Abstract
Acute kidney injury (AKI) is defined as a decrease in kidney function within hours, which encompasses both injury and impairment of renal function. AKI is not considered a pathological condition of single organ failure, but a syndrome in which the kidney plays an active role in the progression of multi-organ dysfunction. The incidence rate of AKI is increasing and becoming a common (8–16% of hospital admissions) and serious disease (four-fold increased hospital mortality) affecting public health costs worldwide. AKI also affects the young and previously healthy individuals affected by infectious diseases in Latin America. Because of the multifactorial pathophysiological mechanisms, there is no effective pharmacological therapy that prevents the evolution or reverses the injury once established; therefore, renal replacement therapy is the only current alternative available for renal patients. The awareness of an accurate and prompt recognition of AKI underlying the various clinical phenotypes is an urgent need for more effective therapeutic interventions to diminish mortality and socio-economic impacts of AKI. The use of biomarkers as an indicator of the initial stage of the disease is critical and the cornerstone to fulfill the gaps in the field. This review discusses emerging strategies from basic science toward the anticipation of features, treatment of AKI, and new treatments using pharmacological and stem cell therapies. We will also highlight bioartificial kidney studies, addressing the limitations of the development of this innovative technology.
Keywords: acute kidney injury, ischemia, reperfusion, renal physiology
1. Introduction
Acute kidney injury (AKI) is characterized by the reduction of renal function during a short period of time, on the order of days or weeks. The renal alterations include a decrease in glomerular filtration rate – leading to an increase in serum concentration of urea nitrogen and creatinine – and proteinuria (Hoste et al., 2018). In general, AKI is associated with decreased renal perfusion pressure, major surgery, radiocontrast exposure, and pharmacological treatments (Basile, Anderson & Sutton, 2012). The incidence of hospital-acquired AKI in the United States is approximately 8 per 1,000 admissions and up to 20% in the intensive care unit (Kam-Tao-Li, Burdman & Mehta, 2013). These patients presented more severe outcomes than patients with community-acquired AKI (Makris & Spanou, 2016).
The incidence of AKI is increasing worldwide and is associated with high medical expenses during the post-AKI course and increased risk of developing an incapacitating chronic kidney disease along with a need for renal replacement therapy (RRT). The outcome for the survivors is early retirement because of work disability. For example, the incremental cost of AKI in Canada is estimated to be over $200 million Canadian dollars per year (Collister et al., 2017). The costs related to AKI were determined by Silver & Chertow (2017) based on the data from the National Inpatient Sample (NIS, the largest all-payer inpatient care database in the United States, containing over seven million hospitalizations from 95% of the United States population). AKI was associated with an increase in hospitalization costs of about $8,000 (~ $1,800 per patient and hospital characteristics) and an increase in about 3 days in the length of stay (LOS). Those numbers can be worse if AKI required dialysis – about $42,000 per patient and 11 days of hospitalization (Silver & Chertow, 2017). In Brazil, as in the rest of Latin America, AKI has a bimodal pattern: in urban areas, AKI is predominantly hospital-acquired as in high-income countries, and in the poorer peripheral areas AKI affects mainly the young and previously healthy individuals also affected by infectious diseases, like malaria and dengue (Makris & Spanou, 2016; Hoste et al., 2018).
Independent of the origin of the disease or the nationality of the patient, the early identification of patients at risk is crucial to prevent the evolution from acute to chronic renal failure, characterized by an irreversible loss of nephrons. There is no successful pharmacological therapy to treat AKI. Currently, RRT is a key component of AKI treatment. This review aims to discuss new strategies emerging from basic science research that generate new pharmacological and non-pharmacological treatment for the prevention and treatment of AKI. We used PubMed, Scopus, and directory of open access journals (DOAJ) to search for the following keywords: acute kidney injury, renal injury, ischemia-reperfusion, acute tubular necrosis, pharmacological treatment, non-pharmacological treatment, cellular therapy, and their equivalents.
2. Acute Kidney Injury
a. History, definition and contrasting epidemiology across economic status
The history and definition of AKI are topics that directly impact in AKI epidemiology. Although the first description of a reduced urine volume dates back to the ancient Greek period; only in the 19th century were the consequences to the body of this reduction, including death, reported (Abercrombie, 1821; Marketos, Eftychiadis & Diamandopoulos, 1993). In the early 20th century, the term acute renal failure characterized the reduction in urine volume. Recent multicenter observational cohorts and administrative database have improved the understanding of the overall disease. Because of that the term “acute renal failure” was preferentially referred to as AKI, which encompasses both structural damage and loss of renal function. The long period of time involving the first descriptions, the knowledge of the consequences of the acute reduced urine volume and the criteria to characterize the insult delayed the development of a consensus standard definition. A survey in 2002 revealed at least 35 definitions in the literature (Kellum, Levin, Bouman & Lameire, 2002).
Several consensus definitions of AKI have been developed based exclusively on the serum creatinine and urine output and are also used in epidemiologic studies to identify the patients. The most used criteria are The Kidney Disease: Improving Global Outcomes (KDIGO), Risk Injury Failure Loss End stage kidney disease (RIFLE) and the Acute Kidney Injury Network (AKIN) (Gameiro, Fonseca, Jorge & Lopes, 2018). The KDIGO guideline is based on the previous two classifications, unifying the AKI definition. In this classification, AKI is determined by an absolute increase in serum creatinine (sCr), at least 0.3 mg/dL within 48 h, by a 50% increase in sCr from baseline in 7 days, or a urine volume of less than 0.5 mL/kg/h for at least 6 h. The small changes in sCr and/or urine output, as it occurs in mild kidney injury, can be a predictor of serious clinical consequences. Thus, the use of novel biomarkers is likely necessary to improve the AKI definition and mainly to help in diagnosis.
After 7 days, if the parameters proposed by KDIGO are still altered, this establishes a critical period of vulnerability for renal patients. When this condition persists for more than 90 days, it will be considered a chronic kidney disease (CKD). The term acute kidney disease (AKD) was proposed to define the period between 7 and 90 days where the pathophysiological processes are ongoing. This period is essential to introduce pharmacological treatment, such as renin angiotensin blockade, to discontinue the AKI-CKD progression (Chou et al., 2017; Chou, Chu & Lin, 2018; Zhang et al., 2016b). The Acute Disease Quality Initiative (ADQI) 16 workgroup reported a series of proposed definitions (a guidance for clinical practice) and recommendations that aim to increase awareness of AKD and encourage research to understand the epidemiology, mechanisms and management of AKD (Chawla et al., 2017).
In developed countries, the prevalence of AKI occurs in the elderly and in consequence to comorbidities and hospitalization (hospital-acquired AKI). It is estimated that the hospital-acquired AKI occurrence is up to 15%, increasing the LOS by several days and being more common in critically ill patients in which the prevalence rises up to 60% (Xue et al., 2006, Lameire et al., 2013, Makris & Spanou, 2016). Concurrently, the proportion of patients surviving after AKI has also been increasing over time (Xue et al., 2006), heightening the risk for chronic kidney disease (CKD) and end-stage renal disease (ESRD) (Makris & Spanou, 2016). The fall in mortality outcome in developed countries may be attributed to the improvement of Nephrology, the intensive care unit and hospital care due to investments in the field (Xue et al., 2006). In developing countries, the investments in research that aim to reduce health expenses in Nephrology are scarce. In this way, health expenses are continuously increased year after year, which makes it difficult for the government’s health budget to follow impacting the mortality rates.
Latin America followed by Southeast Asia present the highest percentage of AKI in the world (Hoste et al., 2018). In the large city centers, AKI is predominantly hospital-acquired affecting the elderly, as occurs in high-income countries. This scenario is worsened because AKI also affects the young and previously healthy individuals. In the poorer peripheral areas, the population is affected by (1) bad sanitation conditions being susceptible to infectious diseases such as leptospirosis, malaria, dengue, yellow fever, and diarrhea; (2) exposure to animal venom and to herbs used in traditional medicine; (3) septic abortion; and (4) natural disasters (Burdmann & Jha, 2017). Under these conditions, the community-acquired form of AKI is prevalent. The occurrence of 1,800,000–2,900,000 new cases of AKI annually has been predicted in Latin America, with a mortality rate between 20 and 60%. Most patients are treated in Nephrology divisions located in teaching hospitals, supported by public health systems (Burdmann & Jha, 2017). For example, in Brazil, 85% of the costs in kidney diseases are supported by the Ministry of Health through the “Sistema Único de Saúde” (Sesso, Lopes, Thomé, Lugon & Martins, 2014). In 2017, the resources used in Nephrology for renal replacement therapy (RRT), hospitalizations, transplants and medications, is about US $ 1 billion. This is the largest resource spent by the Brazilian Ministry of Health for a specific area of care (Brazilian Health Ministry, www.brasil.gov.br/noticias/saude/2017/01/terapia-renal-recebe-investimento-de-r-197-milhoes).
Mesoamerican nephropathy (MeN), a form of CKD of unknown etiology (CKDu), is recognized as a major source of morbidity and mortality in rural workers in Central American countries such as El Salvador and Nicaragua. These individuals are subjected to several hours of hard work at high altitude with limited water intake, which could explain the work-associated AKI (Kupferman et al., 2018). This is supported by increased sCr level observed in sugarcane workers who sought medical attention for acute symptoms including fever, nausea, vomiting, and back pain (Fischer et al., 2017). In the group studied by Kupferman and collaborators, nearly half had established CKD 12 months later (Kupferman et al., 2018). The relevance of these studies identifies a specific group of young and previously healthy workers potentially vulnerable to develop decreased kidney function during work life. More detailed basic and clinical research on MeN will be required for a complete comprehension of the disease and good clinical practice to disrupt the connection between AKI and CKDu. Those practices will certainly attenuate the public health care costs in these countries.
Extracorporeal (hemodialysis) or paracorporeal (peritoneal dialysis) RRT applied intermittently or continuously has been used as a supportive treatment of AKI, focusing on averting metabolic acidosis, hyperkalemia, uremia, fluid overload – derangements associated with kidney failure – allowing time for organ recovery. The health-cost to the overall treatment of complications is about $ 1,800 per patient (Silver, Long, Zheng & Cherton, 2017). Although the restricted availability of organs and transplant rejection limits its widespread use, data from developed and developing countries indicate that kidney transplantation is the most cost-effective modality of RRT (Gouveia et al., 2017; Sánchez-Escuredo et al., 2015). For a better comprehension of the impact cost of RRT and kidney transplantation, please see Caskey & Jager (2014).
b. Physiopathology
For many years the diagnosis and management of AKI were based on the concept of classification to three main categories: pre-renal, renal and post-renal. Pre-renal injury is a consequence of clinical conditions, such as hypovolaemia (provoked by hemorrhage or volume depletion), renal hypoperfusion (caused by the use of drugs, abdominal aortic aneurysm and hepato-renal syndrome) and hypotension (as a consequence of cardiogenic shock, distributive shock, cardiac failure, hepatic cirrhosis and nephrotic syndrome). Sepsis, large surgery and the use of radiocontrast dyes are also common causes of pre-renal injury. The high-risk group is composed of elderly patients with atherosclerotic cardiovascular disease, patients with pre-existing CKD and patients with renal hypoperfusion caused by volume depletion, hypotension or renal artery stenosis (Blantz, 1998). The intrinsic injury (included in the renal category) is often a multifactorial disease caused by glomerular and tubular injury, interstitial nephritis, vascular and thrombotic disorders. Alteration in glomerular function is associated with inflammation and glomerulonephritis as encountered in systemic lupus erythematosus. Acute tubular necrosis, provoked by prolonged ischemia and by xenobiotics, is the most common cause of hospital-acquired AKI (Makris & Spanou, 2016). Post-renal injury is characterized by a secondary obstruction (for example ureter) provoked by intrinsic and extrinsic factors. Intrinsic factors are associated with blood clot, papillary necrosis, urethral structure, prostatic hypertrophy or malignancy, bladder tumor and radiation fibrosis, while extrinsic factors include retroperitoneal fibrosis and pelvic malignancy. In this way, elderly men with prostate disease and patients with intra-abdominal malignancy are considered at higher risk. The high increase in diuresis that generally occurs once obstruction is relieved needs careful monitoring with appropriate fluid replacement to avoid hypovolemia (Basile, Anderson & Sutton, 2012).
Regardless of the AKI classification, ischemia-reperfusion (I/R) is the main pathophysiological mechanism, accounting for 60% of the causes (Basile, Anderson & Sutton, 2012). The kidneys receive 20% of the cardiac output in healthy subjects. Blood flow within the kidneys is selectively distributed with about 90% of the renal blood flow directed to the cortex where glomerular filtration occurs. The remaining 10% of the renal blood flows through peritubular microcirculation in the medulla, off-loads oxygen, delivers nutrients to the tissues and finely adjusts the body homeostasis (returning reabsorbed solutes to systemic circulation and participates in the counter-current mechanisms to allow the conservation of water). Renal blood flow is maintained throughout a wide range of renal perfusion pressures (60–100 mmHg). The renal blood flow is strictly maintained by an autoregulation mechanism mediated by the myogenic mechanism and the tubuloglomerular feedback in order to guarantee an adequate oxygen delivery for the production of mitochondrial adenosine triphosphate (ATP), nitric oxide (NO) and reactive oxygen species (ROS) essential to the homeostatic control of renal function (Just, 2007; Aksu, DemircI & Ince, 2011).
Many observational clinical studies have demonstrated significant associations between systemic hemodynamic instability and the development and progression of AKI, apparently dependent on the severity of illness and patient comorbidities (Legrand, Dupuis, Simon, Gayat, Mateo, Lukaszewicz & Payen, 2013; Ostermann & Liu, 2017; Post, Kellum, Bellomo & Vicent, 2017; Raimundo, Crichton, Syed, Martin, Beale, Treacher & Ostermann, 2015). In summary, the AKI etiology is mainly related to a mismatch between oxygen and nutrient delivery to the nephrons due to an impaired microcirculation and increased energy demands and the subsequent pathogenic effects are hypoxia and oxidative stress (Tögel & Westenfelder, 2014).
The broad range of primary modifiers mentioned above (ischemic insult, toxins, hemodynamic alterations, sepsis) triggers the inflammatory response. The different phases of the inflammatory response during AKI were detailed by Rabb et al. (2015). The primary acute inflammatory response, starting over minutes to hours, recruits resident and infiltrating leukocytes, neutrophils, monocytes/macrophages, dendritic cells, natural killer T cells, natural killer cells and B cells, leading to cell damage and triggering death (Rabb et al., 2016).
In the acute phase, the injured microcirculation provokes the recruitment and adhesion of leukocytes and platelets, amplifying the impairment in renal perfusion and oxygen delivery to produce additional endothelial cell injury and inflammation (Zuk & Bonventre, 2016). The involvement of matrix metalloproteinases (MMPs), a family of endopeptidases, contributes to increased vascular permeability, interstitial edema, affecting even more renal blood flow. Like a domino effect, the remodeling of the extracellular matrix mediated by MMPs boost the inflammatory response and affects the tubular structure (Novak, Le, Christison-Lagay, Nose, Doiron, Moses & Puder, 2010; Rodriguez, Morrison & Overall, 2010; Isaac, Tögel & Westenfelder, 2007; Zhang et al., 2016a)).
Associated with the acute inflammatory response, oxygen deprivation affects glucose metabolism, triggering anaerobic metabolism, which cannot sustain the kidney demand (Malek & Nematbaksh, 2015). ATP deprivation inhibits epithelial Na+ transport, mainly by the medullary (Na++K+)-ATPase, affecting Na+ and solute tubular handling (Cortes et al., 2018). Accumulation of intracellular Na+ induces intracellular edema, resulting in disruption of cellular membranes from the endoplasmic reticulum (ER), Golgi apparatus, mitochondrial membranes, and cytoskeletal microtubules (Mangino, Tian, Ametani, Lindell & Southard, 2008). Endoplasmic-reticulum stress can activate an irreversible phase of renal injury, tubular cell apoptosis and necrosis mediated by caspases and calpains (Ca2+-dependent cysteine proteases). Altered tubular structure – effacement and loss of proximal tubule brush border, patchy loss of tubular cells, focal areas of tubular dilatation and distal tubular casts – provokes derangements in tubular dynamics that include obstruction, back leak and activation of tubuloglomerular feedback (Bonventre & Yang, 2011; Cortes et al., 2018). Those are characteristics of the acute tubular necrosis (ATN) and once established, organ functional impairment of water and electrolyte homeostasis and reduction of the excretion of waste products of metabolism occur (Bonventre & Yang, 2011; Malek & Nematbakhsh, 2015). The damaged tubules produce oxidative stress and vasoconstrictive prostaglandins, further impairing oxygen delivery and leading to a local “no-reflow” phenomenon (Molitoris 2014). I/R injury also contributes to 30% of the kidney delayed graft function (DGF) or primary non-function after kidney transplant (Huart, Krzesinski & Jouret, 2018).
In summary, during the acute phase, the associated pathophysiological mechanisms are inflammatory cell infiltration, mitochondrial dysfunction, oxidative stress and lipid peroxidation, activation of the renin-angiotensin system and impairment of nitric oxide (NO) production. The major part of these pathophysiological mechanisms is orchestrated by the increase of intrarenal angiotensin II levels (Ang II) in the first 24 h of the insult (Kontogiannis & Burns 1998). Moreover, animal and patient studies have shown the beneficial effects of RAS blockade treatment halting CKD and attenuating mortality (Chou et al., 2017; Chou, Chu & Lin, 2018; Zhang et al., 2016b).
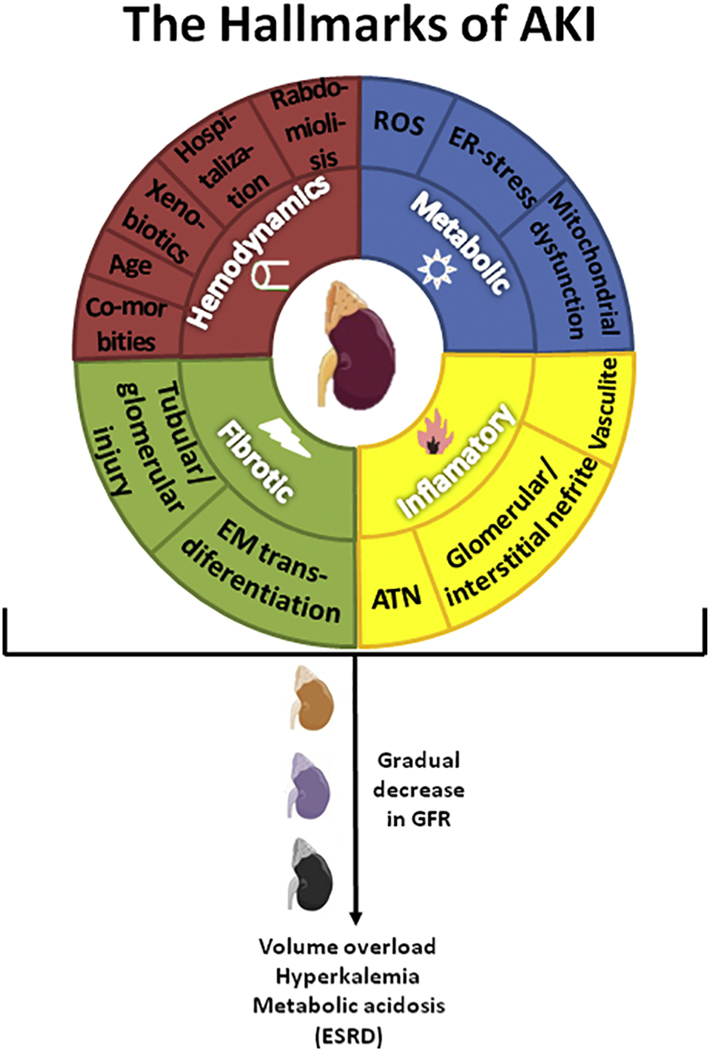
By the action of regulatory and reprogrammed leukocyte subsets the active repair and regeneration phase can occur for several days. This phase is characterized by programmed transitions in the phenotype of immune effector cells (especially mononuclear phagocytes) involving specific alternative intracellular signaling pathways and soluble mediators, such as anti–inflammatory cytokines (e.g., IL-10) (Rabb et al., 2016). This phase has been shown to be essential for optimal recovery of renal function after AKI. Figure 1 shows the hallmarks of AKI, highlighting the main pathophysiological mechanisms.
Figure 1. The hallmarks of acute kidney injury (AKI).
The figure represents a schematic view of the main pathophysiological mechanisms of AKI. The inner circle indicates the four altered processes in the kidney that trigger AKI. The outer circle represents the main events encountered in the clinical practice that alter at least one of the four processes depicted in the inner circle. Independently or together, those alterations will contribute to the gradual decrease in GFR. Late diagnosis and/or non-adequate treatment culminate in ERSD. ROS: reactive oxygen species; ER: endoplasmic reticulum; ATN: acute tubular necrosis; EM: epithelial/mesenchymal; GFR: glomerular filtration rates; ESRD: end stage of renal disease.
The understanding of how these immune factors dictate the course of intrarenal inflammation is a promising pathway toward developing new therapies to attenuate renal structural damage or enhance subsequent repair and regeneration of the kidney. However, it is important to mention that there is a risk to disrupt the natural transition between acute inflammatory phase to the repair phase if the pharmacologic strategy involves inhibiting elements of acute inflammation in an improper time frame.
In most cases of AKI there is complete repair and restoration of normal kidney function. During the recovery phase, several molecular mechanisms counteract by directing the migration, differentiation, and proliferation of renal epithelial cells: (1) M2 macrophage and regulatory T cells (Kumar, 2018), which play a critical role in tissue repair; (2) renal stem cells and the expression of growth hormones and trophic factors; (3) unfolded protein response (UPR) activation – an adaptive response in which the cell attempts to overcome the accumulation of misfolded proteins and ER stress – is activated right after renal ischemia. Studies in mice have shown that pretreatment with ER stress inducers (tunicamycin or thapsigargin) protected tubular cells from renal ischemia-reperfusion injury through enhancement of GRP78 protein expression, and ameliorated renal dysfunction and injury (Prachasilchai et al., 2008); (4) epigenetic regulation involving changes in histone modifications, DNA methylation and the expression of various non-coding RNAs (Guo, Dong, Liang & Dong, 2019).
However, and as discussed above, AKI can elicit permanent changes in kidney function and the development of CKD which can subsequently progress to end-stage renal disease. The maladaptive recovery may lead to a reduction in peritubular capillary density, which is in part a response to decreased VEGF (vascular endothelial growth factor) and increased TGF-β (transforming growth factor beta) signaling, which contributes to ongoing hypoxia, establishes renal fibrosis and, ultimately CKD (Basile, Donohoe, Roethe K & Osborn (2001).
c. Sex-differences in the I/R renal injury physiopathology
Differences in the severity of renal I/R injury among sexes has been demonstrated in experimental models but the role of gender as a risk for AKI in humans is less clear (1) Animal models have consistently shown a protective effect of female sex on the development of AKI after ischemia-reperfusion injury (Hutchens, Fujiyoshi, Komers, Herson & Anderson, 2012; Kang et al., 2014); (2) in humans, female sex is among the shared susceptibility factors that confer a higher risk of AKI, because women are more likely to develop hospital-acquired AKI (cardiac surgery-associated AKI, contrast-induced nephropathy, and aminoglycoside nephropathy) (KDIGO, 2012); (3) Males are more susceptible to develop AKI as result of infections such as HIV, malaria, leptospirosis and other community-acquired forms of AKI (KDIGO, 2012); (4) Other reports describe that males are more susceptible to I/R injury as compared to females (Kher, Meldrum, Wang, Tsai, Pitcher & Meldrum, 2005; Uchino et al., 2009). Hospitalized men have an increased incidence of AKI requiring renal replacement therapy (Neugarten, Golestaneh & Kolhe, 2018; Hellou, Bahouth, Sabo, Abassi & Nativ, 2018). Based on the data available, it seems that the sex differences in AKI resistance are more evident in rodents than in humans.
Given that the mechanistic studies on sexual dimorphism of AKI have predominantly been done on rodents we sought to highlight the most studied molecular mediators of sex differences in I/R injury. These molecular mediators are differently modulated by the levels of the sex hormones, estrogen and testosterone: heat shock protein 27, superoxide dismutase activity, inducible NO synthase (iNOS), endothelial NO synthase (eNOS), phosphorylated extracellular signal-related kinase (ERK), phosphorylated c-jun N-terminal kinase (JNK), and endothelin-1 (Hutchens, Dunlap, Hurn & Jarnberg, 2008; Liu, Meng, Huang, Wang & Liu, 2015; Zhang et al., 2016a). Although more studies are needed to define sex differences in AKI, it has been a consensus that testosterone enhances injury response and renal sympathetic activity. On the contrary, females exhibit a renal sympathetic tolerance in a mechanism mediated by MAO (monoamine oxidase) due to estrogen (Tanaka, Tsutsui, Ohkita, Takaoka, Yukimura & Matsumura, 2013; Tanaka, Yazawa, Morikawa, Tsutsui, Ohkita, Yukimura & Matsumura, 2017).
3. Clinical presentation
Patients with AKI require a careful history, careful interpretation of laboratory tests and imaging, chart review, and a thorough physical examination (Kaushal & Shah, 2014). The clinical symptoms of AKI are dependent on the extent of renal functional damage. During the initial stages the only clinical presentation is the abnormality in renal function as assessed by blood tests (i.e., BUN and Creatinine) and in some cases oliguria. In more severe stages other clinical manifestations may become apparent including nausea, vomiting, bleeding, platelet dysfunction, reduced immune system response, pruritus, dyspnea, edema, hypertension, cardiac insufficiency, pericarditis, pleuritis, pleurisy, tremors, agitation, seizure and coma. These symptoms are consequences of volume overload, hyperkalemia, metabolic acidosis and accumulations of uremic toxins provoked by the reduction renal function (Bouchard et al., 2009; Evans & Greenberg et al., 2005; Bockenkamp & Vyas, 2003). Post-renal AKI can present with low back pain, difficulty with urination and hematuria (Quintavalle, Donnarumma, Fiore, Briguori, & Condorelli, 2013).
The identification of patients presenting with significant risk factors, such as advanced age, diabetes, hypertension, or vascular disease, is essential in the evaluation of patients with suspected AKI. The common diagnostic criteria for AKI are based on guidelines (RIFLE, AKIN and KDIGO) taking into account the rise in sCr in 48 h associated with or without oliguria. Because the rise in sCr may occur when most of the pathophysiologic mechanisms discussed above have been already established, this may make early diagnosis quite difficult (Mcmahon & Koyner, 2016). Urine dipstick and microscopy are useful as they can provide clues on the cause of AKI. The presence of cells, crystals or casts in urine microscopic analyses, mainly red cells, suggests glomerulonephritis (Pickering & Endre, 2014). Kidney histological analysis from rat models of AKI usually detects the presence of red blood cells or protein in the lumen, dysmorphic red cells, red cell casts and eosinophils, suggesting acute interstitial nephritis and alteration in the epithelial cell structure (Cortes et al., 2018; Zhang et al., 2016a). Renal ultrasonography analysis could assist the AKI diagnostic, evaluating the renal size, symmetry and depict any obstruction in the renal system.
4. Characterization of AKI biomarkers
As aforementioned, the sCr concentration is the parameter to assess renal function decay and AKI. However, sCr concentration may not change until approximately 50% of kidney function has been lost (Bennett & Devarajan, 2011). Moreover, the return of sCr to basal levels – reflecting a recovery in renal function – does not necessarily demonstrate a structural recovery and renal integrity (Endre, 2014a). Long term, the impairment of kidney structure may imply a loss of renal function and progression to chronic kidney disease due to a maladaptive repair (Murray et al., 2014). In this context, it is important to evaluate functional and structural markers in a noninvasive way for diagnosing, monitoring, and quantifying kidney damage (Murray et al., 2014). The emerging novel biomarkers specific for kidney damage introduced a new era in Nephrology and became a research priority. The expectations for research on novel biomarkers are related to the improvement of diagnostics, prognostics, decision-making and innovation (Bagshaw, Zappitelli & Chawla, 2013). Different types of molecules have been investigated as biomarkers: lipids, proteins, metabolites and also RNA. The limitations of methodologies discussed below, however, have brought together with these high expectations new challenges, especially on how to optimally interpret the information obtained.
Recently, the “omics” technologies have been used to develop novel biomarkers for kidney diseases, such as functional (1) lipidomic, (2) proteomic and (3) metabolomic analyses. These “omics” biomarkers work as a memory of injury (Liakopoulos, Georgianos, Eleftheriardis & Sarafidis, 2011).
The lipid dysfunction during AKI, especially, in I/R, has shown abnormal lipid accumulation within the kidney (Johnson, Stahl & Zager, 2005; Naito, Bomsztyk & Zager, 2009). For example, in cisplatin-induced AKI the increased levels of ceramide and hexosylceramide in the renal cortex were correlated with the severity of AKI in mice (Dupre et al., 2017). Lipidomic studies have used the (SWATH)-MS (Sequential Window Acquisition of all Theoretical Mass Spectra) to identify real-time lipid changes early following IR-induced kidney injury and correlating with AKI severity (Simons, Kauhanen, Sylvanne, Tarasov, Duchoslav & Ekroos, 2012). MS can be followed by MALDI-IMS (Matrix-Assisted Laser Desorption Ionization Imaging Mass Spectrometry) approach that allows localizing those lipids in the kidney. The use of both techniques allowed the detection of increased phosphatidylcholine (PC) O-38:1 (O-18:0, 20:1) and phosphatidylethanolamine (PE) O-42:3 (O-20:1, 22:2), two abundant ether-linked phospholipids following IR at 6 h post injury. Both of these lipids correlated with the severity of AKI as measured by sCr. Moreover, PC O-38:1 level in the kidney was maintained high at 24 h post-IR, while renal PE O-42:3 levels were decreased. The increase in the PC O-38:1 level occurred in proximal tubules, rich in the rate-limiting enzymes involved in ether-linked phospholipid biosynthesis. The S3 segment of the proximal tubule is the most prone to IR injury (Rao et al., 2016). The goal of proteomics is to identify alterations in specific proteins for renal disease. This technique can be applied in urine, plasma and serum. Proteomic approaches such as 2DE (2 Dimensional Gel Electrophoresis) and MALDI techniques are used to identify larger proteins, and SELDI (Surface-Enhanced Laser Desorption/Ionization) and LC/MS (Liquid Chromatography–Mass Spectrometry) to identify smaller proteins and peptides (Bennett & Devarajan, 2011).
Proteomics revealed interleukin-18, neutrophil gelatinase-associated lipocalin (NGAL, also known as lipocalin 2 or lcn2), kidney injury molecule-1 (KIM-1) and L-type fatty acid binding protein (L-FABP) as important biomarkers of clinical conditions of AKI such as renal ischemia or xenobiotic exposure (Siew et al., 2010; Mussap, Noto, Fanos & Van Den Anker, 2014; Merchant, Brier, Slaugter, Klein & McLeish, 2018). NGAL is an extensively studied novel biomarker of AKI. The protein and mRNA level are augmented after ischemic or toxic kidney injury in human neonates, children and adults and well established in animal models (Mishra et al. 2003; Mishra, Mori, Ma, Kelly, Barasch & Devarajan, 2004; Supavekin, Zhang, Kucherlapati, Kaskel, Moore & Devarajan 2003). NGAL is filtered by the glomerulus and reabsorbed by the proximal tubules (Hvidberg, Jacobsen, Strong, Cowland, Moestrup & Borregaard 2005). The reduction of tubular reabsorption after AKI leads to an augmentation in NGAL urine level. Because it is rapidly expressed during kidney cell stress and/or damage, NGAL is used during admission in the emergency room to predict death, dialysis and AKI risk after cardiac surgery in adult patients (Nickolas et al. 2012; Ho et al. 2015). Patients with NGAL > 104 ng/ml have a 5% probability of death or dialysis during hospitalization. Comparing with sCr, NGAL can rise days before sCr elevation in sepsis condition (Parravicini et al. 2010). According to the criteria proposed by Schmidt-Ott and coworkers (2011), NGAL is more sensitive than sCr to predict AKI, as shown in the nomenclature: NGAL− sCr− (normal), NGAL+ sCr− (damage to <50% of renal mass or early detection of severe disease), NGAL+ sCr+ (damage to >50% of renal mass) and finally NGAL− sCr+ (no renal stress or damage, but functional impairment consistent with pre-renal azotaemia).
KIM-1 and L-FABP can be increased in the urine before elevated sCr levels are detectable (Siew et al., 2010; Parikh et al., 2011; Murray et al., 2014). KIM-1 biomarker is mostly used to detect nephrotoxicity in pre-clinical and early phase I and II clinical studies (Vaidya et al. 2010). The cell cycle inhibitors, TIMP-2 (tissue inhibitor of metalloproteinases-2) and insulin-like growth factor-binding protein (IGF7BP7), could be new biomarkers that present high sensitivity to renal insults compared to other biomarkers of a single mechanism of injury (Endre, 2014b). Recently, Adler et al. (2018) postulated that the ratio of levels of TIMP-2 and IGFBP7 could be used as a biomarker to predict AKI in patients with non-traumatic shock. The increase of [TIMP-2]/[IGFBP7] in urine indicates a high risk of AKI among patients with shock after out-of-hospital cardiac arrest (OHCA), at least 3 hours after the episode.
Metabolomics is an extension of current methods of proteomic analysis. The actual definition is “a global holistic overview of the personal metabolic status.” The aim of metabolomics is to identify and quantify the molecular compounds (proteins and metabolites) found in biological fluids during physiological and physiopathological conditions (Mussap, Noto, Fanos & Van Den Anker, 2014; Marx et al., 2018). The techniques applied are nuclear magnetic resonance (NMR) or MS. The urine of pediatric cardiopulmonary bypass surgery (CPB) patients showed increased levels of homovanillic acid sulfate, a metabolite of dopamine, as a biomarker of AKI after the surgery. This metabolite was upregulated in AKI patients in the early 4 h post-surgery (Beger et al., 2008; Bennet & Devarajan, 2011).
Urine IGFBP-7, TIMP-2 and NGAL are examples of biomarkers that show an inverse relationship with the functional recovery of AKI (Endre, 2014b; Parikh, Moledina, Coca, Thiessen-Philbrook & Garg, 2016). This indicates that these biomarkers are also valuable for monitoring the progression of the disease not only for early diagnosis. Although the advances in “omics” biomarkers in the past few years have improved the early diagnosis of AKI, this novel technology still has many limitations such as the small number of subjects from a single center study; the absence of accuracy and sensitivity of the biomarkers in AKI diagnosis and the absence of studies in AKI that frequently develop chronic kidney disease in clinical practice, which may interfere in the result (Parikh, Moledina, Coca, Thiessen-Philbrook & Garg, 2016; Marx et al., 2018). So far, “omics” biomarkers represent a future prognostic in the clinical diagnosis of AKI, however, more detailed investigations are required.
MicroRNAs (miRNAs) are another type of molecule that could be used as biomarker for AKI. miRNAs are evolutionarily conserved non-coding RNAs formed from 21–25 nucleotides in size and regulate gene expression by binding to the 3′-untranslated region of target mRNAs. The interest in these molecules is mostly because they are very stable in body fluids (blood or urine) when binding to vesicles or proteins. In the kidney, miRNAs have important roles during kidney development, homeostasis and disease. Rudnicki and coworkers observed that miRNA-30d, −140–3p, −532–3p, −194, −190, −204 and −206 were down-regulated in progressive cases of CKD and inversely correlated with 29 up-regulated target mRNAs. These were involved in inflammatory response, cell-cell interaction, apoptosis and intracellular signaling (Rudnicki et al., 2016)
The first evidence of miRNAs having pathological roles in AKI was reported by Wei and coworkers who developed a Dicer-knockout mouse model, in which Dicer (a key enzyme for microRNA synthesis) was specifically deleted from proximal tubular cells. Thus, these mice exhibit a global down-regulation of miRNAs in the renal cortex. They have normal renal function and histology under control conditions but show resistance to the AKI that follows bilateral renal I/R. Under the latter conditions, Dicer-null mice show significantly better renal function, less tissue damage, less tubular apoptosis, and better survival than their wild-type counterparts (Wei, Bhatt, He, Mi, Haase & Dong 2013). Since then, many miRNAs have been implicated in AKI; for a more detailed revision please see Fan, Chen, Chen, Chang & Chu (2016). Some of them contribute to pathogenesis by regulating apoptosis and inflammation, to amplifying or reducing acute injury responses, while others regulate fibrosis and angiogenesis, to participate in renal recovery or the progression to fibrosis. Currently, it is unclear whether these circulating miRNAs are tissue- and disease-specific or represent more general pathologies like inflammation.
Vanmassenhove, Vanholder, Nagler & Biesen (2013) collected the data available in the literature about the diagnostic performance of the different biomarkers and reported that no consistent generalizable conclusions can be drawn on their diagnostic value or that may impact in the correct interpretation of the clinical trials. This may appear a pessimistic view, but it is important to discuss the factors that may lead to a misinterpretation of the results in order to overcome the limitations. The wide range of data on urinary or serum biomarkers can be associated with different explanations: (1) the choice of the study design is widely different, where timing and amount of renal impact can be known (as in post-cardiac bypass) or unknown (as in septic shock) affecting diagnostic performances; (2) most of the biomarkers are not only associated strictly with AKI; (3) statistical pitfalls when translating the clinical trials to the bedside; (4) because of the search for increased levels of biomarkers without an accompanying rise in sCr to characterize early AKI may impact on the lack of specificity to the detriment of increasing sensitivity. Altogether, biomarker development is a long-term investment to be included in the existing consensus definition of AKI (RIFLE, AKIN or KDIGO) but it should be embraced as critical for a successful AKI therapy.
5. What is the available pharmacological arsenal to treat AKI?
Because AKI treatment is not simple, prevention is always critical. Clinical cases with high risk for AKI development (for example, kidney injury provoked by the use of xenobiotics) should be predicted, monitored and prevention should be employed (Zhang et al., 2016b). Preventive actions to avoid AKI are critical for clinical practice because supportive treatment is the mainstay of therapy with few clinically applicable options (Duann, Lianos, Ma & Lin, 2016). Because AKI is a multifactorial disease and it can be associated with co-morbidities, there is no pharmacological approach that reverses the renal injury once established. Patients with AKI may develop hyperkalemia, metabolic acidosis, volume overload, and/or symptoms of uremia due to reduced GFR. Most of the pharmacological treatment is related to attenuation of the symptoms resulting from these alterations.
Patients at an emerging risk for AKI and those with an already established AKI diagnosis should have kidney function monitored closely by sCr concentration and urine output. Careful assessment of volume status and hemodynamics should be undertaken and treatment done with intravenous fluids, diuretics, or other means of hemodynamic support as indicated in Table 1. The eventual use of renal replacement therapy (RRT) is required in most of cases.
Table 1.
Mechanism of action and physiological implications on the pharmacological therapy used to treat AKI.
| Drug | Mechanism | Implications | Reference |
|---|---|---|---|
|
| |||
| Mannitol | Osmotic diuretic (proximal tubule and loop of Henle), extracting the water from the intracellular compartments. | Increases renal blood flow; protector in renal transplantation; | Bragadottir et al. (2012) |
| Furosemide | Inhibitory loop diuretic of simporte Na+−K+−2CI− (thick ascending limb - loop of Henle) | Increases renal blood flow and excretion of Na+ and Cl− | Andreucci et al. (2017) |
| Atrial Natriuretic Peptide | Nonspecific cation channel inhibitor, preventing about 5% of the Na+ reabsorption (collector duct) | Vasodilation of afferent arteriole and vasoconstriction of efferent arteriole (increased glomerular filtration rate) | Mitaka et al. (2017) |
| Dopamine | Paracrine/autocrine agent in the kidneys acts on the receptors dopamine 1 (D1) and dopamine 2 (D2) | In the dose of 1 μg/kg/min, increases renal blood flow and glomerular filtration rate | Joannidis et al. (2017) |
| Fenoldopam | Short-acting selective agonist of dopaminergic Dl receptor | Decreases peripheral vascular resistance, increases renal blood flow | Gillies et al. (2015) |
| Ca2+ Channel inhibitors (verapamil, diltiazem) | Inhibition of T and L-type Ca2+ channels (afferent arteriole) | Increases renal function; renal blood flow, glomerular filtration rate, diuresis and excretion of electrolytes | Quintavalle et al. (2013) |
| RAS inhibitor | Angiotensin converting enzyme inhibitor (iACE) or angiotensin II type 1a receptor blocker (ARB) | Increases renal blood flow, glomerular filtration rate, diuresis. Increased survival time and diminished CKD progression. | Chou et al. (2017) |
The management of the established AKI involves two main strategies: optimizing renal perfusion and modulation of intrarenal pathophysiological mechanisms of AKI. This last strategy is still an open field in Nephrology and is under clinical trials investigation. Improvement of impaired renal perfusion can be achieved by volume expansion. Patients with reduced renal blood flow that can augment their cardiac output by expansion of their intravascular volume would benefit from fluid resuscitation. Major trials of various colloids, physiologic-balanced salt solutions, and saline solution have been used. The use of multiple clinical assessments and repeated measures to assess fluid responsiveness are recommended. Intravenous fluids should be used judiciously in patients with AKI who are not “volume responsive.” After significant volume resuscitation, even if patients remain volume responsive, vasopressor support should be considered to avoid markedly positive fluid balance (Moore, Hsu & Liu, 2018). Several molecules can affect vascular resistance in AKI, such as the decrease in nitric oxide (NO) levels and augmented levels of angiotensin II (Ang II) and endothelin. Appropriate inotropes and/or vasodilators are used to control blood pressure. Losartan (Ang II receptor antagonist) and sodium nitroprusside (NO donor) have been shown to improve renal function after I/R injury. This suggests that changes in renal hemodynamics are partly due to increased Ang II-mediated vasoconstriction and absence of NO activity and the balance of these processes may be useful in preserving renal function in humans (Srisawat, Kongrat, Muanprasat & Chatsudthipong, 2015).
Some differences could be encountered in various countries but in general, the strategy is very similar. After the diagnosis of AKI, it is essential to determine the causes of AKI in order to identify appropriate strategies for minimizing the severity of the injury. Special attention is paid to treatable causes such as hypovolemia and avoidance of nephrotoxins.
The lack of pharmacological options clinically applicable is due to the influence of related organs (lung, heart, liver), comorbidity factors, the complexity of AKI etiology, low statistical power and the lack of consensus for AKI definition in clinical trials (Jo, Rosner & Okusa, 2007). The identification of a single therapy that will benefit all is challenging (Moore, Hsu & Liu 2018).
6. New strategies of AKI treatment and prevention
As shown above, there is no effective pharmacological intervention to prevent or reverse I/R injury. To accelerate the drug development process, reducing the risk of failure and lowering costs, the drug repositioning strategy has been adopted. This means new uses for existing drugs with a well-known mechanism of action associated with low side effects (Ashburn & Thor, 2004). Doxycycline is a tetracycline that has several pharmacological properties in addition to the antimicrobial effect, especially the inhibition of MMPs (mentioned in the topic above). It has been demonstrated that doxycycline prevents oxidative stress provoked by I/R associated with the inhibition of MMPs, leading to the reduction of pro-inflammatory cytokines and the occurrence of apoptosis (Ihtiyar, Yasar, Erkasap, Koken, Tosun, Oner & Erkasap, 2011). In addition, the prevention of MMP activation has been suggested to be a possible mechanism of protection in renal transplantation (Li et al., 2015). By the use of an animal model of AKI (renal ischemia-reperfusion), we provide evidence that intraperitoneal administration of low-dose doxycycline protects kidney function (glomerular filtration and epithelial Na+ transport), beyond its antimicrobial effects (Cortes et al., 2018).
Another emerging pharmacological tool is related to the modulation of oxidative stress. Mitochondria are especially affected in I/R, leading to the production of reactive oxygen species (ROS), which alters all cellular machinery and, especially, renal vascular tone (Majid & Nishiyama, 2002; Silachev et al., 2014). I/R causes depletion of glutathione, the main molecule that participates in defense against oxidative damage and increasing levels of malondialdehyde, indicating the presence of lipid peroxidation (Kucuk, Kabadere, Tosun, Koken, Kinaci, Isikll & Erkasap, 2009; Ihtiyar, Yasar, Erkasap, Koken, Tosun, Oner & Erkasap, 2011). The mitochondrial 10-(6’-plastoquinonyl) decylrhodamine 19 (SkQR1) antioxyporter showed nephroprotective effects, prevention of renal vascular dysfunction and protection of endothelial cell damage (Plotnikov, Chupyrkina, Jankuskas, Pevzner, Silachev, Skulachev & Zorov, 2011; Jankauskas et al., 2012; Jankauskas et al., 2016). During reperfusion, an excessive elevation of free radicals occurs due to the excess of oxygen released in the arterial blood, which may cause diverse tissue lesions (Collard & Gelman, 2001). A study suggests the prevention of oxidative damage caused by I/R through reperfusion with venous blood, which contains less oxygen, revealing an innovative and useful approach to initiate tissue reperfusion (Cetin et al., 2014).
Ischemic preconditioning, in general, involves exposing an organ to sub-lethal ischemia to protect it against subsequent ischemic injury (Zager, Baltes, Sharma & Jurkowitz, 1984). Kidney ischemic pre-conditioning (IPC) is a method that applies intermittent clamping to the renal hilum (or renal artery) resulting in multiple short cycles of ischemia and then reperfusion. It is postulated that the tissue will upregulate protective mechanisms before the main ischemic insult, inducing tolerance or adaptability of the organ (Dirnagl, Simon & Hallenbeck, 2003; Er et al, 2012). Chemical preconditioning methods with the hypoxia mimetic cobalt chloride or zinc chloride in Sprague-Dawley rats has been shown to protect the kidney from increasing hypoxia-inducible factor (HIF1α and 2α) (Rao et al., 2017).
Remote ischemic preconditioning (rIPC) is a novel non-invasive and virtually cost-free strategy to decrease I/R injury. This technique is similar to IPC, but the brief episodes of I/R are applied at a remote site, where modulation of blood flow occurs in a distant anatomical region. For example, rIPC applied before contrast medium use prevents contrast medium-induced acute kidney injury in high-risk patients (Er et al., 2012). Clinical evidence evaluated in nine healthy volunteers suggests that rIPC is a potential renoprotective strategy, due to the release of vasodilatory substances (Robert, Vinet, Jamet & Coudroy, 2017). These conditioning processes activate protective mechanisms, involving mediators such as opioids, adenosine and bradykinin, which result in decreased mitochondrial permeability, reactive oxygen species and inflammatory response (Crowley & McIntyre, 2013).
When a series of alternating intervals of brief ischemia and reperfusion is applied after a prolonged I/R episode, ischemic post-conditioning (POC) is established. Animal models of IPC and POC have been performed to elucidate the beneficial effects of these techniques to prevent I/R injury as shown below. Shokeir and collaborators carried out a study involving protocols of IPC and POC in Sprague-Dawley rats, showing that the former caused an improvement in renal function, while the latter did not (Shokeir, Hussein, Barakat, Abdelaziz, Elgarba & Awadalla, 2014). This protection was related to the activation of the nuclear transcription factor erythroid 2-related factor 2 (Nrf2), a regulator of antioxidant enzyme genes (Zhang, Xiao, Yao, Zhao, Fa & Niu, 2013). Both IPC and POC showed a reduction in the expression of the pro-apoptotic caspase-3 protein. The performance of IPC without ischemia confirmed that the expression of these markers occurs immediately after IPC and not after I/R in the long term, as in POC. The improvement in renal function with the classic and remote POC was associated with reducing levels of oxidative stress markers (Jiang, Chen, Xue, Zhu & Sun, 2015). Not all combinations or durations of I/R and conditioning procedures protect against ischemic injury due to the nature of the adaptive mechanism induced by the adopted protocol (Shokeir, Hussein, Barakat, Abdelaziz, Elgarba & Awadalla, 2014). Therefore, surgical protocols should be adjusted for renal protection.
Kidney transplant is a surgical approach available when renal replacement therapy is not totally efficient at blocking the intensity or progression of AKI, being the only option to extend the patient’s life. The number of kidney transplants per donation after circulatory death has greatly increased in the last decade worldwide, even so, it is still relatively few compared to the number of people awaiting transplant. During the transplant, the kidney undergoes a period of hot ischemia and after transplantation, to reperfusion, when the lesion could be accentuated, and irreversible damage could provoke delayed graft function with a risk of kidney failure (Saat, Van den Akker, Jn, Dor & De Bruin, 2016). The small size of clinical trials and varied definitions of delayed graft function limited the determination of baseline parameters for an optimal kidney transplant (Zhou et al., 2017).
Finally, cell-based therapy is a promising avenue for preventive or curative AKI therapies because it is possible to use the cells from the patient (autologous treatment). This therapy has been employed in many trials of kidney transplantation. Despite the use of Mesenchymal Stem cells (MSCs), Hematopoietic Stem Cells (HSCs) or regulatory T cells, they present a common characteristic to induce immuno-tolerance towards allogeneic cells/antigens in the graft (Dellepiane, Medica, Quercia & Cantaluppi, 2017). However, Patschan and collaborators (Patschan, Buschmann, Ritter & Kribben, 2018) pointed out that for an efficient cell-based AKI therapy, two limitations may arise: the heterogeneity of causes and the highly complex structure of the kidney. The authors also considered that a single population of injected cells may hardly fight on equal terms the multitude of cellular and humoral processes involved.
Barnes and collaborators (Barnes, Distaso, Spitz, Verdun & Haramati, 2016) revised three different types of stem cell therapy: MSCs, induced pluripotent stem cells (iPSCs) and spermatogonial stem cells (SSCs) in order to show the most promising therapy for reparative and regenerative treatment for AKI. MSCs therapies have long been investigated in the preclinical setting and have recently been successful in Phase I clinical trials (Kaushal & Shah, 2014). The beneficial outcomes include anti-inflammatory, mitogenic, anti-apoptotic, and pro-angiogenic effects with no detrimental side effects (Kaushal & Shah, 2014). MSCs can be cultivated from a variety of sources as bone marrow and adipose tissue and further research is needed to determine which type of MSCs are most beneficial for AKI and other forms of acute organ injury. Regarding iPSCs, its use is relatively non-invasive and viable options for most individuals, however, the major concern is the real possibility of teratoma formation. SSCs, an invasive procedure of collection, have shown very limited teratoma formation and do not require the reprogramming factors used in iPSCs to reach a pluripotent state (Barnes, Distaso, Spitz, Verdun & Haramati, 2016). The ability of SSCs to differentiate into renal lineages has been demonstrated, but there are no studies addressing the therapeutic effects of human-derived SSCs for AKI. Because of the insufficient amount of research available, it is not possible to compare the renoprotective effect of iPSCs or SSCs for AKI treatment (Barnes, Distaso, Spitz, Verdun & Haramati, 2016). It seems that so far, the best stem cell-based treatment for AKI is MSC-derived therapy. Because AKI is a silent disease and there are as yet no trusted molecular markers to ensure the exact timing of cell administration, any direct application of cells in daily clinical practice will most likely not become a suitable option (Patschan, Buschmann, Ritter & Kribben, 2018). However, it can be applied in high AKI risk patients such as those post-cardiac surgery, those undergoing chemotherapy with tubular-toxic drugs such as cisplatin, and those administered contrast media. In the case of non-autologous transplant, although it can be an alternative, the compatibility between cells and the recipient must be carefully evaluated.
Ratajczak and collaborators (Ratajczak, Miekus, Kucia, Zhang, Reca, Dvorak & Ratajczak, 2006) first demonstrated that embryonic stem cells may release extracellular vesicles (EVs) capable of reprogramming hematopoietic progenitors. EVs are mainly composed of exosomes, small homogeneous vesicles (50 – 150 nm) formed from the endosomal cell compartment, and by microvesicles, with a more heterogeneous dimension profile, produced by the direct extrusion of the cell plasma membrane. The benefits of the use of EVs derived from adult stem/progenitor cells have been related to the features resembling their cells of origin. Selective macromolecules such as proteins, RNAs and small non-coding RNAs (miRNAs) are compartmentalized inside EVs and may be transferred to target cells via EVs. In particular, EVs derived from mesenchymal stem cells (MSCs) can mediate the paracrine effects of MSCs in different models of tissue regeneration (Bruno et al., 2009; Nakamura et al., 2015). Collino et al. (2017) showed that the proteins and miRNAs shuttled by MSCs provided a collaborative pattern in crucial processes activated after injury in vitro, such as metabolic, stem cell, inflammatory/migration and angiogenic related processes. The contents of EVs could change if the MSCs are cultured in adverse conditions, such as hypoxia mimicking I/R injury (Lindoso et al., 2014).
On the other side of the coin, the EVs released by the organ may also contribute to the development and propagation of inflammatory, vascular, malignant, infectious and degenerative diseases. In this view, EVs might contribute to disease processes in the kidney: such as in AKI and allograft loss and could be considered as biomarkers of renal disease (Karpman, Ståhl & Arvidsson et al., 2017). New pharmacological approaches are also being developed to block the release or uptake of EVs or yet, to use EVs as a new delivery system to specific cells instead of MSC-derived therapy.
7. Is the bioartificial kidney applicable to treat AKI?
Since the advent of peritoneal dialysis (PD) in the 1970s, some advantages were brought to patients, such as decreased “dialysis hangover,” fewer dietary restrictions, direct shipment of PD supplies to the patient’s home and greater flexibility of PD schedule. However, the principles of dialysis have changed very little. The Kidney Project (University of California, San Francisco) aims to develop a wearable and implantable, free-standing artificial kidney to perform the vast majority of the filtration, balancing, and other biological functions of the natural kidney. It is powered by the body’s own blood pressure, without the need for external tubes and tethers or immunosuppressant drugs (Salani, Roy & Fissel, 2018). The optimistic view is that the artificial kidney will give to the renal patient new hope beyond the short-term solution of renal dialysis and the longer-term solution of a living kidney transplant for which donor organs are limited. However, the artificial kidney does not yet fulfill the renal endocrine function.
In general, the big barrier of dialysis is the accumulation of protein-bound toxins that are strongly associated with high morbidity and mortality (Krieter et al., 2010). The bioartificial kidney (BAK) aims at mimicking the functional kidney by making use of proximal tubule epithelial cells (PTECs) equipped with a broad range of transporters that normally mediate the excretion of metabolites (Masereeuw, Mutsaers, Toyohara, Abe, Jhawar, Sweet & Lowenstein, 2014). Chevtchik et al. (2018) reported the use of a conditionally immortalized human PTEC with functional organic cationic transporter (promoting the removing of anionic uremic wastes). Immunosafety was ensured because of the polarized secretion of proinflammatory cytokines to the extraluminal space, not to the interstitium. The biohybrid combining artificial filters and living cells is the edge of innovation. The expectation is that this technique may supply the endocrine function of the kidney (for example the release of erythropoietin and renin). Portable devices reduce the need for large quantities of water and continuous electrical supply, making the device more accessible and reducing health care costs associated with dialysis. The addition of the BAK containing human cells has been shown in preclinical and clinical studies to have the potential to advance AKI treatment as a conventional continuous renal replacement therapy (for a review, see Song & Humes, 2009). The BAK demonstrates metabolic activity with systemic effects, influences systemic leukocyte activation and the balance of inflammatory cytokines. Clinical studies have shown that there is a significant clinical impact on survival with an acceptable safety profile, suggesting that cell therapy combined with the use of the BAK may decrease morbidity and mortality by altering the proinflammatory state of patients with renal failure.
8. Potential limitations of the current studies in the AKI field: why we still face problems with AKI?
In order to understand why we still face problems with AKI, we pointed out the potential limitations encountered in current studies in the AKI field.
AKI is a multifactorial disease that is difficult to represent in a single animal experimental model.
The inaccuracy of diagnosis and absence of reliable early biomarkers for the definition of AKI, AKD and CKD.
In low-income countries, AKI is associated with neglected diseases impacting its epidemiology. Because they are neglected, the lack of resources of these countries in science and technology aggravates the problem.
Successful pharmacological therapies in preclinical studies have not advanced in clinical studies. The main reasons for the failure of drug trials include the heterogeneity of AKI and underpowered studies (Jo, Rosner & Okusa, 2007; Star, 1998). In 2012, using the clinicaltrials.gov electronic database, it was reported that by November 2011, 126 clinical trials regarding the prevention or management of AKI were identified; 118 trials are being performed in adults, and 8 trials are being conducted in children (Faubel, Chawla, Chertow, Goldstein, Jaber & Liu, 2012). Most of the trials (65%) were prevention trials; adequate power, sample size and endpoint to the prevention of AKI were the limitation of these studies. Of the 23 trials addressing general management of AKI, a key element of drug development studies was to establish the therapeutic window for each agent, which depends on good animal models in preclinical studies. The AKI Advisory group proposed that to move straightforward is necessary to the development of a United States-based clinical trials network focused on AKI (Faubel, Chawla, Chertow, Goldstein, Jaber & Liu, 2012). We believe that this model can be extended and applied to any country.
9. Conclusion
A better understanding of the molecular mechanisms and functional defects of AKI is required for an effective treatment. Although distinct research and molecular findings on AKI have been made, the mortality and morbidity of patients with this clinical condition are still high. New management and techniques are urgently needed to improve care with AKI, as well an important health strategy with a focus on prevention. For example, the use of nephrotoxic agents should be performed with caution. New biomarkers are emerging in the early detection of AKI in order to prevent disease progression and increases of health care costs. However, more robust clinical trials with greater statistical power need to be performed. The use of improved surgical conditioning techniques or drug disease management would be feasible for use in humans, avoiding loss of renal function or loss of the transplanted organ. Moreover, MSCs, EVs and the development of a bioartificial kidney offers innovative alternatives with promising results for the treatment of AKI.
Acknowledgments
The authors would like to thank Dr. Edgar A. Jaimes, MD, Nephrologist, for constructive criticism of the manuscript. We also thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for providing fellowships to the graduate students (S.R.G., A.L.C. and R.C.S.) of the Post-Graduation Program in Pharmacology and Medicinal Chemistry (ICB-UFRJ) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for providing Productive fellowship level 2 to L.S.L and J.L. This work was funded by: the Carlos Chagas Filho Rio de Janeiro State Research Foundation (FAPERJ) grant E-26/171.137/2006 and E- 26/111.665/2008 (L.S.L.); the Science Without Borders grant from CNPq-Brazil, Especial Visiting Professor 420584/2013-7 (L.S.L.); and the National Institutes of Health (NIH) though the DK104375, U54 GM104940 and UL1TR001417 grants (M.C.P).
Abbreviations
- AKD
acute kidney disease
- AKI
acute kidney injury
- Ang II
angiotensin II
- ATN
acute tubular necrosis
- ATP
adenosine triphosphate
- BAK
bioartificial kidney
- CBP
pediatrics cardiopulmonary bypass
- CKDu
chronic kidney disease of unknown etiology
- EM
epithelial/mesenchymal
- ER
endoplasmic reticulum
- ESRD
end stage of renal disease
- EVs
extracellular vesicles
- GFR
glomerular filtration rates
- HSCs
hematopoietic stem cells
- IGF7BP7
insulin-like growth factor-binding protein
- IPC
ischemic pre-conditioning
- iPSCs
induced pluripotent stem cells
- IRI
ischemia-reperfusion injury
- KIM-1
kidney injury molecule-1
- L-FABPL
type fatty acid binding protein
- lcn2
lipocalin 2
- LC/MS
liquid chromatography-mass spectrometry
- L-FABPL
type fatty acid binding protein
- LOS
length of stay
- MALDI
matrix-assisted laser desorption ionization imaging mass spectrometry
- MAO
monoamine oxidase
- MMPs
matrix metalloproteinases
- MS
mass spectrometry
- MSCs
mesenchymal stem cells
- NGAL
neutrophil gelatinase-associated lipocalin
- NMR
nuclear magnetic resonance
- NO
nitric oxide
- OHCA
out-of-hospital cardiac arrest
- PD
peritoneal dialysis
- pmp
per million population
- POC
ischemic post-conditioning
- PTECs
proximal tubule epithelial cells
- rIPC
remote ischemic preconditioning
- ROS
reactive oxygen species
- RRT
renal replacement therapy
- sCR
increase in serum creatinine
- SELDI
surface-enhanced laser desorption/ionization
- SSCs
spermatogonial stem cells
- TIMP2
tissue inhibitor of metalloproteinases 2
- TGFβ
transforming growth factor beta
- VEGF
vascular endothelium growth factor
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie J. (1821). Observations on ischuria renalis. Edinburg Medical and Surgical Journal 17, 210–222. [PMC free article] [PubMed] [Google Scholar]
- Adler C, Heller T, Schregel F, Hagmann H, Hellmich M, Adler J. & Reuter H. (2018). TIMP-2/IGFBP7 predicts acute kidney injury in out-of-hospital cardiac arrest survivors. Critical Care 22, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksu U, Demirci C. & Ince C. (2011). The pathogenesis of acute kidney injury and the toxic triangle of oxygen, reactive oxygen species and nitric oxide. Contributions to Nephrology 174, 119–128. [DOI] [PubMed] [Google Scholar]
- Ashburn TT & Thor KB (2004). Drug repositioning: identifying and developing new uses for existing drugs. Nature Reviews Drug Discovery 3, 673–683. [DOI] [PubMed] [Google Scholar]
- Bagshaw SM, Zappitelli M. & Chawla LS (2013) Novel biomarkers of AKI: the challenges of progress ‘Amid the noise and the haste’. Nephrology Dialysis Transplantation 28, 235–238. [DOI] [PubMed] [Google Scholar]
- Barnes CJ, Distaso CT, Spitz KM, Verdun VA & Haramati A. (2016). Comparison of stem cell therapies for acute kidney injury. American Journal of Stem Cells 5, 1–10. [PMC free article] [PubMed] [Google Scholar]
- Basile DP, Donohoe D, Roethe K. & Osborn JL. (2001). Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. The American Journal of Physiolgy Renal Physiology 281, F887–F899. [DOI] [PubMed] [Google Scholar]
- Basile DP, Anderson MD & Sutton TA (2012). Pathophysiology of acute kidney injury. Comprehensive Physiology 2, 1303–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beger RD, Holland RD, Sun J, Schnackenberg LK, Moore PC, Dent CL, Devarajan P, & Portilla D. (2008). Metabonomics of acute kidney injury in children after cardiac surgery. Pediatric Nephrology 23, 977–984. [DOI] [PubMed] [Google Scholar]
- Bennett MR & Devarajan P. (2011). Proteomic analysis of acute kidney injury: biomarkers to mechanisms. Proteomics Clinical Applications 5, 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blantz RC. (1998). Pathophysiology of pre-renal azotemia. Kidney International 53, 512–523. [DOI] [PubMed] [Google Scholar]
- Bockenkamp B & Vyas H. (2003). Understanding and managing acute fluid and electrolyte disturbances. Current Paediatrics 13, 520–528. [Google Scholar]
- Bonventre JV & Yang L. (2011). Cellular pathophysiology of ischemic acute kidney injury. Journal of Clinical Investigation 121, 4210–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard J, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP & Mehta RL Program to Improve Care in Acute Renal Disease (PICARD) Study Group. (2009). Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney International 76, 422–427. [DOI] [PubMed] [Google Scholar]
- Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati B, Tetta C. & Camussi G. (2009). Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. Journal of American Society Nephrology 20, 1053–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdmann EA & Jha V. (2017). Acute kidney injury due to tropical infectious diseases and animal venoms: a tale of 2 continents. Kidney International 91, 1033–1046. [DOI] [PubMed] [Google Scholar]
- Caskey FJ & Jager KJ (2014). A population approach to renal replacement therapy epidemiology: lessons from the EVEREST study. Nephrology Dialysis Transplantion 29, 1494–1499. [DOI] [PubMed] [Google Scholar]
- Cetin N, Suleyman H, Sener E, Demirci E, Gundogdu C. & Akcay F. (2014). The prevention of ischemia/reperfusion induced oxidative damage by venous blood in rabbit kidneys monitored with biochemical, histopatological and immunohistochemical analysis. Journal of Physiology and Pharmacology 65, 383–392. [PubMed] [Google Scholar]
- Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM … Acute Disease Quality Initiative Workgroup 16. (2017). Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nature Reviews Nephrology 13, 241–257. [DOI] [PubMed] [Google Scholar]
- Chou YH, Huang TM, Pan SY, Chang CH, Wu VC, Wu MS, …, Lin SL (2017). Renin-angiotensin system inhibitor is associated with lower risk of ensuing chronic kidney disease after functional recovery from acute kidney injury. Scientific Reports 7, 46518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou YH, Chu TS & Lin SL (2018). Role of renin-angiotensin system in acute kidney injury-chronic kidney disease transition. Nephrology 23, 121–125. [DOI] [PubMed] [Google Scholar]
- Chevtchik NV, Mihajlovic M, Fedecostante M, Bolhuis-Versteeg L, Sastre Toraño J. … Stamatialis D. (2018). A bioartificial kidney device with polarized secretion of immune modulators. Journal of Tissue Engineering Regenerative Medicine 12, 1670–1678. [DOI] [PubMed] [Google Scholar]
- Collard CD & Gelman S. (2001). Pathophysiology, clinical manifestations, and prevention of ischemia-reperfusion injury. Anesthesiology 94, 1133–1138. [DOI] [PubMed] [Google Scholar]
- Collino F, Pomatto M, Bruno S, Lindoso RS, Tapparo M, Sicheng W. … Camussi G. (2017). Exosome and Microvesicle-Enriched Fractions Isolated from Mesenchymal Stem Cells by Gradient Separation Showed Different Molecular Signatures and Functions on Renal Tubular Epithelial Cells. Stem Cell Reviews and Reports 13, 226–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collister D, Pannu N, Ye F, James M, Hemmelgarn B, Chui B, … Klarenbach S. (2017). Health care costs associated with AKI. Clinical Journal of American Society of Nephrology 12, 1733–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes AL, Gonsalez SR, Rioja LS, Oliveira SSC, Santos ALS, Prieto MC … Lara LS (2018). Protective outcomes of low-dose doxycycline on renal function of Wistar rats subjected to acute ischemia/reperfusion injury. Biochimica et Biophysica Acta - Molecular Basis of Disease 1864, 102–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley LE & McIntyre CW (2013). Remote ischaemic conditioning-therapeutic opportunities in renal medicine. Nature Reviews in Nephrology 9, 739–746. [DOI] [PubMed] [Google Scholar]
- Dellepiane S, Medica D, Quercia AD& Cantaluppi V. (2017). The exciting “bench to bedside” journey of cell therapies for acute kidney injury and renal transplantation. Journal of Nephrology 30, 319–336. Erratum in: J Nephrol. (2017) 30, 337–338. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Simon RP & Hallenbeck JM (2003). Ischemic tolerance and endogenous neuroprotection. Trends in Neuroscience 26, 248–254. [DOI] [PubMed] [Google Scholar]
- Duann P, Lianos EA, Ma J. & Lin PH (2016). Autophagy, Innate Immunity and Tissue Repair in Acute Kidney Injury. International Journal of Molecular Science 17, E662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupre TV, Doll MA, Shah PP, Sharp CN, Siow D, Megyesi J. … Siskind LJ (2017). Inhibiting glucosylceramide synthase exacerbates cisplatin-induced acute kidney injury. Journal of Lipid Research 58,1439–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endre ZH (2014a). Recovery from acute kidney injury: the role of biomarkers. Nephron Clinical Practice 127, 101–105. [DOI] [PubMed] [Google Scholar]
- Endre ZH. (2014b). Using biomarkers for acute kidney injury: barriers and solutions. Nephron Clinical Practice 127, 180–184. [DOI] [PubMed] [Google Scholar]
- Er F, Nia AM, Dopp H, Hellmich M, Dahlem KM, Caglayan E, Kubacki T. … Gassanov N. (2012). Ischemic preconditioning for prevention of contrast medium-induced nephropathy: randomized pilot RenPro Trial (Renal Protection Trial). Circulation 126, 296–303. [DOI] [PubMed] [Google Scholar]
- Evans KJ & Greenberg A. (2005). Hyperkalemia: a review. Journal of Intensive Care Medicine 20, 272–290. [DOI] [PubMed] [Google Scholar]
- Fan PC, Chen CC, Chen YC, Chang YS & Chu PH (2016). MicroRNAs in acute kidney injury. Human Genomics 10, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faubel S, Chawla LS, Chertow GM, Goldstein SL, Jaber BL, Liu KD, Acute Kidney Injury Advisory Group of the American Society of Nephrology. (2012). Ongoing clinical trials in AKI. Clinical Journal of American Society Nephrology 7, 861–873. [DOI] [PubMed] [Google Scholar]
- Fischer RSB, Mandayam S, Chavarria D, Vangala C, Nolan MS, Garcia LL, … Murray KO (2017). Clinical evidence of acute Mesoamerican nephropathy. The American Journal of Tropical Medicine and Hygiene, 97 1247–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gameiro J, Fonseca JA, Jorge S. & Lopes JA (2018). Acute Kidney Injury Definition and Diagnosis: A Narrative Review. Journal of Clinical Medicine 7, 307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia DSES, Bignelli AT, Hokazono SR, Danucalov I, Siemens TA, Meyer F. … Furquim R. (2017). Analysis of economic impact between the modality of renal replacement therapy. Brazilian Journal of Nefrolology 39, 162–171. [DOI] [PubMed] [Google Scholar]
- Guo C, Dong G, Liang X. & Dong Z. (2019). Epigenetic regulation in AKI and kidney repair: mechanisms and therapeutic implications. Nature Reviews Nephrology 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellou E, Bahouth Z, Sabo E, Abassi Z. & Nativ O. (2018). The impact of comorbidities, sex and age on the occurrence of acute kidney injury among patients undergoing nephron-sparing surgery. Therapeutics Advances in Urology 10, 103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J, Tangri N, Komenda P, Kaushal A, Sood M, Brar R, …, Rigatto C. (2015). Urinary, plasma, and serum biomarkers’ utility for predicting acute kidney injury associated with cardiac surgery in adults: a meta-analysis. American Journal of Kidney Disease 66, 993–1005. [DOI] [PubMed] [Google Scholar]
- Hoste EAJ, Kellum JA, Selby NM, Zarbock A, Palevsky PM, Bagshaw SM, … Chawla LS (2018). Global epidemiology and outcomes of acute kidney injury. Nature Reviews Nephrology 14, 607–625. [DOI] [PubMed] [Google Scholar]
- Huart J, Krzesinski JM & Jouret F. (2018). Genetic susceptibility to delayed graft function following kidney transplantation: a systematic review of the literature. Clinical Kidney Journal 11, 586–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchens MP, Dunlap J, Hurn PD & Jarnberg PO (2008). Renal ischemia: does sex matter? Anesthesia & Analgesia 107, 239–249. [DOI] [PubMed] [Google Scholar]
- Hutchens MP, Fujiyoshi T, Komers R, Herson PS & Anderson S. (2012). Estrogen protects renal endothelial barrier function from ischemia-reperfusion in vitro and in vivo. American Journal of Physiology Renal Physiology 303, F377–F385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvidberg V, Jacobsen C, Strong RK, Cowland JB, Moestrup SK & Borregaard N. (2005). The endocytic receptor megalin binds the iron transporting neutrophil-gelatinase-associated lipocalin with high affinity and mediates its cellular uptake. FEBS Letter 579, 773–777. [DOI] [PubMed] [Google Scholar]
- Ihtiyar E, Yasar NF, Erkasap N, Koken T, Tosun M, Oner S. & Erkasap S. (2011). Effects of doxycycline on renal ischemia reperfusion injury induced by abdominal compartment syndrome. Journal of Surgical Research 167, 113–120. [DOI] [PubMed] [Google Scholar]
- Isaac J, Tögel FE & Westenfelder C. (2007). Extent of glomerular tubularization is an indicator of the severity of experimental acute kidney injury in mice. Nephron Experimental Nephrology 105, e33–40. [DOI] [PubMed] [Google Scholar]
- Jankauskas SS, Plotnikov EY, Morosanova MA, Pevzner IB, Zorova LD, Skulachev VP, Zorov DB (2012). Mitochondria-targeted antioxidant SkQR1 ameliorates gentamycin-induced renal failure and hearing loss. Biochemistry 77, 666–670. [DOI] [PubMed] [Google Scholar]
- Jankauskas SS, Andrianova NV, Alieva IB, Prusov AN, Matsievsky DD, Zorova LD … Zorov DB (2016). Dysfunction of Kidney Endothelium after Ischemia/Reperfusion and Its Prevention by Mitochondria-Targeted Antioxidant. Biochemistry 81, 1538–1548. [DOI] [PubMed] [Google Scholar]
- Jiang H, Chen R, Xue S, Zhu H. & Sun X. (2015). Protective effects of three remote ischemic conditioning procedures against renal ischemic/reperfusion injury in rat kidneys: a comparative study. Irish Journal of Medical Science 184, 647–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo SK, Rosner MH & Okusa MD (2007). Pharmacologic treatment of acute kidney injury: why drugs haven’t worked and what is on the horizon. Clinical Journal of American Society Nephrology 2, 356–365. [DOI] [PubMed] [Google Scholar]
- Johnson AC, Stahl A. & Zager RA (2005). Triglyceride accumulation in injured renal tubular cells: alterations in both synthetic and catabolic pathways. Kidney International 67, 2196–2209. [DOI] [PubMed] [Google Scholar]
- Just A. (2007). Mechanisms of renal blood flow autoregulation: dynamics and contributions. American Journal of Physiology Regulatory, Integrative and Comparative Physiology 292, R1–17. [DOI] [PubMed] [Google Scholar]
- Kam-Tao-Li P, Burdman EA & Mehta RL (2013). Acute kidney injury: global health alert. Journal of Nephropathology 2, 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang KP, Lee JE, Lee AS, Jung YJ, Kim D, Lee S, …, Park, S.K. (2014). Effect of gender differences on the regulation of renal ischemia-reperfusion- induced inflammation in mice. Molecular Medicine Reports 9, 2061–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpman D, Ståhl AL & Arvidsson I. (2017). Extracellular vesicles in renal disease. Nature Reviews Nephrology 13, 545–562. [DOI] [PubMed] [Google Scholar]
- Kaushal GP & Shah SV (2014). Challenges and advances in the treatment of AKI. Journal of the American Society of Nephrology 25, 877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KDIGO Clinical Practice Guideline for Acute Kidney Injury. (2012) Kidney Int., 2(Supplement 1):1–138; Online Appendices A-F. [Google Scholar]
- Kellum JA, Levin N, Bouman C. & Lameire N. (2002). Developing a consensus classification system for acute renal failure. Current Opinion in Critical Care 8, 509–514. [DOI] [PubMed] [Google Scholar]
- Kher A, Meldrum KK, Wang M, Tsai BM, Pitcher JM & Meldrum DR (2005). Cellular and molecular mechanisms of sex differences in renal ischemia-reperfusion injury. Cardiovascular Reserach 67, 594–603. [DOI] [PubMed] [Google Scholar]
- Kontogiannis J & Burns KD. (1998). Role of AT1 angiotensin II receptors in renal ischemic injury. American Journal Physiology 274, F79–F90. [DOI] [PubMed] [Google Scholar]
- Krieter DH, Hackl A, Rodriguez A, Chenine L, Moragues HL, Lemke HD … Canaud B. (2010). Protein-bound uraemic toxin removal in haemodialysis and post-dilution haemodiafiltration. Nephrology Dialisis Transplantation 25, 212–218. [DOI] [PubMed] [Google Scholar]
- Kucuk A, Kabadere S, Tosun M, Koken T, Kinaci MK, Isiskll B. & Erkasap N. (2009). Protective effects of doxycycline in ischemia/reperfusion injury on kidney. Journal of Physiology and Biochemistry 65, 183–191. [DOI] [PubMed] [Google Scholar]
- Kumar S. (2018). Cellular and molecular pathways of renal repair after acute kidney injury. Kidney International 93, 27–40. [DOI] [PubMed] [Google Scholar]
- Kupferman J, Ramírez-Rubio O, Amador JJ, López-Pilarte D, Wilker EH, Laws RL … Friedman DJ (2018). Acute Kidney Injury in Sugarcane Workers at Risk for Mesoamerican Nephropathy. American Journal of Kidney Disease 72, 475–482. [DOI] [PubMed] [Google Scholar]
- Legrand M, Dupuis C, Simon C, Gayat E, Mateo J, Lukaszewicz AC & Payen D. (2013). Association between systemic hemodynamics and septic acute kidney injury in critically ill patients: a retrospective observational study. Critical Care 17, R278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Liu J, Wang W, Zhang Z, Li D, Lin K, … Lin W. (2015). Matrix Metalloproteinase 9 and Vasodilator-Stimulated Phosphoprotein Related to Acute Kidney Injury in Severe Acute Pancreatitis Rats. Digestive Disease and Science 60, 3647–3655. [DOI] [PubMed] [Google Scholar]
- Liakopoulos V, Georgianos PI, Eleftheriadis T. & Sarafidis PA (2011). Epigenetic mechanisms and kidney diseases. Current Medicinal Chemistry 18, 1733–1739. [DOI] [PubMed] [Google Scholar]
- Lindoso RS, Collino F, Bruno S, Araujo DS, Sant’Anna JF, Tetta C Camussi G. (2014). Extracellular vesicles released from mesenchymal stromal cells modulate miRNA in renal tubular cells and inhibit ATP depletion injury. Stem Cells and Development 23, 1809–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HB, Meng QH, Huang C, Wang JB & Liu XW (2015). Nephroprotective Effects of Polydatin against Ischemia/Reperfusion Injury: A Role for the PI3K/Akt Signal Pathway. Oxidative Medicine and Cellular Longevity 2015, 362158–362171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majid DS & Nishiyama A. (2002). Nitric oxide blockade enhances renal responses to superoxide dismutase inhibition in dogs. Hypertension 39, 293–297. [DOI] [PubMed] [Google Scholar]
- Makris K. & Spanou L. (2016). Acute kidney injury: definition, pathophysiology and clinical phenotypes. The Clinical Biochemist Reviews 37, 85–98. [PMC free article] [PubMed] [Google Scholar]
- Malek M. & Nematbakhsh M. (2015). Renal ischemia/reperfusion injury; from pathophysiology to treatment. Journal of Renal Injury Prevention 4, 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangino MJ, Tian T, Ametani M, Lindell S. & Southard JH (2008). Cytoskeletal involvement in hypothermic renal preservation injury. Transplantation 85, 427–436. [DOI] [PubMed] [Google Scholar]
- Marketos SG, Eftychiadis AG & Diamandopoulos A. (1993). Acute renal failure according to ancient Greek and Byzantine medical writers. Journal of the Royal Society of Medicine 86, 290–293. [PMC free article] [PubMed] [Google Scholar]
- Marx D, Metzger J, Pejchinovski M, Gil RB, Frantzi M, Latosinska A, … Herget-Rosenthal S. (2018). Proteomics and Metabolomics for AKI Diagnosis. Seminars in Nephrology 38, 63–87. [DOI] [PubMed] [Google Scholar]
- Masereeuw R, Mutsaers HA, Toyohara T, Abe T, Jhawar S, Sweet DH, Lowenstein J. (2014). The kidney and uremic toxin removal: glomerulus or tubule? Seminars in Nephrology 34, 191–208. [DOI] [PubMed] [Google Scholar]
- McMahon BA & Koyner JL (2016). Risk Stratification for Acute Kidney Injury: Are Biomarkers Enough? Advances in Chronic Kidney Disease 23, 167–178. [DOI] [PubMed] [Google Scholar]
- Merchant ML, Brier ME, Slaughter MS, Klein JB & McLeish KR (2018). Biomarker enhanced risk prediction for development of AKI after cardiac surgery. BMC Nephrology 19, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, …, Devarajan P. (2003). [DOI] [PubMed] [Google Scholar]
- Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. Journal of the American Society Nephrology 14, 2534–2543. [DOI] [PubMed] [Google Scholar]
- Mishra J, Mori K, Ma Q, Kelly C, Barasch J. & Devarajan P. (2004). Neutrophil gelatinase-associated lipocalin: a novel early urinary biomarker for cisplatin nephrotoxicity. American Journal of Nephrology 24, 307–315. [DOI] [PubMed] [Google Scholar]
- Molitoris BA. (2014). Therapeutic translation in acute kidney injury: the epithelial/endothelial axis. Journal of Clinical Investigation 124, 2355–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PK, Hsu RK & Liu KD (2018). Management of Acute Kidney Injury: Core Curriculum 2018. American Journal of Kidney Diseases 72, 136–148. [DOI] [PubMed] [Google Scholar]
- Murray PT, Mehta RL, Shaw A, Ronco C, Endre Z, Kellum JA, … Workgroup A. (2014). Potential use of biomarkers in acute kidney injury: report and summary of recommendations from the 10th Acute Dialysis Quality Initiative consensus conference. Kidney International 85, 513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussap M, Noto A, Fanos V. & Van Den Anker JN (2014). Emerging biomarkers and metabolomics for assessing toxic nephropathy and acute kidney injury (AKI) in neonatology. BioMed Research International 2014, 602526–602532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito M, Bomsztyk K. & Zager RA (2009). Renal ischemia-induced cholesterol loading: transcription factor recruitment and chromatin remodeling along the HMG CoA reductase gene. The American Journal of Pathology 174, 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Miyaki S, Ishitobi H, Matsuyama S, Nakasa T, Kamei N. … Ochi M. (2015). Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Letters 589, 1257–1265. [DOI] [PubMed] [Google Scholar]
- Neugarten J, Golestaneh L. & Kolhe NV (2018). Sex differences in acute kidney injury requiring dialysis. BMC Nephrology 19, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickolas TL, Forster CS, Sise ME, Barasch N, Solá-Del Valle D, Viltard M, …, Barasch J. (2012). NGAL (Lcn2) monomer is associated with tubulointerstitial damage in chronic kidney disease. Kidney International 82, 718–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak KB, Le HD, Christison-Lagay ER, Nose V, Doiron RJ, Moses MA & Puder M. (2010). Effects of metalloproteinase inhibition in a murine model of renal ischemia-reperfusion injury. Pediatric Research 67, 257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermann M. & Liu K. (2017). Pathophysiology of AKI. Best Practice & Research Clinical Anaesthesiology 31, 305–314. [DOI] [PubMed] [Google Scholar]
- Parikh CR, Devarajan P, Zappitelli M, Sint K, Thiessen-Philbrook H, …, TRIBE-AKI Consortium. (2011). Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. Journal of the American Society of Nephrology 22,1737–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh CR, Moledina DG, Coca SG, Thiessen-Philbrook HR & Garg AX (2016). Application of new acute kidney injury biomarkers in human randomized controlled trials. Kidney International 89, 1372–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parravicini E, Nemerofsky SL, Michelson KA, Huynh TK, Sise ME, Bateman DA, …, Barasch JM (2010). Urinary neutrophil gelatinase-associated lipocalin is a promising biomarker for late onset culture-positive sepsis in very low birth weight infants. Pediatric Research 67, 636–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patschan D, Buschmann I, Ritter O. & Kribben A. (2018). Cell-Based Therapies in Acute Kidney Injury (AKI). Kidney Blood Pressure Research 43, 673–681. [DOI] [PubMed] [Google Scholar]
- Pickering JW & Endre ZH (2014). The definition and detection of acute kidney injury. Journal of Renal Injury Prevention 3, 21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikov EY, Chupyrkina AA, Jankauskas SS, Pevzner IB, Silachev DN, Skulachev VP & Zorov DB (2011). Mechanisms of nephroprotective effect of mitochondria-targeted antioxidants under rhabdomyolysis and ischemia/reperfusion. Biochimica et Biophysica Acta, 1812: 77–86. [DOI] [PubMed] [Google Scholar]
- Post EH, Kellum JA, Bellomo R. & Vincent JL (2017). Renal perfusion in sepsis: from macro- to microcirculation. Kidney International 91, 45–60. [DOI] [PubMed] [Google Scholar]
- Prachasilchai W, Sonoda H, Yokota-Ikeda N, Oshikawa S, Aikawa C, Uchida K, …, Ikeda M. (2008). A protective role of unfolded protein response in mouse ischemic acute kidney injury. European Journal of Pharmacology 592, 138–145. [DOI] [PubMed] [Google Scholar]
- Quintavalle C, Donnarumma E, Fiore D, Briguori C. & Condorelli G. (2013). Therapeutic strategies to prevent contrast-induced acute kidney injury. Current Opinion in Cardiology 28, 676–682. [DOI] [PubMed] [Google Scholar]
- Rabb H, Griffin MD, McKay DB, Swaminathan S, Pickkers P, …, Acute Dialysis Quality Initiative Consensus XIII Work Group (2015). Inflammation in AKI: Current Understanding, Key Questions, and Knowledge Gaps. Journal of the American Society of Nephrology 27, 371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabb H, Griffin MD, McKay DB, Swaminathan S, Pickkers P, Rosner MH …, Ronco C. (2016). Inflammation in AKI: Current understanding, key questions, and knowledge gaps. Journal of the American Society Nephrology 27, 371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimundo M, Crichton S, Syed Y, Martin JR, Beale R, Treacher D. & Ostermann M. (2015). Low systemic oxygen delivery and BP and risk of progression of early AKI. Clinical Journal of American Society Nephrology 10, 1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S, Walters KB, Wilson L, Chen B, Bolisetty S, Graves D, … Kabarowski JH (2016). Early lipid changes in acute kidney injury using SWATH lipidomics coupled with MALDI tissue imaging. American Journal Physiology Renal Physiology 310, F1136–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao K, Sethi K, Ischia J, Gibson L, Galea L, Xiao L, … Patel O. (2017). Protective effect of zinc preconditioning against renal ischemia reperfusion injury is dose dependent. PLoS One 12, e0180028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P. & Ratajczak MZ (2006). Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 20, 847–856. [DOI] [PubMed] [Google Scholar]
- Rudnicki M, Perco P, D Haene B, Leierer J, Heinzel A, Mühlberger I, …, Mayer G. (2016). Renal microRNA- and RNA-profiles in progressive chronic kidney disease. European Journal of Clinical Investigation 46, 213–226. [DOI] [PubMed] [Google Scholar]
- Robert R, Vinet M, Jamet A. & Coudroy R. (2017). Effect of non-invasive remote ischemic preconditioning on intra-renal perfusion in volunteers. Journal of Nephrology 30, 393–395. [DOI] [PubMed] [Google Scholar]
- Rodriguez D, Morrison CJ & Overall CM (2010). Matrix metalloproteinases: what do they not do? New substrates and biological roles identified by murine models and proteomics. Biochimica et Biophysica Acta1803, 39–54. [DOI] [PubMed] [Google Scholar]
- Saat TC, Van den Akker EK, Jn IJ, Dor FJ & De Bruin RW (2016). Improving the outcome of kidney transplantation by ameliorating renal ischemia reperfusion injury: lost in translation? Journal of Translational Medicine 14, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salani M, Roy S. & Fissell WH (2018). Innovations in wearable and implantable artificial kidneys. American Journal of Kidney Diseases 72, 745–751. [DOI] [PubMed] [Google Scholar]
- Sánchez-Escuredo A, Alsina A, Diekmann F, Revuelta I, Esforzado N, Ricart MJ … Fernandez E. (2015). Economic analysis of the treatment of end-stage renal disease treatment: living-donor kidney transplantation versus hemodialysis. Transplantation Proceedings 47, 30–33. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ott KM (2011). Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury--where do we stand today? Nephrology and Dialysis Transplantation. 26, 762–764. [DOI] [PubMed] [Google Scholar]
- Schrezenmeier EV, Barasch J, Budde K, Westhoff T. & Schmidt-Ott KM (2018). Biomarkers in acute kidney injury in preclinical biomarker qualification studies. Nature Biotechnol 28, 478–485. [Google Scholar]
- Sesso RC, Lopes AA, Thomé FS, Lugon JR & Martins CT (2014). Inquérito Brasileiro de Diálise Crônica 2014. Jornal Brasileiro de Nefrologia 38, 54–61. [DOI] [PubMed] [Google Scholar]
- Shokeir AA, Hussein AM, Barakat N, Abdelaziz A, Elgarba M. & Awadalla A. (2014). Activation of nuclear factor erythroid 2-related factor 2 (Nrf2) and Nrf-2-dependent genes by ischaemic pre-conditioning and post-conditioning: new adaptive endogenous protective responses against renal ischaemia/reperfusion injury. Acta Physiologica 210, 342–353. [DOI] [PubMed] [Google Scholar]
- Siew ED, Ikizler TA, Gebretsadik T, Shintani A, Wickersham N, Bossert F, …Ware LB (2010). Elevated urinary IL-18 levels at the time of ICU admission predict adverse clinical outcomes. Clinical Journal of the American Society of Nephrology 5, 1497–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silachev DN, Plotnikov EY, Pevzner IB, Zorova LD, Babenko VA; Zorov SD,… Zorov DB (2014). The mitochondrion as a key regulator of ischaemic tolerance and injury. Heart, Lung and Circulation 23, 897–904. [DOI] [PubMed] [Google Scholar]
- Silva GBDJ, Vasconcelos AGJ, Rocha AMT, Vasconcelos VR, Barros JN, Fujima JS, … Daher EF (2017). Acute kidney injury complicating bee stings - a review. Revista do Instituto de Medicina Tropical de São Paulo 59, e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver SA & Chertow GM (2017). The economic consequences of acute kidney injury. Nephron 137, 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver SA, Long J, Zheng Y. & Chertow GM (2017). Cost of acute kidney injury in hospitalized patients. Journal of Hospital Medicine 12, 70–76. [DOI] [PubMed] [Google Scholar]
- Simons B, Kauhanen D, Sylvanne T, Tarasov K, Duchoslav E. & Ekroos K. (2012). Shotgun Lipidomics by Sequential Precursor Ion Fragmentation on a Hybrid Quadrupole Time-of-Flight Mass Spectrometer. Metabolites 2, 195–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH & Humes HD (2009). The bioartificial kidney in the treatment of acute kidney injury. Current Drug Targets 10, 1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srisawat U, Kongrat S, Muanprasat C. & Chatsudthipong V. (2015). Losartan and Sodium Nitroprusside Effectively Protect against Renal Impairments after Ischemia and Reperfusion in Rats. Biological and Pharmaceutical Bulletin 38, 753–762. [DOI] [PubMed] [Google Scholar]
- Star RA (1998). Treatment of acute renal failure. Kidney International 54, 1817–1831. [DOI] [PubMed] [Google Scholar]
- Supavekin S, Zhang W, Kucherlapati R, Kaskel FJ, Moore LC & Devarajan P. (2003). Differential gene expression following early renal ischemia/reperfusion. Kidney International 63, 1714–1724. [DOI] [PubMed] [Google Scholar]
- Tanaka R, Tsutsui H, Ohkita M, Takaoka M, Yukimura T. & Matsumura Y. (2013) Sex differences in ischemia/reperfusion-induced acute kidney injury are dependent on the renal sympathetic nervous system. European Journal of Pharmacology 714, 397–404. [DOI] [PubMed] [Google Scholar]
- Tanaka R, Yazawa M, Morikawa Y, Tsutsui H, Ohkita M, Yukimura T. & Matsumura Y. (2017). Sex differences in ischaemia/reperfusion-induced acute kidney injury depends on the degradation of noradrenaline by monoamine oxidase. Clin. Exp. Pharmacol. Physiol 44, 371–377. [DOI] [PubMed] [Google Scholar]
- Tögel F. & Westenfelder C. (2014). Recent advances in the understanding of acute kidney injury. F1000Prime Reports 6, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, … Kellum JA (2009). Discontinuation of continuous renal replacement therapy: a post hoc analysis of a prospective multicenter observational study. Critical Care Medicine 37, 2576–2582. [DOI] [PubMed] [Google Scholar]
- Vaidya VS, Ozer JS, Dieterle F, Collings FB, Ramirez V. Troth S, …, Bonventre JV, (2010). Kidney injury molecule-1 outperforms traditional biomarkers of kidney. Nature Biotechnology 28, 478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanmassenhove J, Vanholder R, Nagler E. & Biesen WV (2013). Urinary and serum biomarkers for the diagnosis of acute kidney injury: an in-depth review of the literature. Nephrology Dialysis Transplantation 28, 254–273. [DOI] [PubMed] [Google Scholar]
- Wei Q, Bhatt K, He HZ, Mi QS, Haase VH & Dong Z. (2010). Targeted deletion of Dicer from proximal tubules protects against renal ischemia-reperfusion injury. Journal of the American Society Nephrology 21, 756–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA … Collins AJ (2006). Incidence and mortality of acute renal failure in medicare beneficiaries, 1992 to 2001. Journal of American Society Nephrology 17, 1135–1142. [DOI] [PubMed] [Google Scholar]
- Zager RA, Baltes LA, Sharma HM & Jurkowitz MS (1984). Responses of the ischemic acute renal failure kidney to additional ischemic events. Kidney International 26, 689–700. [DOI] [PubMed] [Google Scholar]
- Zhang G, Wang Q, Zhou Q, Wang R, Xu M, Wang H, … Lai EY (2016a). Protective Effect of Tempol on Acute Kidney Injury Through PI3K/Akt/Nrf2 Signaling Pathway. Kidney and Blood Pressure Research 41, 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Rudemiller NP, Patel MB, Wei QQ, Karlovich NS, Jeffs AD, …, Crowley AD (2016b). Competing actions of type 1 angiotensin II receptors expressed on T lymphocytes and kidney epithelium during cisplatin-induced AKI. Journal of the American Society Nephrology 27, 2257–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Xiao Z, Yao J, Zhao G, Fa X. & Niu J. (2013). Participation of protein kinase C in the activation of Nrf2 signaling by ischemic preconditioning in the isolated rabbit heart. Molecular and Cellular Biochemistry 372, 169–179. [DOI] [PubMed] [Google Scholar]
- Zhou CC, Ge YZ, Yao WT, Wu R, Xin H, Lu TZ, …, Jia RP (2017). Limited Clinical Utility of Remote Ischemic Conditioning in Renal Transplantation: A Meta-Analysis of Randomized Controlled Trials. PLoS One 12, e0170729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk A, & Bonventre JV (2016). Acute Kidney Injury. Annual Review of Medicine, 67, 293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]