Key Points
Question
Does a repeated low-level red-light (RLRL) intervention prevent incident myopia among children with premyopia?
Findings
In this randomized clinical trial including 278 school-aged children with premyopia, the incidence of myopia was lower among children receiving RLRL therapy than among controls.
Meaning
Exposure to RLRL is a novel and effective intervention for myopia prevention among children with premyopia, with good user acceptability and safety.
Abstract
Importance
Myopia is a global concern, but effective prevention measures remain limited. Premyopia is a refractive state in which children are at higher risk of myopia, meriting preventive interventions.
Objective
To assess the efficacy and safety of a repeated low-level red-light (RLRL) intervention in preventing incident myopia among children with premyopia.
Design, Setting, and Participants
This was a 12-month, parallel-group, school-based randomized clinical trial conducted in 10 primary schools in Shanghai, China. A total of 139 children with premyopia (defined as cycloplegic spherical equivalence refraction [SER] of −0.50 to 0.50 diopter [D] in the more myopic eye and having at least 1 parent with SER ≤−3.00 D) in grades 1 to 4 were enrolled between April 1, 2021, and June 30, 2021; the trial was completed August 31, 2022.
Interventions
Children were randomly assigned to 2 groups after grade stratification. Children in the intervention group received RLRL therapy twice per day, 5 days per week, with each session lasting 3 minutes. The intervention was conducted at school during semesters and at home during winter and summer vacations. Children in the control group continued usual activities.
Main Outcomes and Measures
The primary outcome was the 12-month incidence rate of myopia (defined as SER ≤−0.50 D). Secondary outcomes included the changes in SER, axial length, vision function, and optical coherence tomography scan results over 12 months. Data from the more myopic eyes were analyzed. Outcomes were analyzed by means of an intention-to-treat method and per-protocol method. The intention-to-treat analysis included participants in both groups at baseline, while the per-protocol analysis included participants in the control group and those in the intervention group who were able to continue the intervention without interruption by the COVID-19 pandemic.
Results
There were 139 children (mean [SD] age, 8.3 [1.1] years; 71 boys [51.1%]) in the intervention group and 139 children (mean [SD] age, 8.3 [1.1] years; 68 boys [48.9%]) in the control group. The 12-month incidence of myopia was 40.8% (49 of 120) in the intervention group and 61.3% (68 of 111) in the control group, a relative 33.4% reduction in incidence. For children in the intervention group who did not have treatment interruption secondary to the COVID-19 pandemic, the incidence was 28.1% (9 of 32), a relative 54.1% reduction in incidence. The RLRL intervention significantly reduced the myopic shifts in terms of axial length and SER compared with the control group (mean [SD] axial length, 0.30 [0.27] mm vs 0.47 [0.25] mm; difference, 0.17 mm [95% CI, 0.11-0.23 mm]; mean [SD] SER, –0.35 [0.54] D vs –0.76 [0.60] D; difference, –0.41 D [95% CI, –0.56 to –0.26 D]). No visual acuity or structural damage was noted on optical coherence tomography scans in the intervention group.
Conclusions and Relevance
In this randomized clinical trial, RLRL therapy was a novel and effective intervention for myopia prevention, with good user acceptability and up to 54.1% reduction in incident myopia within 12 months among children with premyopia.
Trial Registration
ClinicalTrials.gov Identifier: NCT04825769
This randomized clinical trial assesses the efficacy and safety of a repeated low-level red-light intervention in preventing incident myopia among children in China with premyopia.
Introduction
Myopia is one of the most common eye diseases and is of worldwide concern.1,2,3 In some parts of East and Southeast Asia, myopia prevalence among high school students reaches 80% to 90%.2,3,4,5 Myopia poses a burden on individuals and society for refractive error correction.6 In addition, early-onset myopia is more likely to progress to high myopia,7,8,9 which significantly increases the risk of potential vision-threatening conditions, such as myopic macular degeneration.10 Therefore, myopia prevention is of significance.
Currently, several interventions have been proposed to prevent the onset of myopia, such as increased outdoor time,11,12,13,14,15,16,17,18,19,20,21,22,23 reducing activities done at a short working distance,18,19,20,23,24,25,26,27,28 Chinese eye exercises,29,30,31,32,33 and low-dose atropine.34,35 The efficacy rate of these interventions at preventing myopia within 1 year ranged from 11.0% to 54.3%.11,12,13,14,15,16,17,18,19,20,21,22
Repeated low-level red-light (RLRL) therapy delivered by a device emitting 650-nm visible red light has been proposed as an alternative myopia control intervention.36,37,38,39,40,41 Recent research has reported increased blood flow and stabilization of axial elongation among children after the RLRL intervention, which might suggest that RLRL therapy could ameliorate scleral hypoxia and thus prevent the development of myopia.36 Despite that, its efficacy at preventing myopia remains unknown. Given that RLRL intervention requires dedicated devices and an investment of time, the technology is most useful for children who are at high risk of developing myopia. In this trial, we intended to enroll children with premyopia, who were normally considered as having a greater risk of developing myopia,42,43,44,45,46 to further assess the efficacy and safety of RLRL therapy for myopia prevention among them.
Methods
This 12-month, 2-group, single-blinded, school-based randomized clinical trial was conducted in Shanghai, China. Children from 10 primary schools were enrolled between April 1, 2021, and June 30, 2021. Examinations were conducted at baseline and at the 3-, 6-, 9-, and 12-month follow-up visits. This trial was completed August 31, 2022. The study was approved by the Shanghai General Hospital Ethics Committee and adhered to the tenets of the Declaration of Helsinki.47 Written informed consent was obtained from participants’ guardians. This trial was registered with ClinicalTrials.gov (NCT04825769) and followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline (see the trial protocol in Supplement 1).
Eligibility Criteria
Eligible participants were primary school students in grades 1 to 4 (aged 6-11 years) with premyopia, defined as a cycloplegic spherical equivalent refraction (SER) of the more myopic eye in the range of −0.50 to 0.50 (inclusive) diopters (D) and having at least 1 parent with an SER in either eye of −3.00 D or less. Children were excluded if they had astigmatism of 1.50 D or more, anisometropia of 1.50 D or more, strabismus and other ocular abnormalities, any systemic diseases, or a history of any myopia interventions.
Randomization and Masking
The randomization was stratified by grade and allocated to individuals at a ratio of 1:1. The randomization list was generated using R software, version 3.6 (R Group for Statistical Computing). To avoid stigmatization for being selected or not, children, parents, and teachers were educated on the purpose of the study and randomization. They were also informed of the right to withdraw at any time. Children and their guardians were aware of the study allocation due to the nature of the intervention. Outcome assessors and statisticians were masked.
Intervention
Children in the intervention group received the RLRL intervention, while those in the control group did not. The RLRL intervention was provided by a desktop device (Eyerising, Suzhou Xuanjia Optoelectronics Technology; eFigure 1 in Supplement 2), which consists of semiconductor laser diodes and delivers low-level red light with a mean (SD) wavelength of 650 (10) nm. The device is certified as a class IIa device by the China National Medical Products Administration.36,37 The RLRL intervention was conducted twice per day, 5 days per week, with each session lasting 3 minutes and with an interval of at least 4 hours between 2 sessions.
Intervention Compliance Monitoring
During semesters, children in the intervention group completed treatment at school. During summer and winter vacations, guardians were trained by teachers to supervise the intervention at home. The device was linked to the internet with an automated diary function to record the treatment history. In addition, 2 investigators (X.H. and J.W.) were responsible for the intervention compliance management. School teachers or parents or legal guardians were reminded about the intervention to improve treatment compliance every week.
Data Collection
Clinical examinations were performed at baseline and at 3-, 6-, 9-, and 12-month follow-up visits. Baseline demographic characteristics were collected via questionnaire; the information was obtained from school teachers with permission from the participants and their guardians.
Uncorrected visual acuity (UCVA) and best-corrected visual acuity (BCVA) were assessed at 4 m by trained optometrists using the Early Treatment Diabetic Retinopathy Study (ETDRS) logMAR chart (Guangzhou Xieyi Weishikang). Axial length (AL) was measured before cycloplegia using the IOLMaster (Carl Zeiss Meditec) and averaged until the maximum error did not exceed 0.05 mm. Cycloplegia was induced half-yearly using 2 drops of 1% cyclopentolate (Alcon) 5 minutes apart. One additional drop was administered 5 minutes later if cycloplegia was insufficient. After an additional 30 minutes, full cycloplegia was present if the pupil diameter reached at least 6 mm and the pupillary reaction to light was absent. Refraction data were measured using an autorefractor (KR8800; Topcon) 3 times after cycloplegia and averaged until the desired precision (0.25 D) was achieved. Optical coherence tomography (OCT) scans and fundus images were obtained from swept-source OCT (DRI OCT Triton; Topcon) under radiographic scanning (radial 9 mm). Choroidal thickness was obtained automatically from the built-in segmentation software.
Outcomes
The primary outcome was the 12-month cumulative incidence of myopia in both groups. Myopia was defined as the cycloplegic SER (sphere plus half of cylinder) of −0.50 D or less in the more myopic eye. Secondary outcomes of the study were the changes in SER, AL, choroidal thickness, UCVA, and BCVA over 1 year. Data from the more myopic eye at baseline were also used for secondary outcomes analysis.
Adverse Events
At baseline, all children in the intervention group completed 1 session of the RLRL intervention. The children were asked to close their eyes until the bright spot, also known as the afterimage, disappeared, then the duration of the afterimage was documented. If the afterimage duration exceeded 6 minutes, the child was clinically considered too sensitive to the intervention and was excluded (n = 2). In addition, eye discomforts, including but not limited to pain, itching, dryness, dazzling, short-term glare, and flash blindness, were reported to investigators or supervisors. The treatment was stopped immediately if the child in the intervention group had any severe adverse events, including sudden visual loss of 2 or more lines or a scotoma perceived in the center of the viewing field by the child.
Fundus images by swept-source OCT were independently evaluated by 2 accredited senior ophthalmologists. Any fundus abnormality and its grading, including but not limited to vitreomacular traction, macular schisis, macular hole, intraretinal fluid, subretinal fluid, hemorrhage, retinal pigment epithelium proliferation, and atrophy, would be recorded. Any difference between the 2 graders was adjudicated by a third senior ophthalmologist (X.X.).
Sample Size
The sample size estimation was conducted based on the assumption of a 2-sided α level of .05, 90% power, and the expected effect size. The incidence of myopia among primary school students with premyopia was approximately 42% per year,46,48,49 and the expected reduction was set at 50%. This would require a total of 202 participants. Adjusting for 20% loss to follow-up yielded a total sample size of 254 participants.
Statistical Analysis
Statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc). Outcomes were analyzed by means of an intention-to-treat method and a per-protocol method. The intention-to-treat analysis included participants in both groups at baseline, while the per-protocol analysis included participants in the control group and those in the intervention group who were able to continue the intervention without interruption by the COVID-19 pandemic. A comparison between the 2 groups was performed using the χ2 test. The unpaired t test was used to compare continuous outcomes with normal distribution, while the nonparametric statistical method was used if otherwise. All P values were from 2-sided tests and results were deemed statistically significant at P < .05.
Results
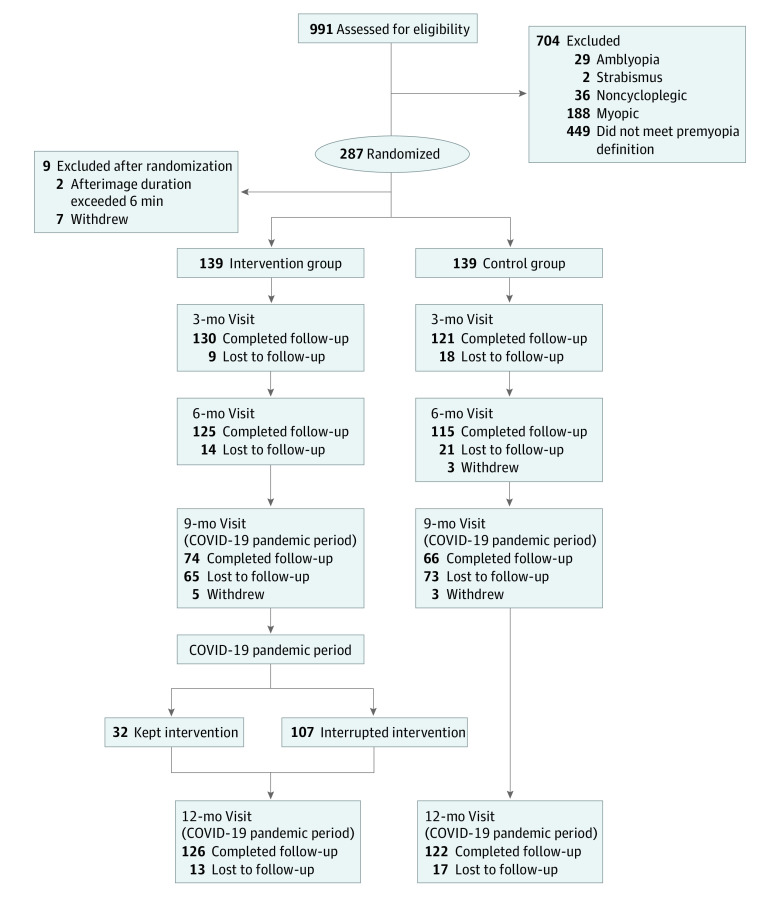
A total of 278 children (28.1%) were included in the trial: 139 children (mean [SD] age, 8.3 [1.1] years; 71 boys [51.1%]) in the intervention group and 139 children (mean [SD] age, 8.3 [1.1] years; 68 boys [48.9%]) in the control group (Figure 1; eTable 1 in Supplement 2). Figure 1 summarizes the number of the participants who completed enrollment, baseline examination, and intervention at each visit. Ocular characteristics, including UCVA, AL and SE, are presented in eTable 1 in Supplement 2.
Figure 1. Flowchart of the Study.
Because of the COVID-19 pandemic and the associated sudden lockdown in Shanghai from March to May 2022, the number of participants at the 9-month visit and compliance with the treatment during the last 3 months in the intervention group were affected significantly when the children did not bring the devices home from school. A total of 65 of 139 children (46.8%) in the intervention group and 73 of 139 children (52.5%) in the control group did not attend the 9-month follow-up visit. For the last 3 months of the study, 32 of 139 children (23.0%) in the intervention group were able to continue the RLRL treatment. Despite this, we continued the trial and tried our best to complete the examination at the 12-month visit. Of 278 included children, 248 (89.2%) participated in the 12-month visit, consisting of 126 children (90.6%) in the intervention group and 122 children (87.8%) in the control group. The median compliance rate in the intervention group was 60.0% (IQR, 54.2%-64.8%).
Primary Outcome
The 12-month incidence rate of myopia was 40.8% (49 of 120) in the intervention group and 61.3% (68 of 111) in the control group (Table 1). The absolute mean difference between the 2 groups was 20.4% (95% CI, 7.9%-33.1%; P = .003), representing a 33.4% reduction in incidence (Table 1; eFigure 2 in Supplement 2). The relative risk between the 2 groups was 0.67 (95% CI, 0.51-0.86) (Table 1). The 6-month incidence rates of myopia were 10.0% (12 of 120) for the intervention group and 22.5% (23 of 102) for the control group, indicating a 55.6% reduction in incidence (eTable 2 and eFigure 2 in Supplement 2).
Table 1. Refractive and Biometric Outcomes at 12-Month Follow-up (Intention-to-Treat Analysis).
| Outcome | Students, % (No./total No.) | Risk difference, (95% CI)b | Relative risk (95% CI)c | Relative efficacyd | P valuee | |
|---|---|---|---|---|---|---|
| Interventiona | Control | |||||
| Incidence of myopia, % | 40.8 (49/120) | 61.3 (68/111) | 20.4 (7.9 to 33.1) | 0.67 (0.51 to 0.86) | 33.4 | .003 |
| Mean (SD), change | ||||||
| SER, D | −0.35 (0.54) | −0.76 (0.60) | −0.41 (−0.56 to –0.26) | NA | 53.9 | <.001 |
| AL, mm | 0.30 (0.27) | 0.47 (0.25) | 0.17 (0.11 to 0.23) | NA | 36.2 | <.001 |
Abbreviations: AL, axial length; D, diopters; NA, not applicable; SER, spherical equivalent refraction.
The intervention group included those who continued the intervention and those with interrupted intervention.
Risk difference, absolute efficacy = value in control group − value in intervention group.
Relative risk = value in intervention group/value in control group.
Relative efficacy = (value in control group − value in intervention group)/value in control group.
The t test was used for change of AL and SER, and the χ2 test was used for incidence.
Secondary Outcomes
For the intervention group, the mean (SD) SER change over 12 months was –0.35 (0.54) D. For the control group, the corresponding mean (SD) change was –0.76 (0.60) D. The absolute mean difference in SER change between the 2 groups was –0.41 D (95% CI, –0.56 to –0.26 D; P < .001) (Table 1), representing a 53.9% reduction in SER shift. The 6-month SER change for each group and the mean difference between the intervention and control groups are presented in eTable 2 in Supplement 2. Spaghetti plots of longitudinal SER data are presented in eFigure 3 in Supplement 2.
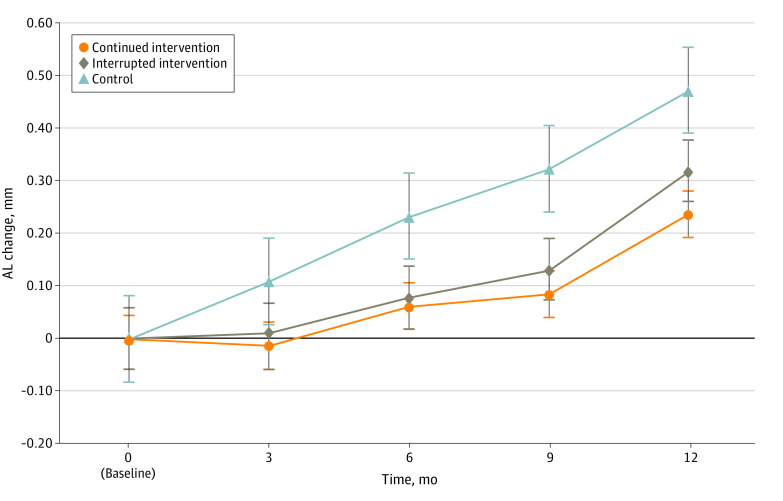
The 12-month mean (SD) AL increase was 0.30 (0.27) mm for the intervention group and 0.47 (0.25) for the control group, with an absolute mean difference in AL changes of 0.17 mm (95% CI, 0.11-0.23 mm; P < .001) representing a 36.2% reduction in AL changes (Table 1). The 3-, 6-, 9-, and 12- month AL changes for each group and the mean difference between the 2 groups are presented in Figure 2.
Figure 2. Axial Length (AL) Change Between the Intervention and Control Groups Over 12 Months.
The proportions of children showing SER regression (hyperopic shift of >0.25 D, which cannot be explained as the measurement errors in refraction device) in the intervention group were 51.2% (62 of 121) at the 6-month follow-up visit and 19.0% (23 of 121) at the 12-month follow-up visit, while the corresponding proportions in the control group were 24.5% (25 of 102) at 6 months and 2.7% (3 of 111) at 12 months (eTable 3 in Supplement 2). In addition, 3.2% of children (4 of 126) in the intervention group at the 12-month follow-up achieved AL shortening of more than 0.05 mm. The proportions of children having clinically significant AL shortening in the intervention group at the 3-, 6-, and 9-month follow-up were 23.1% (30 of 130), 12.5% (15 of 120), and 9.5% (7 of 74), respectively, while the corresponding proportions in the control group were 4.1% (5 of 122), 0.9% (1 of 115), and 0%, respectively.
At the 12-month follow-up visit, the proportion of children whose UCVA decreased by at least 2 lines was significantly greater in the control group than in the intervention group (17.5% [22 of 126] vs 32.0% [39 of 122]; P = .02) (Table 2). The proportion of children achieving a BCVA of at least 0.8 was similar between the intervention and control groups (100.0% vs 99.2% [121 of 122]; P = .50). For the intervention group, the mean (SD) change in choroidal thickness over 12 months was 3.0 (16.9) μm, while for the control group, the mean (SD) change in choroidal thickness was −9.2 (22.3) μm (P < .001).
Table 2. Vision Function and Choroidal Thickness at the 12-Month Follow-up (Intention-to-Treat Analysis).
| Outcome | Students, % (No./total No.) | Absolute efficacy, % (95% CI) | P valueb | |
|---|---|---|---|---|
| Interventiona | Control | |||
| Change in UCVA | ||||
| 2 Lines worsening | 17.5 (22/126) | 32.0 (39/122) | 14.5 (3.9 to 25.1) | .02 |
| Within 1 line | 77.0 (97/126) | 65.6 (80/122) | 11.4 (0.2 to 22.6) | |
| 2 Lines improvement | 5.6 (7/126) | 2.5 (3/122) | 3.1 (1.8 to 8.0) | |
| BCVA <0.8 | 0 (0/123) | 0.8 (1/122) | 0.8 (−0.7 to 2.3) | .50 |
| CT change, mean (SD), μm | 3.0 (16.9) | −9.2 (22.3) | 12.3 (7.3 to 17.2) | <.001 |
Abbreviations: BCVA, best corrected visual acuity; CT, choroidal thickness; UCVA, uncorrected visual acuity.
The intervention group included those who continued the intervention and those with interrupted intervention.
The t test was used for CT change, and the χ2 test was used for change of UCVA and BCVA.
Effect of the COVID-19 Pandemic
The baseline characteristics between the continued intervention group and the interrupted intervention group were well balanced (eTable 4 in Supplement 2). The continued intervention group had a greater reduction in the incidence rate of myopia compared with those in the control group (28.1% [9 of 32] vs 61.3% [68 of 111]) (eTable 5 in Supplement 2), representing a 54.1% reduction in incident myopia within 12 months. Similarly, the mean SER change and the mean AL change in the continued intervention group were –0.18 (0.61) D and 0.24 (0.23) mm, respectively. When compared with the mean changes in the control group, the continued intervention group achieved 76.3% and 48.9% efficacy in slowing SER and AL shifts, respectively (eTable 5 in Supplement 2).
Adverse Events
Two participants withdrew from the study because the afterimage duration exceeded 6 minutes at baseline. According to swept-source OCT images, no other structural damage was noted in the intervention and control groups.
Sensitivity and Subgroup Analyses
Subgroup analyses comparing the efficacy of intervention for myopia prevention and control (incidence, SER changes, and AL changes) by different baseline SER groups and age groups were performed. Better efficacy was observed among children with an SER of 0.01 to 0.50 D than among those with an SER of −0.50 to 0.00 D (relative efficacy, 64.0% vs 14.0%; SER changes, 69.0% vs 39.8%; AL changes, 45.7% vs 23.4%) (Table 3). Children of different age groups did not show significant differences in the efficacy of myopia prevention (eTable 6 in Supplement 2).
Table 3. Refractive and Biometric Outcomes at 12-Month Follow-up in Different Baseline SER Groups (Adjusted for Baseline Age).
| Outcome and SER group | No. | Interventiona | Control | Absolute efficacy (relative efficacy)b | Difference of efficacy (95% CI)c |
|---|---|---|---|---|---|
| Incidence of myopia, % | |||||
| 0.01 to 0.50 D | 140 | 17.7 | 49.1 | 31.4 (64.0) | 19.9 (−1.2 to 41.0) |
| −0.50 to 0.00 D | 91 | 71.5 | 83.1 | 11.% (14.0) | |
| SER change, D | |||||
| 0.01 to 0.50 D | 140 | −0.22 | −0.71 | −0.49 (69.0%) | 0.17 (−0.13 to 0.46) |
| −0.50 to 0.00 D | 91 | −0.50 | −0.83 | −0.33 (39.8%) | |
| AL change, mm | |||||
| 0.01 to 0.50 D | 150 | 0.25 | 0.46 | 0.21 (45.7%) | 0.10 (−0.02 to 0.23) |
| −0.50 to 0.00 D | 98 | 0.36 | 0.47 | 0.11 (23.4%) |
Abbreviations: AL, axial length; D, diopters; SER, spherical equivalent refraction.
The intervention group included those who continued the intervention and those with interrupted intervention.
Absolute efficacy = value in control group − value in intervention group; relative efficacy = (value in control group − value in intervention group)/value in control group.
Difference of efficacy = the efficacy difference between 2 subgroups.
Discussion
To our knowledge, this was the first trial to date to investigate the efficacy of the RLRL intervention in myopia prevention. In this 12-month randomized clinical trial, the RLRL intervention achieved an absolute difference of 20.4% in incidence of myopia, representing a 33.4% relative reduction in incident myopia. For children without treatment interruption due to the COVID-19 pandemic, the absolute difference increased to 33.2%, representing a 54.1% relative reduction in incident myopia over 12 months.
Effect of RLRL Intervention on Myopia Incidence
The efficacy of a 33.4% to 54.1% relative reduction in the incidence of myopia with the RLRL intervention should be interpreted carefully. Increased outdoor time has been consistently shown to have an efficacy rate ranging from 11.0% to 54.3% in preventing myopia onset within 1 year.11,12,13,14,50,51 However, our study population is substantially different than those in the outdoor trials; the children without myopia at baseline were enrolled in the outdoor trials, whereas our study enrolled the children with premyopia who had a much higher risk of developing myopia. As demonstrated in our subgroup analysis, for the children with premyopia, in particular those whose SER was very close to −0.50 D (the cutoff for myopia), the prophylactic effect was much lower because the intervention was introduced too late. Considering the difference in study participants, the RLRL prophylactic effect could be even stronger if the children who had an SER of 0.01 to 0.50 D at baseline were enrolled.
Effect of RLRL Intervention on Myopic Shift
We found that the RLRL intervention significantly reduced the myopic shifts in terms of AL and SER compared with the control group (difference, 0.17 mm and –0.41 D, respectively). In the subgroup analysis of individuals with premyopia from a randomized clinical trial in Taiwan, a 1-year outdoor intervention achieved reductions of myopia shift by 0.04 mm for AL and 0.11 D for SER.13 A recent study investigated the effect of 0.01% atropine on myopic shift and indicated that 0.01% atropine could reduce myopic shift by 0.09 mm in AL and 0.45 D in SER.34 Our study observed regression in AL (by >0.05 mm) and SER (by >0.25 D) by 3.2% and 19.0%, respectively, at 12-month follow-up, which was consistent with the report by Jiang et al36 of a 21.6% AL regression after an RLRL intervention among children with myopia. The effect of RLRL therapy might originate from increasing blood flow and metabolism of the fundus, as well as ameliorating scleral hypoxia, which had been proved to be a promoter for myopia development.52,53
Influencing Factors of RLRL Intervention Efficacy
Our results showed that the effect of the RLRL intervention on myopia prevention was different among baseline SER groups. The RLRL intervention was more effective among children with an SER of 0.01 to 0.50 D at baseline than those with an SER of −0.50 to 0.00 D at baseline. In 2021, the International Myopia Institute defined premyopia as a refractive state of an eye of +0.75 D or less and more than −0.50 D in children.42 Based on our data, it might already be too late to treat those with an SER of −0.50 to 0.00 D because their incidence of myopia in 12 months could be as high as 83.1%.42 Therefore, prophylactic intervention, including RLRL therapy and increased outdoor time, may need to be implemented earlier, at least among those with an SER in the range of 0.01 to 0.50 D, or even earlier.
Safety
After this 12-month RLRL intervention, no functional damage as indicated by BCVA was observed. No children reported having glare, flash blindness, or afterimages longer than 6 minutes after treatment. An afterimage induced by prior adaptation to a visual stimulus is believed to be due to bleaching of photochemical pigments or neural adaptation in the retina. Participants with an afterimage duration exceeding 6 minutes were clinically considered too sensitive to visual stimulus.54 No other structural changes were noted in this study, consistent with other RLRL studies.36,40
Limitations
This study had several limitations. First, the open-label design may bias the results. Future studies comparing a light treatment simulator with a much lower power should exclude potential placebo effects. Second, because of the outbreak of the COVID-19 pandemic in Shanghai, approximately 70% of the children in the intervention group discontinued treatment from 9 to 12 months. Nevertheless, we tried to maximize the follow-up retention rate for the 12-month visit. Furthermore, children who continued treatment and those who discontinued treatment were well balanced for baseline characteristics. Third, the observed efficacy of the intervention in preventing myopia was generalizable only to the device used in the present study. It is unproven that other wavelengths, power intensities, or frequencies of intervention may have a similar or even better efficacy.
Conclusions
This randomized clinical trial found that RLRL is a novel and effective intervention for myopia prevention, with good user acceptability and a 54.1% reduction in incident myopia within 12 months for children with premyopia. Our findings have public health significance, especially for myopia prevention in countries with a high incidence of myopia. More studies are needed to understand the long-term efficacy and safety, optimal intervention dose, and potential underlying mechanisms of the RLRL intervention.
Trial Protocol
eFigure 1. Schematic Diagram of Red Light Spectrum and Intervention Instrument
eFigure 2. Myopia Incidence in 6- and 12-Month Between the Intervention and Control Groups
eFigure 3. Spaghetti Plots of Longitudinal Data on SER
eTable 1. Baseline Characteristics of the Included Participants
eTable 2. Refractive and Biometric Outcomes at 6-Month Follow-up
eTable 3. Percentages of SER Regression in 6 and 12 Months
eTable 4. Baseline Characteristics Between Continued and Interrupted Intervention Groups
eTable 5. Refractive and Biometric Outcomes at 12-Month Follow-up Between Continued Intervention and Control Group
eTable 6. Refractive and Biometric Outcomes at 12-Month Follow-up in Different Baseline Age Groups
Data Sharing Statement
References
- 1.Bullimore MA, Ritchey ER, Shah S, Leveziel N, Bourne RRA, Flitcroft DI. The risks and benefits of myopia control. Ophthalmology. 2021;128(11):1561-1579. doi: 10.1016/j.ophtha.2021.04.032 [DOI] [PubMed] [Google Scholar]
- 2.Jonas JB, Ang M, Cho P, et al. IMI prevention of myopia and its progression. Invest Ophthalmol Vis Sci. 2021;62(5):6. doi: 10.1167/iovs.62.5.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baird PN, Saw SM, Lanca C, et al. Myopia. Nat Rev Dis Primers. 2020;6(1):99. doi: 10.1038/s41572-020-00231-4 [DOI] [PubMed] [Google Scholar]
- 4.Wu LJ, You QS, Duan JL, et al. Prevalence and associated factors of myopia in high-school students in Beijing. PLoS One. 2015;10(3):e0120764. doi: 10.1371/journal.pone.0120764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding BY, Shih YF, Lin LLK, Hsiao CK, Wang IJ. Myopia among schoolchildren in East Asia and Singapore. Surv Ophthalmol. 2017;62(5):677-697. doi: 10.1016/j.survophthal.2017.03.006 [DOI] [PubMed] [Google Scholar]
- 6.Sankaridurg P, Tahhan N, Kandel H, et al. IMI impact of myopia. Invest Ophthalmol Vis Sci. 2021;62(5):2. doi: 10.1167/iovs.62.5.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chua SYL, Sabanayagam C, Cheung YB, et al. Age of onset of myopia predicts risk of high myopia in later childhood in myopic Singapore children. Ophthalmic Physiol Opt. 2016;36(4):388-394. doi: 10.1111/opo.12305 [DOI] [PubMed] [Google Scholar]
- 8.Pärssinen O, Kauppinen M. Risk factors for high myopia: a 22-year follow-up study from childhood to adulthood. Acta Ophthalmol. 2019;97(5):510-518. doi: 10.1111/aos.13964 [DOI] [PubMed] [Google Scholar]
- 9.Hu Y, Ding X, Guo X, Chen Y, Zhang J, He M. Association of age at myopia onset with risk of high myopia in adulthood in a 12-year follow-up of a Chinese cohort. JAMA Ophthalmol. 2020;138(11):1129-1134. doi: 10.1001/jamaophthalmol.2020.3451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohno-Matsui K, Wu PC, Yamashiro K, et al. IMI pathologic myopia. Invest Ophthalmol Vis Sci. 2021;62(5):5. doi: 10.1167/iovs.62.5.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He M, Xiang F, Zeng Y, et al. Effect of time spent outdoors at school on the development of myopia among children in China: a randomized clinical trial. JAMA. 2015;314(11):1142-1148. doi: 10.1001/jama.2015.10803 [DOI] [PubMed] [Google Scholar]
- 12.Wu PC, Tsai CL, Wu HL, Yang YH, Kuo HK. Outdoor activity during class recess reduces myopia onset and progression in school children. Ophthalmology. 2013;120(5):1080-1085. doi: 10.1016/j.ophtha.2012.11.009 [DOI] [PubMed] [Google Scholar]
- 13.Wu PC, Chen CT, Lin KK, et al. Myopia prevention and outdoor light intensity in a school-based cluster randomized trial. Ophthalmology. 2018;125(8):1239-1250. doi: 10.1016/j.ophtha.2017.12.011 [DOI] [PubMed] [Google Scholar]
- 14.He X, Sankaridurg P, Wang J, et al. Time Outdoors in Reducing Myopia: a school-based cluster randomized trial with objective monitoring of outdoor time and light intensity. Ophthalmology. 2022;129(11):1245-1254. doi: 10.1016/j.ophtha.2022.06.024 [DOI] [PubMed] [Google Scholar]
- 15.Xiong S, Sankaridurg P, Naduvilath T, et al. Time spent in outdoor activities in relation to myopia prevention and control: a meta-analysis and systematic review. Acta Ophthalmol. 2017;95(6):551-566. doi: 10.1111/aos.13403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bilbao-Malavé V, González-Zamora J, Gándara E, et al. A cross-sectional observational study of the relationship between outdoor exposure and myopia in university students, measured by conjunctival ultraviolet autofluorescence (CUVAF). J Clin Med. 2022;11(15):4264. doi: 10.3390/jcm11154264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukazhanova A, Aldasheva N, Iskakbayeva J, et al. Prevalence of refractive errors and risk factors for myopia among schoolchildren of Almaty, Kazakhstan: a cross-sectional study. PLoS One. 2022;17(6):e0269474. doi: 10.1371/journal.pone.0269474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pärssinen O, Lassila E, Kauppinen M. Associations of children’s close reading distance and time spent indoors with myopia, based on parental questionnaire. Children (Basel). 2022;9(5):632. doi: 10.3390/children9050632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu LJ, Wang YX, You QS, et al. Risk factors of myopic shift among primary school children in Beijing, China: a prospective study. Int J Med Sci. 2015;12(8):633-638. doi: 10.7150/ijms.12133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.French AN, Morgan IG, Mitchell P, Rose KA. Patterns of myopigenic activities with age, gender and ethnicity in Sydney schoolchildren. Ophthalmic Physiol Opt. 2013;33(3):318-328. doi: 10.1111/opo.12045 [DOI] [PubMed] [Google Scholar]
- 21.Guggenheim JA, Northstone K, McMahon G, et al. Time outdoors and physical activity as predictors of incident myopia in childhood: a prospective cohort study. Invest Ophthalmol Vis Sci. 2012;53(6):2856-2865. doi: 10.1167/iovs.11-9091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones LA, Sinnott LT, Mutti DO, Mitchell GL, Moeschberger ML, Zadnik K. Parental history of myopia, sports and outdoor activities, and future myopia. Invest Ophthalmol Vis Sci. 2007;48(8):3524-3532. doi: 10.1167/iovs.06-1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.French AN, Morgan IG, Mitchell P, Rose KA. Risk factors for incident myopia in Australian schoolchildren: the Sydney Adolescent Vascular and Eye Study. Ophthalmology. 2013;120(10):2100-2108. doi: 10.1016/j.ophtha.2013.02.035 [DOI] [PubMed] [Google Scholar]
- 24.You X, Wang L, Tan H, et al. Near work related behaviors associated with myopic shifts among primary school students in the Jiading district of Shanghai: a school-based one-year cohort study. PLoS One. 2016;11(5):e0154671. doi: 10.1371/journal.pone.0154671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu CC, Huang N, Lin PY, et al. Risk factors for myopia progression in second-grade primary school children in Taipei: a population-based cohort study. Br J Ophthalmol. 2017;101(12):1611-1617. doi: 10.1136/bjophthalmol-2016-309299 [DOI] [PubMed] [Google Scholar]
- 26.Huang HM, Chang DST, Wu PC. The association between near work activities and myopia in children—a systematic review and meta-analysis. PLoS One. 2015;10(10):e0140419. doi: 10.1371/journal.pone.0140419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ip JM, Saw SM, Rose KA, et al. Role of near work in myopia: findings in a sample of Australian school children. Invest Ophthalmol Vis Sci. 2008;49(7):2903-2910. doi: 10.1167/iovs.07-0804 [DOI] [PubMed] [Google Scholar]
- 28.Saw SM, Chua WH, Hong CY, et al. Nearwork in early-onset myopia. Invest Ophthalmol Vis Sci. 2002;43(2):332-339. [PubMed] [Google Scholar]
- 29.Kang MT, Li SM, Peng X, et al. Chinese eye exercises and myopia development in school age children: a nested case-control study. Sci Rep. 2016;6:28531. doi: 10.1038/srep28531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin Z, Vasudevan B, Fang SJ, et al. Eye exercises of acupoints: their impact on myopia and visual symptoms in Chinese rural children. BMC Complement Altern Med. 2016;16(1):349. doi: 10.1186/s12906-016-1289-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin Z, Vasudevan B, Jhanji V, et al. Eye exercises of acupoints: their impact on refractive error and visual symptoms in Chinese urban children. BMC Complement Altern Med. 2013;13:306. doi: 10.1186/1472-6882-13-306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin Y, Qiu C, Qi Y. Myopia in Chinese adolescents: its influencing factors and correlation with physical activities. Comput Math Methods Med. 2022;2022:4700325. doi: 10.1155/2022/4700325 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Zhuang M, Xie H, Zhang Y, et al. Prevalence and influence factors for myopia and high myopia in schoolchildren in Shandong, China. Cent Eur J Public Health. 2022;30(3):190-195. doi: 10.21101/cejph.a7158 [DOI] [PubMed] [Google Scholar]
- 34.Jethani J. Efficacy of low-concentration atropine (0.01%) eye drops for prevention of axial myopic progression in premyopes. Indian J Ophthalmol. 2022;70(1):238-240. doi: 10.4103/ijo.IJO_1462_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang PC, Chung MY, Yu HJ, Wu PC. Prevention of myopia onset with 0.025% atropine in premyopic children. J Ocul Pharmacol Ther. 2010;26(4):341-345. doi: 10.1089/jop.2009.0135 [DOI] [PubMed] [Google Scholar]
- 36.Jiang Y, Zhu Z, Tan X, et al. Effect of repeated low-level red-light therapy in myopia control in children: a multicenter randomized controlled trial. Ophthalmology. 2022;129(5):509-519. doi: 10.1016/j.ophtha.2021.11.023 [DOI] [PubMed] [Google Scholar]
- 37.Xiong F, Mao T, Liao H, et al. Orthokeratology and low-intensity laser therapy for slowing the progression of myopia in children. Biomed Res Int. 2021;2021:8915867. doi: 10.1155/2021/8915867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou L, Xing C, Qiang W, Hua C, Tong L. Low-intensity, long-wavelength red light slows the progression of myopia in children: an Eastern China–based cohort. Ophthalmic Physiol Opt. 2022;42(2):335-344. doi: 10.1111/opo.12939 [DOI] [PubMed] [Google Scholar]
- 39.Chen H, Wang W, Liao Y, et al. Low-intensity red-light therapy in slowing myopic progression and the rebound effect after its cessation in Chinese children: a randomized controlled trial. Graefes Arch Clin Exp Ophthalmol. 2023;261(2):575-584. doi: 10.1007/s00417-022-05794-4 [DOI] [PubMed] [Google Scholar]
- 40.Dong J, Zhu Z, Xu H, He M. Myopia control effect of repeated low-level red-light therapy in Chinese children: a randomized, double-blind, controlled clinical trial. Ophthalmology. 2023;130(2):198-204. doi: 10.1016/j.ophtha.2022.08.024 [DOI] [PubMed] [Google Scholar]
- 41.Tian L, Cao K, Ma DL, et al. Investigation of the efficacy and safety of 650 nm low-level red light for myopia control in children: a randomized controlled trial. Ophthalmol Ther. 2022;11(6):2259-2270. doi: 10.1007/s40123-022-00585-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jong M, Jonas JB, Wolffsohn JS, et al. IMI 2021 yearly digest. Invest Ophthalmol Vis Sci. 2021;62(5):7. doi: 10.1167/iovs.62.5.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rozema J, Dankert S, Iribarren R, Lanca C, Saw SM. Axial growth and lens power loss at myopia onset in Singaporean children. Invest Ophthalmol Vis Sci. 2019;60(8):3091-3099. doi: 10.1167/iovs.18-26247 [DOI] [PubMed] [Google Scholar]
- 44.Mutti DO, Hayes JR, Mitchell GL, et al. ; CLEERE Study Group . Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2007;48(6):2510-2519. doi: 10.1167/iovs.06-0562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang SK, Guo Y, Liao C, et al. Incidence of and factors associated with myopia and high myopia in Chinese children, based on refraction without cycloplegia. JAMA Ophthalmol. 2018;136(9):1017-1024. doi: 10.1001/jamaophthalmol.2018.2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma Y, Zou H, Lin S, et al. Cohort study with 4-year follow-up of myopia and refractive parameters in primary schoolchildren in Baoshan District, Shanghai. Clin Exp Ophthalmol. 2018;46(8):861-872. doi: 10.1111/ceo.13195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 48.Qi LS, Yao L, Wang XF, et al. Risk factors for incident myopia among teenaged students of the experimental class of the Air Force in China. J Ophthalmol. 2019;2019:3096152. doi: 10.1155/2019/3096152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao L, Qi LS, Wang XF, et al. Refractive change and incidence of myopia among a group of highly selected senior high school students in China: a prospective study in an aviation cadet prerecruitment class. Invest Ophthalmol Vis Sci. 2019;60(5):1344-1352. doi: 10.1167/iovs.17-23506 [DOI] [PubMed] [Google Scholar]
- 50.Hua WJ, Jin JX, Wu XY, et al. Elevated light levels in schools have a protective effect on myopia. Ophthalmic Physiol Opt. 2015;35(3):252-262. doi: 10.1111/opo.12207 [DOI] [PubMed] [Google Scholar]
- 51.Jin JX, Hua WJ, Jiang X, et al. Effect of outdoor activity on myopia onset and progression in school-aged children in northeast China: the Sujiatun Eye Care Study. BMC Ophthalmol. 2015;15:73. doi: 10.1186/s12886-015-0052-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu H, Chen W, Zhao F, et al. Scleral hypoxia is a target for myopia control. Proc Natl Acad Sci U S A. 2018;115(30):E7091-E7100. doi: 10.1073/pnas.1721443115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Y, Wang L, Xu Y, Pang Z, Mu G. The influence of the choroid on the onset and development of myopia: from perspectives of choroidal thickness and blood flow. Acta Ophthalmol. 2021;99(7):730-738. doi: 10.1111/aos.14773 [DOI] [PubMed] [Google Scholar]
- 54.Shimojo S, Kamitani Y, Nishida S. Afterimage of perceptually filled-in surface. Science. 2001;293(5535):1677-1680. doi: 10.1126/science.1060161 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure 1. Schematic Diagram of Red Light Spectrum and Intervention Instrument
eFigure 2. Myopia Incidence in 6- and 12-Month Between the Intervention and Control Groups
eFigure 3. Spaghetti Plots of Longitudinal Data on SER
eTable 1. Baseline Characteristics of the Included Participants
eTable 2. Refractive and Biometric Outcomes at 6-Month Follow-up
eTable 3. Percentages of SER Regression in 6 and 12 Months
eTable 4. Baseline Characteristics Between Continued and Interrupted Intervention Groups
eTable 5. Refractive and Biometric Outcomes at 12-Month Follow-up Between Continued Intervention and Control Group
eTable 6. Refractive and Biometric Outcomes at 12-Month Follow-up in Different Baseline Age Groups
Data Sharing Statement