Morphea, also known as localized scleroderma (LS), is an inflammatory connective tissue disease characterized by the insidious onset of thickened, scar-like, oval patches of skin. Various interventions have been used to treat morphea. Oral methotrexate and oral prednisone are reportedly effective, but may cause adverse events (1). Tofacitinib is a Janus tyrosine kinase (JAK) inhibitor, recently approved as treatment for patients with autoimmune disorders (2); however, its utility in paediatric patients with morphea is unclear (3). We report the successful use of tofacitinib in 2 paediatric patients with morphea.
CASE REPORTS
Case 1. A 6-year-old girl presented with a 4-year history of localized, indurated, atrophic plaques with undefined margins. She had previously been prescribed methotrexate and topical glucocorticoid ointment; however, as post-treatment laboratory tests revealed elevated concentrations of alanine transaminase (82 U/L) and aspartate transaminase (86 U/L), methotrexate was discontinued. Upon presentation, physical examinations revealed hyperpigmented, scaly, shiny plaques on her right abdomen and left forearm (Fig. 1A and B). Auto-antibody-, liver-, and muscle enzyme test results were negative. Skin biopsy shows a pattern of morphea (Fig. 1E and G). A diagnosis of linear morphea was made. Taking into consideration the patient’s side-effects to methotrexate, she was initially treated with glycyrrhizin (4) tablets (25 mg, twice daily), topical tacrolimus ointment (0.03%, twice daily), and ultraviolet radiation A1 (UVA1; 30 J/cm2, every other day). After 3 weeks, the UVA1 treatment was terminated because the patient was unable to return to hospital on a regular basis. Thereafter, the patient’s parents provided informed consent for initiation of tofacitinib therapy, as they declined methotrexate and mycophenolate. Taking into consideration that the patient’s body weight was 16 kg, the initial dose of tofacitinib was 2.5 mg twice daily. After 2 months of treatment with tofacitinib, her sclerotic lesions resolved dramatically. The tofacitinib regimen was decreased to 2.5 mg, once daily for 4 months followed by 2.5 mg, every other day, for 2 months, as her lesions had stopped progressing and exhibited no signs of activity. Abdominal lesion biopsy specimens obtained before and after tofacitinib therapy from the same lesion revealed that the improvement was accompanied by concomitant histological changes (Fig. 1C and D). Skin biopsy shows significant improvement in sclerosis of the dermis (Fig. 1F and H). The Localized Scleroderma Cutaneous Assessment Tool (LoSCAT) (5) was used to assess disease-related activity and damage at each visit after the start of tofacitinib treatment. From before tofacitinib treatment to 6 months thereafter, the patient’s modified Localized Scleroderma Skin Severity Index (mLoSSI) score improved from 6 to 1, while her Localized Scleroderma Damage Index (LosDi) score improved from 7 to 2 (Table I). The skin biopsy and final LoSCAT scoring were performed at the same visit.
Fig. 1.
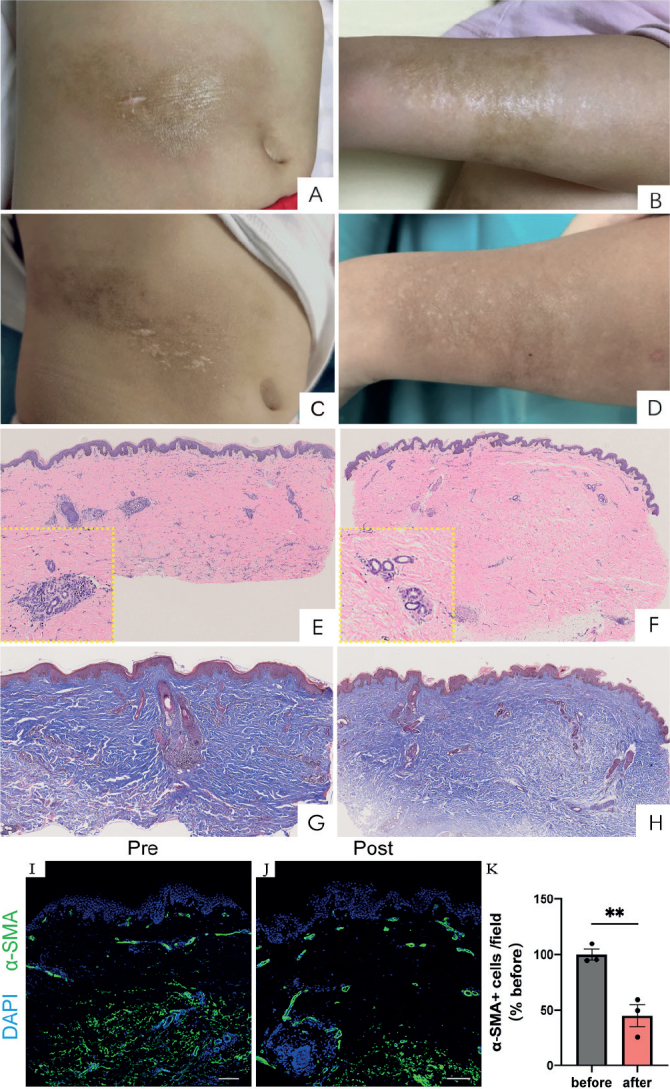
Clinical and histological features for case 1: (A, B, E, G, I) before and (C, D, F, H, J) after tofacitinib treatment for 6 months. Before treatment, brown patches with a waxy gloss were observed on (A) the right abdomen and (B) left forearm. After treatment, the hardness of skin lesions was alleviated on (C) the right abdomen and (D) the left forearm. (E, G) Histopathological examination of an abdominal skin biopsy revealed lymphocytes and plasma cell infiltration in the peripheral blood vessels, with dermal collagen fibres increased and thickened before therapy. Immunofluorescent staining for α-smooth muscle actin (α-smooth muscle actin (α-SMA), green) expression diffuse positivity through the deep dermis (I). Scale bar = 100 μm. After treatment, histopathological examination from the lesion showed (F, H) minor lymphocytic infiltration of the peripheral tissue, with moderate amount of collagen fibres in the dermis (haematoxylin and eosin, original magnification ×4, enlarged view × 20 in yellow boxes of E and F; Masson trichrome, original magnification ×4). Immunofluorescent staining revealed a decreased α-SMA expression in the deep dermis and mainly located at perivascular area (J). Scale bar = 100 μm. Quantification of α-SMA+ cells (defined as relative mean grey density) per visual field normalized before treatment (K). n = 3 random field of view for each group. Mean±SD. **p < 0.01.
Table I.
Localized Scleroderma Cutaneous Assessment Tool (LoSCAT) of 2 cases
| Before | After 2 months | At withdrawal | ||
|---|---|---|---|---|
| Case1 | mLoSSI | 6 | 3 | 1 |
| LosDi | 7 | 5 | 2 | |
| Case2 | mLoSSI | 7 | 3 | 1 |
| LosDi | 7 | 4 | 2 | |
The LoSCAT scale was used to assess disease-related activity and damage, performed independently by 2 clinicians. All of the scores were considerably reduced after medication.
mLoSSI: modified LS Skin Severity Index, which measures disease activity; LosDi: Localized scleroderma Damage Index, which measures damage.
Case 2. A 13-year-old boy presented with a 4-year history of brown and oedematous plaques on the right abdomen. Physical examinations revealed sharply demarcated, hyperpigmented, indurated patches on his right abdomen, with a slight depression of the skin that formed a confluent area with atrophy (Fig. 2A). His erythrocyte sedimentation rate, C-reactive protein levels, and antinuclear antibody test results were unremarkable.Skin biopsy shows a pattern of morphea (Fig. 2C). A diagnosis of linear morphea was made. Similar to case 1, the parents in case 2 declined the use of methotrexate and corticosteroids. The initial treatment was a combination of hydroxychloroquine sulphate (6) (100 mg, twice daily), topical tacrolimus ointment (0.03%, twice daily), and UVA1 (40 J/cm2, 3 times a week for 12 weeks). However, rapid progression of widespread, circumferential thickening and tightening of the trunk skin occurred. After obtained informed consent, tofacitinib therapy was initiated (5 mg, twice daily, based on his weight of 65 kg). After 2 months of treatment, the patient’s lesions had not progressed and there were no adverse effects. Within months, the sclerotic plaques exhibited substantial softening (Fig. 2B). Therefore, tofacitinib was decreased to 5 mg, daily. Normalization of lesions continued during 3 months of tofacitinib treatment. Skin biopsy shows significant improvement in the dermis (Fig. 2D). From before tofacitinib treatment to 5 months thereafter, his mLoSSI and LosDi scores improved from 7 to 1 and from 7 to 2, respectively (Table I).
Fig. 2.
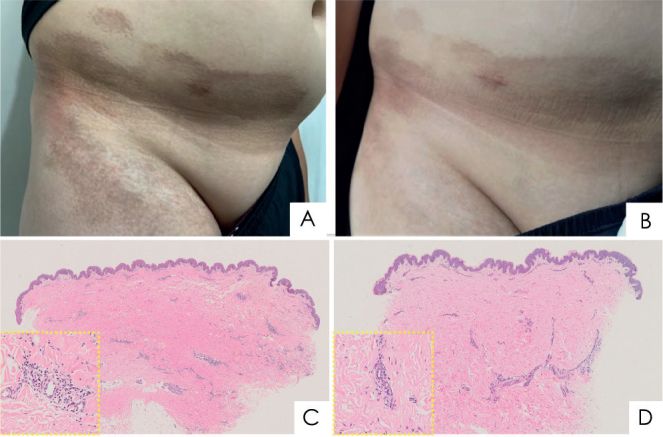
Clinical and histological features for case 2: (A, C) before and (B, D) after tofacitinib treatment for 5 months. (A) Before treatment, brown patches were observed on the right lower abdomen, groin and thigh. (B) After treatment, the skin lesions became softer and pigmentation considerably improved. (C) Histopathological examination of an abdominal skin biopsy revealed thickened and homogenous collagen bundles in the mid- and reticular dermis, with sparse deep lymphoplasmacytic infiltrate. Loss of adventitial fat resulted in a lack of eccrine glands. (D) Post-treatment biopsy specimens revealed decreased collagen bundle size and collagen fibres in the lower dermis (haematoxylin–eosin, original magnification ×4, enlarged view ×20 in yellow boxes of C and D).
To better understand the mechanism of antifibrotic treatment of tofacitinib, proliferation of myofibroblasts was analysed with immunofluorescence staining for case 1 (Fig. 1 I, J). Only an initial skin biopsy was taken from case 2. Excessive deposition of extracellular matrix in the dermis is reflected by an increased number of α-smooth muscle actin (α-SMA). Activated, α - SMA+ myofibroblasts were markedly decreased after tofacitinib treatment, mostly in the dermal papillary layer. Specifically, 2 blinded evaluators each manually quantified 3 regions of interest under high-power fields (×20) to assess differentiated fibroblasts numbers, as reported previously (7) (Fig. 1K).
DISCUSSION
To our knowledge, these are the first 2 reported cases of significant improvement following treatment with tofacitinib in patients with paediatric morphea. The European Pediatric Rheumatology Group recommended methotrexate and prednisone as first-line treatment for morphea, although the evidence base is weak (1). Hydroxychloroquine and the herb extract glycyrrhizin are reportedly valuable and well-tolerated, reducing inflammation in patients with morphea (8, 9). The standard therapies methotrexate or prednisone (1) were not used in our 2 cases, because their parents did not allow the use of these compounds due to possible side-effects.
An imbalance between different subsets of helper T cells (Th cells) drives inflammation of scleroderma (10). Multiple lines of evidence indicate that the innate and adaptive immune responses require JAK-STAT signalling to mediate cytokine function (11). Profibrotic cytokines, such as TGF-β, are considered involved in pathological fibrosis via fibroblast activation, induction of myofibroblast differentiation, and α-SMA expression. More specifically, the JAK-STAT pathway is considered the downstream mediator in the promotion of fibrosis by TGF-β, IL-6, and other factors, and plays a signal transduction role in the process of fibrosis (12, 13) JAK inhibitors, such as tofacitinib, targeting the JAK-STAT pathway, have been widely used for a variety of immune-mediated diseases, such as rheumatoid arthritis (14). Of note, tofacitinib has recently been reported to reverse fibrosis in 3 patients with recalcitrant morphea (3). How-ever, morphea usually has a self-limited evolution, with a high rate of spontaneous regression within 3–5 years (3). Hence, existing research cannot rule out the possibility of self-remission of the disease. Clinicians should monitor paediatric patients’ adverse effects of JAK inhibitors according to morphea subtypes (15). In the current case reports, no secondary outcome measures, such as thermography or durometry, were used to document clinical improvement of the patients’ scleroderma. However, the results illustrate the benefits of tofacitinib, which may be useful in paediatric patients with treatment-refractory morphea.
In conclusion, we present here the successful use of tofacitinib in 2 paediatric patients with morphea. The appearance of the skin improved markedly, and the sclerosis resolved. Further pathology and immunofluorescence staining indicated that regulation of myofibroblast activity and collagen synthesis may underlie these effects.
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Albuquerque JVD, Andriolo BN, Vasconcellos MR, Civile VT, Lyddiatt A, Trevisani VF. Interventions for morphea. Cochrane Database Syst Rev 2019; 7: CD005027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.You H, Xu D, Zhao J, Li J, Wang Q, Tian X, et al. JAK inhibitors: prospects in connective tissue diseases. Clin Rev Allergy Immunol 2020; 59: 334–351. [DOI] [PubMed] [Google Scholar]
- 3.Damsky W, Patel D, Garelli CJ, Garg M, Wang A, Dresser K, et al. JAK inhibition prevents bleomycin-induced fibrosis in mice and is effective in patients with morphea. J Invest Dermatol 2020; 140: 1446–1449. [DOI] [PubMed] [Google Scholar]
- 4.Richard SA. Exploring the pivotal immunomodulatory and anti-inflammatory potentials of glycyrrhizic and glycyrrhetinic acids. Mediators Inflamm 2021; 2021: 6699560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelsey CE, Torok KS. The localized scleroderma cutaneous assessment tool: responsiveness to change in a pediatric clinical population. J Am Acad Dermatol 2013; 69: 214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groot N, de Graeff N, Avcin T, Bader-Meunier B, Brogan P, Dolezalova P, et al. European evidence-based recommendations for diagnosis and treatment of childhood-onset systemic lupus erythematosus: the SHARE initiative. Ann Rheum Dis 2017; 76: 1788–1796. [DOI] [PubMed] [Google Scholar]
- 7.Stevens WG, Gould DJ, Pham LD, Jimenez Lozano JN. Molecular and histological evidence detailing clinically observed skin improvement following cryolipolysis. Aesthet Surg J 2022; 42: 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang P, Guo W, Liu S. A rare case of juvenile localised scleroderma with intra-oral and dental involvement. Exp Ther Med 2015; 10: 2213–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar AB, Blixt EK, Drage LA, El-Azhary RA, Wetter DA. Treatment of morphea with hydroxychloroquine: a retrospective review of 84 patients at Mayo clinic, 1996–2013. J Am Acad Dermatol 2019; 80: 1658–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker D, Susa JS, Currimbhoy S, Jacobe H. Histopathological changes in morphea and their clinical correlates: results from the morphea in adults and children cohort V. J Am Acad Dermatol 2017; 76: 1124-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torok KS, Kurzinski K, Kelsey C, Yabes J, Magee K, Vallejo AN, et al. Peripheral blood cytokine and chemokine profiles in juvenile localized scleroderma: T-helper cell-associated cytokine profiles. Semin Arthritis Rheu 2015; 45: 284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakraborty D, Šumová B, Mallano T, Chen C, Distler A, Bergmann C, et al. Activation of STAT3 integrates common profibrotic pathways to promote fibroblast activation and tissue fibrosis. Nat Commun 2017; 8: 1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Celada LJ, Kropski JA, Herazo-Maya JD, Luo W, Creecy A, Abad AT, et al. PD-1 up-regulation on CD4+ T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-β1 production. Sci Transl Med 2018; 10: eaar8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seif F, Khoshmirsafa M, Aazami H, Mohsenzadegan M, Sedighi G, Bahar M. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal 2017; 15: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGaugh S, Kallis P, De Benedetto A, Thomas RM. Janus kinase inhibitors for treatment of morphea and systemic sclerosis: a literature review. Dermatol Ther 2022; 35: e15437. [DOI] [PubMed] [Google Scholar]


