Abstract
Background
Late gadolinium enhancement (LGE) on cardiac magnetic resonance is a predictor of adverse events in patients with nonischemic cardiomyopathy (NICM).
Objective
This meta-analysis evaluated the correlation between LGE and mortality, ventricular arrhythmias (VAs) and sudden cardiac death (SCD), and heart failure (HF) outcomes.
Methods
A literature search was conducted for studies reporting the association between LGE in NICM and the study endpoints. The primary endpoint was mortality. Secondary endpoints included VA and SCD, HF hospitalization, improvement in left ventricular ejection fraction (LVEF) to >35%, and heart transplantation referral. The search was not restricted to time or publication status. The minimum follow-up duration was 1 year.
Results
A total of 46 studies and 10,548 NICM patients (4610 with LGE, 5938 without LGE) were included; mean follow-up was 3 years (range 13–71 months). LGE was associated with increased mortality (odds ratio [OR] 2.9; 95% confidence interval [CI] 2.3–3.8; P < .01) and VA and SCD (OR 4.6; 95% CI 3.5–6.0; P < .01). LGE was associated with an increased risk of HF hospitalization (OR 3.4; 95% CI 2.3–5.0; P < .01), referral for transplantation (OR 5.1; 95% CI 2.5–10.4; P < .01), and decreased incidence of LVEF improvement to >35% (OR 0.2; 95% CI 0.03–0.85; P = .03).
Conclusion
LGE in NICM patients is associated with increased mortality, VA and SCD, and HF hospitalization and heart transplantation referral during long-term follow up. Given these competing risks of mortality and HF progression, prospective randomized controlled trials are required to determine if LGE is useful for guiding prophylactic implantable cardioverter-defibrillator placement in NICM patients.
Keywords: LGE, CMR, Mortality, Ventricular arrhythmia
Key Findings.
-
▪
Late gadolinium enhancement in nonischemic cardiomyopathy (NICM) patients is associated with increased mortality, ventricular arrythmia and sudden cardiac death, heart failure hospitalization, and heart transplantation referral during long-term follow up.
-
▪
Prospective randomized controlled trials are required to determine if late gadolinium enhancement is useful for guiding prophylactic implantable cardioverter-defibrillator placement in NICM patients who meet current guideline indications and NICM patients with less severe left ventricular dysfunction.
Introduction
Nonischemic cardiomyopathy (NICM) is a highly prevalent chronic disease that has been associated with increased morbidity and mortality through progressive pump failure and life-threatening arrhythmias.1 With an estimated disease prevalence ranging between 0.05% to 5% of all patients seen in the inpatient and outpatient settings and accounting for 1% to 2% of all annual healthcare costs, NICM places a large burden on the healthcare system in the United States and worldwide.1,2 It is imperative to identify patients who are at elevated risk for disease progression and mortality.
Late gadolinium enhancement (LGE) on cardiac magnetic resonance (CMR) is a promising technique for risk stratification of patients with NICM. LGE is useful for the detection of myocardial scar and fibrosis in patients with ischemic cardiomyopathy (ICM).3,4 While LGE is present in approximately 30% to 35% of patients with NICM, studies evaluating its association with clinical outcomes have mostly been limited to single-center observational studies.5
The goal of this systematic review and meta-analysis was to conduct a comprehensive evaluation of the association between LGE and clinical outcomes in patients with NICM. We examined the association of LGE with all-cause mortality, ventricular arrhythmias (VAs) and sudden cardiac death (SCD), heart failure (HF) hospitalization, improvement of left ventricular ejection fraction (LVEF) to >35%, and referral for heart transplantation in NICM patients.
Methods
Data search
This systematic review was performed in adherence to the guidelines of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) statement. The review was performed using a preplanned protocol in January 2022. The primary endpoint was mortality. Secondary endpoints included the composite of VA and SCD, HF hospitalization, interval improvement in LVEF to >35%, and heart transplantation referral. VAs were defined as the combined incidence of premature ventricular contractions (PVCs), nonsustained and sustained ventricular tachycardia, and appropriate implantable cardioverter-defibrillator (ICD) shocks. The studies were inconsistent regarding the amount of PVCs that qualified as VAs.
Search strategy
A systematic search was conducted using Ovid MEDLINE, EMBASE, Scopus, Web of Science, and Google Scholar for relevant literature that reported an association between LGE in CMR and VA, SCD, mortality, and HF outcomes. The search was not restricted to time or publication status. Two independent reviewers (M.A.-S. and M.T.) performed an electronic search using the following keywords: “late,” “gadolinium,” “enhancement,” “enhanced,” “enhance,” “enhancer,” “enhancers,” “enhances,” “enhancing,” “nonischaemic,” “nonischemic,” “nonischemics,” “cardiomyopathy,” “dilated,” “sensitivity,” “specificity,” “Predictive Value of Tests,” “Diagnostic Value,” and “Prediction.” The references of the included studies, other systematic reviews, and meta-analyses were also manually reviewed to obtain a comprehensive list of studies. After identifying relevant studies, the full texts of the selected articles were examined by both reviewers based on inclusion criteria. Disagreements were resolved by consensus.
Study selection
Studies were selected using the PICO (patient/population, intervention, comparison and outcomes) format to include those that studied patients with NICM (population), comparing LGE present (intervention) with LGE absent (comparison), and assessing for all-cause mortality, SCD, VA, appropriate ICD shock, SCD, HF hospitalization, referral for heart transplantation, and improvement in LVEF to >35% in subjects with baseline LVEF ≤35% (outcomes). Studies that did not separate mixed ICM and NICM patient populations were excluded. LGE presence was assessed either by visual estimation (present/absent) or quantitatively. When quantitative analysis was performed, the mean signal intensity and standard deviation of the region of interest were measured, and enhanced myocardium was defined as myocardium with signal intensity >5 SD above the remote normal myocardial signal. Patients with hypertrophic cardiomyopathy were excluded.
Data extraction
Two reviewers (M.A.-S. and M.T.) independently extracted the study data using a predefined data extraction sheet. Variables that were extracted from the studies included lead author, year of publication, study design, all-cause mortality, SCD, total patients with LGE, total patients without LGE, VAs, HF hospitalization, referral for transplantation, mean follow-up, mean age, mean LVEF, sex, left ventricular end-diastolic volume, and qualitative vs quantitative interpretation of LGE.
Statistical analysis
Meta-analysis was performed using Comprehensive Meta-Analysis software, version 3.6 We used a random-effects model to examine the association between LGE and outcomes, which were presented with an odds ratio (OR) with 95% confidence interval (CI) and Z value. The extent of heterogeneity was determined by I2 (ranging from 0% to 100%). Statistical significance was considered with a P value <.05, and all tests were 2-sided.
Results
Literature search and study selection
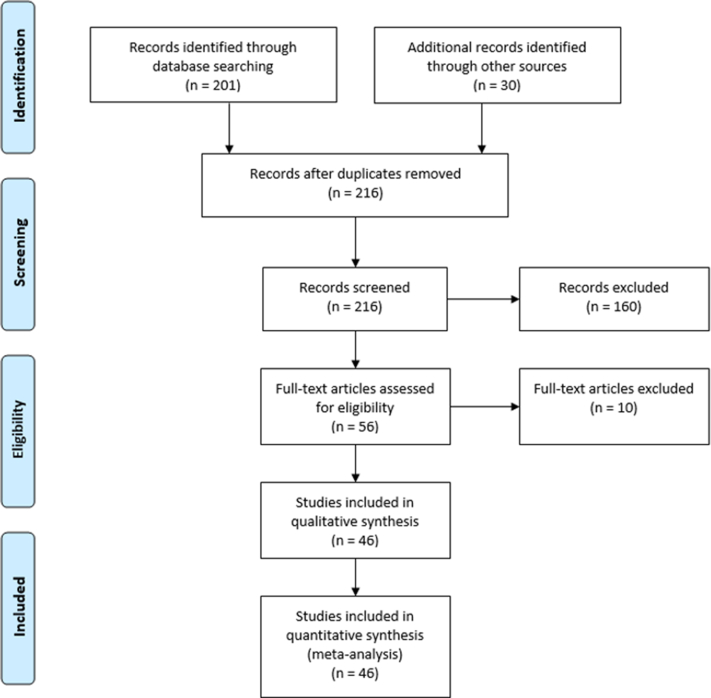
We identified 216 eligible studies from our literature search. After reviewing all studies in full text for relevance, 46 studies were identified to be eligible for meta-analysis for the outcomes of all-cause mortality (primary endpoint) and the composite of VAs, SCD, and appropriate ICD therapy (secondary endpoint). For the secondary endpoints of HF hospitalization, referral for heart transplantation, and LVEF improvement to >35%, 25 studies met inclusion criteria (Figure 1).
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) flow chart. The flow diagram depicts study selection for inclusion in the meta-analysis according to the PRISMA statement for reporting systematic reviews and meta-analyses.
Study and patient characteristics
This meta-analysis included prospective and retrospective (Table 1). A total of 10,548 patients (4610 with LGE and 5938 without LGE) were reported in the studies evaluating the association between LGE and all-cause mortality, and the combined incidence of VAs, SCD, and appropriate ICD shocks. A total of 3039 patients (1265 with LGE and 1774 without LGE) were reported in the studies evaluating the association between LGE and HF hospitalization, referral for heart transplantation, and LVEF improvement. The mean duration of follow-up was 36 (range 13–71) months (Figure 1).
Table 1.
Demographic data of the included studies
| First author | Year | Type | LGE | No LGE | Mean follow-up (mo) | Mean Age (y) | Mean LVEF (%) | Male (%) | LGE reading |
|---|---|---|---|---|---|---|---|---|---|
| Park37 | 2006 | pro | 24 | 22 | 8.1 | 55.9 | 26.3 | 58.7 | V |
| Assomull33 | 2006 | pro | 35 | 66 | 32 | 51 | 35.6 | 69.3 | V |
| Wu3 | 2008 | pro | 27 | 38 | 17 | 55 | 24 | 64.6 | SD |
| Cheong38 | 2009 | retro | 37 | 178 | 52.8 | 51 | 52 | 57 | V |
| Yokokawa39 | 2009 | retro | 18 | 11 | 20 | 65 | 24 | 58.6 | SD |
| Cho40 | 2010 | retro | 42 | 37 | 33.4 | 56 | 26.6 | 60.8 | V |
| Kono41 | 2010 | pro | 18 | 14 | 30.8 | 61 | 21.3 | 59.4 | SD |
| Looi42 | 2010 | pro | 31 | 72 | 32 | 58 | 32 | 75.7 | V |
| Shimizu31 | 2010 | pro | 11 | 40 | 14.1 | 59 | 30 | 76.7 | V |
| Iles43 | 2011 | retro | 31 | 30 | 19 | 53 | 25 | 68.9 | SD |
| Lehrke20 | 2011 | retro | 72 | 112 | 22 | 52 | 39 | 75 | SD |
| Fernández-Armenta44 | 2012 | pro | 15 | 22 | 25 | 64 | 22 | 83 | SD |
| Gao45 | 2012 | pro | 46 | 19 | 21 | 61 | 26.2 | 81 | V |
| Klem46 | 2012 | pro | 37 | 27 | 24 | 52 | 41 | 50 | V |
| Leyva47 | 2012 | pro | 20 | 77 | 35 | 66 | 22.3 | 61.9 | V |
| Masci48 | 2012 | pro | 50 | 75 | 14.2 | 58.2 | 34 | 65.6 | SD |
| Gulati19 | 2013 | pro | 142 | 330 | 64 | 51 | 37.2 | 68.6 | V |
| Kubanek49 | 2013 | pro | 30 | 14 | 12 | 43 | 23 | 71 | V |
| Masci50 | 2013 | pro | 26 | 32 | 24 | 55 | 37 | 33 | SD |
| Müller51 | 2013 | pro | 94 | 91 | 21 | 51 | 43.3 | 71.4 | V |
| Neilan21 | 2013 | pro | 81 | 81 | 29 | 55 | 26 | 65 | V |
| Šramko52 | 2013 | retro | 28 | 14 | 25 | 44 | 26 | 68.2 | V |
| Almehmadi53 | 2014 | retro | 107 | 62 | 15.6 | 62 | 33 | 73 | SD |
| Hasselberg54 | 2014 | retro | 4 | 9 | 29 | 52 | 32 | — | V |
| Machii55 | 2014 | retro | 48 | 24 | 36.2 | 64 | 24.8 | 72 | V |
| Perazzolo Marra56 | 2014 | pro | 76 | 61 | 36 | 49 | 32.5 | 78.8 | V |
| Masci57 | 2014 | pro | 61 | 167 | 23 | 50 | 43 | 79 | V |
| Mordi58 | 2014 | pro | 76 | 20 | 30.5 | 46 | 27 | 78.1 | SD |
| Nabeta59 | 2014 | pro | 36 | 39 | 11 | 56 | 30.2 | 65 | SD |
| Rodríguez-Capitán60 | 2014 | retro | 23 | 41 | 31.5 | 56.2 | 29.1 | 75 | V |
| Yamada61 | 2014 | pro | 25 | 32 | 71 | 55 | 33.5 | 70 | V |
| Amzulescu62 | 2015 | pre | 63 | 99 | 41 | 55 | 25 | 63 | V |
| Barison63 | 2015 | pro | 39 | 50 | 24 | 59 | 41 | X | V |
| Chimura64 | 2015 | retro | 122 | 53 | 61 | 60 | 29 | 63 | V |
| Piers65 | 2015 | pro | 55 | 32 | 45 | 56 | 29 | 62 | V |
| Tateishi66 | 2015 | pro | 105 | 102 | 44 | 50 | 27 | 80 | V |
| Venero67 | 2015 | pro | 21 | 10 | 12 | 45 | 17.6 | 67.7 | V |
| Gaztanaga68 | 2016 | retro | 71 | 34 | 27 | 50 | 25.3 | 56.2 | SD |
| Hu69 | 2016 | pro | 35 | 50 | 42.7 | 55 | 84 | 75.3 | V |
| Ishii70 | 2016 | retro | 37 | 41 | 47.7 | 56 | 31 | 68 | SD |
| Mikami71 | 2016 | pro | 66 | 52 | 25.2 | 57 | 32 | 57.6 | SD |
| Shin72 | 2016 | retro | 261 | 104 | 44.3 | 54.1 | 26.5 | 61.9 | SD |
| Tachi73 | 2016 | pro | 22 | 19 | — | 60 | 19.5 | 83 | SD |
| Voskoboinik74 | 2016 | retro | 17 | 11 | 32 | 44.2 | 20.3 | 64 | V |
| Riffel75 | 2016 | retro | 64 | 82 | 51.6 | 53 | 29.3 | 80 | V |
| Halliday28 | 2017 | pro | 101 | 298 | 55.2 | 49.9 | 49.6 | 63.7 | V |
| Chimura77 | 2017 | retro | 100 | 79 | 45.6 | 61 | 33 | 68 | V |
| Acosta76 | 2018 | pros | 22 | 109 | 35.5 | 65.1 | 24 | 72 | SD |
| Marume78 | 2018 | pro | 118 | 162 | 45.6 | 52.2 | 27.6 | 73.6 | V |
| Muthalaly79 | 2018 | retro | 62 | 68 | 38.4 | 54.8 | 29.4 | 83 | V |
| Voskoboinik80 | 2018 | retro | 147 | 189 | 39 | 50.7 | 36.8 | 67.3 | SD |
| Zhang81 | 2018 | pro | 101 | 119 | 61 | 49.6 | 25.6 | 73.2 | SD |
| Gutman12 | 2019 | retro | 174 | 72 | 37.9 | 52.4 | 24.3 | 74.8 | V |
| Halliday34 | 2019 | pro | 300 | 574 | 58.8 | 52.1 | 39 | 67.3 | V |
| Alba82 | 2020 | retro | 650 | 1022 | 60 | 57 | 33 | 71 | V |
| Barison83 | 2020 | pro | 116 | 77 | 30 | 66 | 27 | 73.2 | V |
| Behera22 | 2020 | pro | 44 | 68 | 24.8 | 43.3 | 24.6 | 64.2 | V |
| Yi84 | 2020 | pro | 258 | 120 | 40.8 | 55 | 24.1 | 62.7 | SD |
| Chen85 | 2021 | retro | 121 | 36 | 13 | 52.3 | 27 | 70.7 | SD |
| Di Marco14 | 2021 | retro | 486 | 679 | 36 | 58 | 39 | 66 | V |
| Guarici86 | 2021 | pro | 457 | 543 | 32 | 56.7 | 33 | 68.6 | V |
LGE = late gadolinium enhancement; LVEF = left ventricular ejection fraction; pro = prospective; retro = retrospective; V = visual estimation.
Association of LGE with all-cause mortality and VAs, SCD, and appropriate ICD shocks
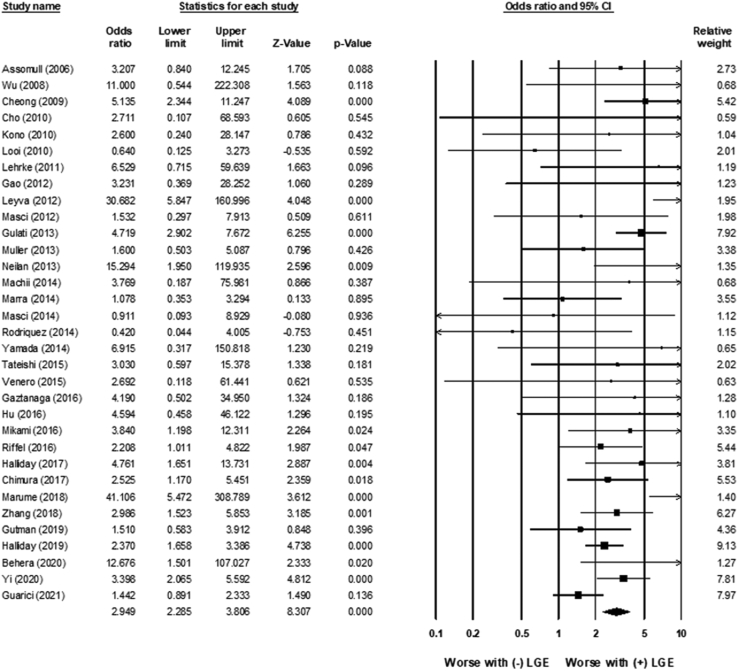
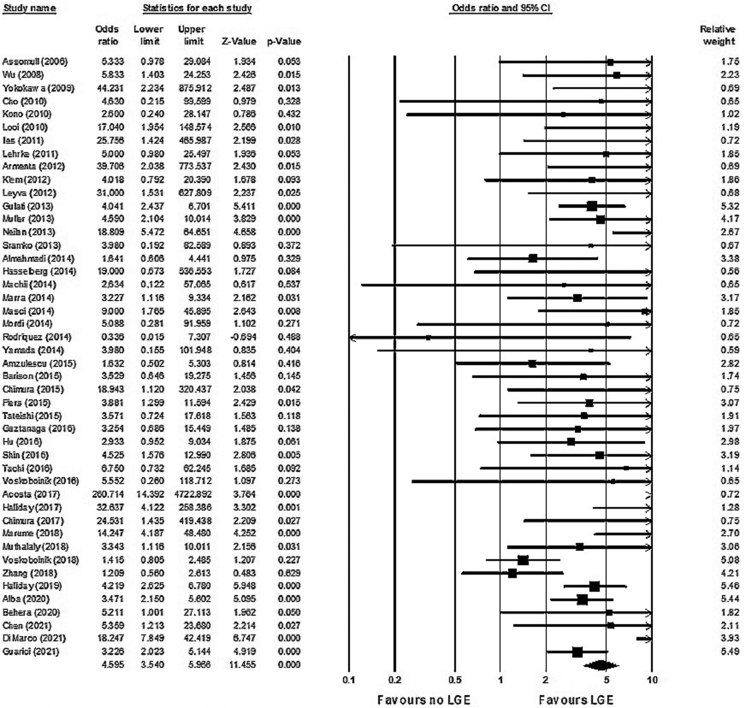
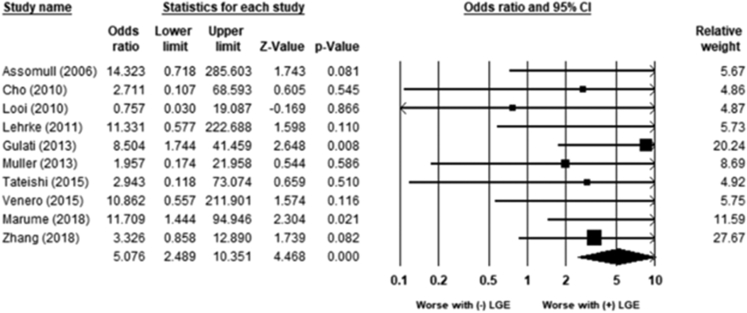
LGE was associated with an increased risk of all-cause mortality (OR 2.9; 95% CI 2.3–3.8; P < .01) (Figure 2). There was low heterogeneity (χ232 = 51.26; P = .017; I2 = 37%). LGE was also associated with an increased risk for the combined incidence of VAs, SCD, and appropriate ICD shocks (OR 4.6; 95% CI 3.5–6.0; P < .01) (Figure 3). There was low to moderate heterogeneity (χ245 = 82.2; P = .001; I2 = 45%).
Figure 2.
Association between late gadolinium enhancement (LGE) and mortality. LGE was associated with an increased risk of all-cause mortality (odds ratio 2.9; 95% confidence interval [CI] 2.3–3.8; P < .01). There was low heterogeneity (χ232 = 51.26; P = .017; I2 = 37%).
Figure 3.
Association between late gadolinium enhancement (LGE) and ventricular arrhythmias/sudden cardiac death. LGE was associated with an increased risk for the combined incidence of ventricular arrhythmias, sudden cardiac death, and appropriate implantable cardioverter-defibrillator shocks (odds ratio 4.6; 95% confidence interval [CI] 3.5–6.0; P < .01). Heterogeneity was low to moderate (χ245 = 82.2; P = .001; I2 =45%).
Association of LGE with HF hospitalization, referral for transplantation, and recovery of LVEF
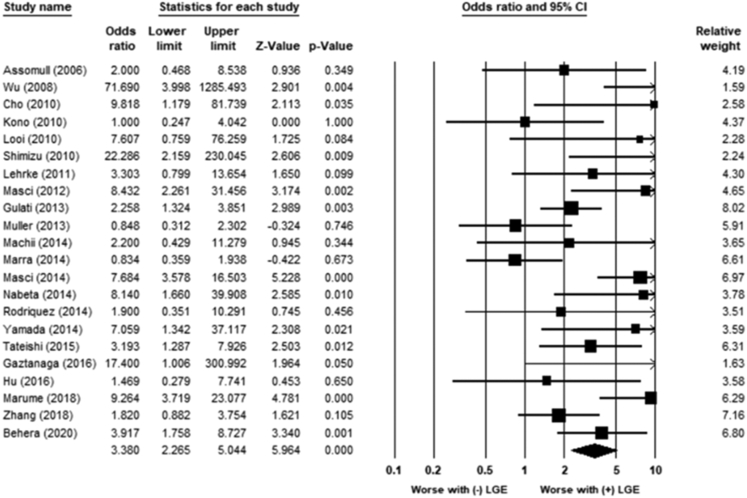
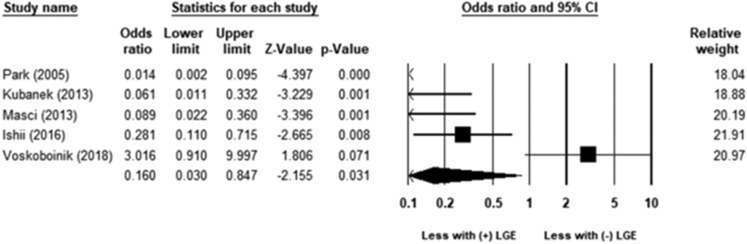
LGE was associated with an increased risk of HF hospitalization (OR 3.4; 95% CI 2.3–5.0; P < .01) (Figure 4). The heterogeneity was moderate (χ221 = 49.5; P = .001; I2 = 57%). LGE was associated with increased referral for heart transplantation (OR 5.1; 95% CI 2.5–10.4; P < .01) (Figure 5). The heterogeneity was low (χ29 = 4; P = .87; I2 = 0%). LGE was associated with an increased risk for lack of improvement in LVEF to >35% (OR 0.2; 95% CI 0.03–0.85; P = .03) (Figure 6). The heterogeneity was moderate to high (χ24 = 30; P = .001; I2 = 86%).
Figure 4.
Association between late gadolinium enhancement (LGE) and heart failure hospitalization. LGE was associated with an increased risk of heart failure hospitalization (odds ratio 3.4; 95% confidence interval [CI] 2.3–5.0; P < .01). The heterogeneity was moderate (χ221 = 49.5; P = .001; I2 = 57%).
Figure 5.
Association between late gadolinium enhancement (LGE) and referral for heart transplantation. LGE was associated with increased referral for heart transplantation (odds ratio 5.1; 95% confidence interval [CI] 2.5–10.4; P < .01). The heterogeneity was low (χ29 = 4; P = .87; I2 = 0%).
Figure 6.
Association between late gadolinium enhancement (LGE) and ejection fraction improvement to >35%. LGE was associated with an increased risk for lack of improvement in left ventricular ejection fraction to >35% (odds ratio 0.2; 95% confidence interval [CI] 0.03–0.85; P = .03). The heterogeneity was moderate to high (χ24 = 30; P = .001; I2 = 86%).
Discussion
The major findings of this study are that LGE identifies NICM patients who are at increased risk for all-cause mortality and the combined incidence of VAs, SCD, and appropriate ICD shocks. LGE also identified NICM patients who are at increased risk for HF hospitalization, referral for heart transplantation, and lack of improvement in LVEF. To our knowledge, this meta-analysis is the most comprehensive evaluation to date of the association of LGE and clinical outcomes in NICM.
The initial American Heart Association/American College of Cardiology/Heart Rhythm Society guidelines recommending defibrillator implantation in NICM were primarily based on the results of The Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) published over a decade ago.7,8 However, the SCD-HeFT trial was conducted on a mixed population (52% ICM and 48% NICM). At 10-year follow-up of the SCD-HeFT population, there was no mortality benefit for ICD placement in the patients with NICM.9 Similarly, the Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure (DANISH) trial demonstrated no significant difference in all-cause mortality with ICD implantation in patients with NICM.10 However, in the Defibrillators in Nonischemic Cardiomyopathy Treatment Evaluation (DEFINITE) trial, ICD placement did not reduce mortality but was associated with a reduction in sudden death from arrhythmia.11 One possible explanation for these findings may be that NICM represents a heterogeneous group of diseases in which certain disease etiologies place patients at higher cardiovascular risk than others.10 The lack of benefit of prophylactic ICD implantation in these studies highlights the need for additional risk stratification beyond LVEF, such as LGE. In one study, ICD placement was associated with a reduction in mortality only in patients with LGE (hazard ratio 0.45 vs 1.22 for LGE and no LGE, respectively; P < .05).12
LGE may also be utilized to identify high-risk patients that are excluded from current guidelines for ICD implantation. Although LVEF <35% is the current standard for recommending ICD implantation in NICM patients, it has low sensitivity (71.7%) and specificity (50.5%) for identifying patients at risk for SCD.13 As a result, some high-risk patients are not receiving ICD implantation due to not meeting LVEF criteria, while other low-risk patients with LVEF <35% and no LGE are having ICDs implanted and are exposed to device complications such as inappropriate shocks, lead or pulse generator malfunction, and infection. One study demonstrated that LGE is associated with VAs and SCD even in patients with LVEF >35%.14
The stark contrast in the utility of LVEF for predicting risk in NICM and ICM may be due to the fact that in ICM, a significant reduction in LVEF represents more extensive myocardial injury and scar formation. Several previous studies have suggested a strong correlation between reduction in LVEF and the extent of myocardial scarring in patients with ICM.15,16 In comparison, the pathogenesis of myocardial fibrosis in NICM remains unclear and may occur in varying distributions of myocardial tissue.17 While this development of fibrosis may not significantly impact LVEF, it may still place patients at risk for adverse events. In one study, the presence of LGE was not associated with initial low LVEF, but it predicted subsequent worsening of LVEF over time.18
The existing literature has been mixed regarding whether LGE is associated with adverse left ventricular remodeling and differences in left ventricular dimensions.5,19, 20, 21, 22 In the present study, LGE identified NICM patients who are at increased risk for HF hospitalization, referral for heart transplantation, and lack of improvement in LVEF. The clinical implications of these results are 2-fold. First, patients at high risk for HF progression may require close monitoring by an HF specialist and earlier referral to specialty centers for evaluation of advanced therapeutic options. Second, patients with LVEF <35% who do not have LGE may not need a prophylactic ICD or could be considered for a cardiac resynchronization therapy (CRT) pacemaker if they meet CRT criteria, given the higher likelihood for left ventricular reverse remodeling. These findings await confirmation in adequately powered, prospective studies before withholding ICD therapy from patients that meet current guidelines. The available data on the utility of adding ICD therapy to CRT in NICM patients is conflicting, as several studies have demonstrated no added mortality benefit,23, 24, 25 while the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) trial reported the opposite result.26
Study limitations
There are several limitations that need to be taken into consideration when assessing the results of this present study. Given that currently there are no standardized methods for defining the presence or extent of LGE, the interpretation of LGE varied across the studies included in this meta-analysis. The presence of LGE was defined in several ways, ranging from visual estimation to different threshold-based methods of analysis where the signal intensity of contrast-enhanced areas was compared with nonenhanced areas of myocardium.
While our results suggest that the presence of LGE has significant associations with clinical outcomes, we did not evaluate whether patterns of LGE result in differences in associated risk. There have been several studies demonstrating septal, subepicardial, and multiple LGE lesions to be independent predictors of cardiovascular outcomes.22,27,28 However, we could not identify enough current literature on this topic to further investigate in this meta-analysis.
Because there is a lack of standardization for defining the extent of LGE, we could not evaluate whether the extent of LGE correlates with differences in clinical outcomes. LGE extent can be interpreted in various ways, including summation of segments with hyperenhancement, percentage of involved myocardium, or absolute weight of enhanced myocardium.29 Interpretation is further complicated, as different LGE quantification techniques have been shown to cause wide variations in results in a single patient.30 Perhaps it is because of these reasons that there is no current consensus on what extent of LGE is predictive of clinical events. Cutoff values of significance for LGE extent range as broadly as >5% to >17%, and even results on the clinical significance of small areas of LGE have been mixed.22,31, 32, 33, 34
LGE on CMR is only able to detect regional myocardial fibrosis. While this pattern is typical in ICM, in which regional fibrosis is present, fibrosis patterns in NICM can occur either regionally or diffusely.22,35 Studies that utilized T1 mapping and extracellular volume fraction to detect diffuse myocardial fibrosis have shown this pattern to be significantly associated with adverse cardiovascular outcomes as well.36 Future studies should examine whether combined assessment of regional and diffuse fibrosis is useful for risk stratifying NICM patients.
The definition of VAs varied between studies, and some studies included VAs that are not life threatening in the composite endpoint, such as PVCs and nonsustained ventricular tachycardia.
Conclusion
LGE in NICM patients is associated with increased mortality, VA and SCD, HF hospitalization, and heart transplantation referral during long-term follow up. Given these competing risks of mortality and HF progression, prospective randomized controlled trials are required to determine if LGE is useful for guiding prophylactic implantable cardioverter-defibrillator placement in NICM patients who meet current guideline indications and NICM patients with less severe left ventricular dysfunction.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures
The authors report no relevant conflicts of interest.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Ethics Statement
This systematic review was performed in adherence to the guidelines of the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-analyses).
References
- 1.Berry C., Murdoch D.R., McMurray J.J.V. Economics of chronic heart failure. Eur J Heart Fail. 2001;3:283–291. doi: 10.1016/s1388-9842(01)00123-4. [DOI] [PubMed] [Google Scholar]
- 2.Follath F. Nonischemic heart failure: epidemiology, pathophysiology, and progression of disease. J Cardiovasc Pharmacol. 1999;33:S31–S35. doi: 10.1097/00005344-199906003-00004. [DOI] [PubMed] [Google Scholar]
- 3.Wu K.C., Weiss R.G., Thiemann D.R., et al. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:2414–2421. doi: 10.1016/j.jacc.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catalano O., Moro G., Perotti M., et al. Late gadolinium enhancement by cardiovascular magnetic resonance is complementary to left ventricle ejection fraction in predicting prognosis of patients with stable coronary artery disease. J Cardiovasc Magn Reson. 2012;14:29. doi: 10.1186/1532-429X-14-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang E.Y., Shah D.J. Cardiac magnetic resonance in nonischemic cardiomyopathies. Methodist Debakey Cardiovasc J. 2020;16:97–105. doi: 10.14797/mdcj-16-2-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comprehensive Meta-Analysis Software (CMA) https://www.meta-analysis.com/?gclid=CjwKCAjwhNWZBhB_EiwAPzlhNtFfAiO5PI-wyIXrg8LfjOGG_Bi0X4Y9Ryt-FMOlwfERKT67Dh4ncRoC4fYQAvD_BwE Available at:
- 7.Al-Khatib S.M., Stevenson W.G., Ackerman M.J., et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation. 2018;138:e272–e391. doi: 10.1161/CIR.0000000000000549. [DOI] [PubMed] [Google Scholar]
- 8.Bardy G.H., Lee K.L., Mark D.B., et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 9.Poole J.E., Olshansky B., Mark D.B., et al. Long-term outcomes of implantable cardioverter-defibrillator therapy in the SCD-HeFT. J Am Coll Cardiol. 2020;76:405–415. doi: 10.1016/j.jacc.2020.05.061. [DOI] [PubMed] [Google Scholar]
- 10.Køber L., Thune J.J., Nielsen J.C., et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375:1221–1230. doi: 10.1056/NEJMoa1608029. [DOI] [PubMed] [Google Scholar]
- 11.Kadish A., Dyer A., Daubert J.P., et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–2158. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 12.Gutman S.J., Costello B.T., Papapostolou S., et al. Reduction in mortality from implantable cardioverter-defibrillators in non-ischaemic cardiomyopathy patients is dependent on the presence of left ventricular scar. Eur Heart J. 2019;40:542–550. doi: 10.1093/eurheartj/ehy437. [DOI] [PubMed] [Google Scholar]
- 13.Goldberger J.J., Subačius H., Patel T., Cunnane R., Kadish A.H. Sudden cardiac death risk stratification in patients with nonischemic dilated cardiomyopathy. J Am Coll Cardiol. 2014;63:1879–1889. doi: 10.1016/j.jacc.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 14.di Marco A., Brown P.F., Bradley J., et al. Improved risk stratification for ventricular arrhythmias and sudden death in patients with nonischemic dilated cardiomyopathy. J Am Coll Cardiol. 2021;77:2890–2905. doi: 10.1016/j.jacc.2021.04.030. [DOI] [PubMed] [Google Scholar]
- 15.Nijveldt R., Beek A.M., Hirsch A., et al. Functional recovery after acute myocardial infarction. comparison between angiography, electrocardiography, and cardiovascular magnetic resonance measures of microvascular injury. J Am Coll Cardiol. 2008;52:181–189. doi: 10.1016/j.jacc.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Nijveldt R., van der Vleuten P.A., Hirsch A., et al. Early electrocardiographic findings and MR imaging-verified microvascular injury and myocardial infarct size. J Am Coll Cardiol Img. 2009;2:1187–1194. doi: 10.1016/j.jcmg.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Zeppenfeld K. Ventricular tachycardia ablation in nonischemic cardiomyopathy. J Am Coll Cardiol EP. 2018;4:1123–1140. doi: 10.1016/j.jacep.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Nabeta T., Ishii S., Ikeda Y., et al. Late gadolinium enhancement for re-worsening left ventricular ejection fraction in patients with dilated cardiomyopathy. ESC Heart Fail. 2021;8:615–624. doi: 10.1002/ehf2.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gulati A., Jabbour A., Ismail T.F., et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013;309:896–908. doi: 10.1001/jama.2013.1363. [DOI] [PubMed] [Google Scholar]
- 20.Lehrke S., Lossnitzer D., Schöb M., et al. Use of cardiovascular magnetic resonance for risk stratification in chronic heart failure: prognostic value of late gadolinium enhancement in patients with non-ischaemic dilated cardiomyopathy. Heart. 2011;97:727–732. doi: 10.1136/hrt.2010.205542. [DOI] [PubMed] [Google Scholar]
- 21.Neilan T.G., Coelho-Filho O.R., Danik S.B., et al. CMR quantification of myocardial scar provides additive prognostic information in nonischemic cardiomyopathy. J Am Coll Cardiol Img. 2013;6:944–954. doi: 10.1016/j.jcmg.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Behera D.R., Ajit Kumar V.K., Narayanan Namboodiri K.K., et al. Prognostic value of late gadolinium enhancement in cardiac MRI of non-ischemic dilated cardiomyopathy patients. Indian Heart J. 2020;72:362–368. doi: 10.1016/j.ihj.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cleland J.G.F., Daubert J.C., Erdmann E., et al. The CARE-HF study (cardiac resynchronisation in heart failure study): rationale, design and end-points. Eur J Heart Fail. 2001;3:481–489. doi: 10.1016/s1388-9842(01)00176-3. [DOI] [PubMed] [Google Scholar]
- 24.Looi K.L., Gajendragadkar P.R., Khan F.Z., et al. Cardiac resynchronisation therapy: pacemaker versus internal cardioverter-defibrillator in patients with impaired left ventricular function. Heart. 2014;100:794–799. doi: 10.1136/heartjnl-2014-305537. [DOI] [PubMed] [Google Scholar]
- 25.Reitan C., Chaudhry U., Bakos Z., et al. Long-term results of cardiac resynchronization therapy: a comparison between CRT-pacemakers versus primary prophylactic CRT-defibrillators. Pacing Clin Electrophysiol. 2015;38:758–767. doi: 10.1111/pace.12631. [DOI] [PubMed] [Google Scholar]
- 26.Doran B., Mei C., Varosy P.D., et al. The addition of a defibrillator to resynchronization therapy decreases mortality in patients with nonischemic cardiomyopathy. J Am Coll Cardiol HF. 2021;9:439–449. doi: 10.1016/j.jchf.2021.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Ota S., Orii M., Nishiguchi T., et al. Implications of multiple late gadolinium enhancement lesions on the frequency of left ventricular reverse remodeling and prognosis in patients with non-ischemic cardiomyopathy. J Cardiovasc Magn Reson. 2021;23:32. doi: 10.1186/s12968-021-00734-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halliday B.P., Gulati A., Ali A., et al. Association between midwall late gadolinium enhancement and sudden cardiac death in patients with dilated cardiomyopathy and mild and moderate left ventricular systolic dysfunction. Circulation. 2017;135:2106–2115. doi: 10.1161/CIRCULATIONAHA.116.026910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keil L., Chevalier C., Kirchhof P., et al. CMR-based risk stratification of sudden cardiac death and use of implantable cardioverter-defibrillator in non-ischemic cardiomyopathy. Int J Mol Sci. 2021;22:7115. doi: 10.3390/ijms22137115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flett A.S., Hasleton J., Cook C., et al. Evaluation of techniques for the quantification of myocardial scar of differing etiology using cardiac magnetic resonance. J Am Coll Cardiol Img. 2011;4:150–156. doi: 10.1016/j.jcmg.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 31.Shimizu I., Iguchi N., Watanabe H., et al. Delayed enhancement cardiovascular magnetic resonance as a novel technique to predict cardiac events in dilated cardiomyopathy patients. Int J Cardiol. 2010;142:224–229. doi: 10.1016/j.ijcard.2008.12.189. [DOI] [PubMed] [Google Scholar]
- 32.Pöyhönen P., Kivistö S., Holmström M., Hänninen H. Quantifying late gadolinium enhancement on CMR provides additional prognostic information in early risk-stratification of nonischemic cardiomyopathy: a cohort study. BMC Cardiovasc Disord. 2014;14:110. doi: 10.1186/1471-2261-14-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Assomull R.G., Prasad S.K., Lyne J., et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977–1985. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 34.Halliday B.P., Baksi A.J., Gulati A., et al. Outcome in dilated cardiomyopathy related to the extent, location, and pattern of late gadolinium enhancement. J Am Coll Cardiol Img. 2019;12:1645–1655. doi: 10.1016/j.jcmg.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuruvilla S., Adenaw N., Katwal A.B., Lipinski M.J., Kramer C.M., Salerno M. Late gadolinium enhancement on cardiac magnetic resonance predicts adverse cardiovascular outcomes in nonischemic cardiomyopathy: a systematic review and meta-analysis. Circ Cardiovasc Imaging. 2014;7:250–258. doi: 10.1161/CIRCIMAGING.113.001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puntmann V.O., Carr-White G., Jabbour A., et al. T1-mapping and outcome in nonischemic cardiomyopathy: all-cause mortality and heart failure. J Am Coll Cardiol Img. 2016;9:40–50. doi: 10.1016/j.jcmg.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Park S., Choi B.W., Rim S.J., et al. Delayed hyperenhancement magnetic resonance imaging is useful in predicting functional recovery of nonischemic left ventricular systolic dysfunction. J Card Fail. 2006;12:93–99. doi: 10.1016/j.cardfail.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Cheong B.Y.C., Muthupillai R., Wilson J.M., et al. Prognostic significance of delayed-enhancement magnetic resonance imaging. Circulation. 2009;120:2069–2076. doi: 10.1161/CIRCULATIONAHA.109.852517. [DOI] [PubMed] [Google Scholar]
- 39.Yokokawa M., Tada H., Koyama K., et al. The characteristics and distribution of the scar tissue predict ventricular tachycardia in patients with advanced heart failure. Pacing Clin Electrophysiol. 2009;32:314–322. doi: 10.1111/j.1540-8159.2008.02238.x. [DOI] [PubMed] [Google Scholar]
- 40.Cho J.R., Park S., Choi B.W., et al. Delayed enhancement magnetic resonance imaging is a significant prognostic factor in patients with non-ischemic cardiomyopathy. Circ J. 2010;74:476–483. doi: 10.1253/circj.cj-09-0446. [DOI] [PubMed] [Google Scholar]
- 41.Kono A.K., Ishii K., Kumagai H., Taniguchi Y., Kajiya T., Sugimura K. Late gadolinium enhancement on cardiac magnetic resonance imaging: is it associated with a higher incidence of nonsustained ventricular tachycardia in patients with idiopathic dilated cardiomyopathy? Jpn J Radiol. 2010;28:355–361. doi: 10.1007/s11604-010-0433-1. [DOI] [PubMed] [Google Scholar]
- 42.Looi J.L., Edwards C., Armstrong G.P., et al. Characteristics and prognostic importance of myocardial fibrosis in patients with dilated cardiomyopathy assessed by contrast-enhanced cardiac magnetic resonance imaging. Clin Med Insights Cardiol. 2010;4:129–134. doi: 10.4137/CMC.S5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iles L., Pfluger H., Lefkovits L., et al. Myocardial fibrosis predicts appropriate device therapy in patients with implantable cardioverter-defibrillators for primary prevention of sudden cardiac death. J Am Coll Cardiol. 2011;57:821–828. doi: 10.1016/j.jacc.2010.06.062. [DOI] [PubMed] [Google Scholar]
- 44.Fernández-Armenta J., Berruezo A., Mont L., et al. Use of myocardial scar characterization to predict ventricular arrhythmia in cardiac resynchronization therapy. Europace. 2012;14:1578–1586. doi: 10.1093/europace/eus104. [DOI] [PubMed] [Google Scholar]
- 45.Gao P., Yee R., Gula L., et al. Prediction of arrhythmic events in ischemic and dilated cardiomyopathy patients referred for implantable cardiac defibrillator. Circ Cardiovasc Imaging. 2012;5:448–456. doi: 10.1161/CIRCIMAGING.111.971549. [DOI] [PubMed] [Google Scholar]
- 46.Klem I., Weinsaft J.W., Bahnson T.D., et al. Assessment of myocardial scarring improves risk stratification in patients evaluated for cardiac defibrillator implantation. J Am Coll Cardiol. 2012;60:408–420. doi: 10.1016/j.jacc.2012.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leyva F., Taylor R.J., Foley P.W.X., et al. Left ventricular midwall fibrosis as a predictor of mortality and morbidity after cardiac resynchronization therapy in patients with nonischemic cardiomyopathy. J Am Coll Cardiol. 2012;60:1659–1667. doi: 10.1016/j.jacc.2012.05.054. [DOI] [PubMed] [Google Scholar]
- 48.Masci P.G., Barison A., Aquaro G.D., et al. Myocardial delayed enhancement in paucisymptomatic nonischemic dilated cardiomyopathy. Int J Cardiol. 2012;157:43–47. doi: 10.1016/j.ijcard.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 49.Kubanek M., Sramko M., Maluskova J., et al. Novel predictors of left ventricular reverse remodeling in individuals with recent-onset dilated cardiomyopathy. J Am Coll Cardiol. 2013;61:54–63. doi: 10.1016/j.jacc.2012.07.072. [DOI] [PubMed] [Google Scholar]
- 50.Masci P.G., Schuurman R., Andrea B., et al. Myocardial fibrosis as a key determinant of left ventricular remodeling in idiopathic dilated cardiomyopathy: a contrast-enhanced cardiovascular magnetic study. Circ Cardiovasc Imaging. 2013;6:790–799. doi: 10.1161/CIRCIMAGING.113.000438. [DOI] [PubMed] [Google Scholar]
- 51.Müller K.A.L., Müller I., Kramer U., et al. Prognostic value of contrast-enhanced cardiac magnetic resonance imaging in patients with newly diagnosed non-ischemic cardiomyopathy: cohort study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0057077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Šramko M., Kubánek M., Tintěra J., et al. Utility of combination of cardiac magnetic resonance imaging and high-sensitivity cardiac troponin T assay in diagnosis of inflammatory cardiomyopathy. Am J Cardiol. 2013;111:258–264. doi: 10.1016/j.amjcard.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 53.Almehmadi F., Joncas S.X., Nevis I., et al. Prevalence of myocardial fibrosis patterns in patients with systolic dysfunction: prognostic significance for the prediction of sudden cardiac arrest or appropriate implantable cardiac defibrillator therapy. Circ Cardiovasc Imaging. 2014;7:593–600. doi: 10.1161/CIRCIMAGING.113.001768. [DOI] [PubMed] [Google Scholar]
- 54.Hasselberg N.E., Edvardsen T., Petri H., et al. Risk prediction of ventricular arrhythmias and myocardial function in lamin A/C mutation positive subjects. Europace. 2014;16:563–571. doi: 10.1093/europace/eut291. [DOI] [PubMed] [Google Scholar]
- 55.Machii M., Satoh H., Shiraki K., et al. Distribution of late gadolinium enhancement in end-stage hypertrophic cardiomyopathy and dilated cardiomyopathy: differential diagnosis and prediction of cardiac outcome. Magn Reson Imaging. 2014;32:118–124. doi: 10.1016/j.mri.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 56.Perazzolo Marra M., de Lazzari M., Zorzi A., et al. Impact of the presence and amount of myocardial fibrosis by cardiac magnetic resonance on arrhythmic outcome and sudden cardiac death in nonischemic dilated cardiomyopathy. Heart Rhythm. 2014;11:856–863. doi: 10.1016/j.hrthm.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 57.Masci P.G., Doulaptsis C., Bertella E., et al. Incremental prognostic value of myocardial fibrosis in patients with non-ischemic cardiomyopathy without congestive heart failure. Circ Heart Fail. 2014;7:448–456. doi: 10.1161/CIRCHEARTFAILURE.113.000996. [DOI] [PubMed] [Google Scholar]
- 58.Mordi I., Jhund P.S., Gardner R.S., et al. LGE and NT-proBNP identify low risk of death or arrhythmic events in patients with primary prevention ICDs. J Am Coll Cardiol Img. 2014;7:561–569. doi: 10.1016/j.jcmg.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 59.Nabeta T., Inomata T., Iida Y., et al. Baseline cardiac magnetic resonance imaging versus baseline endomyocardial biopsy for the prediction of left ventricular reverse remodeling and prognosis in response to therapy in patients with idiopathic dilated cardiomyopathy. Heart Vessels. 2014;29:784–792. doi: 10.1007/s00380-013-0415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodríguez-Capitán J., García-Pinilla J.M., Ruiz-Zamora I., et al. Long-term prognostic value of late gadolinium enhancement in a cohort of patients with nonischemic dilated cardiomyopathy. Int J Cardiol. 2014;177:17–19. doi: 10.1016/j.ijcard.2014.09.110. [DOI] [PubMed] [Google Scholar]
- 61.Yamada T., Hirashiki A., Okumura T., et al. Prognostic impact of combined late gadolinium enhancement on cardiovascular magnetic resonance and peak oxygen consumption in ambulatory patients with nonischemic dilated cardiomyopathy. J Card Fail. 2014;20:825–832. doi: 10.1016/j.cardfail.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 62.Amzulescu M.S., Rousseau M.F., Ahn S.A., et al. Prognostic impact of hypertrabeculation and noncompaction phenotype in dilated cardiomyopathy: a CMR study. J Am Coll Cardiol Img. 2015;8:934–946. doi: 10.1016/j.jcmg.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 63.Barison A., del Torto A., Chiappino S., et al. Prognostic significance of myocardial extracellular volume fraction in nonischaemic dilated cardiomyopathy. J Cardiovasc Med (Hagerstown) 2015;16:681–687. doi: 10.2459/JCM.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 64.Chimura M., Kiuchi K., Okajima K., et al. Distribution of ventricular fibrosis associated with life-threatening ventricular tachyarrhythmias in patients with nonischemic dilated cardiomyopathy. J Cardiovasc Electrophysiol. 2015;26:1239–1246. doi: 10.1111/jce.12767. [DOI] [PubMed] [Google Scholar]
- 65.Piers S.R.D., Everaerts K., van der Geest R.J., et al. Myocardial scar predicts monomorphic ventricular tachycardia but not polymorphic ventricular tachycardia or ventricular fibrillation in nonischemic dilated cardiomyopathy. Heart Rhythm. 2015;12:2106–2114. doi: 10.1016/j.hrthm.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 66.Tateishi E., Noguchi T., Goto Y., et al. Prognostic impact of blood pressure response plus gadolinium enhancement in dilated cardiomyopathy. Heart. 2015;101:774–780. doi: 10.1136/heartjnl-2014-307007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Venero J.V., Doyle M., Shah M., et al. Mid wall fibrosis on CMR with late gadolinium enhancement may predict prognosis for LVAD and transplantation risk in patients with newly diagnosed dilated cardiomyopathy—preliminary observations from a high-volume transplant centre. ESC Heart Fail. 2015;2:150. doi: 10.1002/ehf2.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gaztanaga J., Paruchuri V., Elias E., et al. Prognostic value of late gadolinium enhancement in nonischemic cardiomyopathy. Am J Cardiol. 2016;118:1063–1068. doi: 10.1016/j.amjcard.2016.06.059. [DOI] [PubMed] [Google Scholar]
- 69.Hu D.J., Xu J., Du W., Zhang J.X., Zhong M., Zhou Y.N. Cardiac magnetic resonance and galectin–3 level as predictors of prognostic outcomes for non-ischemic cardiomyopathy patients. Int J Cardiovasc Imaging. 2016;32:1725–1733. doi: 10.1007/s10554-016-0958-1. [DOI] [PubMed] [Google Scholar]
- 70.Ishii S., Inomata T., Fujita T., et al. Clinical significance of endomyocardial biopsy in conjunction with cardiac magnetic resonance imaging to predict left ventricular reverse remodeling in idiopathic dilated cardiomyopathy. Heart Vessels. 2016;31:1960–1968. doi: 10.1007/s00380-016-0815-0. [DOI] [PubMed] [Google Scholar]
- 71.Mikami Y., Cornhill A., Heydari B., et al. Objective criteria for septal fibrosis in non-ischemic dilated cardiomyopathy: validation for the prediction of future cardiovascular events. J Cardiovasc Magn Reson. 2016;18:82. doi: 10.1186/s12968-016-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shin D.G., Lee H.J., Park J., et al. Pattern of late gadolinium enhancement predicts arrhythmic events in patients with non-ischemic cardiomyopathy. Int J Cardiol. 2016;222:9–15. doi: 10.1016/j.ijcard.2016.07.122. [DOI] [PubMed] [Google Scholar]
- 73.Tachi M., Amano Y., Inui K., et al. Relationship of postcontrast myocardial T1 value and delayed enhancement to reduced cardiac function and serious arrhythmia in dilated cardiomyopathy with left ventricular ejection fraction less than 35. Acta Radiol. 2016;57:430–436. doi: 10.1177/0284185115580840. [DOI] [PubMed] [Google Scholar]
- 74.Voskoboinik A., Bloom J., Taylor A., Mariani J. Early implantation of primary prevention implantable cardioverter defibrillators for patients with newly diagnosed severe nonischemic cardiomyopathy. Pacing Clin Electrophysiol. 2016;39:992–998. doi: 10.1111/pace.12911. [DOI] [PubMed] [Google Scholar]
- 75.Riffel J.H., Keller M.G.P., Rost F., et al. Left ventricular long axis strain: a new prognosticator in non-ischemic dilated cardiomyopathy? J Cardiovasc Magn Reson. 2016;18:36. doi: 10.1186/s12968-016-0255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Acosta J., Fernández-Armenta J., Borràs R., et al. Scar characterization to predict life-threatening arrhythmic events and sudden cardiac death in patients with cardiac resynchronization therapy: the GAUDI-CRT study. J Am Coll Cardiol Img. 2018;11:561–572. doi: 10.1016/j.jcmg.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 77.Chimura M., Onishi T., Tsukishiro Y., et al. Longitudinal strain combined with delayed-enhancement magnetic resonance improves risk stratification in patients with dilated cardiomyopathy. Heart. 2017;103:679–686. doi: 10.1136/heartjnl-2016-309746. [DOI] [PubMed] [Google Scholar]
- 78.Marume K., Noguchi T., Tateishi E., et al. Mortality and sudden cardiac death risk stratification using the noninvasive combination of wide QRS duration and late gadolinium enhancement in idiopathic dilated cardiomyopathy. Circ Arrhythm Electrophysiol. 2018;11 doi: 10.1161/CIRCEP.117.006233. [DOI] [PubMed] [Google Scholar]
- 79.Muthalaly R.G., Kwong R.Y., John R.M., et al. Left ventricular entropy is a novel predictor of arrhythmic events in patients with dilated cardiomyopathy receiving defibrillators for primary prevention. J Am Coll Cardiol Img. 2019;12:1177–1184. doi: 10.1016/j.jcmg.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 80.Voskoboinik A., Wong M.C.G., Elliott J.K., et al. Absence of late gadolinium enhancement on cardiac magnetic resonance imaging in ventricular fibrillation and nonischemic cardiomyopathy. Pacing Clin Electrophysiol. 2018;41:1109–1115. doi: 10.1111/pace.13426. [DOI] [PubMed] [Google Scholar]
- 81.Zhang K., Wang W., Zhao S., et al. Long-term prognostic value of combined free triiodothyronine and late gadolinium enhancement in nonischemic dilated cardiomyopathy. Clin Cardiol. 2018;41:96–103. doi: 10.1002/clc.22858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alba A.C., Gaztañaga J., Foroutan F., et al. Prognostic value of late gadolinium enhancement for the prediction of cardiovascular outcomes in dilated cardiomyopathy: an international, multi-institutional study of the MINICOR group. Circ Cardiovasc Imaging. 2020;13 doi: 10.1161/CIRCIMAGING.119.010105. [DOI] [PubMed] [Google Scholar]
- 83.Barison A., Aimo A., Mirizzi G., et al. The extent and location of late gadolinium enhancement predict defibrillator shock and cardiac mortality in patients with non-ischaemic dilated cardiomyopathy. Int J Cardiol. 2020;307:180–186. doi: 10.1016/j.ijcard.2020.02.028. [DOI] [PubMed] [Google Scholar]
- 84.Yi J.E., Lee H.J., Kim Y.J., Kim Y., Joung B., Park J. Additive prognostic value of red cell distribution width over late gadolinium enhancement on CMR in patients with non-ischemic dilated cardiomyopathy. Sci Rep. 2020;10:9212. doi: 10.1038/s41598-020-66198-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen W., Qian W., Zhang X., et al. Ring-like late gadolinium enhancement for predicting ventricular tachyarrhythmias in non-ischaemic dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2021;22:1130–1138. doi: 10.1093/ehjci/jeab117. [DOI] [PubMed] [Google Scholar]
- 86.Guaricci A.I., Masci P.G., Muscogiuri G., et al. CarDiac magnEtic Resonance for prophylactic Implantable-cardioVerter defibrillAtor ThErapy in Non-Ischaemic dilated CardioMyopathy: an international registry. Europace. 2021;23:1072–1083. doi: 10.1093/europace/euaa401. [DOI] [PubMed] [Google Scholar]