diagnostic criteria and severity score for triglyceride deposit cardiomyovasculopathy. Ann Nucl Cardiol 2018; 4: 94–100. [Google Scholar]
- 17.Fischer J, Lefèvre C, Morava E, Mussini JM, Laforêt P, Negre-Salvayre A, et al. The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nat Genet 2007; 39: 28–30. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi K, Inoguchi T, Maeda Y, Nakashima N, Kuwano A, Eto E, et al. The lack of the C-terminal domain of adipose triglyceride lipase causes neutral lipid storage disease through impaired interactions with lipid droplets. J Clin Endocrinol Metab 2008; 93: 2877–84. [DOI] [PubMed] [Google Scholar]
- 19.Dorfman ML, Hershko C, Eisenberg S, Sagher F. Ichthyosi-form dermatosis with systemic lipidosis. Arch Dermatol 1974; 110: 261–6. [PubMed] [Google Scholar]
- 20.Chanarin I, Patel A, Slavin G, Wills EJ, Andrews TM, Stewart G. Neutral-lipid storage disease: a new disorder of lipid metabolism. Br Med J 1975; 1: 553–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hara Y, Kawasaki N, Hirano K, Hashimoto Y, Adachi J, Watanabe S, et al. Quantitative proteomic analysis of cultured skin fibroblast cells derived from patients with triglyceride deposit cardiomyovasculopathy. Orphanet J Rare Dis 2013; 8: 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirano K, Tanaka T, Ikeda Y, Yamaguchi S, Zaima N, Kobayashi K, et al. Genetic mutations in adipose triglyceride lipase and myocardial up-regulation of peroxisome proliferated activated receptor-gamma in patients with triglyceride deposit cardiomyovasculopathy. Biochem Biophys Res Commun 2014; 443: 574–9. [DOI] [PubMed] [Google Scholar]
- 23.Nagasaka H, Hirano K, Ohtake A, Miida T, Takatani T, Murayama K, et al. Improvements of hypertriglyceridemia and hyperlacticemia in Japanese children with glycogen storage disease type Ia by medium-chain triglyceride milk. Eur J Pediatr 2007; 166: 1009–16. [DOI] [PubMed] [Google Scholar]
- 24.Labarthe F, Gélinas R, Des Rosiers C. Medium-chain fatty acids as metabolic therapy in cardiac disease. Cardiovasc Drugs Ther 2008; 22: 97–106. [DOI] [PubMed] [Google Scholar]
- 25.Takagi A, Ikeda Y, Kobayashi K, Kobayashi K, Ikeda Y, Kozawa J, et al. Newly developed selective immunoinactivation assay revealed reduction in adipose triglyceride lipase activity in peripheral leucocytes from patients with idiopathic triglyceride deposit cardiomyovasculopathy. Biochem Biophys Res Commun 2018; 495: 646–51. [DOI] [PubMed] [Google Scholar]


