Abstract
Introduction:
Structural characterization of Low Molecular Weight Heparin (LMWH) is critical to meet biosimilarity standards. In this context, the review focuses on structural analysis of labile sulfates attached to the side-groups of LMWH using mass spectrometry. A comprehensive review of this topic will help readers to identify key strategies for tackling the problem related to sulfate loss. At the same time, various mass spectrometry techniques are presented to facilitate compositional analysis of LMWH, mainly Enoxaparin.
Areas Covered:
This review summarizes findings on mass spectrometry application for LMWH, including modulation of sulfates, using enzymology and sample preparation approaches. Popular open-source software packages for automated spectral data interpretation are also discussed. Successful use of LC/MS can decipher structural composition for LMWH and help evaluate their sameness or biosimilarity with the innovator molecule. Overall, the literature has been searched using PubMed by typing various search queries such as “enoxaparin”, “mass spectrometry”, “low molecular weight heparin”, “structural characterization”, etc.
Expert Commentary:
This section highlights clinically relevant areas that need improvement to achieve satisfactory commercialization of LMWHs. It also primarily emphasizes the advancements in instrumentation related to mass spectrometry, and discusses building automated software for data interpretation and analysis.
Introduction
Heparin is a molecule belonging to the family of Glycosaminoglycans (GAGs), and consists of the heterogeneous mixture of polymers ranging from 5,000–30,000 Da. Due to the presence of heterogeneity, this molecule has been referred to as “Unfractionated Heparin (UFH)” amongst clinicians and researchers [1]. Heparin possesses biological functionality towards angiogenesis and host-pathogen interactions [2,3,4]. Furthermore, Heparin is popular in the pharmaceutical industry for its anti-coagulant properties, and its depolymerized version, termed Low Molecular Weight Heparins (LMWH), has gained much attention in the recent past. Different types of LMWH are derived based on differing depolymerization processes. These molecules are similar to UFH in monosaccharide composition and oligosaccharide sequence. LMWH possess several advantages over UFH due to their lower molecular mass, including prolonged antithrombotic effect and better bioavailability. Given that LMWH do not bind to plasma proteins and endothelial cells, they have a longer half-life in circulation [5].
Due to the often-reported side effects causing Heparin Induced Thrombocytopenia (HIT), LMWHs have been explored as anticoagulants [6]. These molecules are considered more potent compared to unfractionated heparin (UFH) [7]. Herein, the choice of depolymerization process dictates the composition percentage of pentasaccharide units involved in antithrombin activity. It has been shown that the LMWH having longer chains have higher antithrombin activity compared to shorter chains [8]. This indicates a major challenge in associating functional relevance to LMWH from its heterogeneous polysaccharide structures. Understanding charge distribution from the varying sulfation, carboxylation units on specific saccharide provides knowledge on active pharmaceutical ingredient (API) [9,10]. The chemo-enzymatic process resulting in depolymerization of heparin also becomes important to study due to its regulatory effect on physico-chemical properties. Amongst currently marked LMWH, the present review focuses on Enoxaparin as it is used to treat a multitude of disorders. Enoxaparin has the highest Anti-Xa: Anti-IIa ratio and its plasma half-life is second only to Tinzaparin. Enoxaparin also reports the highest concentration of free Tissue Factor Pathway Inhibitor (TFPI) when injected subcutaneously [11].
As Enoxaparin is one of the most commonly prescribed LMWH for many disorders [12,13,14,15,16], it becomes critical to identify methods that could be used to determine the sameness of biosimilarity in enoxaparin compared with that of an innovator molecule. In this regard, FDA guidelines suggest five different criteria for demonstrating sameness of an in-house product to that of an innovator molecule [17]. In addition to biological function (phenotypic sameness) and in vivo pharmacodynamics, the following listed characterization instruments are used to associate sameness. Various analytical techniques include but are not limited to RPIP-LC-MS (Reverse Phase Ion Paired – Liquid Chromatography – Mass Spectrometry), HILIC-MS (Hydrophilic Liquid Chromatography-Mass Spectrometry), SAX (Strong Anion Exchange Chromatography), CZE (Capillary Zone Electrophoresis), and SEC (Size Exclusion Chromatography) have been recently adopted (Fig. 1). Combinatorial usage of instruments has also been applied for mass spectral characterization of LMWH. Thus, this review focuses primarily on structural verification and attempts to identify a strategy that may be used to comprehensively characterize sulfates on LMWH, mainly Enoxaparin (Table I).
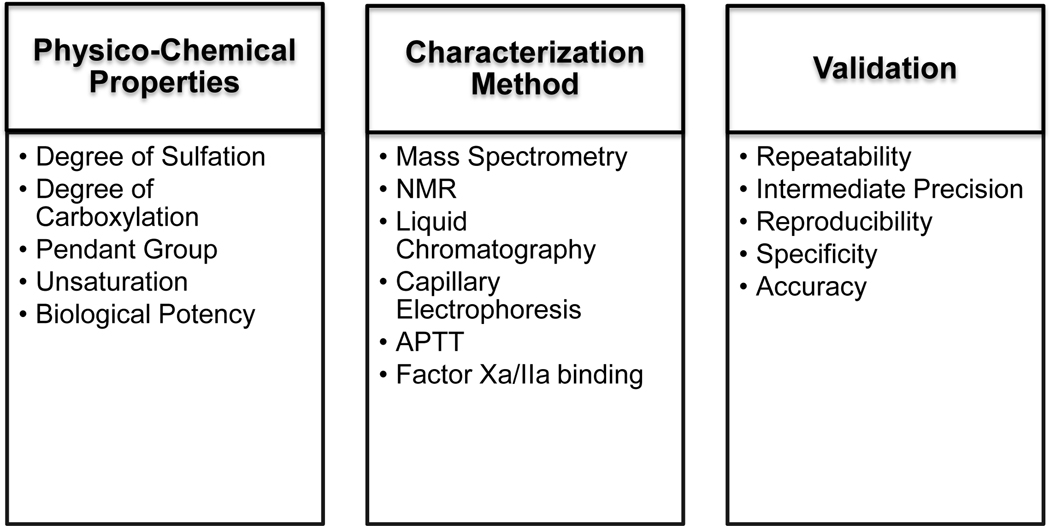
Figure 1:
The schema here describes various parameters that need to be evaluated using various instrumentation approaches to validate LMWH. Also, shown herein are different tests performed for method validation. These aspects are covered for evaluating sameness for any biosimilar or generic molecule.
Table I:
Physico-chemical properties of Heparin
| Heparin Type | Molecule Name | Molecular Weight (kDa) | Characteristic structural feature |
|---|---|---|---|
| Unfractionated Heparin | Heparin | 8 – 15 | IdoA-2S-(1→4)-GlcNS,6S |
| Low Molecular Weight Heparin | Enoxaparin | 4.5 | 4,5 uronic acid on non-reducing end; 1,6-anhydro ring on the reducing terminus (15 – 25 %) |
| Low Molecular Weight Heparin | Dalteparin | 6.0 | anhydromannitol ring at the reducing end |
| Low Molecular Weight Heparin | Tinzaparin | 6.5 | Non-reducing end with unsaturated uronate residue |
Heparin structure and its implications for antithrombin activity
Heparin is a polysaccharide with characteristic trisulfated disaccharide units of IdoA2S-GlcNS6S. Its synthesis begins via chain extension of a tetrasaccharide linker O-linked to serine residue on a protein backbone [18]. In the presence of nucleotide-sugar substrates such as uridinediphosphate-N-acetylglucosamine (UDP-GlcNAc) and uridinediphosphate-glucose (UDP-Glc), monosaccharide addition occurs via the exostosin glycosyltransferase (EXT) enzyme [19]. Heparin enhances ~1000-fold anticoagulation activity of antithrombin-III (AT) through complex formation. This interaction results in the inhibition of two coagulation proteases, thrombin (Factor IIa) and Factor Xa (FXa) [20]. This catalytic action by Heparin-bound AT is facilitated by the presence of a pentasaccharide unit as a part of heparin’s polysaccharide backbone. This unit consists of carbohydrates with varying degrees of sulfation, and is represented as: GlcNAc6S-GlcA-GlcNS3S6S*-IdoA2S-GlcNS6S (abbreviated as ANAGA*ISA). The presence of 2-O-sulfate on IdoA (Iduronic acid) monosaccharide increases affinity towards AT, whereas the presence of 6-O-sulfate on glucosamine indirectly contributes to AT binding by proportionately increasing the 2S0 conformation [21]. Because LMWH are derived from Heparin, the structure-function analysis of Heparin would help to establish the functional role of heterogeneous chains present on LMWH. Moreover, an in-depth structure-function analysis of Heparin would also help to identify key design strategies for synthesizing Heparin mimetics beyond LMWH. Initial scientific steps have already been taken in this direction [22]. Polymer Chain and Extension Units on LMWH
Enoxaparin is derived from Heparin, extracted from porcine intestinal mucosa, by depolymerization that leads to lower molecular weight fractions. Enoxaparin is further obtained via alkaline β-elimination of the UFH benzyl ester. By contrast, the other two LMWH, i.e. Dalteparin and Tinzaparin, are chemically derived by nitrous acid depolymerization and enzymatically by heparinase from F. heparinum, respectively [23]. Enoxaparin contains variants of uronic acid on its non-reducing end, whereas the reducing end consists of a 1,6 anhydro closed ring-like structure, or possesses an open conformation consisting of a hydroxyl group [24]. The non-reducing end contains either 4,5 unsaturated or a saturated form of uronic acid. These terminal groups are flanked by a repeating unit of GlcNAc (and variants) and IdoA/ GlcA with β1,4 linkage, which extends from n=1 to 22. The degree of sulfation on the repeating unit primarily determines the potency of LMWH and its binding to Factor Xa/IIa in an in vitro assay [25]. For example, a compositional analysis of acetyl groups, sulfate to carboxylate ratio, 1,6 anhydro, active pentasaccharide unit determines sameness between the generic and innovator molecule. Due to its inherent complexity and inbuilt heterogeneity in the structural composition of Enoxaparin, it is indispensable to conduct a comprehensive analysis of its characterization. In this regard, structural characterization between the biosimilar and innovator Enoxaparin will be required to meet regulatory guidelines for generic drug approval.
Latest therapeutic uses of Enoxaparin
Enoxaparin is majorly used to treat Deep Venous Thrombosis (DVT) and pulmonary embolism (PE) which constitutes Venous Thromboembolism (VTE). In regard to treatment, adequate dosages of Enoxaparin have still not been finalized. There are patients for whom 40mg dosages of Enoxaparin are given once or twice daily, or 0.4 – 0.5 mg/kg daily dosages are also recommended. A recent study by Pannucci et al. attempted to compare Enoxaparin dose response in the plastic and reconstructive surgery population [26]. Baumgartner et al. presented yet another study wherein they suggest that prophylactic dosages of Enoxaparin may not serve patients who undergo abdominal cancer surgery. Given that cancer results in VTE due to malignancy and major surgery, anticoagulants find tremendous uses in the treatment of such patients. But the right dosage of Enoxaparin is essential for the treatment of VTE in cancer patients, which this study found to be inadequate [27]. Alnatsheh et al. further studied the occurrence of VTE in obese and non-obese patients who were given prophylactic dosages of Enoxaparin. In this study, no differences were observed in VTE occurrence between the two cohorts, but the authors suggest conducting randomized clinical trials to confirm these findings [28]. Due to the popularity of Enoxaparin in treating VTE, it has become critical to perform randomized clinical trials of various diseased conditions to identify disease-specific dosages for effective administration. Thus, collective efforts by clinicians, researchers and doctors will be required to determine the right dosages under different circumstances, and finally to individualize Enoxaparin use amongst patients.
Heparinase digestion of LMWH
The digestion protocol resulting in Heparin depolymerization to LMWH is as illustrated in Table I. Three different types of heparinase results in specific cleavage onto the repeating unit of LMWH [29]. Heparinase obtained from Flavobacterium Heparinum (F. Heparinum) primarily cleaves between GlcN and uronic acid (Iduronic acid/Glucoronic acid) [30]. Heparinase I and III cleave to LMWH based on the decreasing degree of sulfation, whereas Heparinase II cleaves almost at all sites [31,32,33,34,35,36]. Thus, the choice of Heparinase II for digestion appeals as regards introducing homogeneity in molecular fragments. However, this simultaneously results in a highly fragmented species that is much more tedious to characterize. Heparinase obtained from Prevotella Heparinolytica (P. Heparinolytica) also efficiently cleaves LMWH similar to Heparinase I from F. Heparinum. Furthermore, F. Heparinum contains Heparinase II that interferes with the cleavage of LMWH treated with Heparinase I. Such interference is not observed when Heparinase from P. Heparinolytica is used, due to lack of other enzymes present in the organism [37]. However, the KM for cleavage of the latter is slightly larger than that obtained for Heparinase I from F. Heparinum. Thus, to obtain complete digestion, it is indispensable to have an enzyme which possesses high VMax and low KM properties. Furthermore, in regard to reaction conditions, the enzymatic buffer resulting in the cleavage requires Bovine Serum Albumin (BSA) as the control protein. While buffer conditions can be adjusted such that complete digestion results in disaccharide, tetrasaccharide, and hexasaccharide fragments, the presence of extraneous protein causes contamination. Thus, additional sample preparation steps can be incorporated involving treatment with either acetone or ethanol for protein precipitation. These steps may be added after digestion of heparinase such that the carbohydrate is finally suspended in a protein-free buffer solution for sameness evaluation.
Regulating Sulfation for Structural Characterization
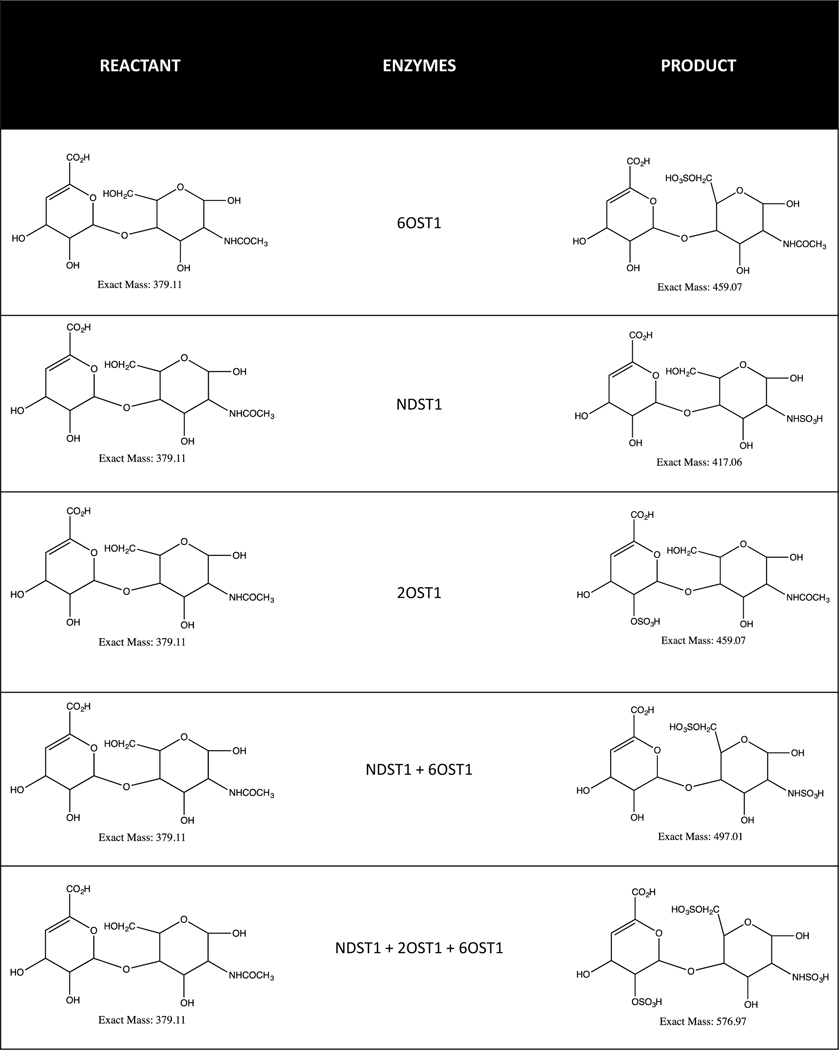
Due to its efficacy and anticoagulant properties, Enoxaparin is popular as an LMWH-based therapeutic drug (Fig. 2). Moreover, Enoxaparin digestion by Heparinase II results in characteristics tetrasaccharide fragments, including, but not limited to, deltaUA-GlcNS6S-GlcA-GlcNS3S, deltaUA-GlcNS6S-GlcA-GlcNS3S6S, deltaUA-GlcNAc6S-GlcA-GlcNS3S6S, and deltaUA-GlcNAc-GlcA-GlcNS3S. The disaccharide structure mostly released contains deltaUA2S-GlcNS6S. These characteristic structures exhibit a prominence of sulfate groups on carbohydrates and introduce complexity in structural verification [38,39,40]. Furthermore, the sulfate groups are critical for an anti-coagulant property, and thus are relevant from a functional perspective [9,41,42]. Because sulfation is primarily involved in introducing structural variability in Enoxaparin, the specificity analysis of sulfolyltransferases in catalyzing sulfolyl groups onto various carbohydrates requires assessment (Table II). Each enzyme adds a mole of sulfate per mole of PAPS (3’-phosphoadenosine 5’-phosphosulfate) in a bi-bi substrate reaction. The addition of a sulfate group results in a bulky molecule and reduces heterogeneity that would otherwise have been introduced from variable sulfation (Fig. 3). Taken together, a strategy whereby enoxaparin is treated with sulfolyltransferase enzymes for maximizing sulfation, and thus reducing heterogeneity on carbohydrate substrates, may simplify structural characterization amongst various enoxaparin grades. While this approach will change the overall sulfation patterns on Enoxaparin and result in changes in the native structure, it will nevertheless reduce variability due to sulfation. Such treatments can be applied only when a comparative analysis is performed. For example, biosimilar and innovator Enoxaparin can be treated under the identical enzymatic conditions and compared for their structural identity. Thus, the methodology can be adopted for structurally comparing Enoxaparin obtained from various sources.
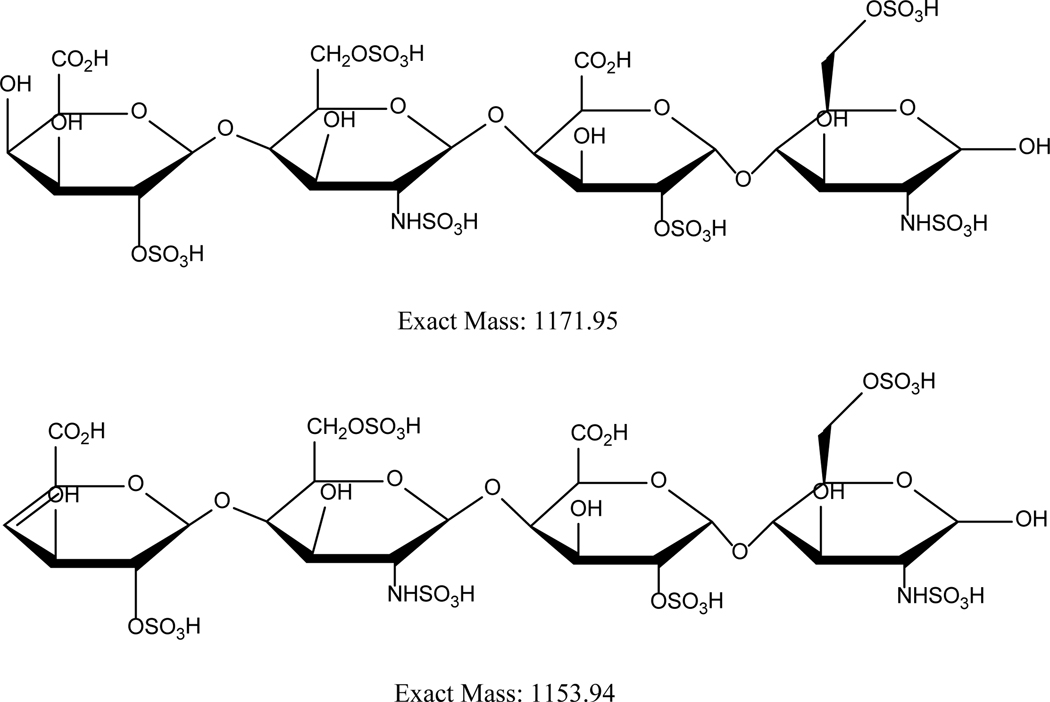
Figure 2:
These structures here illustrate two of the tetrasaccharide moieties obtained for Enoxaparin. Particularly, here 2 variants with or without 4,5 unsaturation on the non-reducing terminus of enoxaparin is exhibited. Such highly sulfated structures introduce heterogeniety in characterization.
Table II:
Substrate specificity of sulfolyltransferase acting on GAGs
| S. No. | Substrate(s) | Sulfolyltransferase | Enzyme Specificity |
|---|---|---|---|
| 1 | GlcNAc | NDST1 and NDST2 N-deacetylase/N-sulfotransferase |
Specific |
| 2 | GlcNS | 6OST-1 6O-sulfotransferase1 |
Non-specific |
| 3 | GlcNS6S | 3OST-1 3O-sulfotransferase1 |
Specific |
| 4 | IdoA | 2OST-1, 6OST-1 2O-sulfotransferase1, 6 O-sulfotransferase1 |
Specific/Non-specific |
Figure 3:
This schema lists products formed in the presence of specific sulfolyltransferases and specific reactant.
Sample preparation of Enoxaparin for Structural Characterization
Sample preparation for LMWH or Enoxaparin has traditionally depended on the source of its origin. Usually, the active pharmaceutical ingredients (API) in Enoxaparin are pressure dialysed against a 1000 Da molecular weight cutoff membrane, and then lyophilized. These samples are then solubilized in the variety of buffers compatible with the enzymatic digestion protocol. For structural characterization, different enzymes such as Heparinase I, II and III are usually pooled together, and Enoxaparin is incubated with them for 16 – 18 h at 37°C. The optimal concentration of Heparinase mixture for treatment means complete digestion of oligosaccharides into disaccharide units [43]. Appropriate sized molecular weight cutoff membrane is further used to remove intact Heparinases from the digested product, or the sample is heated at 100°C for 5 min to quench the reaction [38]. Since the introduction of Heparinase in the final mixture could increase sample noise, it is desirable to use an appropriate molecular weight cutoff membrane to remove these enzymes from the digested product. This procedure will increase the S/N ratio and further clean the experimental output. Downstream analytical applications, such as MS, demand purity in the compound to remove any unwanted m/z signal on the total ion chromatogram (TIC). Thus, an overall strategy whereby contaminating additives are removed serves as a prerequisite for the sample preparation step.
Protecting Sulfation for Intact MS
Dell et al. have identified β-elimination as a chemical methodology for peeling O-glycans from the cell surface or circulatory glycoproteins. While the approach results in the analysis of cleaved O-glycans, a part of the procedure involves modification of –OH groups on carbohydrate sidechains into –OMethyl, such that their damage or loss is reduced during passage from the high-resolution mass spectrometry instrument [44]. Because methyl groups are not cleaved in spite of high ion source temperature, analysis of intact molecules is possible. Similarly, sulfoesters present on the carbohydrate units of LMWH become labile at high temperature, and are prone to the hydrolysis that results in the loss of a side-group. Various protecting groups have been suggested for use in the preparation of sulfoesters, but none has been classified for protecting sulfate groups while running for spectral analysis. Some of these suggested include phenol, trichloroethyl, neopentyl, isobutyl, and others that form sulfate diester [45,46]. However, in the presence of nucleophiles, these sulfate diesters are prone to attack at either the carbon or the sulfur atom, resulting in dissociated molecules. In this context, therefore, the identification of an effective methyl group as a protecting agent for hydroxyl might well work for sulfoesters. Some other binding agents, such as basic peptides, have also been used to circumvent sulfate loss. This has been shown in various studies wherein analysis of a Heparin-peptide mixture resulted in the identification of an intact molecular mass of complex molecules without loss of sulfate [47,48].
Recent advancements in the field of mass spectrometry have also shown Matrix Assisted Laser Desorption/Ionization (MALDI) or its variant, surface-enhance LDI, as a promising tool for reducing sulfate loss. This reduction stems from usage of correct insource conditions, both related to the instrument parameter and that of the buffer employed [49]. However, application of FT-ICR MS-based mass spectrometry has not prevented sulfate loss, but has nevertheless allowed identification of sites of sulfation by conducting cross-ring cleavages, which it is able to do via the negative electron transfer dissociation (NETD) technique employed using FT-ICR MS [50]. Kailemia et al. recently investigated the use of sodium hydroxide as a spray solution to circumvent sulfate loss during mass spectrometry analysis. In this study, exhaustive deprotonation of acidic groups in precursor ions was performed using 1 or 2 mM sodium hydroxide, and resulted in stabilization of sulfo groups in those oligosaccharides which are either lowly or heavily sulfated. This use results in adequate fragmentation of the glycosidic bonds and cross-ring cleavages, in addition to retaining the site of sulfate loss. Overall, this methodology is presented as demonstrating a comprehensive structural characterization for sulfated oligosaccharides [51].
Mass Spectrometry for LMWH
Enoxaparin is a highly emerging LMWH, annotated as a category B pregnancy drug. Typically, enoxaparin sodium contains eight disaccharide units that are analyzed by various chromatographic techniques [52]. For structural verification of enoxaparin or LMWH, various tandem mass spectrometry techniques have been recently used ,including, but not limited to, high performance liquid chromatography (HPLC) with quadrupole time-of-flight (Q-TOF) mass spectrometry (MS) and diode array detector (DAD) MS, capillary electrophoresis (CE) with MS, HILIC Fourier transform (FT) MS, ultra performance (UP) SEC-QTOF-MS, RPIP-UPLC-MS, and others. [39,40,53,54,55,56,57]. In a recent study, oligosaccharides resistant to Heparinase II digestion were also analyzed using LC-MS [58]. Li et al. reported a fingerprinting methodology using RPIP-ESI-MS, in which an ion-trap TOF MS was used tandem with capillary HPLC for enoxaparin oligosaccharide assignment. The authors used 15mM pentylamine (PTA) as an MS compatible ion-pairing agent to segregate different Enoxaparin oligosaccharide, and then matched it with in-house developed software. Using this software, the authors report identification of 150 oligosaccharides belonging to enoxaparin [59]. The same group later reported further characterization of Enoxaparin digested separately with Heparinase I, II, and III. In total, 200 different oligosaccharides ranging between a degree of depolymerization (dp) 2 to dp10 were found, using three different enzymatic treatments of Enoxaparin [38]. Liu et al. reported a top-down and bottom-up approach to compare innovator and biosimilar version of Enoxaparin by carefully selecting mass spectrometry approaches. This article covers results wherein intact Enoxaparin chains, Heparinase II digested oligosaccharides were utilized in hydrophilic interaction chromatography (HILIC) Fourier Transform (FT) MS. Completely digested disaccharides were further used in RPIP-LC-ITMS for bottom-up analysis of Enoxaparin drugs. A comprehensive analysis of four Enoxaparin drugs depicted similar structural features. The major difference observed in the top-down approach between these four drugs was due to the difference in the parent heparin used by different manufacturers [60].
In another study published by the same group, a structural correlation was developed between parent heparins and daughter Enoxaparin using an integrated analytical approach. Parent heparin as used by two different manufacturers in the US and Europe were compared here [61]. The same group later identified sameness in the enoxaparin obtained using heparins from bovine intestinal and bovine lung. In vitro potency and structural characterization assays depicted comparable results in enoxaparin obtained from different heparin sources. Thus, the bottom-up and top-down approach adopted by this group helped to establish sameness in enoxaparin obtained from different heparin sources [62,63]. Based on the strategy adopted for deriving LMWH from heparins, their molecular weight (MW) properties will differ. In this context, an article by Ouyang et al. suggests the use of size exclusion chromatography (SEC) inductively coupled plasma (ICP) MS for determining accurate MW of enoxaparin and other compounds. To do this, the authors used SEC-ICP-MS for accurately determining the ion-pairing cation which forms part of the mobile phase. In this work, a specific mobile phase was used as the source of cation and their accurate MW along with the enoxaparin’s MW was successfully determined [64].
The advancement in the field of spectroscopy has enabled precise identification of species of interest from a sample containing a complex mixture. To annotate different polysaccharide fragments present in Enoxaparin, the choice of the right mass spectrometry ionization source serves as a useful initial step (Fig. 4). Accumulating evidence suggests electrospray ionization (ESI) is a useful analyzer due to its soft ionization tendency [65,66]. To this end, use of negative polarity serves to establish correct mass determination of sulfate groups without using an ion-pairing agent. It has also become a general practice to use arixtra as a calibration molecule for optimization of ion source parameters. This molecule consists of octasulfated penta-saccharide moieties, and thus allows for optimized instrument settings for highly sulfated polysaccharides.
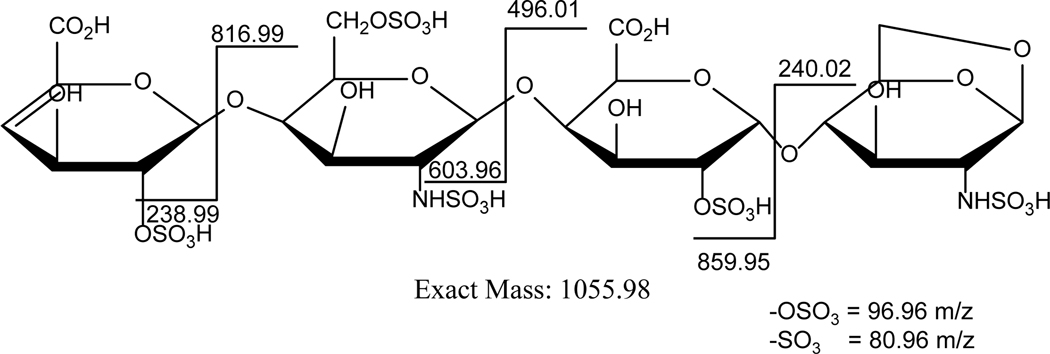
Figure 4:
The LC-MS based fragmentation pattern for a 1,6 anhydro terminated enoxaparin grade. This fragmentation profile illustrates different m/z obtained on cleaving enoxaparin into disaccharides, trisaccharides and tetrasaccharides.
Any mass spectrometry-led experiment can be carried out in tandem with liquid chromatography (LC) or by direct infusion. Experiments are initiated by identification of any mixture by first infusing the sample. ESI-led infusion experiments have been used to quantify LMWH disaccharide mixtures, and collisional induced dissociation (CID) has been performed tandem to LC, enabling identification of isomers. Furthermore, ESI-MS tandem to nano-LC has allowed for experiments with nano-litres sample-volume, thus increasing permutations of experiments for optimizing methods [67]. Various other LC use, including reverse-phase ion pairing (RPIP) and Size Exclusion Chromatography (SEC), has enabled identification of complex LMWH or enoxaparin fragments using ESI-MS [68,69]. This has further indicated the usefulness of column compatibility with different ion pairing agents, buffer conditions, solvent requirement, and the like. Such analysis overall has resulted in qualitative identification of molecules. Furthermore, specifically for Heparin Sulfate (HS), synthesis of a labeled molecule such as 13C/15N has enabled quantitative estimation of this GAG [70].
Various other LC-MS methods have been employed for qualitative and quantitative estimation of LMWH species. In addition to the RPIP and SEC, HILIC provides a chemically modified matrix wherein the species binds to the column based on charges [71]. In this case, the mobile phase contains a water gradient with decreasing organic phase content. HILIC LC-MS has also been employed using a chip-based LC/ MS interface for analysis of CS/DS [72]. Unlike HILIC, graphitized carbon chromatography (GCC) LC-MS produces high resolution from its ability to withstand the wide range of pH and physio-chemical conditions [73]. In this regard, the negative ion mode GCC has been employed for heparinase digested heparin or LMWH. Given that Heparin or LMWH contains diverse sulfate groups, an introduction of negative charge is handled using high pH conditions in the presence of ammonium buffers [74].
Exhaustive structural characterization may for analysis also require use of more than one column connected in tandem with MS. Because LMWH isomer analysis is of considerable interest, using multiple LC columns together with MS has enormous potential. To this end, an independent study subjected a digested HS sample to an SEC column, and thereby onto Strong Anion Exchange (SAX) chromatography for wholesome separation. MS analysis resulted in two different isoforms of a hexasaccharide but with a common set of m/z values [75].
Post-experiment Processing for Spectral Analysis
Identification of LMWH composition benefits exploring their biological and therapeutic potentials. The biological and therapeutic potential of LMWH will benefit by an exploration and identification of its composition. The application of various mass spectrometry instruments, such as ESI and MALDI, has revolutionized structural characterization of complex molecules such as Heparin and LMWH [76]. Furthermore, an addition of tandem LC features has already dramatically improved sample analysis for a given set of carbohydrates. However, a major challenge in using tandem LC-MS for GAGs arises from their ability to lose sulfate because they are collisionally-activated molecules. It has been suggested that the application of various ion modes and/or adducts that result in differing charge and adduct states may reduce sulfate loss. This ability prevents or simplifies complexity arising in the fragmentation pattern, but together makes data interpretation highly cumbersome. Towards this end, deconvolution has been proposed as an algorithm that simplifies data interpretation [77]. Deconvolution results in merging various charges states (z) for a given molecular mass (m). m/z for the same sample further differs from multiplying differing absolute masses (m) due to adduct addition or loss. In the former case, assignment of z from various isotopic peaks is simple in cases where z < 10 because the reciprocal of spacing between adjacent peaks provides z. In the latter case, building a theoretical database is required for knowledge of various m/z or intact masses either with or without adduct. For z > 10 wherein the required mass resolution < 3 ppm at an m/z of 1000, the instrument-specific z interpretation becomes tedious [78]. To relieve this problem, various deconvolution or pattern recognition techniques are employed. In an independent study, Fourier transform, Patterson function, and a combination of these were used to automate determination of various charge states (z). This required information obtained from the isotopic peak envelopes to calculate the spacing between peaks with high accuracy [79]. For using deconvolution methods, Decon2LS provides an open-source software package for automated progression of mass spectral data, whereby deisotoping masses using the THRASH (Thorough High-Resolution Analysis of Spectral by Horn) algorithm can be performed.
Software tools for LMWH Spectral Interpretation
With increasing interest in the field of mass spectrometry, many software packages have been developed over the last two decades. The glycoworkbench software can draw structures that correspond to glycans, GAG, etc. It draws mass composition of structures modified with methylation, and implies structures that are permethylated and modified on the hydroxyl-end. glycoworkbench also allows annotation of carbohydrate composition (MS) and product ions (MS/MS) from tandem mass spectra [80]. Glycomod program does carbohydrate annotation using mass spectral data but is not automated [81]. The Heparin oligosaccharide sequencing tool (HOST) is another software package that provides automated data interpretation, but is limited in its ability to analyze homogeneous samples and highly digested LMWH [65]. GlycReSoft open-source software is specific to glycan analysis, but ranks peaks based on the composition of multiple charged MS ions. It is limited in that it cannot analyze oligosaccharide sequence, in that MS/MS product ion spectra are not accounted for [82]. Recently, Wang et al. designed another software, GlycCompSoft, which takes input data from GlycReSoft and computes results within minutes. It is advantageous over GlycReSoft because the labour-intensive annotation of output is completely automated in this software [83].
In comparison, software such as MyriMatch and GAG-ID have been developed for automated data interpretation of complex molecules. Various scoring algorithms have been proposed for automating spectral data, including cross-correlation, hypergeometric distribution, Poisson distributions, Bayesian statistics, dot products, and many others. Most of these algorithms function by matching product ions from either peptide or glycans to that of the observed or database-contained spectrum without distinguishing major versus minor peaks. Software packages such as GAG-ID and Myrimatch use a novel algorithm of multivariate hypergeometric distribution [84]. This analysis relies on distributing peaks found in the spectra in intensity classes such that the known or reported m/z can be categorized based on intensity fingerprints. For GAG-ID, the advantage of multivariate hypergeometric distribution is readily apparent in the way the authors designed a theoretical sequence database to contain every possible derivation of Heparin and HS. GAG-ID further provides a user-friendly GUI and makes feasible high-throughput sequencing of LMWH [85]. Together, amongst known software packages currently available, GAG-ID identifies enoxaparin structures with greater ease.
Conclusion
Amongst known LMWH, Enoxaparin is the most promising anticoagulant, owing to its prescribed utility over Dalteparin and Tinzaparin. Although Enoxaparin is preferred over the other two LMWH, their functional efficacy is not entirely different. In their efficacy towards Factor Xa/IIa, the degree of sulfate-to-carboxylate ratio acts as an important parameter. Thus, in this review sulfolyltransferases and heparinase are recommended to reduce heterogeneity on Enoxaparin and digesting substrates for structural characterization. Following USFDA guidelines, the application of various analytical instruments for sameness evaluation is simplified because the sample becomes homogeneous and enzymatic treatment introduces ease of analysis.
Amongst known instruments of choice, mass spectrometry is popular due to its ability to perform structural characterization and compositional breakup of LMWH. Such mass spectrum evaluation can be obtained by using either ESI or MALDI instrument with various chromatography techniques. Various LC, including SEC, RP, RPIP, HILIC, SAX, CE, and GCC, have been proposed for use. Because compositional analysis of sulfates is also required for understanding key API amongst the pool of heterogeneous mixtures, automation of spectral data introduces yet another ease of functioning. Here, various online tools are referred to, with GAG-ID most prominent for LMWH analysis. Mass spectrometry instrumentation and data interpretation techniques are evaluated in this review as a comprehensive way to structurally analyze Enoxaparin.
Expert Commentary
Low Molecular Weight Heparins (LMWH) are structurally complex, heterogeneous, polydisperse, and highly charged mixtures of chemically or enzymatically treated Unfractionated Heparin (UFH). Due to their less adverse side effects, LMWHs have seen precedence in many thrombotic disorders over UFH. Owing to these factors, LMWHs have been evaluated in multiple randomized clinical trials and found to be safe and effective for the treatment of venous thrombosis, pulmonary embolism, and unstable angina. For patient treatment, they have been found to be equally effective as UFHs, the current treatment choice globally for venous thromboembolism. However, specifically for LMWH, the major caveat in clinical management is that LMWH cannot be tried on patients with a history of heparin-induced thrombocytopenia (HIT), osteopenia, and or on those with LMWH allergy [86]. Moreover, since LMWH are less potent than UFH or Heparin, there is no standardization or dosage information available for their clinical use. The longer-term treatment of LMWH has also not been established, and thus its acceptance as a replacement for UFH or Heparin is limited.
These are the current clinically relevant limitations in promoting LMWH as a therapeutic. Ongoing efforts in this direction will certainly ameliorate these factors, and currently are an area of active research. The overall role of LMWH is very promising in thrombotic disorders, and thus it is of keen interest to keep working in this area for the coming years. Many new pharmacology firms have begun to develop drugs biosimilar to LMWH, and some have succeeded in marketing them in developing nations. Certainly, since biosimilar demands extensive physico-chemical, structural, functional, pharmacokinetic, and pharmacodynamic characterization, it is of the utmost importance that researchers continue to develop tools that are user-friendly and provide extensive characterization of LMWH. Owing to the inherent complexity of its structural features, the use of advanced analytical techniques such as mass-spectrometry (MS) becomes indispensable. In this regard, this review highlights the use of MS in analyzing LMWH. With the advent of high resolution and highly sensitive MS, there is a better understanding of the structural features of LMWH. The FDA previously approved a method for assessing the structural features of LMWH using low-resolution MS, but currently available instrumentation has enhanced our understanding of the structural characterization of LMWH [43]. Major progress has been achieved on account of the optimization of the enzymatic digestion protocol and the development of liquid chromatography (LC) methods. Since MS is mostly used with LC, optimal LC methods are critical for gathering structural attributes. Here, major advancements in MS research will drive a better understanding of the composition of these LMWH and help assess their biosimilarity to innovator drugs. The greatest emphasis should be laid on advanced analytical techniques for comprehensive characterization of these biosimilars, such that more and more pharma companies are able to commercialize these products. This will not only make the drug cheaper (the cost of LMWH is high), but make them more accessible to developing nations as well. Thus, automation of MS data and user-friendly interpretation will provide an integrated approach to MS data analysis and will drive characterization of biosimilar LMWH for drug develpment.
Five-year Viewpoint
Low molecular weight heparins have long been prescribed as an alternative to UFH due to the multiple advantages it carries. Amongst known LMWH, Enoxaparin is considered to be the most-prescribed LMWH as an anticoagulant, both in the US and Europe. Despite having major advantages over the UFH, very few LMWH biosimiliars are available in the market. In the near future, a few if not many biosimilars for LMWH, including Enoxaparin, Dalteparin, and Tinzaparin, will be commercialized, thus further reducing cost per dosage. This will require Himalayan efforts from the pharma industry to demonstrate sameness in the analytical assays and potency tests of these biosimilars. Because currently there are not major available biosimilars for LMWH, there likely are not many well-developed methodologies available globally that can be used to determine sameness of a biosimilar to an innovator drug. In our opinion, more user-friendly techniques employing simpler instrumentation will be developed in the near future. There are various HPLC and tandem MS techniques currently used for the structural analysis of Enoxaparin and other LMWHs. With the advent of ESI-MS, major challenges in the comprehensive structural characterization of sulfates and carboxylates on LMWH are being circumvented. More literature will be available illustrating the identification of sulfates and carboxylates on LMWH using MS. Major emphasis is currently given to Enoxaparin due to large market share, and therefore other LMWH will also be comprehensively characterized for their unique sulfation and carboxylate patterns. Current MS techniques also use either high-end MS instruments such as Orbitrap, or use ion-pairing agents that contaminate MS instrument. Thus, better methods will be developed without affecting instrument performance, simultaneously providing cleaner and more comprehensive structural data for evaluation of sameness. In order to develop an analytical method for complete characterization of chemical structure, better separation techniques of LMWH oligosaccharides are also required. Thus, significant work will be pursued in this direction to completely characterize structures [87]. Given that current efforts are also being directed toward characterization of the monosaccharides, disaccharides, and oligosaccharides involved in LMWH, not much emphasis has been given to the analysis of contaminants using MS. Thus, studies will be conducted using MS and other analytical techniques to determine percentage of contaminants and to structurally determine their composition, so as to identify strategies to rectify them.
Focus will be placed on the changes in clinical practices in identifying the dose of LMWH for individual patients. Current practices include measuring anti-factor Xa and activated partial thromboplastin time (APTT). For LMWH dosing, this method needs to be replaced with anti-thrombin activity to assess the potency of these drugs. Patient-specific dosage and type of LMWH will be further prescribed in the future. Because the thrombin generation capacity of individual patients varies significantly in within a given population, measurement of anti-thrombin activity will be used as a criteria for individualizing the drugs [88].
Another main emphasis will be on the consideration of the effects of alternative medication as anticoagulants in place of LMWH. Because these LMWH drugs are injected, many patients with cancer related thrombosis do not use of them as anticoagulants. The competition from other drugs, such as direct oral anticoagulants (DOACs), will have a significant effect on treatment. Trials have already been conducted using random introduction of LMWH and DOACs in patients to observe their efficacy in treating venous thromboembolism (VTE) and major bleeding [89]. It is easy to see that introduction of such alternative drugs, although important from the patient perspective, will affect overall market share of LMWH as the current standard of care in treatment of VTE. Although this will not affect the role of LMWH in other antithrombotic disorders, relevance in cancer-associated VTE will likely be reduced.
Table III:
Different buffer conditions corresponding to LC-MS instrumentation
| LC-MS Type | Condition Used | GAG Studied | Fraction Analyzed |
|---|---|---|---|
| Reversed Phase (RP) | 1-phenyl-3-methyl pyrazolone (PMP) derivatization | HS and DS | NR |
| Reverse phase ion pairing (RPIP) | Dibutylamine as IPA | Unsulfated Heparosan | Upto dp40 |
| Reverse phase ion pairing (RPIP) | Tributylamine as IPA | Unsulfated Heparosan | dp 2 – 20 |
| Reverse phase ion pairing (RPIP) | Tripropylamine as IPA | Partially depolymerized Heparin | Upto 200 components |
| Hydrophilic interaction (HILIC) | Negative ESI with Ammonium formate modifier | Heparin | Upto dp18 |
| Size Exclusion (SEC) | Use online ion suppressor to reduce ammonium salts | Partially depolymerized CS | Upto dp14 |
| Graphitized Carbon (GCC) | Negative mode with ammonium bicarbonate modifier | Hyaluronan, KS, Heparin and HS | Isomeric disaccharides |
| Multidimensional LC | SEC and SAX chromatogrpahy | Heparinase treated HS | Hexasaccharide isomer separated |
| Capillary Electrophoresis (CE) | Reverse CE with Negative ESI | HS | Disaccharide separation |
Key issues.
Enoxaparin is amongst the most well-studied and characterized low molecular weight heparin drugs. There are many studies on its structural characterization.
The application of enzymes is towards not only enzymatically deriving LMWH (e.g. Enoxaparin) from UFH or Heparin, but also towards having available a sulfated molecular species to reduce structural heterogeneity in the pool of variable compositions of LMWH. Sample preparation techniques are also presented that conserve sulfates on enoxaparin. Such methods are immensely helpful in retaining the structural integrity of these LMWHs.
Determination of structural composition is presented in the review by advanced analytical technique such as mass spectrometry (MS). Various examples of the use of liquid chromatography (LC) with MS are presented, along with the latest automated software for MS data interpretation.
Acknowledgments
The authors on this manuscript are, in part, supported by grants from the National Institutes of Health RO1 CA210637 and the Nebraska Department of Health and Human Services LB595. The authors acknowledge support from Biological E Limited. The authors thank Dr. Adrian Koesters, Research Editor at UNMC, for her editorial contribution to the manuscript.
References
- [1].Guerrini M, Bisio A, Low-molecular-weight heparins: differential characterization/physical characterization, Handb Exp Pharmacol (2012) 127–157. [DOI] [PubMed] [Google Scholar]
- [2].Alam F, Hwang SR, Al-Hilal TA, et al. , Safety studies on intravenous infusion of a potent angiogenesis inhibitor: taurocholate-conjugated low molecular weight heparin derivative LHT7 in preclinical models, Drug Dev Ind Pharm 42 (2016) 1247–1257. [DOI] [PubMed] [Google Scholar]
- [3].Thacker BE, Seamen E, Lawrence R, et al. , Expanding the 3-O-Sulfate Proteome-Enhanced Binding of Neuropilin-1 to 3-O-Sulfated Heparan Sulfate Modulates Its Activity, ACS Chem Biol 11 (2016) 971–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Xu Y, Martinez P, Seron K, et al. , Characterization of hepatitis C virus interaction with heparan sulfate proteoglycans, J Virol 89 (2015) 3846–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Aguilar OM, Kleiman NS, Low molecular weight heparins, Expert Opin Pharmacother 1 (2000) 1091–1103. [DOI] [PubMed] [Google Scholar]
- [6].Chandarajoti K, Liu J, Pawlinski R, The design and synthesis of new synthetic low molecular weight heparins, J Thromb Haemost (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Casu B, Naggi A, Torri G, Re-visiting the structure of heparin, Carbohydr Res 403 (2015) 60–68. [DOI] [PubMed] [Google Scholar]
- [8].Chen Y, Zhao J, Yu Y, et al. , Antithrombin III-Binding Site Analysis of Low-Molecular-Weight Heparin Fractions, J Pharm Sci (2018). [DOI] [PubMed] [Google Scholar]
- [9].Chandarajoti K, Xu Y, Sparkenbaugh E, et al. , De novo synthesis of a narrow size distribution low-molecular-weight heparin, Glycobiology 24 (2014) 476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mourier PA, Guichard OY, Herman F, et al. , Isolation of a pure octadecasaccharide with antithrombin activity from an ultra-low-molecular-weight heparin, Anal Biochem 453 (2014) 7–15. [DOI] [PubMed] [Google Scholar]
- [11].Racine E, Differentiation of the low-molecular-weight heparins, Pharmacotherapy 21 (2001) 62S–70S; discussion 71S–72S. [DOI] [PubMed] [Google Scholar]
- [12].Lee YR, Vega JA, Duong HN, et al. , Monitoring Enoxaparin with Antifactor Xa Levels in Obese Patients, Pharmacotherapy 35 (2015) 1007–1015. [DOI] [PubMed] [Google Scholar]
- [13].Lai S, Coppola B, Use of enoxaparin in end-stage renal disease, Kidney Int 84 (2013) 433–436. [DOI] [PubMed] [Google Scholar]
- [14].Huang J, Li N, Li Z, et al. , Low-Dose Unfractionated Heparin with Sequential Enoxaparin in Patients with Diabetes Mellitus and Complex Coronary Artery Disease during Elective Percutaneous Coronary Intervention, Chin Med J (Engl) 131 (2018) 764–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Green MS, Tellor KB, Buckallew AR, Safety and Efficacy of Enoxaparin Compared With Unfractionated Heparin for Venous Thromboembolism Prophylaxis in Hemodialysis Patients, Hosp Pharm 52 (2017) 623–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dragoni F, Kaarniranta K, Individual benefits of enoxaparin treatment in branch vein occlusion, Graefes Arch Clin Exp Ophthalmol (2017). [DOI] [PubMed] [Google Scholar]
- [17].Imberti D, Marietta M, Polo Friz H, et al. , The introduction of biosimilars of low molecular weight heparins in Europe: a critical review and reappraisal endorsed by the Italian Society for Haemostasis and Thrombosis (SISET) and the Italian Society for Angiology and Vascular Medicine (SIAPAV), Thromb J 15 (2017) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Suflita M, Fu L, He W, et al. , Heparin and related polysaccharides: synthesis using recombinant enzymes and metabolic engineering, Appl Microbiol Biotechnol 99 (2015) 7465–7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sugahara K, Kitagawa H, Heparin and heparan sulfate biosynthesis, IUBMB Life 54 (2002) 163–175. [DOI] [PubMed] [Google Scholar]
- [20].Atkinson HM, Mewhort-Buist TA, Berry LR, et al. , Anticoagulant mechanisms of covalent antithrombin-heparin investigated by thrombelastography. Comparison with unfractionated heparin and low-molecular-weight heparin, Thromb Haemost 102 (2009) 62–68. [DOI] [PubMed] [Google Scholar]
- [21].Viskov C, Elli S, Urso E, et al. , Heparin dodecasaccharide containing two antithrombin-binding pentasaccharides: structural features and biological properties, J Biol Chem 288 (2013) 25895–25907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nahain AA, Ignjatovic V, Monagle P, et al. , Heparin mimetics with anticoagulant activity, Med Res Rev (2018). [DOI] [PubMed] [Google Scholar]
- [23].Fareed J, Jeske W, Hoppensteadt D, et al. , Low-molecular-weight heparins: pharmacologic profile and product differentiation, Am J Cardiol 82 (1998) 3l–10l. [DOI] [PubMed] [Google Scholar]
- [24].Guerrini M, Elli S, Gaudesi D, et al. , Effects on molecular conformation and anticoagulant activities of 1,6-anhydrosugars at the reducing terminal of antithrombin-binding octasaccharides isolated from low-molecular-weight heparin enoxaparin, J Med Chem 53 (2010) 8030–8040. [DOI] [PubMed] [Google Scholar]
- [25].Zhu H, Liu YJ, Han XW, et al. , [Comparison of structural characteristics and anticoagulation activity of enoxaparin sodium with different degree of 1,6-anhydro derivatives], Yao Xue Xue Bao 49 (2014) 1049–1053. [PubMed] [Google Scholar]
- [26].Pannucci CJ, Fleming KI, Agarwal J, et al. , The impact of once versus twice daily enoxaparin prophylaxis on risk for venous thromboembolism and clinically relevant bleeding, Plast Reconstr Surg (2018). [DOI] [PubMed] [Google Scholar]
- [27].Baumgartner JM, McKenzie S, Block S, et al. , Prophylactic enoxaparin doses may be inadequate in patients undergoing abdominal cancer surgery, J Surg Res 221 (2018) 183–189. [DOI] [PubMed] [Google Scholar]
- [28].Alnatsheh AH, Beckett RD, Waterman S, Comparison of the effectiveness of venous thromboembolism prophylaxis with enoxaparin between obese and non-obese patients, J Oncol Pharm Pract (2018) 1078155218760159. [DOI] [PubMed] [Google Scholar]
- [29].Wu J, Zhang C, Mei X, et al. , Controllable production of low molecular weight heparins by combinations of heparinase I/II/III, Carbohydr Polym 101 (2014) 484–492. [DOI] [PubMed] [Google Scholar]
- [30].Myette JR, Shriver Z, Kiziltepe T, et al. , Molecular cloning of the heparin/heparan sulfate delta 4,5 unsaturated glycuronidase from Flavobacterium heparinum, its recombinant expression in Escherichia coli, and biochemical determination of its unique substrate specificity, Biochemistry 41 (2002) 7424–7434. [DOI] [PubMed] [Google Scholar]
- [31].Bohmer LH, Pitout MJ, Steyn PL, et al. , Purification and characterization of a novel heparinase, J Biol Chem 265 (1990) 13609–13617. [PubMed] [Google Scholar]
- [32].Lohse DL, Linhardt RJ, Purification and characterization of heparin lyases from Flavobacterium heparinum, J Biol Chem 267 (1992) 24347–24355. [PubMed] [Google Scholar]
- [33].Ernst S, Venkataraman G, Winkler S, et al. , Expression in Escherichia coli, purification and characterization of heparinase I from Flavobacterium heparinum, Biochem J 315 ( Pt 2) (1996) 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wang Z, Zhang T, Xie S, et al. , Sequencing the oligosaccharide pool in the low molecular weight heparin dalteparin with offline HPLC and ESI-MS/MS, Carbohydr Polym 183 (2018) 81–90. [DOI] [PubMed] [Google Scholar]
- [35].Mourier PAJ, Herman F, Sizun P, et al. , Analytical comparison of a US generic enoxaparin with the originator product: The focus on comparative assessment of antithrombin-binding components, J Pharm Biomed Anal 129 (2016) 542–550. [DOI] [PubMed] [Google Scholar]
- [36].Shaya D, Zhao W, Garron ML, et al. , Catalytic mechanism of heparinase II investigated by site-directed mutagenesis and the crystal structure with its substrate, J Biol Chem 285 (2010) 20051–20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Watanabe M, Tsuda H, Yamada S, et al. , Characterization of heparinase from an oral bacterium Prevotella heparinolytica, J Biochem 123 (1998) 283–288. [DOI] [PubMed] [Google Scholar]
- [38].Xu X, Li D, Chi L, et al. , Fragment profiling of low molecular weight heparins using reversed phase ion pair liquid chromatography-electrospray mass spectrometry, Carbohydr Res 407 (2015) 26–33. [DOI] [PubMed] [Google Scholar]
- [39].Li G, Steppich J, Wang Z, et al. , Bottom-up low molecular weight heparin analysis using liquid chromatography-Fourier transform mass spectrometry for extensive characterization, Anal Chem 86 (2014) 6626–6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ouyang Y, Wu C, Sun X, et al. , Development of hydrophilic interaction chromatography with quadruple time-of-flight mass spectrometry for heparin and low molecular weight heparin disaccharide analysis, Rapid Commun Mass Spectrom 30 (2016) 277–284. [DOI] [PubMed] [Google Scholar]
- [41].Chandarajoti K, Liu J, Pawlinski R, The design and synthesis of new synthetic low-molecular-weight heparins, J Thromb Haemost 14 (2016) 1135–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Achour O, Bridiau N, Godhbani A, et al. , Ultrasonic-assisted preparation of a low molecular weight heparin (LMWH) with anticoagulant activity, Carbohydr Polym 97 (2013) 684–689. [DOI] [PubMed] [Google Scholar]
- [43].Wang B, Buhse LF, Al-Hakim A, et al. , Characterization of currently marketed heparin products: analysis of heparin digests by RPIP-UHPLC-QTOF-MS, J Pharm Biomed Anal 67–68 (2012) 42–50. [DOI] [PubMed] [Google Scholar]
- [44].Stolfa G, Mondal N, Zhu Y, et al. , Using CRISPR-Cas9 to quantify the contributions of O-glycans, N-glycans and Glycosphingolipids to human leukocyte-endothelium adhesion, Sci Rep 6 (2016) 30392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Miller SC, Profiling sulfonate ester stability: identification of complementary protecting groups for sulfonates, J Org Chem 75 (2010) 4632–4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ali AM, Hill B, Taylor SD, Trichloroethyl group as a protecting group for sulfonates and its application to the synthesis of a disulfonate analog of the tyrosine sulfated PSGL-1(43–50) peptide, J Org Chem 74 (2009) 3583–3586. [DOI] [PubMed] [Google Scholar]
- [47].Rhomberg AJ, Shriver Z, Biemann K, et al. , Mass spectrometric evidence for the enzymatic mechanism of the depolymerization of heparin-like glycosaminoglycans by heparinase II, Proc Natl Acad Sci U S A 95 (1998) 12232–12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rhomberg AJ, Ernst S, Sasisekharan R, et al. , Mass spectrometric and capillary electrophoretic investigation of the enzymatic degradation of heparin-like glycosaminoglycans, Proc Natl Acad Sci U S A 95 (1998) 4176–4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zaia J, Glycosaminoglycan glycomics using mass spectrometry, Mol Cell Proteomics 12 (2013) 885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Leach FE 3rd, Wolff JJ, Xiao Z, et al. , Negative electron transfer dissociation Fourier transform mass spectrometry of glycosaminoglycan carbohydrates, Eur J Mass Spectrom (Chichester) 17 (2011) 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kailemia MJ, Li L, Xu Y, et al. , Structurally informative tandem mass spectrometry of highly sulfated natural and chemoenzymatically synthesized heparin and heparan sulfate glycosaminoglycans, Mol Cell Proteomics 12 (2013) 979–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ingle RG, Agarwal AS, A world of low molecular weight heparins (LMWHs) enoxaparin as a promising moiety--a review, Carbohydr Polym 106 (2014) 148–153. [DOI] [PubMed] [Google Scholar]
- [53].Ouyang Y, Zeng Y, Rong Y, et al. , Profiling analysis of low molecular weight heparins by multiple heart-cutting two dimensional chromatography with quadruple time-of-flight mass spectrometry, Anal Chem 87 (2015) 8957–8963. [DOI] [PubMed] [Google Scholar]
- [54].Wang Z, Li D, Sun X, et al. , Liquid chromatography-diode array detection-mass spectrometry for compositional analysis of low molecular weight heparins, Anal Biochem 451 (2014) 35–41. [DOI] [PubMed] [Google Scholar]
- [55].Sun X, Lin L, Liu X, et al. , Capillary Electrophoresis-Mass Spectrometry for the Analysis of Heparin Oligosaccharides and Low Molecular Weight Heparin, Anal Chem 88 (2016) 1937–1943. [DOI] [PubMed] [Google Scholar]
- [56].Zhang Q, Chen X, Zhu Z, et al. , Structural analysis of low molecular weight heparin by ultraperformance size exclusion chromatography/time of flight mass spectrometry and capillary zone electrophoresis, Anal Chem 85 (2013) 1819–1827. [DOI] [PubMed] [Google Scholar]
- [57].Langeslay DJ, Urso E, Gardini C, et al. , Reversed-phase ion-pair ultra-high-performance-liquid chromatography-mass spectrometry for fingerprinting low-molecular-weight heparins, J Chromatogr A 1292 (2013) 201–210. [DOI] [PubMed] [Google Scholar]
- [58].Li G, Li L, Tian F, et al. , Glycosaminoglycanomics of cultured cells using a rapid and sensitive LC-MS/MS approach, ACS Chem Biol 10 (2015) 1303–1310. [DOI] [PubMed] [Google Scholar]
- [59].Li D, Chi L, Jin L, et al. , Mapping of low molecular weight heparins using reversed phase ion pair liquid chromatography-mass spectrometry, Carbohydr Polym 99 (2014) 339–344. [DOI] [PubMed] [Google Scholar]
- [60].Liu X, St Ange K, Lin L, et al. , Top-down and bottom-up analysis of commercial enoxaparins, J Chromatogr A 1480 (2017) 32–40. [DOI] [PubMed] [Google Scholar]
- [61].Liu X, St Ange K, Wang X, et al. , Parent heparin and daughter LMW heparin correlation analysis using LC-MS and NMR, Anal Chim Acta 961 (2017) 91–99. [DOI] [PubMed] [Google Scholar]
- [62].Guan Y, Xu X, Liu X, et al. , Comparison of Low-Molecular-Weight Heparins Prepared From Bovine Lung Heparin and Porcine Intestine Heparin, J Pharm Sci 105 (2016) 1843–1850. [DOI] [PubMed] [Google Scholar]
- [63].Liu X, St Ange K, Fareed J, et al. , Comparison of Low-Molecular-Weight Heparins Prepared From Bovine Heparins With Enoxaparin, Clin Appl Thromb Hemost 23 (2017) 542–553. [DOI] [PubMed] [Google Scholar]
- [64].Ouyang Y, Zeng Y, Yi L, et al. , Qualitative and quantitative analysis of heparin and low molecular weight heparins using size exclusion chromatography with multiple angle laser scattering/refractive index and inductively coupled plasma/mass spectrometry detectors, J Chromatogr A 1522 (2017) 56–61. [DOI] [PubMed] [Google Scholar]
- [65].Saad OM, Leary JA, Heparin sequencing using enzymatic digestion and ESI-MSn with HOST: a heparin/HS oligosaccharide sequencing tool, Anal Chem 77 (2005) 5902–5911. [DOI] [PubMed] [Google Scholar]
- [66].Saad OM, Ebel H, Uchimura K, et al. , Compositional profiling of heparin/heparan sulfate using mass spectrometry: assay for specificity of a novel extracellular human endosulfatase, Glycobiology 15 (2005) 818–826. [DOI] [PubMed] [Google Scholar]
- [67].Flangea C, Schiopu C, Sisu E, et al. , Determination of sulfation pattern in brain glycosaminoglycans by chip-based electrospray ionization ion trap mass spectrometry, Anal Bioanal Chem 395 (2009) 2489–2498. [DOI] [PubMed] [Google Scholar]
- [68].Lawrence R, Olson SK, Steele RE, et al. , Evolutionary differences in glycosaminoglycan fine structure detected by quantitative glycan reductive isotope labeling, J Biol Chem 283 (2008) 33674–33684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Shi X, Zaia J, Organ-specific heparan sulfate structural phenotypes, J Biol Chem 284 (2009) 11806–11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zhang Z, Xie J, Liu H, et al. , Quantification of heparan sulfate disaccharides using ion-pairing reversed-phase microflow high-performance liquid chromatography with electrospray ionization trap mass spectrometry, Anal Chem 81 (2009) 4349–4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Alpert AJ, Hydrophilic-interaction chromatography for the separation of peptides, nucleic acids and other polar compounds, J Chromatogr 499 (1990) 177–196. [DOI] [PubMed] [Google Scholar]
- [72].Staples GO, Bowman MJ, Costello CE, et al. , A chip-based amide-HILIC LC/MS platform for glycosaminoglycan glycomics profiling, Proteomics 9 (2009) 686–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Davies M, Smith KD, Harbin AM, et al. , High-performance liquid chromatography of oligosaccharide alditols and glycopeptides on a graphitized carbon column, J Chromatogr 609 (1992) 125–131. [DOI] [PubMed] [Google Scholar]
- [74].Karlsson NG, Schulz BL, Packer NH, et al. , Use of graphitised carbon negative ion LC-MS to analyse enzymatically digested glycosaminoglycans, J Chromatogr B Analyt Technol Biomed Life Sci 824 (2005) 139–147. [DOI] [PubMed] [Google Scholar]
- [75].Schenauer MR, Meissen JK, Seo Y, et al. , Heparan sulfate separation, sequencing, and isomeric differentiation: ion mobility spectrometry reveals specific iduronic and glucuronic acid-containing hexasaccharides, Anal Chem 81 (2009) 10179–10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Laremore TN, Murugesan S, Park TJ, et al. , Matrix-assisted laser desorption/ionization mass spectrometric analysis of uncomplexed highly sulfated oligosaccharides using ionic liquid matrices, Anal Chem 78 (2006) 1774–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Chen L, Yap YL, Automated charge state determination of complex isotope-resolved mass spectra by peak-target Fourier transform, J Am Soc Mass Spectrom 19 (2008) 46–54. [DOI] [PubMed] [Google Scholar]
- [78].Senko MW, Beu SC, McLafferty FW, Automated assignment of charge states from resolved isotopic peaks for multiply charged ions, J Am Soc Mass Spectrom 6 (1995) 52–56. [DOI] [PubMed] [Google Scholar]
- [79].Horn DM, Zubarev RA, McLafferty FW, Automated reduction and interpretation of high resolution electrospray mass spectra of large molecules, J Am Soc Mass Spectrom 11 (2000) 320–332. [DOI] [PubMed] [Google Scholar]
- [80].Ceroni A, Maass K, Geyer H, et al. , GlycoWorkbench: a tool for the computer-assisted annotation of mass spectra of glycans, J Proteome Res 7 (2008) 1650–1659. [DOI] [PubMed] [Google Scholar]
- [81].Cooper CA, Gasteiger E, Packer NH, GlycoMod--a software tool for determining glycosylation compositions from mass spectrometric data, Proteomics 1 (2001) 340–349. [DOI] [PubMed] [Google Scholar]
- [82].Maxwell E, Tan Y, Tan Y, et al. , GlycReSoft: a software package for automated recognition of glycans from LC/MS data, PLoS One 7 (2012) e45474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Wang X, Liu X, Li L, et al. , GlycCompSoft: Software for Automated Comparison of Low Molecular Weight Heparins Using Top-Down LC/MS Data, PLoS One 11 (2016) e0167727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Tabb DL, Fernando CG, Chambers MC, MyriMatch: highly accurate tandem mass spectral peptide identification by multivariate hypergeometric analysis, J Proteome Res 6 (2007) 654–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Chiu Y, Huang R, Orlando R, et al. , GAG-ID: Heparan Sulfate (HS) and Heparin Glycosaminoglycan High-Throughput Identification Software, Mol Cell Proteomics 14 (2015) 1720–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Hirsh J, Low-molecular-weight heparin : A review of the results of recent studies of the treatment of venous thromboembolism and unstable angina, Circulation 98 (1998) 1575–1582. [DOI] [PubMed] [Google Scholar]
- [87].Sadowski R, Gadzala-Kopciuch R, Buszewski B, Recent developments in separation of low molecular weight heparin anticoagulants, Curr Med Chem (2017). [DOI] [PubMed] [Google Scholar]
- [88].Hemker HC, A century of heparin: past, present and future, J Thromb Haemost 14 (2016) 2329–2338. [DOI] [PubMed] [Google Scholar]
- [89].Smrke A, Gross PL, Cancer-Associated Venous Thromboembolism: A Practical Review Beyond Low-Molecular-Weight Heparins, Front Med (Lausanne) 4 (2017) 142. [DOI] [PMC free article] [PubMed] [Google Scholar]