This phase 3 randomized clinical trial assesses locoregional control rates after hyperthermic intraperitoneal chemotherapy with mitomycin C during cytoreductive surgery among adults with locally advanced colorectal cancer in Spain.
Key Points
Question
Does the use of hyperthermic intraperitoneal chemotherapy (HIPEC) with mitomycin C along with complete cytoreduction improve locoregional control in patients with locally advanced colorectal cancer?
Findings
This phase 3 randomized clinical trial of 184 adults in Spain showed an improvement in the locoregional control rate at 3 years in the HIPEC group (97%) compared with the surgery alone group (87%), without an increase in morbidity.
Meaning
The use of HIPEC with mitomycin C along with complete cytoreduction improved locoregional control in patients with locally advanced colorectal cancer and should be considered for treatment of locally advanced colorectal cancer.
Abstract
Importance
Peritoneal metastasis in patients with locally advanced colon cancer (T4 stage) is estimated to recur at a rate of approximately 25% at 3 years from surgical resection and is associated with poor prognosis. There is controversy regarding the clinical benefit of prophylactic hyperthermic intraperitoneal chemotherapy (HIPEC) in these patients.
Objective
To assess the efficacy and safety of intraoperative HIPEC in patients with locally advanced colon cancer.
Design, Setting, and Participants
This open-label, phase 3 randomized clinical trial was conducted in 17 Spanish centers from November 15, 2015, to March 9, 2021. Enrolled patients were aged 18 to 75 years with locally advanced primary colon cancer diagnosed preoperatively (cT4N02M0).
Interventions
Patients were randomly assigned 1:1 to receive cytoreduction plus HIPEC with mitomycin C (30 mg/m2 over 60 minutes; investigational group) or cytoreduction alone (comparator group), both followed by systemic adjuvant chemotherapy. Randomization of the intention-to-treat population was done via a web-based system, with stratification by treatment center and sex.
Main Outcomes and Measures
The primary outcome was 3-year locoregional control (LC) rate, defined as the proportion of patients without peritoneal disease recurrence analyzed by intention to treat. Secondary end points were disease-free survival, overall survival, morbidity, and rate of toxic effects.
Results
A total of 184 patients were recruited and randomized (investigational group, n = 89; comparator group, n = 95). The mean (SD) age was 61.5 (9.2) years, and 111 (60.3%) were male. Median duration of follow-up was 36 months (IQR, 27-36 months). Demographic and clinical characteristics were similar between groups. The 3-year LC rate was higher in the investigational group (97.6%) than in the comparator group (87.6%) (log-rank P = .03; hazard ratio [HR], 0.21; 95% CI, 0.05-0.95). No differences were observed in disease-free survival (investigational, 81.2%; comparator, 78.0%; log-rank P = .22; HR, 0.71; 95% CI, 0.41-1.22) or overall survival (investigational, 91.7%; comparator, 92.9%; log-rank P = .68; HR, 0.79; 95% CI, 0.26-2.37). The definitive subgroup with pT4 disease showed a pronounced benefit in 3-year LC rate after investigational treatment (investigational: 98.3%; comparator: 82.1%; log-rank P = .003; HR, 0.09; 95% CI, 0.01-0.70). No differences in morbidity or toxic effects between groups were observed.
Conclusions and Relevance
In this randomized clinical trial, the addition of HIPEC to complete surgical resection for locally advanced colon cancer improved the 3-year LC rate compared with surgery alone. This approach should be considered for patients with locally advanced colorectal cancer.
Trial Registration
ClinicalTrials.gov Identifier: NCT02614534
Introduction
Colorectal cancer is a highly prevalent disease, with an incidence of more than 1.1 million cases worldwide in 2020.1 The peritoneum is a common site of dissemination, with approximately 10% of patients developing peritoneal metastases.2 Patients with peritoneal metastatic colorectal cancer have significantly shorter overall survival than those with other isolated sites of metastases.3 Peritoneal metastases are often diagnosed at an advanced and symptomatic stage and are associated with poor prognosis.4,5 A locoregional approach to treating peritoneal metastases led to the development of radical cytoreductive surgery with adjuvant hyperthermic intraperitoneal chemotherapy (HIPEC). Results from randomized clinical trials (RCTs) have yielded mixed results regarding the clinical efficacy of HIPEC.4,6,7,8
Many strategies are aimed at prevention or early detection of peritoneal metastases. Patients at high risk are typically defined as those with locally advanced disease (T4 stage), tumor perforation, mucinous or signet ring cell histologic findings, advanced nodal stage, right-sided tumor location, and incomplete resection.2,6 Locally advanced colon cancer (stage T4) is recognized as an independent factor associated with peritoneal recurrence.9 Notably, the prognosis of patients with pT4 colon cancer is similar to that of patients with N2 and M1 stages, with a 5-year overall survival rate of approximately 20%.10,11 The incidence of metachronous peritoneal metastases in patients with pT4 colon cancer has been reported to be up to 36%.2,9
Patients with pT4 stage colon cancer (both stage II and III) are commonly treated with adjuvant systemic chemotherapy after surgical resection. Nevertheless, peritoneal metastases develop in many patients. The use of postoperative HIPEC to prevent metachronous peritoneal metastases has been evaluated in comparative studies with promising results,12 but there was a substantial risk of bias. The only phase 3 RCT,13 to our knowledge, of prophylactic HIPEC with oxaliplatin in patients with T4 or perforated colon cancer (the COLOPEC trial) found no benefit in the prevention of peritoneal metastases. To investigate the effect of administering HIPEC as adjuvant therapy, we conducted a multicenter phase 3 RCT (HIPECT4).14 The primary aim of this trial was to determine the efficacy of intraoperative HIPEC with mitomycin C with complete resection of locally advanced colon cancer (cT4). Here, we report the primary end point of locoregional control rate and the secondary end points of disease-free survival and overall survival.
Methods
Trial Design and Participants
HIPECT4 (NCT02614534) was an open-label, phase 3 randomized clinical trial conducted from November 15, 2015 to March 9, 2021, at 17 cancer centers in Spain. This trial was conducted in accordance with the Declaration of Helsinki15 and Good Clinical Practice requirements. Independent local ethics committees at each trial site approved the trial protocol. This trial has been reported in accordance with the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline. All patients provided written informed consent.
Eligible patients were aged 18 to 75 years and had histologically confirmed adenocarcinoma of the colon and rectum above the peritoneal reflection at stage cT4N02M0 based on preoperative imaging. Lymph node involvement (N0-2) was allowed when complete resection was performed. A Karnofsky Performance Scale Index score greater than 70 or an Eastern Cooperative Oncology Group performance status score of 2 or lower was required. The exclusion criteria were presence of metastases (M1); incomplete resection or presence of unresectability criteria; urgent management for obstruction or perforation removing the primary tumor; extraperitoneal rectal carcinoma; preoperative chemotherapy or radiotherapy; coexistence with other malignant neoplastic disease; severely altered liver, kidney, or cardiovascular functions; intolerance to the treatment; and pregnancy or breastfeeding (the trial protocol is provided in Supplement 1).
Randomization and Masking
Eligible patients were recruited by the trial investigators and centrally randomly assigned (1:1) preoperatively to receive either surgery plus HIPEC (investigational group) or surgery alone (comparator group). The clinical team was masked to the randomization process. The Maimónides Biomedical Research Institute of Córdoba generated an allocation table using R, version 4.2.0 (R Foundation for Statistical Computing) with the parameters required for the study and equitable stratification by sex and by treatment center. The table was uploaded in .csv format to the REDCap, version 10.6.27 platform (Vanderbilt University). Users assigned for data entry pressed only 1 button to randomly assign a patient to a treatment group. The registries were anonymized through the use of automatically assigned identification numbers. This trial was open label, and thus, neither investigators nor patients were masked to group assignment.
Procedures
All patients received extensive cytoreductive and target surgery (laparoscopic or open), including complete tumor resection, extensive lymphatic nodes resection, omentectomy, appendicectomy, and bilateral oophorectomy (in postmenopausal women). In the investigational group, immediately after cytoreduction, patients received HIPEC with mitomycin C, 30 mg/m2, diluted in 4 L of dextrose 1.5% solution over 60 minutes. The temperature was required to be between 42 °C and 43 °C during perfusion into the abdominal cavity. No drug substitutions or dose modifications were permitted for HIPEC. Patients in the investigational and comparator groups received routine adjuvant systemic chemotherapy (folinic acid, fluorouracil, and oxaliplatin [FOLFOX] with oxaliplatin and capecitabine [XELOX]) within 12 weeks of surgery according to local treatment protocols (Figure 1). For all time-to-event analyses, patients without events at the time of analysis were censored at the date of their last informative follow-up.
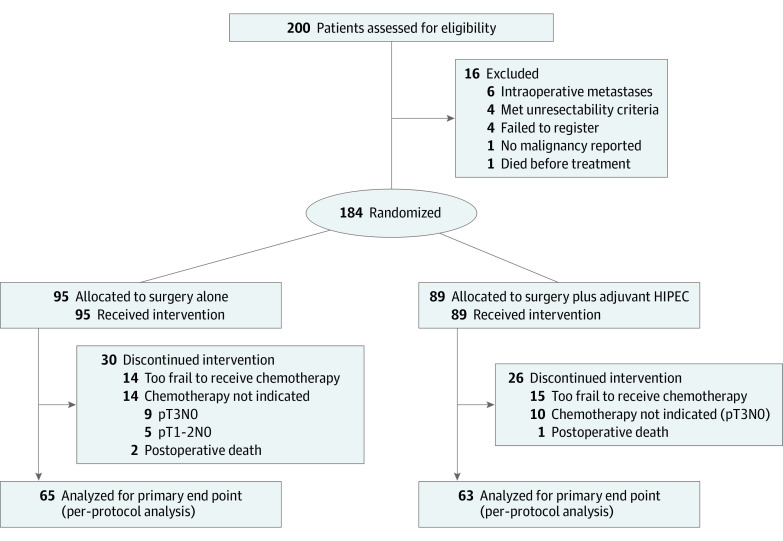
Figure 1. CONSORT Diagram.
HIPEC indicates hyperthermic intraperitoneal chemotherapy.
Outcomes
The primary outcome was the locoregional control rate evaluated at 3 years, defined as the proportion of patients without pathologic or radiologic diagnosis of locoregional recurrence. Locoregional recurrence was defined as relapse of the disease in the abdominal cavity involving the peritoneal surfaces or the tumor bed. Systemic and lymphatic recurrences were defined separately. The secondary end points were disease-free survival, overall survival, morbidity, and mortality. Locoregional recurrence and survival were analyzed from the surgical treatment up to the event or end of follow-up; the last patient included in this analysis had at least 12 months of follow-up. The definitive diagnosis of suspicious peritoneal relapse was assessed by additional imaging tests (computed tomography [CT], magnetic resonance imaging [MRI], or positron emission tomography) with or without percutaneous or surgical biopsy. Follow-up was performed every 6 months with clinical imaging tests (thorax and abdominal CT mainly or MRI if allergy to intravenous contrast was present) and evaluation of tumor markers (carcinoembryonic antigen and cancer antigen 19-9). Safety was evaluated through the analysis of perioperative morbidity according to Common Terminology Criteria for Adverse Events, version 4.0, and perioperative mortality at 30 and 90 days after treatment.
Statistical Analysis
The sample size calculation was based on the locoregional control rate at 36 months after treatment using the intention-to-treat (ITT) population. The investigational treatment was expected to result in an 82% locoregional control rate at 36 months after surgery (absolute risk of recurrence of 18% at 36 months), and the comparator treatment was expected to result in a 64% locoregional control rate at 36 months after surgery (absolute risk of recurrence of 36% at 36 months). To detect an 18% reduction in the proportion of locoregional relapses, a total of 190 patients (95 in each group) was needed (2-sided α = .05; power = 0.80); to accommodate a potential dropout rate of 5%, the definitive number of patients was estimated to be 200 (100 in each group). No continuity correction was applied.
A Shapiro-Wilk test and Q-Q plots were used to establish the goodness of fit to the normality of the variables studied. The Levene test was used to compare the equality of variances. Continuous variables were analyzed with an independent-samples t test (parametric) or the Mann-Whitney U test (nonparametric). A χ2 test and a Fisher exact test were used. Kaplan-Meier survival analysis with a log-rank test was used to compare the 2 study groups. We used an adjusted Cox proportional hazards regression model to estimate hazard ratios (HRs) with 95% CIs and the proportional hazards assumption test. Data were analyzed using IBM SPSS Statistics, version 26.0.0.0 (IBM). A 2-sided P value ≤.05 was considered statistically significant. A data and safety monitoring board performed interim reviews after inclusion of 100 patients and advised on trial continuation based on the incidence of serious adverse events.
Results
From November 15, 2015, to March 9, 2021, 200 patients were recruited and randomized. Sixteen patients were excluded from the trial, and 184 were included in the ITT analysis (mean [SD] age, 61.5 [9.2] years; 73 [39.7%] female, 111 [60.3%] male); 89 patients were in the surgery plus HIPEC group and 95 were in the comparator group (Figure 1). At baseline, the demographic characteristics, tumor characteristics, tumor molecular profiles, surgical management, and final tumor pathology stages reported were similar in both groups (Table 1). In total, 128 patients in the per-protocol population (69.6%) received adjuvant postoperative chemotherapy: 63 in the HIPEC group (70.8%) and 65 in the comparator group (68.4%). The accuracy in preoperative diagnosis of definitive pT4 colon cancer from a preoperative radiologic stage of cT4 was 67.9%.
Table 1. Demographic Characteristics and Pathologic Features.
| Characteristic or feature | Patients a | ||
| Total (N = 184) | HIPEC group (n = 89) | Surgery only group (n = 95) | |
| Age, mean (SD), y | 61.5 (9.2) | 60 (8.7) | 62 (10.6) |
| Sex | |||
| Female | 73 (39.7) | 33 (37.1) | 40 (42.1) |
| Male | 111 (60.3) | 56 (62.9) | 55 (57.9) |
| ECOG performance status score | |||
| 0 | 127 (69.0) | 63 (70.8) | 64 (67.4) |
| 1 | 49 (26.6) | 23 (25.8) | 26 (27.4) |
| 2 | 8 (4.3) | 3 (3.4) | 5 (5.3) |
| BMI ≥30 | 41 (22.3) | 20 (22.5) | 21 (22.1) |
| ASA score ≥3b | 56 (30.4) | 28 (31.5) | 28 (29.5) |
| PSS ≥1c | 40 (21.7) | 19 (21.3) | 21 (22.1) |
| Tumor location | |||
| Right colon | 70 (38.0) | 35 (39.3) | 35 (36.8) |
| Transverse colon | 8 (4.3) | 3 (3.4) | 5 (5.3) |
| Left colon | 31 (16.8) | 17 (19.1) | 14 (14.7) |
| Sigmoid rectum | 75 (40.8) | 34 (38.2) | 41 (43.2) |
| Preoperative CEA level, mean (SD), ng/mL | 17.7 (49.2) | 13 (41.2) | 22 (56.5) |
| Preoperative CA 19-9 level, mean (SD), U/mL | 26 (56.2) | 21 (37.9) | 30 (69.3) |
| T stage | |||
| T1-2 | 5 (2.7) | 0 | 5 (5.3) |
| T3a | 18 (9.8) | 9 (10.1) | 9 (9.5) |
| T3b | 35 (19.0) | 17 (19.1) | 18 (18.9) |
| T4a | 77 (41.8) | 36 (40.4) | 41 (43.2) |
| T4b | 48 (26.1) | 27 (30.3) | 21 (22.1) |
| N stage, No./total No. (%) | |||
| N0 | 97/182 (53.3) | 45/88 (51.1) | 52/94 (55.3) |
| N1 | 43/182 (23.6) | 23/88 (26.1) | 20/94 (21.3) |
| N2 | 42/182 (23.1) | 20/88 (22.7) | 22/94 (23.4) |
| Low-grade differentiation, No./total No. (%) | 119/177 (67.2) | 58/88 (65.9) | 61/90 (67.8) |
| Type of adenocarcinoma, No./total No. (%) | |||
| Mucinous | 32/183 (17.5) | 15 (16.9) | 17/94 (18.1) |
| Signet ring | 3/183 (1.6) | 2 (2.2) | 1/94 (1.1) |
| Tumor perforation, No./total No. (%) | 31/184 (16.8) | 14 (15.7) | 17 (17.9) |
| Pathology lymphatic invasion, No./total No. (%) | 80/183 (43.7) | 41 (46.1) | 39/94 (41.5) |
| Pathology perineural invasion, No./total No. (%) | 77/182 (42.3) | 36/88 (40.9) | 41/94 (43.6) |
| Pathology vascular invasion, No./total No. (%) | 66/182 (36.3) | 31 (34.8) | 35/93 (37.6) |
| Microsatellite instability, No./total No. (%) | 34/180 (18.9) | 22/88 (25.0) | 12/92 (13.0) |
| Tumor size, mean (SD), cm | 6.9 (3.8) | 6.5 (3.2) | 7.2 (4.4) |
| BRAF variant, No./total No. (%) | 8/37 (21.6) | 4/17 (23.5) | 4/18 (22.2) |
| KRAS wild type, No./total No. (%) | 26/44 (59.0) | 11/22 (50.0) | 15/22 (68.2) |
| Lymph nodes isolated, mean (SD), No. | 25 (12.6) | 25 (12.8) | 26 (12.5) |
| Blood needed, No./total No. (%) | 23 (12.5) | 15 (16.9) | 8 (8.4) |
| Laparoscopic approach | 29 (15.8) | 17 (19.1) | 12 (12.6) |
| Operative time, mean (SD), min | 250 (97.3) | 311 (81.8) | 193 (73.2) |
| Ostomy | 12 (6.5) | 4 (4.5) | 8 (8.4) |
| Intraoperative vasoactive drugs | 10 (5.4) | 7 (7.9) | 3 (3.2) |
| Adjuvant therapy with FOLFOX and XELOX | 128 (69.6) | 63 (70.8) | 65 (68.4) |
| Adjuvant therapy cycles of FOLFOX and XELOX, mean (SD), No. | NA | 7.2 (3.0) | 6.9 (3.4) |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CA, cancer antigen; CEA, carcinoembryonic antigen; ECOG, Eastern Cooperative Oncology Group; FOLFOX, folinic acid, fluorouracil, and oxaliplatin; HIPEC, hyperthermic intraperitoneal chemotherapy; NA, not applicable; PSS, previous surgical score; XELOX, oxaliplatin and capecitabine.
SI conversion factors: To convert CEA to micrograms per liter, multiply by 1.0.
Data are presented as the number (percentage) of patients unless otherwise indicated.
Physical status score range of 1 to 5, with higher scores indicating worse overall health.
Score range, 1 to 3, with higher scores indicating greater previous surgical intervention.
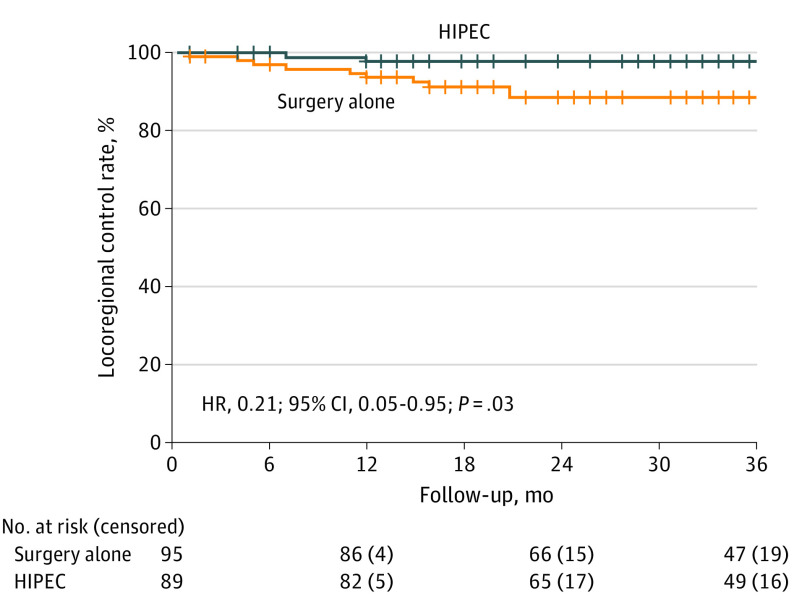
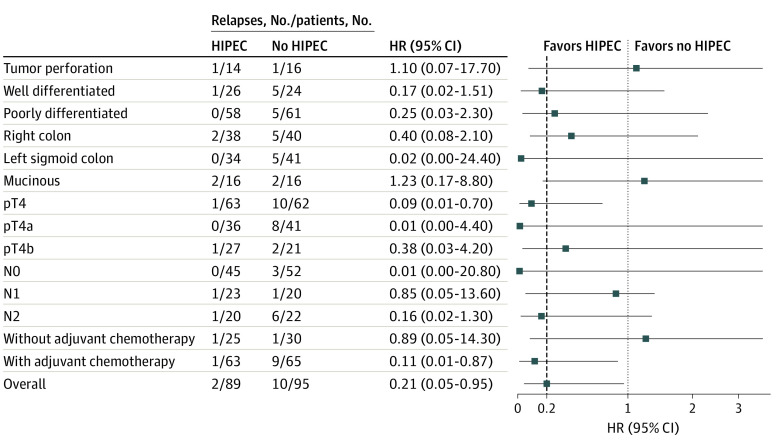
After a median follow-up of 36 months (IQR, 27-36 months), 12 patients had locoregional relapse: 2 patients in the HIPEC group (2.2%) and 10 patients in the comparator group (10.5%). In the ITT population, the 3-year locoregional control rates were 97.6% in the HIPEC group and 87.6% in the comparator group (log-rank P = .03; HR, 0.21; 95% CI, 0.05-0.95) (Figure 2 and Figure 3). The 3-year disease-free survival rates were 81.2% in the HIPEC group and 78.0% in the comparator group (log-rank P = .22; HR, 0.71; 95% CI, 0.41-1.22) (eFigure 1 in Supplement 2). Recurrence in the HIPEC group was most frequently in the liver in contrast with the comparator group, in which recurrence was frequently observed in the peritoneum (eFigure 2 in Supplement 2). The 3-year overall survival rates were 91.7% in the HIPEC group and 92.9% in the comparator group (log-rank P = .68; HR, 0.79; 95% CI, 0.26-2.37) (eFigure 3 in Supplement 2). In an exploratory analysis of patients in the subgroup with pT4 disease, the HIPEC group (n = 62) showed a benefit, with a 3-year locoregional control rate of 98.3% in contrast with the comparator group (n = 62) (82.1%) (log-rank P = .003; HR, 0.09; 95% CI, 0.01-0.70), resulting in an adjusted rate of peritoneal relapse of 16.2% (eFigure 4 in Supplement 2). Furthermore, the HIPEC group showed an improvement in locoregional control in the per-protocol population (patients who received adjuvant systemic chemotherapy) (HR, 0.11; 95% CI, 0.01-0.87) (Figure 3).
Figure 2. Kaplan-Meier Estimates of Locoregional Control Rate in the Surgery Plus Hyperthermic Intraperitoneal Chemotherapy (HIPEC) and Surgery Alone Groups.
Hash marks represent censored data. HR indicates hazard ratio.
Figure 3. Forest Plot of Locoregional Relapse Hazard Ratios (HRs) by Subgroup.
Squares indicate HRs, with horizontal lines indicating 95% CIs. Dashed line represents the HR for the overall comparison. HIPEC indicates hyperthermic intraperitoneal chemotherapy.
No increase in morbidity was observed between the groups postoperatively or at 30 days or 90 days after treatment (Table 2). Three patients died within 30 days of cytoreductive surgery: 1 in the HIPEC group (1.1%) and 2 in the comparator group (2.1%). The causes of death were intestinal leak and sepsis in 2 cases, and COVID-19 was the primary cause of the third (Table 2). The incidence of neutropenia in the HIPEC and comparator groups was 9.0% and 2.2%, respectively; the difference was not significant (Table 2). Severe neutropenia occurred in only 2 patients in the HIPEC group (2.2%).
Table 2. Posttreatment Morbidity at 30 and 90 daysa.
| Adverse event | Patients, No. (%) | ||
|---|---|---|---|
| Total (N = 184) | HIPEC group (n = 89) | Surgery only group (n = 95) | |
| Ostomy | 12/182 (6.6) | 4 (4.5) | 8/93 (8.6) |
| Anemia, CTCAE grade | |||
| 1 | 25/182 (13.7) | 13 (14.6) | 12/93 (12.9) |
| 2 | 26/182 (14.3) | 14 (15.7) | 12/93 (12.9) |
| 3 | 12/182 (6.6) | 7 (7.9) | 5/93 (5.4) |
| Neutropenia, CTCAE grade | |||
| 1 | 4/182 (2.2) | 2 (2.2) | 2/93 (2.2) |
| 2 | 4/182 (2.2) | 4 (4.5) | 0 |
| 3 | 1/182 (0.5) | 1 (1.1) | 0 |
| 4 | 1/182 (0.5) | 1 (1.1) | 0 |
| Thrombocytopenia, CTCAE grade | |||
| 1 | 3/182 (1.6) | 2/87 (2.3) | 1 (1.1) |
| 2 | 2/182 (1.1) | 2/87 (2.3) | 0 |
| Kidney failure, RIFLE criteria | |||
| Risk | 2/182 (1.1) | 1 (1.1) | 1/93 (1.1) |
| Injury | 1/182 (0.5) | 1 (1.1) | 0 |
| Reintervention | 22 (12.0) | 11 (12.4) | 11 (11.6) |
| Major morbidity | |||
| At 30 d | 38 (20.7) | 21 (23.6) | 17 (17.9) |
| At 90 d | 40 (21.7) | 23 (25.8) | 17 (17.9) |
| Mortality at 30 d | 3 (1.6) | 1 (1.1) | 2 (2.1) |
| Adynamic ileus | 19 (10.3) | 9 (10.1) | 10 (10.5) |
| Pleural effusion | 1 (0.5) | 1 (1.1) | 0 |
| Wound infection | 13 (7.1) | 3 (3.4) | 10 (10.5) |
| Urinary infection | 5 (2.7) | 3 (3.4) | 2 (2.1) |
| Anastomosis dehiscence | 15 (8.2) | 6 (6.7) | 9 (9.5) |
| Urinary leak | 1 (0.5) | 1 (1.1) | 0 |
| Intraabdominal collection | 7 (3.8) | 3 (3.4) | 4 (4.2) |
| Phlebitis | 2 (1.1) | 0 | 2 (2.1) |
| Cholecystitis | 1 (0.5) | 0 | 1 (1.1) |
| Evisceration | 4 (2.2) | 1 (1.1) | 3 (3.2) |
| Intestinal subocclusion | 1 (0.5) | 0 | 1 (1.1) |
| Lymphatic leak | 1 (0.5) | 1 (1.1) | 0 |
| COVID-19 | 1 (0.5) | 1 (1.1) | 0 |
| DVT | 1 (0.5) | 0 | 1 (1.1) |
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events; DVT, deep venous thrombosis; HIPEC, hyperthermic intraperitoneal chemotherapy; RIFLE, risk, injury, failure, loss of kidney function, and end-stage kidney disease.
No significant differences were found between the 2 groups.
Discussion
The results of the HIPECT4 trial demonstrated that cytoreductive surgery plus HIPEC provides a clinical benefit in terms of 3-year locoregional control compared with cytoreductive surgery alone in patients with locally advanced colorectal cancers (cT4). To our knowledge, this trial is the first to investigate the specific role of HIPEC with mitomycin C in reducing the risk of peritoneal relapse after resection in patients with locally advanced colorectal cancer. These results suggest that this HIPEC regimen confers an additional benefit to cytoreductive surgery and thus has important implications for clinical practice.
The T4 category is an important factor associated with developing metachronous peritoneal metastases.2,9,16 The overall reported proportion of metachronous peritoneal metastases varies from 17.9% to 36% at 3 years.9,16,17 In our trial, the proportion of patients experiencing peritoneal relapse by 3 years after surgery was 10.5% in the comparator group (10 of 95). This comparatively low incidence of peritoneal relapse in our trial could have several causes. First, the definitive cohort with pT4 disease in the comparator group comprised 62 patients, resulting in an adjusted rate of peritoneal relapse of 16.2%, which is in accordance with the literature.2,6,9 Another reason could be that this trial included only patients who had elective surgeries and not those who underwent urgent surgical procedures or who had perforated tumors. Additionally, the trial protocol took a targeted approach to surgery, focusing on locations where tumor cells frequently seed, which could have contributed to the overall improvement in rates of peritoneal relapse.
Despite the potential benefit of prophylactically treating patients at high risk of developing peritoneal metastases, there is currently only 1 preventive strategy used in this context: the administration of adjuvant systemic chemotherapy.18 This strategy is controversial because no studies, to our knowledge, have demonstrated that its use for high-risk, stage II (pT4) colon cancer reduces the rate of peritoneal relapse or improves disease-free survival. Post hoc analyses in the MOSAIC19 and SACURA20 trials did not show a benefit in patients with high-risk, stage II disease. Our protocol included the administration of adjuvant chemotherapy (FOLFOX and XELOX) after surgical procedures for patients with high-risk, stage II (pT4N0M0) and stage III (any T with N1-2) disease (Table 1).
These findings support the need for different strategies to prevent peritoneal relapse in patients at high risk. Several comparative studies have shown a benefit of HIPEC in preventing peritoneal carcinomatosis in T4 colorectal cancers.12,21,22,23,24,25,26 The reported rates of major complications related to adjuvant HIPEC were low.12 A recent retrospective comparative trial27 that included 352 patients with pT4N02M0 disease compared postoperative HIPEC with surgery alone in which the HIPEC protocol was lobaplatin in the early postoperative period (days 2-7). The rates of peritoneal recurrence were significantly lower for the HIPEC group than for the comparator group (26.1% vs 37.4%).27 Baratti et al28 conducted a comparative trial and reported a 9.3% incidence of peritoneal metastases at 5 years after surgery compared with a 42.5% incidence in case-matched controls. Substantial biases in the methods were observed among the aforementioned studies27,28; however, their promising results have encouraged other teams to design RCTs. Two multi-institutional RCTs13,29 have evaluated the role of prophylactic HIPEC in reducing the incidence of peritoneal metastases in patients with high-risk colon cancer. The COLOPEC13 trial did not demonstrate a reduction in the risk of peritoneal relapse in patients with pT4 and perforated colon cancer when HIPEC (fluorouracil, 400 mg/m2, and leucovorin, 20 mg/m2, administered intravenously, followed by intraperitoneal delivery of oxaliplatin, 460 mg/m2, for 30 minutes at 42 °C) was delivered intraoperatively or staged as an additional intervention within 5 to 8 weeks after the primary tumor resection. The primary end point (locoregional control rate) was evaluated at 18 months after the procedure using laparoscopic surgical exploration in only 62% of patients. This represented a limitation because the requirement to undergo another surgical intervention under general anesthesia might have been a reason for patients to decline this procedure. There was no difference in locoregional control rate at 18 months for the HIPEC group (80.9%) vs the comparator group (76.2%). Furthermore, 14% of patients who received adjuvant HIPEC developed postoperative major complications. In our trial, although the accuracy of preoperative diagnosis of definitive pT4 was limited to 67.9%, HIPEC was administered simultaneously with surgery to avoid the need for an additional surgical procedure, which may have resulted in the observed benefit in locoregional control rate. The second RCT, PROPHYLOCHIP,29 investigated another strategy to prevent peritoneal carcinomatosis in patients at high risk, defined as those with ovarian metastases, resected localized peritoneal disease, or perforated tumors in the first surgery. After adjuvant chemotherapy, the patients were followed up for 6 months, and if no disease was reported in the imaging tests, they were randomized to a second-look surgery plus HIPEC (oxaliplatin) vs observation. This strategy did not achieve disease-free survival benefits for these patients compared with the observation protocol; furthermore, the authors described an increase in major morbidity (41%) secondary to this additional prophylactic surgical procedure. In our trial, simultaneous adjuvant HIPEC avoided the inherent morbidity associated with a secondary procedure, and no differences were observed between groups in postoperative complications or toxic effects.
Mitomycin C is appealing as a HIPEC agent owing to its molecular features and a pharmacokinetic profile showing a rapid rise in tissue concentration in residual tumor deposits and the peritoneum over prolonged periods.30 Furthermore, the cytotoxicity of mitomycin C is synergistic with hyperthermia.31 However, in spite of these pharmacokinetic advantages, intraperitoneal mitomycin C is not devoid of systemic toxicity.32,33 In our trial, the incidence of neutropenia in the HIPEC and comparator groups was 9.0% and 2.2%, respectively, and severe neutropenia occurred in only 2 patients in the HIPEC group (2.2%) without severe effects on their outcomes. This low rate of neutropenia might have been because mitomycin C was administered in a prophylactic context at a smaller dose and faster rate than in a therapeutic context. Oxaliplatin and mitomycin C are the most frequently used chemotherapy agents. No difference in efficacy has been observed, but mitomycin C has demonstrated an improved safety profile compared with oxaliplatin (21% vs 30% of severe postoperative complications).34 Our trial protocol administered a fixed dose of mitomycin C (30 mg/m2 diluted in 4 L of dextrose 1.5% for 60 minutes). Although this is a lower dose compared with other protocols,34 it fits the safety profile used in a previous study35 with the aim of providing tolerable and effective treatment in a prophylactic context.
Strengths and Limitations
The HIPECT4 trial has several strengths. First, all patients were recruited in the setting of the Grupo Español de Carcinomatosis Peritoneal, which provides certified experience in HIPEC administration and ensures high-quality, complete tumor resection (100% R0), whereas a previously published trial reported a 75% rate of complete cytoreduction.36 Furthermore, to our knowledge, this is the first phase 3 RCT using HIPEC with mitomycin C in patients with colon cancer, an important point of differentiation compared with previous HIPEC trials with oxaliplatin. As discussed, mitomycin C has a pharmacodynamic profile that is well suited to HIPEC.
A potential limitation of this study is the preoperative selection for simultaneous HIPEC, which was complicated by the restricted sensitivity and specificity of imaging methods used to diagnose definitive pT4 disease. However, in our trial, the accuracy in preoperative diagnosis of definitive pT4 colon cancer from a preoperative radiologic stage of cT4 was 67.9%. This is in accordance with the literature, in which about 40% of patients classified as having cT4 disease were definitively classified as having pT2-3 disease.36 This possible overtreatment for the nondefinitive population with pT4 disease (32% in our trial) and the low risk of adverse events associated with HIPEC using mitomycin C must be balanced against the importance of decreasing the risk of peritoneal relapse by up to 80% over 3 years without increasing morbidity.
Conclusions
In this RCT, use of HIPEC with mitomycin C after complete cytoreductive surgery in patients with locally advanced colorectal cancer improved the locoregional control rate without increasing morbidity compared with surgery alone. This clinical benefit was greatest in the definitive exploratory analysis of a subpopulation with pT4 disease. Longer follow-up is required to evaluate disease-free survival and overall survival after HIPEC with cytoreductive surgery vs surgery alone.
Trial Protocol
eFigure 1. Three-Year Disease-Free Survival Rates in the HIPEC Group and Comparator Group
eFigure 2. Recurrence Sites in the HIPEC Group and Control Group
eFigure 3. Three-Year Overall Survival Rates in the HIPEC Group and Comparator Group
eFigure 4. Three-Year Locoregional Control Rate in the HIPEC Group and Comparator Group
Grupo Español de Carcinomatosis Peritoneal
Data Sharing Statement
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Segelman J, Granath F, Holm T, Machado M, Mahteme H, Martling A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2012;99(5):699-705. doi: 10.1002/bjs.8679 [DOI] [PubMed] [Google Scholar]
- 3.Franko J, Shi Q, Meyers JP, et al. ; Analysis and Research in Cancers of the Digestive System (ARCAD) Group . Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016;17(12):1709-1719. doi: 10.1016/S1470-2045(16)30500-9 [DOI] [PubMed] [Google Scholar]
- 4.Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21(20):3737-3743. doi: 10.1200/JCO.2003.04.187 [DOI] [PubMed] [Google Scholar]
- 5.Franko J, Shi Q, Goldman CD, et al. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of North Central Cancer Treatment Group phase III trials N9741 and N9841. J Clin Oncol. 2012;30(3):263-267. doi: 10.1200/JCO.2011.37.1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klaver CEL, van Huijgevoort NCM, de Buck van Overstraeten A, et al. Locally advanced colorectal cancer: true peritoneal tumor penetration is associated with peritoneal metastases. Ann Surg Oncol. 2018;25(1):212-220. doi: 10.1245/s10434-017-6037-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quénet F, Elias D, Roca L, et al. ; UNICANCER-GI Group and BIG Renape Group . Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):256-266. doi: 10.1016/S1470-2045(20)30599-4 [DOI] [PubMed] [Google Scholar]
- 8.Baratti D, Kusamura S, Pietrantonio F, Guaglio M, Niger M, Deraco M. Progress in treatments for colorectal cancer peritoneal metastases during the years 2010-2015: a systematic review. Crit Rev Oncol Hematol. 2016;100:209-222. doi: 10.1016/j.critrevonc.2016.01.017 [DOI] [PubMed] [Google Scholar]
- 9.Bastiaenen VP, Aalbers AGJ, Arjona-Sánchez A, et al. Risk of metachronous peritoneal metastases in patients with pT4a versus pT4b colon cancer: an international multicentre cohort study. Eur J Surg Oncol. 2021;47(9):2405-2413. doi: 10.1016/j.ejso.2021.05.009 [DOI] [PubMed] [Google Scholar]
- 10.Snaebjornsson P, Coupe VMH, Jonasson L, Meijer GA, van Grieken NC, Jonasson JG. pT4 stage II and III colon cancers carry the worst prognosis in a nationwide survival analysis: Shepherd’s local peritoneal involvement revisited. Int J Cancer. 2014;135(2):467-478. doi: 10.1002/ijc.28676 [DOI] [PubMed] [Google Scholar]
- 11.Amin MB, Edge S, Greene F, et al. AJCC Cancer Staging Manual. 8th ed. Springer International Publishing; 2017. doi: 10.1007/978-3-319-40618-3 [DOI] [Google Scholar]
- 12.Sloothaak DAM, Mirck B, Punt CJ, et al. Intraperitoneal chemotherapy as adjuvant treatment to prevent peritoneal carcinomatosis of colorectal cancer origin: a systematic review. Br J Cancer. 2014;111(6):1112-1121. doi: 10.1038/bjc.2014.369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klaver CEL, Wisselink DD, Punt CJA, et al. ; COLOPEC collaborators group . Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): a multicentre, open-label, randomised trial. Lancet Gastroenterol Hepatol. 2019;4(10):761-770. doi: 10.1016/S2468-1253(19)30239-0 [DOI] [PubMed] [Google Scholar]
- 14.Arjona-Sánchez A, Barrios P, Boldo-Roda E, et al. HIPECT4: multicentre, randomized clinical trial to evaluate safety and efficacy of hyperthermic intra-peritoneal chemotherapy (HIPEC) with mitomycin C used during surgery for treatment of locally advanced colorectal carcinoma. BMC Cancer. 2018;18(1):183. doi: 10.1186/s12885-018-4096-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 16.Uppal A, Helmink B, Grotz TE, et al. What is the risk for peritoneal metastases and survival afterwards in T4 colon cancers? Ann Surg Oncol. 2022;29:4224-4233. doi: 10.1245/s10434-022-11472-w [DOI] [PubMed] [Google Scholar]
- 17.Hompes D, Tiek J, Wolthuis A, et al. HIPEC in T4a colon cancer: a defendable treatment to improve oncologic outcome? Ann Oncol. 2012;23(12):3123-3129. doi: 10.1093/annonc/mds173 [DOI] [PubMed] [Google Scholar]
- 18.Benson AB, Venook AP, Al-Hawary MM, et al. Colon cancer, version 2.2021: NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(3):329-359. doi: 10.6004/jnccn.2021.0012 [DOI] [PubMed] [Google Scholar]
- 19.Tournigand C, André T, Bonnetain F, et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J Clin Oncol. 2012;30(27):3353-3360. doi: 10.1200/JCO.2012.42.5645 [DOI] [PubMed] [Google Scholar]
- 20.Matsuda C, Ishiguro M, Teramukai S, et al. ; SACURA Study Group . A randomised-controlled trial of 1-year adjuvant chemotherapy with oral tegafur-uracil versus surgery alone in stage II colon cancer: SACURA trial. Eur J Cancer. 2018;96:54-63. doi: 10.1016/j.ejca.2018.03.009 [DOI] [PubMed] [Google Scholar]
- 21.Sugarbaker PH, Gianola FJ, Speyer JL, Wesley R, Barofsky I, Myers CE. Prospective randomized trial of intravenous v intraperitoneal 5-FU in patients with advanced primary colon or rectal cancer. Semin Oncol. 1985;12(3)(suppl 4):101-111. [PubMed] [Google Scholar]
- 22.Graf W, Westlin JE, Påhlman L, Glimelius B. Adjuvant intraperitoneal 5-fluorouracil and intravenous leucovorin after colorectal cancer surgery: a randomized phase II placebo-controlled study. Int J Colorectal Dis. 1994;9(1):35-39. doi: 10.1007/BF00304298 [DOI] [PubMed] [Google Scholar]
- 23.Scheithauer W, Kornek GV, Marczell A, et al. Combined intravenous and intraperitoneal chemotherapy with fluorouracil + leucovorin vs fluorouracil + levamisole for adjuvant therapy of resected colon carcinoma. Br J Cancer. 1998;77(8):1349-1354. doi: 10.1038/bjc.1998.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaillant JC, Nordlinger B, Deuffic S, et al. Adjuvant intraperitoneal 5-fluorouracil in high-risk colon cancer: a multicenter phase III trial. Ann Surg. 2000;231(4):449-456. doi: 10.1097/00000658-200004000-00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noura S, Ohue M, Shingai T, et al. Effects of intraperitoneal chemotherapy with mitomycin C on the prevention of peritoneal recurrence in colorectal cancer patients with positive peritoneal lavage cytology findings. Ann Surg Oncol. 2011;18(2):396-404. doi: 10.1245/s10434-010-1319-2 [DOI] [PubMed] [Google Scholar]
- 26.Sammartino P, Sibio S, Biacchi D, et al. Prevention of peritoneal metastases from colon cancer in high-risk patients: preliminary results of surgery plus prophylactic HIPEC. Gastroenterol Res Pract. 2012;2012:141585. doi: 10.1155/2012/141585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li T, Yu J, Chen Y, et al. Preventive intraperitoneal hyperthermic perfusion chemotherapy for patients with T4 stage colon adenocarcinoma. Tech Coloproctol. 2021;25(6):683-691. doi: 10.1007/s10151-020-02270-1 [DOI] [PubMed] [Google Scholar]
- 28.Baratti D, Kusamura S, Iusco D, et al. Hyperthermic intraperitoneal chemotherapy (HIPEC) at the time of primary curative surgery in patients with colorectal cancer at high risk for metachronous peritoneal metastases. Ann Surg Oncol. 2017;24(1):167-175. doi: 10.1245/s10434-016-5488-5 [DOI] [PubMed] [Google Scholar]
- 29.Goéré D, Glehen O, Quenet F, et al. ; BIG-RENAPE group . Second-look surgery plus hyperthermic intraperitoneal chemotherapy versus surveillance in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP-PRODIGE 15): a randomised, phase 3 study. Lancet Oncol. 2020;21(9):1147-1154. doi: 10.1016/S1470-2045(20)30322-3 [DOI] [PubMed] [Google Scholar]
- 30.Kuzuya T, Yamauchi M, Ito A, Hasegawa M, Hasegawa T, Nabeshima T. Pharmacokinetic characteristics of 5-fluorouracil and mitomycin C in intraperitoneal chemotherapy. J Pharm Pharmacol. 1994;46(8):685-689. doi: 10.1111/j.2042-7158.1994.tb03883.x [DOI] [PubMed] [Google Scholar]
- 31.Teicher BA, Kowal CD, Kennedy KA, Sartorelli AC. Enhancement by hyperthermia of the in vitro cytotoxicity of mitomycin C toward hypoxic tumor cells. Cancer Res. 1981;41(3):1096-1099. [PubMed] [Google Scholar]
- 32.Sugarbaker PH, Alderman R, Edwards G, et al. Prospective morbidity and mortality assessment of cytoreductive surgery plus perioperative intraperitoneal chemotherapy to treat peritoneal dissemination of appendiceal mucinous malignancy. Ann Surg Oncol. 2006;13(5):635-644. doi: 10.1245/ASO.2006.03.079 [DOI] [PubMed] [Google Scholar]
- 33.Lambert LA, Armstrong TS, Lee JJ, et al. Incidence, risk factors, and impact of severe neutropenia after hyperthermic intraperitoneal mitomycin C. Ann Surg Oncol. 2009;16(8):2181-2187. doi: 10.1245/s10434-009-0523-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wisselink DD, Braakhuis LLF, Gallo G, et al. Systematic review of published literature on oxaliplatin and mitomycin C as chemotherapeutic agents for hyperthermic intraperitoneal chemotherapy in patients with peritoneal metastases from colorectal cancer. Crit Rev Oncol Hematol. 2019;142:119-129. doi: 10.1016/j.critrevonc.2019.06.014 [DOI] [PubMed] [Google Scholar]
- 35.Arjona-Sanchez A, Rodriguez-Ortiz L, Baratti D, et al. RAS mutation decreases overall survival after optimal cytoreductive surgery and hyperthermic intraperitoneal chemotherapy of colorectal peritoneal metastasis: a modification proposal of the peritoneal surface disease severity score. Ann Surg Oncol. 2019;26(8):2595-2604. doi: 10.1245/s10434-019-07378-9 [DOI] [PubMed] [Google Scholar]
- 36.Klaver CEL, Gietelink L, Bemelman WA, et al. ; Dutch Surgical Colorectal Audit Group . Locally advanced colon cancer: evaluation of current clinical practice and treatment outcomes at the population level. J Natl Compr Canc Netw. 2017;15(2):181-190. doi: 10.6004/jnccn.2017.0019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure 1. Three-Year Disease-Free Survival Rates in the HIPEC Group and Comparator Group
eFigure 2. Recurrence Sites in the HIPEC Group and Control Group
eFigure 3. Three-Year Overall Survival Rates in the HIPEC Group and Comparator Group
eFigure 4. Three-Year Locoregional Control Rate in the HIPEC Group and Comparator Group
Grupo Español de Carcinomatosis Peritoneal
Data Sharing Statement