SUMMARY
Signals from the surrounding niche drive proliferation and suppress differentiation of intestinal stem cells (ISCs) at the bottom of intestinal crypts. Among sub-epithelial support cells, deep sub-cryptal CD81+ PDGFRAlo trophocytes capably sustain ISC functions ex vivo. Here we show that mRNA and chromatin profiles of abundant CD81− PDGFRAlo mouse stromal cells resemble those of trophocytes and that both populations provide crucial canonical Wnt ligands. Mesenchymal expression of key ISC-supportive factors extends along a spatial and molecular continuum from trophocytes into peri-cryptal CD81− CD55hi cells, which mimic trophocyte activity in organoid co-cultures. Graded expression of essential niche factors is not cell-autonomous but dictated by the distance from bone morphogenetic protein (BMP)-secreting PDGFRAhi myofibroblast aggregates. BMP signaling inhibits ISC-trophic genes in PDGFRAlo cells near high crypt tiers; that suppression is relieved in stromal cells near and below the crypt base, including trophocytes. Cell distances thus underlie a self-organized and polar ISC niche.
Keywords: Mesenchymal niche compartments, intestinal crypt structure and function, colonic crypts, intestinal Wnt source
eTOC
Shivdasani et al assign niche functions to specific mesenchymal cell types near stem cells at the base of intestinal crypts. They identify PDGFRAlo fibroblasts as a substantial source of requisite Wnt ligands and show that BMP signaling from crypt tops organizes functional heterogeneity of mesenchyme along the crypt vertical axis.
Graphical Abstract
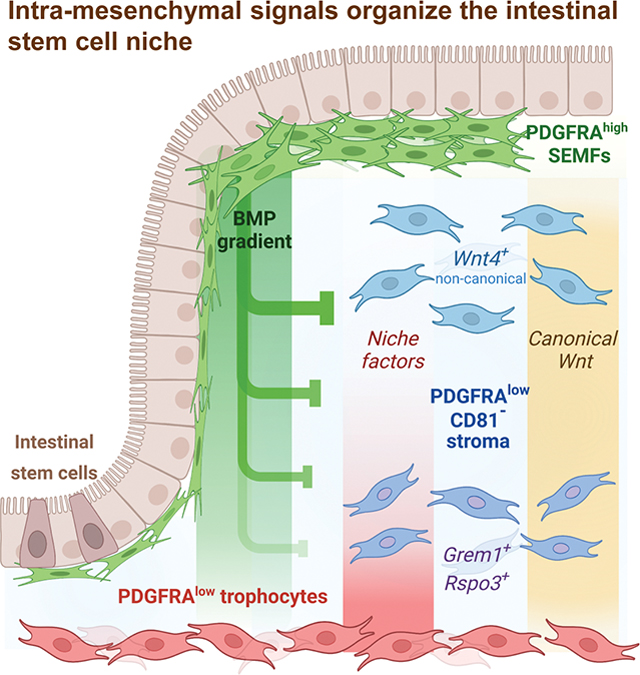
INTRODUCTION
Lgr5+ intestinal stem cells (ISCs) self-renew at the bottom of intestinal crypts, while their progeny proliferate and differentiate in higher crypt tiers;1,2 epithelial 3D organoids replicate these polar functions. Canonical Wnt signaling and bone morphogenetic protein inhibitors (BMPi) underlie intestinal tumors,3 reinforcing the dependence of proliferating cells on canonical Wnt/R-spondin (Rspo) signaling and BMPi (Refs. 4–6). However, the extent and determinants of crypt polarity are incompletely understood. Epithelial Paneth cells, which lie near ISCs, express canonical Wnt3 (Ref. 7) but ISCs are intact in the absence of Paneth cells8,9 and the sub-epithelial mesenchyme likely dominates in sustaining ISCs and crypt polarity.10
Small intestine (SI) mesenchyme, once regarded as a loose collection of “myofibroblasts,”11,12 is an organized tissue with distinct cell types that express multiple Wnt/Rspo and BMP agonists and antagonists.13–18 Foxl1+ Pdgfrahi sub-epithelial myofibroblasts (SEMFs, also called telocytes) envelop all crypts and villi. SEMFs are proposed as a crucial Wnt source,19,20 but they concentrate near SI crypt tops, express abundant BMPs and only non-canonical Wnts, and fail to support ISCs in culture.17 In contrast, Pdgfralo CD34+ stromal cells support ISCs in vitro.13–15,21 One spatially segregated subpopulation, sub-cryptal CD81+ trophocytes that express Rspo3 and the BMPi Grem1, uniquely expands ISCs in the absence of exogenous factors.22 SEMFs and trophocytes hence provide opposing cues for ISC self-renewal at the bottom and BMP-mediated epithelial differentiation at the top of SI crypts (Figure 1A).
Figure 1. Relationship of mesenchymal cell populations across the intestinal length.
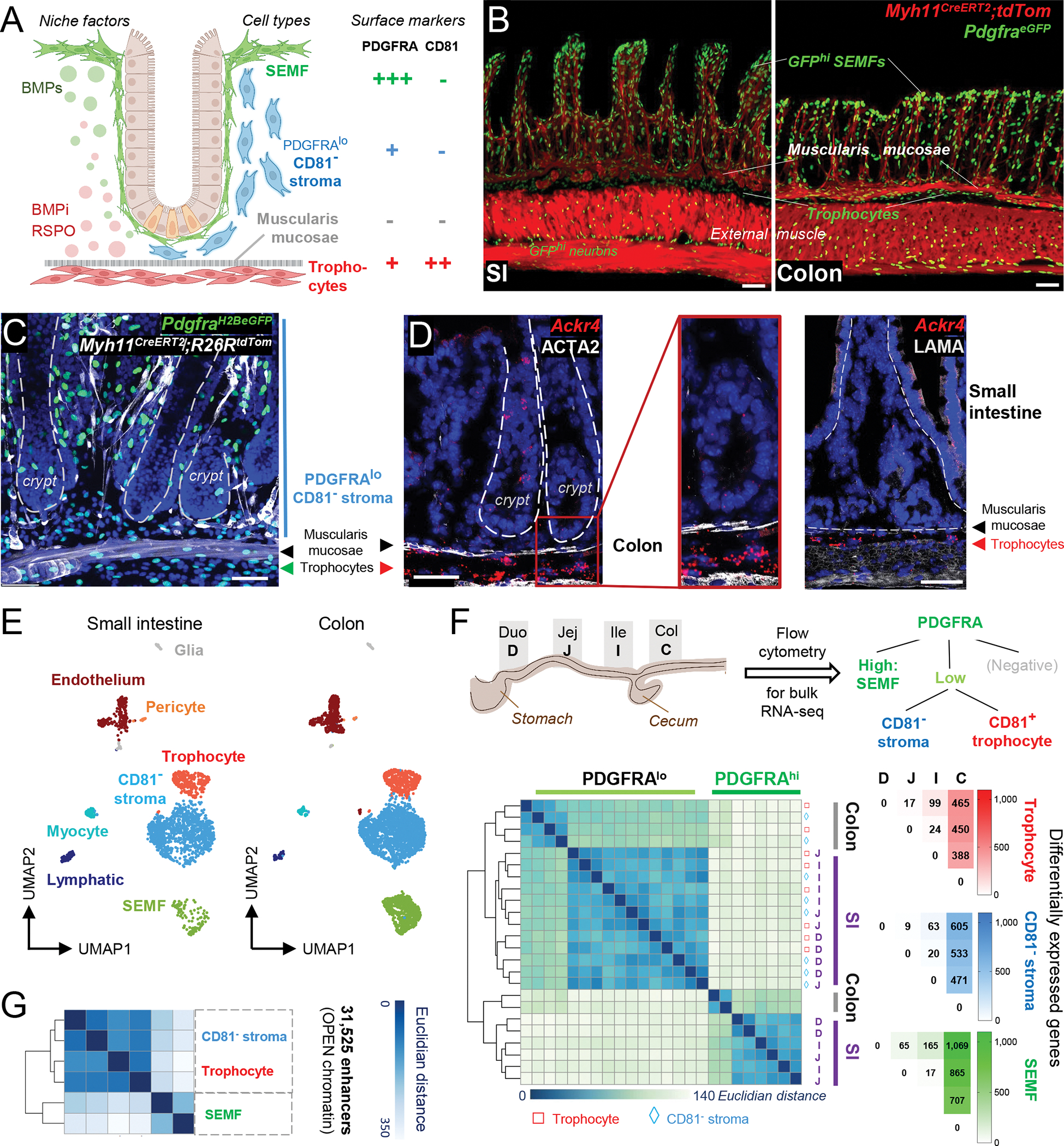
A) Cell populations and signaling in SI crypts. BMPi provided by sub-cryptal trophocytes (red, PDGFRAlo CD81+) oppose agonists provided by dense SEMF (green, PDGFRAhi) aggregates at the crypt-villus junction. The function of supra-muscularis PDGFRAlo cells (blue), which lack CD81 expression, is unknown and is the topic of this study.
B) Confocal images of Myh11CreERT2;R26RLSL-tdTom;PdgfraH2BeGFP mouse ileum (SI, left, 24 μm z-stack) and colon (right, 15 μm z-stack). Scale bars 50 μm.
C) Left, whole-mount confocal micrograph (26-μm z-stack) of colonic tissue from PdgfraH2B-eGFP;Myh11CreERT2;R26RtdTom mice. White: Myh11Cre-marked smooth muscle, green: GFP+ nuclei, blue (DAPI): cell nuclei. Dashed lines demarcate crypts. Scale bar 50 μm.
D) In situ hybridization for trophocyte marker Ackr4 (red) in colonic crypts counterstained with ACTA2 antibody (white). Rare epithelial Ackr4+ cells are tuft cells. Dashed lines demarcate crypts. Scale bars 50 μm.
E) scRNAseq-derived UMAP clustering of unfractionated SI (n=2,042)17 and colonic (n=3,940) EPCAM− CD45− mesenchymal cell types identified using known cell-specific markers (Figure S1B).
F) Differential PDGFRA and CD81 expression allow purification of 3 dominant PDGFRA+ cell types from each intestinal segment: duodenum (D), jejunum (J), ileum (I), and colon (C). Left, Unsupervised hierarchical clustering of duplicate bulk RNA-seq profiles of SEMFs (green), trophocytes (red), and CD81− stromal cells (blue) from each intestinal segment; the latter two populations cluster together. Color scale: Euclidean distance of samples. Right, Numbers of differentially expressed genes (Padj <0.01, log2 fold-difference >|1.5|) in each cell type and intestinal segment.
G) Hierarchical clustering of duplicate PDGFRA+ cell samples from whole SI by similarities in open chromatin reads (ATAC-Seq) at the union set of 31,525 enhancers (> −1 kb, > 2 kb from transcription start sites). Color scale: Euclidean distance.
See also Figure S1.
In this model, Pdgfralo cells other than trophocytes appear possibly inert because they support organoid growth far less robustly.17 However, the stromal CD81− fraction outnumbers CD81+ trophocytes and together the two cell types are the major source of canonical Wnt ligands. Here we report that both Pdgfralo subpopulations harbor supportive activity in the SI and colon, together constituting a finely graded and self-organized niche. Spatial and molecular distinction of Cd81− Pdgfralo cells, coupled with reliable ex vivo assays for ISC support, reveal this activity to include both functional Wnt signaling and extension of ISC-sustaining signals beyond trophocytes, into the immediate ISC vicinity. Intra-mesenchymal BMP signaling is prominent among a likely range of cues to generate diversity among Pdgfralo cells, driving functional polarity of the peri-cryptal ISC niche.
RESULTS
Distinction and fundamental similarity of intestinal Pdgfralo subpopulations
Across independent studies,17,23 three cell populations dominate in the mouse SI mesenchyme (Figure 1A): BMP-secreting PDGFRAhi SEMFs embedded in the basement membrane; abundant scattered sub-epithelial PDGFRAlo cells of unknown function; and PDGFRAlo trophocytes, which lie beneath the muscularis mucosae and express Ackr4 (Ref. 24), CD81, BMPi, and RSPOs.17,22 GFP+ cells in the adult PdgfraeGFP mouse colon are similarly distributed.25 GFPhi (PDGFRAhi) SEMFs envelop each crypt (Figure 1B–C), GFPlo Ackr4+ trophocytes localize under the muscularis mucosae, and additional GFPlo Ackr4− cells are present throughout the stroma (Figure 1C–D), interspersed between Myh11+ lamina propria myocytes and CD31+ blood vessels (Figure S1A). Beyond these anatomic features shared with the SI, graph-based cell clustering26 of single-cell (sc) RNA-seq data from colonic CD45− EPCAM− mesenchyme (anchored by known molecular markers – Figure S1B) revealed identical population structures in the colon and SI, with CD81− PDGFRAlo stromal cells abundant in both organs (Figures 1E and S1C). Because scRNA data might conceal regional differences, we profiled mRNAs in purified bulk GFP+ cell fractions from adult PdgfraeGFP mouse colon and distinct SI segments. Dividing the SI into 5 equal parts, we regard the first 1/5 as duodenum, the 3rd of 5 pieces as jejunum, the most distal 1/5 as ileum, and ~3 cm distal to the cecum as ascending colon (Figure 1F). As with their SI counterparts, flow cytometry readily separated colonic CD81+ trophocytes from SEMFs and other PDGFRAlo (GFPlo) cells (Figure S1D). Overall, flow cytometry for Pdgfra+ (GFP+) cells in this study yielded ~23% GFPhi SEMFs and ~77% GFPlo cells in both SI and colon (Figure S1E). Bulk RNA-seq data from duplicate samples isolated from each intestinal segment were highly concordant (Figure S1F), marker genes confirmed accurate cell purification (Figure S1G), and Hox gene expression varied along the rostro-caudal axis as expected (Figure S1H). Lower difference in Cd81 mRNA levels between colonic and SI PDGFRAlo cells did not compromise separation of colonic CD81− stromal cells from CD81+ trophocytes by flow cytometry (Figure S1D).
Unsupervised hierarchical analysis of bulk transcriptomes first clustered SEMFs apart from PDGFRAlo cells, irrespective of intestinal segment, followed by distinction of colonic from SI cell types (Figure 1F). SEMFs showed substantial segmental variation (Padj <0.01, log2 fold-difference >1.5), whereas PDGFRAlo populations (CD81+ or CD81−) from different SI segments showed few differences (Figure 1F and Table S1). Moreover, although CD81+ trophocytes are superior to CD81− PDGFRAlo cells in supporting ISCs in organoid assays22 and the two cell types cluster separately in scRNA analysis (Figure 1E), mRNA differences between them are small in any segment (Table S1). We verified this similarity using the assay for transposase-accessible chromatin (ATAC-seq), which distinguishes cell types accurately and identifies loci poised for transcription.27,28 Among cis-regulatory elements accessible in purified SI mesenchymal cells, 77% of 13,749 promoters and 21% of 31,525 enhancers (≥−1 kb or ≥2 kb from transcription start sites) were open in all three PDGFRA+ populations. At the remaining accessible sites, chromatin was differentially open or closed in SEMFs but at least 94% of enhancers open in trophocytes were also open in CD81− cells (Figures 1G and S2A). Chromatin was accessible at the same sites in both PDGFRAlo cell fractions at loci where mRNA levels robustly distinguish the two, such as Ackr4 and Cd81, and at loci expressed equally in both cell types, such as Sfrp1 (Figure S2A–B). Thus, whereas PDGFRAhi and PDGFRAlo cells differ in several ways, including ISC support,17 PDGFRAlo subsets are similar and key genes that distinguish the two PDGFRAlo cell fractions may be controlled by extrinsic factors that operate differently above and below the muscularis mucosae.
CD81− PDGFRAlo stromal cells are a key source of canonical Wnts
Among secreted factors implicated in ISC self-renewal, canonical Wnts are paramount29,30 and ISCs depend critically on a mesenchymal source of ligands.31 Two findings merit note. First, expression of Wnt-modulating genes varies across cell types and regions. Wif1 is expressed only in SEMFs, while Wnt-sequestering factors expressed in PDGFRAlo cells, such as Sfrp1 and Frzb, decrease along the rostro-caudal axis. Other antagonists, such as Dkk2 and Apcdd1,32 are absent in the colon, while Wls, which is required for Wnt secretion33,34 and expressed in all PDGFRA+ cells, is highest in that segment (Figure S2C). Although many factors determine tissue Wnt signaling, these mRNA findings point to a higher Wnt “tone” in the colon than in the SI. Second, among all 19 Wnt genes, bulk and scRNA-seq data identified only canonical Wnt2, Wnt2b and Wnt9a and non-canonical Wnt4, Wnt5a and Wnt5b in any PDGFRA+ cell type (Figures S2D–E). Wnt2 is expressed mainly in SI lymphatic vessels35–37 and at low levels in colonic PDGFRAlo cells (Figure S2D). The most prevalent canonical ligand, Wnt2b, is 10–15 times more abundant in PDGFRAlo cells in all intestinal segments than in SEMFs, and its locus is accessible in PDGFRAlo cells but not in SEMFs; conversely, the most prevalent non-canonical ligand, Wnt5a, is 8–9 times more abundant in SEMFs (Figures S2D and S3A). This distribution of Wnt ligand transcripts contradicts the view that SEMFs are a critical source of canonical Wnt ligands.19
That view rests in part on the finding that disruption of Porcn, a gene required for secretion of all Wnts,38 in SEMFs triggered ISC and crypt failure.19 As Cre recombinase activity in the study was driven by an ostensibly SEMF-specific Foxl1 transgene that is vulnerable to silencing, the authors generated mice with a tandem Cre-tdTomato cassette knocked in to the Foxl1 locus and found tdTomato principally in villus tip SEMFs.39 Our examination of Foxl1Cre-tdTomato mouse colon also revealed tdTomato only in SEMFs at crypt apices, with rare recombination of the reporter gene Rosa26YFP (Figure S3B); flow cytometry identified ~1% tdTomato+ cells (Figure S3C). We therefore evaluated PdgfraCreERT2 mice,40 crossing them first with Rosa26mG/mT reporter mice41 so that tamoxifen would convert baseline red membrane fluorescence in all cells into membrane GFP in Cre+ cells (Figure S3D). Flow cytometry revealed GFP in 13% of mesenchymal cells, about half of which (6%) expressed CD34, a marker specific to Pdgfralo cells, and the other half represented CD34− SEMFs (Figure S3E, see also Figures S1B and S1G). Based on cell fractions in CD34 flow cytometry of PdgfraH2BeGFP mesenchyme (Figure S3E), we conclude that PdgfraCreERT2 marks the majority of SEMFs and a considerably smaller fraction of Pdgfralo cells. Indeed, tamoxifen elicited robust GFP signals in SI and colonic SEMFs, notably including those at the crypt base; scattered Pdgfralo cells also turned green, but the majority did not (Figures 2A and S3D). Ten days after the last of 5 daily injections of tamoxifen, PdgfraCreERT2 mice crossed with the PorcnFl strain42 (Porcn-null) showed Porcn gene disruption in cells isolated by PDGFRA+ flow cytometry, but no overt intestinal histopathology or defects in crypt cell replication and expression of ISC or Wnt target genes (Figures 2B–C and S3F). Although a small fraction of SEMFs may have evaded Porcn deletion, these findings coupled with their negligible levels of canonical Wnt transcripts argue against SEMFs as a critical Wnt source. Conversely, intestinal PDGFRAlo cells express canonical Wnts and largely resist perturbation in PdgfraCreERT2 mice, suggesting that they are the predominant source.
Figure 2. PDGFRAlo intestinal cells are a key source of canonical Wnts.
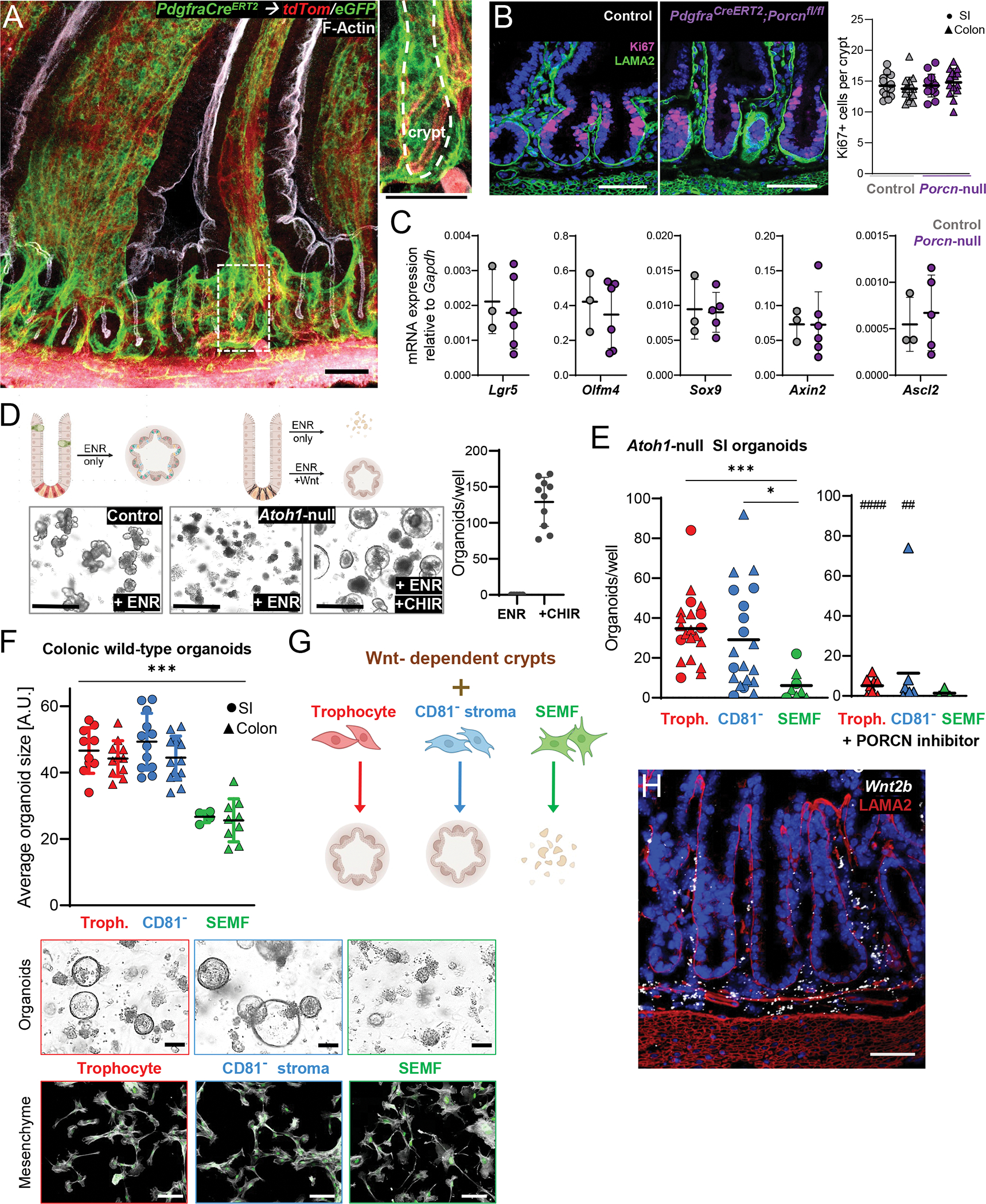
A) Whole-mount images of SI from PdgfraCreERT2;R26RmT/mG mice, where Cre-expressing cells convert ubiquitous tdTom fluorescence (red) to eGFP signal (green), counterstained for F-Actin (phalloidin, white). 30 μm z-stack overlay. The dashed box is enlarged to the right and the crypt in that image is marked with a dashed white line. Scale bars 50 μm.
B) Ki67 immunofluorescence (magenta) in control (left) and PdgfraCreERT2;Porcnfl/fl mouse (Porcn-null) SI after 5 daily tamoxifen injections and 10 days recovery, counterstained for laminin (LAMA2, green) and nuclei (DAPI, blue). Representative images and quantitation of Ki67+ stained cells in SI (circles) and colonic (triangles) crypts from n=4 (control) and n=6 (Porcn-null) animals. At least 10 crypts per sample counted. Lines indicate Mean ±SD.
C) mRNA expression of ISC-associated marker genes in crypt epithelium isolated from control (left, n=3) and Porcn-null (right, n=6) mice 10 days after 5 daily tamoxifen injections, measured by quantitative real-time PCR and expressed relative to Gapdh levels (2− ΔΔCt). Lines indicate Mean ±SD. Additional makers are shown in Figure S3F.
D) Exogenous Wnt requirement for organoid growth from Atoh1-null SI crypts. Representative brightfield images of organoids from control (Atoh1fl/fl) or Atoh1-null (Vil1-CreERT2;Atoh1fl/fl) SI crypts grown in ENR medium only or with the GSK3 inhibitor CHIR99021 (CHIR). Scale bar 200 μm. Right, Atoh1-null organoid numbers per 10 μl Matrigel droplet on the 4th day of culture. Each dot represents one sample, lines indicate Mean ±SD, n=4 independent experiments.
E) Organoid counts from Atoh1-null SI crypts cultured in ENR with duodenal (circles) or colonic (triangles) mesenchymal cells (10,000 cells per 10 μl Matrigel droplet) 4 days after plating without (left graph) or with (right graph) 100 nM Wnt-C59, a Porcupine inhibitor. Each dot represents one sample, lines indicate mean counts from 5 independent experiments. Kruskal-Wallis ANOVA, SEMFs vs. trophocytes Padj 0.0004 (***); SEMF vs. CD81− stroma cells Padj 0.0016 (*). Comparisons of the same cell type with or without Wnt-C59 were made using the Mann-Whitney test, Padj 0.008 (##), Padj <0.0001 (###).
F) Organoid size 4 days after wild-type colonic crypts were co-cultured with mesenchymal cells (2,000 cells per 10 μl Matrigel droplet in ENR medium) isolated from SI (circles) or colon (triangles). Values are in arbitrary units (A.U.) and lines indicate Mean ±SD. Padj <0.0005 (***) for any trophocyte or CD81− stromal cell sample vs. any SEMF sample (one-way ANOVA, Tukey- corrected). Micrographs: Top row, representative bright-field images of organoids from colonic crypts cultured with the indicated colonic mesenchymal cell type. Bottom row, corresponding mesenchymal cells on day 4 of co-culture, showing PdgfraeGFP expression (green) and F-Actin (phalloidin, grey) counterstain as indicators of cell viability. Scale bars 50μm.
G) Summary of experimental results from panels E and F.
H) Wnt2b in situ hybridization (white) in colon tissue sections counterstained with laminin antibody (LAMA2, red) and DAPI (blue). Scale bar 50 μm. Representative image from n= 4 mice. Additional images are shown in Figure S4F.
See also Figures S2–S4.
We used Wnt-dependent functional assays to test this possibility in vitro. Wnt3+ Paneth cells, which lie near ISCs in vivo, enable organoid growth from SI crypts or isolated ISC-Paneth doublets in the absence of supplemental Wnt.7 In contrast, organoid growth from Atoh1-null crypts, which lack Paneth cells,8,43,44 or from colonic crypts requires exogenous Wnt9 or pharmacologic activation of the Wnt pathway with, for example, glycogen synthase kinase 3 inhibitor CHIR99021 (CHIR). Indeed, Paneth cell-depleted crypts isolated from Vil1-CreERT2;Atoh1fl/fl mice (Atoh1-null, Figure S4A) and cultured in EGF, Noggin, and RSPO1 (ENR medium)4 required CHIR to spawn organoids (Figure 2D). Substituting CHIR with purified SI or colonic CD81− stromal cells or trophocytes potently rescued organoid formation in ENR medium, while SEMFs from either location did not (Figures 2E and S4B). To confirm that this PDGFRAlo cell effect reflects Wnt secretion, we added Wnt-C59, a PORCN inhibitor that blocks Wnt secretion.45 Wnt-C59 abrogated organoid formation (Figures 2E and S4B) and this effect did not represent generic toxicity because the compound did not affect organoids formed in the presence of CHIR (Fig. S4C). SI and colonic PDGFRAlo cells, but not SEMFs, also enabled Wnt-dependent organoid growth from wild-type colonic crypts (Figure 2F–G), similar to CHIR (Figure S4D). Mesenchymal cells retained pertinent transcripts during their brief in vitro expansion before crypt co-culture (Figure S4E) and they remained viable, with intact GFP (PDGFRA) expression, during co-culture (Figure 2F). Thus, both CD81− and CD81+ PDGFRAlo cells are bona fide sources of canonical Wnt. Of note, and in line with a previous report,46 Wnt2b is expressed evenly over the length of colonic crypts and SI crypt-villus units (Figures. 2H and S4F), paralleling the distribution of PDGFRAlo cells.
Molecular heterogeneity of CD81− PDGFRAlo stroma
Although Wnt2b is expressed equally in both PDGFRAlo populations, canonical Wnt signaling would concentrate near ISCs because limiting RSPO co-factors47 are abundant in trophocytes17,22 (Figure 3A). Potent ISC-directed activity of the latter cells is attributed to expression of both Rspo3 and Grem1,17,22 which are highest in bulk trophocytes from all intestinal segments, but also expressed at lower levels in CD81− stromal cells (Figures 3B and S5A). Moreover, in situ hybridization (ISH) signals for both factors extend above the muscularis mucosae, into Ackr4− stromal cells (Figure 3B), suggesting that cells other than trophocytes may also harbor niche activity.
Figure 3. Molecular heterogeneity of CD81− stromal cells.
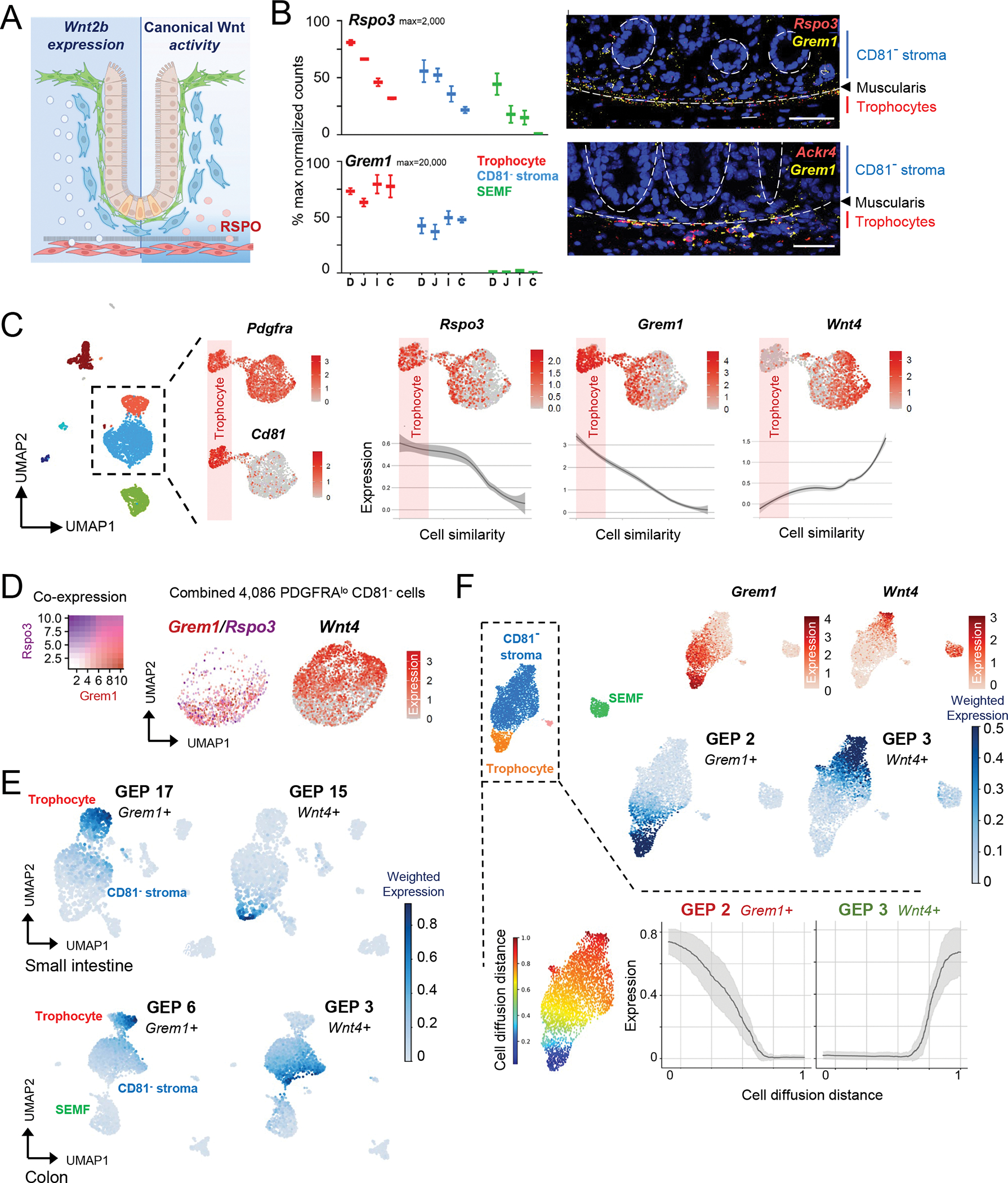
A) Model for Wnt2b expression domain (left, broad) and predicted signal activity (right, spatially restricted by RSPO localization).
B) Left, normalized bulk RNA-seq counts for Rspo3 and Grem1 in PDGFRA+ cells from each intestinal region. n=2 biological replicates per cell type and region. Right, in situ hybridization of colonic tissue for Rspo3 (red - top), Grem1 (yellow), and Ackr4 (red - bottom) counterstained with DAPI (blue). Scale bars 50 μm. Dashed circles: crypts, dashed lines: muscularis mucosae.
C) Feature plots of selected markers in scRNA-seq analysis of Pdgfralo cells extracted in silico from whole mesenchyme data (Fig. 1E, n=2,472); Cd81 marks trophocytes. Rspo3, Grem1, and Wnt4 expression is quantified below in Pdgfralo cells ranked (pseudotime algorithm) by similarity to trophocytes (shaded in pink). Expression means are smoothened and shaded regions on the curves represent the 95% confidence interval.
D) Grem1 and Rspo3 (overlay, left) and Wnt4 (right) expression on an aggregate feature plot from all 4,086 Pdgfralo CD81− cells. In addition to those extracted in silico as above, these include cells from 2 independent replicates (CD81− fractions of 1,184 cells and 865 cells, respectively) purified by PdgfraeGFP flow cytometry.
E) Consensus non-negative matrix factorization (cNMF)-based average expression of gene expression programs (GEPs) containing Grem1 (module 17 in SI, module 6 in colon) or Wnt4 (GEP 15 in SI, GEP 3 in colon) among the top-weighted genes.
F) scRNA-seq feature plots for Grem1 and Wnt4 in 3,370 isolated from wild-type mice by flow cytometry using PDGFRA antibody. Leiden clusters identified by known marker genes (see Fig. S1B) are shown alongside feature plots for Grem1 and Wnt4 (top) and cNMF plots for the modules that contain these markers (middle) and below a map of color-coded cell diffusion distances along the Pdgfralo subset, calculated by diffusion pseudotime51 relative to trophocytes as the root. Moving average expression and standard deviation (grey) are shown for GEPs 2 (Grem1+) and 3 (Wnt4+), calculated using a sliding window of 200 cells and ordered by diffusion distance (0=most similar; 1=most different).
See also Figure S5.
In scRNA-seq data from whole colonic mesenchyme, Rspo3 and Grem1 mapped principally to CD81+ trophocytes, but also to a CD81− PDGFRAlo cell subset, distinct from a complementary subset expressing non-canonical Wnt4 (Figure 3C). Indeed, a pseudotime algorithm48 that ranked all PDGFRAlo cells according to their transcriptional similarity to trophocytes revealed gradients of decreasing Grem1 and Rspo3 and increasing Wnt4 expression (Figure 3C), hence identifying useful proxies for seemingly distinct PDGFRAlo cell states. To confirm this duality, we purified 2,040 additional CD81− PDGFRAlo colonic cells by flow cytometry. Aggregate scRNA analysis of all CD81− PDGFRAlo cells from bulk mesenchyme (Figure 1E) and this additional isolation of CD81− stroma verified non-overlapping expression of Grem1 and Rspo3 in one subpopulation and Wnt4 in the other (Figure 3D). This mRNA reciprocity was also evident in independent data23,49 from adult mouse SI and colonic mesenchymal cells (Figure S5B).
To determine if these select gene markers reflect distinct cell states, we examined differential gene expression without marker supervision and independent of graph-based cell clustering. In scRNA data from colonic (this study) or SI (Ref. 17) mesenchyme, consensus non-negative matrix factorization (cNMF)50 identified distinct gene co-expression programs (GEPs) in various cell populations (Figure S5C and Table S2). Projecting these GEP modules onto UMAP plots revealed the expressing populations, e.g., SEMFs, myocytes and endothelial cells, each displaying unique GEPs (Figure S5D). Although modules 17 (SI) and 6 (colon), which contain Grem1 and Rspo3 among the top-weighted genes, mapped predominantly to trophocytes, expression stretched into a fraction of CD81− PDGFRAlo cells; conversely, GEPs 15 (SI) and 3 (colon), which contain Wnt4 and other genes, projected onto a distinct, non-overlapping subset of CD81− stroma (Figure 3E).
To further confirm stromal heterogeneity, we fractionated wild-type SI mesenchyme by flow cytometry with PDGFRA antibody. Among the 3,370 cells recovered for scRNA analysis, both a marker-based supervised analysis and cNMF identified Grem1, Rspo3, and the corresponding GEPs extending from trophocytes into the CD81− stroma, with Wnt4 expressed in a different Cd81− cell fraction (Figure 3F). As expected from scRNA data (Figure S2D–E), Wnt4 is also present in SEMFs. cNMF identified the respective Grem1+ or Wnt4+ modules across independent datasets of whole mesenchyme from SI (Ref. 17), colon, and SI PDGFRA+ cells, with large overlap among the top 100 genes (Figure S5E). Across PDGFRAlo cells ranked by overall transcriptional similarity (diffusion pseudotime method51), the Grem1+ and Wnt4+ modules were expressed reciprocally (Figure 3F). Importantly, genes in the respective modules, such as Aebp1, Cxcl14, and Lpl, are distributed variably across Cd81− cells (Figure S5F) and the Grem1/Rspo3+ fraction is not a trophocyte replica: ISC-trophic mRNA levels are lower than in trophocytes (Figure 3B), where transcripts such as Ackr4 are highly restricted.
Spatial and functional heterogeneity of CD81− PDGFRAlo stroma
ISH signals for Rspo3 were limited (Figure 3B) by low mRNA levels and probe efficiency, but Grem1 signals were consistent and showed asymmetric distribution. In both SI and colon, Grem1+ cells above the muscularis were restricted to the lower crypt and excluded from villi or upper crypt regions, while Wnt4 was reciprocally expressed in Grem1− cells located high in crypts and in SI villus stroma (Figures 4A and S6A). We verified this asymmetry in two independent mouse models. First, we crossed Wnt4eGFP-CreERT2 knock-in mice52 with the Rosa26LSL-TdTom strain53, hence marking Wnt4+ cells with fluorescence; RT-PCR analysis of FACS-purified tdTom+ cells confirmed that they represent the Wnt4+ fraction (Figure S6B). tdTom+ cells included SEMFs, as expected from scRNA data (see Figure S2E), as well as stromal cells in the lamina propria, with a firm boundary in upper crypt regions (Figures 4B and S6C). Along the radial axis, these Wnt4+ stromal cells lie farther from the epithelium than SEMFs and, distinct from SEMFs, they co-express Sfrp1 and stain poorly with PDGFRA antibody (Ab, Figure S6C). Second, we generated a Grem1 reporter allele with in-frame insertion of a DTR-tdTomato cassette, which places fluorescence in Grem1-expressing cells (Experimental Methods). Smooth muscle cells showed bright tdTom signals, as expected; additional non-muscle Grem1+ cells were confined to a few peri-cryptal mesenchymal tiers above the muscularis (Figure 4C). Thus, trophic factor expression extends beyond sub-muscularis trophocytes, into PDGFRAlo cells that concentrate near the crypt base, distinct from Wnt4+ cells in higher mesenchymal tiers (Figure 4D).
Figure 4. Spatial segregation of heterogenous CD81− stromal cells.
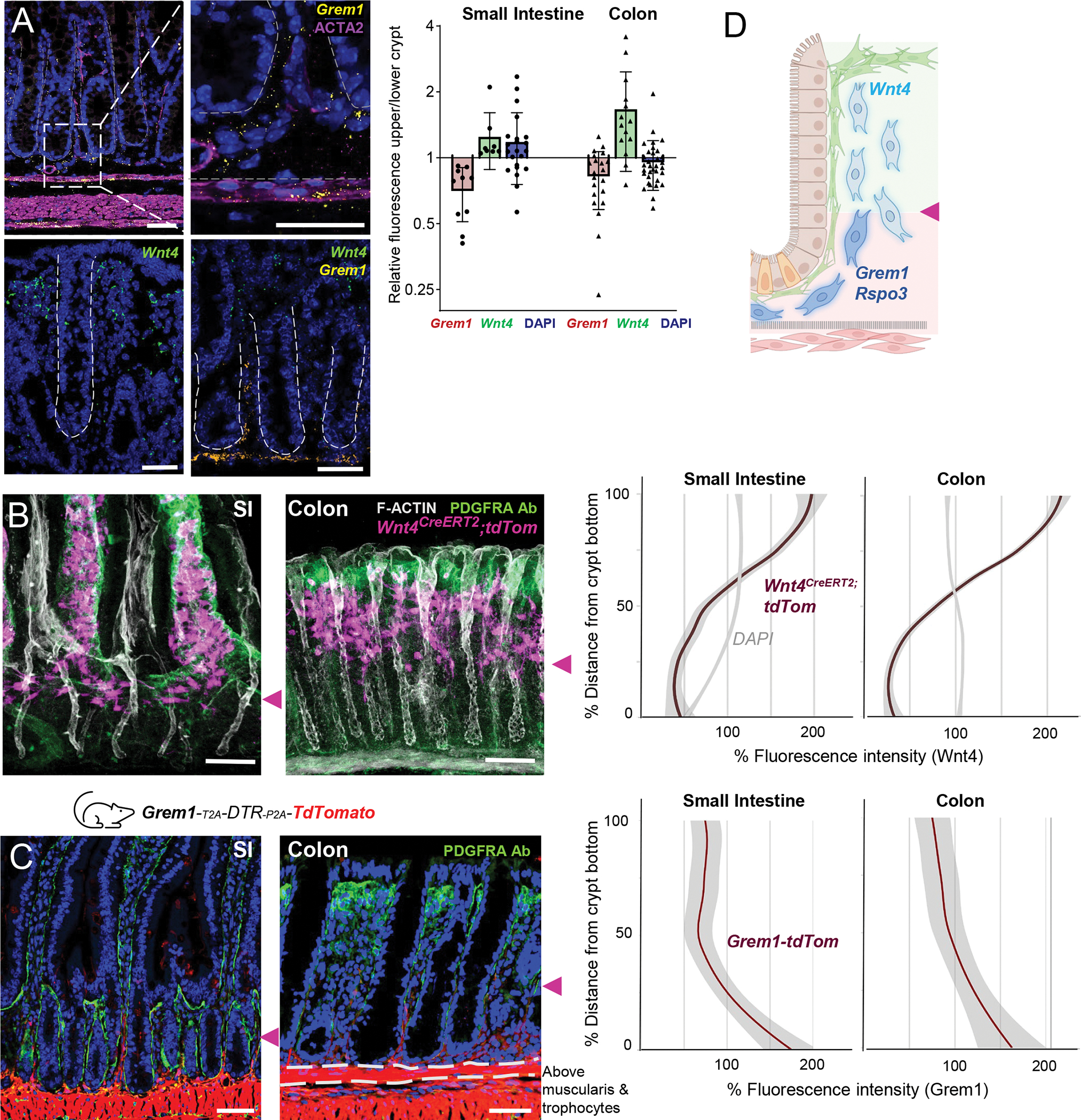
A) Grem1 and Wnt4 ISH in colon, stained with ACTA2 Ab (magenta in Grem1 ISH) or both probes together. Graph: ratios of RNAscope fluorescence intensity for each marker in sub-epithelial cells in the upper (luminal) and lower (serosal) crypt halves (equal measurement areas, mean fluorescence measured above the muscularis mucosae, DAPI signals control for cell density). Each dot represents one image measurement, sampled across multiple tissue sections from n=3 animals.
B) Wnt4 expression detected by the tdTom fluorescence proxy in Wnt4CreERT2;R26RtdTom SI (left) and colon (right) 2 weeks after tamoxifen injection. Tissue whole-mounts were counterstained with PDGFRA Ab to highlight SEMFs (green), phalloidin to demarcate boundaries (F-Actin, white), and DAPI (blue). Confocal 40-μm z-stacks are shown; see Figure S6C for single cross-sections without phalloidin staining. Scale bars 50 μm. Purple arrowheads point to lower edges of the Wnt4+ stromal domain. Graphs quantify the percent of average tdTom (red line) and DAPI (grey line) fluorescence intensity along the length from crypt bottom (0) to crypt top (100: full crypt length). The graphs display smoothened mean values with 95% confidence interval (shaded grey) from n=3 mice.
C) SI and colonic tissue sections from Grem1tdTom (red) reporter mice, stained with PDGFRA antibody (green) and DAPI (nuclei, blue). Dashed lines indicate muscularis mucosae. Purple arrowheads point to upper edges of the Grem1+ stromal domain. Scale bars 50 μm. Graphs quantify average tdTom fluorescence (red line) along the crypt, from bottom (0) to top (100), as in panel B. The graphs display smoothened mean values with 95% confidence interval (shaded grey) from n=3 mice.
D) Schema of heterogeneous CD81− PDGFRAlo cell states along the crypt axis marked by factor expression. Cell symbols as in Fig. 1.
See also Figure S6.
This mesenchymal polarity suggests that the Grem1+ Rspo3+ subset may provide the limited organoid support from unfractionated CD81− PDGFRAlo cells and we therefore sought to separate them from the putative inactive (Wnt4+) fraction. Projection of differentially expressed genes in colonic GEP module 6 (which contains Grem1 and Rspo3 – Table S2) revealed higher Cd55 mRNA levels in the corresponding CD81− stromal cell fraction (Figure 5A). Cd55 encodes a cell surface protein, is also represented in SI GEP module 17 (though not among the 100 top-weighted genes), and was recently reported to predominate in colonic Grem1+ cells near the crypt base.54 Indeed, our separation of CD55lo from CD55hi cells by flow cytometry revealed asymmetric Wnt4 and Grem1/Rspo3 expression (Figure 5B). Testing these mesenchymal fractions for differential niche activity required a sensitive in vitro assay, and because colonic crypts form small organoids in variable numbers and require large number of mesenchymal cells, which hampers organoid quantitation. We therefore co-cultured duodenal crypts with colonic or duodenal CD55lo or CD55hi cells. The latter cells consistently spawned larger organoids in substantially larger numbers (Figure 5C). Thus, the CD55hi subset of CD81− stromal cells provides demonstrable niche support; when crypts are cultured with unfractionated PDGFRAlo cells, the complementary Wnt4+ cell fraction likely masks this activity.
Figure 5. Functional distinction of heterogenous CD81− stromal cells.
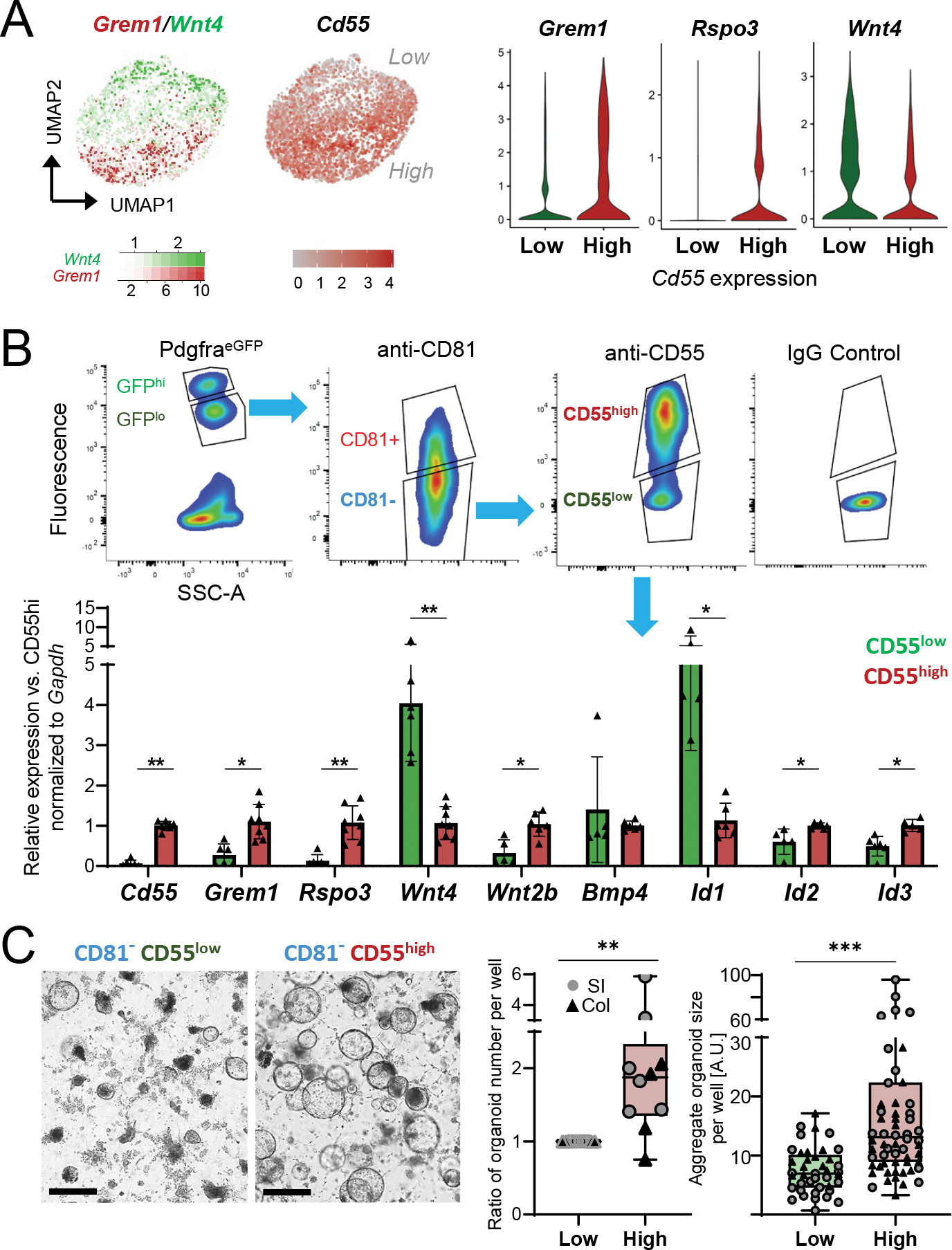
A) Left, Grem1 (red) and Wnt4 (green) expression overlay and Cd55 levels in single Cd81− stromal cells aggregated from independent collections, as in Figure 3D. Right, marker expression in scRNA-seq data in cells separated in silico for high (SCT-transformed counts >1, n= 1,756 cells) or low (n=2,322 cells) Cd55 expression.
B) Top, representative side-scatter area (SSC-A) and fluorescence plots from FACS isolation of PDGFRAlo (based on native PdgfraeGFP signal) CD81− (fluorophore-conjugated 2nd Ab) cells with high or low surface CD55 expression (conjugated Ab, FACS gating with IgG isotype control). Below, qRT-PCR for selected mRNAs in primary colonic PDGFRAlo CD81− stromal cells sorted for low (green) or high (red) surface CD55. mRNA expression is normalized to Gapdh and displayed relative to average expression in CD55hi cells (ΔΔCt). Bars: Mean ±SD from n=5–8 independent cell isolations.
C) Organoids from duodenal crypts co-cultured with CD55lo or CD55hi cells without added growth factors 4 days after seeding at 10,000 stromal cells/10 μl Matrigel droplet; brightfield images, scale bars 200 μm. Left graph shows fold-difference of average organoid number per well 4 days after the start of co-culture with CD55hi cells, relative to CD55lo cell co-culture (=1). Each point represents the organoid count (average of biological replicates) from each independent experiment with stromal cells from SI (circles, n=6 experiments) or colon (triangles, n=4 experiments). Two-tailed Wilcoxon matched-pairs signed rank test, **Padj 0.006. Right graph shows the sum of all organoid diameters per well in arbitrary units (A.U.) from the same experiments. Box: 25th-75th percentile with median, whiskers: minimum to maximum values. Two-tailed Mann-Whitney test, **Padj <0.001.
BMP-mediated self-organization underlies ISC niche polarity
These findings together suggest that local cues might influence the niche potential of sub-cryptal CD81+ trophocytes and peri-cryptal CD81− CD55hi cells; specifically, that these PDGFRAlo cells may realize latent niche potential as a function of their locations. As all functional data point to similar PDGFRAlo cell activity in the SI and colon, putative inductive signals must be present in both organs. Thus, although lymphatic vessels are implicated in ISC support,35 they are poor candidates for niche organization because lacteals are only present in SI lamina propria and large sub-mucosal lymphatic vessels are irregularly spaced, so many crypts lie far from them (Figure S7A). In contrast, PDGFRAhi SEMFs are uniformly present in the SI and colon, aggregating densely at two SI sites: villus tips and bases.17 Similarly, colonic SEMFs are abundant under the luminal epithelial cuff and consistently less dense below that region, as seen both in PdgfraeGFP colon (Figure 6A) and in wild-type mice using PDGFRA Ab (Figure S7B). These cells are the predominant BMP source in both SI and colon (Figure S7C – PDGFRAlo cells express low levels, especially Bmp4) and, in line with that observation, colonic ISH signals for Bmp4 and Bmp5 concentrate at sub-luminal SEMF aggregates (Figure 6B). Furthermore, the BMP-responsive gene Id1 is expressed at higher levels in functionally inert CD81− CD55lo stromal cells compared to CD55hi cells, similar to Wnt4 (Figure 5B). SEMF-derived BMPs are thus candidate self-organizing signals for the ISC niche.
Figure 6. BMPs as candidates for intra-mesenchymal signaling in crypt niche zonation.
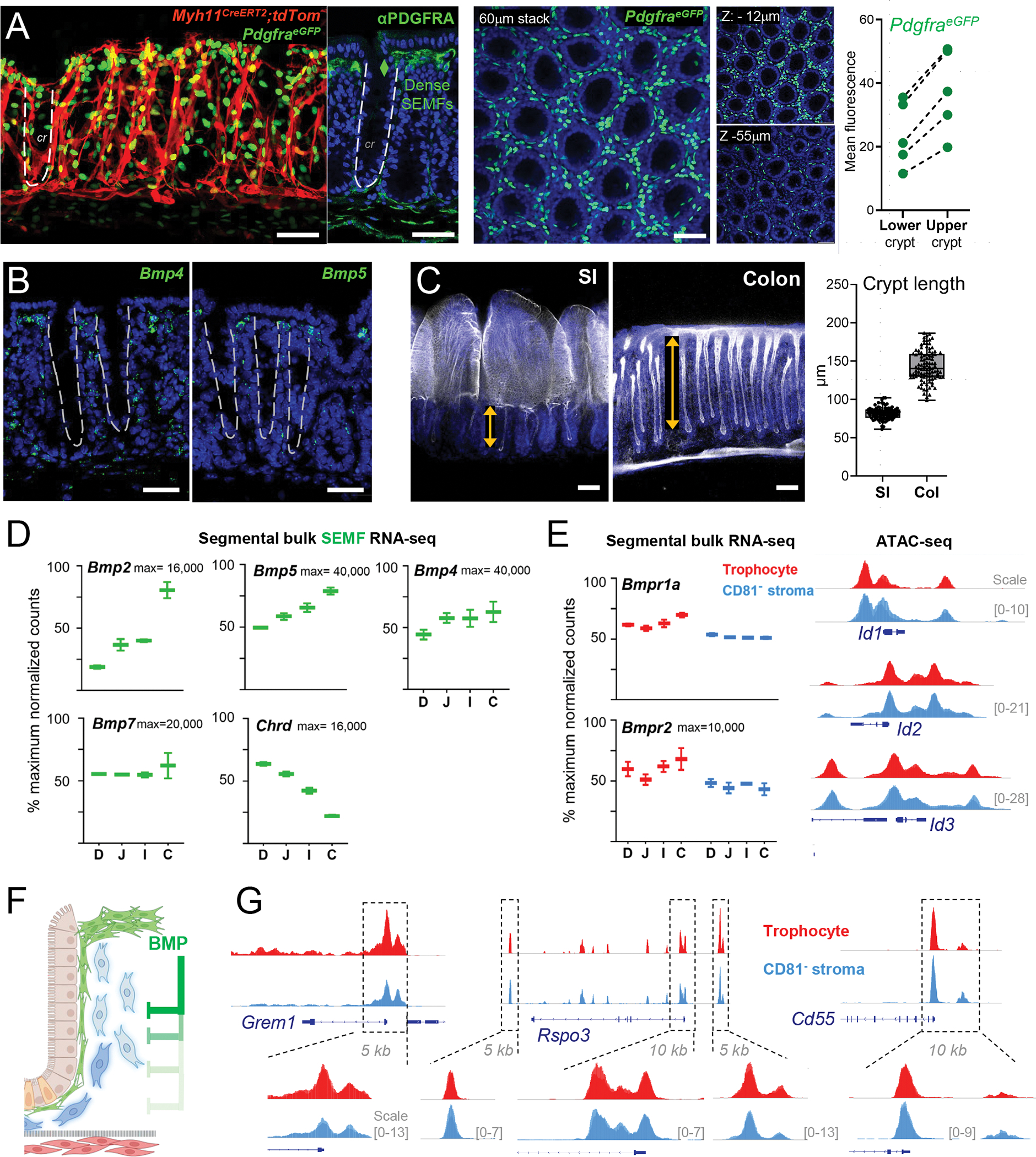
A) Left: Myh11CreERT2;R26RtdTom;PdgfraeGFP mouse colon showing SEMF (PDGFRAhi cells, 60 μm stack, en face view) distribution along the crypt length demarcated by tdTom+ myocytes. Adjacent image shows a z-slice of the same tissue stained with PDGFRA Ab (green) and DAPI (nuclei, blue); the dashed line demarcates one crypt. Center, Whole-mount confocal microscopy of colonic PdgfraeGFP crypts counterstained with DAPI (blue). A 60-μm stack is shown with 6-μm sub-stacks at z= −9 to −15 μm (top) and z= −52 to −58 μm (below) shown alongside. Right, eGFP signals were quantified in crypt upper and lower halves as mean fluorescence values in cross-sections of whole-mount images (5 images each from n=3 mice); lines connect paired values from each image. All scale bars 50 μm.
B) In situ hybridization (RNAscope, green) for Bmp4 and Bmp5 in adult mouse colon sections co-stained with DAPI (blue). Dashed lines demarcate crypts. Scale bars 50 μm.
C) Whole-mount confocal images of SI and colon after stripping external smooth muscle layers and staining with phalloidin (F-actin, white) and DAPI (blue). SI: single whole-mount micrograph, colon: 60-μm z-stack. Double arrows outline crypt heights and the graph depicts the heights of ≥200 crypts measured on tissue sections from n=3 wild-type adult mice stained with hematoxylin and eosin (see Figure S7D).
D) Normalized bulk RNA-seq counts for BMP ligands and the BMPi Chordin (Chrd) in SEMFs from each intestinal segment: duodenum (D), jejunum (J), ileum (I), and colon (C) from n=2 mice. Y-axis: percent of maximum normalized read counts (as indicated) for each gene. Box: 25th- 75th percentile, whiskers: minimum to maximum values.
E) Left, normalized bulk RNA-seq counts of BMP receptors 1a and 2 (Bmpr1a and Bmpr2) in SI and colonic trophocytes and CD81− stromal cells (both max=10,000 read counts). Bmpr1b mRNA was not detected. Right, IGV tracks of open chromatin (ATAC-seq signals overlaid from duplicate SI samples) at BMP-responsive loci Id1, Id2, and Id3 (10-kb regions, y-axis scales as indicated).
F) Schema of the hypothesis that BMPs from SEMF aggregates at crypt tops restrain the niche properties of PDGFRAlo cells. BMP-mediated gene suppression is relieved with increasing distance from those aggregates, allowing PDGFRAlo cells toward the crypt bottom to express niche factors and functions.
G) IGV tracks (ATAC-seq signal overlay from duplicate samples) from PDGFRAlo mesenchymal cells at the Grem1, Rspo3, and Cd55 loci. Boxed 5–10 kb regions are magnified to highlight trophocyte and CD81− stromal cell similarities. Y-axis scales are indicated.
See also Figure S7.
Crypts are nearly twice as long in the adult rat and weanling mouse colon than in the SI (Refs. 55,56). We confirmed that the significant difference in crypt height persists in adult mice (Figures 6C and S7D). Therefore, ISCs in the colonic crypt base and the colonic niche are farther from SEMF-derived products than their SI counterparts, so if SEMF-derived BMPs organize the niche, then colonic SEMFs must produce larger amounts than those in the SI. Bmp2 and Bmp5 levels are indeed highest in colonic SEMFs, while levels of the BMPi Chrd are lowest (Figure 6D and Table S1 – Bmp4 levels in PDGFRAlo cells, albeit lower than BMP gene levels in SEMFs, are also highest in the colon). Moreover, both trophocytes and CD81− PDGFRAlo stromal cells express abundant Bmpr1a and Bmpr2 receptor transcripts and have accessible chromatin at key BMP-responsive Id1-3 loci (Figure 6E), consistent with their potential to react to BMP signaling. Together, these findings support the idea that SEMF-derived BMPs inhibit PDGFRAlo cell expression of ISC niche genes, such as BMPi, hence positioning functional niche cells at set distances from the BMP source (Figure 6F). In this model, PDGFRAlo cell heterogeneity is not cell-intrinsic but locally determined, and the requisite epigenetic plasticity is reflected in near-identical configurations of open chromatin at niche-relevant loci, such as Grem1, Rspo3, and Cd55 (Figure 6G).
To test this model, first we treated primary intestinal CD81− CD55hi PDGFRAlo cells in culture with mixtures of recombinant (r) BMPs (rBMP2/4/7) or BMPi (rGREM1, rNOG). Elevated phosphor-SMAD1/5 levels (pSMAD, Figure 7A) and nuclear pSMAD immunofluorescence (Figure 7B), confirmed BMP signaling in vitro. In response to this stimulation of SI and colonic stromal cells, Wnt4 mRNA, which marks PDGFRAlo cells closest to SEMF aggregates (and farthest from ISCs) in vivo, increased 16-fold on average compared to untreated controls, whereas Rspo3, Grem1 and Cd55, which mark PDGFRAlo cells near the crypt base and farthest from SEMF aggregates in vivo, consistently declined 1.5- to 8-fold (Figure 7C). Of note, rBMP exposure markedly reduced endogenous Bmp4 expression and, as a sign of specificity, other genes such as the EGF ligands Ereg or Nrg1 were unaffected. Conversely, treatment with rBMPi increased expression of Bmp4 and Cd55; because BMPi effects in vitro rely on inhibition of likely weak native BMP signals, they were predictably less potent (Figure 7C). Trophocytes reacted similarly to rBMP exposure, with notable induction of Wnt4 and suppression of niche-relevant factors, including trophocyte-specific marker Cd81 (Figure 7D). To assess the functional consequences of these effects, we introduced rBMP-treated CD81− CD55hi PDGFRAlo cells into crypt co-cultures, anticipating that prolonged withdrawal of BMP might attenuate the phenotype. Nevertheless, treated cells in each experiment yielded smaller organoids than untreated cells (Figures 7E and S7E).
Figure 7. BMP-responsive gene regulation in CD81− stromal cells.
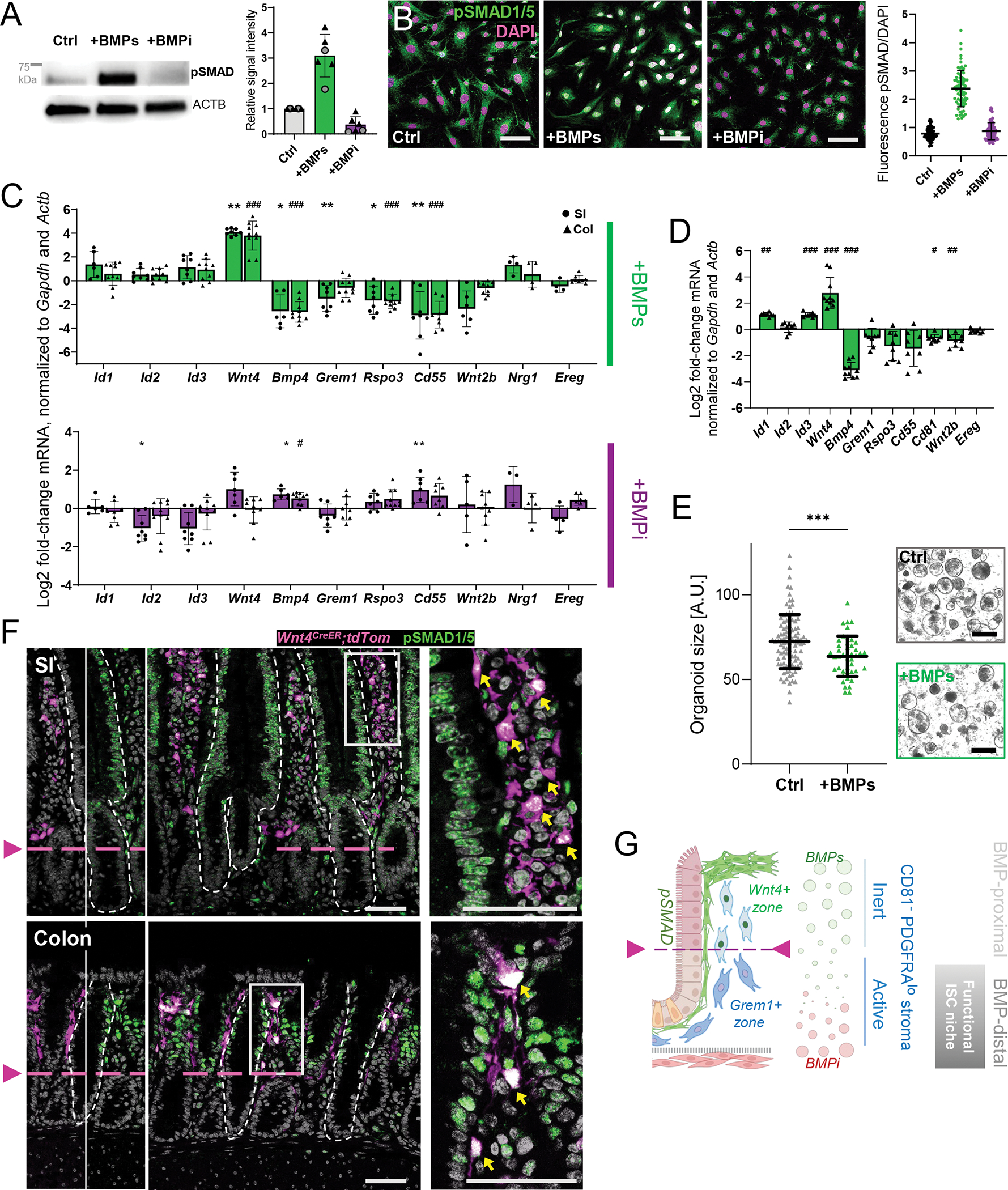
A) Phospho-SMAD1/5 and ACTB (loading control of the same lane) immunoblots from cultured PDGFRAlo CD81− CD55hi stromal cells treated for 5 days with rBMP2/4/7 (100 ng/mL each, combined) or BMPi (rGREM1 and rNOG, 100 ng/mL each, combined) in serum-free medium. The position of a 75-kDa size marker is indicated. Graph: pSMAD1/5 chemiluminescence signals quantified relative to ACTB in SI (circles) or colonic (triangles) cells with respect to untreated cells (Ctrl). Bars represent the Mean±SD from n=6 independent blots.
B) pSMAD1/5 immunofluorescence (green) in cultured PDGFRAlo CD81− CD55hi stromal cells after treatment with rBMPs or rBMPi. Scale bars 100 μm. Representative images from n=4 independent cell isolations and experiments. Graph: nuclear pSMAD fluorescence intensity normalized to DAPI signals. Each dot represents one cell. Lines= Mean ±SD.
C) qRT-PCR assessment of selected transcripts after treatment of primary SI (circles) and colonic (triangles) PDGFRAlo CD81− CD55hi stromal cells for 5 days with rBMPs (top) or rBMPi (bottom) in serum-free media. Expression was normalized to Gapdh and Actb RNA levels. Results are represented as log2 fold-change of normalized expression compared to control cultures of the same region (ΔΔCt). Bar graphs: Mean ±SD of individual samples, derived from n=4 (SI) or n=5 (colon) experiments with 1–2 biological replicates per experiment. Two-tailed Mann-Whitney test with Holm-Sidak correction, SI: Padj <0.05 (*), Padj <0.01 (**), colon: Padj <0.05 (#), Padj <0.01 (##), Padj <0.001 (###).
D) qRT-PCR data for colonic trophocytes cultured and treated as in Figure 7C. Bar graphs: Mean ±SD of individual samples, derived from n=4 experiments with 2 biological replicates per experiment. Two-tailed Mann-Whitney test with Holm-Sidak correction, Padj <0.05 (#), Padj <0.01 (##), Padj <0.001 (###).
E) Organoid size 4 days after SI crypts were co-cultured with PDGFRAlo CD81− CD55hi stromal cells (10,000 cells/10 μl Matrigel) pre-treated for 5 days with rBMP2/4/7 (+BMPs) or untreated (Ctrl, serum-free medium only). Values are in arbitrary units (A.U.) and lines indicate mean±SD. Data are collated from n=3 independent experiments (1–3 biological replicates in each; the individual experiments are shown in Figure S7E) and each value represents one organoid. Unpaired t-test with Welch-correction Padj=0.0004 (***).
F) pSMAD1/5 immunofluorescence (green) and native tdTom signal (magenta) in Wnt4eGFP-CreERT2);R26RtdTom mouse SI (top) and colon (bottom). Left, Single-color view of overlay image in the center. Arrowheads and dashed magenta lines indicate the lower pSMAD/Wnt4 expression boundary. Dashed white lines demarcate epithelium from mesenchyme. Right, magnified insets of boxed areas, with yellow arrows pointing to pSMAD+ (nuclear signal) Wnt4+ (cytoplasmic signal) stromal cells. Scale bars 50 μm.
G) Model for BMP signaling, with supra-cryptal SEMFs providing BMP ligands that are opposed by sub-cryptal BMPi sources, as one basis for heterogenous PDGFRAlo stromal cell states and consequent self-organization of the ISC niche.
See also Figure S7.
If SEMF-derived BMP signaling indeed influence phenotypes of other sub-epithelial cells, then nuclear pSMAD should localize in roughly the same tiers in the mesenchyme as in BMP-responsive epithelium. Thus, Wnt4+ stroma should express pSMAD and localize in close alignment with epithelial pSMAD+ cells, which extend from villus base to tip in the SI and across the top half of colonic crypts. Immunostaining of Wnt4CreERT2;R26RtdTom mouse intestines revealed exactly this pattern: both Wnt4+ stromal cells and adjoining epithelial cells showed nuclear pSMAD, while both epithelial and stromal cells in lower tiers lacked pSMAD (Figure 7F). Together, these findings implicate BMP signaling in regulation of niche factor genes in PDGFRAlo cells. They suggest that the position of ISC-supporting mesenchymal cells near the crypt base is determined, at least in part, by their distance from supra-cryptal SEMF aggregates, which provide BMP agonists, and their proximity to various sub-cryptal sources of BMPi (Figure 7G).
DISCUSSION
Countervailing Wnt and BMP gradients underlie intestinal crypt homeostasis. ISCs require high Wnt/Rspo and absent BMP activity,57–60 and sub-epithelial mesenchyme is the predominant source of Wnt/Rspo and BMP ligands and inhibitors.10,13–15,17,18,61, 63 Signal gradients could arise because key mesenchymal cells occupy opposite sides of the crypt base or because cells express agonists and antagonists in a graded fashion; recent investigation of the intestinal mesenchyme has brought these possibilities into focus.15–17,19,20,61–63 PDGFRAlo CD81+ Ackr4+ trophocytes lie beneath the muscularis mucosae, express Grem1 and Rspo3, and robustly support organoid growth from isolated crypts.17 Conversely, SEMFs concentrate near crypt tops and the BMP gradient is thought to reflect opposing foci of agonist-secreting SEMFs at crypt tops and antagonist-secreting trophocytes near the crypt base.17 Another mesenchymal population of PDGFRAlo CD81− Ackr4− cells is abundant, but because these cells populate the whole crypt-villus axis, express little BMPi or RSPO, and support ISCs poorly ex vivo, their contribution to the ISC niche previously seemed insignificant.17 Previous identification of PDGFRAlo cell population substructure by scRNA-seq25,49 also appears unrelated to known niche properties, e.g., extracellular matrix.54 We show here that CD81− PDGFRAlo cells located above the muscularis mucosae are a potent source of canonical Wnt and that a CD55hi fraction bathing crypt bottoms expresses key niche factors and promotes organoid growth ex vivo. The roles of molecular markers such as CD81 and ACKR4 remain unknown and CD81 expression in various PDGFRA− mesenchymal cells limits its utility to isolating trophocytes from other PDGFRA+ cells (see Figures S1 and S4).
Our study highlights four salient features of the ISC niche: extension beyond sub-mucosal trophocytes, into a defined peri-cryptal domain; functional cellular heterogeneity along the crypt vertical axis; overlapping, graded sources of diffusible supportive factors; and, most notably, self-organization. The findings deliver a coherent picture in which SI and colonic ISCs draw support, including canonical Wnts, from transcriptionally, anatomically, and functionally distinct CD81+ (trophocyte) and CD81− pools of PDGFRAlo cells. Ablation of leptin-receptor (Lepr)-expressing cells, which fortuitously encompass Grem1+ PDGFRAlo cells, was recently reported to reduce ISC numbers, distort crypts, and impair their regeneration after γ-irradiation;21 our findings are consistent with these observations. Goto et al. also found recently that cells expressing Rspo3 and Grem1 include trophocytes as well as CD81− stromal cells,36 and other spatial analyses have identified asymmetric distribution of selected mRNAs along the crypt axis.35,64,65
Nearly identical configurations of chromatin in CD81+ (trophocyte) and CD81− PDGFRAlo cells imply that their differences are not intrinsic and likely imparted by local determinants of niche self-organization. Our findings nominate SEMF-derived BMPs as a principal stimulus for the distinction between stromal cells that provide or do not provide key niche factors. Exposure to BMPs reduces Grem1 and Rspo3 mRNA levels in PDGFRAlo cells; these factors are accordingly expressed as a function of the cells’ distance from crypt tops, where SEMFs aggregate. We therefore propose that SEMFs provide BMPs not only as a cue for epithelial cells to mature near crypt tops66 but also to inhibit niche activity in neighboring mesenchymal cells. Circumstantial (Figure 6) and experimental (Figure 7) evidence support a model in which BMPs repress niche genes at the tops of crypts. Increasing distance from this concentrated BMP source, likely coupled with proximity to sources of BMPi, relieves that repression modestly in CD81− stroma near the crypt base and robustly in CD81+ trophocytes, the PDGFRAlo cells that lie farthest from SEMF aggregates (Figure 7G). Whereas colonic SEMFs aggregate only at crypt tops, those in the SI additionally aggregate at the tops of villi.17 This additional BMP reserve in the SI can explain why Wnt4+ cells, i.e., those subject to BMP-mediated repression of niche factors, are not confined to the tops of crypts but extend throughout the villus lamina propria.
Beyond this proposed simple mechanism for mesenchymal self-organization, other signals likely help craft a complex ISC niche. Wnt4+ stromal cells, for example, are well positioned to provide factors that also suppress niche activity at crypt tops and in villi; Wnt4 is a transcriptional target of BMP signaling in PDGFRAlo stromal cells and it or other genes might help restrict niche size and ISC location. Conversely, although BMPi do not activate niche factor genes in vitro as potently as BMPs repress them (Figure 7C), that finding may reflect absence of supplemental BMP and other, unknown variables in primary cell cultures; in vivo, various sub-cryptal sources of BMPi, such as trophocytes and smooth muscle, may enhance niche activity of PDGFRAlo stroma. Furthermore, while our studies focus on mRNA levels of genes known to support ISCs – not only Grem1 and Rspo3 but also ligands and modulators of canonical Wnt signaling– post-transcriptional and post-translational effects in each signaling pathway likely refine the milieu further for optimal ISC support. Finally, mesenchymal cell support of organoid growth in Matrigel droplets belies their layered arrangement in vivo. Note that crypts cultured with mesenchymal cells form spheroids and not budding organoids (e.g., Figures 5C and 7E); moreover, overt polarity of organoids cultured in soluble niche factors4 implies that crypt epithelium itself harbors some degree of self-organization, a property that may well contribute to mesenchymal cell heterogeneity. For example, epithelial Hedgehog signals to mesenchymal and smooth muscle cells67 might influence niche properties. Limited recovery of mesenchymal cells after crypt co-culture precluded critical assessment of their response to epithelial cell exposure.
From imaging studies in reporter mice (Fig. 4), the boundary between non-overlapping Wnt4+ and Grem1/Rspo3+ subpopulations of peri-cryptal PDGFRAlo cells appears to lie near the crypt equator. As both ISH and reporter gene activity have limits in sensitivity, we cannot exclude low expression of genes from each cell population in the other. The scRNA expression domain of Cd55, a target of BMP repression similar to Grem1 and Rspo3, accentuates this point because it tracks expression of the latter genes imprecisely (Figure 5A). Nevertheless, CD55 flow cytometry is effective at separating the active Grem1+ Rspo3+ fraction of CD81− stromal cells from the inert Wnt4+ fraction. Thus, although the significance of Cd55 and non-canonical Wnt4 expression in distinct PDGFRAlo stromal cells is unknown, they are useful molecular markers of cells that support (Cd55) or do not support (Wnt4) ISC functions.
Limitations of the study
Genetic ablation of discrete cell populations or depletion of candidate genes from those cells could help refine emerging models of the ISC niche, but neither approach is currently practical because candidate niche factors are generally redundant with each other, and molecular markers are not strictly restricted to single cell types. For example, Cd81 and Ackr4 accurately distinguish trophocytes from other PDGFRAlo cells, and Cd55 levels separate active from inactive PDGFRAlo stromal cells, but expression of these markers outside the PDGFRAlo compartment limits experimental possibilities and interpretations. Moreover, intestinal mesenchymal cells expel tamoxifen, a conventional agent to activate Cre recombinase.68 We overcome this limitation in part using organoid assays, which reveal crucial differences, for example between PDGFRAlo cells and SEMFs as Wnt sources and between CD55hi and CD55lo cells in ISC support; however, ex vivo interrogation of niche functions lacks the context of in vivo cell interactions. Furthermore, although we handled diverse mesenchymal cells in parallel for organoid assays and they retain the tested native transcripts in vitro, the degree of inevitable phenotypic drift in culture is unknown. Finally, our focus on BMP-mediated transcriptional control as an organizing principle for the ISC niche is necessarily reductionist. Other signaling pathways, epithelial-stromal interactions, and post-transcriptional effects must also contribute toward ISC support in vivo. Our delineation of BMP effects provides a foundation to investigate additional niche determinants, not only in the resting state we investigated in the current study but also in disease states.
STAR METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact.
Materials availability
Materials used in this study are listed in the Key Resources Table.
Key Resource Table.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| AlexaFluor 594 donkey anti-goat IgG | Invitrogen | A11058 |
| AlexaFluor Goat 546 anti-rat IgG | Invitrogen | A11081 |
| AlexaFluor Goat 633 anti-rabbit IgG | Invitrogen | A21071 |
| Alpha-smooth muscle actin | Abcam | 5694; RRID: AB_2223021 |
| Armenian Hamster IgG Isotype Control | eBioscience | eBio299Arm |
| Beta-Actin | Sigma | A5441 |
| CD31 | BD | 557355; RRID: AB_396660 |
| CD34 | BioLegend | 119310 |
| CD45-APC | invitrogen | 17045182 |
| CD55 clone RIKO-3 | BioLegend | 131803 |
| CD81-biotin Clone EAT2 | eBioscience | 13-0811-81 |
| EpCAM-PE clone G8.8 | eBioscience | 12-5791-83 |
| GFP | Abcam | 6556/6662RRIDs: AB_305564 and AB_305635 |
| Goat anti-mouse IgG HRP conjugate | Invitrogen | 62-6520 |
| Goat anti-rabbit IgG HRP-conjugate | Invitrogen | 656120 |
| Laminin | Sigma | L9393; RRID: AB_477163 |
| Lysozyme | Dako | A0099; RRID: AB_2341230 |
| MKi67 | Invitrogen | MA5-14520 |
| Pdgfra (IHC) | R&D Systems | AF1062; RRID: AB_2236897 |
| Pdgfra (CD140a-Ab) for FACS | BioLegend | 135905 |
| PE Armenian Hamster IgG Isotype Ctrl | BioLegend | 400907 |
| Phospho-Smad 1/5(ser463/465) (41D10) Rabbit mAb | Cell Signaling Technology | 9516 RRID: AB_491015 |
| Streptavidin-APC | eBioscience | 17431782 |
| anti-rabbit biotinylated 2nd antibody | Jackson Immuno Research | 111-065-003 |
| Streptavidin-HRP | Jackson Immuno Research | 016-030-084 |
| Tyramide-C3 | Perkin Elmer | NEL744E001KT |
| Chemicals, peptides, and recombinant proteins | ||
| Agarose | A1705Z | Azura Genomics |
| Alexa Fluor 647 Phalloidin | Life Technologies | A22287 |
| AMPure beads | Beckman Coulter | A63880 |
| B-27 Supplement (50X) | Life Technologies | 17504001 |
| Blocking Reagent | Perkin Elmer | FP1020 |
| Bovine Serum Albumin | Sigma | A9647 |
| CHIR99021 (Chir) | Sigma-Aldrich | SML1046-5MG |
| Chlorophorm | Sigma-Aldrich | C2432 |
| Collagenase II | Worthington | LS004176 |
| Collagenase XI | Sigma | C7657 |
| Complete (Protease Inhibitor Cocktail Tablets) | Sigma Aldrich | 11697498001 |
| DAPI | BD PharMingen | 5649097 |
| DAPI solution | BD Bioscience | 564907 |
| Dispase II | Sigma | D4693 |
| Dulbecco’s Modified Eagle Medium (DMEM) | Corning | 17-205-CV |
| DMEM/F12 Media | GIBCO | 12634-010 |
| EDTA | Gibco | AM9260G |
| Fetal Bovine Serum | Corning | 35-010-CV |
| FocusClear | CelExplorer | FC-101 |
| Glutamax | GIBCO | 35050-661 |
| Hanks’ Balanced Salt Solution (HBSS, 10X) | GIBCO | 14065-056 |
| HEPES | Sigma | H3375 |
| 30% H2O2 | Electron Microscopy Science | 2621-02 |
| Matrigel | Corning | 356231 |
| Mini-Protean TGX Gels | BioRad | 4561084 |
| N-2 Supplement (100X) | Life Technologies | 17502001 |
| N-Acetylcysteine | Sigma | A9165 |
| Normal Goat Serum | Cell Signaling | 5425S |
| Paraformaldehyde | EMS | 15714-S |
| PBS (10X) | Corning | 46-013-CM |
| Penicillin/Streptomycin | Life Technologies | 15140163 |
| Pierce Bovine Serum Albumin Standard Ampules | Life Technologies | 23209 |
| Recombinant EGF | ThermoFisher | PHG0311 |
| Recombinant Gremlin-1 | ThermoFisher | 956GR050 |
| Recombinant Human/Murine/Rat BMP-2 | Peprotech | 120-02C-10UG |
| Recombinant Murine BMP-4 | Peprotech | 315-27-10UG |
| Recombinant Murine BMP-7 | R&D | 5666-BP/CF |
| Recombinant Noggin | Peprotech | 120-10C |
| Sodium azide | Sigma | S2002 |
| T ransfer Buffer (10x) | Boston BioProducts | BP-190 |
| Tris-Glycine-SDS Running buffer (10x) | Boston BioProducts | BP-150 |
| Triton X-100 | Sigma | T8787 |
| Trizol reagent | ThermoFisher | 15596026 |
| T rypLE Express | Thermo Fisher Scientific | 12604-021 |
| Trypsin 0.25% EDTA | Life Technologies | 25200114 |
| VectaShield mounting medium | Vector Laboratories | H-1000 |
| Wnt-C59 | ThermoFisher | NC0186427 |
| Critical commercial assays | ||
| BCA PROTEIN ASSAY KIT | Thermo Fisher | PI23227 |
| Agilent High Sensitivity DNA Kit | Agilent | 5067-4626 |
| Agilent RNA 6000 Pico Kit | Agilent | NoImage 5067-1513 |
| Alcian Blue Counterstain | Thermo Fisher | NC1318236 |
| Chromium Next GEM Chip G Single Cell Kit | 10X Genomics | PN-1000120 |
| Chromium Next GEM Single Cell 3’ GEM, Library & Gel Bead Kit v3.1, 4 rxns PN-1000128 | 10X Genomics | PN-1000128 |
| DNA-purification kit | Qiagen | 28004 |
| ECL Prime Western Blotting Detection Reagents | GE Healthcare | RPN2232 |
| High-Fidelity 2X PCR master mix | New England Biolabs | M0541S |
| Nextera Tn5 Transposase | Illumina | FC-121-1030 |
| Power SYBR Green PCR MasterMix | Life Technologies | 4367659 |
| PureLink RNA mini kit | Life Technologies | 12183018A |
| Qubit dsDNA high-sensitivity assay | Life Technologies | Q32851 |
| RNAscope Hydrogen Peroxide | ACD | 322335 |
| RNAscope Multiplex Fluorescent Reagent Kit v2 | ACD | 323100 |
| RNAscope Protease III | ACD | 322337 |
| RNeasy Plus micro kit | Qiagen | 74034 |
| SapphireAmp Fast PCR Mastermix | Takara | RR350 |
| Single Index Kit T Set A | 10X Genomics | PN-100021 |
| SMART-Seq v4 Ultra Low Input RNA kit | Clontech | 634890 |
| SUPERSCRIPT III 1ST STRAND | Life Technologies | 18080051 |
| Deposited data | ||
| Bulk RNA-seq mesenchymal cells | This study | GEO accession: GSE211275 |
| ATAC-seq mesenchymal cells | This study | GEO accession: GSE211275 |
| scRNA-seq whole mesenchyme colon | This study | GEO accession: GSE211275 |
| scRNA-seq PdgfraLow cells colon | This study | GEO accession: GSE211275 |
| scRNA-seq Pdgfra+ cells small intestine | This study | GEO accession: GSE212601 |
| scRNA-seq whole mesenchyme small Intestine | McCarthy et al | GEO: GSE130681 GSM4196131, GSM4196131 |
| scRNA-seq whole mesenchyme colon | Kinchen et al | GEO: GSE114374 |
| scRNA-seq whole mesenchyme small Intestine | Kim et al | GEO: GSE116514 |
| Mouse reference genome Mm10, GRCm38 | Genome Reference Consortium | https://www.ncbi.nlm.nih.gov/grc/mouse |
| Experimental models: Organisms/strains | ||
| Atoh1fl/fl (B6.129S7-Atoh1tm3Hzo/J) | The Jackson Laboratory | 008681 RRID:IMSR_JAX:008681 |
| FoxL1-CreERT2-2A-tdTomato (B6.Cg-Foxl1tm2(cre/ERT2)Khk) | The Jackson Laboratory | RRID: MMRRC_068163-JAX |
| Grem1tdTom (Grem1-P2A-DTR-P2A-TdTomato) | This study | |
| Wnt4-CreERT2 (B6.Cg-Wnt4tm2(EGFP/cre/ERT2)Amc/J) | The Jackson Laboratory | 032489 RRID: IMSRJAX:032489 |
| Myh11-CreERT2 (B6.FVB-Tg(Myh11-icre/ERT2)1Soff/J) | The Jackson Laboratory | 19079 RRID:IMSR_JAX:019079 |
| PdgfraCreERT2 B6.129S-Pdgfratm1.1(cre/ERT2)Blh/J | The Jackson Laboratory | 032770 RRID:IMSR_JAX:032770 |
| PdgfraH2BeGFP (B6.129S4-Pdgfratm11(EGFP)Sor/J) | The Jackson Laboratory | 007669 RRID:IMSR_JAX:007669 |
| PorcnFl | L. Charles Murtaugh, University of Utah, United States | |
| Rosa26-TdTomato B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J | The Jackson Laboratory | 007909 RRID:IMSR_JAX:007909 |
| Rosa mT/mG (B6.129(Cg)-Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J) | The Jackson Laboratory | 007676 RRID:IMSR_JAX:007676 |
| Rosa26-EYFP (B6.129X1-Gt(ROSA)26Sortm1(EYFP)Cos/J) | The Jackson Laboratory | 006148 RRID:IMSR_JAX:006148 |
| Vil1-Cre(ER-T2) | Sylvie Robine, Institut Pasteur, Paris, France | |
| Oligonucleotides | ||
| RNAscope® Ackr4 probes | ACD | 1081831 |
| RNAscope® Bmp4 probes | ACD | 454301 |
| RNAscope® Bmp5 probes | ACD | 401241 |
| RNAscope® Grem1 probes | ACD | 314741 |
| RNAscope® Olfm4 probes | ACD | 311831 |
| RNAscope® Rspo3 probes | ACD | 483781 |
| RNAscope® Sfrp1 probes | ACD | 404981 |
| RNAscope® Wnt2b probes | ACD | 405031 |
| RNAscope® Wnt4 probes | ACD | 401101 |
| Software and algorithms | ||
| Cell Ranger v2.1.1 | 10X Genomics | https://support.10xgenomics.com/single-cell-gene-expression/software/pipelines/latest/installation |
| BioRender | BioRender.com | |
| cnmf v1.3.2 | Kotliar et al., 2019 | https://github.com/dylkot/cNMF |
| Corrplot | Wei et al., 2017 | https://github.com/taiyun/corrplot |
| DeepTools | Ramírez et al., 2016 | https://github.com/deeptools/deepTools |
| DESeq2_1.26.0 | Love et al., 2014 | https://bioconductor.org/packages/release/bioc/html/DESeq2.html |
| Diffusion Pseudotime (dpt) | Haghverdi et al., 2016 | https://www.nature.com/articles/nmeth.3971 |
| Fiji (ImageJ 1.52p) | Schindelin et al., 2012 | https://imagej.net/Fiji/ |
| FlowJo v10.6 | BD Biosciene | |
| ggplot2_3.3.2 | Wickham H., 2016 | ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York. ISBN 978-3-319-24277-4, https://ggplot2.tidyverse.org |
| GraphPad Prism | GraphPad, Inc | |
| Integrated Genomics Viewer, ver 2.9.4 | Robinson et al., 2011 | http://software.broadinstitute.org/software/igv/ |
| LAS X Version 3.5.7.23225 | Leica Microsystems CMS GmbH | |
| MAST algorithm | Finak et al., 2015 | https://github.com/RGLab/MAST |
| Morpheus | Broad Institute | https://software.broadinstitute.org/morpheus/ |
| Python functions for scRNA-seq | Wager Lab, UCSF | DOI: 10.5281/zenodo.7686568 |
| R software for statistical computing | R Development Core Team, 2013 | https://cran.r-project.org/bin/windows/ |
| RSeQC | Wang et al., 2012 | http://rseqc.sourceforge.net/ |
| RStudio | RStudio Team (2020). | RStudio: Integrated Development for R. RStudio, PBC, Boston, MA URL http://www.rstudio.com |
| Seurat_3.2.2 | Butler et al., 2018 | https://github.com/satijalab/seurat |
| slingshot_1.4.0 | Street et al., 2018 | https://bioconductor.org/packages/release/bioc/html/slingshot.html |
| STAR | Dobin et al., 2013 | https://github.com/alexdobin/STAR/releases |
| t-SNE algorithm | van der Maaten, 2014 | https://lvdmaaten.github.io/tsne/ |
| VIPER | Cornwell et al., 2018 | https://bitbucket.org/cfce/viper/ |
| Zen blue 3.5 (Zen lite) Version 3.5.093 | Carl Zeiss Microscopy GmbH | |
| Other | ||
| ACD HybEZ II Hybridization System | ACD | 321710 |
| 10X Genomics Controller | 10X Genomics | 1000204 |
| Leica cryostat | Leica Microsystems | CM-1510-3 |
| Slide Spacers | Grace Bio-Labs | 654002 |
Data and code availability
Sequencing data have been deposited in the Gene Expression Omnibus (GEO) repository under the accession numbers GSE211275 and GSE212601 as listed in the Key Resources Table. Data are publicly available as of the date of publication.
This study includes analysis of previously published data, referenced with the accession numbers in the Key Resources Table.
This study used custom functions in a Python environment (3.7.13) available at Zenodo, DOI:10.5281/zenodo.7686568
Any additional information required to reanalyze data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL DETAILS
Mouse strains and husbandry
All animal procedures were approved by Animal Care and Use Committees at Dana-Farber Cancer Institute or Columbia University Medical Center. Mice were housed in 12-hour light/dark cycles with constant temperature and ad libitum access to food and water. Strains were maintained on a mixed C57BL/6 background and both male and female mice aged 8–16 weeks were used. PdgfraH2BeGFP (JAX strain 007669)69, Myh11CreERT2 (JAX strain 019079)70, Atoh1fl/fl (JAX strain 008681)44, Wnt4CreERT2 (JAX strain 032489)52, Rosa26LSL-TdTomato (JAX strain 007909)53, PdgfraCreERT2 (JAX strain 032770)40, Rosa26mT/mG (JAX strain 007676)41, FoxL1CreERT2−2A-tdTomato (RRID: MMRRC_068163-JAX)39 and Rosa26EYFP (JAX strain 006148)71 mice were purchased from the Jackson Laboratories. Porcnfl/fl mice42 and Vil1CreERT2 mice72 were generous gifts from C. Murtaugh (University of Utah) and from S. Robine (Institut Curie, Paris), respectively. For Grem1-DTRtdTom mice, a targeting vector pMCS-Grem1-P2A-DTR-P2A-TdTomato was created to replacing the translational stop codon TAA in the Grem1 locus. Male chimeras were made by injecting correctly targeted FL19 (C57BL/6N) embryonic stem cells into C57BL/6J blastocysts.
Tamoxifen (Sigma, 1 mg) dissolved in sterile sunflower oil (Sigma) was injected intraperitoneally for 3 or 5 days (as specified) to induce cell-type-specific gene recombination via CreERT2. Injected Cre-negative littermates were used as controls. Vil1-CreERT2;Atoh1fl/fl (Atoh1-null) mice were sampled 3 weeks after the tamoxifen injection to ensure abolition of secretory cells, including long-lived Paneth cells. For imaging studies, PdgfraH2BeGFP;Myh11CreERT2;R26RLSL-TdTomato mice were harvested 5 days after 2 daily tamoxifen injections. Wnt4CreERT2;Rosa26RLSL-TdTomato mice were given tamoxifen, 6 mg dissolved in corn oil, by oral gavage on 3 consecutive days. FoxL1CreERT2−2A-tdTomato;Rosa26EYFP mice used for whole-mount imaging and all PdgfraCreERT2 mice were injected on 5 consecutive days with 1 mg tamoxifen daily. PdgfraCreERT2;Porcnfl/fl (Porcn-null) female mice were injected for 5 consecutive days with 1 mg tamoxifen daily and examined 10 days later. Injected Cre-negative littermates served as controls.
METHOD DETAILS
Sampling and cell isolation
To sample distinct intestinal regions, we divided the small intestine (SI) from stomach end to ileocecal junction into 5 segments of equal length, designated the first segment as Duodenum, the third segment as Jejunum, and the fifth segment as Ileum; intermediate regions were discarded. The proximal half of colon (~3 cm) was also used. Littermates of PdgfraH2BeGFP mice lacking this Gfp allele were used to isolate intestinal crypts.
Mesenchymal cell isolation
Mesenchymal cells were isolated as described previously17. Briefly, mice were sacrificed and perfused with phosphate-buffered saline (PBS) by cardiac injection. Intestines were harvested, washed in PBS, and the outer muscle layer was removed with tweezers under stereomicroscope guidance. Tissue pieces were incubated twice, shaking at 300 rpm at 37°C for 15 min each time, in 10 mM EDTA in Hank’s Balanced Salt Solution (HBSS, Wisent) +10 mM HEPES (Gibco) +5% fetal bovine serum (FBS, Sigma). Epithelium-depleted pieces were then washed in HBSS +10 mM HEPES and digested with 3 mg/ml Collagenase 2 (Worthington) for 20 min under rotation at 30 rpm at 37°C. The resulting cell suspension was filtered through a 40 μm mesh (Falcon), centrifuged at 300 g for 10 min at 4°C, and washed again in HBSS +10 mM HEPES before staining procedures and flow cytometry.
Unfractionated mesenchyme isolates for expansion culture were centrifuged at 300 g for 10 min at room temperature and kept in Advanced DMEM (Life Technologies) supplemented with 10% FBS (Sigma) for 3 days.
Flow cytometry for Foxl1tdTom, PdgfraCreERT2 and PdgfraH2BeGFP was performed on cells stained for CD34 (Biolegend, 119310, 1:100) or PDGFRA (CD140a, BioLegend 135905, 1:100), as indicated, for 20 min at 4°C in FACS buffer (HBSS, 10 mM HEPES, 2 mM EDTA, 0.5% bovine serum albumin (Sigma)). Single cell (sc)RNA-seq of PdgfraH2BeGFP cells proceeded on unstained cells sorted by GFP levels. For colonic whole mesenchyme scRNA-seq, cells were stained with EpCAM (eBioscience, 12-5791-83, 1:100) and CD45 (Invitrogen, 17045182, 1:100) antibodies (Ab) to exclude epithelial and immune cells.
For scRNA-seq of small intestinal PDGFRA+ stromal cells,10 cm of jejunum from a male 8-week-old mouse were dissected, flushed with cold PBS, and opened longitudinally. Tissue was cut into small pieces and incubated in cold PBS +10 mM EDTA to release epithelial cells and the remaining fragments were digested using Collagenase XI (Sigma) and Dispase II (Sigma) for 30 min at 37°C. Filtered single cells were stained with PDGFRA (Biolegend, 135905) and CD31− Ab for 20 min on ice and live (DAPI−) CD31− PDGFRA+ cells were isolated.
For all other experiments, cells were stained with biotinylated anti-CD81 (eBiosciences 13-0811-81, 1:50) or control biotinylated IgG for 25 min at 4°C in FACS buffer, washed, and incubated with streptavidin-APC (eBiosciences, 17431782, 1:100). For CD55 sorting, anti-CD55-PE (Biolegend, 131803, 1:100) or control IgG-PE (Biolegend, 400907, 1:100) Ab was added to streptavidin-APC during the second incubation. Each staining step was followed by centrifugation (300 g, 5 min, 4°C) and washing in FACS buffer.
Fluorescence-activated cell sorting
Mesenchymal cells were sorted using a BD FACS M Aria II instrument with 100 μm nozzle. Cells were collected in FACS buffer and used directly to extract RNA or washed with Dulbecco’s Modified Eagle Medium (DMEM, Gibco) for organoid co-culture and sub-culture.
Bulk RNA-sequencing
Freshly sorted mesenchymal cells from different intestinal regions were used immediately for RNA extraction with RNAeasy Plus micro kit (Qiagen). RNA integrity and concentration were determined using Agilent Bioanalyzer High Sensitivity RNA 6000 pico kits (Agilent). For samples with RNA-integrity number (RIN) >8.5, libraries were constructed using SMART-Seq v4 Ultra Low Input RNA kits and sequenced on the Novaseq platform (Illumina).
Single-cell RNA sequencing
8,000 cells were loaded onto a 10X Chromium controller followed by the Single Cell 3′ V3.1 assay (10X Genomics PN-120237). Libraries were constructed according to the manufacturer’s recommendations and sequenced on the NovaSeq platform (Illumina).
Assay for Transposase-Accessible Chromatin (ATAC-seq)
ATAC-seq was performed on 50,000 live cells, sorted for PDGFRA level and presence of CD81, using the “Omni-ATAC” protocol73 and library preparation as described previously74. After quantification by Qubit dsDNA high-sensitivity assay (Life Technologies) and assessment of library quality using a High Sensitivity DNA Kit on a Bioanalyzer 2100 instrument (both Agilent), residual primers were excluded using AMPure beads (Beckman Coulter). Libraries were sequenced on the NovaSeq platform (Illumina).
Mesenchymal cell culture
As freshly sorted SEMFs grow poorly in isolation, we obtained adequate numbers for Wnt experiments by first culturing whole unfractionated mesenchyme up to 3 days in Advanced DMEM/F12 with 10% FBS before sorting (“expanded”). Cells were washed in PBS, digested with 0.25% Trypsin-EDTA (Gibco) for 5 min at 37°C, neutralized with DMEM +10% FBS, and centrifuged at 300 g for 5 min at room temperature before staining for CD81 and sorting. Cells were plated immediately after sorting with Atoh1-null (or control) SI crypts or wild-type colonic crypts and cultured in ENR medium or RNA was extracted to assess potential transcriptional drift.
Freshly isolated PDGFRAlo cells sorted for CD81 and CD55, as described above, were expanded in vitro for 2–7 days in MesenCult Expansion medium (Stem Cell Technologies). For primary cell treatments, serum-free Advanced/DMEM/F12 medium was supplemented with a mix of BMP-2 (Peprotech), BMP-4 (Peprotech) and BMP-7 (R&D Systems) or a mix of GREM1 (Thermo Fisher) and NOG (Peprotech) at 100 ng/ml each. Cells were treated for 5 days, with replacement of the medium every 2 days, before harvest for qRT-PCR analysis or further assays.
For subsequent co-culture, sorted and expanded mesenchymal cells were washed in PBS, digested with 0.25% Trypsin-EDTA for 5 min at 37°C, neutralized with Advanced DMEM/F12 medium (Gibco) containing 10% FBS and centrifuged at 300 g for 5 min. Cells were transferred to ice, counted with a hemocytometer and pelleted by centrifugation before adding to freshly isolated intestinal crypts mixed in Matrigel (Corning) droplets (1,000 mesenchymal cells per μl Matrigel (i.e. 10,000 cells per 10 μl Matrigel droplet) for SI crypts or 2,000 mesenchymal cells per μl Matrigel (i.e. 20,000 cells per 10 μl Matrigel droplet) for colonic crypts).
Crypt isolation and Intestinal organoid culture
Crypt epithelium from dissected intestines was enriched by 2 successive incubations for 15 min in 2 mM EDTA (SI) or for 45 min in 5 mM EDTA (colon) in HBSS at 4°C on a tube rotator at 30 rpm. Washed crypts were shaken 30 times manually and filtered through a 70 μm mesh (Falcon) to separate crypts from villi (SI only). Crypt suspensions were centrifuged at 80 g at 4°C for 8 min, washed in DMEM, and centrifuged again. Between 50 and 100 crypts were added to 10 μl or 20 μl Matrigel (Corning) droplets, respectively, and mixed with mesenchymal cells (when applicable) before culture in 24-well plates or chamber slides (Nunc) to facilitate imaging.
Standard organoid medium (ENR) as described previously4,17 contained Advanced DMEM, 1% B27 (Gibco), 0.5% N2-supplement (Gibco), 125 mM N-Acetylcysteine (Sigma), 10% Rspo1-conditioned medium (Harvard Digestive Disease Center), 100 ng/ml rNOG (Peprotech), 100 ng/ml rEGF (Peprotech) and 10 μM Y-27632 (Sigma). Co-cultures with Atoh1-null crypts occurred in organoid medium lacking R-spondin, rEGF, and rNOG unless otherwise specified. The GSK3-inhibitor CHIR99021 (Sigma, 5 μM) served as a positive control for canonical Wnt-activation. Porcupine inhibitor Wnt-C59 (Thermo Fisher, 100 nM/ml) was added immediately after plating. Co-cultures were maintained at 37°C and 5% CO2 and the medium replaced every two days.
Brightfield images were acquired with a CKX53 microscope (Olympus) using 4X or 2X objectives to count structures. Organoids were counted manually across the entire well using Fiji (ImageJ 1.52p) software and the Multi-point tool to mark counted structures. Organoid diameters were measured by drawing mid-organoid lines that we quantified using the Measure tool in Fiji. Measurements were summarized in Excel (Microsoft) and plotted using Prism9 (Graph Pad).
RNA extraction, reverse transcription, and qRT-PCR
Freshly sorted cells were pellet and lysed in RLT Plus buffer (Qiagen). DNA elimination and RNA extraction were achieved using RNeasy Plus Micro kit (Qiagen).
Cultured cells or isolated crypts were lysed in TRIZOL reagent (Life Technologies) and RNA was extracted by combining TRIZOL-chloroform extraction with RNA-binding columns (PureLink kit). RNA (50–200 ng, quantified by NanoDrop spectrophotometer (Thermo Fisher) was reverse transcribed using SuperScript kit (Life Technologies) according to the manufacturer’s instructions. Gene expression levels were measured by real-time PCR using Power SYBR reagents (Life Technologies) on a BioRad instrument.
Tissue processing and immunofluorescence
Mice were perfused with 4% paraformaldehyde and intestines were fixed overnight at 4 °C. After washing in PBS, samples were placed in sucrose gradients of 10% (6 h, 4°C), 20% overnight, and 30% overnight at 4°C. Tissue rolls were embedded in OCT medium (Sakura) and stored at −80°C until cryo-sectioning of 10 μm slices onto Superfrost Plus microscope slides (Thermo Fisher). Goblet cells were visualized using the Alcian Blue stain kit (H3501, Vector Laboratories) according to the manufacturer’s recommendations. Paneth cells were visualized after blocking slides for 1 h with 5% goat serum in PBST followed by incubations with anti-LYZ Ab (Dako, A009, 1:100 dilution in blocking buffer) at 4°C overnight, AlexaFluor anti-rabbit secondary antibody (Life Technologies), and Vectashield+DAPI mounting medium (Vector Laboratories).
For hematoxylin and eosin (H&E) images, formaldehyde-fixed tissues were processed to paraffin-embedded (FFPE) blocks, followed by cutting 10 μm sections, H&E staining, and imaging (all performed by Servicebio).
For Phospho-SMAD and Ki67 staining, fixed frozen tissue sections (10 μm) were washed with PBS and antigens were retrieved by boiling for 1 min in 10 mM Citrate buffer (pH 6.0). Primary Pdgfralo CD81− CD55hi mesenchymal cells were cultured for 4 days in MesenCult on chamber slides and treated with rBMPs or rBMPi as above. Cells were fixed with 4% paraformaldehyde for 30 min at room temperature, permeabilized for 30 min with PBS +0.3% Triton-X100, and washed twice in PBS. Endogenous peroxidase activity in both tissue sections and fixed cells was blocked by incubation for 1 h with 0.3% H202 in TN buffer (0.1 M Tris-HCl, pH 7.5; 0.15 M NaCl). Slides were washed twice in TN buffer, blocked for 1 h at room temperature with 0.5% Blocking Reagent (Perkin Elmer) in TN buffer +0.05% Tween-20 (BioRad), and incubated overnight with 1:300 dilution of anti-pSMAD1/5 (Cell Signaling Technologies, 9516) or anti-MKI67 (Invitrogen, MA5-14520) Ab at 4°C. Slides were washed 3 times in TN buffer +Tween-20, incubated for 1 h at room temperature with biotinylated 2nd antibody (1:300 in blocking buffer), washed 4 times, incubated with Streptavidin-HRP in blocking buffer, washed 3 times, incubated with Tyramide signal amplification (TSA) solution + 1μg/ml DAPI for 15 min, washed in PBS, and mounted as above.
RNAscope in situ hybridization (ISH), confocal imaging, and quantitation
Probes for RNAscope (catalog numbers in the Key Resources Table) were used as described previously (McCarthy et al., 2020a) except for 12 min target retrieval, based on the manufacturer’s recommendations with the RNAscope Multiplex Fluorescent Reagent Kit v2 (ACD). After the last hybridization, additional antibody staining and mounting were performed as above for ACTA2 (abcam, ab5694) or LAMA2 (Sigma, L9393). Slides were imaged using one of two confocal microscopes: Leica Sp5 for which image processing and export were performed in LAS X Version 3.5.7.23225 (Leica Microsystems CMS GmbH); or Zeiss Lsm900 using Zeiss ZEN blue 3.5 lite software (Carl Zeiss Microscopy GmbH).
Relative fluorescence intensities were quantified in these systems, summarized in Microsoft Excel, and plotted using Prism9 (GraphPad Inc) as follows: Regions of interest (ROI) were drawn along the crypt length above the muscularis mucosae, fluorescent channel intensity was quantified using the Profile function (grid distance=10) in Zeiss ZEN blue 3.5 lite. Values were calculated as % of total distance from crypt bottoms per image (crypt bottom to top =100%). To normalize for variation in crypt length or signal brightness, for each measurement, we calculated the % of average channel fluorescence per image. Intensities were measured in at least 3 different images from 3 different Wnt4CreERT2;tdTom or Grem1tdTom mice. Values were processed in R using reshape2 and ggplot2 to plot the smoothened mean with 95% confidence interval via function (geom_smooth, method=loess).
Whole mount sample preparation
The protocol for intestinal whole-mount imaging adapted from 75 was described previously 17. In brief, mouse tissue was perfused and fixed overnight in 4% paraformaldehyde in PBS, washed and incubated overnight in 10% sucrose +10% glycerol before blocking for 6 h and staining with anti-PDGFRA Ab (R&D Systems, AF1062) for 3 days at 4°C. After 5 washes, secondary antibody, phalloidin (Life Technologies), and DAPI were applied for 2 days at 4°C, followed by 10 washes in PBS. Small tissue sections were manually cut, cleared with FocusClear for 30 min at room temperature, and mounted in 97% Thiodiethanol (TDE) onto slides with a space holder (Grace Bio-Labs) before sealing with a coverslip.
Protein extraction and immunoblotting
Cells were lysed in RIPA buffer (Sigma) with Complete Protease Inhibitor (Roche) for 20 min at 4°C and 30 min on ice. Lysates were centrifuged at 12,000 g for 15 min at 4°C to remove cellular debris and supernatants were stored at −80°C until further use. Protein extracts were quantified using a BCA kit (Pierce). 5–7 μg protein was boiled for 5 min at 95°C in 4x Laemmli buffer (BioRad), loaded onto 5–12% gradient gels (BioRad), and electrophoresed at 100 V for 45 min in Running buffer (BioRad). Protein was transferred onto PVDF membranes at 55 V for 90 min at 4°C in transfer buffer (BioRad) containing 10% methanol (Sigma). Phospho-Smad 1/5 (1:1000, Cell Signaling Technology, 9516) and ACTB (loading control, 1:500, Sigma, A5441) were detected with HRP-coupled secondary antibodies (Invitrogen, 656120 and 626520) and chemiluminescence products were imaged by LS Quant 4000 (GE healthcare). Signal intensities were quantified in Fiji (ImageJ 1.52p). pSMAD1/5 band intensity was normalized to that of ACTB control from the same sample lane and blot.
Data processing and analysis
Bulk RNA-sequencing
RNA-seq samples were aligned to the mm10 genome and processed using the Viper pipeline 76 with default parameters. Differential gene expression analysis was performed in R software (R Core Team (2015)) version 3.6.0 and the Bioconductor (Biobase_2.46.0, BiocParallel_1.20.1) package DESeq2 ver1.26.0 78 using |log2FC| cut-off >1.5 and Padj <0.01. Normalized counts extracted from DEseq2 and log2FC values were plotted in GraphPad Prism9. Related samples were clustered by Euclidian distance, method=”complete”, and plotted with pHeatmap ver 1.0.12.
Single-cell RNA-sequencing
Samples were aligned to the mm10 genome using 10x Genomics Cell Ranger 3.0.2 using default parameters79. Bioinformatic analyses were conducted via R version 3.6.0. Single-cell analyses used Seurat_3.2.2.80. Cells were filtered for a minimum of 1,000 and a maximum of 3,500 reads and <5% mitochondrial reads and data were log-normalized (factor=10,000). Cell features were detected with the FindVariableFeatures function (2,000 features, selection method “vst”). The first 15 principal components were taken to run uniform manifold approximation and projection (UMAP) algorithms81. Cell types were identified using established marker genes detected by the “FindAllMarkers” function in Seurat2 (min.pct=0.25, test “roc”). Data from SI (sample GSM3242185 from GEO series GSE116514)23 and colon (GSE114374, Mouse_HC_expression_matrix)49 were processed in the same manner. Multiple samples were merged using Seurat’s integration function over 2,000 features with SCT-normalization.
Some scRNA-seq data from SI were generated previously17: GEO accession nos. GSM4196131 and GSM4196132. Two libraries of unfractionated SI mesenchyme were processed and combined by integration in Seurat as above. As these samples had not excluded CD45+ cells, we filtered these out by Ptprc (Cd45) expression in the respective clusters. Pearson correlation was calculated in R based on AverageExpression of all SCT-normalized gene counts per cell type cluster and plotted with the package pheatmap_1.0.12. Exported raw RNA-seq counts from this dataset were subsequently used in cNMF analysis for the SI.
Consensus non-negative matrix factorization (cNMF)
cNMF analyses were performed in a Python environment (3.7.13) using custom functions (https://github.com/wagnerde/scTools-py.git), Scrublet (0.2.3), and ScanPy (1.8.2). Colonic scRNA-seq data were filtered for cells with at least 3,000 detected transcripts, <5% mitochondrial reads, and doublet scores <0.20. cNMF analyses were performed with the cnmf package (v1.3.2) using the prepare, factorize, combine, and consensus subroutines with n=100 iterations and n=2,000 variable genes, as described50. For each dataset, k values were determined empirically to maximize silhouette scores (stability) relative to the Frobenius reconstruction error through the use of a k-selection plot. The values determined for each sample were k=22 (SI, whole mesenchyme), k=18 (colon, whole mesenchyme), k=16 (SI, PDGFRA+ cells). UMAP plots for GEP score overlays were constructed within ScanPy using n=2,000 variable genes and n=50 principal components.
Trajectory Analysis
Expression over pseudotime (Fig. 3C) was calculated using Slingshot48. Graph diffusion distance-based ordering was performed on a subset of 3,370 Pdgfralo cells by randomly selecting a root cell in the Grem1+ trophocyte cluster and calculating the diffusion pseudotime (dpt) distance of all remaining cells relative to that root 51. Trajectories were assembled for paths through specified clusters, with cells ordered by dpt values. Mean and SD values for select genes were plotted along sliding windows of 200 cells (Fig. 3F).
ATAC-sequencing
Reads were trimmed using trim_galore v0.4.5, using a stringency of 3 for adapter trimming and a minimum trimmed read length of 30 (Krueger et al., 2021). Alignment to the mm10 genome was done using bowtie2 v2.3.4.3 with default parameters83. Samtools v1.9 was used for file processing, including the removal of duplicate reads84. Sample quality was assessed using the RSeqQC v4.0.0 suite85. Peaks were called using MACS2 v2.1.1 (Feng et al., 2012), with q-value cut-off 0.001, lower Mfold=5, upper Mfold=50. Bigwig files were generated using bamCoverage from the DeepTools v3.0.2 package, with bin size of 10 bp, smoothing of 30 bp, and normalizing using reads per genomic content. Unstranded peak overlap of biological replicates was further processed excluding the mm10 blacklist. A universal peak set was obtained using the Merge tool in bedtools v2.29.087 to merge the peak overlap in each biological group and Bamliquidator (https://github.com/linlabcode/bamliquidator-batch) to aggregate counts within the universal peak set. The resulting matrix was used to perform a gap statistic calculation with clusGap from the cluster library in R v3.6.0, resulting in an optimal k=6. Enhancers were defined as open chromatin peak regions <1 kb and >2 kb from transcription start sites. ATAC-seq heatmaps (peak centers ±1.5 kb) were produced using the computeMatrix and plotHeatmap functions in DeepTools v3.3.2.0.188 using k=6.
QUANTIFICATION AND STATISTICAL ANALYSIS
Prism9 (GraphPad Inc) was used for statistical analysis of all data other than nucleotide sequences. Specific tests and significance levels are indicated in the respective figure legends. Non-parametric tests were chosen when data was not normally distributed based on D’Agostino & Pearson, Anderson-Darling, Shapiro-Wilk, and Kolmogorov-Smirnov tests with alpha=0.05. No prior determination of sample size or power calculation was performed.
Graphical illustrations were created with BioRender.com.
Supplementary Material
Table S1. Related to Figure1. Differentially expressed genes between different mesenchymal cell types. Comparisons as indicated, adj. P-value <0.01 and log2FC >±1.5. DESeq2-output, only genes with BaseMean expression >50 are shown.
Table S2. Related to Figure3. Top100 ranked genes in cNMF gene expression programs (GEPs) in small intestinal and colonic mesenchyme.
Highlights.
In addition to CD81+ trophocytes, other PDGFRAlo cells support intestinal stem cells
The CD81− fraction of PDGFRAlo cells is a principal source of canonical Wnt ligands
These cells segregate along the crypt length into niche-active and inert fractions
Distance from a BMP signaling center organizes that spatially segregated niche
Acknowledgments
Supported by NIH awards R01DK121540 (R.A.S.) and K01DK125639 (N.M.) and the Intestinal Stem Cell Consortium of NIDDK and NIAID: grants U01DK103152 (R.A.S.), UO1DK103155 (T.C.W.) and U01DK103147 (D.E.W.). J.K. is supported by the DFG, German Research Foundation (fellowship 444801317). We gratefully acknowledge the contributions of imaging, flow cytometry, and organoid core facilities at the Dana-Farber Cancer Institute, Harvard Digestive Diseases Center (P30DK034854) and Columbia University. We thank Dr. Charles Murtaugh (University of Utah) for the gift of PorcnFl mice42 and Dr. Carla Kim (Boston Children’s Hospital) for sharing Foxl1tdTom-Cre mice from the Jackson Laboratories.
Footnotes
Declaration of interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. (2007). Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007. 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 2.Barker N (2014). Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol 15, 19–33. 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 3.Kinzler KW, Nilbert MC, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hamilton SR, Hedge P, Markham A, et al. (1991). Identification of a gene located at chromosome 5q21 that is mutated in colorectal cancers. Science (1979) 251, 1366–1370. 10.1126/science.1848370. [DOI] [PubMed] [Google Scholar]
- 4.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, et al. (2009). Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265. 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 5.Sato T, Stange DE, Ferrante M, Vries RGJ, van Es JH, van den Brink S, van Houdt WJ, Pronk A, van Gorp J, Siersema PD, et al. (2011). Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141, 1762–1772. 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 6.Jung P, Sato T, Merlos-Suárez A, Barriga FM, Iglesias M, Rossell D, Auer H, Gallardo M, Blasco MA, Sancho E, et al. (2011). Isolation and in vitro expansion of human colonic stem cells. Nat Med 17, 1225–1227. 10.1038/nm.2470. [DOI] [PubMed] [Google Scholar]
- 7.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, and Clevers H (2011). Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418. 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim T-HHT-H, Escudero S, and Shivdasani RA (2012). Intact function of Lgr5 receptor-expressing intestinal stem cells in the absence of Paneth cells. Proc Natl Acad Sci U S A 109, 3932–3937. 10.1073/pnas.1113890109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durand A, Donahue B, Peignon G, Letourneur F, Cagnard N, Slomianny C, Perret C, Shroyer NF, and Romagnolo B (2012). Functional intestinal stem cells after Paneth cell ablation induced by the loss of transcription factor Math1 (Atoh1). Proc Natl Acad Sci U S A 109, 8965–8970. 10.1073/pnas.1201652109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farin HF, van Es JH, and Clevers H (2012). Redundant sources of Wnt regulate intestinal stem cells and promote formation of paneth cells. Gastroenterology 143, 1518–1529.e7. 10.1053/j.gastro.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 11.Powell DW, Adegboyega PA, di Mari JF, and Mifflin RC (2005). Epithelial cells and their neighbors: I. Role of intestinal myofibroblasts in development, repair, and cancer. Am J Physiol Gastrointest Liver Physiol 289. 10.1152/ajpgi.00075.2005. [DOI] [PubMed] [Google Scholar]
- 12.Powell DW, Pinchuk IV, Saada JI, Chen X, and Mifflin RC (2011). Mesenchymal Cells of the Intestinal Lamina Propria. Annu Rev Physiol 73, 213–237. 10.1146/annurev.physiol.70.113006.100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greicius G, Kabiri Z, Sigmundsson K, Liang C, Bunte R, Singh MK, and Virshup DM (2018). PDGFRα + pericryptal stromal cells are the critical source of Wnts and RSPO3 for murine intestinal stem cells in vivo. Proc Natl Acad Sci U S A 115, E3173–E3181. 10.1073/pnas.1713510115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kabiri Z, Greicius G, Madan B, Biechele S, Zhong Z, Zaribafzadeh H, Edison, Aliyev J, Wu Y, Bunte R, et al. (2014). Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development (Cambridge) 141, 2206–2215. 10.1242/dev.104976. [DOI] [PubMed] [Google Scholar]
- 15.Stzepourginski I, Nigro G, Jacob J-MM, Dulauroy S, Sansonetti PJ, Eberl G, and Peduto L (2017). CD34+ mesenchymal cells are a major component of the intestinal stem cells niche at homeostasis and after injury. Proc Natl Acad Sci U S A 114, E506–E513. 10.1073/pnas.1620059114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karpus ON, Westendorp BF, Vermeulen JLM, Meisner S, Koster J, Muncan V, Wildenberg ME, and van den Brink GR (2019). Colonic CD90+ Crypt Fibroblasts Secrete Semaphorins to Support Epithelial Growth. Cell Rep 26, 3698–3708.e5. 10.1016/j.celrep.2019.02.101. [DOI] [PubMed] [Google Scholar]
- 17.McCarthy N, Manieri E, Storm EE, Saadatpour A, Luoma AM, Kapoor VN, Madha S, Gaynor LT, Cox C, Keerthivasan S, et al. (2020). Distinct Mesenchymal Cell Populations Generate the Essential Intestinal BMP Signaling Gradient. Cell Stem Cell 26, 391–402.e5. 10.1016/j.stem.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valenta T, Degirmenci B, Moor AE, Herr P, Zimmerli D, Moor MB, Hausmann G, Cantù C, Aguet M, Basler K, et al. (2016). Wnt Ligands Secreted by Subepithelial Mesenchymal Cells Are Essential for the Survival of Intestinal Stem Cells and Gut Homeostasis. Cell Rep 15, 911–918. 10.1016/j.celrep.2016.03.088. [DOI] [PubMed] [Google Scholar]
- 19.Shoshkes-Carmel M, Wang YJ, Wangensteen KJ, Tóth B, Kondo A, Massassa EE, Itzkovitz S, Kaestner KH, Massasa EE, Itzkovitz S, et al. (2018). Subepithelial telocytes are an important source of Wnts that supports intestinal crypts. Nature 557, 242–246. 10.1038/s41586-018-0084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aoki R, Shoshkes-Carmel M, Gao N, Shin S, May CL, Golson ML, Zahm AM, Ray M, Wiser CL, Wright CVE, et al. (2016). Foxl1-Expressing Mesenchymal Cells Constitute the Intestinal Stem Cell Niche. Cell. Mol. Gastroenterol. Hepatol. 2, 175–188. 10.1016/j.jcmgh.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng M, Guerrero-Juarez CF, Sheng X, Xu J, Wu X, Yao K, Li M, Yang X, Li G, Xiao J, et al. (2022). Lepr + mesenchymal cells sense diet to modulate intestinal stem/progenitor cells via Leptin-Igf1 axis. Cell Res. 10.1038/S41422-022-00643-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarthy N, Kraiczy J, and Shivdasani RA (2020). Cellular and molecular architecture of the intestinal stem cell niche. Nat Cell Biol 22, 1033–1041. 10.1038/s41556-020-0567-z. [DOI] [PubMed] [Google Scholar]
- 23.Kim JE, Fei L, Yin WC, Coquenlorge S, Rao-Bhatia A, Zhang X, Shi SSW, Lee JH, Hahn NA, Rizvi W, et al. (2020). Single cell and genetic analyses reveal conserved populations and signaling mechanisms of gastrointestinal stromal niches. Nat Commun 11. 10.1038/s41467-019-14058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomson CA, van de Pavert SA, Stakenborg M, Labeeuw E, Matteoli G, Mowat AM, and Nibbs RJB (2018). Expression of the Atypical Chemokine Receptor ACKR4 Identifies a Novel Population of Intestinal Submucosal Fibroblasts That Preferentially Expresses Endothelial Cell Regulators. The Journal of Immunology 201, 215–229. 10.4049/jimmunol.1700967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brügger MD, Valenta T, Fazilaty H, Hausmann G, and Basler K (2020). Distinct populations of crypt-associated fibroblasts act as signaling hubs to control colon homeostasis. PLoS Biol 18, e3001032. 10.1371/journal.pbio.3001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butler A, Hoffman P, Smibert P, Papalexi E, and Satija R (2018). Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol 36, 411–420. 10.1038/nbt.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buenrostro JD, Giresi PG, Zaba LC, Chang HY, and Greenleaf WJ (2013). Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods 10, 1213–1218. 10.1038/nmeth.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klemm SL, Shipony Z, and Greenleaf WJ (2019). Chromatin accessibility and the regulatory epigenome. Nat Rev Genet 20, 207–220. 10.1038/S41576-018-0089-8. [DOI] [PubMed] [Google Scholar]
- 29.Mah AT, Yan KS, and Kuo CJ (2016). Wnt pathway regulation of intestinal stem cells. J Physiol 594, 4837. 10.1113/JP271754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, and Clevers H (1998). Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet 19, 379–383. 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 31.Farin HF, Jordens I, Mosa MH, Basak O, Korving J, Tauriello DVFF, de Punder K, Angers S, Peters PJ, Maurice MM, et al. (2016). Visualization of a short-range Wnt gradient in the intestinal stem-cell niche. Nature 530, 340–343. 10.1038/nature16937. [DOI] [PubMed] [Google Scholar]
- 32.Shimomura Y, Agalliu D, Vonica A, Luria V, Wajid M, Baumer A, Belli S, Petukhova L, Schinzel A, Brivanlou AH, et al. (2010). APCDD1 is a novel Wnt inhibitor mutated in hereditary hypotrichosis simplex. Nature 464, 1043–1047. 10.1038/NATURE08875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartscherer K, Pelte N, Ingelfinger D, and Boutros M (2006). Secretion of Wnt Ligands Requires Evi, a Conserved Transmembrane Protein. Cell 125, 523–533. 10.1016/J.CELL.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 34.Bänziger C, Soldini D, Schütt C, Zipperlen P, Hausmann G, and Basler K (2006). Wntless, a Conserved Membrane Protein Dedicated to the Secretion of Wnt Proteins from Signaling Cells. Cell 125, 509–522. 10.1016/J.CELL.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 35.Niec RE, Chu T, Schernthanner M, Gur-Cohen S, Hidalgo L, Pasolli HA, Luckett KA, Wang Z, Bhalla SR, Cambuli F, et al. (2022). Lymphatics act as a signaling hub to regulate intestinal stem cell activity. Cell Stem Cell. 10.1016/J.STEM.2022.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goto N, Goto S, Imada S, Hosseini S, Deshpande V, and Yilmaz ÖH (2022). Lymphatics and fibroblasts support intestinal stem cells in homeostasis and injury. Cell Stem Cell 29, 1246–1261.e6. 10.1016/J.STEM.2022.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palikuqi B, Rispal J, Reyes EA, Vaka D, Boffelli D, and Klein O (2022). Lymphangiocrine signals are required for proper intestinal repair after cytotoxic injury. Cell Stem Cell 29, 1262–1272.e5. 10.1016/J.STEM.2022.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Proffitt KD, and Virshup DM (2012). Precise regulation of porcupine activity is required for physiological Wnt signaling. Journal of Biological Chemistry 287, 34167–34178. 10.1074/JBC.M112.381970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kolev HM, Tian Y, Kim MS, Leu NA, Adams-Tzivelekidis S, Lengner CJ, Li N, and Kaestner KH (2021). A FoxL1-CreERT-2A-tdTomato Mouse Labels Subepithelial Telocytes. Cell. Mol. Gastroenterol. Hepatol. 12, 1155–1158.e4. 10.1016/J.JCMGH.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung MI, Bujnis M, Barkauskas CE, Kobayashi Y, and Hogan BLM (2018). Niche-mediated BMP/SMAD signaling regulates lung alveolar stem cell proliferation and differentiation. Development 145. 10.1242/DEV.163014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muzumdar MD, Tasic B, Miyamichi K, Li N, and Luo L (2007). A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605. 10.1002/DVG.20335. [DOI] [PubMed] [Google Scholar]
- 42.Barrott JJ, Cash GM, Smith AP, Barrow JR, and Charles Murtaugh L Deletion of mouse Porcn blocks Wnt ligand secretion and reveals an ectodermal etiology of human focal dermal hypoplasia/Goltz syndrome. 10.1073/pnas.1006437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Q, Bermingham NA, Finegold MJ, and Zoghbi HY (2001). Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science (1979) 294, 2155–2158. 10.1126/SCIENCE.1065718/SUPPL_FILE/1065718S4_THUMB.GIF. [DOI] [PubMed] [Google Scholar]
- 44.Shroyer NF, Helmrath MA, Wang VYC, Antalffy B, Henning SJ, and Zoghbi HY (2007). Intestine-specific ablation of mouse atonal homolog 1 (Math1) reveals a role in cellular homeostasis. Gastroenterology 132, 2478–2488. 10.1053/J.GASTRO.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 45.Proffitt KD, Madan B, Ke Z, Pendharkar V, Ding L, Lee MA, Hannoush RN, and Virshup DM (2013). Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of WNT-driven mammary cancer. Cancer Res 73, 502–507. 10.1158/0008-5472.CAN-12-2258. [DOI] [PubMed] [Google Scholar]
- 46.Gregorieff A, Pinto D, Begthel H, Destrée O, Kielman M, and Clevers H (2005). Expression Pattern of Wnt Signaling Components in the Adult Intestine. Gastroenterology 129, 626–638. 10.1053/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 47.Yan KS, Janda CY, Chang J, Zheng GXYY, Larkin KA, Luca VC, Chia LA, Mah AT, Han A, Terry JM, et al. (2017). Non-equivalence of Wnt and R-spondin ligands during Lgr5 + intestinal stem-cell self-renewal. Nature 545, 238–242. 10.1038/nature22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Street K, Risso D, Fletcher RB, Das D, Ngai J, Yosef N, Purdom E, and Dudoit S (2018). Slingshot: Cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genomics 19. 10.1186/S12864-018-4772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kinchen J, Chen HH, Parikh K, Antanaviciute A, Jagielowicz M, Fawkner-Corbett D, Ashley N, Cubitt L, Mellado-Gomez E, Attar M, et al. (2018). Structural Remodeling of the Human Colonic Mesenchyme in Inflammatory Bowel Disease. Cell 175, 372–386.e17. 10.1016/j.cell.2018.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kotliar D, Veres A, Nagy MA, Tabrizi S, Hodis E, Melton DA, and Sabeti PC (2019). Identifying gene expression programs of cell-type identity and cellular activity with single-cell RNA-Seq. Elife 8. 10.7554/eLife.43803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haghverdi L, Büttner M, Wolf FA, Buettner F, and Theis FJ (2016). Diffusion pseudotime robustly reconstructs lineage branching. Nature Methods 2016 13:10 13, 845–848. 10.1038/nmeth.3971. [DOI] [PubMed] [Google Scholar]
- 52.Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, and McMahon AP (2008). Six2 Defines and Regulates a Multipotent Self-Renewing Nephron Progenitor Population throughout Mammalian Kidney Development. Cell Stem Cell 3, 169–181. 10.1016/J.STEM.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. (2010). A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13, 133–140. 10.1038/NN.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jasso GJ, Jaiswal A, Varma M, Laszewski T, Grauel A, Omar A, Silva N, Dranoff G, Porter JA, Mansfield K, et al. (2022). Colon stroma mediates an inflammation-driven fibroblastic response controlling matrix remodeling and healing. PLoS Biol 20, e3001532. 10.1371/JOURNAL.PBIO.3001532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sunter JP, Wright NA, and Appleton DR (1978). Cell population kinetics in the epithelium of the colon of the male rat. Virchows Arch B Cell Pathol 26, 275–287. 10.1007/BF02889556. [DOI] [PubMed] [Google Scholar]
- 56.Cheng H, and Bjerknes M (1985). Whole population cell kinetics and postnatal development of the mouse intestinal epithelium. Anat Rec 211, 420–426. 10.1002/AR.1092110408. [DOI] [PubMed] [Google Scholar]
- 57.Clevers H, Loh KM, and Nusse R (2014). An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science (1979) 346, 1248012–1248012. 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- 58.Qi Z, Li Y, Zhao B, Xu C, Liu Y, Li H, Zhang B, Wang X, Yang X, Xie W, et al. (2017). BMP restricts stemness of intestinal Lgr5 + stem cells by directly suppressing their signature genes. Nat Commun 8, 13824. 10.1038/ncomms13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pinto D, Gregorieff A, Begthel H, and Clevers H (2003). Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev 17, 1709–1713. 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J, Nusse R, and Kuo CJ (2004). Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci U S A 101, 266–271. 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Degirmenci B, Valenta T, Dimitrieva S, Hausmann G, and Basler K (2018). GLI1-expressing mesenchymal cells form the essential Wnt-secreting niche for colon stem cells. Nature 558, 449–453. 10.1038/s41586-018-0190-3. [DOI] [PubMed] [Google Scholar]
- 62.Roulis M, Kaklamanos A, Schernthanner M, Bielecki P, Zhao J, Kaffe E, Frommelt L-S, Qu R, Knapp MS, Henriques A, et al. (2020). Paracrine orchestration of intestinal tumorigenesis by a mesenchymal niche. Nature 580, 524–529. 10.1038/s41586-020-2166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCarthy N, Tie G, Madha S, Ruiyang H, Kraiczy J, Maglieri A, and Shivdasani R (2023). Smooth muscle contributes to the development and function of a layered intestinal stem cell niche. Dev Cell. 10.2139/SSRN.4149949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kobayashi H, Gieniec KA, Wright JA, Wang TCT, Asai N, Mizutani Y, Lida T, Ando R, Suzuki N, Lannagan TRM, et al. (2021). The Balance of Stromal BMP Signaling Mediated by GREM1 and ISLR Drives Colorectal Carcinogenesis. Gastroenterology 160, 1224–1239.e30. 10.1053/j.gastro.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bahar Halpern K, Massalha H, Zwick RK, Moor AE, Castillo-Azofeifa D, Rozenberg M, Farack L, Egozi A, Miller DR, Averbukh I, et al. (2020). Lgr5+ telocytes are a signaling source at the intestinal villus tip. Nat Commun 11. 10.1038/s41467-020-15714-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haramis APG, Begthel H, van den Born M, van Es J, Jonkheer S, Offerhaus GJA, and Clevers H (2004). De Novo Crypt Formation and Juvenile Polyposis on BMP Inhibition in Mouse Intestine. Science (1979) 303, 1684–1686. 10.1126/science.1093587. [DOI] [PubMed] [Google Scholar]
- 67.Walton KD, and Gumucio DL (2020). Hedgehog Signaling in Intestinal Development and Homeostasis. Annual Review of Physiology Annu. Rev. Physiol 2021 83, 359–380. 10.1146/annurev-physiol-031620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chee YC, Pahnke J, Bunte R, Adsool VA, Madan B, and Virshup DM (2018). Intrinsic Xenobiotic Resistance of the Intestinal Stem Cell Niche. Dev Cell 46, 681–695.e5. 10.1016/j.devcel.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 69.Hamilton TG, Klinghoffer RA, Corrin PD, and Soriano P (2003). Evolutionary Divergence of Platelet-Derived Growth Factor Alpha Receptor Signaling Mechanisms. Mol Cell Biol 23, 4013–4025. 10.1128/mcb.23.11.4013-4025.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wirth A, Benyó Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Rsy PO+, Horváth B, Maser-Gluth C, Greiner E, et al. (2008). G 12-G 13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- 71.Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, and Costantini F (2001). Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 1, 1–8. 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.el Marjou F, Janssen KP, Chang BHJ, Li M, Hindie V, Chan L, Louvard D, Chambon P, Metzger D, and Robine S (2004). Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 39, 186–193. 10.1002/gene.20042. [DOI] [PubMed] [Google Scholar]
- 73.Buenrostro JD, Wu B, Chang HY, and Greenleaf WJ (2015). ATAC-seq: A Method for Assaying Chromatin Accessibility Genome-Wide. Curr Protoc Mol Biol 109, 21.29.1–9. 10.1002/0471142727.mb2129s109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jadhav U, Cavazza A, Banerjee KK, Xie H, O’Neill NK, Saenz-Vash V, Herbert Z, Madha S, Orkin SH, Zhai H, et al. (2019). Extensive Recovery of Embryonic Enhancer and Gene Memory Stored in Hypomethylated Enhancer DNA. Mol Cell 74, 542–554.e5. 10.1016/j.molcel.2019.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bernier-Latmani J, Cisarovsky C, Demir CS, Bruand M, Jaquet M, Davanture S, Ragusa S, Siegert S, Dormond O, Benedito R, et al. (2015). DLL4 promotes continuous adult intestinal lacteal regeneration and dietary fat transport. Journal of Clinical Investigation 125, 4572–4586. 10.1172/JCI82045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cornwell MI, Vangala M, Taing L, Herbert Z, Köster J, Li B, Sun H, Li T, Zhang J, Qiu X, et al. (2018). VIPER: Visualization Pipeline for RNA-seq, a Snakemake workflow for efficient and complete RNA-seq analysis. BMC Bioinformatics 19. 10.1186/S12859-018-2139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Team, R.C. R Core Team (2015). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. [Google Scholar]
- 78.Love MI, Huber W, and Anders S (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550. 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zheng GXY, Terry JM, Belgrader P, Ryvkin P, Bent ZW, Wilson R, Ziraldo SB, Wheeler TD, Mcdermott GP, Zhu J, et al. (2017). ARTICLE Massively parallel digital transcriptional profiling of single cells. Nat Commun. 10.1038/ncomms14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM, Hao Y, Stoeckius M, Smibert P, and Satija R (2019). Comprehensive Integration of Single-Cell Data. Cell 177, 1888–1902.e21. 10.1016/j.cell.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McInnes L, and Healy J (2018). UMAP: Uniform Manifold Approximation and Projection for Dimension Reduction. 10.21105/joss.00861. [DOI] [Google Scholar]
- 82.Krueger F, James F, Ewels P, Afyounian E, and Schuster-Boeckler B (2021). FelixKrueger/TrimGalore: v0.6.7 - DOI via Zenodo. 10.5281/ZENODO.5127899. [DOI] [Google Scholar]
- 83.Langmead B, and Salzberg SL (2012). Fast gapped-read alignment with Bowtie 2. Nat Methods 9, 357–359. 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Danecek P, Bonfield JK, Liddle J, Marshall J, Ohan V, Pollard MO, Whitwham A, Keane T, McCarthy SA, Davies RM, et al. (2021). Twelve years of SAMtools and BCFtools. Gigascience 10, 1–4. 10.1093/GIGASCIENCE/GIAB008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang L, Wang S, and Li W (2012). RSeQC: Quality control of RNA-seq experiments. Bioinformatics 28, 2184–2185. 10.1093/BIOINFORMATICS/BTS356. [DOI] [PubMed] [Google Scholar]
- 86.Feng J, Liu T, Qin B, Zhang Y, and Liu XS (2012). Identifying ChIP-seq enrichment using MACS. Nat Protoc 7, 1728–1740. 10.1038/nprot.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Quinlan AR, and Hall IM (2010). BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842. 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ramírez F, Ryan DP, Bj¨ B, Grüning B, Grüning G, Bhardwaj V, Kilpert F, Richter AS, Heyne S, Undar FD¨, et al. (2016). deepTools2: a next generation web server for deep-sequencing data analysis. Web Server issue Published online 44. 10.1093/nar/gkw257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Related to Figure1. Differentially expressed genes between different mesenchymal cell types. Comparisons as indicated, adj. P-value <0.01 and log2FC >±1.5. DESeq2-output, only genes with BaseMean expression >50 are shown.
Table S2. Related to Figure3. Top100 ranked genes in cNMF gene expression programs (GEPs) in small intestinal and colonic mesenchyme.
Data Availability Statement
Sequencing data have been deposited in the Gene Expression Omnibus (GEO) repository under the accession numbers GSE211275 and GSE212601 as listed in the Key Resources Table. Data are publicly available as of the date of publication.
This study includes analysis of previously published data, referenced with the accession numbers in the Key Resources Table.
This study used custom functions in a Python environment (3.7.13) available at Zenodo, DOI:10.5281/zenodo.7686568
Any additional information required to reanalyze data reported in this paper is available from the lead contact upon request.


