Abstract
Atopic dermatitis is a chronic skin condition for which a range of systemic treatments have recently been approved. A treat-to-target strategy has been developed previously alongside an algorithm to guide the management of patients with atopic dermatitis. Here, we review the strategy and algorithm in the context of the evolving therapeutic landscape, and identify areas for further refinement and development.
Key words: atopic dermatitis, consensus, systemic treatment, treat-to-target
SIGNIFICANCE.
As the atopic dermatitis treatment landscape evolves rapidly, a treat-to-target strategy may become an important part of the clinical decision-making process. We have identified areas for further refinement within a published treat-to-target consensus document.
Atopic dermatitis (AD) is a chronic skin condition driven by underlying type 2 inflammation. The burdensome signs and symptoms, including intense itch, disfiguring lesions, and skin pain, are associated with multidimensional impacts on quality of life and may require long-term management. Moderate-to-severe AD that is insufficiently controlled by topical or phototherapy may require systemic treatment to reduce the debilitating clinical manifestations and control the disease. Until recently, systemic therapies were limited to traditional immunosuppressive therapies (e.g. cyclosporine, methotrexate), but these treatments have safety profiles that limit long-term use. A range of new systemic treatment options have been approved recently for AD, including biologics (e.g. dupilumab, tralokinumab) and Janus kinase (JAK) inhibitors (e.g. baricitinib, abrocitinib, upadacitinib), with more approvals expected over the next few years. These therapies provide physicians with new tools that could potentially transform the lives of many patients with moderate-to-severe disease. Guidelines for the use of systemic therapies in AD have reviewed treatment options and their use in the clinic, but have not provided criteria with which to judge treatment success (1, 2). However, with a rapidly evolving range of treatment options, there is increasing focus on the assessment of treatment outcomes in the clinic to support treatment choices, and to guide the development of short- and long-term management goals, including eventual disease control.
A recent consensus publication, guided by the authors and published in 2021, proposed a treat-to-target strategy like those already established for the management of a range of immune-mediated/inflammatory conditions, such as rheumatoid arthritis, spondyloarthropathies, systemic lupus erythematosus, and psoriasis. The consensus publication proposed treatment goals in AD (target thresholds using clinically appropriate instruments) and embedded them in an algorithm that could guide the management of adults with AD requiring systemic therapy (3). The current report evaluates that proposal against the background of the evolving therapeutic landscape, reviews its strengths, explores potential for improvement, and proposes a path for evolution of the concept.
METHODS
Consensus statements were developed using a modified Delphi process implemented using an anonymized online platform. An initial statement set, developed by a 14-member Steering Committee, was evaluated by a mixed group of dermatologists, nurses, and patient representatives (the Extended Panel) using a 9-point Likert scale, with consensus on any given statement requiring the highest level of agreement from 75% or more of respondents; this is known as a “consensus in” approach. While the Delphi methodology was generally robust, limitations of the proposal have been identified.
When the treat-to-target project was initiated, the evidence base for systemic therapies was sparse, and skewed toward randomized clinical trials rather than the real-world evidence (RWE) that better reflects outcomes in the clinic. Therefore, the initial statements concerning thresholds had to be based on the expert opinion of the Steering Committee. In the period since publication, the real-world evidence base has expanded (e.g. SCRATCH, PROSE, TREAT, BIODAY, TARGET-DERM) (4–8) and thus could inform a review of treatment goals. Considering representation in the consensus process, the Extended Panel comprised 87 participants: 74 physicians (mostly dermatologists; 3 were allergists/immunologists), 3 nurses, and 10 patient representatives. The original publication accepted and reported that the low number of nurses and patients/patient representatives who participated in the eDelphi process was a limitation. In addition, the expert physician group would have benefited from the inclusion of office-based dermatologists to ensure that their everyday clinical practice perspective was acknowledged. Finally, although the initial panel included members from 28 countries, the great majority were from Europe, with smaller numbers from Australia, Canada, and Japan; some other regions, such as the USA, Latin America, and China, were not represented at all.
THE TREAT-TO-TARGET ALGORITHM
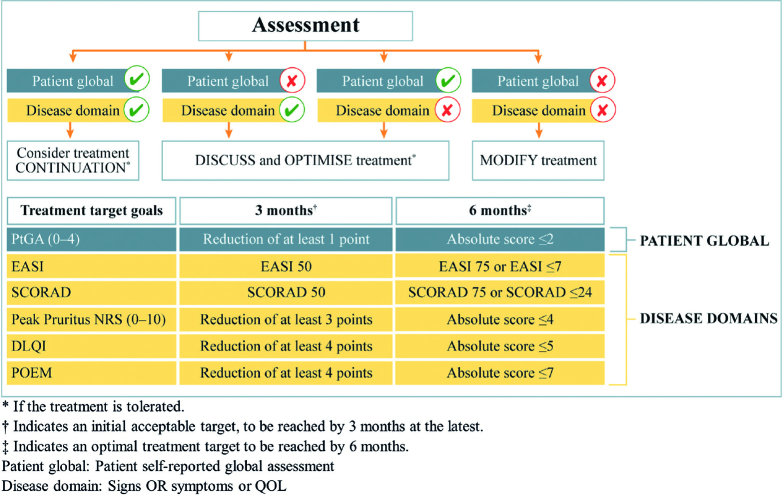
The final consensus statements form the basis of a simple but treat-to-target algorithm (Fig. 1). The primary strength of the algorithm is that it offers a flexible approach to produce customized targets according to the needs of individual patients, irrespective of the medication selected. The recommended assessment instruments aimed to take into account the constraints of the everyday clinical setting, allowing customization of treatment goals to the needs of the patient. Particularly important is the role of a global patient assessment instrument, which recognizes the central importance of the patient’s experience of the disease. The instrument included was the self-reported Patient Global Assessment of disease severity (PtGA), which allows the patient to estimate their disease severity as clear, almost clear, mild, moderate, or severe. However, this scale does not consider patients’ symptoms or well-being. An argument could be made for using other instruments instead, such as the Patient Global Assessment of Disease Status (PGADS), or the Patient Global Assessment of Treatment Effect (PGATE), which allows patients to provide a more holistic picture of their disease status or their perception of treatment effect (poor, fair, good, very good or excellent). We note, however, that the role of the patient extends beyond the choice of assessment instrument and requires further consideration.
Fig. 1.
Algorithm for decision-making in treating moderate-to-severe atopic dermatitis (AD) to target with systemic treatments based on the consensus. DLQI: Dermatology Life Quality Index; EASI: Eczema Area and Severity Index; NRS: numerical rating scale; POEM: Patient-Oriented Eczema Measure; QOL: quality of life; SCORAD: SCORing Atopic Dermatitis. Published in Acta Dermato-Venereologica (3).
The assessment time-points built into the algorithm (3 and 6 months) reflect typical time-points used to evaluate disease control in everyday clinical practice. However, they do not consider life-long disease management, nor early assessment of treatment effect where it might be relevant. Considering thresholds, there is a concentration on relative (e.g. Eczema Area and Severity Index (EASI) 75) rather than absolute outcomes (e.g. EASI ≤ 7, or Itch Numerical Rating Scale (NRS) 0 or 1), particularly at the 3-month time-point. Relative response criteria are commonly used in clinical trials (where a wash-out period of prior systemic treatment is common), and for comparing newer and older treatments, but have limitations when applied in daily clinical practice. For example, patients with severe disease may achieve large improvements relative to their initial disease status, while retaining significant disease activity and health-related quality of life (HRQoL) deficits; for example, itch and mental health issues. Conversely, patients with less severe disease may experience improvements over time that are not captured by relative outcomes. In addition, relative outcomes are not suitable for patients receiving bridging therapy or switching therapy, due to safety concerns. Safety assessments could also be built into the algorithm in a more systematic way.
Finally, a concern was found amongst some colleagues that the algorithm was potentially time-consuming for use in daily clinical practice. It is necessary to better understand to what extent this perception is attributable to situational factors; for example, a lack of everyday familiarity with the instruments included in the algorithm.
PROPOSALS FOR EVOLUTION
As discussed above, the systemic treatment landscape in AD is evolving rapidly, and we consider that an evolution of the published consensus is necessary and potentially valuable. While the basic Delphi methodology was robust, there are a number of areas where improvements could be made. Firstly, the process should include contributions from more office-based dermatologists and more nurses and patients/patient representatives, with a broader international representation.
Considering the algorithm itself, we may assess the potential for the algorithm to be streamlined or simplified, if further evaluation suggests the need. The menu of assessment instruments will be reviewed: (i) for ease of use in daily clinical practice; (ii) to accommodate regional preferences (e.g. the common use of the Patient-Oriented Eczema Measure (POEM) in the UK); (iii) to provide potentially more valuable metrics (e.g. substitution of PtGA by PGADS or PGATE); (iv) to better reflect outcomes possible with currently available treatments; (v) to increase the value of the algorithm in lifelong disease management (e.g. by incorporation of the Atopic Dermatitis Control Test (ADCT) or Recap of Atopic Eczema (RECAP) to assess long-term disease control). A “treatable traits” approach may be helpful in guiding this review (9). The thresholds should be reviewed carefully, in terms of their definition (absolute vs relative) and their interchangeability, so that where alternatives are offered, they are equivalent. This evaluation should be supported by real-world data: it may be possible to collaborate with registries to acquire the necessary information. As part of this review, it may be useful to build a specific process for assessing treatment responses in patients transitioning from other systemics for reasons other than loss of efficacy. Safety assessments could be elaborated within the algorithm, considering the profiles of systemic treatments in daily clinical practice. Finally, we recognize the need to review the assessment time-points, to accommodate the need for assessment of disease control in long-term therapy.
In conclusion, a treat-to-target strategy offers the potential for disease management that optimizes treatment outcomes for the patient, although no supporting evidence currently exists in AD.
In conclusion, the previously published algorithm represents a solid foundation on which to build an even more robust and flexible tool for use in everyday clinical practice, which accommodates the expanding range of systemic treatments approved for AD. To this end, we will establish an extended steering committee to execute the recommendations discussed, so that a new consensus document can be produced.
ACKNOWLEDGEMENTS
Stephen McGrath (IntraMed Communications) provided support in manuscript development. Manuscript development was financially supported by Sanofi.
Conflicts of interest
MdB-W is co-principal investigator of the Dutch BioDay Registry and serves as a principal investigator in a number of multi-center clinical trials in atopic dermatitis sponsored by a wide range of pharmaceutical companies; has attended advisory boards and educational events sponsored by Sanofi, Regeneron, AbbVie and Galderma; has received institutional research grants from Sanofi; and has performed consultancy roles with Sanofi, Regeneron, LEO Pharma, Eli Lilly, AbbVie, Pfizer, Galderma and UCB, outside the submitted work. TB has received speaker and consultancy fees and has received institutional research grants from Alk-Abelló, Amgen, Almirall, Celgene, Galderma, LEO Pharma, Eli Lilly, Mylan, Novartis, Phadia-Thermo-Fisher, Regeneron, Sanofi-Genzyme, and Viatris outside the submitted work. RB is an employee and shareholder of Innovaderm Research; has received speaker and consultancy fees, and has received institutional research grants from AbbVie, Arcutis, Arena Pharma, Asana BioSciences, Bellus Health, Bluefin Biomedicine, BioMimetix, Boehringer-Ingelheim, Boston, Brickell, CARA Therapeutic, Clexio, Dermavant, Eli Lilly, EMD Serono, Evidera, Galderma, GlaxoSmithKline, Incyte, Inmagene Bio, Janssen, Kiniksa, Kyowa Kirin, LEO Pharma, Novan, Novartis, Pfizer, Ralexar, RAPT Therapeutic, Regeneron, Respivant, Sanofi, Sienna, Target RWE and Vyne Therapeutics. MD has received speaker fees, served as a consultant, and/or has received institutional research grants from AbbVie, Eli Lilly, Aslan Pharmaceuticals, Arena Pharmaceuticals, Incyte, LEO Pharma, Pfizer, La Roche Posay, Pierre Fabre, Regeneron Pharmaceuticals, Inc., and Sanofi, outside the submitted work. PF has attended advisory boards for AbbVie, Amgen, Aslan, Boehringer-Ingelheim, Bristol Myers Squibb, Celgene, Galderma, GSK, Janssen, LEO Pharma, Eli Lilly, Mayne Pharma, Merck, Novartis, Pfizer, Sanofi, Sun Pharma, UCB Pharma, and Valeant; has served as a consultant to Aslan, Bristol Myers Squibb, Galderma, GenesisCare, Hexima, Janssen, LEO Pharma, Eli Lilly, Mayne Pharma, MedImmune, Novartis, Pfizer, Roche, and UCB Pharma; has acted as an investigator in clinical trials for AbbVie, Akaal, Amgen, Argenx, Aslan, Boehringer Ingelheim, Celgene, Janssen, LEO Pharma, Eli Lilly, Merck, Novartis, Pfizer, Sun Pharma, UCB Pharma, Valeant, Astra Zeneca, BMS, Botanix, Celtaxsys, CSL, Cutanea, Dermira, Evelo, Galderma, Genentech, GSK, Hexima, Kymab, Regeneron Pharmaceuticals Inc., Roche, Teva, and Sanofi; has received institutional research grants from AbbVie, Amgen, Celgene, Janssen, Eli Lilly, Merck, Novartis, Pfizer, Sun Pharma, Galderma, and Sanofi; has received speaker fees or other honoraria from AbbVie, Amgen, Celgene, Janssen, LEO Pharma, Eli Lilly, Merck, Novartis, Pfizer, UCB Pharma, Valeant, Galderma, GSK, Roche, and Sanofi; and has received travel expenses for attendance at educational meetings from AbbVie, Janssen, LEO Pharma, Eli Lilly, Merck, Novartis, Pfizer, Sun Pharma, Galderma, Roche, and Sanofi, outside the submitted work. GG has received personal fees from AbbVie, Almirall, Amgen, Biogen, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Fresenius Kabi, Galderma, Genzyme, LEO Pharma, Novartis, Pfizer, Regeneron, Samsung and Sanofi. JH has attended advisory boards for Sanofi Genzyme, LEO Pharma and UCB; has acted as an investigator in clinical studies sponsored by Diasorin and Eli Lilly; and has received speaker fees from UCB, Janssen, Sanofi-Aventis, Eli Lilly, Novartis and AbbVie. C-HH has received honoraria as a speaker/consultant for AbbVie, Amgen, Bausch Health, Celgene, Eli Lilly, Galderma, Glaxo-Smith-Kline, Janssen, LEO Pharma, Merck, Novartis, Pfizer, Regeneron, Sanofi, and UCB; and has received grants as an investigator from AbbVie, Amgen, Bausch Health, Boehringer Ingelheim, Bristol Meyers Squibb, Celgene, Dermavant, Eli Lilly, Galderma, Glaxo-Smith-Kline, Incyte, Janssen, LEO Pharma, MedImmune, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi, and UCB, outside the submitted work. NK has received honoraria as a speaker/consultant for AbbVie, Celgene Japan, Janssen Pharma, Kyowa Kirin, LEO Pharma, Eli Lilly Japan, Maruho, Mitsubishi Tanabe Pharma, Sanofi, Taiho Pharmaceutical, and Torii Pharmaceutical, and has received grants as an investigator from AbbVie, A2 Healthcare, Boehringer Ingelheim Japan, Eisai, Janssen Pharma, Kyowa Kirin, LEO Pharma, Eli Lilly Japan, Maruho, Sun Pharma, Taiho Pharmaceutical, and Torii Pharmaceutical. AEP has acted as an advisor, speaker, investigator or received educational support from: Sanofi, AbbVie, Eli Lilly, Pfizer, Galderma, Novartis, Almirall, La-Roche Posay, LEO, UCB, BMS, Amgen, Celgene, Novartis, and Janssen. SS has received honoraria as a speaker/consultant for Sanofi and has received institutional research grants as an investigator from Sanofi, outside of submitted work. JS reports institutional grants for investigator-initiated research from the German GBA, the BMG, BMBF, EU, Federal State of Saxony, Novartis, Sanofi, ALK, and Pfizer. He also participated in advisory board meetings as a paid consultant for Sanofi, Eli Lilly, and ALK. JT is an advisor for AbbVie, Almirall, Arena Pharmaceuticals, Coloplast, OM Pharma, Aslan Pharmaceuticals, Union Therapeutics, Eli Lilly, LEO Pharma, Pfizer, Regeneron, and Sanofi-Genzyme, a speaker for AbbVie, Almirall, Eli Lilly, LEO Pharma, Pfizer, Regeneron, and Sanofi-Genzyme, and received research grants from Pfizer, Regeneron, and Sanofi-Genzyme. SW is the co-principal investigator of the German Atopic Eczema Registry (TREATgermany) and coordinator of the EU/IMI project BIOMAP (BIOMarkers in Atopic dermatitis and Psoriasis) projects and is an investigator in a number of clinical trials in atopic dermatitis and psoriasis sponsored by a wide range of pharmaceutical companies; has received institutional research grants from Sanofi, LEO Pharma and L’Oréal; has acted as a consultant for Sanofi, Regeneron, LEO Pharma, Eli Lilly, AbbVie, Pfizer, Incyte and Novartis; and has lectured at educational events sponsored by Sanofi, Regeneron, LEO Pharma, AbbVie and Galderma, outside of submitted work.
REFERENCES
- 1.Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol 2018; 32: 657–682. [DOI] [PubMed] [Google Scholar]
- 2.Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol 2018; 32: 850–878. [DOI] [PubMed] [Google Scholar]
- 3.De Bruin-Weller M, Biedermann T, Bissonnette R, Deleuran M, Foley P, Girolomoni G, et al. Treat-to-target in atopic dermatitis: an international consensus on a set of core decision points for systemic therapies. Acta Derm Venereol 2021; 101: adv00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larsen HH, Vittrup I, Ruge IF, Elberling J, Skov L, Ibler K, et al. Severe and ChRonic Atopic dermatitis Treatment CoHort (SCRATCH): a Danish real-world evidence atopic dermatitis treatment registry. Acta Derm Venereol 2022; 102: adv00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagel J, Nguyen TQ, Lima H, Jain N, Pariser DM, Hsu S, et al. Baseline Demographics and Severity and Burden of Atopic Dermatitis in Adult Patients Initiating Dupilumab Treatment in a Real-World Registry (PROSE). Dermatol Ther (Heidelb) 2022; 12: 1417–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegels D, Haufe E, Heinrich L, Werfel T, Weidinger S, Schmitt J; TREATgermany Study Group . Status report on the atopic dermatitis registry TREATgermany. Allergol Select 2021; 5: 274–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ariëns LFM, van der Schaft J, Spekhorst LS, Bakker DS, Romeijn GLE, Kouwenhoven TA, et al. Dupilumab shows long-term effectiveness in a large cohort of treatment-refractory atopic dermatitis patients in daily practice: 52-week results from the Dutch BioDay registry. J Am Acad Dermatol 2021; 84: 1000–1009. [DOI] [PubMed] [Google Scholar]
- 8.Abuabara K, Silverberg JI, Simpson EL, Paller AS, Eichenfield LF, Bissonnette R, et al. International observational atopic dermatitis cohort to follow natural history and treatment course: TARGET-DERM AD study design and rationale. BMJ Open 2020; 10: e039928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thyssen JP, Vestergaard C, Deleuran M, de Bruin-Weller MS, Bieber T, Taieb A, et al. European Task Force on Atopic Dermatitis (ETFAD): treatment targets and treatable traits in atopic dermatitis. J Eur Acad Dermatol Venereol 2020; 34: e839–e842. [DOI] [PubMed] [Google Scholar]