Abstract
Limited studies assess the efficacy of vitamin K administration in patients with chronic liver disease (CLD). However, vitamin K is commonly used to treat elevations in international normalized ratio (INR) in these patients with the intended benefit of reducing bleeding risk. This retrospective, single-center cohort study aimed to evaluate the impact of vitamin K administration on INR in patients with CLD. Hospitalized patients ≥ 18 years of age with a diagnosis of CLD or cirrhosis and received vitamin K were included. The primary outcome was the absolute change in INR from baseline to 24 to 48 h after vitamin K administration. Secondary endpoints included subgroup analyses of the primary outcome by route of administration and single versus multidose administration, and incidence of in-hospital venous thromboembolism (VTE) or major bleeding. A total of eighty-five patients, primarily with Child–Pugh class C (76.5%), were included. Route of vitamin K administration included oral (PO) (72%) and intravenous (IV) (26%) with a mean daily dose of 8.5 ± 2.3 mg. The absolute change in INR was −0.07 ± −0.35 following vitamin K administration. There was no difference in absolute INR change between single versus multiple dose administration (−0.16 ± −0.35 and −0.03 ± −0.35; P= .13) or between PO versus IV administration (−0.06 ± −0.23 and −0.18 ± −0.48; P = .11). The incidences of in-hospital VTE and major bleeding were 2.4% and 3.5%, respectively. The administration of vitamin K in hospitalized patients with CLD resulted in minimal INR change, suggesting this intervention may not have the intended benefit of reducing bleeding risk.
Keywords: chronic liver disease, cirrhosis, INR, vitamin K, international normalized ratio, bleeding
Introduction
Approximately 4.5 million patients are diagnosed with chronic liver disease (CLD) annually. 1 CLD or cirrhosis results in greater than 400,000 hospital admissions and approximately 44,000 deaths annually.1–3 The liver plays a key role in the coagulation process by synthesizing both procoagulant and anticoagulant factors from phytonadione (vitamin K); however, both are decreased in CLD, creating a rebalanced, more fragile hemostasis than in patients without CLD. 4 Thus, coagulation abnormalities are expected in CLD. Patients with CLD often have an elevated international normalized ratio (INR) due to disease-associated coagulopathy resulting from a decrease in the production of vitamin K-dependent coagulation factors II, VII, IX, and X. Due to these INR elevations, clinicians frequently administer vitamin K in an attempt to lower the INR and protect the patient from a perceived bleeding risk.
Vitamin K is a fat-soluble vitamin that promotes synthesis of the active forms of coagulation factors in healthy individuals.5–7 However, supplementation in CLD is likely to achieve only partial, if any, correction of these factors due to the underlying mechanism. In patients with CLD, there is decreased synthesis of factors II, VII, IX, and X in hepatocytes, not a deficiency in vitamin K.5–7
Although inexpensive and readily available, the conventional INR laboratory test was developed and calibrated for patients receiving vitamin K antagonist therapy, such as warfarin; thus, its use as a marker of anticoagulation and bleeding risk in CLD is unknown.4,8–11 Furthermore, the incidence of venous thromboembolism (VTE) in CLD patients has been reported to be 0.5% to 6.3%.4–6,8,9,12–17 This suggests that CLD not only causes an acquired bleeding disorder as once traditionally believed but can also be complicated by thrombosis. Tripodi et al. suggest that bleeding in patients with CLD is more likely related to hemodynamic instability related to the pathophysiology of the disease, rather than hypocoagulabilty.4,11
Current American Association for the Study of Liver Diseases (AASLD) guidelines do not address the use of vitamin K in CLD, and evidence to support this practice is lacking. Guidelines for acute liver failure (ALF) recommend vitamin K administration (5-10 mg subcutaneously for a 3-day course) in the setting of underlying vitamin K deficiency. 18 Limited studies have addressed the administration of vitamin K in CLD, and those that have did not find significant improvements in the majority of coagulation parameters.5,8,9,19,20 Despite the lack of evidence, vitamin K administration remains a commonly used pharmacotherapy in the management of CLD when an elevated INR is present. Thus, the objective of this study was to determine the impact of vitamin K administration on INR in patients with CLD.
Methods
This study was approved by the institutional review board, complying with all standards of the US Federal Policy for the Protection of Human Subjects, and was conducted at a large community teaching hospital. Vitamin K use at this facility is not guided by a protocol. The dose, number of doses, and route of vitamin K ordered for patients with CLD re based on the primary provider preference, though the practice is often guided by a gastrointestinal (GI) specialist.
This was a retrospective, single-center observational cohort study. Patients were identified through the corporate patient financial services database using the ICD-9-CM code 571.XX for CLD or cirrhosis and/or if they had an order placed for vitamin K during hospital admission. Patients were only eligible for inclusion once. Patients were included if they were admitted to the hospital, were 18 years of age or older at the time of admission, received at least 1 dose of intravenous (IV) or oral (PO) vitamin K, and a diagnosis of CLD or cirrhosis. CLD was defined based on the following clinical and pathological criteria: previous histories or clinical presentations of past variceal bleed, presence of varices based on endoscopy report, hepatic encephalopathy, spontaneous bacterial peritonitis, ascites, liver biopsy-proven cirrhosis, or imaging consistent with cirrhotic liver changes. Exclusion criteria were pregnancy, ALF, active bleeding or thrombotic disorder on admission, prior or current liver transplantation, missing INR data surrounding vitamin K administration, subcutaneous vitamin K, and/or transfer from an outside hospital. ALF was defined per AASLD guidelines as an INR ≥ 1.5 and any degree of mental alteration in a patient without preexisting cirrhosis and with an illness of < 26 weeks duration. Subcutaneous administration of vitamin K was excluded because this formulation is associated with erratic, unpredictable absorption which would not allow for a clean follow-up INR window of 24 to 48 h. Patients were also excluded if they received any anticoagulant (warfarin, DOACs [edoxaban, apixaban, rivaroxaban, dabigatran], argatroban, fondaparinux, bivalirudin, enoxaparin, or heparin) prior to or during admission. Lastly, patients were excluded if they had a history of transfusion of any blood product preceding 1 month or received any fresh frozen plasma (FFP) within the period 12 h prior to baseline INR through the time of follow-up INR.
INR values obtained between 24 and 48 h after documentation of vitamin K administration were recorded. If a patient had more than 1 follow-up INR reading during that period, the second of the 2 readings was recorded. Baseline INR included the most recent INR prior to vitamin K administration. All INR values were assessed and reported by the inpatient lab following in-hospital venipuncture; none were obtained via a point-of-care (POC) device. In-hospital VTE was defined as a positive Venous Doppler, CT chest, or V/Q scan finding during the admission. Major bleeding was defined as any clinically overt bleeding documented in the medical record plus the administration of PRBC, FFP, rFVIIa, and/or PCC.
The primary outcome was absolute change in INR from baseline to 24 to 48 h after last vitamin K administration. Secondary outcomes included subgroup analyses of the primary outcome by route of administration and dosing frequency (single vs multiple dose administration), incidence of in-hospital VTE, incidence of major bleeding, hospital length of stay, and in-hospital mortality.
Descriptive statistics were used to summarize baseline characteristics. Student's t-test was used to compare the subgroup analyses of the primary outcome by route of administration and dosing frequency.
Results
A total of 1102 patient charts were screened. Eighty-five patients met the eligibility criteria and were included in the analysis. Common reasons for exclusion were admission with an active bleed, missing INR data, or receipt of blood products within the time period specified previously, anticoagulation, and/or subcutaneous or intramuscular vitamin K. The majority of patients were White (85.9%), male (71.8%) with Child–Pugh Class C (76.5%), and alcoholic cirrhosis etiology (56.5%). Table 1 includes complete information about patient demographic factors.
Table 1.
Baseline Characteristics.
| Characteristic | n = 85 |
|---|---|
| Age (years)—avg. ± SD | 56.6 ± 13.5 |
| Male sex—n, (%) | 61 (71.8) |
| Body mass index [kg/m2]—avg. ± SD | 27.40 ± 6.05 |
| Race—n, (%) | |
| White | 73 (85.9) |
| Black | 5 (5.9) |
| Other | 7 (8.2) |
| Cirrhosis etiology—n, (%) | |
| Alcoholic | 48 (56.5) |
| Hepatitis B or C | 12 (14.1) |
| NASH | 12 (14.1) |
| Other | 13 (15.3) |
| Major bleeding risk—n, (%) | |
| Active gastroduodenal ulcer | 3 (3.5) |
| Bleeding 3 months prior to admission | 13 (15.3) |
| Platelet count < 50 K/µL on admission | 26 (30.6) |
| VTE prophylaxis—n, (%) | 65 (76.5) |
| SCDs | 54 (63.5) |
| Chemoprophylaxisa | 11 (12.9) |
| Baseline medications—n, (%) | |
| Antiplatelet | 10 (11.8) |
| NSAID | 12 (14.1) |
| Baseline laboratory results—avg. ± SD | |
| Lowest albumin (g/dL) | 2.39 ± 0.54 |
| Highest total bilirubin (mg/dL) | 9.04 ± 8.64 |
| Baseline serum creatinine (mg/dL) | 0.90 ± 0.41 |
| Platelets on admission (K/µL)—avg. ± SD | 108.5 ± 76.4 |
| Baseline INR—avg. ± SD | 1.90 ± 0.49 |
| Child–Pugh Class—n, (%) | |
| A | 2 (2.3) |
| B | 18 (21.2) |
| C | 65 (76.5) |
| MELD score—avg. ± SD | 18.18 ± 5.23 |
VTE chemoprophylaxis was given after/outside of vitamin K administration and INR window.
Abbreviations: INR, international normalized ratio; MELD, Model for End-Stage Liver Disease; NASH, non-alcoholic steatohepatitis; NSAID, non-steroidal anti-inflammatory drug; SD, standard deviation; SCD, sequential compression device; VTE, venous thromboembolism.
Route of vitamin K administration was primarily PO (71.8%) with less patients receiving the IV (25.9%) formulation. Two patients received a combination of both PO and IV vitamin K (2.4%). Individual doses were variable, ranging from 2.5 mg to 15 mg, but the average daily and total dose of vitamin K was 8.5 ± 2.3 mg and 19.8 ± 12.1 mg, respectively. The average number of doses administered was 2.31 ± 1.25, with the most common dosing frequency being one-time doses (58.9%).
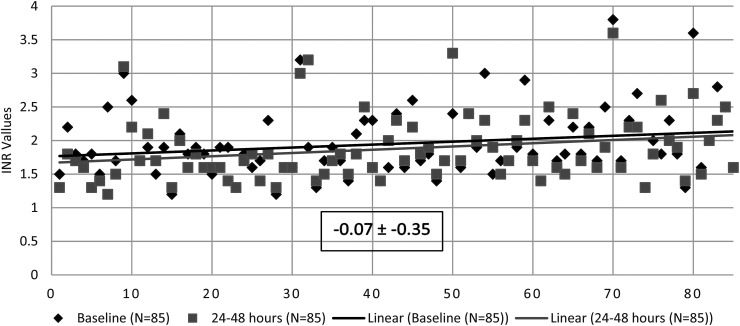
The primary outcome of absolute change in INR from baseline to 24 to 48 h was −0.07 ± −0.35 following last vitamin K administration (Figure 1). Absolute change in INR at 24 and 48 h was −0.08 ± −0.32 (n = 81) and −0.10 ± −0.40 (n = 49), respectively. The subgroup analyses of the primary outcome by route of administration and dosing frequency demonstrated no differences between groups (Table 2). There was no difference in absolute INR change between single versus multiple dose administration (−0.16 ± −0.35 and −0.03 ± −0.35; P = .13). Absolute change in INR after PO versus IV administration was −0.06 ± −0.23 and −0.18 ± −0.48, respectively (P = .11). Other key secondary outcomes (Table 3) including the incidence of in-hospital VTE and major bleeding were 2.35% and 3.53%, respectively. Incidence of in-hospital mortality was 7.06% (n = 6) with an average length of stay of 8.1 ± 5.9 days.
Figure 1.
Absolute change in international normalized ratio (INR) from baseline to 24 to 48 h after last vitamin K administration—avg. ± SD.
Table 2.
Subgroup Analyses of the Primary Outcome by Route of Administration and Dosing Frequency.
| Comparator Groups | n = 85 | Absolute Change in INR ± SD from Baseline to 24 to 48 h after last Vitamin K | P value |
|---|---|---|---|
| Route of administration, n, (%) | |||
| PO | 61 (71.8) | −0.06 ± −0.23 | .13 |
| IV | 22 (25.9) | −0.18 ± −0.48 | |
| Dosing frequency, n, (%) | |||
| Single dose | 30 (35.3) | −0.16 ± −0.35 | .11 |
| Multiple doses | 55 (64.7) | −0.03 ± −0.35 | |
Abbreviations: IV, intravenous; PO, by mouth; SD, standard deviation.
Table 3.
Secondary Outcomes.
| Outcomes | n = 85 |
|---|---|
| Incidence of in-hospital VTE—n, (%) | 2 (2.4) |
| Incidence of in-hospital major bleeding—n, (%) | 3 (3.5) |
| Length of stay (days)—avg. ± SD | 8.1 ± 5.9 |
| In-hospital mortality—n, (%) | 6 (7.1) |
Abbreviations: SD, standard deviation; VTE, venous thromboembolism.
Discussion
The administration of vitamin K in hospitalized patients with CLD resulted in minimal INR change, which was hypothesized based on the mechanism of coagulopathy in patients with CLD not being based on vitamin K deficiency. Minimal incidences of in-hospital major bleeding were seen among study patients, and similar rates were seen when compared to incidences of in-hospital VTE. These results support that this intervention may not have the intended benefit of reducing bleeding risk in these patients. However, given the small sample population in this study and lack of a comparator group, it is difficult to draw any conclusion about bleeding risk in patients with CLD receiving vitamin K supplementation.
There was no difference found between the route of administration or dosing frequency with regards to change in INR, suggesting there is not a preferred route or frequency of vitamin K administration to aid in the intended benefit of reducing bleeding risk. Among the subgroups, IV vitamin K demonstrated a relatively larger decrease in INR compared to PO administration. This is likely due to the faster onset of action of IV vitamin K compared to PO. Nonetheless, the difference between both subgroup analyses was not significant.
Results from this study are consistent with previous retrospective, single-center cohort studies. Meyer et al. found that vitamin K administration was not associated with a decrease in INR in 276 patients with CLD/cirrhosis (Adjusted OR, 1.17; 95% CI, 0.66–2.08; P = .59). 19 Vitamin K was also found to have no impact on bleeding (Adjusted OR 4.90; 95% CI 0.56–43.0; P = .15). 19 Additionally, Rivosecchi et al. found no statistically significant response in INR after IV vitamin K administration in 96 patients with CLD and a baseline INR greater than 1.5. 20 While it was noted that patients with higher baseline INR were more likely to respond, this was not statistically significant. 20
The strict exclusion criteria decreased the number of potential confounders, which was a strength of this study. PO and IV vitamin K have more predictable pharmacokinetics with a relatively short onset of action and minimal side effects. Exclusion of patients who received subcutaneous and intramuscular vitamin K administration decreased the likelihood of erratic absorption and unfavorable side effects, respectively. This allowed for a shorter follow-up INR window of 24 to 48 h based on the more predictable onset of action and half-life of PO and IV vitamin K. Exclusion of patients who received FFP and other blood products within a prespecified time window and those who received anticoagulation helped to eliminate pharmacotherapy that could have affected INR data. Patients with active bleeding on admission were excluded from the study which aided in limiting a potential confounder of patients with a higher risk of bleeding at baseline.
A limitation was the retrospective, single-center observational study design. Due to the retrospective nature of the study, many patients were missing INR data and thus were excluded. Diet was not collected and may influence INR depending on the content of vitamin K-containing foods consumed. The single-center nature of this study also was a limitation, making it more difficult to extrapolate these results to outside patient populations. Although the exclusion criteria of the study limited potential confounders, it led to a smaller sample size and the possibility of type II error. Lastly, there was a lack of standardization in dosing regimens between prescribers, which could have impacted study results.
Conclusion and Relevance
Overall, PO and IV vitamin K administration in hospitalized adult patients with CLD resulted in minimal change in INR, suggesting this intervention may not have the intended benefit of reducing bleeding risk. Decreasing the potentially unnecessary administration of vitamin K could lead to decreased health care costs and adverse drug reactions for patients diagnosed with CLD who have an elevated INR. Larger studies are needed to evaluate the impact of vitamin K on clinical outcomes.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Carmen B. Smith https://orcid.org/0000-0003-2399-3714
References
- 1.Chronic liver disease and cirrhosis mortality by state: CDC National Health Interview Survey .U.S. Department of Health & Human Services;2018. [Google Scholar]
- 2.Kozak LJ, Owings MF, Hall MJ. National hospital discharge survey: 2002 annual summary with detailed diagnosis and procedure data. Vital Health Stat 2005;158:1‐199. [PubMed] [Google Scholar]
- 3.Heron M. Deaths: Leading causes for 2010. Natl Vital Stat Rep. 2013;62(6):1‐96. [PubMed] [Google Scholar]
- 4.Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med 2011;365(2):147‐156. [DOI] [PubMed] [Google Scholar]
- 5.Blanchard RA, Furie BC, Jorgensen BA, Kruger SF, Furie B. Acquired vitamin K-dependent carboxylation deficiency in liver disease. N Engl J Med .1981;305(5):242‐248. [DOI] [PubMed] [Google Scholar]
- 6.Feldshon SD, Earnest DL, Corrigan JJ. Impaired coagulant factor synthesis is more important than impaired carboxylation in the coagulopathy of liver disease. Hepatology .1983;3(5):858. [Google Scholar]
- 7.Northup PG, Caldwell SH. Coagulation in liver disease: A guide for the clinician. Clin Gastroenterol Hep .2013;11(9):1064‐1074. [DOI] [PubMed] [Google Scholar]
- 8.Gursoy S, Baskol M, Torun E, et al. Importance of anticoagulant proteins in chronic liver diseases. Turk J Gastroenterol. 2005;16(3):129‐133. [PubMed] [Google Scholar]
- 9.Tripodi A, Caldwell SH, Hoffman M, Trotter JF, Sanyal AJ. Review article: The prothrombin time test as a measure of bleeding risk and prognosis in liver disease. Aliment Pharmacol Ther 2007; 26(2):141‐148. [DOI] [PubMed] [Google Scholar]
- 10.Saja MF, Abdo AA, Sanai FM, Shaikh SA, Abdel Gader AGM. The coagulopathy of liver disease: Does vitamin K help? Blood Coagul Fibrinolysis .2013;24(1):10‐17. [DOI] [PubMed] [Google Scholar]
- 11.Tripodi A, Salerno F, Chantarangkul V, et al. Evidence of normal thrombin generation in cirrhosis despite abnormal conventional coagulation tests. Hepatology 2005;41(3):553‐558. [DOI] [PubMed] [Google Scholar]
- 12.Dabbagh O, Oza A, Prakash S, et al. Coagulopathy does not protect against venous thromboembolism in hospitalized patients with chronic liver disease. Chest 2010;137(5):1145‐1149. [DOI] [PubMed] [Google Scholar]
- 13.Aldawood A, Arabi Y, Aljumah A, et al. The incidence of venous thromboembolism and practice of deep venous thrombosis prophylaxis in hospitalized cirrhotic patients. Thromb J. 2011;9(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Northup PG, McMahon MM, Ruhl AP, et al. Coagulopathy does not fully protect hospitalized cirrhosis patients from peripheral venous thromboembolism. Am J Gastroenterol 2006;101(7):1524‐1528. [DOI] [PubMed] [Google Scholar]
- 15.Gully D, Teal E, Suvannasankha A, et al. Deep vein thrombosis and pulmonary embolism in cirrhosis patients. Dig Dis Sci 2008;53(11):3012‐3017. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Fuster MJ, Abdilla N, Fabia MJ, et al. Venous thromboembolism and liver cirrhosis .Rev Esp Enferm Dig 2008;100(5):259‐262. [DOI] [PubMed] [Google Scholar]
- 17.Smith CB, Hurdle AC, Kemp LO, Sands CD, Twilla JD. Evaluation of venous thromboembolism prophylaxis in patients with chronic liver disease. J Hosp Med 2013;8(10):569‐573. [DOI] [PubMed] [Google Scholar]
- 18.Polson J, Lee WM. AASLD Position paper: The management of acute liver failure. Hepatology 2005;41(5):1179‐1197. [DOI] [PubMed] [Google Scholar]
- 19.Meyer AV, Green M, Pautler HM, et al. Impact of vitamin K administration on INR changes and bleeding events among patients with cirrhosis. Ann Pharmacother. 2016;50(2):113‐117. [DOI] [PubMed] [Google Scholar]
- 20.Rivosecchi RM, Kane-Gill SL, Garavaglia J, MacLasco A, Johnson H. The effectiveness of intravenous vitamin K in correcting cirrhosis-associated coagulopathy. International J of Pharm Prac .2017;25(6):463‐465. [DOI] [PubMed] [Google Scholar]