Abstract
Objectives
Large hepatocellular carcinoma (LHCC) is prone to short‐term recurrence and poor long‐term survival after hepatectomy, and there is still a lack of effective neoadjuvant treatments to improve recurrence‐free survival (RFS) and overall survival (OS). We retrospectively analyzed the efficacy of preoperative transcatheter arterial chemoembolization (TACE) in solitary LHCC (≥5 cm).
Materials and Methods
A multicenter medical database was used to analyze preoperative TACE's effects on RFS, OS, and perioperative complications in patients with solitary LHCC who received surgical treatment from January 2005 to December 2015. The patients were divided into Group A (5.0–9.9 cm) and Group B (≥10 cm), with 10 cm as the critical value, and the effect of preoperative TACE on RFS, OS and perioperative complications was assessed in each subgroup.
Results
In the overall population, patients with preoperative TACE had better RFS and OS than those without preoperative TACE. However, after stratifying the patients into the two HCC groups, preoperative TACE only improved the survival outcomes of patients with Group B (≥10 cm). Multivariate Cox‐regression analysis showed that lack of preoperative TACE was an independent risk factor for RFS and OS in the overall population and in Group B but not in Group A.
Conclusions
Preoperative TACE is beneficial for patients with solitary HCC (≥10 cm).
Keywords: hepatectomy, hepatocellular carcinoma, recurrence survival, transcatheter arterial chemoembolization
First, preoperative imaging evaluation of HCC patients was performed to evaluate tumor size and location.Second, whether or not a patient should receive TACE before surgical resection is determined according to tumor diameter.Finally, with the assistance of interventional equipment, the blood vessels of nutritional tumors are identified and embolized.
1. INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most common malignancy worldwide and the third leading cause of cancer‐related death. 1 For HCC with a relatively early stage of disease, hepatectomy has been considered a radical treatment that can achieve a good survival prognosis. 2 , 3 , 4 However, the tumor is highly prone to recurrence after hepatectomy, resulting in the patient's death, particularly in HCC patients with larger tumor diameters and microvascular invasion (MVI). 5 , 6 , 7 Therefore, we should take appropriate measures to reduce recurrence and improve the overall survival (OS) of patients. 8
Transcatheter arterial chemoembolization (TACE), as an effective local treatment, can improve the OS of patients with unresectable HCC, and thus is often used for the treatment of advanced HCC. 9 , 10 , 11 , 12 , 13 A large number of studies in the past have confirmed that postoperative TACE can reduce recurrence and prolong OS of patients, 14 , 15 , 16 , 17 and can TACE also be used as a neoadjuvant treatment for HCC? There is still controversy on the benefits of using TACE as a neoadjuvant therapy. 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 Some scholars have proposed that preoperative TACE is not beneficial for all of patients with HCC, and whether it can improve the long‐term survival mainly depends on the diameter of the tumor. 17 , 18 , 20 , 25 , 27 To explore whether the efficacy of preoperative TACE depends on the tumor diameter, we used a multicenter database to stratify patients according to tumor diameter and, for the first time, explored the efficacy of preoperative TACE in patients with large hepatocellular carcinoma (LHCC) in different tumor diameter groups.
2. MATERIALS AND METHODS
2.1. Patient population
This study was based on patients who underwent curative resection of HCC in Zhongshan People's Hospital, Hubei Xiaogan Central Hospital, People's Hospital of Wuhan University, Qinghai University Affiliated Hospital, Huangshi Central Hospital, and Wuhan Tongji Hospital from January 2005 to December 2015. Inclusion criteria (1) a solitary HCC with tumor diameter ≥5 cm; (2) a postoperative pathological diagnosis of HCC; (3) no extrahepatic metastasis; (4) no radiologic evidence of invasion into the major portal/hepatic vein branches; (5) radical resection of HCC (R0), that is, no residual tumor tissue under direct observation or microscopy; (6) no previous treatment of HCC. Exclusion criteria: (1) younger than 18 years old; (2) poor liver function with Pugh‐Child Class C; (3) missing prognosis and follow‐up information. The ethics committees of the six medical centers approved the study, and the study complied with the Helsinki Declaration and local laws.
2.2. Data collection
All patients underwent contrast‐enhanced computed tomography (CT), magnetic resonance imaging (MRI), or chest X‐ray scanning upon admission to the hospital. Preoperative Information on baseline patient characteristics includes age, sex, diabetes mellitus, etiology of liver diseases, cirrhosis, Child‐Pugh grade, platelets count, international normalized ratio (INR), alpha‐fetoprotein (AFP) level, the presence or absence of postoperative adjuvant TACE, maximum tumor size, MVI, satellite nodules, tumor differentiation, and tumor capsule. Continuous variables, such as age, are transformed into binary variables according to recognized cut‐off values or upper and lower lines of normal values. 8 , 18 , 42 Anatomic resection refers to the resection of one or more adjacent hepatic sections along the hepatic vasculature and includes segmentectomy, subsegmentectomy, sectionctomy, and hemihepatectomy. Non‐anatomic resection is defined as local resection or enucleation regardless of the anatomical segment or section of the lobar anatomy. 43 , 44
2.3. Preoperative TACE
Considering that this was a retrospective study, the decision to use TACE prior to surgery was left to the discretion of the treating surgeon and the patient at that time. The patient was placed supine, locally disinfected, draped, and given local anesthetized. The puncture site was chosen to be 2 cm below the inguinal ligament, and the catheter sheath was placed into the femoral artery using the Seldinger technique. First, the DSA technique helps with abdominal trunk and standard hepatic artery angiography to determine the tumor's location, size, and condition of the tumor. Once the tumor is understood, the catheter sheath is advanced deeper into the left or right hepatic artery or the vessel that feeds the tumor, 5‐fluorouracil (500 mg/m2) or oxaliplatin (100 mg/m2) was injected into the proper hepatic artery, and embolization was performed using different embolization materials. The embolization materials used were iodized oil and gelatin sponge cubes, or iodized oil only, which was entirely mixed with these chemotherapeutic drugs as an emulsion and injected. Because TACE was performed at different hospitals, embolization materials varied. Patients were asked to return to the hospital 4–8 weeks after embolization for follow‐up investigations, including routine blood tests, liver, and kidney function, coagulation function, AFP, and imaging. Imaging included abdominal enhanced CT, MRI, or chest X‐ray scans. The above procedures were performed by highly qualified attending physicians who received relevant interventional medicine training.
2.4. Stratification according to the initial maximum diameter of the tumor
The maximum tumor diameters were measured by enhanced CT or MRI before surgical resection or preoperative TACE in all patients. According to the maximum tumor diameter, all HCC patients were divided into the 5–9.9 cm group and the ≥10 cm group, which were then defined as Group A and Group B, respectively. 18
2.5. Postoperative follow‐up and study endpoints
The reexamination frequency of all patients after the operation was once every 2–3 months in the first 6 months, once every 3–6 months in the following 18 months, and then once every 6–9 months if there was no recurrence. The postoperative follow‐up included liver biochemistry, routine blood tests, coagulation function, AFP, chest X‐ray or chest CT scans, abdominal B ultrasound, abdominal enhanced CT or MRI. Radiofrequency ablation, TACE, chemotherapy, molecular targeted therapy, surgical re‐resection, or liver transplantation were performed according to the recurrence and the patient's wishes when the patient was diagnosed with recurrence. Life‐supporting treatment was given to the end‐stage patient.
Study endpoints included complications within 30 days, recurrence‐free survival (RFS), and OS. Postoperative liver failure (PLF) was defined as serum TBIL >50 μoml/L and prothrombin activity (PTA) <50% on day 5 after hepatectomy, 45 postoperative bile leakage was defined as ≥3 days after surgery with a bilirubin concentration in the drain exceeding three times the normal bilirubin concentration in plasma. 46 OS was defined as the time from the date of surgery to the date of patient death or last follow‐up, and RFS was defined as the time from the date of surgery to the date of first postoperative tumor recurrence or last follow‐up. The cut‐off last date was July 1, 2021.
2.6. Statistical analysis
Continuous variables were expressed as median (range) or mean ± standard deviation (SD), categorical variables were reported as number (n) or percentages of patients (%). Continuous variables were compared by the Student's t‐test or Mann–Whitney U‐test. Categorical variables were compared by the χ2 test or Fisher's exact test. The survival curves of RFS and OS of patients who received or did not receive TACE before surgery were generated by the Kaplan–Meier method, and the log‐rank test was used to compare the differences. The Cox proportional hazard regression analyses were used to adjust for other prognostic factors associated with RFS and OS. All statistical analyses and visualizations of this study were obtained by R version 3.6.1 with the SVA. A p value <0.05 was considered statistically significant.
3. RESULTS
3.1. Baseline clinicopathological and postoperative complications
During the study period, 2560 HCC patients underwent radical HCC resection, of which 556 solitary HCC patients with diameter ≥5 cm were included in the study cohort. The baseline characteristics, clinicopathological features, and postoperative complications of the entire population are presented in Table 1.
TABLE 1.
Comparison of clinicopathological characteristics and perioperative outcomes between patients with and without preoperative TACE in the total population
| Variable | Overall (556) | Non‐TACE (n = 406) | TACE (n = 150) | p | |
|---|---|---|---|---|---|
| Age (%) | <60 years | 318 (57.2) | 228 (56.2) | 90 (60.0) | 0.474 |
| ≥60 years | 238 (42.8) | 178 (43.8) | 60 (40.0) | ||
| Gender (%) | Female | 137 (24.6) | 106 (26.1) | 31 (20.7) | 0.226 |
| Male | 419 (75.4) | 300 (73.9) | 119 (79.3) | ||
| HBV (%) | No | 23 (4.1) | 18 (4.4) | 5 (3.3) | 0.735 |
| Yes | 533 (95.9) | 388 (95.6) | 145 (96.7) | ||
| HCV (%) | No | 548 (98.6) | 400 (98.5) | 148 (98.7) | 1.000 |
| Yes | 8 (1.4) | 6 (1.5) | 2 (1.3) | ||
| Cirrhosis (%) | No | 181 (32.6) | 133 (32.8) | 48 (32.0) | 0.946 |
| Yes | 375 (67.4) | 273 (67.2) | 102 (68.0) | ||
| Child‐Pugh (%) | A | 490 (88.1) | 362 (89.2) | 128 (85.3) | 0.275 |
| B | 66 (11.9) | 44 (10.8) | 22 (14.7) | ||
| ALT (%) | <50 U/L | 354 (63.7) | 259 (63.8) | 95 (63.3) | 0.999 |
| ≥50 U/L | 202 (36.3) | 147 (36.2) | 55 (36.7) | ||
| AST (%) | <40 U/L | 198 (35.6) | 145 (35.7) | 53 (35.3) | 1.000 |
| ≥40 U/L | 358 (64.4) | 261 (64.3) | 97 (64.7) | ||
| GGT (%) | <45 U/L | 116 (20.9) | 85 (20.9) | 31 (20.7) | 1.000 |
| ≥45 U/L | 440 (79.1) | 321 (79.1) | 119 (79.3) | ||
| ALP (%) | <125 U/L | 420 (75.5) | 306 (75.4) | 114 (76.0) | 0.966 |
| ≥125 U/L | 136 (24.5) | 100 (24.6) | 36 (24.0) | ||
| Alb (%) | <35 g/L | 94 (16.9) | 65 (16.0) | 29 (19.3) | 0.423 |
| ≥35 g/L | 462 (83.1) | 341 (84.0) | 121 (80.7) | ||
| TBIL (%) | <20.4 μmol/L | 466 (83.8) | 341 (84.0) | 125 (83.3) | 0.955 |
| ≥20.4 μmol/L | 90 (16.2) | 65 (16.0) | 25 (16.7) | ||
| DBIL (%) | <6.8 μmol/L | 446 (80.2) | 327 (80.5) | 119 (79.3) | 0.843 |
| ≥6.8 μmol/L | 110 (19.8) | 79 (19.5) | 31 (20.7) | ||
| CR (%) | <84 μmol/L | 474 (85.3) | 349 (86.0) | 125 (83.3) | 0.522 |
| ≥84 μmol/L | 82 (14.7) | 57 (14.0) | 25 (16.7) | ||
| INR (%) | <1.15 | 379 (68.2) | 280 (69.0) | 99 (66.0) | 0.573 |
| ≥1.15 | 177 (31.8) | 126 (31.0) | 51 (34.0) | ||
| PLT (%) | <100 | 160 (28.8) | 115 (28.3) | 45 (30.0) | 0.778 |
| ≥100 | 396 (71.2) | 291 (71.7) | 105 (70.0) | ||
| AFP (%) | <400 μg/L | 355 (63.8) | 258 (63.5) | 97 (64.7) | 0.885 |
| ≥400 μg/L | 201 (36.2) | 148 (36.5) | 53 (35.3) | ||
| Maximum tumor size | Mean ± SD | 9.92 (2.48) | 9.85 (2.52) | 10.10 (2.39) | 0.304 |
| Size group (%) | Group A | 241 (43.3) | 169 (41.6) | 72 (48.0) | 0.211 |
| Group B | 315 (56.7) | 237 (58.4) | 78 (52.0) | ||
| Edmondson grade (%) | I + II | 79 (14.2) | 59 (14.5) | 20 (13.3) | 0.824 |
| III + IV | 477 (85.8) | 347 (85.5) | 130 (86.7) | ||
| MVI (%) | No | 197 (35.4) | 144 (35.5) | 53 (35.3) | 1.000 |
| Yes | 359 (64.6) | 262 (64.5) | 97 (64.7) | ||
| Satellite nodules | No | 336 (60.4) | 247 (60.8) | 89 (59.3) | 0.823 |
| Yes | 220 (39.6) | 159 (39.2) | 61 (40.7) | ||
| Tumor capsule (%) | Absent or partial | 432 (77.7) | 321 (79.1) | 111 (74.0) | 0.247 |
| Complete | 124 (22.3) | 85 (20.9) | 39 (26.0) | ||
| Type of liver resection | Non‐anatomical | 339 (61.0) | 246 (60.6) | 93 (62.0) | 0.838 |
| Anatomical | 217 (39.0) | 160 (39.4) | 57 (38.0) | ||
| Postoperative adjuvant TACE | No | 280 (50.4) | 205 (50.5) | 75 (50.0) | 0.994 |
| Yes | 276 (49.6) | 201 (49.5) | 75 (50.0) | ||
| Perioperative mortality | No | 552 (99.3) | 404 (99.5) | 148 (98.7) | 0.295 |
| Yes | 4 (0.7) | 2 (0.5) | 2 (1.3) | ||
| PLF (%) | No | 534 (96.0) | 388 (95.6) | 146 (97.3) | 0.482 |
| Yes | 22 (4.0) | 18 (4.4) | 4 (2.7) | ||
| Abdominal hemorrhage (%) | No | 549 (98.7) | 401 (98.8) | 148 (98.7) | 1.000 |
| Yes | 7 (1.3) | 5 (1.2) | 2 (1.3) | ||
| Bile leakage (%) | No | 543 (97.7) | 396 (97.5) | 147 (98.0) | 0.996 |
| Yes | 13 (2.3) | 10 (2.5) | 3 (2.0) | ||
| Incisional infection (%) | No | 516 (92.8) | 374 (92.1) | 142 (94.7) | 0.397 |
| Yes | 40 (7.2) | 32 (7.9) | 8 (5.3) | ||
| Organ/space infection (%) | No | 524 (94.2) | 381 (93.8) | 143 (95.3) | 0.642 |
| Yes | 32 (5.8) | 25 (6.2) | 7 (4.7) | ||
| Respiratory infection (%) | No | 545 (98.0) | 399 (98.3) | 146 (97.3) | 0.715 |
| Yes | 11 (2.0) | 7 (1.7) | 4 (2.7) | ||
| Pleural effusion (%) | No | 495 (89.0) | 365 (89.9) | 130 (86.7) | 0.352 |
| Yes | 61 (11.0) | 41 (10.1)9 | 20 (13.3) | ||
| Ascites (%) | No | 507 (91.2) | 370 (91.1) | 137 (91.3) | 1.000 |
| Yes | 49 (8.8) | 36 (8.9) | 13 (8.7) | ||
| Other complications (%) | No | 539 (96.9) | 395 (97.3) | 144 (96.0) | 0.612 |
| Yes | 17 (3.1) | 11 (2.7) | 6 (4.0) | ||
Abbreviations: AFP, alpha‐fetoprotein; Alb, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CR, creatinine; DBIL, direct bilirubin; GGT, gamma‐glutamy transpeptidase; HBV, hepatitis B virus; HCV, hepatitis C virus; INR, international normalized ratio; PLF, postoperative liver failure; PLT, blood platelet; TACE, transcatheter arterial chemoembolization; TBIL, total bilirubin.
Out of the 556 HCC patients, 150 (27.0%) were treated with preoperative TACE and 406 (73.0%) were not treated with preoperative TACE. For patients who only had one TACE session, the median interval between the TACE and surgery was 5 weeks (range 4–8), and for patients who had multiple preoperative TACE sessions, the median interval between the last TACE and surgery was 4 weeks (range 3–6). There were no significant differences in age, sex, cirrhosis, tumor diameter, Child‐Pugh classification, MVI, pathological grade, postoperative complications, and other variables between the two groups (p > 0.05). The baseline characteristics, clinicopathological features, and postoperative complications of each subgroup are listed in Table 2.
TABLE 2.
Comparison of clinicopathological characteristics and perioperative outcomes between patients with and without preoperative TACE in Group A and Group B
| Variable | Group A (n = 315) | Group B (n = 241) | |||||
|---|---|---|---|---|---|---|---|
| Non‐TACE (n = 237) | TACE (n = 78) | p | Non‐TACE (n = 169) | TACE (n = 72) | p | ||
| Age (%) | <65 years | 132 (55.7) | 50 (64.1) | 0.241 | 96 (56.8) | 40 (55.6) | 0.970 |
| ≥65 years | 105 (44.3) | 28 (35.9) | 73 (43.2) | 32 (44.4) | |||
| Gender (%) | Female | 59 (24.9) | 17 (21.8) | 0.687 | 47 (27.8) | 14 (19.4) | 0.228 |
| Male | 178 (75.1) | 61 (78.2) | 122 (72.2) | 58 (80.6) | |||
| HBV (%) | No | 9 (3.8) | 0 (0.0) | 0.176 | 9 (5.3) | 5 (6.9) | 0.849 |
| Yes | 228 (96.2) | 78 (100.0) | 160 (94.7) | 67 (93.1) | |||
| HCV (%) | No | 235 (99.2) | 78 (100.0) | 1.000 | 165 (97.6) | 70 (97.2) | 1.000 |
| Yes | 2 (0.8) | 0 (0.0) | 4 (2.4) | 2 (2.8) | |||
| Cirrhosis (%) | No | 75 (31.6) | 32 (41.0) | 0.168 | 58 (34.3) | 16 (22.2) | 0.087 |
| Yes | 162 (68.4) | 46 (59.0) | 111 (65.7) | 56 (77.8) | |||
| Child‐Pugh (%) | A | 214 (90.3) | 66 (84.6) | 0.239 | 148 (87.6) | 62 (86.1) | 0.920 |
| B | 23 (9.7) | 12 (15.4) | 21 (12.4) | 10 (13.9) | |||
| ALT (%) | <50 U/L | 157 (66.2) | 49 (62.8) | 0.679 | 102 (60.4) | 46 (63.9) | 0.710 |
| ≥50 U/L | 80 (33.8) | 29 (37.2) | 67 (39.6) | 26 (36.1) | |||
| AST (%) | <40 U/L | 85 (35.9) | 26 (33.3) | 0.788 | 60 (35.5) | 27 (37.5) | 0.882 |
| ≥40 U/L | 152 (64.1) | 52 (66.7) | 109 (64.5) | 45 (62.5) | |||
| GGT (%) | <45 U/L | 51 (21.5) | 11 (14.1) | 0.206 | 34 (20.1) | 20 (27.8) | 0.256 |
| ≥45 U/L | 186 (78.5) | 67 (85.9) | 135 (79.9) | 52 (72.2) | |||
| ALP (%) | <125 U/L | 177 (74.7) | 64 (82.1) | 0.239 | 129 (76.3) | 50 (69.4) | 0.338 |
| ≥125 U/L | 60 (25.3) | 14 (17.9) | 40 (23.7) | 22 (30.6) | |||
| Alb (%) | <35 g/L | 41 (17.3) | 15 (19.2) | 0.829 | 24 (14.2) | 14 (19.4) | 0.407 |
| ≥35 g/L | 196 (82.7) | 63 (80.8) | 145 (85.8) | 58 (80.6) | |||
| TBIL (%) | <20.4 μmol/L | 201 (84.8) | 65 (83.3) | 0.895 | 140 (82.8) | 60 (83.3) | 1.000 |
| ≥20.4 μmol/L | 36 (15.2) | 13 (16.7) | 29 (17.2) | 12 (16.7) | |||
| DBIL (%) | <6.8 μmol/L | 193 (81.4) | 64 (82.1) | 1.000 | 134 (79.3) | 55 (76.4) | 0.741 |
| ≥6.8 μmol/L | 44 (18.6) | 14 (17.9) | 35 (20.7) | 17 (23.6) | |||
| CR (%) | <84 μmol/L | 203 (85.7) | 66 (84.6) | 0.968 | 146 (86.4) | 59 (81.9) | 0.491 |
| ≥84 μmol/L | 34 (14.3) | 12 (15.4) | 23 (13.6) | 13 (18.1) | |||
| INR (%) | <1.15 | 163 (68.8) | 49 (62.8) | 0.405 | 117 (69.2) | 50 (69.4) | 1.000 |
| ≥1.15 | 74 (31.2) | 29 (37.2) | 52 (30.8) | 22 (30.6) | |||
| PLT (%) | <100 | 67 (28.3) | 24 (30.8) | 0.781 | 48 (28.4) | 21 (29.2) | 1.000 |
| ≥100 | 170 (71.7) | 54 (69.2) | 121 (71.6) | 51 (70.8) | |||
| AFP (%) | <400 μg/L | 161 (67.9) | 52 (66.7) | 0.946 | 97 (57.4) | 45 (62.5) | 0.552 |
| ≥400 μg/L | 76 (32.1) | 26 (33.3) | 72 (42.6) | 27 (37.5) | |||
| Maximum tumor size | Mean ± SD | 8.06 (0.85) | 8.21 (0.91) | 0.183 | 12.37 (1.82) | 12.41 (1.71) | 0.366 |
| Edmondson grade (%) | I + II | 39 (16.5) | 12 (15.4) | 0.964 | 20 (11.8) | 8 (11.1) | 1.000 |
| III + IV | 198 (83.5) | 66 (84.6) | 149 (88.2) | 64 (88.9) | |||
| MVI (%) | No | 111 (46.8) | 38 (48.7) | 0.874 | 33 (19.5) | 15 (20.8) | 0.955 |
| Yes | 126 (53.2) | 40 (51.3) | 136 (80.5) | 57 (79.2) | |||
| Satellite nodules | No | 150 (63.3) | 53 (67.9) | 0.543 | 97 (57.4) | 36 (50.0) | 0.360 |
| Yes | 87 (36.7) | 25 (32.1) | 72 (42.6) | 36 (50.0) | |||
| Tumor capsule (%) | Absent or partial | 181 (76.4) | 60 (76.9) | 1.000 | 140 (82.8) | 51 (70.8) | 0.054 |
| Complete | 56 (23.6) | 18 (23.1) | 29 (17.2) | 21 (29.2) | |||
| Type of liver resection | Non‐anatomical | 147 (62.0) | 47 (60.3) | 0.885 | 99 (58.6) | 46 (63.9) | 0.531 |
| Anatomical | 90 (38.0) | 31 (39.7) | 70 (41.4) | 26 (36.1) | |||
| Postoperative adjuvant TACE | No | 120 (50.6) | 45 (57.7) | 0.341 | 85 (50.3) | 30 (41.7) | 0.277 |
| Yes | 117 (49.4) | 33 (42.3) | 84 (49.7) | 42 (58.3) | |||
| Perioperative mortality | No | 237 (100) | 78 (100) | 167 (98.8) | 70 (97.2) | 0.585 | |
| Yes | 0 | 0 | 1.000 | 2 (1.2) | 2 (2.8) | ||
| PLF, n (%) | No | 228 (96.2) | 76 (97.4) | 0.874 | 160 (94.7) | 70 (97.2) | 0.596 |
| Yes | 9 (3.8) | 2 (2.6) | 9 (5.3) | 2 (2.8) | |||
| Abdominal hemorrhage (%) | No | 236 (99.6) | 77 (98.7) | 0.994 | 165 (97.6) | 71 (98.6) | 1.000 |
| Yes | 1 (0.4) | 1 (1.3) | 4 (2.4) | 1 (1.4) | |||
| Bile leakage (%) | No | 232 (97.9) | 76 (97.4) | 1.000 | 164 (97.0) | 71 (98.6) | 0.792 |
| Yes | 5 (2.1) | 2 (2.6) | 5 (3.0) | 1 (1.4) | |||
| Incisional infection (%) | No | 221 (93.2) | 74 (94.9) | 0.809 | 153 (90.5) | 68 (94.4) | 0.452 |
| Yes | 16 (6.8) | 4 (5.1) | 16 (9.5) | 4 (5.6) | |||
| Organ/space infection (%) | No | 224 (94.5) | 76 (97.4) | 0.457 | 157 (92.9) | 67 (93.1) | 1.000 |
| Yes | 13 (5.5) | 2 (2.6) | 12 (7.1) | 5 (6.9) | |||
| Respiratory infection (%) | No | 232 (97.9) | 76 (97.4) | 1.000 | 167 (98.8) | 70 (97.2) | 0.737 |
| Yes | 5 (2.1) | 2 (2.6) | 2 (1.2) | 2 (2.8) | |||
| Pleural effusion (%) | No | 216 (91.1) | 67 (85.9) | 0.266 | 149 (88.2) | 63 (87.5) | 1.000 |
| Yes | 21 (8.9) | 11 (14.1) | 20 (11.8) | 9 (12.5) | |||
| Ascites (%) | No | 210 (88.6) | 73 (93.6) | 0.295 | 160 (94.7) | 64 (88.9) | 0.183 |
| Yes | 27 (11.4) | 5 (6.4) | 9 (5.3) | 8 (11.1) | |||
| Other complications (%) | No | 233 (98.3) | 75 (96.2) | 0.497 | 162 (95.9) | 69 (95.8) | 1.000 |
| Yes | 4 (1.7) | 3 (3.8) | 7 (4.1) | 3 (4.2) | |||
Abbreviations: AFP, alpha‐fetoprotein; Alb, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CR, creatinine; DBIL, direct bilirubin; GGT, gamma‐glutamy transpeptidase; HBV, hepatitis B virus; HCV, hepatitis C virus; INR, international normalized ratio; PLF, postoperative liver failure; PLT, blood platelet; TACE, transcatheter arterial chemoembolization; TBIL, total bilirubin.
3.2. The effects of preoperative TACE on the prognosis of HCC in two groups
The median follow‐up time of the overall HCC population was 41 months. The mortality (25.3% vs. 39.4%, p < 0.05) and recurrence (38.0% vs. 58.4%, p < 0.05) rates of patients undergoing surgical resection with TACE were lower than those without TACE, showing statistical differences. The median OS and RFS of patients with TACE before surgery were 76 months and 37 months, respectively, longer than those without TACE (73 months and 32 months, respectively, p = 0.044 and 0.025) (Figure 1A,B). Then, we stratified according to the tumor diameter and found that there was no significant difference in OS and RFS between patients with TACE and those without TACE in Group A (p = 0.88 and p = 0.81, respectively) (Figure 2A,B). However, at Group B, the OS and RFS of patients with preoperative TACE were significantly better than those without preoperative TACE (p < 0.05 and p < 0.05, respectively) (Figure 3A,B).
FIGURE 1.
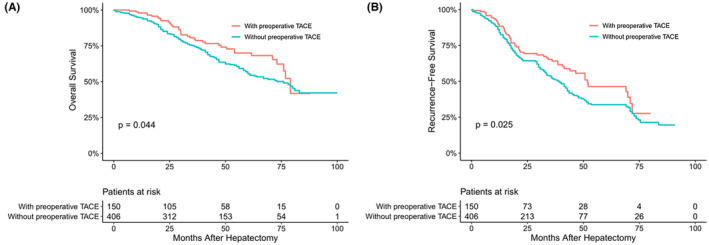
Overall (A) and recurrence‐free (B) survival curves of entire hepatocellular carcinoma patients with or without preoperative transcatheter arterial chemoembolization (TACE)
FIGURE 2.
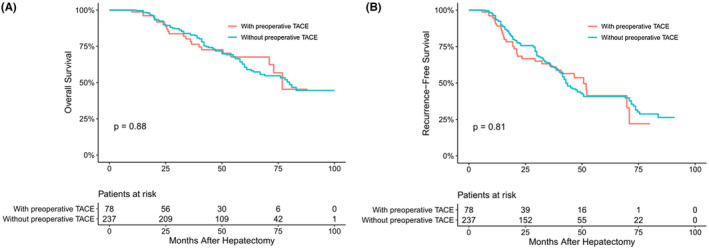
Overall (A) and recurrence‐free (B) survival curves of hepatocellular carcinoma patients in Group A with or without preoperative transcatheter arterial chemoembolization (TACE)
FIGURE 3.
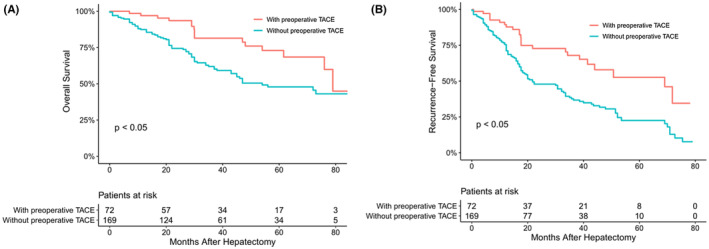
Overall (A) and recurrence‐free (B) survival curves of hepatocellular carcinoma patients in Group B with or without preoperative transcatheter arterial chemoembolization (TACE)
3.3. Univariable and multivariable analyses of OS and RFS
Results of univariate and multivariate analyses for the entire study cohort's overall and recurrence‐free survivals are presented in Tables 3 and 4, respectively. Univariate and multivariate Cox‐regression analysis showed that preoperative TACE could reduce postoperative recurrence (HR = 0.658, 95% CI: 0.490–0.885; p = 0.006) and prolong the survival (HR = 0.673, 95% CI: 0.470–0.963; p = 0.030). In the study cohort of each subgroup, the univariate and multivariate analysis results were listed in Table 5; Tables S1–S3, respectively. At Group A, the result showed that preoperative TACE had no statistical significance with RFS (HR = 1.045, 95% CI: 0.71–1.538; p = 0.823) and OS (HR = 0.961, 95% CI: 0.601–1.537; p = 0.869). However, in the Group B, the result reported that preoperative TACE could improve OS (HR = 0.448, 95% CI: 0.260–0.773; p = 0.004).and RFS (HR = 0.419, 95% CI: 0.269–0.652; p < 0.005).
TABLE 3.
Univariate and multivariate Cox‐regression analyses for overall survival in the total population
| Variables | HR comparison | UV HR (95% CI) | UV p | MV HR (95% CI) | MV p * |
|---|---|---|---|---|---|
| Preoperative TACE | Yes versus no | 0.696 (0.489–0.993) | 0.045 | 0.673 (0.470–0.963) | 0.030 |
| Age | ≥60 versus <60 years | 1.339 (1.013–1.77) | 0.040 | 1.434 (0.99–1.905) | 0.065 |
| Gender | Male versus female | 1.321 (0.934–1.869) | 0.116 | ||
| HBV | Yes versus no | 1.588 (0.746–3.38) | 0.230 | ||
| HCV | Yes versus no | 0.567 (0.141–2.286) | 0.425 | ||
| Cirrhosis | Yes versus no | 0.95 (0.703–1.283) | 0.737 | ||
| Child‐Pugh | B versus A | 1.237 (0.793–1.929) | 0.348 | ||
| ALT | ≥50 versus <50 U/L | 1.059 (0.796–1.409) | 0.695 | ||
| AST | ≥40 versus <40 U/L | 1.096 (0.816–1.471) | 0.543 | ||
| GGT | ≥45 versus <45 U/L | 1.222 (0.844–1.769) | 0.288 | ||
| ALP | ≥40 versus <40 U/L | 0.871 (0.621–1.222) | 0.424 | ||
| Alb | ≥35 versus <35 g/L | 0.82 (0.571–1.178) | 0.284 | ||
| TBIL | ≥20.4 versus <20.4 μmol/L | 0.907 (0.615–1.338) | 0.622 | ||
| DBIL | ≥6.8 versus <6.8 μmol /L | 0.982 (0.692–1.395) | 0.919 | ||
| CR | ≥80.4 versus <80.4 μmol /L | 0.748 (0.488–1.146) | 0.182 | ||
| INR | ≥1.15 versus <1.15 | 0.899 (0.661–1.224) | 0.500 | ||
| PLT | ≥100 versus <100 × 109/L | 1.391 (0.992–1.95) | 0.056 | ||
| AFP | ≥400 versus <400 ng/L | 2.026 (1.528–2.685) | 0.000 | 1.878 (1.41–2.501) | 0.000 |
| Maximum tumor size | Group A versus Group B | 0.685 (0.517–0.908) | 0.008 | 0.766 (0.57–0.929) | 0.045 |
| Edmondson grade | III + IV versus. I + II | 3.195 (1.955–5.224) | 0.000 | 3.211 (1.959–5.261) | 0.000 |
| MVI | Yes versus no | 1.979 (1.452–2.697) | 0.000 | 1.824 (1.310–2.542) | 0.000 |
| Satellite nodules | Yes versus no | 1.543 (1.322–2.996) | 0.000 | 1.721 (1.112–2.673) | 0.020 |
| Tumor capsule | Complete versus incomplete | 0.883 (0.639–1.221) | 0.451 | ||
| Type of liver resection | Anatomical versus non‐anatomical | 1.237 (0.424–2.342) | 0.453 | ||
| Postoperative adjuvant TACE | Yes versus no | 0.850 (0.632–1.768) | 0.654 |
Abbreviations: AFP, alpha‐fetoprotein; Alb, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; CR, creatinine; DBIL, direct bilirubin; GGT, gamma‐glutamy transpeptidase; HBV, hepatitis B virus; HCV, hepatitis C virus; HR, hazard ratio; INR, international normalized ratio; MV, multivariable; PLT, blood platelet; TACE, transcatheter arterial chemoembolization; TBIL, total bilirubin; UV, univariable.
Those variables found significant at p < 0.05 in univariable analyses were entered into multivariable Cox‐regression analyses.
TABLE 4.
Univariate and multivariate Cox‐regression analyses for recurrence‐free survival in the total population
| Variables | HR comparison | UV HR (95% CI) | UV p | MV HR (95% CI) | MV p * |
|---|---|---|---|---|---|
| Preoperative TACE | Yes versus no | 0.719 (0.538–0.961) | 0.026 | 0.658 (0.49–0.885) | 0.006 |
| Age | ≥60 versus <60 years | 1.092 (0.866–1.377) | 0.456 | 1.434 (0.98–1.905) | 0.073 |
| Gender | Male versus female | 1.115 (0.85–1.462) | 0.432 | ||
| HBV | Yes versus no | 1.108 (0.658–1.865) | 0.699 | ||
| HCV | Yes versus no | 0.936 (0.386–2.268) | 0.884 | ||
| Cirrhosis | Yes versus no | 1.008 (0.784–1.295) | 0.951 | ||
| Child‐Pugh | B versus A | 1.508 (1.071–2.121) | 0.018 | 1.136 (0.8–1.612) | 0.476 |
| ALT | ≥50 versus <50 U/L | 1.086 (0.86–1.372) | 0.486 | ||
| AST | ≥40 versus <40 U/L | 1.032 (0.812–1.312) | 0.795 | ||
| GGT | ≥45 versus <45 U/L | 1.188 (0.88–1.604) | 0.261 | ||
| ALP | ≥40 versus <40 U/L | 0.95 (0.725–1.247) | 0.713 | ||
| Alb | ≥35 versus <35 g/L | 0.93 (0.68–1.271) | 0.647 | ||
| TBIL | ≥20.4 versus <20.4 μmol/L | 0.869 (0.629–1.202) | 0.397 | ||
| DBIL | ≥6.8 versus <6.8 μmol /L | 1.013 (0.761–1.349) | 0.928 | ||
| CR | ≥80.4 versus <80.4 μmol /L | 0.877 (0.632–1.217) | 0.434 | ||
| INR | ≥1.15 versus <1.15 | 1.08 (0.845–1.379) | 0.540 | ||
| PLT | ≥ 100 versus <100 × 109/L | 1.059 (0.817–1.372) | 0.663 | ||
| AFP | ≥400 versus <400 ng/L | 3.283 (2.595–4.153) | 0.000 | 2.936 (2.303–3.743) | 0.000 |
| Maximum tumor size | Group A versus Group B | 0.615 (0.488–0.775) | 0.000 | 0.702 (0.551–0.895) | 0.004 |
| Edmondson grade | III + IV versus I + II | 2.807 (1.94–4.062) | 0.000 | 3.024 (2.075–4.407) | 0.000 |
| MVI | Yes versus no | 2.522 (1.933–3.29) | 0.000 | 1.982 (1.456–2.699) | 0.000 |
| Satellite nodules | Yes versus no | 2.563 (1.322–3.235) | 0.000 | 1.871 (1.345–2.677) | 0.003 |
| Tumor capsule | Complete versus incomplete | 0.582 (0.433–0.783) | 0.000 | 1.016 (0.726–1.423) | 0.925 |
| Type of liver resection | Anatomical versus non‐anatomical | 1.048 (0.316–2.441) | 0.753 | ||
| Postoperative adjuvant TACE | Yes versus no | 0.651 (0.424–1.427) | 0.435 |
Abbreviations: AFP, alpha‐fetoprotein; Alb, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; CR, creatinine; DBIL, direct bilirubin; GGT, gamma‐glutamy transpeptidase; HBV, hepatitis B virus; HCV, hepatitis C virus; HR, hazard ratio; INR, international normalized ratio; MV, multivariable; PLT, blood platelet; TACE, transcatheter arterial chemoembolization; TBIL, total bilirubin; UV, univariable.
Those variables found significant at p < 0.05 in univariable analyses were entered into multivariable Cox‐regression analyses.
TABLE 5.
Univariate and multivariate Cox‐regression analyses for overall survival in the Group A
| Variables | HR comparison | UV HR (95% CI) | UV p | MV HR (95% CI) | MV p * |
|---|---|---|---|---|---|
| Preoperative TACE | Yes versus no | 0.961 (0.601–1.537) | 0.869 | ||
| Age | ≥60 versus <60 years | 1.182 (0.812–1.720) | 0.384 | ||
| Gender | Male versus female | 1.131 (0.719–1.778) | 0.594 | ||
| HBV | Yes versus no | 0.949 (0.349–2.579) | 0.919 | ||
| HCV | Yes versus no | 2.168 (0.301–15.623) | 0.442 | ||
| Cirrhosis | Yes versus no | 0.825 (0.558–1.220) | 0.335 | ||
| Child‐Pugh | B versus A | 2.197 (1.288–3.749) | 0.004 | 1.786 (1.033–3.091) | 0.038 |
| ALT | ≥50 versus <50 U/L | 1.156 (0.789–1.694) | 0.457 | ||
| AST | ≥40 versus <40 U/L | 1.152 (0.771–1.721) | 0.489 | ||
| GGT | ≥45 versus <45 U/L | 1.149 (0.707–1.867) | 0.574 | ||
| ALP | ≥40 versus <40 U/L | 1.036 (0.667–1.609) | 0.876 | ||
| Alb | ≥35 versus <35 g/L | 0.786 (0.492–1.258) | 0.316 | ||
| TBIL | ≥20.4 versus <20.4 μmol/L | 0.912 (0.537–1.551) | 0.735 | ||
| DBIL | ≥6.8 versus <6.8 μmol /L | 1.109 (0.695–1.772) | 0.663 | ||
| CR | ≥80.4 versus <80.4 μmol /L | 0.814 (0.465–1.427) | 0.472 | ||
| INR | ≥1.15 versus <1.15 | 1.124 (0.758–1.666) | 0.561 | ||
| PLT | ≥ 100 versus <100 × 109/L | 1.195 (0.778–1.835) | 0.416 | ||
| AFP | ≥400 versus <400 ng/L | 1.771 (1.206–2.601) | 0.004 | 1.517 (1.026–2.244) | 0.037 |
| Edmondson grade | III + IV versus I + II | 2.615 (1.457–4.693) | 0.001 | 2.524 (1.400–4.551) | 0.002 |
| MVI | Yes versus no | 1.694 (1.158–2.477) | 0.007 | 1.572 (1.065–2.316) | 0.023 |
| Satellite nodules | Yes versus no | 1.563 (1.265–3.286) | 0.000 | 1.844 (1.045–3.677) | 0.003 |
| Tumor capsule | Complete versus incomplete | 0.844 (0.553–1.288) | 0.433 | ||
| Type of liver resection | Anatomical versus non‐anatomical | 1.246 (0.411–2.631) | 0.651 | ||
| Postoperative adjuvant TACE | Yes versus no | 0.543 (0.278–1.524) | 0.543 |
Abbreviations: AFP, alpha‐fetoprotein; Alb, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; CR, creatinine; DBIL, direct bilirubin; GGT, gamma‐glutamy transpeptidase; HBV, hepatitis B virus; HCV, hepatitis C virus; HR, hazard ratio; INR, international normalized ratio; MV, multivariable; PLF, postoperative liver failure; PLT, blood platelet; TACE, transcatheter arterial chemoembolization; TBIL, total bilirubin; UV, univariable.
Those variables found significant at p < 0.05 in univariable analyses were entered into multivariable Cox‐regression analyses.
3.4. Comparison of the clinicopathological features between Group A and Group B
The comparison of clinicopathological features between Group A and Group B is shown in Table S4.
Of the 315 patients in the Group A, 239 (75.9%) were male and 76 (24.1%) were female; 306 patients (97.1%) had chronic HBV infection and two patients (0.6%) were positive for hepatitis C virus RNA.
Among 241 patients with a maximum tumor diameter ≥10 cm (Group B), 180 (74.7%) patients were male, and 227 (94.2%) patients had chronic HBV infection. Patients who received preoperative TACE in the Group A less than those with preoperative TACE in the Group B (78/315 [24.8%] vs. 72/241 [29.9%]), but the difference was not statistically significant (p = 0.211). The proportion of patients with AFP ≥400 μg/L (41.1% vs. 32.4%, p = 0.043), MVI (80.1% vs. 52.7%, p < 0.001) and satellite nodules (44.8% vs. 35.6%, p = 0.034) in Group B was higher than that in Group A, while other clinicopathological indicators were not significantly different.
4. DISCUSSION
Transcatheter arterial chemoembolization is one of the most widely used non‐surgical therapeutic modalities for HCC. It mainly causes ischemic necrosis of the tumor by blocking the blood vessels feeding tumor, and at the same time delivers chemotherapy drugs through the artery to the target region to further promote tumor necrosis and tumor shrinkage. Recently, some researchers will consider it as a means of neoadjuvant therapy, the aim of which is mainly to improve the detection rate of latent intrahepatic metastasis or increase the resectability rate by decreasing the tumor diameter, and finally to improve the postoperative RFS and OS of HCC patients. 25 , 35 , 47 , 48 , 49 However, it is controversial whether preoperative TACE is effective in reducing recurrence and prolonging survival. 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 41 , 50
Interestingly, we found that the HCC population included in studies against preoperative adjuvant TACE generally had smaller tumor diameters (<5 cm), and that patients with preoperative TACE had much larger tumor diameters than those without preoperative TACE. 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 Therefore, the validity of their conclusions remained questionable, although they performed a propensity matched score (PSM) analysis. 31 , 33 , 35 , 36 In contrast, studies asserting that preoperative TACE was valuable usually included patients with LHCC (>5 cm) with more balanced clinicopathological data. 17 , 18 , 20 , 21 , 25 So, HCC patients with a maximum tumor diameter of 5 cm or more were selected as our study population.
Some reports suggest that preoperative TACE may only be significant in patients with an excessively large tumor diameter, 17 , 18 , 19 , 20 , 21 , 25 , 26 especially for patients with tumor diameter ≥10 cm. 18 We then divided the population into Groups A (5–9.9 cm) and B (≥10 cm) and explored the efficacy of preoperative TACE in each subgroup of the HCC population separately.
The results of this study showed that preoperative TACE benefited HCC patients and improved their RFS and OS in the overall population. However, after stratification, it was clear that the benefit was only significant for patients with tumor diameters ≥10 cm. In recent years, two studies on preoperative TACE from the same medical center, they just will be different in the setting of the inclusion criteria, and then the conclusion is different, and Zhou et al. included the inclusion criterion of tumor diameter ≥5 cm, which concluded that preoperative TACE had no effect on RFS and OS of patient. 39 Whereas, Li et al. set the cut‐off value of tumor diameter at 10 cm, their conclusion was quite different. 18 This latter study illustrated that the benefit population of preoperative TACE might be patients with huge HCC (≥10 cm), which is consistent with our current results.
We speculate that the possible reason for this stratification effect is that the corresponding tumor vascularization of Group B is more severe. Since the degree of tumor vascularization is positively correlated with the effectiveness of TACE, preoperative TACE could play a more critical role in Group B and ultimately benefit the survival of patients. 19 , 51 , 52 Second, as far as the tumor's biological characteristics are concerned, AFP level, MVI, and satellite nodules were higher in the Group B than in the Group A (p < 0.05), reflecting that the tumor biological characteristics of Group B were more aggressive. In other words, preoperative TACE could effectively inhibit the growth and proliferation of highly invasive HCC. Toshiya et al. suggested that preoperative TACE may be more suitable for more aggressive tumor populations. 22 , 53 Based on the above lines, we have reason to believe that for patients with solitary HCC with a diameter of ≥10 cm, preoperative TACE can improve the survival prognosis of HCC patients.
In terms of perioperative complications and 30‐day mortality, previous studies have shown that preoperative TACE could increase the intraoperative difficulty and perioperative complications. 26 , 36 , 38 In our study, this view is not tenable, which is consistent with the result of Li et al. 18 , 54 The surgical procedure does have a small portion of patients with necrosis tumors adherent to the surrounding tissues. But our chief surgeons are experienced and can completely overcome the adhesions caused by TACE. Again, it has been reported that as long as the interval between preoperative TACE and the operation is long enough, the negative impact of preoperative TACE on operation can be controllable. 18 , 54 In our study, the interval is at least 4 weeks. Therefore, as long as the patients are appropriately managed during perioperative period, the obstruction of TACE to surgery can be eliminated.
The results of this study revealed that tumor diameter ≥10 cm, AFP ≥ 400 μg/L, MVI, Edmondson grade, PLT level, and satellite nodules were independent risk factors for postoperative OS and RFS, which were also confirmed by previous studies. 18 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62
Our research has limitations. First, our study was a multicenter retrospective study that did not have a uniform standard for preoperative TACE. Second, considering that this study is a multi‐center study, the embolic materials and chemotherapeutic drugs used in each center are different. Third, most of the people we include are infected with HBV, while the majority of HCC patients in western countries are caused by factors such as HCV or alcohol. This study may not be suitable for western populations.
In conclusion, our study demonstrated that preoperative TACE is a safe neoadjuvant that does not increase perioperative complications and mortality. There was a stratification effect on the efficacy of preoperative TACE, and the beneficiary population is HCC patients with tumor diameter ≥10 cm. This study provides further guidance for the treatment of patients with large and huge solitary HCC to avoid unnecessary preoperative TACE.
AUTHOR CONTRIBUTIONS
Ali Mo: Conceptualization (equal). Qiao Zhang: Data curation (equal). Feng Xia: Conceptualization (equal); data curation (equal). Zhiyuan Huang: Resources (equal); software (equal). Shasha Peng: Supervision (equal); validation (equal). Wenjing Cao: Supervision (equal); validation (equal). Hongliang Mei: Data curation (equal); software (equal). Li Ren: Conceptualization (equal); resources (equal). Yang Su: Investigation (equal); methodology (equal). Hengyi Gao: Writing – original draft (equal); writing – review and editing (equal). Weiqiang Chen: Resources (equal); software (equal).
ETHICS STATEMENT
The ethics committees of the six medical centers approved the study, and the study complied with the Helsinki Declaration and local laws.
INFORMED CONSENT
The patient provided informed consent, which was registered in the medical record.
Supporting information
Appendix S1.
Mo A, Zhang Q, Xia F, et al. Preoperative transcatheter arterial chemoembolization and prognosis of patients with solitary large hepatocellular carcinomas (≥5 cm): Multicenter retrospective study. Cancer Med. 2023;12:7734‐7747. doi: 10.1002/cam4.5529
Ali Mo, Qiao Zhang, Feng Xia, and Zhiyuan Huang contributed equally to this work and share first authorship.
Contributor Information
Hengyi Gao, Email: 173178053@qq.com.
Weiqiang Chen, Email: cwq20138@aliyun.com.
DATA AVAILABILITY STATEMENT
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. [DOI] [PubMed] [Google Scholar]
- 2. Agrawal S, Belghiti J. Oncologic resection for malignant tumors of the liver. Ann Surg. 2011;253(4):656‐665. [DOI] [PubMed] [Google Scholar]
- 3. Zhong JH, Ke Y, Gong WF, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced‐stage hepatocellular carcinoma. Ann Surg. 2014;260(2):329‐340. [DOI] [PubMed] [Google Scholar]
- 4. Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261(5):947‐955. [DOI] [PubMed] [Google Scholar]
- 5. Pawlik TM, Delman KA, Vauthey JN, et al. Tumor size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11(9):1086‐1092. [DOI] [PubMed] [Google Scholar]
- 6. Poon RT, Fan ST, Wong J. Selection criteria for hepatic resection in patients with large hepatocellular carcinoma larger than 10 cm in diameter. J Am Coll Surg. 2002;194(5):592‐602. [DOI] [PubMed] [Google Scholar]
- 7. Pandey D, Lee KH, Wai CT, Wagholikar G, Tan KC. Long term outcome and prognostic factors for large hepatocellular carcinoma (10 cm or more) after surgical resection. Ann Surg Oncol. 2007;14(10):2817‐2823. [DOI] [PubMed] [Google Scholar]
- 8. Yang Y, Lin K, Liu L, et al. Impact of preoperative TACE on incidences of microvascular invasion and long‐term post‐hepatectomy survival in hepatocellular carcinoma patients: a propensity score matching analysis. Cancer Med. 2021;10(6):2100‐2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hu HT, Kim JH, Lee LS, et al. Chemoembolization for hepatocellular carcinoma: multivariate analysis of predicting factors for tumor response and survival in a 362‐patient cohort. J Vasc Interv Radiol. 2011;22(7):917‐923. [DOI] [PubMed] [Google Scholar]
- 10. Rahbari NN, Mehrabi A, Mollberg NM, et al. Hepatocellular carcinoma: current management and perspectives for the future. Ann Surg. 2011;253(3):453‐469. [DOI] [PubMed] [Google Scholar]
- 11. Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19(3):329‐338. [DOI] [PubMed] [Google Scholar]
- 12. Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734‐1739. [DOI] [PubMed] [Google Scholar]
- 13. Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(5):1164‐1171. [DOI] [PubMed] [Google Scholar]
- 14. Zheng Z, Liang W, Wang D, et al. Adjuvant chemotherapy for patients with primary hepatocellular carcinoma: a meta‐analysis. Int J Cancer. 2015;136(6):E751‐E759. [DOI] [PubMed] [Google Scholar]
- 15. Zhong JH, Li H, Li LQ, et al. Adjuvant therapy options following curative treatment of hepatocellular carcinoma: a systematic review of randomized trials. Eur J Surg Oncol. 2012;38(4):286‐295. [DOI] [PubMed] [Google Scholar]
- 16. Chen W, Ma T, Zhang J, et al. A systematic review and meta‐analysis of adjuvant transarterial chemoembolization after curative resection for patients with hepatocellular carcinoma. HPB. 2020;22(6):795‐808. [DOI] [PubMed] [Google Scholar]
- 17. Liao M, Zhu Z, Wang H, Huang J. Adjuvant transarterial chemoembolization for patients after curative resection of hepatocellular carcinoma: a meta‐analysis. Scand J Gastroenterol. 2017;52(6–7):624‐634. [DOI] [PubMed] [Google Scholar]
- 18. Li C, Wang MD, Lu L, et al. Preoperative transcatheter arterial chemoembolization for surgical resection of huge hepatocellular carcinoma (≥10 cm): a multicenter propensity matching analysis. Hepatol Int. 2019;13(6):736‐747. [DOI] [PubMed] [Google Scholar]
- 19. Nishikawa H, Arimoto A, Wakasa T, Kita R, Kimura T, Osaki Y. Effect of transcatheter arterial chemoembolization prior to surgical resection for hepatocellular carcinoma. Int J Oncol. 2013;42(1):151‐160. [DOI] [PubMed] [Google Scholar]
- 20. Yamashita Y, Takeishi K, Tsuijita E, et al. Beneficial effects of preoperative lipiodolization for resectable large hepatocellular carcinoma (≥5 cm in diameter). J Surg Oncol. 2012;106(4):498‐503. [DOI] [PubMed] [Google Scholar]
- 21. Chen XP, Hu DY, Zhang ZW, et al. Role of mesohepatectomy with or without transcatheter arterial chemoembolization for large centrally located hepatocellular carcinoma. Dig Surg. 2007;24(3):208‐213. [DOI] [PubMed] [Google Scholar]
- 22. Ochiai T, Sonoyama T, Hironaka T, Yamagishi H. Hepatectomy with chemoembolization for treatment of hepatocellular carcinoma. Hepatogastroenterology. 2003;50(51):750‐755. [PubMed] [Google Scholar]
- 23. Zhang Z, Liu Q, He J, Yang J, Yang G, Wu M. The effect of preoperative transcatheter hepatic arterial chemoembolization on disease‐free survival after hepatectomy for hepatocellular carcinoma. Cancer. 2000;89(12):2606‐2612. [PubMed] [Google Scholar]
- 24. Gerunda GE, Neri D, Merenda R, et al. Role of transarterial chemoembolization before liver resection for hepatocarcinoma. Liver Transpl. 2000;6(5):619‐626. [DOI] [PubMed] [Google Scholar]
- 25. Lu CD, Peng SY, Jiang XC, Chiba Y, Tanigawa N. Preoperative transcatheter arterial chemoembolization and prognosis of patients with hepatocellular carcinomas: retrospective analysis of 120 cases. World J Surg. 1999;23(3):293‐300. [DOI] [PubMed] [Google Scholar]
- 26. Majno PE, Adam R, Bismuth H, et al. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg. 1997;226(6):688‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheng X, Sun P, Hu QG, Song ZF, Xiong J, Zheng QC. Transarterial (chemo)embolization for curative resection of hepatocellular carcinoma: a systematic review and meta‐analyses. J Cancer Res Clin Oncol. 2014;140(7):1159‐1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Si T, Chen Y, Ma D, et al. Preoperative transarterial chemoembolization for resectable hepatocellular carcinoma in Asia area: a meta‐analysis of random controlled trials. Scand J Gastroenterol. 2016;51(12):1512‐1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu CC, Ho YZ, Ho WL, Wu TC, Liu TJ, P'Eng FK. Preoperative transcatheter arterial chemoembolization for resectable large hepatocellular carcinoma: a reappraisal. Br J Surg. 1995;82(1):122‐126. [DOI] [PubMed] [Google Scholar]
- 30. Jianyong L, Jinjing Z, Lunan Y, et al. Preoperative adjuvant transarterial chemoembolization cannot improve the long term outcome of radical therapies for hepatocellular carcinoma. Sci Rep. 2017;7:41624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ha TY, Hwang S, Lee YJ, et al. Absence of benefit of transcatheter arterial chemoembolization (TACE) in patients with resectable solitary hepatocellular carcinoma. World J Surg. 2016;40(5):1200‐1210. [DOI] [PubMed] [Google Scholar]
- 32. Shi HY, Wang SN, Wang SC, Chuang SC, Chen CM, Lee KT. Preoperative transarterial chemoembolization and resection for hepatocellular carcinoma: a nationwide Taiwan database analysis of long‐term outcome predictors. J Surg Oncol. 2014;109(5):487‐493. [DOI] [PubMed] [Google Scholar]
- 33. Lee KT, Lu YW, Wang SN, et al. The effect of preoperative transarterial chemoembolization of resectable hepatocellular carcinoma on clinical and economic outcomes. J Surg Oncol. 2009;99(6):343‐350. [DOI] [PubMed] [Google Scholar]
- 34. Kim IS, Lim YS, Lee HC, Suh DJ, Lee YJ, Lee SG. Pre‐operative transarterial chemoembolization for resectable hepatocellular carcinoma adversely affects post‐operative patient outcome. Aliment Pharmacol Ther. 2008;27(4):338‐345. [DOI] [PubMed] [Google Scholar]
- 35. Choi GH, Kim DH, Kang CM, et al. Is preoperative transarterial chemoembolization needed for a resectable hepatocellular carcinoma? World J Surg. 2007;31(12):2370‐2377. [DOI] [PubMed] [Google Scholar]
- 36. Sasaki A, Iwashita Y, Shibata K, Ohta M, Kitano S, Mori M. Preoperative transcatheter arterial chemoembolization reduces long‐term survival rate after hepatic resection for resectable hepatocellular carcinoma. Eur J Surg Oncol. 2006;32(7):773‐779. [DOI] [PubMed] [Google Scholar]
- 37. Sugo H, Futagawa S, Beppu T, Fukasawa M, Kojima K. Role of preoperative transcatheter arterial chemoembolization for resectable hepatocellular carcinoma: relation between postoperative course and the pattern of tumor recurrence. World J Surg. 2003;27(12):1295‐1299. [DOI] [PubMed] [Google Scholar]
- 38. Paye F, Jagot P, Vilgrain V, Farges O, Borie D, Belghiti J. Preoperative chemoembolization of hepatocellular carcinoma: a comparative study. Arch Surg. 1998;133(7):767‐772. [DOI] [PubMed] [Google Scholar]
- 39. Zhou WP, Lai EC, Li AJ, et al. A prospective, randomized, controlled trial of preoperative transarterial chemoembolization for resectable large hepatocellular carcinoma. Ann Surg. 2009;249(2):195‐202. [DOI] [PubMed] [Google Scholar]
- 40. Tao Q, He W, Li B, et al. Resection versus resection with preoperative transcatheter arterial chemoembolization for resectable hepatocellular carcinoma recurrence. J Cancer. 2018;9(16):2778‐2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kaibori M, Tanigawa N, Kariya S, et al. A prospective randomized controlled trial of preoperative whole‐liver chemolipiodolization for hepatocellular carcinoma. Dig Dis Sci. 2012;57(5):1404‐1412. [DOI] [PubMed] [Google Scholar]
- 42. Chen MY, Juengpanich S, Hu JH, et al. Prognostic factors and predictors of postoperative adjuvant transcatheter arterial chemoembolization benefit in patients with resected hepatocellular carcinoma. World J Gastroenterol. 2020;26(10):1042‐1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Qi LN, Ma L, Chen YY, et al. Outcomes of anatomical versus non‐anatomical resection for hepatocellular carcinoma according to circulating tumour‐cell status. Ann Med. 2020;52(1–2):21‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wakabayashi G, Cherqui D, Geller DA, et al. The Tokyo 2020 terminology of liver anatomy and resections: updates of the Brisbane 2000 system. J Hepatobiliary Pancreat Sci. 2022;29(1):6‐15. [DOI] [PubMed] [Google Scholar]
- 45. Balzan S, Belghiti J, Farges O, et al. The "50‐50 criteria" on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242(6):824‐828. discussion 828–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149(5):680‐688. [DOI] [PubMed] [Google Scholar]
- 47. Yamada R, Kishi K, Sato M, et al. Transcatheter arterial chemoembolization (TACE) in the treatment of unresectable liver cancer. World J Surg. 1995;19(6):795‐800. [DOI] [PubMed] [Google Scholar]
- 48. Sitzmann JV. Conversion of unresectable to resectable liver cancer: an approach and follow‐up study. World J Surg. 1995;19(6):790‐794. [DOI] [PubMed] [Google Scholar]
- 49. Bismuth H, Morino M, Sherlock D, et al. Primary treatment of hepatocellular carcinoma by arterial chemoembolization. Am J Surg. 1992;163(4):387‐394. [DOI] [PubMed] [Google Scholar]
- 50. Zhong C, Guo RP, Li JQ, et al. A randomized controlled trial of hepatectomy with adjuvant transcatheter arterial chemoembolization versus hepatectomy alone for stage III a hepatocellular carcinoma. J Cancer Res Clin Oncol. 2009;135(10):1437‐1445. [DOI] [PubMed] [Google Scholar]
- 51. Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103(4):914‐921. [DOI] [PubMed] [Google Scholar]
- 52. Zhang Z, Wu M, Liu Q. The effect of preoperative transcatheter hepatic arterial chemoembolization on disease‐free survival after hepatectomy for hepatocellular carcinoma. Zhonghua Zhong Liu Za Zhi. 1999;21(3):214‐216. [PubMed] [Google Scholar]
- 53. Rong‐Ping G, Wu‐Sheng Y, Wei W. Expression and prognostic value of proliferating cell nuclear antigen in hepatocellular carcinoma patients received preoperative transcatheter arterial chemoembolization. Chin J Cancer. 2008;27(2):5. [PubMed] [Google Scholar]
- 54. Nagasue N, Galizia G, Kohno H, et al. Adverse effects of preoperative hepatic artery chemoembolization for resectable hepatocellular carcinoma: a retrospective comparison of 138 liver resections. Surgery. 1989;106(1):81‐86. [PubMed] [Google Scholar]
- 55. Roayaie S, Blume IN, Thung SN, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137(3):850‐855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mehta N, Heimbach J, Harnois DM, et al. Validation of a Risk Estimation of Tumor Recurrence After Transplant (RETREAT) score for hepatocellular carcinoma recurrence after liver transplant. JAMA Oncol. 2017;3(4):493‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cong WM, Bu H, Chen J, et al. Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol. 2016;22(42):9279‐9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang XF, Meng B, Qi X, et al. Prognostic factors after liver resection for hepatocellular carcinoma with hepatitis B virus‐related cirrhosis: surgeon's role in survival. Eur J Surg Oncol. 2009;35(6):622‐628. [DOI] [PubMed] [Google Scholar]
- 59. Chen LP, Li C, Wang C, Wen TF, Yan LN, Li B. Risk factors of ascites after hepatectomy for patients with hepatocellular carcinoma and hepatitis B virus‐associated cirrhosis. Hepatogastroenterology. 2012;59(113):292‐295. [DOI] [PubMed] [Google Scholar]
- 60. Zhou L, Rui JA, Wang SB, Chen SG, Qu Q. Prognostic factors of solitary large hepatocellular carcinoma: the importance of differentiation grade. Eur J Surg Oncol. 2011;37(6):521‐525. [DOI] [PubMed] [Google Scholar]
- 61. Zhou L, Rui JA, Ye DX, Wang SB, Chen SG, Qu Q. Edmondson‐Steiner grading increases the predictive efficiency of TNM staging for long‐term survival of patients with hepatocellular carcinoma after curative resection. World J Surg. 2008;32(8):1748‐1756. [DOI] [PubMed] [Google Scholar]
- 62. Xu XF, Xing H, Han J, et al. Risk factors, patterns, and outcomes of late recurrence after liver resection for hepatocellular carcinoma: a multicenter study from China. JAMA Surg. 2019;154(3):209‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.


