Abstract
Background:
Infrapatellar fat pad (IFP) fibrosis is reportedly associated with anterior knee pain and the progression of patellofemoral osteoarthritis after anterior cruciate ligament reconstruction (ACLR). However, causes of IFP fibrosis after ACLR have not been sufficiently investigated.
Purpose:
To compare the descriptive characteristics, clinical outcomes, and inflammatory cytokine levels in the synovial fluid between patients who underwent ACLR with versus without severe IFP fibrosis.
Study Design:
Cohort study; Level of evidence, 3.
Methods:
Patients who underwent primary ACLR using autologous hamstring tendon were divided into 2 groups based on magnetic resonance imaging IFP fibrosis scoring (grades 0-5) at 3 months after surgery: the severe fibrosis group (grades 4 and 5) and mild fibrosis group (grades 0-3). Synovial fluid was aspirated on postoperative day 3 or 4 to measure inflammatory cytokine levels. Patient characteristics, clinical outcomes at 3 and 12 months after surgery, and inflammatory cytokine (interleukin [IL]-1β, IL-2, IL-6, IL-8, IL-10, tumor necrosis factor–α, and interferon-γ) levels were compared between the groups.
Results:
Of the 36 patients included, 7 were allocated to the severe fibrosis group and 29 were allocated to the mild fibrosis group. The severe fibrosis group had a significantly longer operation time (153.0 vs 116.5 minutes for mild fibrosis; P = .007). Compared with the mild fibrosis group, the severe fibrosis group had greater pain during stair climbing (2.0 vs 0.7; P = .01) and a lower extension muscle strength ratio (operated/healthy side, 52.9% vs 76.1%; P < .001) at 3 months, and the severe fibrosis group had a lower Lysholm score (93.7 vs 97.3; P = .026) and greater knee extension (0.3° vs 1.9°; P = .043) and flexion angle restriction (142.9° vs 149.0°; P = .013) at 12 months. The severe fibrosis group demonstrated higher IL-1β (2.6 vs 1.4 pg/mL; P = .022), IL-6 (2.0 vs 1.1 ng/mL; P = .029), and interferon-γ levels (11.3 vs 4.0 pg/mL; P = .044).
Conclusion:
Severe IFP fibrosis was associated with a longer operation time, higher inflammatory cytokine level in the synovial fluid, and worse clinical outcomes at 3 and 12 months after ACLR.
Keywords: fibrosis, infrapatellar fat pad, anterior cruciate ligament reconstruction, magnetic resonance imaging, inflammatory cytokines
Complications after anterior cruciate ligament (ACL) reconstruction (ACLR) include intra-articular fibrosis leading to residual pain and delayed functional recovery. 8 The infrapatellar fat pad (IFP) is a somatic fat tissue structure located between the patellar tendon and intra-articular space. It is extrasynovial; however, it is located in the intracapsular region of the knee joint. 9 It has proximal extensions that wrap around the patella. Furthermore, it is a dynamic and mobile structure that deforms during knee motion.27,41
Functions of IFP include lubrication of joints, 29 abruption of loading, mechanoreceptor/proprioceptor, 28 and vascular supply of the patella. 36 Additionally, IFP plays a central nociceptive role and contains abundant substance P nerves. It has been suggested that IFP can be a source of pain.3,5,7
Anterior knee pain after ACLR leads to delayed muscle recovery and poor clinical outcomes. 35 Among the reasons for anterior knee pain, pathologies of IFP, such as Hoffa disease (IFP impingement) or inflammation, play an important role.9,15 IFP fibrosis has been associated with anterior knee pain after ACLR. 32 In animal studies, knee arthritis induced by intra-articular injection of monosodium iodoacetate (MIA) caused joint inflammation. This was followed by extensive fibrotic changes in the IFP, which possibly played a crucial role in prolonged pain development in the knee joint.16,18 In addition, chronic IFP fibrosis leads to residual pain, 37 whereas antifibrotic treatment decreased IFP fibrosis and alleviated persistent pain in a rat arthritis model induced by MIA. 2 Accordingly, IFP fibrosis may result in unfavorable clinical outcomes.
The elevation of several cytokines has been found in the synovial fluid after ACL injury. The concentration of inflammatory cytokines, such as interleukin (IL)–6 and IL-8, was elevated during the early (between 0 and 48 hours after injury) 4 and late (>3 months after injury) phases of ACL injury. 1 Higher IL-1β and tumor necrosis factor (TNF)–α levels are associated with chondral damage at the time of ACLR. 30 Moreover, inflammatory cytokines are negatively associated with functional recovery after ACLR. 19 In fact, a recent study demonstrated that the degree of IFP synovitis, which was evaluated based on magnetic resonance imaging (MRI), 38 was correlated with several inflammatory cytokines including IL-6 and interferon (IFN)–γ in patients with an ACL injury. 14 However, the relationship between the elevation of inflammatory cytokines and IFP fibrosis in patients who had an ACLR has not been elucidated.
In this study, we aimed to (1) compare the clinical outcomes of ACLR between patients with or without IFP fibrosis and (2) investigate the association between IFP fibrosis and inflammatory cytokine levels in the synovial fluid in patients after ACLR. Our hypotheses were that patients with IFP fibrosis would show delayed muscle strength recovery and greater pain and that there would be several inflammatory cytokines associated with IFP fibrosis.
Methods
Participants
Patients who underwent primary double-bundle ACLR with an autologous semitendinosus tendon between 2012 and 2014 and who agreed to participate in the study were included. Patients with revision ACLR, multiple ligament reconstruction, bone–patellar tendon–bone graft use, prior injuries, or surgeries in the contralateral knee; aged >40 or <15 years; and lost to follow-up before 1 year were excluded. Based on the study inclusion and exclusion criteria, 36 patients were enrolled.
The study protocol was approved by the ethics committee at our institution. All data were collected in a prospective manner, and all patients included in this study gave their full written informed consent for participation in this study before ACLR.
Surgical Procedures
The procedures were performed by 1 of 2 attending surgeons (T.N. and H.Ko) or under their supervision. First, standard arthroscopy was undertaken through anterolateral and anteromedial portals to confirm the ruptured ACL and to evaluate the status of the menisci and articular cartilage. The mucosal ligament was removed before performing the meniscal surgery and ACLR. The fat pad and synovial membrane around the portals were removed as minimally as possible and only to obtain the clear visual field. If meniscal injury was identified, it was repaired if appropriate. Basically, tears in the posterior, middle, and anterior parts of the meniscus were repaired using the all-inside, inside-out, and outside-in techniques, respectively. Longitudinal tears including bucket-handle tears were generally repaired with vertical mattress sutures. Radial tears were mostly repaired with tie-grip sutures: the vertical sutures were first positioned near both edges of the tear site, and then 2 or 3 horizontal mattress sutures were placed perpendicular to and over the vertical sutures and the tear site. These sutures were tied over the joint capsule. Most of the lateral posterior root tears were repaired using a pullout technique. Centralization of the meniscus was added if extrusion of the lateral meniscus (LM) was confirmed by pushing the midbody of the meniscus out of the lateral tibial plateau using a probe. 22
ACLR was performed using a remnant-preserved anatomic double-bundle technique using the semitendinosus tendon as described previously. 31 Namely, the anteromedial and posterolateral bundles were created using the autologous semitendinosus tendon, which was cut into halves and folded to create 2 double-stranded bundles. Both femoral and tibial tunnels were created at the anatomic position of the insertion sites of each bundle. 34 The femoral sides of both grafts were fixed with the EndoButton CL (Smith & Nephew) and were then fixed to an anchor staple (Meira Corp) with sutures at the tibial site, at 20° of knee flexion with the applied initial tension adjusted to be equal per cross-sectional area on a basis of 25 N for a graft diameter of 6 mm. 23
Postoperative Management
The postoperative rehabilitation protocol was the same for all patients, except that weightbearing at >90° of flexion was prohibited in patients with meniscal repair until 3 months after surgery. From the day after the surgery, range of motion exercises were initiated, and walking exercises with a knee immobilizer and crutches was permitted. Crutches were used for 4 weeks. Patients who recovered >65% of muscle strength compared with the healthy side began running at 3 months after surgery. Patients who recovered >90% muscle strength were permitted full athletic activities at 6 months after surgery.
Clinical Evaluation
Clinical evaluations were performed preoperatively and at 3 and 12 months postoperatively. The Lysholm knee score was used to evaluate the general knee condition, and the Tegner scale was used to assess preinjury sports activity. 43 Subjective evaluation of patient satisfaction and sports performance was assessed on a scale from 0 to 100 (highest). Pain during walking and stair climbing was assessed on a numeric rating scale (NRS) from 0 to 10 (worst pain). Knee extension, measured in 1° increments, and flexion, measured in 5° increments, were assessed using a goniometer. When the knee extension angle was measured, the leg was passively raised in a supine position to maintain the knee in full extension, and the maximum extension strength (in kg·m) was measured in both knees using a Cybex machine (Lumex; accuracy up to 1 decimal place) at 60 deg/s. Extension strength was also recorded as a percentage of the unoperated knee. The test-retest reliability (standard error of measurement) of maximum knee extension strength using a Cybex machine ranged from 4.0% to 5.3% for maximum strength and 3.2% to 6.5% for ratio (right:left of the maximum knee extension strength). 17 Finally, physical activity at 3 months postoperatively was evaluated using a 4-point scoring system: 1 = hard to walk, 2 = walking, 3 = climbing stairs, and 4 = jogging. 19
MRI Evaluation
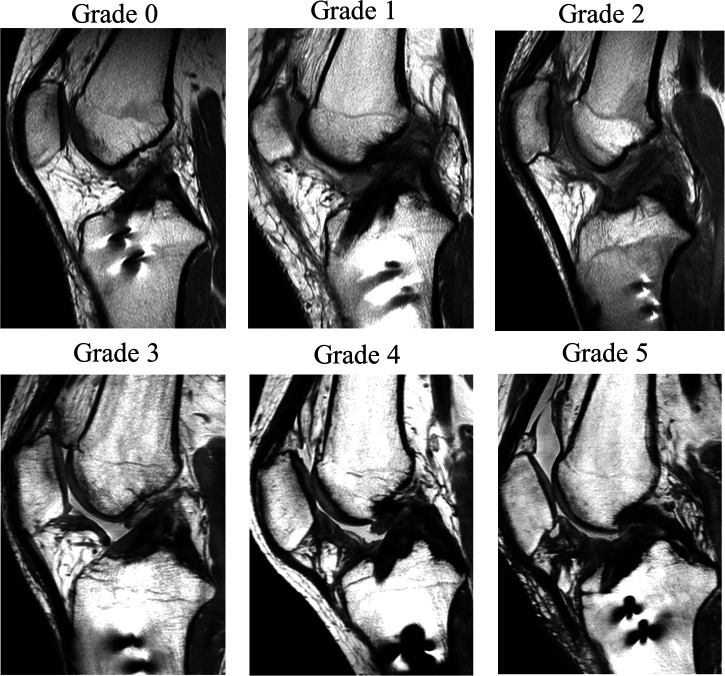
MRI was performed with the patient in a nonweightbearing supine position before and at 3 months after surgery using either a 1.5-T or 3.0-T MRI scanner. Proton density–weighted images in the sagittal plane at the slice of the reconstructed ACL were used. IFP fibrosis on MRI was classified into 6 grades based on fibrosis pattern: grade 0 = none; grade 1 = focal fibrosis; grade 2 = incomplete band fibrosis (no connection between the lower pole of the patella and the tibial plateau); grade 3 = complete band fibrosis (connection between the lower pole of the patella and the tibial plateau); grade 4 = infiltrated fibrosis; and grade 5 = diffuse and infiltrated fibrosis (occupying >75% area) (Figure 1). This grading system was developed by modifying the classification system of Yoon et al. 47 The patients were divided into 2 groups according to the IFP fibrosis grade: the severe fibrosis group (grades 4 and 5) and the mild fibrosis group (grades 0-3). Two independent investigators (M.S. and R.Y.) who were blinded to the patient characteristics, clinical outcomes, and cytokine data determined the IFP fibrosis grade. The interobserver reliability between these 2 investigators was 0.817, which indicated almost perfect agreement according to the Landis and Koch 25 criteria.
Figure 1.
Proton density–weighted sagittal magnetic resonance imaging scans showing infrapatellar fat pad fibrosis classifications: grade 0, none; grade 1, focal fibrosis; grade 2, incomplete band fibrosis; grade 3, complete band fibrosis; grade 4, infiltrated fibrosis; and grade 5, diffuse and infiltrated fibrosis.
Cytokine Measurement and Blood Testing
Synovial fluid was aspirated 3 or 4 days after surgery and was stored at –80°C until measurement. Inflammatory cytokine levels in the synovial fluid were measured using a multiplex enzyme-linked immunosorbent assay system (V-PLEX human proinflammatory panel; Meso Scale Discovery) and MESO Quick Plex SQ 120 MM Reader (Meso Scale Discovery). IL-1β, IL-2, IL-6, IL-8, IL-10, TNF-α, and IFN-γ levels were quantified according to the manufacturer’s protocol. Nondiluted synovial fluid was transferred into 96-well plates and then measured.
Before surgery and at 3 or 4 days after surgery, the C-reactive protein (CRP) level and erythrocyte sedimentation rate (ESR) in the blood were measured.
Statistical Analysis
Statistical analyses were performed using the Stata Version 15.0 software (StataCorp). Patient background, inflammatory cytokine levels in the synovial fluid, and clinical outcomes were compared between the severe fibrosis group and mild fibrosis group with the Mann-Whitney U test for continuous data and the chi-square test for categorical data. For all analyses, a P value <.05 was considered significant.
Based on a pilot study, the sample size calculation was performed using IL-6 levels in the synovial fluid. We estimated IL-6 levels of 2.0 ± 1.0 ng/mL in the severe fibrosis group and 0.8 ± 1.0 ng/mL in the mild fibrosis group. The ratio of group sizes was set at 1:3 for the severe fibrosis to mild fibrosis groups. Given these values, the estimation of the minimum sample size with an adequate power of an alpha of .05 and a power of 0.8 was 28 patients.
The post hoc power analysis for nonparametric tests based on our sample size (N = 36) was conducted using the G*Power 3.1 calculation software (Kiel University), which revealed that with an alpha of .05, a power of 0.85 was achieved for the preoperative Lysholm score. A power of 1.0 was achieved for extension muscle strength ratio; 0.83, for knee extension angle; 0.76, for knee flexion angle; and 0.71, for NRS score of pain while stair climbing at 3 months postoperatively. A power of 0.66 was achieved for Lysholm score; 0.55, for knee extension angle; and 0.77, for knee flexion angle at 12 months postoperatively. The power of IL-1β was 0.35, while the power of IL-6 was 0.43.
Results
Of the 36 study patients, 7 patients were allocated to the severe fibrosis group and 29 patients to the mild fibrosis group. None of the patients had a reinjury within 12 months after surgery.
Patient Characteristics and Preoperative Clinical Findings
Table 1 shows the preoperative characteristics of the patients. There was no significant difference between the severe fibrosis and mild fibrosis groups except for operation time (P = .007). In terms of concomitant procedures with ACLR, repair of the medial meniscus (MM) was performed in 4 patients in the severe fibrosis group and in 9 patients in the mild fibrosis group, whereas repair of the LM was performed in 2 and 10 patients, respectively. LM partial meniscectomy was performed in 1 patient in the mild fibrosis group. No additional procedure for cartilage injuries was performed. Table 2 shows the clinical findings before surgery. There was no significant difference between the 2 groups except for the Lysholm score (P = .045).
Table 1.
Comparison of Patient Characteristics a
| Severe Fibrosis (n = 7) |
Mild Fibrosis (n = 29) |
P | |
|---|---|---|---|
| Age, y | 22.9 ± 7.1 | 21.7 ± 6.7 | .446 |
| Sex | .271 | ||
| Male | 5 (71.4) | 14 (48.3) | |
| Female | 2 (28.6) | 15 (51.7) | |
| Height, cm | 166.4 ± 7.2 | 167.1 ± 6.9 | .984 |
| Body weight, kg | 62.9 ± 8 | 65.0 ± 11.5 | .794 |
| BMI | 22.7 ± 2.7 | 23.3 ± 3.5 | .617 |
| Tegner activity score | 7.1 ± 0.7 | 6.9 ± 0.8 | .400 |
| Side affected | .797 | ||
| Right | 4 (57.1) | 15 (48.3) | |
| Left | 3 (42.9) | 14 (48.3) | |
| Time from injury to surgery, d | 74.7 ± 40.0 | 115.8 ± 68.5 | .299 |
| Preoperative IFP fibrosis grade | 0.6 ± 0.8 | 0.5 ± 0.5 | .942 |
| Operation time, min | 153.0 ± 16.3 | 116.5 ± 44.2 | .007 |
| Tourniquet | .081 | ||
| Yes | 3 (42.9) | 4 (13.8) | |
| No | 4 (57.1) | 25 (86.2) | |
| Harvest of gracilis tendon | .261 | ||
| Present | 1 (14.3) | 1 (3.4) | |
| Absent | 6 (85.7) | 28 (96.6) | |
| Meniscal injury | .925 | ||
| Present | 4 (57.1) | 16 (55.2) | |
| Absent | 3 (42.9) | 13 (44.8) | |
| Cartilage injury | .973 | ||
| Present | 1 (14.3) | 4 (13.8) | |
| Absent | 6 (85.7) | 25 (86.2) |
a Data are reported as mean ± SD or n (%). Bolded P value indicates a statistically significant difference between groups (P < .05). BMI, body mass index; IFP, infrapatellar fat pad.
Table 2.
Comparison of Preoperative Clinical Findings a
| Severe Fibrosis (n = 7) |
Mild Fibrosis (n = 29) |
P | |
|---|---|---|---|
| IFP fibrosis grade | 0.6 ± 0.8 | 0.5 ± 0.5 | .942 |
| Lysholm score | 87.2 ± 7.8 | 75.6 ± 13.5 | .045 |
| Patient satisfaction | 43.3 ± 20.7 | 45.9 ± 27.9 | .829 |
| NRS pain: walking | 0.4 ± 0.8 | 1.4 ± 1.7 | .058 |
| NRS pain: stair climbing | 1.0 ± 1.2 | 2.5 ± 2.3 | .087 |
| Knee extension angle, deg | 1.1 ± 1.1 | 2.1 ± 2.2 | .176 |
| Knee flexion angle, deg | 147.9 ± 2.7 | 146.3 ± 6.4 | .495 |
| Extension muscle strength | |||
| Operated side, kg·m | 9.5 ± 4.0 | 11.4 ± 4.5 | .368 |
| Healthy side, kg·m | 13.0 ± 6.2 | 14.4 ± 5.2 | .500 |
| Ratio, operated/healthy side, % | 75.4 ± 10.7 | 80.3 ± 17.1 | .368 |
a Data are reported as mean ± SD. Bolded P value indicates a statistically significant difference between groups (P < .05). IFP, infrapatellar fat pad; NRS, numeric rating scale.
Clinical Outcomes
The clinical outcomes at 3 months postoperatively are described in Table 3. The patient satisfaction and physical activity score in the severe fibrosis group were lower than those in the mild fibrosis group (P = .014, and 0.003, respectively). The NRS score for pain during stair climbing was higher in the severe fibrosis group than in the mild fibrosis group (P = .01). Patients in the severe fibrosis group had greater extension and flexion angle restriction (P = .003 and .026, respectively) and lower knee extension muscle strength ratio (P < .001) compared with patients in the mild fibrosis group.
Table 3.
Comparison of 3-Month Postoperative Clinical Outcomes a
| Severe Fibrosis (n = 7) |
Mild Fibrosis (n = 29) |
P | |
|---|---|---|---|
| IFP fibrosis grade | 4.1 ± 0.4 | 2.0 ± 1.0 | <.001 |
| Patient satisfaction | 48.6 ± 18.6 | 66.7 ± 15.2 | .014 |
| NRS pain: walking | 0.7 ± 0.8 | 0.5 ± 0.8 | .302 |
| NRS pain: stair climbing | 2.0 ± 1.3 | 0.7 ± 0.9 | .010 |
| Knee extension angle, deg | –1.0 ± 2.1 | 1.4 ± 1.3 | .003 |
| Knee flexion angle, deg | 140.0 ± 5.0 | 146.0 ± 6.6 | .026 |
| Extension muscle strength | |||
| Operated side, kg·m | 7.3 ± 3.2 | 10.0 ± 3.5 | .047 |
| Healthy side, kg·m | 13.7 ± 5.0 | 13.2 ± 3.6 | .944 |
| Ratio, operated/healthy side, % | 52.9 ± 7.5 | 76.1 ± 14.6 | <.001 |
| Physical activity score | 2.6 ± 0.5 | 3.6 ± 0.7 | .003 |
a Data are reported as mean ± SD. Bolded P values indicate a statistically significant difference between groups (P < .05). IFP, infrapatellar fat pad; NRS, numeric rating scale.
Clinical outcomes at 12 months after surgery are described in Table 4. The Lysholm score in the severe fibrosis group was lower than that in the mild fibrosis group (P = .026). Patients in the severe fibrosis group had greater extension and flexion angle restriction than those in the mild fibrosis group (P = .043 and .013, respectively), while the significant between-group difference in knee extensor muscle strength at 3 months postoperatively became nonsignificant at 12 months.
Table 4.
Comparison of 12-Month Postoperative Clinical Outcomes a
| Severe Fibrosis (n = 7) |
Mild Fibrosis (n = 29) |
P | |
|---|---|---|---|
| Lysholm score | 93.7 ± 3.6 | 97.3 ± 3.6 | .026 |
| Patient satisfaction | 87.1 ± 8.1 | 92.4 ± 6.0 | .067 |
| Sports performance evaluation | 89.3 ± 12.4 | 91.9 ± 8.4 | .763 |
| NRS pain: walking | 0.0 ± 0.0 | 0.1 ± 0.3 | .472 |
| NRS pain: stair climbing | 0.7 ± 1.2 | 0.2 ± 0.4 | .330 |
| Knee extension angle, deg | 0.3 ± 1.8 | 1.9 ± 1.9 | .043 |
| Knee flexion angle, deg | 142.9 ± 4.9 | 149.0 ± 6.9 | .013 |
| Extension muscle strength | |||
| Operated side, kg·m | 11.7 ± 5.0 | 13.5 ± 5.3 | .419 |
| Healthy side, kg·m | 13.5 ± 5.3 | 14.5 ± 5.3 | .726 |
| Ratio, operated/healthy side, % | 86.8 ± 11.7 | 93.5 ± 15.7 | .419 |
| Returned to preoperative sports level | .211 | ||
| Yes | 5 (71.4) | 26 (89.7) | |
| No | 2 (28.6) | 3 (10.3) |
a Data are reported as mean ± SD or n (%). Bolded P values indicate a statistically significant difference between groups (P < .05). NRS, numeric rating scale.
Inflammatory Cytokines and Blood Test Results
The inflammatory cytokine levels in the synovial fluid at 3 or 4 days after surgery are shown in Table 5. The severe fibrosis group demonstrated statistically higher levels of inflammatory cytokines, particularly IL-1β, IL-6, and IFN-γ (P = .022, .029, and .044, respectively).
Table 5.
Inflammatory Cytokines a
| Severe Fibrosis (n = 7) |
Mild Fibrosis (n = 29) |
P | |
|---|---|---|---|
| IL-1β, pg/mL | 2.6 ± 1.8 | 1.4 ± 1.9 | .022 |
| IL-2, pg/mL | 1.2 ± 1.3 | 0.4 ± 0.8 | .082 |
| IL-6, ng/mL | 2.0 ± 1.2 | 1.1 ± 1.2 | .029 |
| IL-8, ng/mL | 4.2 ± 3.3 | 1.7 ± 2.1 | .302 |
| IL-10, pg/mL | 1.6 ± 1.0 | 1.1 ± 1.6 | .106 |
| TNF-α, pg/mL | 3.8 ± 4.9 | 2.4 ± 2.3 | .348 |
| IFN-γ, pg/mL | 11.3 ± 11.7 | 4.0 ± 5.0 | .044 |
a Data are reported as mean ± SD. Bolded P values indicate a statistically significant difference between groups (P < .05). IFN, interferon; IL, interleukin; TNF, tumor necrosis factor.
There were no statistically significant differences between the severe fibrosis and mild fibrosis groups in mean CRP (0.08 ± 0.08 vs 0.08 ± 0.15 mg/dL, respectively; P = .545) or ESR (3.6 ± 3.6 vs 6.9 ± 5.8 mm/h, respectively; P = .093) before surgery or in CRP (1.7 ± 1.5 vs 2.3 ± 1.9 mg/dL, respectively; P = .603) or ESR (26.0 ± 10.0 vs 31.8 ± 18.1 mm/h, respectively; P = .087) at 3 or 4 days after surgery.
Discussion
The most important findings of this study were that patients with severe IFP fibrosis on MRI at 3 months after ACLR had significantly longer operation times, greater pain, and slower recovery of range of motion and extensor muscle strength at 3 months postoperatively and worse range of motion and Lysholm scores at 12 months postoperatively versus patients with mild IFP fibrosis. Inflammatory cytokine levels in the synovial fluid at 3 or 4 days postoperatively were significantly higher in the severe fibrosis group than in the mild fibrosis group.
IFP has a high sensitivity to mechanical stimulation and a large number of sensory neuron fiber endings. 7 Murakami et al 32 demonstrated an association between IFP fibrosis and knee pain or stiffness in squatting after quantitative analysis of IFP fibrosis in patients after ACLR. In the current study, patients with severe IFP fibrosis experienced greater pain than those with mild IFP fibrosis during stair climbing but not while walking on flat ground. We did not evaluate the location of knee pain; however, in general, anterior knee pain is more relevant with stair climbing than during walking.
Tang et al 42 observed IFP fibrosis (scarring) on MRI at various time points after ACLR. They reported that it occurred within 6 months, peaked, and disappeared thereafter in half of the cases within 1 year after surgery. Therefore, we considered that it was desirable to perform MRI within 6 months. In this study, we selected 3 months as the time point of MRI evaluation; however, MRI evaluation at a later point may be useful. Yoon et al 47 reported that there was an association between IFP fibrosis and patellofemoral osteoarthritis progression as assessed by MRI at a mean of 28.7 months (range, 7-111 months) postoperatively. In their study, severe IFP fibrosis was found in <10% of patients (7/107), which was lower than the finding in the present study (7/36; 19%). However, there were fewer patients with long-term residual IFP fibrosis in the Yoon et al study. It would be interesting to examine the difference in clinical outcomes between patients with severe fibrosis persisting for a long period and those with improvement in a short period.
Ueda et al 45 found after logistic regression analysis that older age, female sex, preoperative extension muscle strength, and pain were risk factors for delayed extension muscle recovery at 6 months after ACLR with hamstring tendon. However, other studies have indicated that anterior knee pain might result in a delayed recovery of muscle strength after ACLR.11,35 Based on the results of the present study, greater knee pain associated with IFP fibrosis may have led to poor muscle recovery and delayed functional recovery in the early phase of ACLR (3 months postoperatively), and even though knee extensor muscle strength recovers over time, its adverse effect on knee function lasts for a longer period.
According to LaPrade et al, 26 ACLR with MM repair led to worse short-term patient-reported outcomes. Although the number of patients who underwent MM repair was not significant between the 2 groups in the current study, there is a possibility that intra-articular procedures such as MM repair during ACLR might have affected the results. In this study, 13 of 36 patients underwent MM repair, and no differences were observed in the clinical outcomes at 3 or 12 months postoperatively between patients with and without MM repair (AppendixTable A1). Further studies are needed to examine the relationship between IFP fibrosis and various factors that affect clinical outcomes of ACLR.
An association between arthrofibrosis, global scarring of the joint soft tissues, and acute ACLR has been reported.6,39 In terms of IFP fibrosis, although the time from injury to surgery was not significantly different between the 2 groups in the current study, the timing of surgery in the severe fibrosis group tended to be earlier than that in the mild fibrosis group. Further studies with a larger sample size are needed to clarify if ACLR in an acute phase is associated with IFP fibrosis.
The preoperative IFP fibrosis grade was not different between the 2 study groups, and only low-grade fibrosis was detected. The ACL injury itself did not cause significant IFP fibrosis among the 36 study patients; moreover, the preoperative Lysholm score was better in the severe fibrosis group than in the mild fibrosis group. The IFP fibrosis, therefore, seems directly related to the surgery rather than the preoperative condition. It could have resulted from direct IFP trauma or a longer operation time, which could be associated with a greater inflammatory response secondary to a bigger surgical trauma.
Synovial fluid in the acute phase after ACLR includes blood from the bone marrow associated with the creation of a bone tunnel. Bone marrow–derived blood contains stem cells and growth factors that promote biological healing. The failure rate of meniscal repair during ACLR is lower than that of isolated meniscal repair. 46 A systematic review of biomarkers in patients with ACLR showed higher inflammatory cytokine levels in the synovial fluid after surgery than before, while inflammatory cytokine levels in the serum did not change. 12 Hayward et al 13 compared synovial fluid before and at 1 and 6 hours after surgery and found that monocyte-derived IL-1 and IL-6 were elevated and T cell–derived IL-2 was mildly elevated. They also demonstrated that growth factors such as transforming growth factor (TGF)–β and platelet-derived growth factor (PDGF) increased 6 hours after surgery.
Growth factors such as TGF-β may be essential for IFP fibrosis, as TGF-β was shown to be upregulated in the IFP at 2 weeks after ACLR in a sheep model. 40 It has also been reported through immunohistochemical analysis that expression of the fibrogenic cytokines TGF-β and PDGF was found in fibrotic sites of IFP in patients with ACLR. 33
Katagiri et al 21 compared synovial fluid before surgery and at 4 and 21 days after surgery and reported more granulocytes and fewer T cells at 4 days than at 21 days. In the current study, the synovial fluid in the severe fibrosis group contained higher inflammatory cytokine levels, suggesting that intra-articular inflammation in the acute phase indicates the onset of IFP fibrosis after ACLR.
It has been shown that in early osteoarthritic knee joints, the mediators involved in the inflammatory process of the joints also affect the development of structural changes in the IFP, characterized by an increase in the infiltration of inflammatory cells, new vascular formation, and fibrotic change at both cellular and histological levels. 20 Synovial tissues around the knee, particularly the IFP, produce and store inflammatory cytokines.40,44 Furthermore, immune cells can be locally activated by tissue damage to secrete inflammatory cytokines, particularly TNF-α and IL-6. 20 IL-1β is an important mediator of fibrosis by influencing the migration of cells, adhesion, matrix metalloproteinase production, and the expression of immunomodulatory genes. 10 IL-1β has been reported to strongly induce TGF-β and PDGF, leading to the development of lung fibrosis after injury or infection. 24 In this study, IL-1β and IL-6 levels were significantly more elevated in the severe fibrosis group than the mild fibrosis group. These findings, in conjunction with those from previous studies, indicate that these 2 cytokines may be involved in the formation of IFP fibrosis and may be predictors of short-term clinical outcomes after ACLR.
Limitations
There are some limitations to this study. First, the number of patients was too small to draw any robust conclusions. Several outcomes, even those exhibiting statistical significance, were of low power. Further studies with a larger number of cases are necessary to validate the results obtained here. Second, MRI evaluation for IFP fibrosis was only conducted at a single time point 3 months after surgery. Third, the cytokine measurement was also performed at only 1 time point, which was 3 or 4 days after the surgery. These data obtained at preoperative or longer time points postoperatively could add further information. Fourth, this study assessed inflammatory cytokines alone. A more comprehensive measurement of cytokines would be necessary to elucidate the mechanisms of IFP fibrosis after ACLR.
Conclusion
Severe IFP fibrosis was associated with longer operation time, higher inflammatory cytokine levels in the synovial fluid, and unfavorable clinical outcomes at 3 and 12 months after ACLR. Inflammatory cytokines in the synovial fluid, such as IL-1β, IL-6, and IFN-γ, may be involved in the development of IFP fibrosis.
Acknowledgment
The authors thank Atsushi Okawa, MD, PhD, for continuous support; Miho Okada, Masayo Tsukamoto, and Miyoko Ojima for data registration; and Editage (www.editage.jp) for the English-language editing.
Appendix
Table A1.
Comparison of 3-Month and 12-Month Postoperative Outcomes Between Patients With Versus Without MM Repair a
| With MM Repair (n = 13) |
Without MM Repair (n = 23) |
P | |
|---|---|---|---|
| 3 mo postoperative | |||
| Patient satisfaction | 63.9 ± 22.0 | 62.7 ± 14.7 | .604 |
| NRS pain: walking | 0.3 ± 0.5 | 0.7 ± 0.9 | .175 |
| NRS pain: stair climbing | 0.9 ± 1.0 | 1.0 ± 1.2 | .605 |
| Knee extension angle, deg | 0.5 ± 2.1 | 1.2 ± 1.5 | .477 |
| Knee flexion angle, deg | 143.9 ± 2.1 | 145.4 ± 6.0 | .666 |
| Extension muscle strength ratio, operated/healthy side, % | 72.3 ± 17.8 | 71.2 ± 16.1 | .746 |
| 12 mo postoperative | |||
| Lysholm score | 96.0 ± 4.4 | 96.9 ± 3.6 | .453 |
| Patient satisfaction | 90.1 ± 7.7 | 92.1 ± 6.2 | .489 |
| Sports performance evaluation | 90.9 ± 11.1 | 91.4 ± 8.4 | .808 |
| NRS pain: walking | 0.0 ± 0.0 | 0.1 ± 0.4 | .274 |
| NRS pain: stair climbing | 0.3 ± 0.4 | 0.3 ± 0.8 | .805 |
| Knee extension angle, deg | 1.9 ± 2.8 | 1.4 ± 1.9 | .834 |
| Knee flexion angle, deg | 146.6 ± 8.7 | 148.5 ± 6.9 | .684 |
| Extension muscle strength ratio, operated/healthy side, % | 93.0 ± 15.8 | 91.7 ± 15.0 | .719 |
a Data are reported as mean ± SD. MM, medial meniscus; NRS, numeric rating scale.
Footnotes
Final revision submitted December 13, 2022; accepted January 17, 2023.
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Tokyo Medical and Dental University (protocol No. 1146).
References
- 1.Alonso B, Bravo B, Mediavilla L, et al. Osteoarthritis-related biomarkers profile in chronic anterior cruciate ligament injured knee. Knee. 2020;27(1):51–60. [DOI] [PubMed] [Google Scholar]
- 2.An JS, Tsuji K, Onuma H, et al. Inhibition of fibrotic changes in infrapatellar fat pad alleviates persistent pain and articular cartilage degeneration in monoiodoacetic acid-induced rat arthritis model. Osteoarthritis Cartilage. 2021;29(3):380–388. [DOI] [PubMed] [Google Scholar]
- 3.Belluzzi E, Stocco E, Pozzuoli A, et al. Contribution of infrapatellar fat pad and synovial membrane to knee osteoarthritis pain. Biomed Res Int. 2019;2019:6390182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bigoni M, Sacerdote P, Turati M, et al. Acute and late changes in intraarticular cytokine levels following anterior cruciate ligament injury. J Orthop Res. 2013;31(2):315–321. [DOI] [PubMed] [Google Scholar]
- 5.Bohnsack M, Meier F, Walter GF, et al. Distribution of substance-P nerves inside the infrapatellar fat pad and the adjacent synovial tissue: a neurohistological approach to anterior knee pain syndrome. Arch Orthop Trauma Surg. 2005;125(9):592–597. [DOI] [PubMed] [Google Scholar]
- 6.DeHaven KE, Cosgarea AJ, Sebastianelli WJ. Arthrofibrosis of the knee following ligament surgery. Instr Course Lect. 2003;52:369–381. [PubMed] [Google Scholar]
- 7.Dye SF, Vaupel GL, Dye CC. Conscious neurosensory mapping of the internal structures of the human knee without intraarticular anesthesia. Am J Sports Med. 1998;26(6):773–777. [DOI] [PubMed] [Google Scholar]
- 8.Ekhtiari S, Horner NS, de Sa D, et al. Arthrofibrosis after ACL reconstruction is best treated in a step-wise approach with early recognition and intervention: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2017;25(12):3929–3937. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher J, Tierney P, Murray P, O’Brien M. The infrapatellar fat pad: anatomy and clinical correlations. Knee Surg Sports Traumatol Arthrosc. 2005;13(4):268–272. [DOI] [PubMed] [Google Scholar]
- 10.Gasse P, Mary C, Guenon I, et al. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J Clin Invest. 2007;117(12):3786–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gobbi A, Domzalski M, Pascual J. Comparison of anterior cruciate ligament reconstruction in male and female athletes using the patellar tendon and hamstring autografts. Knee Surg Sports Traumatol Arthrosc. 2004;12(6):534–539. [DOI] [PubMed] [Google Scholar]
- 12.Harkey MS, Luc BA, Golightly YM, et al. Osteoarthritis-related biomarkers following anterior cruciate ligament injury and reconstruction: a systematic review. Osteoarthritis Cartilage. 2015;23(1):1–12. [DOI] [PubMed] [Google Scholar]
- 13.Hayward AL, Deehan DJ, Aspden RM, Sutherland AG. Analysis of sequential cytokine release after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19(10):1709–1715. [DOI] [PubMed] [Google Scholar]
- 14.Heilmeier U, Mamoto K, Amano K, et al. Infrapatellar fat pad abnormalities are associated with a higher inflammatory synovial fluid cytokine profile in young adults following ACL tear. Osteoarthritis Cartilage. 2020;28(1):82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffa A. The influence of the adipose tissue with regard to the pathology of the knee joint. J Am Med Assoc. 1904;XLIII(12):795–796. [Google Scholar]
- 16.Hoshino T, Tsuji K, Onuma H, et al. Persistent synovial inflammation plays important roles in persistent pain development in the rat knee before cartilage degradation reaches the subchondral bone. BMC Musculoskelet Disord. 2018;19(1):291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Impellizzeri FM, Bizzini M, Rampinini E, Cereda F, Maffiuletti NA. Reliability of isokinetic strength imbalance ratios measured using the Cybex NORM dynamometer. Clin Physiol Funct Imaging. 2008;28(2):113–119. [DOI] [PubMed] [Google Scholar]
- 18.Inomata K, Tsuji K, Onuma H, et al. Time course analyses of structural changes in the infrapatellar fat pad and synovial membrane during inflammation-induced persistent pain development in rat knee joint. BMC Musculoskelet Disord. 2019;20(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inoue M, Muneta T, Ojima M, et al. Inflammatory cytokine levels in synovial fluid 3, 4 days postoperatively and its correlation with early-phase functional recovery after anterior cruciate ligament reconstruction: a cohort study. J Exp Orthop. 2016;3(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ioan-Facsinay A, Kloppenburg M. An emerging player in knee osteoarthritis: the infrapatellar fat pad. Arthritis Res Ther. 2013;15(6):225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katagiri H, Nakamura K, Muneta T, et al. Inflammatory and healing environment in synovial fluid after anterior cruciate ligament reconstruction: granulocytes and endogenous opioids as new targets of postoperative pain. Biochem Biophys Rep. 2021;26:100981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koga H, Muneta T, Yagishita K, et al. Arthroscopic centralization of an extruded lateral meniscus. Arthrosc Tech. 2012;1(2):e209–e212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koga H, Muneta T, Yagishita K, et al. Effect of initial graft tension on knee stability and graft tension pattern in double-bundle anterior cruciate ligament reconstruction. Arthroscopy. 2015;31(9):1756–1763. [DOI] [PubMed] [Google Scholar]
- 24.Kolahian S, Fernandez IE, Eickelberg O, Hartl D. Immune mechanisms in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2016;55(3):309–322. [DOI] [PubMed] [Google Scholar]
- 25.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 26.LaPrade CM, Dornan GJ, Granan LP, LaPrade RF, Engebretsen L. Outcomes after anterior cruciate ligament reconstruction using the Norwegian Knee Ligament Registry of 4691 patients: how does meniscal repair or resection affect short-term outcomes? Am J Sports Med. 2015;43(7):1591–1597. [DOI] [PubMed] [Google Scholar]
- 27.Leese J, Davies DC. An investigation of the anatomy of the infrapatellar fat pad and its possible involvement in anterior pain syndrome: a cadaveric study. J Anat. 2020;237(1):20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macchi V, Porzionato A, Sarasin G, et al. The infrapatellar adipose body: a histotopographic study. Cells Tissues Organs. 2016;201(3):220–231. [DOI] [PubMed] [Google Scholar]
- 29.MacConaill MA. The movements of bones and joints; the synovial fluid and its assistants. J Bone Joint Surg Br. 1950;32-B(2):244–252. [DOI] [PubMed] [Google Scholar]
- 30.Marks PH, Donaldson ML. Inflammatory cytokine profiles associated with chondral damage in the anterior cruciate ligament-deficient knee. Arthroscopy. 2005;21(11):1342–1347. [DOI] [PubMed] [Google Scholar]
- 31.Muneta T, Koga H, Nakamura T, et al. Behind-remnant arthroscopic observation and scoring of femoral attachment of injured anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2016;24(9):2906–2914. [DOI] [PubMed] [Google Scholar]
- 32.Murakami S, Muneta T, Ezura Y, Furuya K, Yamamoto H. Quantitative analysis of synovial fibrosis in the infrapatellar fat pad before and after anterior cruciate ligament reconstruction. Am J Sports Med. 1997;25(1):29–34. [DOI] [PubMed] [Google Scholar]
- 33.Murakami S, Muneta T, Furuya K, et al. Immunohistologic analysis of synovium in infrapatellar fat pad after anterior cruciate ligament injury. Am J Sports Med. 1995;23(6):763–768. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura K, Nakamura T, Horie M, et al. Anatomic femoral tunnel placement is difficult by the transtibial technique: comparison of three different femoral tunnel drilling techniques in double-bundle anterior cruciate ligament reconstructions. Knee Surg Sports Traumatol Arthrosc. 2020;28(2):584–593. [DOI] [PubMed] [Google Scholar]
- 35.Natri A, Järvinen M, Latvala K, Kannus P. Isokinetic muscle performance after anterior cruciate ligament surgery: long-term results and outcome predicting factors after primary surgery and late-phase reconstruction. Int J Sports Med. 1996;17(3):223–228. [DOI] [PubMed] [Google Scholar]
- 36.Nemschak G, Pretterklieber ML. The patellar arterial supply via the infrapatellar fat pad (of Hoffa): a combined anatomical and angiographical analysis. Anat Res Int. 2012;2012:713838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Onuma H, Tsuji K, Hoshino T, et al. Fibrotic changes in the infrapatellar fat pad induce new vessel formation and sensory nerve fiber endings that associate prolonged pain. J Orthop Res. 2020;38(6):1296–1306. [DOI] [PubMed] [Google Scholar]
- 38.Roemer FW, Frobell R, Lohmander LS, Niu J, Guermazi A. Anterior Cruciate Ligament OsteoArthritis Score (ACLOAS): longitudinal MRI-based whole joint assessment of anterior cruciate ligament injury. Osteoarthritis Cartilage. 2014;22(5):668–682. [DOI] [PubMed] [Google Scholar]
- 39.Shelbourne KD, Wilckens JH, Mollabashy A, DeCarlo M.Arthrofibrosis in acute anterior cruciate ligament reconstruction. the effect of timing of reconstruction and rehabilitation. Am J Sports Med. 1991;19(4):332–336. [DOI] [PubMed] [Google Scholar]
- 40.Solbak NM, Heard BJ, Achari Y, et al. Alterations in Hoffa’s fat pad induced by an inflammatory response following idealized anterior cruciate ligament surgery. Inflamm Res. 2015;64(8):615–626. [DOI] [PubMed] [Google Scholar]
- 41.Stephen JM, Sopher R, Tullie S, et al. The infrapatellar fat pad is a dynamic and mobile structure, which deforms during knee motion, and has proximal extensions which wrap around the patella. Knee Surg Sports Traumatol Arthrosc. 2018;26(11):3515–3524. [DOI] [PubMed] [Google Scholar]
- 42.Tang G, Niitsu M, Ikeda K, Endo H, Itai Y.Fibrous scar in the infrapatellar fat pad after arthroscopy: MR imaging. Radiat Med. 2000;18(1):1–5. [PubMed] [Google Scholar]
- 43.Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;198:43–49. [PubMed] [Google Scholar]
- 44.Turhan E, Doral MN, Atay AO, Demirel M. A giant extrasynovial osteochondroma in the infrapatellar fat pad: end stage Hoffa’s disease. Arch Orthop Trauma Surg. 2008;128(5):515–519. [DOI] [PubMed] [Google Scholar]
- 45.Ueda Y, Matsushita T, Araki D, et al. Factors affecting quadriceps strength recovery after anterior cruciate ligament reconstruction with hamstring autografts in athletes. Knee Surg Sports Traumatol Arthrosc. 2017;25(10):3213–3219. [DOI] [PubMed] [Google Scholar]
- 46.Wasserstein D, Dwyer T, Gandhi R, et al. A matched-cohort population study of reoperation after meniscal repair with and without concomitant anterior cruciate ligament reconstruction. Am J Sports Med. 2013;41(2):349–355. [DOI] [PubMed] [Google Scholar]
- 47.Yoon KH, Tak DH, Ko TS, et al. Association of fibrosis in the infrapatellar fat pad and degenerative cartilage change of patellofemoral joint after anterior cruciate ligament reconstruction. Knee. 2017;24(2):310–318. [DOI] [PubMed] [Google Scholar]