Abstract
Background:
There is a paucity of literature reporting clinical and magnetic resonance imaging (MRI) outcomes after allogeneic umbilical cord blood–derived mesenchymal stem cell (UCB-MSC) implantation for chondral defects of the knee.
Purpose:
To report clinical and MRI outcomes after UCB-MSC implantation for chondral lesions of the knee.
Study Design:
Case series; Level of evidence, 4.
Methods:
Inclusion criteria were patients aged between 40 and 70 years with focal chondral lesions of grade 3 or 4 on the medial femoral condyle, defect sizes >4 cm2, and intact ligaments. Exclusion criteria were patients who required realignment osteotomy or who had a meniscal deficiency, ligamentous instability, or a concomitant full-thickness chondral defect in the lateral or patellofemoral compartment. A mixture of human UCB-MSCs and sodium hyaluronate was implanted into the chondral defect through mini-arthrotomy. MRI at 1-year follow-up was performed to evaluate repaired cartilage hypertrophy. Repaired cartilage thickness was measured, and hypertrophy was classified as grade 1 (<125%), grade 2 (<150%), or grade 3 (<200%). Patient-reported outcomes (PROs; International Knee Documentation Committee, visual analog scale for pain, and Western Ontario and McMaster Universities Osteoarthritis Index) were evaluated preoperatively and at 1, 2, and 3 years postoperatively. Repaired cartilage hypertrophy was evaluated for a correlation with PRO scores.
Results:
Enrolled were 85 patients with a mean age of 56.8 ± 6.1 years and a mean chondral defect size of 6.7 ± 2.0 cm2. At follow-up, a significant improvement in all PRO scores was seen compared with preoperatively (P < .001 for all). MRI at 1-year follow-up demonstrated that 28 patients had grade 1 repaired cartilage hypertrophy, 41 patients had grade 2, and 16 patients had grade 3. MRI performed in 11 patients at 2 years after surgery indicated no difference in repaired cartilage hypertrophy between the 1- and 2-year time points. The grade of repaired cartilage hypertrophy did not correlate with PRO scores.
Conclusion:
Clinical outcomes improved significantly at short-term follow-up after UCB-MSC implantation. Although all patients showed repaired cartilage hypertrophy, it did not correlate with clinical outcomes.
Keywords: chondral defect, graft hypertrophy, mesenchymal stem cell, MRI, umbilical cord blood
The treatment of large chondral defects in the knee is challenging. Several surgical options exist to address these lesions. Autologous chondrocyte implantation (ACI) has been a treatment option since the late 1980s. 1 First-generation ACI using a periosteal flap (ACI-P) has demonstrated good long-term results over 10 years. 26 However, ACI-P has been blamed for a high rate of graft hypertrophy that is reported as being up to 36% in the literature. 18 Coverage of the chondral lesion using a periosteal flap has been known to be the primary cause of graft hypertrophy. Second-generation ACI using a type I or III collagen membrane (ACI-C) next emerged in the market and decreased graft hypertrophy significantly. 16 Matrix-induced ACI (MACI) is a third-generation technique in which chondrocytes are seeded onto an absorbable matrix. However, graft hypertrophy is still observed after MACI but does not affect the clinical outcome. 27
Mesenchymal stem cell (MSC) treatment is an alternative option for cartilage repair. 20 MSCs can be collected from the bone marrow, adipose tissue, and the synovial membrane. 20 Intra-articular injections of MSCs are popular because their harvest is relatively easy and also MSCs have the potential to differentiate into chondral tissue. 25 However, a recent meta-analysis demonstrated that an intra-articular MSC injection has limited evidence for pain relief and functional improvement in knee osteoarthritis. 14
Umbilical cord blood is another source of MSCs. Human umbilical cord blood–derived MSCs (UCB-MSCs) exhibit high proliferation rates, can be expanded for many population doublings, and are hypoimmunogenic and nontumorigenic. 6 Harvesting from the patient and isolation procedures are unnecessary because UCB-MSCs are allogeneic. A collagen membrane or matrix is not used in UCB-MSC implantation. This procedure has demonstrated satisfactory clinical outcomes at short-term follow-up2,3,29; however, there is a paucity of literature reporting clinical and magnetic resonance imaging (MRI) outcomes after UCB-MSC implantation.
The purpose of this study was to report clinical and MRI outcomes after UCB-MSC implantation for chondral lesions of the knee. It was hypothesized that clinical and MRI outcomes after UCB-MSC implantation would be satisfactory.
Methods
Study Patients
This study retrospectively evaluated patients who underwent UCB-MSC implantation between January 2014 and November 2016. Patients who had a large chondral defect of the medial femoral condyle and were symptomatic after nonoperative treatment were indicated for UCB-MSC implantation. Inclusion criteria were patients aged between 40 and 70 years, chondral lesions of Outerbridge grade 3 or 4 on the medial femoral condyle, defect sizes >4 cm2, and intact ligaments. We excluded patients who required realignment osteotomy or who had a meniscal deficiency, ligamentous instability, or a concomitant full-thickness chondral defect in the lateral or patellofemoral compartment. Small chondral lesions of the medial tibial plateau (<1-2 cm2) were included; however, lesions >2 cm2 were excluded. The protocol for this retrospective study received institutional review board approval, and all patients signed an informed consent form.
Preparation of Allogeneic MSCs
Human UCB was collected from umbilical veins after neonatal delivery, with informed consent from pregnant mothers. The mean donor age was 31 years. Cell isolation and culture procedures were performed at a commercial company (Medipost). UCB was isolated by separating mononuclear cells with Ficoll-Hypaque solution (Sigma-Aldrich). The separated mononuclear cells were washed and suspended in minimum essential medium alpha (Gibco/Invitrogen) supplemented with 10% fetal bovine serum (Gibco/Invitrogen). Cultures were maintained at 37°C in a humidified atmosphere containing 5% CO2, and the culture medium was changed twice a week. Cells were verified for positive (CD29, CD73, CD90, CD105, CD166) and negative (CD14, CD45) surface markers by flow cytometry. Human UCB-MSCs at passage 6 were used as a stem cell drug (CARTISTEM; Medipost). The mean viability of UCB-MSCs was 95%. The prepared stem cell drug was implanted after a mean of 5 days. The therapeutic dosage of the stem cell drug was 500 μL/cm2 of the defect area, with a cell concentration of 0.5 × 107 cells/mL. There were 3 types of stem cells used for the patients enrolled in this study. Before implantation, UCB-MSCs and sodium hyaluronate were mixed according to the manufacturer’s instructions.
Surgical Procedure
Routine anteromedial and anterolateral portals were created, and meniscectomy was performed if necessary. Although meniscectomy may not improve clinical outcomes in knees with chondral lesions, unstable meniscal fragments were removed concomitantly. After mini-arthrotomy was performed, the chondral defect was debrided using a motorized shaver, and the size of the defect was measured. Multiple holes with a 4-mm diameter were created approximately 2 mm apart using a drill bit described by Park et al. 24 A mixture of sodium hyaluronate and UCB-MSCs was implanted into the holes and trimmed to the height of the surrounding cartilage (Figure 1). The arthrotomy site was closed 5 minutes after implantation. Continuous passive motion exercise was recommended after postoperative day 1 and was allowed for 30 minutes per day for 6 weeks. Partial weightbearing was recommended until 6 weeks postoperatively, and gradual increases in weightbearing were allowed thereafter.
Figure 1.
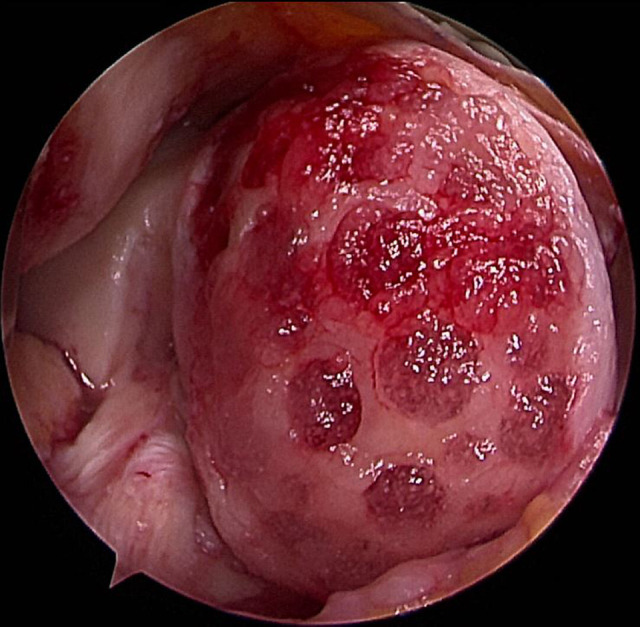
A mixture of sodium hyaluronate and umbilical cord blood–derived mesenchymal stem cells was implanted into the prepared lesion of the medial femoral condyle in a right knee.
MRI Evaluation
MRI (1.5 T) at 1-year follow-up was performed to evaluate repaired cartilage hypertrophy. The imaging sequences were axial T2-weighted images, sagittal short-tau inversion recovery (STIR) and proton density–weighted images, and coronal STIR and proton density–weighted images. Structural repair was evaluated using the modified MOCART (magnetic resonance observation of cartilage repair tissue) score. 13 Repaired cartilage thickness was measured by an experienced musculoskeletal radiologist (K.T.H.) as described by Kreuz et al 18 (Figure 2). The thickest areas of the repaired cartilage were measured in 3 regions, and the thickness of the healthy surrounding cartilage was measured in the same manner. A mean value was calculated from the 3 measurements. The relative repaired cartilage thickness was calculated as a ratio between the repaired cartilage thickness and the thickness of the healthy surrounding cartilage. Hypertrophy was classified as grade 1 (<125%), grade 2 (<150%), or grade 3 (<200%) according to Kreuz et al. 18 The measurements were performed on a picture archiving and communication system (GE Healthcare).
Figure 2.
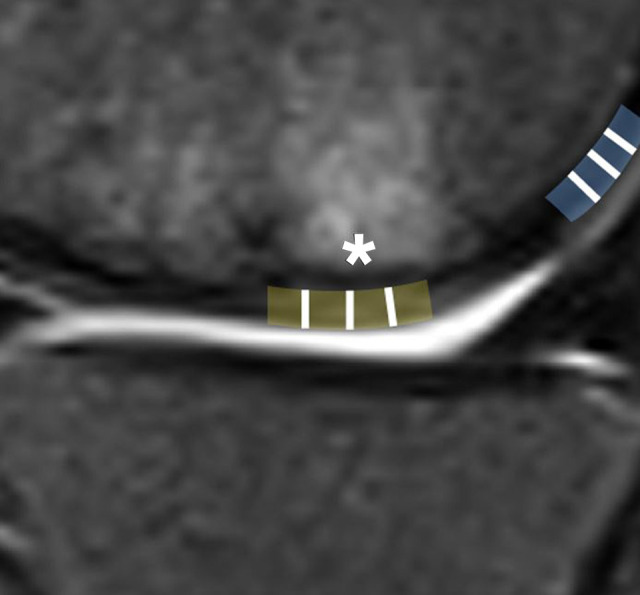
The relative repaired cartilage thickness was calculated as a ratio between the repaired cartilage thickness (asterisk) and the thickness of the healthy surrounding cartilage on the sagittal view. The brown and blue areas represent repaired and healthy cartilage, respectively. The height of the cartilage was measured at each white line and the mean was calculated.
Patient-Reported Outcomes and Second-Look Arthroscopic Examination
Patient-reported outcomes (PROs) including the International Knee Documentation Committee (IKDC), visual analog scale (VAS) for pain, and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores were evaluated preoperatively and at 1, 2, and 3 years postoperatively. A second-look arthroscopic examination at 1 year postoperatively was performed. Consent for second-look arthroscopic surgery was received from all patients before UCB-MSC implantation. The cartilage repair site was evaluated according to the International Cartilage Regeneration & Joint Preservation Society (ICRS) classification. 11 The ICRS ratings are normal (grade 1), nearly normal (grade 2), abnormal (grade 3), and severely abnormal (grade 4). The ICRS grade was determined based on the agreement of 2 orthopaedic surgeons who were not involved in surgery.
Statistical Analysis
The IKDC, VAS, and WOMAC scores preoperatively and at 1, 2, and 3 years after surgery were compared using the Mann-Whitney test. The Pearson correlation test was used between repaired cartilage hypertrophy and patient factors (size of the chondral defect, body mass index [BMI], and age). Analysis of variance was performed to compare postoperative PRO scores among 3 groups according to repaired cartilage hypertrophy. All analyses were performed using SPSS software (SPSS for Windows version 12.0; IBM), and significance was assumed at P < .05.
Results
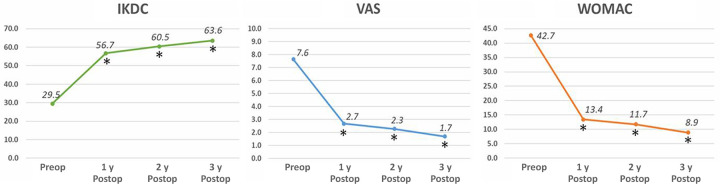
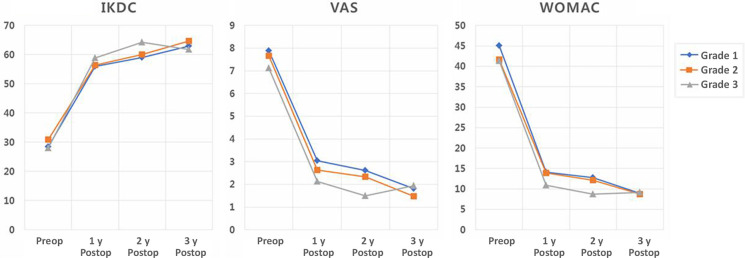
A total of 85 patients were enrolled in this study. There were 20 (23.5%) male and 65 (76.5%) female patients. The mean age of the patients at the time of surgery was 56.8 ± 6.1 years. The mean BMI was 25.7 ± 2.6 kg/m2. The mean size of the chondral defect was 6.7 ± 2.0 cm2. Chondromalacia of the lateral and palletofemoral compartment was a concomitant lesion. Eight patients had grade 2 chondromalacia in the lateral compartment, and 50 patients had grade 2 chondromalacia and 20 patients had grade 3 chondromalacia in the patellofemoral compartment. Meniscectomy was performed in 28 medial menisci and 11 lateral menisci. Arthroscopic lateral release was performed in 70 patients. Lateral retinacular release was performed to alleviate symptoms during deep flexion of the knee. On 1-year MRI, the mean modified MOCART score was 56.0 ± 3.5 of a maximum score of 100. All patients demonstrated repaired cartilage hypertrophy: 28 (33.0%) patients had grade 1 repaired cartilage hypertrophy, 41 (48.2%) had grade 2, and 16 (18.8%) had grade 3 (Figure 3). There were no patients with grade 4 hypertrophy. There were 3 patients with a subchondral cyst and 1 patient with subchondral sclerosis. There was no patient showing intralesional osteophytes. Repaired cartilage hypertrophy did not correlate with the size of the chondral defect, BMI, or age. The IKDC, VAS, and WOMAC scores at 3 years after surgery improved significantly compared with those before surgery (P < .001 for all). These scores improved significantly every year (Figure 4). The postoperative IKDC, VAS, and WOMAC scores did not differ significantly among the 3 groups according to repaired cartilage hypertrophy (Figure 5), and repaired cartilage hypertrophy was not correlated with these scores. MRI was performed in 11 (12.9%) patients at 2 years after surgery. In these patients, repaired cartilage hypertrophy did not differ between 1 and 2 years after surgery (P = .953). The second-look arthroscopic examination at 1 year after surgery revealed that the repaired cartilage was classified as grade 1 in 49 patients, grade 2 in 26 patients, and grade 3 in 10 patients. None of the patients complained of persistent popping, clicking, or catching, and none of the patients had infections or underwent additional arthroscopic surgery after CARTISTEM implantation.
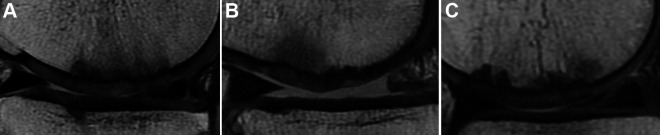
Figure 3.
Repaired cartilage hypertrophy on magnetic resonance imaging at 1 year after surgery: (A) grade 1, (B) grade 2, and (C) grade 3.
Figure 4.
International Knee Documentation Committee (IKDC), visual analog scale (VAS), and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores preoperatively and at 1, 2, and 3 years postoperatively. Each asterisk means that each score improved significantly compared to the previous year. Postop, postoperatively; Preop, preoperatively.
Figure 5.
International Knee Documentation Committee (IKDC), visual analog scale (VAS), and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores preoperatively and at 1, 2, and 3 years postoperatively according to the grade of repaired cartilage hypertrophy. Postop, postoperatively; Preop, preoperatively.
Discussion
The most important finding of this study was that all patients who underwent human UCB-MSC implantation showed satisfactory clinical and MRI outcomes. Postoperative IKDC, VAS, and WOMAC scores improved significantly, regardless of the degree of repaired cartilage hypertrophy. Repaired cartilage hypertrophy was not correlated with these scores, the size of the chondral defect, or age.
ACI is an effective therapeutic option for chondral defects of the knee. However, the difficulty in obtaining a suitable number of chondrocytes, the slow rate of cartilage cell proliferation, and the high rate of graft hypertrophy are limitations. 28 Compared with UCB-MSCs, primary cells from aged patients show impaired proliferation capacity and the chondrogenic commitment of cultured chondrocytes. 7 Therefore, UCB-MSC implantation may be an alternative treatment method for chondral defects of the knee in older patients.
Clinical outcomes after UCB-MSC implantation have been recently reported in the literature. Song et al 29 performed UCB-MSC implantation in 25 patients, with a mean age of 64.9 ± 4.4 years and a mean chondral defect size in the medial femoral condyle of 7.2 ± 1.9 cm2. They found that age <65 years and a larger chondral defect size were associated with a significantly greater improvement in IKDC, VAS, and WOMAC scores at 2 years. Dilogo et al 3 found that the IKDC score increased significantly and the WOMAC score decreased significantly at 6 months after an injection of UCB-MSCs and sodium hyaluronate. Chung et al 2 performed 93 UCB-MSC implantation procedures and followed patients for a mean of 1.7 years. They found that the median IKDC subjective score significantly improved from 39.0 to 71.3 and the WOMAC score from 44.5 to 11.0. Cartilage regeneration at second-look arthroscopic surgery showed that 8.2% of patients improved to ICRS grade 1 and 69.3% to ICRS grade 2. In the current study, PROs improved significantly every year and were comparable with those in previous ACI studies.19,21,22
To the best of our knowledge, repaired cartilage hypertrophy after UCB-MSC implantation has not been reported in the literature. Graft hypertrophy is the most common complication of ACI-P.27,30 The periosteum is thought to be the major cause of graft hypertrophy. The periosteum can promote cartilage formation because the cambium layer contains growth factors and chondrogenic precursor cells. 30 Kreuz et al 18 reported that the incidence of graft hypertrophy on MRI at 6 months after ACI-P was 28%. In a multicenter study, Gomoll et al 8 reported that 25.7% of patients treated with ACI-P required a reoperation for hypertrophy. Gooding et al 9 reported that 36.4% of the ACI-P group required debridement of graft hypertrophy during second-look arthroscopic surgery. The use of a collagen membrane decreased the incidence of graft hypertrophy. Gomoll et al reported that 5% of patients required a reoperation for hypertrophy after ACI-C within 1 year of surgery. Gooding et al reported that none of the 35 patients in the ACI-C group required debridement of graft hypertrophy during second-look arthroscopic surgery. However, the incidence of graft hypertrophy after MACI was similar to that after ACI-P. Ebert et al 4 reported that 22.6% of patients had asymptomatic graft hypertrophy at 5 years after MACI. Pietschmann et al 27 reported that graft hypertrophy developed in 25% of patients treated with MACI within a postoperative course of 1 year.
In the current study, 28 (33.0%) patients showed grade 1 repaired cartilage hypertrophy, 41 (48.2%) showed grade 2, and 16 (18.8%) showed grade 3. All patients showed repaired cartilage hypertrophy at 1 year after surgery. Compared with many studies on ACI-P, ACI-C, and MACI, the incidence of repaired cartilage hypertrophy was high.4,8,9,16,26,27,30 The exact reason why UCB-MSC implantation revealed a high incidence of repaired cartilage hypertrophy is not known yet. First, multiple 4-mm holes made on the subchondral bone might be a reason. However, there is controversy concerning the diameter of microfracture holes. A translational animal model study showed that a small-diameter microfracture awl led to significantly improved histological repair tissue quality and surface grading compared with larger awls. 23 Another animal model study reported that the bone marrow stimulation technique did not improve by changing the depth or diameter of the holes. 15 Second, the use of UCB-MSCs rather than autologous chondrocytes for the treatment of chondral defects may be an explanation for the high incidence of repaired cartilage hypertrophy. UCB-MSCs have a high rate of cell proliferation and low senescence. Jin et al 12 compared human bone marrow–derived MSCs, adipose tissue–derived MSCs, and UCB-MSCs for surface antigen expression, differentiation ability, proliferation capacity, clonality, tolerance for aging, and paracrine activity. Although MSCs from different types of tissue have similar levels of surface antigen expression, immunosuppressive activity, and differentiation ability, UCB-MSCs have the highest rate of cell proliferation and clonality and significantly lower expression of markers for senescence.
The timing of graft hypertrophy development was not completely established. Most authors reported the presence of hypertrophy within the first year postoperatively.10,18,27 Pietschmann et al 27 reported that graft hypertrophy occurred primarily in the first 12 months and regressed in most cases within 2 years. All cases of graft hypertrophy in their study developed within 12 months postoperatively and in 75% of the cases within the first 6 months. All cases of grade 1 graft hypertrophy regressed within the second postoperative year. A progression to grade 2 graft hypertrophy was observed in only 1 case. In patients who developed grade 2 graft hypertrophy, hypertrophy persisted within the 24-month postoperative phase. Kreuz et al 18 reported that graft hypertrophy of grade 3 and 4 was no longer detectable after 36 months and that the number of patients with grade 2 hypertrophy decreased from 11 to 5. They suggested that minimal grade 1 and 2 hypertrophic changes may diminish over time without a surgical intervention. However, Ebert et al 5 reported that hypertrophic grafts after MACI continued to develop through 24 months after surgery. In their study, 50 of 180 patients demonstrated graft hypertrophy: 9 grafts (5.0%) at 3 months, 32 grafts (18.7%) at 12 months, and 47 grafts (26.1%) at 24 months.
Limitations
We acknowledge some limitations in this study. First, the repaired cartilage thickness was measured 1 year after surgery. New repaired cartilage hypertrophy may be found after 1 year. Niethammer et al 22 reported that 75% of their patients developed graft hypertrophy in the first year after ACI. In 25%, additional graft hypertrophy was found at 24 months postoperatively. Changes in graft thickness were not evaluated on serial MRI. Second, there was no control group treated by arthroscopic debridement or marrow stimulation. However, clinical outcomes after microfracture in older patients, especially with large chondral lesions, have been unsatisfactory. 17 Therefore, a comparison between UCB-MSC implantation and microfracture in older patients seems to be inappropriate. Third, the quality of the repaired cartilage was not investigated by a biopsy or T2 mapping. The quality of the repaired cartilage can be an important factor to long-term durability. The durability of the repaired cartilage was not proven at midterm or long-term follow-up. Fourth, the timing of follow-up MRI did not match that of the collection of PRO scores. Fifth, younger patients were excluded from this study. Autologous osteochondral graft transfer or matrix-induced chondrogenesis is a preferred treatment method for young patients with large chondral defects. ACI and osteochondral allografts are not available in our country. HCB-MSC implantation is usually not recommended for young patients. Finally, lateral retinacular release was performed to alleviate symptoms during deep flexion of the knee, although its value has been questioned in the literature. Partial meniscectomy of the lateral meniscus was performed to prevent potential symptoms, although patients were asymptomatic at the time of HCB-MSC implantation. Concomitant surgery at the time of UCB-MSC implantation could affect PROs at follow-up and was a bias of this study.
Conclusion
Clinical outcomes improved significantly at short-term follow-up after UCB-MSC implantation. Although all patients showed repaired cartilage hypertrophy, it did not correlate with clinical outcomes.
Footnotes
Final revision submitted December 7, 2022; accepted January 17, 2023.
The authors declared that there are no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from a public institutional review board designated by the Korean Ministry of Health and Welfare (No. 2020-2659-001).
References
- 1.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–895. [DOI] [PubMed] [Google Scholar]
- 2.Chung YW, Yang HY, Kang SJ, Song EK, Seon JK. Allogeneic umbilical cord blood-derived mesenchymal stem cells combined with high tibial osteotomy: a retrospective study on safety and early results. Int Orthop. 2021;45(2):481–488. [DOI] [PubMed] [Google Scholar]
- 3.Dilogo IH, Canintika AF, Hanitya AL, Pawitan JA, Liem IK, Pandelaki J. Umbilical cord-derived mesenchymal stem cells for treating osteoarthritis of the knee: a single-arm, open-label study. Eur J Orthop Surg Traumatol. 2020;30(5):799–807. [DOI] [PubMed] [Google Scholar]
- 4.Ebert JR, Fallon M, Wood DJ, Janes GC. A prospective clinical and radiological evaluation at 5 years after arthroscopic matrix-induced autologous chondrocyte implantation. Am J Sports Med. 2017;45(1):59–69. [DOI] [PubMed] [Google Scholar]
- 5.Ebert JR, Smith A, Fallon M, et al. Incidence, degree, and development of graft hypertrophy 24 months after matrix-induced autologous chondrocyte implantation: association with clinical outcomes. Am J Sports Med. 2015;43(9):2208–2215. [DOI] [PubMed] [Google Scholar]
- 6.Fong CY, Subramanian A, Gauthaman K, et al. Human umbilical cord Wharton’s jelly stem cells undergo enhanced chondrogenic differentiation when grown on nanofibrous scaffolds and in a sequential two-stage culture medium environment. Stem Cell Rev Rep. 2012;8(1):195–209. [DOI] [PubMed] [Google Scholar]
- 7.Giannoni P, Pagano A, Maggi E, et al. Autologous chondrocyte implantation (ACI) for aged patients: development of the proper cell expansion conditions for possible therapeutic applications. Osteoarthritis Cartilage. 2005;13(7):589–600. [DOI] [PubMed] [Google Scholar]
- 8.Gomoll AH, Probst C, Farr J, Cole BJ, Minas T. Use of a type I/III bilayer collagen membrane decreases reoperation rates for symptomatic hypertrophy after autologous chondrocyte implantation. Am J Sports Med. 2009;37(suppl 1):20S–23S. [DOI] [PubMed] [Google Scholar]
- 9.Gooding CR, Bartlett W, Bentley G, Skinner JA, Carrington R, Flanagan A. A prospective, randomized study comparing two techniques of ACI for osteochondral defects in the knee: periosteum covered versus type I/III collage covered. Knee. 2006;13(3):203–210. [DOI] [PubMed] [Google Scholar]
- 10.Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation: a systematic review. Osteoarthritis Cartilage. 2011;19(7):779–791. [DOI] [PubMed] [Google Scholar]
- 11.International Cartilage Regeneration & Joint Preservation Society. ICRS Clinical Cartilage Injury Evaluation System. Accessed November 1, 2020. https://cartilage.org/society/publications/icrs-score
- 12.Jin HJ, Bae YK, Kim M, et al. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci. 2013;14(9):17986–18001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karnovsky SC, DeSandis B, Haleem AM, Sofka CM, O’Malley M, Drakos MC. Comparison of juvenile allogenous articular cartilage and bone marrow aspirate concentrate versus microfracture with and without bone marrow aspirate concentrate in arthroscopic treatment of talar osteochondral lesions. Foot Ankle Int. 2018;39(4):393–405. [DOI] [PubMed] [Google Scholar]
- 14.Kim SH, Ha CW, Park YB, Nam EW, Lee JE, Lee HJ. Intra-articular injection of mesenchymal stem cells for clinical outcomes and cartilage repair in osteoarthritis of the knee: a meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg. 2019;139(7):971–980. [DOI] [PubMed] [Google Scholar]
- 15.Kok AC, Tuijthof GJ, den Dunnen S, et al. No effect of hole geometry in microfracture for talar osteochondral defects. Clin Orthop Relat Res. 2013;471(11):3653–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kon E, Gobbi A, Filardo G, Delcogliano M, Zaffagnini S, Marcacci M. Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee: prospective nonrandomized study at 5 years. Am J Sports Med. 2009;37(1):33–41. [DOI] [PubMed] [Google Scholar]
- 17.Kreuz PC, Erggelet C, Steinwachs MR, et al. Is microfracture of chondral defects in the knee associated with different results in patients aged 40 years or younger? Arthroscopy. 2006;22(11):1180–1186. [DOI] [PubMed] [Google Scholar]
- 18.Kreuz PC, Steinwachs M, Erggelet C, et al. Classification of graft hypertrophy after autologous chondrocyte implantation of full-thickness chondral defects in the knee. Osteoarthritis Cartilage. 2007;15(12):1339–1347. [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Alcorocho JM, Aboli L, Guillen-Vicente I, et al. Cartilage defect treatment using high-density autologous chondrocyte implantation: two-year follow-up. Cartilage. 2018;9(4):363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McIntyre JA, Jones IA, Han B, Vangsness CT, Jr. Intra-articular mesenchymal stem cell therapy for the human joint: a systematic review. Am J Sports Med. 2018;46(14):3550–3563. [DOI] [PubMed] [Google Scholar]
- 21.Minas T, Gomoll AH, Solhpour S, Rosenberger R, Probst C, Bryant T. Autologous chondrocyte implantation for joint preservation in patients with early osteoarthritis. Clin Orthop Relat Res. 2010;468(1):147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niethammer TR, Loitzsch A, Horng A, et al. Graft hypertrophy after third-generation autologous chondrocyte implantation has no correlation with reduced cartilage quality: matched-pair analysis using T2-weighted mapping. Am J Sports Med. 2018;46(10):2414–2421. [DOI] [PubMed] [Google Scholar]
- 23.Orth P, Duffner J, Zurakowski D, Cucchiarini M, Madry H. Small-diameter awls improve articular cartilage repair after microfracture treatment in a translational animal model. Am J Sports Med. 2016;44(1):209–219. [DOI] [PubMed] [Google Scholar]
- 24.Park YB, Ha CW, Lee CH, Yoon YC, Park YG. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cells Transl Med. 2017;6(2):613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pas HI, Winters M, Haisma HJ, Koenis MJ, Tol JL, Moen MH. Stem cell injections in knee osteoarthritis: a systematic review of the literature. Br J Sports Med. 2017;51(15):1125–1133. [DOI] [PubMed] [Google Scholar]
- 26.Peterson L, Vasiliadis HS, Brittberg M, Lindahl AJ. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38(6):1117–1124. [DOI] [PubMed] [Google Scholar]
- 27.Pietschmann MF, Niethammer TR, Horng A, et al. The incidence and clinical relevance of graft hypertrophy after matrix-based autologous chondrocyte implantation. Am J Sports Med. 2012;40(1):68–74. [DOI] [PubMed] [Google Scholar]
- 28.Song JS, Hong KT, Kim NM, et al. Implantation of allogenic umbilical cord blood-derived mesenchymal stem cells improves knee osteoarthritis outcomes: two-year follow-up. Regen Ther. 2020;14:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song JS, Hong KT, Kim NM, Park HS, Choi NH. Human umbilical cord blood-derived mesenchymal stem cell implantation for osteoarthritis of the knee. Arch Orthop Trauma Surg. 2020;140(4):503–509. [DOI] [PubMed] [Google Scholar]
- 30.Steinwachs M, Kreuz PC. Autologous chondrocyte implantation in chondral defects of the knee with a type I/III collagen membrane: a prospective study with a 3-year follow-up. Arthroscopy. 2007;23(4):381–387. [DOI] [PubMed] [Google Scholar]