Abstract
Background
Transcatheter closure of perimembranous ventricular septal defect (VSD) is a promising alternative to surgical closure but has been associated with conduction disorders. Vascular access via multiple large vessels is associated with procedure-related complications, undermining the benefit of percutaneous approaches. In this case, we present the first-in-man transcatheter closure of a perimembranous VSD with an Amplatzer Duct Occluder IΙ in an adult patient via a single transradial artery access.
Case summary
A 62-year-old female was admitted to the hospital due to gradually worsening fatigue and shortness of breath on exertion. Transoesophageal echocardiogram (TOE) revealed a VSD size of 4–6 mm and a left ventricular ampulla size of 12 mm. A percutaneous VSD closure with the Amplatzer Duct Occluder II was decided. The angiography and TOE showed successful device placement and excellent procedural results. The patient was discharged home the next day after the procedure. The patient did not report any post-procedural complications during the 8-month follow-up. Echocardiographic assessment showed a gradual decrease in left ventricular dimensions.
Discussion
Transcatheter closure of perimembranous VSD is a promising alternative to surgical closure, but it is not free of complications. Traditional VSD occluders rely on multivessel access and complex formation of arteriovenous loops. In this case, we report the feasibility of perimembranous VSD closure with an Amplatzer Duct Occluder IΙ via a single radial artery access in an adult patient. This approach is a much simpler technique with several potential advantages and should be considered in selected adult patients and in similar clinical scenarios.
Keywords: Perimembranous ventricular septal defect, Amplatzer Duct Occluder II, Single transradial access
Learning points.
Even though percutaneous closure is a promising alternative to surgical intervention for ventricular septal defect (VSD) closure, conduction disorders and procedure-related complications from conventional perimembranous VSD occluders are not infrequent.
Transcatheter closure of a perimembranous VSD in adult patients, using an Amplatzer Duct Occluder IΙ and a single transradial artery access is a feasible technique.
Improved post-procedural outcomes using transradial artery approaches may provide a promising procedural alternative.
Introduction
Surgical closure had been accepted as the standard treatment for perimembranous ventricular septal defect (VSD), while percutaneous closure has emerged as a promising alternative.1 However, transcatheter VSD closure has been associated with conduction disorders, generating concerns regarding the benefit of the percutaneous approach over surgery.2 Meanwhile, procedure-related complications urge a viable solution over the traditional approaches that opt for vascular access via multiple large vessels.3 We report the first-in-man perimembranous VSD closure with an Amplatzer Duct Occluder IΙ in an adult patient performed through a single radial artery access.
Timeline
| Date | Event |
|---|---|
| 1 week prior to hospitalization |
|
| Hospital admission |
|
| |
| Hospitalization day 2 |
|
| Hospitalization day 3 |
|
| |
| 8-Month follow-up |
|
|
Case presentation
A 62-year-old female was admitted to the hospital due to gradually worsening fatigue, shortness of breath on exertion, and frequent palpitations. Vital signs were normal, and clinical examination did not reveal any significant findings. The patient had a history of perimembranous VSD with a membranous septum aneurysm, arterial hypertension, and occasional premature ventricular contractions. Recent echocardiography revealed that left ventricular end-diastolic and end-systolic diameters had increased compared to prior examinations. The patient had mild aortic and mitral regurgitation, with no aortic cusp prolapse.
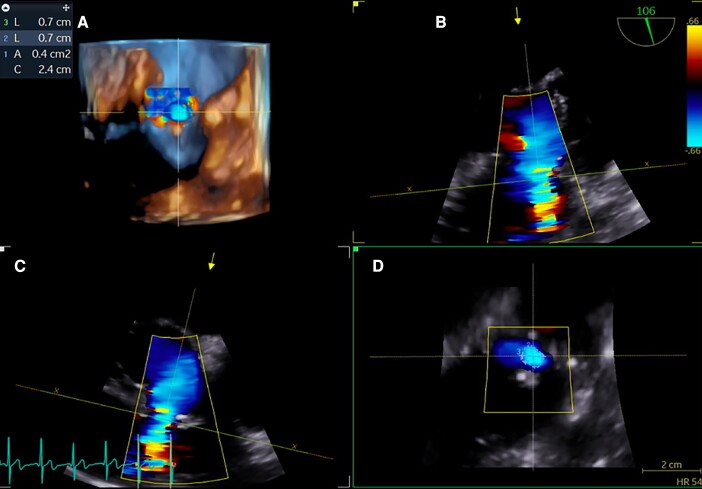
Transoesophageal echocardiogram (TOE) revealed a VSD size of 4–6 mm and a left ventricular ampulla size of 12 mm (Figure 1). The Qp/Qs ratio was 1.5 after complete heart catheterization. Pulmonary vascular resistances were within normal range. In view of the new left ventricular morphology, deteriorating symptoms and TOE findings, a percutaneous VSD closure with the Amplatzer Duct Occluder II was decided (Figure 2).
Figure 1.
TOE imaging of the VSD. Three-dimensional TOE reconstruction (A). Colour flow Doppler of the septum (90° view, B and 106° view, C). En-face view of the defect (D). TOE, transoesophageal echocardiography; VSD, ventricular septal defect.
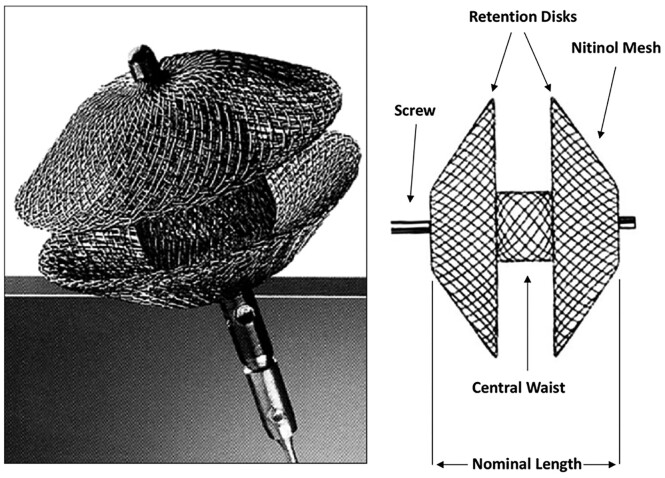
Figure 2.
Design of an Amplatzer Duct Occluder II. The device has a self-expanding nitinol mesh device and two retention discs positioned on either side of the duct, connected by the waist.
The operation was performed under general anaesthesia and TOE guidance. Intravenous low molecular weight heparin (7000 IU) was administered, aiming for an activated clotting time >250 s. A single left radial access was performed. Left cardiac ventriculography was performed with a 5 French pigtail catheter (Figure 3A) and, selectively, with a 6 French JR4 diagnostic catheter to confirm the VSD dimensions. Under fluoroscopic guidance, the VSD was crossed using the JR4 catheter and an angled hydrophilic 0.035 inch, 260 cm glidewire (Terumo, Somerset, NJ). The latter was advanced in the pulmonary artery. The JR4 catheter was then advanced over the wire in the right ventricular outflow tract. A 6/4 mm Amplatzer Duct Occluder II (St. Jude Medical, St. Paul, Minnesota Amplatzer) was deployed under angiographic and TOE guidance (Figure 3B). The angiography and TOE showed successful device placement and excellent procedural results, with almost complete VSD occlusion (Figures 3D, 4B and C). Pre-device release imaging revealed no disruption of the aortic valve, no aortic regurgitation, and only minimal residual shunt. No heart block or other conduction disturbances were observed. After performing a vigorous stability test (‘tug test’), the device was released (Figure 3C). The left radial sheath was removed, and the puncture site was closed uneventfully, without haematomas or other complications. The patient was extubated and fully recovered. Post-procedural TOE revealed a well-seated Amplatzer Duct Occluder II device with excellent alignment to the ventricular septum and no signs of residual shunt, thrombosis, or pericardial fusion (Figure 4D). The patient was discharged home the next day after the procedure. The patient did not report any post-procedural complications during the 8-month follow-up. Echocardiographic assessment showed gradual decrease of left ventricular dimensions. Specifically, the end-diastolic LV diameter was 5.9 cm at pre-procedural echocardiographic assessment. At 3 and 8 months after the procedure, the end-diastolic LV diameter was 5.6 and 5.4 cm, respectively. At the 3-month echocardiographic assessment, a small residual leak with a peak gradient of 83 mmHg was reported. At the 8-month assessment, a residual leak was still visible but so small that a flow gradient could not be acquired.
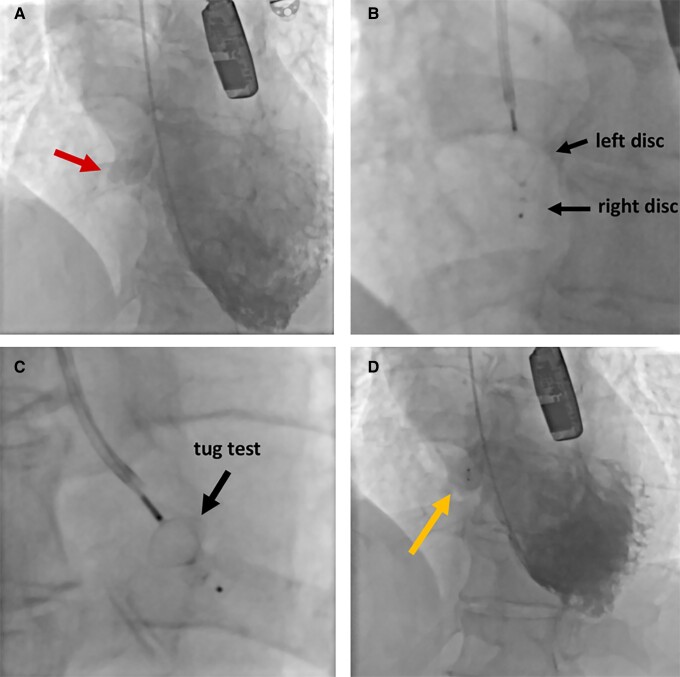
Figure 3.
Angiographic images of perimembranous VSD closure with the Amplatzer Duct Occluder II. Pre-implantation left ventriculography showing the VSD (arrow) (A). Left disc following right disc deployment (B). Stability test (‘tug’ test) after complete device deployment (C). Left ventriculography after device release showing minor leak (arrow) (D). VSD, ventricular septal defect.
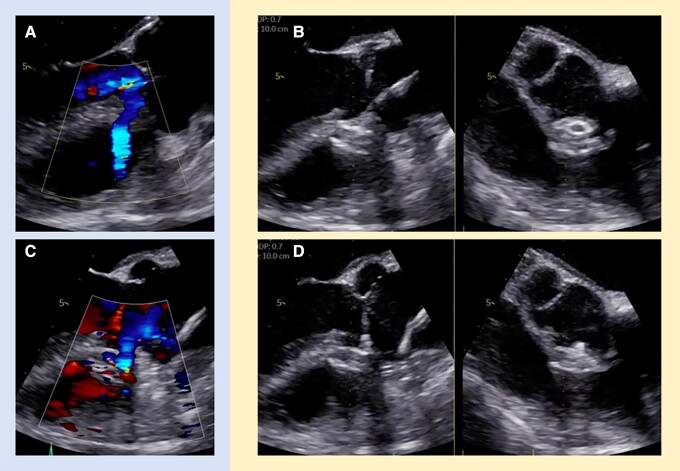
Figure 4.
TOE imaging of perimembranous VSD closure with the Amplatzer Duct Occluder II. Colour flow Doppler before (A) and after (C) device implantation. TOE before (B) and after (D) device release. TOE, transoesophageal echocardiography; VSD, ventricular septal defect.
Discussion
Complications of percutaneous ventricular septal defect closure
Percutaneous approaches using conventional perimembranous VSD occluders have not been accepted yet as the gold standard of VSD closure due to the associated high rates of complete heart block, either early or late, that may become persistent and require permanent pacemaker insertion.3,4 They may be caused by fibrosis, compression, or inflammation of the conduction system, as well as due to direct periprocedural mechanical trauma by the device delivery system.4 Indeed, the conventional perimembranous VSD occluders can cause damage to the ventricular septum and the adjacent structures due to their large delivery systems, and their high stiffness and clamp force resulting in high radial stress.5
Conventional vs. off-label device use for percutaneous ventricular septal defect closure approach
The traditional approach to percutaneous VSD closure involves primary access via a central vein to load the closure device and secondary access via an artery to capture the guide wire. Hence, the defect is crossed in a retrograde fashion from the left to the right ventricle, and the guide wire is snared in the venous system to make an arteriovenous loop.6 Double-disc devices such as the Amplatzer Membranous VSD Occluders (St. Jude Medical) are mainly used for perimembranous VSD closure.7 However, the use of off-label devices, such as the Amplatzer Duct Occluder II, has shown very promising results lately by significantly decreasing complication rates.8 The ease of its implantation could reduce complications by reducing clamp force, tissue trauma, and radial stress to the ventricular septum and adjacent structures. However, VSDs >6.5 mm and a distance <3 mm between the aortic valve and the upper VSD margin are two limitations for Amplatzer Duct Occluder II implantation.8
Percutaneous ventricular septal defect closure through transradial artery access
Although approaches through the femoral artery, femoral vein, of internal jugular vein access are arguably safe, access-related complications still exist, ranging from 2% to 2.5%.3 Alternatively, radial access bears many advantages over traditional approaches, such as lower access-related complications, improved morbidity, overwhelming patient preference, reduced procedural cost, and early ambulation.9
In the present paper, we reported the first-in-man transcatheter closure of a perimembranous VSD with an Amplatzer Duct Occluder IΙ through a single transradial artery access in an adult patient. We believe that the use of a duct occluder over conventional VSD occluders, combined with adoption of a single radial artery access instead of the traditional multivessel approach, could offer significant procedural benefit. Our approach has only been reported once in the past using both an Amplatzer Duct Occluder IΙ and a single transradial artery access in an adolescent patient, showing great procedural results.10
Conclusions
Percutaneous closure of perimembranous VSD is a promising alternative to surgical closure, but it is not free of complications, mainly attributed to conduction system disturbances. Conventional VSD occluders rely on multivessel access and complex formation of arteriovenous loops. In our case, we highlight the feasibility of perimembranous VSD closure with an Amplatzer Duct Occluder IΙ via a single radial artery access in an adult patient. This approach is a much simpler technique with several potential advantages and should be considered in selected adult patients and in similar clinical scenarios.
Supplementary Material
Acknowledgements
None.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient, in line with the COPE guidance.
Funding: None declared.
Contributor Information
Athanasios Samaras, Department of Cardiology, AHEPA University Hospital, St. Kiriakidi 1, 54636 Thessaloniki, Greece.
Konstantinos Papadopoulos, Department of Cardiology, Interbalkan European Medical Center, Asklipiou 10, Pylaia, 55535 Thessaloniki, Greece.
George Giannakoulas, Department of Cardiology, AHEPA University Hospital, St. Kiriakidi 1, 54636 Thessaloniki, Greece.
Apostolos Tzikas, Department of Cardiology, Interbalkan European Medical Center, Asklipiou 10, Pylaia, 55535 Thessaloniki, Greece.
Lead Author biography
 Athanasios Samaras, MD, graduated from the Medical School of the Aristotle University of Thessaloniki, where he is currently a PhD candidate and, also, an MSc student in Health Statistics & Data Analytics. He completed a research fellowship at the Cardiology Department of Aarhus University Hospital, Denmark. He is currently working as a Cardiology Resident in Thessaloniki, Greece. His research areas of interest are interventional cardiology, digital health, artificial intelligence, and data analytics.
Athanasios Samaras, MD, graduated from the Medical School of the Aristotle University of Thessaloniki, where he is currently a PhD candidate and, also, an MSc student in Health Statistics & Data Analytics. He completed a research fellowship at the Cardiology Department of Aarhus University Hospital, Denmark. He is currently working as a Cardiology Resident in Thessaloniki, Greece. His research areas of interest are interventional cardiology, digital health, artificial intelligence, and data analytics.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Fu YC, Bass J, Amin Z, Radtke W, Radtke W, Cheatham JP, et al. Transcatheter closure of perimembranous ventricular septal defects using the new Amplatzer membranous VSD occluder: results of the U.S. phase I trial. J Am Coll Cardiol 2006;47:319–325. [DOI] [PubMed] [Google Scholar]
- 2. Sullivan ID. Transcatheter closure of perimembranous ventricular septal defect: is the risk of heart block too high a price? Heart 2007;93:284–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang R, Kong XQ, Sheng YH, Zhou L, Xu D, Yong YH, et al. Risk factors and outcomes of post-procedure heart blocks after transcatheter device closure of perimembranous ventricular septal defect. JACC Cardiovasc Interv 2012;5:422–427. [DOI] [PubMed] [Google Scholar]
- 4. Butera G, Carminati M, Chessa M, Piazza L, Micheletti A, Negura DG, et al. Transcatheter closure of perimembranous ventricular septal defects: early and long-term results. J Am Coll Cardiol 2007;50:1189–1195. [DOI] [PubMed] [Google Scholar]
- 5. Chungsomprasong P, Durongpisitkul K, Vijarnsorn C, Soongswang J, Lê TP. The results of transcatheter closure of VSD using Amplatzer® device and Nit Occlud® Lê coil. Catheter Cardiovasc Interv 2011;78:1032–1040. [DOI] [PubMed] [Google Scholar]
- 6. Pineda AM, Mihos CG, Singla S, Santana O, Rhodes J >3rd, Sommer RJ, et al. Percutaneous closure of intracardiac defects in adults: state of the art. J. Invasive Cardiol 2015;27:561–572. [PubMed] [Google Scholar]
- 7. Tzikas A, Ibrahim R, Velasco-Sanchez D, Freixa X, Alburquenque M, Khairy P, et al. Transcatheter closure of perimembranous ventricular septal defect with the Amplatzer(®) membranous VSD occluder 2: initial world experience and one-year follow-up. Catheter Cardiovasc Interv 2014;83:571–580. [DOI] [PubMed] [Google Scholar]
- 8. Koneti NR, Penumatsa RR, Kanchi V, Arramraj SK SJ, Bhupathiraju S. Retrograde transcatheter closure of ventricular septal defects in children using the Amplatzer Duct Occluder II. Catheter and Cardiovasc Interv 2011;77:252–259. [DOI] [PubMed] [Google Scholar]
- 9. Hamon M, Pristipino C, Di Mario C, Nolan J, Ludwig J, Tubaro M, et al. Consensus document on the radial approach in percutaneous cardiovascular interventions: position paper by the European Association of Percutaneous Cardiovascular Interventions and working groups on acute cardiac care and thrombosis of the European Society of Cardiology. Eurointervention 2013;8:1243–1251. [DOI] [PubMed] [Google Scholar]
- 10. Podhar AM, Pahwa JS, Patil DV, Phatarpekar A. Single access transcatheter ventricular septal defect closure through trans radial approach. J Cardiol Clin Res 2016;4:1089. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.