Abstract
Utilizing the Andersen Healthcare Utilization Model, we examined the role of neighborhood context on pre-exposure prophylaxis (PrEP) utilization among a sample of Black men who have sex with men (MSM) residing in a medium-sized city in the Deep South. Data were derived from a sample of 142 Black MSM aged 18–64 years who were eligible for PrEP from a community-based study known as “ACCELERATE!.” We used multi-level structural equation modeling to assess PrEP use. Social support, sexual risk, and healthcare access were predictive of PrEP use. Notably, residing in a neighborhood with concentrated poverty was associated with decreased PrEP use. Our findings reveal neighborhood structural disadvantage is associated with decreased PrEP use among Black MSM, after adjusting of individual-level socio-demographic characteristics. There is an urgent need to develop HIV prevention interventions and programs that explicitly address structural level factors to eliminate racial/ethnic differences in HIV.
Keywords: Black MSM, PrEP, HIV, Macrosocial Determinants, Neighborhood Structural Disadvantage, Structural Discrimination
INTRODUCTION
Racial/ethnic and sexual minorities in the United States (U.S.) bear the brunt of the HIV epidemic (CDC, 2018). Notably, African-Americans represent less than 13% of the population, but Black men who have sex with men (MSM) account for 42% of all new HIV infections (CDC, 2019). It is estimated half of Black MSM in the U.S. can be expected to become HIV-positive in their lifetime (Hess, Hu, Lansky et al., 2017). Current surveillance data show that most of the HIV cases are clustered in the Southern U.S., where there is a large Black population (CDC 2020a). In 2017, the South accounted for more than half of the 38,739 new HIV diagnoses, including Mississippi which has the 9th highest rate (17%) of new HIV infections (CDC, 2020b, 2020c). According to the Centers for Disease Control and Prevention, Mississippi ranked 8th in AIDS death rate in the nation. Among Metropolitan Statistical Areas in 2018, Jackson, the capital of Mississippi, was ranked 8th for HIV diagnoses (CDC, 2020d). Also, data from the Mississippi Department of Health reveal that male-to-male sexual contact account for 71% of all new diagnosis among males (MSDH, 2020).
While pre-exposure prophylaxis (PrEP) has been shown to be highly efficacious in reducing risk of acquisition of HIV (Desai, Field, Grant & MCCormack, 2017), uptake among African-Americans/ Blacks remains alarming low. It is estimated that approximately 500,000 African Americans could benefit from PrEP, but only 7,000 prescriptions (0.014%) were filled (Bowen, Eikmeier, Talley et al., 2015). The literature on PrEP use reveals that Black MSM are less likely to use PrEP than their white counterparts (Liu, Cohen Vittinghoff, et al., 2016; Snowden, Chen, McFarland & Raymond, 2017). For example, a study utilizing the National HIV Behavioral Surveillance survey conducted in San Francisco among MSM showed only 7.7% of Blacks used PrEP compared to 22.9% of their white counterparts.
While there is a growing body of literature examining PrEP use among MSM, most of these studies focus on white MSM with scant attention to Black MSM in the South, the region where the majority of Black MSM reside (Arnold, Brinkley-Rubinstein, Chan et al., 2017; Chan, Mena Patel, et al., 2016; Strauss, Greene, Phillips, et al., 2017; Bauermeister, Meanley, Pingel, Soler, Harper, 2013;). Furthermore, many of these studies tend to prioritize individual-level factors, with little consideration of the effects of macro-level factors related to PrEP engagement.
Meanwhile, despite tremendous progress in the area of civil rights, African-Americans continue to face significant socioeconomic disparities in education, employment, and healthcare. American cities remain profoundly segregated and unequal, reflecting previously codified racial divisions derived out of the historical legacies of chattel slavery and Jim Crow and contemporary forms of structural racism, which have resulted in a range of adverse social conditions and economic deprivation (e.g. residential segregation, concentrated poverty, low educational attainment, high rates of incarceration, unemployment, and limited access to healthcare due to lack of Medicaid expansion) (Williams and Mohammed, 2013; Williams, Neighbors and Jackson, 2003). Systematic disinvestments in black neighborhoods along with race-based policies and practices (e.g., urban renewal, white flight, redlining, and predatory lending) have severely disadvantaged black neighborhoods and its residents (Diez Roux, 2001; Krieger, Waterman, Picket & Pearl 2001; Rehkoof, 2005; Williams & Collins, 2001). Consequently, living in a poor, black neighborhood limits an individual’s social and economic opportunities and reduces their access to healthcare (CDC, 2021).
Recent data show that 21.2% of African Americans are living below the poverty compared to 9.0 of whites; 22.6% of Blacks compared to 36.9% of whites have a bachelor’s degree or higher, 7.7% of Blacks are unemployed compared to 3.7 for whites; and 10.1% of Blacks have no health insurance compared to 6.3 for whites (HHS, 2021). Given the persistent and profound racial/ethnic disparities in social and structural determinants of health in the U.S., more attention is warranted to better understand how the structural character of neighborhoods manifest in the material landscape either promoting or constraining PrEP engagement. The dearth of studies that examine the role of macro-level factors on PrEP utilization highlight the significant gaps in our knowledge regarding how specific structural level factors associated with high poverty urban neighborhoods may influence PrEP utilization. We know even less about the mechanisms underlying the ways that living in concentrated poverty in poor urban neighborhoods affect PrEP utilization among Black MSM. The lack of such studies is especially notable in the U.S., given that the highest rates of new infections occur among racial and ethnic minorities, specifically Black MSM, living in urban areas in the South. Further examination of these factors is critical in elucidating how specific risk environments may promote utilization patterns, which drive the transmission of HIV and STIs, and in advancing our understanding of the multi-level factors that determine population-level HIV disease burden.
In brief, this knowledge gap is a major impediment for developing tailored, culturally appropriate HIV prevention and control interventions for vulnerable populations such as Black MSM. Consequently, there is an urgent need to better understand the influence of both individual- and structural-level factors in explain high rates of HIV incidence among racial and sexual minorities, particularly young, Black MSM. Therefore, we conducted this study to determine if structural level factors, independent of individual-level characteristics, predict PrEP engagement among a sample of Black MSM residing in a medium-sized city in the South.
Race, Neighborhood Structural Disadvantage, and HIV
Over the last two decades, we have seen an increased interest in race/ethnicity, neighborhoods, and health (Diez Roux & Mair, 2010). The association of structural- and neighborhood-level determinants have been widely documented for a variety of health outcomes including obesity, diabetes, hypertension, mental health, and cardiovascular disease, and HIV (Borell, Diez Roux, Rose, Catellier, Clark, 2004; Christine, Auchincloss, Bertoni, Carnethon, Sanchez, Moore et al., 2015; Diez Roux, Mujahid, Hirsch, Moore K, & Moore LV, 2016; Morenoff, House, Williams, Hansen, Kaplan, & Hunte, 2007; Truong & Ma, 2006; Pickett, 2001). A growing body of literature has begun to examine associations of structural and neighborhood physical environments and HIV vulnerability (Bauermeister, Zimmerman & Caldwell, 2011; Billy, Brewster, & Grady, 1995; Burgard & Lee-Rife, 2009; Burns P. & Snow, 2012; Cohen, Spear, Scribner, Kissinger, Mason & Wildgen, 2000). A seminal study of gonorrhea using Broken Windows Theory found a robust association between deteriorated physical conditions and increased gonorrhea rates, independent of socioeconomic status (Cohen, Spear, Scribner, et al., 2000). Burns and Snow found youth residing in informal settlements in South Africa characterized by substandard housing without basic services (e.g., water, electricity, indoor plumbing) were more likely to engage in sexual risk-taking behavior (Burns P & Snow, 2012). Using a social disorder index to capture community context, concentrated neighborhood disadvantage has been linked to increased risk of unprotected sex among young men in South Africa (Burgard and Rife, 2009).
Despite the presence of debilitating structural inequities in poor, black urban neighborhoods and documented impact on HIV-related outcomes, few studies have examined how structural level features of neighborhoods affect the social patterning of PrEP engagement, among Black MSM (Ransome, Bogart, Kawachi, Kaplan, Mayer, & Ojikutu, 2020; Grant, Lama, Anderson, McMahan, Liu, Vargas et al 2010; Finlayson, Cha, Xia, Trujillo, Denson, Prejean, et al., 2019; Parsons, Rendina, Lassiter, Whitfield, Starks, Grov, 2017; Fallon, Park, Ogbue, Flynn, & German, 2017). A recent study found that higher area-level HIV risk was more significantly associated with increased willingness to use PrEP among a sample of African Americans (Ransome, Bogart, Kawachi et al., 2020). Liu et al.; (2016) found that individuals who reported having stable housing and at least 2 condomless anal sex partners in the past 3 months are more likely to have protective levels of tenofovir-diphosphate (TFV-DP), the active ingredients of PrEP. Also, a qualitative study of young MSM found that having access to payment assistance programs and access to healthcare is associated with PrEP engagement (Fallon, Park, Ogbue et al, 2017).
Andersen Model of Healthcare Utilization
In this study we utilize the Andersen Healthcare Utilization Model (AHUM) to inform how structural level factors influence PrEP use. AHUM provides a useful heuristic framework for the systematic identification of social and structural factors that influence an individual’s decision to use available health care services (Andersen, 1995; Andersen & Newman, 1973). AHUM is a multilevel model that incorporates both individual and of structural-level factors which allows us to contend with some of the larger macrolevel factors that may create barriers to PrEP uptake. The model posits utilization of health services is determined by multiple and multilevel factors including macrolevel factors at the structural-level and perceived and evaluated factors and the individual level. AHUM is divided into three major components: (1) Environmental factors include external or macrosocial forces such as poverty that might influence an individual’s health-seeking behavior, (2) Predisposing factors are those socio-demographic characteristics such as age of the individual that exist prior to their health condition(s), and (3) Enabling factors reflect the means or logistics required to obtain the services. We selected this framework for two primary reasons: (1) AHUM has been used widely in the U.S. and United Kingdom to study healthcare utilization in a number of settings, however to our knowledge this is the first study to utilize AHUM to examine PrEP utilization; and (2) the majority of studies on PrEP use has focused primarily on individual level factors (e.g. sociodemographic characteristics and behavioral factors). Guided by the modified AHUM (see Figure), the purpose of this paper is to identify factors associated with PrEP utilization among a sample of Black MSM residing in a medium-sized city in the Deep South.
Figure 1.
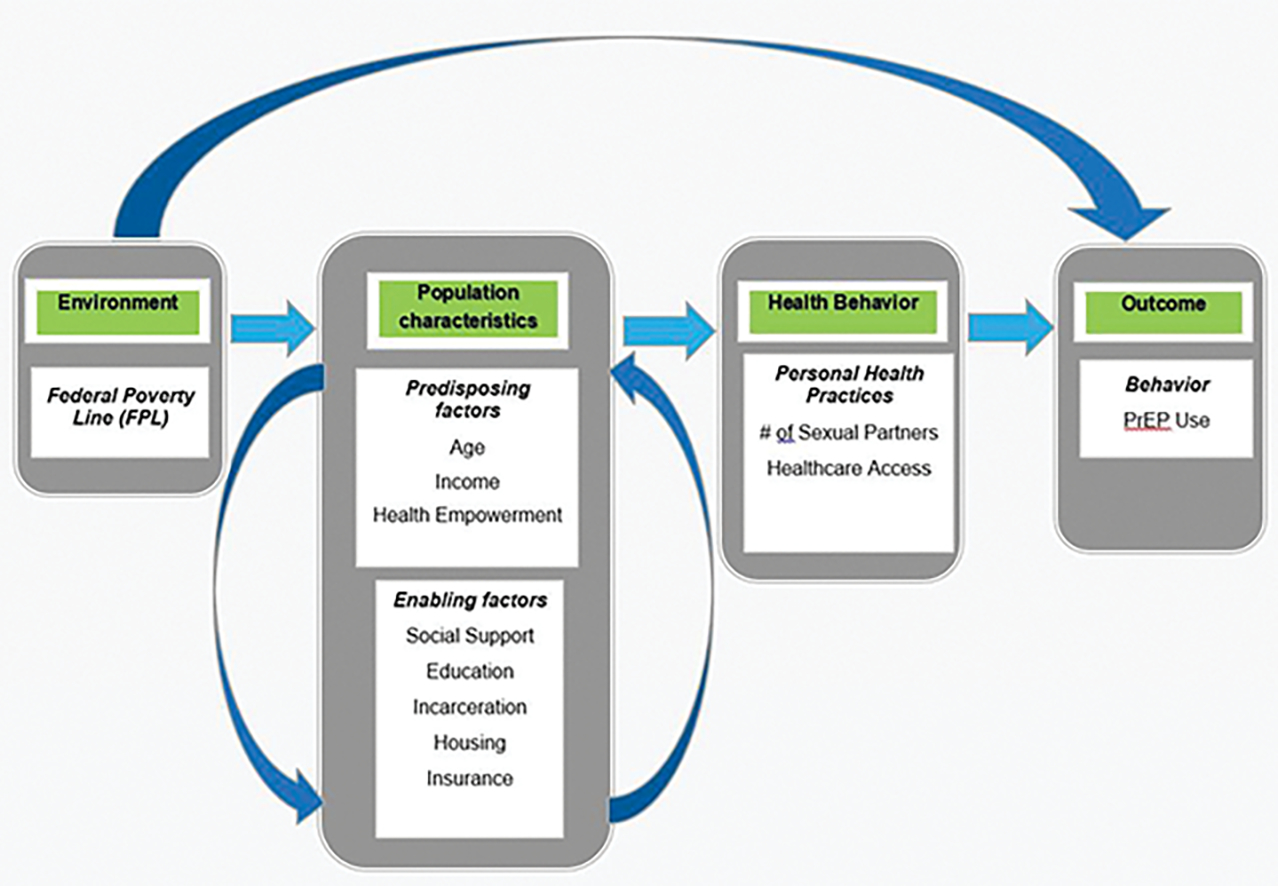
Conceptual Model for PrEP Use Among Black MSM
METHODS
Setting
Data for this study were drawn from a sub-sample (Jackson, MS) of the study known as “ACCELERATE!,” which was a two-city, multisite HIV prevention intervention tailored to Black MSM in Jackson, MS and Baltimore, MD (Burns, Williams Mena et al 2020). ACCELERATE! Initiative sought to develop innovative and sustainable community-driven projects to improve health outcomes among Black MSM in geographic areas most affected by HIV/AIDS. The program utilizes three primary strategies to expand access to and retention in HIV prevention, care, and treatment: (1) engaging Black MSM directly in identifying their unique priorities and concerns, (2) activating and supporting community partnerships that foster innovation, and (3) translating key lessons learned towards the development of new and innovative approaches to reduce the numbers of new infections among Black MSM. This cross-sectional study utilized both convenient sampling and respondent-driven sampling (RDS) methods (Heckathorn, 1997). Data for these analyses were collected between December 2018 and May 2019. Study protocols performed for the evaluation were reviewed and approved by both the University of Mississippi Medical Center and Johns Hopkins University Institutional Review Boards.
Study Population
Initially, individuals were screened for their eligibility for participating in the study if they: (1) self-identified as African-American/Black, (2) aged 18–64, (3) designated as male at birth, (4) having had sex with another man within the last 12 months, and (5) resided in the Jackson Metropolitan Area. Exclusion criteria included being female, transgender; less than 18 years or other race (excluding non-Hispanic Black). Analytic inclusion required the participants to report having received a negative test at their most recent HIV test. The parent study consisted of 322 MSM; however, 134 (41.6%) were determined as not HIV-negative, 122 were living with HIV, seven had missing data for this variable, four never received the results of their test, and one received an “undetermined” result). Of the 188 HIV-negative MSM an additional 46 (24%) were excluded by list-wise deletion due to missing values on one or more of the key constructs included in this analysis. The final analytic sample included 142 HIV-negative MSM were included in this analysis.
Recruitment
Participants were recruited across diverse community settings and medium, including: (1) HIV clinics, (2) AIDS Service Organizations, (3) other community-based organizations, (4) social media, and (5) word of mouth. Participants were given an incentive of $50 in form of a gift card for completing the survey. Participants were invited to recruit individuals in their social networks and were compensated an additional $15 per person recruited. All participants gave written informed consent and the Institutional Review Boards of University of Mississippi Medical Center approved all study protocols.
Data Collection: AHUM Domains and Variables
Data were collected using a computer-assisted self-interviewing survey, including a battery of socio-demographic characteristics (e.g., age, education, income, insurance), knowledge, attitudes and beliefs regarding HIV, sexual risk behavior, HIV testing use and PrEP use.
Measures
Guided by AHUM domains and constructs, we used the following variables to assess PrEP utilization among a sample of Black MSM.
Environment:
Poverty.
The primary variable measured from the Andersen construct of “environment” was zip code level poverty. The median household income at the county level was used to assess the larger socio-economic character of the neighborhood in which participants resided. Structural level variables may influence the level of availability and access to opportunity structures such as healthcare and access to HIV prevention services (e.g., PrEP). These data were based on median household income from 2014–2018 obtained from the U.S. Census Data.
Population Characteristics:
Predisposing Characteristics.
Age was treated as continuous variable in years. Health Beliefs were assessed using an 8-item “Health Empowerment Scale” (e.g., “I accept that the future of any health issues I experience may be unknown.”) on a 4-point Likert format (1 = strongly disagree to 4 = strongly agree) (Johnson Rose, Dilworth, et al., 2012). This previously validated scale (Johnson Rose, Dilworth, et al., 2012) has a theoretical minimum of 8 and a theoretical maximum of 32. In this sample the min=10 and max=32 and Cronbach alpha=.80.
Enabling Characteristics:
Several measures were used that are classified as enabling characteristics/resources by the Andersen Model. Individual level poverty was measured as having a self-reported income below the federal poverty line. Education was a categorical variable defined as highest level of education completed (e.g., “Did not complete high school, diploma or GED, vocational, some college or Associates, Bachelors, Graduate School?”). Homelessness was having been homeless in last 12 months (yes/no). Incarceration was defined as having been detained in a detention center, jail or prison in last year (yes/no). Insurance was assessed as a 3-category variable (i.e., private, public, none). Social support was a 4-item “Emotional Support Scale” (e.g. “Others are there with me during a difficult time.”) utilizing a 4-point Likert format (1–4 from strongly disagree to strongly agree) (Krause, 1995). This previously validated scale (Krause,1995) has a theoretical minimum of 4 and a theoretical maximum of 16. In this sample the min = 4 and the max = 16 with a Cronbach α of .84.
Health Behavior Variables:
Health behavior was assessed using two variables: (1) use of General Health Services (e.g., “Have you ever used health services not related to HIV?”) coded (0 = no, 1 = yes) and (2) Number of sexual partners coded as a continuous variable.
Outcome Variable:
PrEP Utilization was defined as those respondents who used PrEP in the last 12 months (0 = no; 1 = yes). All PrEP eligible participants were included in the sample.
Analytic Strategy
Data cleaning, univariate and bivariate analyses were conducted in SAS version 9.4. Multi-level logistic regressions were performed utilizing MPlus version 7. Models were estimated at two levels: (1) person level (N = 142), which were nested within (2) zip code level (n = 31). Missing data (n = 13) were handled with leastwise deletion. To determine if multi-level modeling was a suitable approach the intra-class correlation coefficient (ICC) was first assessed to establish the percent of variance captured at the zip code level. The ICC provides information about the degree of independence of residuals by cluster and ranges from 0 to 1 where 0 indicates perfect independence of residuals and 1 indicates perfect dependence of residuals (Sommet & Morselli, 2017). Variables measured at the individual level were age, income below the poverty line, social support, education, incarceration, housing insecurity, insurance, number of sexual partners in the past year, and healthcare access with outcome at the individual level (PrEP use). Only one variable was measured at the zip code level, which was percent of inhabitants below the poverty line; however, cluster means for individual-level variables were included at the zip code level. Continuous variables at the individual level were cluster-mean centered. To determine which variables were allowed for random effects, the variance of individual-level variables was assessed for significance at the 0.05 level.
RESULTS
Table 1 is a summary of the characteristics of the sample. At the cluster level, 31 zip codes were included with an average percent of residents below the federal poverty line of 18.8%. The average age was 27.6 years. The majority of participants had an income below the poverty line (60.6%), a high school diploma or less education (53.5%), some form of insurance (69.0%), had accessed healthcare in the past year (54.9%), did not have a history of incarceration (93.7%), and did not experience housing insecurity in the past year (83.8%).
Table 1.
Descriptive statistics for sample (n=142)
| Level | Andersen Model Construct | Variable | n (%) | x (SD) |
|---|---|---|---|---|
| 2-Zipcode-Level (n=31) | Environment (Macro) | % below poverty line | 18.8 (7.0) | |
| 1-Individual-Level (n=142) | Predisposing Characteristics | Age | 27.5 (8.3) | |
| Income below poverty line | ||||
| Yes | 86 (60.6%) | |||
| No | 56 (39.4%) | |||
| Health Empowerment Scale | 26.7 (3.9) | |||
| Enabling Resources | Emotional Social Support | 11.2 (4.2) | ||
| Education | ||||
| Up to a High School Diploma | 76 (55.5 %) | |||
| Some college or more | 66 (46.5%) | |||
| Incarceration | ||||
| Yes | 9 (6.3%) | |||
| No | 133 (93.7%) | |||
| Housing Insecurity (12 months) | ||||
| Yes | 23 (16.2%) | |||
| No | 119 (83.8%) | |||
| Insurance | ||||
| Yes | 98 (69.0%) | |||
| No | 44 (31.0%) | |||
| Personal Health Practices | Number of Sexual (12 months) | 4.9 (8.5) | ||
| Use of Health Services | Healthcare access (12 months) | |||
| Yes | 78 (54.9%) | |||
| No | 64 (45.1%) | |||
| Health Outcome | PrEP Use (6 months) | |||
| Yes | 59 (41.6%) | |||
| No | 83 (58.4%) |
The unconditional model predicting PrEP use yielded an ICC of 0.22 indicating that approximately 22% of variance is accounted for by zip code level differences. This suggests that a 2-level model is appropriate for this data as there is evidence of partial dependence by zip code. Random coefficients models were estimated to assess the significance of the variance for each individual-level predictor to assess the need for random slopes in the final model. This resulted in no level-1 predictor with significant variance and therefore, no random slopes in the final model.
Unadjusted Estimates
Unadjusted odds ratio (OR) estimates are presented in Table 2. We found residing in a zip code with higher percentages of residents with income below the poverty line was correlated with lower odds of PrEP use (OR = 0.83, 95%CI 0.75, 0.91, p < 0.001). Age was correlated suggesting that older participants had lower odds of PrEP use (OR = 0.93, 95%CI 0.87, 0.98, p < 0.01). Higher health empowerment scores were associated with an increased odds of PrEP use (OR = 1.18, 95%CI 1.06, 1.29, p < 0.001). Experience of housing insecurity was associated with lower odds of PrEP use (OR = 0.20, 95%CI 0.06, 0.59, p < 0.01). Lastly, having insurance was associated with a higher odds of PrEP use (OR = 4.01, 95%CI 1.61, 10.01, p < 0.01).
Table 2.
Unadjusted OR and Adjusted OR using (n=142)
| Level | Construct | Variable | Unadjusted Estimates | Full Model | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | aOR (95% CI) | p-value | |||
| 2-Zipcode | Environment (Macro) | % below poverty line | 0.83 (0.75, 0.91) | <0.001 | 0.73 (0.62, 0.85) | <0.001 |
| Age cluster mean | 1.01 (0.85, 1.98) | 0.92 | 1.01 (0.9, 1.13) | 0.89 | ||
| Health empowerment cluster mean | 1.03 (0.76, 1.40) | 0.85 | 0.93 (0.74, 1.18) | 0.57 | ||
| Social support cluster mean | 0.76 (0.57, 1.01) | 0.06 | 0.78 (0.6, 1.01) | 0.06 | ||
| Education cluster mean | 0.63 (0.08, 5.06) | 0.36 | 1.58 (0.11, 23.36) | 0.74 | ||
| Incarceration cluster mean | 0.06 (0.00, 2.95) | 0.16 | 1.12 (0.02, 60.02) | 0.96 | ||
| Housing insecurity cluster mean | 0.63 (0.05, 8.02) | 0.73 | 0.81 (0.04, 15.18) | 0.89 | ||
| Insurance cluster mean | 2.83 (0.31, 25.92) | 0.36 | 1.01 (0.05, 19.54) | 0.99 | ||
| Sexual Risk cluster mean | 1.04 (0.95, 1.14) | 0.36 | 1.15 (1.01, 1.3) | 0.04 | ||
| Healthcare access cluster mean | 0.17 (0.02, 1.47) | 0.11 | 0.02 (0, 0.32) | <0.01 | ||
| 1-Individual | Predisposing Characteristics |
Age | 0.93 (0.87, 0.98) | <0.01 | 0.92 (0.88, 0.97) | <0.01 |
| Income below poverty line | 0.51 (0.24, 1.07 ) | 0.08 | 0.39 (0.14, 1.09) | 0.08 | ||
| Health empowerment | 1.18 (1.06, 1.29 ) | <0.001 | 1.15 (1.01, 1.30) | 0.04 | ||
| Enabling Resources | Social Support | 0.94 (0.83, 1.05) | 0.25 | 0.93 (0.81, 1.07) | 0.31 | |
| Education | 0.96 (0.48, 1.94) | 0.91 | 0.3 (0.06, 1.4) | 0.13 | ||
| Incarceration | 0.89 (0.32, 2.51) | 0.83 | 5.31 (1.32, 21.36) | 0.02 | ||
| Housing Insecurity | 0.20 (0.06, 0.59) | <0.01 | 0.14 (0.05, 0.42) | <0.001 | ||
| Insurance status | 4.01 (1.61, 10.01) | <0.01 | 3.26 (0.56, 19.01) | 0.19 | ||
| Personal Health Practices | Sexual Risk | 0.96 (0.90, 1.02) | 0.18 | 0.97 (0.92, 1.03) | 0.34 | |
| Use of Health Services | Healthcare access | 1.04 (0.42, 2.59) | 0.93 | 1.38 (0.46, 4.12) | 0.57 | |
Final Model
The results of the final full model are presented in Table 2. The AIC was 165.42 and the BIC was 230.45 for the final model. A Chi-squared difference test comparing the full model to the null model was 157.1404 (19), p < 0.001.
After adjusting for co-variates, living in a zip code with higher percentages of residents with income below the poverty line was correlated with lower odds of PrEP use (adjusted OR [aOR] = 0.73, 95%CI 0.62, 0.85, p < 0.001). Being in a zip code with higher cluster mean for number of male sexual partners in the past year was correlated with higher odds of PrEP use (aOR = 1.15, 95%CI 1.01, 1.30, p < 0.05). Counterintuitively, being in a zip code with higher cluster mean for accessing healthcare in the past year was correlated with lower odds of PrEP use (aOR = 0.02, 95%CI 0.00, 0.32, p < 0.01). Higher age was correlated with lower odds of PrEP use (aOR = 0.92, 95%CI 0.88, 0.97, p < 0.01). Higher health empowerment scores were associated with an increased odds of PrEP use (aOR = 1.18, 95%CI 1.01, 1.30, p < 0.05). Experience of housing insecurity was associated with lower odds of PrEP use (aOR = 0.14, 95% CI 0.05, 0.42, p <0.01). Lastly, after adjusting for other covariates experience of incarceration was associated with a higher likelihood of PrEP use (aOR = 0.14, 95%CI 0.05, 0.42, p < 0.001).
DISCUSSION
In this study, we used the Andersen Model of Health Utilization (Andersen, 1995) -- a theory-driven socio-ecological framework -- to elucidate challenges and opportunities that may mitigate or promote an individual’s ability to access PrEP and/or disrupt their ability access and use PrEP. The model constructs of macro-level environment, socio-demographic characteristics and health behavior provides a useful framework to better understand the syndemic nature of PrEP use among Black MSM (Singer, 2009; Stall, Mills, Williamson, Hart, Greenwood Paul et al., 2003). As Singer posits, a syndemic is the interaction and intersecting of multiple diseases (e.g., HIV, STD, etc.) and the noxious structural environment (e.g., poverty, poor housing, etc.) that create the conditions ripe for HIV. Our findings show that Black MSM who are older and those who are housing insecure are less likely to consider using PrEP. Also, respondents with a higher health empowerment score are more likely to consider using PrEP. These multiple and intersecting social and structural factors and behaviors create barriers to the uptake of PrEP among Black MSM and must be addressed when developing HIV prevention interventions.
The study points to the importance of other structural variables such as housing and incarceration. Those individuals who reported experiencing homelessness in the last 12 months were less like to use PrEP. This finding is consistent with prior studies examining the influence of housing on ART adherence (Milloy, Kerr, Bangsberg. Buxton, Parashar; Guillemi, Montaner et al., 2012; Palepu, Milloy, Kerr, Zhang & Wood 2011). One study found access to housing is a structural barrier and that being unhoused was associated with sub-optimal ART adherence. Moreover, we found that individuals who reported a history of incarceration are more likely to use PrEP. One explanation for this finding might be this adverse life experience (incarceration) is a significant source of motivation for using HIV prevention services such as PrEP. Research shows compared to the general population, HIV prevalence is considerably higher among incarcerated persons. In 2017, Blacks represented 12% of the U.S. adult population but it is estimated that Black men are 6 times more likely to be incarcerated compared to their white counterparts (The Sentencing Project, 2020). Given the alarming statistics in HIV rates in prison and the racial disparities in sentencing, Black MSM with a history of incarceration may be more willing to use PrEP (Golrokhi, Farhoudi, Taj; Pahlaviani, Mazaheri-Tehrani, Cossarizza et al., 2018). However, additional research is warranted to better understand health-seeking behavior as it relates to PrEP use among incarcerated vs. non-incarcerated populations, particularly Black MSM who have prior experience within the criminal justice system.
This study shows that above and beyond individual level socio-demographic level factors, structural factors specifically neighborhood poverty rates influence PrEP utilization. Studies have shown the negative impacts of neighborhood maybe become embodied within individuals generating poor health outcomes (Diez Roux 2001; Geronimus, Hicken, Keene, Bound, 2006; Krieger, 2003). Neighborhood structural disadvantage may be especially relevant in the context of increasing clustering of geographic concentrations of poverty with other forms of intersectional stigma (e.g., race, gender, and sexual identity) that exacerbate existing forms of structural discrimination. (Bowleg 2019; Hirsch, 2017; Parker CM, Garcia, Philbin, Wilson, Parker RG et al., 2017; Poteat 2021; Turan JM, Elafros, Logie, Swagata, Turan B, Crockett et al., 2019). National HIV/AIDS Strategy has emphasized the need to adopt structural level approaches to reducing the number of new infections (HRSA, NHAS, 2021). Our findings underscore the importance of this overarching goal in addressing the social and structural determinants of health for racial ethnic and sexual minority populations. We argue neighborhoods are socially constructed environments of unequal distribution of power relations and resources which contribute to the production of “HIV riskscapes” or spaces of increased HIV vulnerability (e.g. concentrated poverty, lack of culturally-appropriate HIV prevention services and high levels of community-level HIV); and shape an individual’s ability to avoid risk or minimize the effects of structural discrimination and in turn can have profound consequences on PrEP engagement.
This study has several limitations. First, this was a purposive sample of Black MSM residing in Jackson, MS, a medium-size city in the Deep South, thus the findings may not be generalizable to other cities or settings. Second, we acknowledge there are inherent limitations in an ecological study design, particularly ecological fallacy (Lavrakas, PJ, 2008). Furthermore, neighborhood-level socioeconomic status was obtained using zip-code tabulated area which is an arbitrary boundary with heterogeneity (Duncan and Kawachi, 2018; Krieger, Williams & Moss, 1997. We used zip code as a geographical unit of measure to define neighborhoods. While neighborhoods are a unit that can be used to describe the local accessibility context that shapes health access and utilization, it does not necessarily imply causality. Additionally, we acknowledge percent of residents below the federal poverty at the neighborhood level may not fully capture the complexity of the concept of structural disadvantage of poor, urban neighborhoods. Future analyses should consider examining additional dimensions of neighborhoods (e.g., percent of unemployed, owner occupied houses, incarcerated persons) and different geographic boundaries (e.g. neighborhoods or census tracts). Finally, the use of AHUM to explain multi-level influences of PrEP use is a strength of this analysis. However, the model used (Andersen Model) was not considered in the development of the data collection instrument and therefore the measures included in this analysis are proximal measures of the constructs of the model. Future analyses should incorporate understandings of theory in the development of the data collection instrument.
Despite these limitations, the findings suggest that macro-determinants of health defined as median household income at the zip code level may partly explain decreased rates of PrEP uptake and use among Black MSM. Thus, these neighborhoods characterized by high levels of concentrated poverty may create vulnerability for Black MSM increasing their risk of acquiring HIV. Therefore, increasing the number of PrEP providers and HIV-related services without simultaneously addressing social determinants of health, particularly neighborhood structural inequalities, may be insufficient to affect health-seeking behavior of Black MSM vis-a-vis PrEP use.
CONCLUSIONS
In sum, the socioeconomic character of a neighborhood is associated with PrEP use among a sample of Black MSM. The results of the study show that explanations of PrEP use that rely solely on individual-level socio demographic characteristics and behavioral factors are incomplete; neighborhood structural poverty, in particular, the distribution and concentration of poverty, contributes significantly to the perpetuation of HIV vulnerability among Black MSM in urban cities. There is an urgent need to recognize the role of structural discrimination plays in shaping the social and structural character of poor, urban neighborhoods and increasing HIV vulnerability for vulnerable populations, particularly Black MSM. Healthcare providers, researchers, and policymakers should adopt structural approaches that mitigate the negative impacts of neighborhood structural disadvantage including: (1) incorporating screening and treatment for HIV-related structural risk factors; (2) investing in the development of novel and innovative structural-level HIV prevention interventions (e.g., workforce development, education, and housing programs) that complement biomedical strategies, such as PrEP; and (3) implementation of policies that address social and structural determinants of health to ensure we achieve EHE goal of ending the epidemic by 2030.
REFERENCES
- Andersen RM (1995). Revisiting the behavioral model and access to medical care: does it matter?. J Health Soc Behav. 1995;36(1):1–10. [PubMed] [Google Scholar]
- Andersen R, Newman JF (1973). Societal and Individual Determinants of Medical Care Utilization in the United States. Milbank Memorial Fund Quarterly. 1973;51(1):95–124. [PubMed] [Google Scholar]
- Arnold T, Brinkley-Rubinstein L, Chan PA, Perez-Brumer A, Bologna ES, Beauchamps L, Johnson K, Mena L, Nunn A. (2017). Social, structural, behavioral and clinical factors influencing retention in Pre-Exposure Prophylaxis (PrEP) care in Mississippi. PLoS One. 2017;12(2):e0172354. Published 2017 Feb 21. doi: 10.1371/journal.pone.017235420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsberg D, Haberer J, Psaros C, Baeten J, Katabira E, Tumwesigye E (2012). High adherence and high effectiveness observed in HIV discordant couples: Partners PrEP Study, adherence monitoring and counseling substudy.. Paper presented at: The Conference on Retroviruses and Opportunistic Infections (CROI); Seattle. 2012. [Google Scholar]
- Bauermeister JA, Meanley S, Pingel E, Soler JH, Harper GW. (2013). PrEP awareness and perceived barriers among single young men who have sex with men. Curr HIV Res. 2013 Oct;11(7):520–7. doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauermeister JA, Zimmerman MA, Caldwell CH (2011).. Neighborhood disadvantage and changes in condom use among African American adolescents. J Urban Health. 2011;88(1):66–83. doi: 10.1007/s11524-010-9506-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell LN, Diez Roux AV, Rose K, Catellier D, Clark BL (2004). Neighbourhood characteristics and mortality in the Atherosclerosis Risk in Communities Study. Int J Epidemiol. 2004;33:398–407. [DOI] [PubMed] [Google Scholar]
- Bowen A, Eikmeier D, Talley P, Siston A, Smith S, Hurd J, Smith K, Leano F, Bicknese A, Norton JC, Campbell D (2015).Centers for Disease Control and Prevention (CDC). MMWR Morb Mortal Wkly Rep. 2015 Jun 5; 64(21): 597–8. [PMC free article] [PubMed] [Google Scholar]
- Bowleg L. The problem with the phrase women and minorities: intersectionality-an important theoretical framework for public health. Am J Public Health. 2012;102(7):1267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braveman PA, Cubbin C, Egerter S, Williams DR, Pamuk E (2010). Socioeconomic disparities in health in the United States: what the patterns tell us. Am J Public Health. 2010;100 Suppl 1(Suppl 1):S186–S196. doi: 10.2105/AJPH.2009.166082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson J and Carson EA (2019). Prisoners in 2017. Age, 500, p.400. [Google Scholar]
- Burgard SA, Lee-Rife SM (2019). Community characteristics, sexual initiation, and condom use among young Black South Africans. J Health Soc Behav. 2009;50(3):293–309. doi: 10.1177/002214650905000304. [DOI] [PubMed] [Google Scholar]
- Burns PA, Snow RC (2012). The built environment & the impact of neighborhood characteristics on youth sexual risk behavior in Cape Town, South Africa. Health Place. 2012;18(5):1088–1100. doi: 10.1016/j.Health and Place.2012.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns PA, Williams MS, Mena LA, Bruce MA, Bender M, Burton ET, Beech BM (2020). Leveraging Community Engagement: The Role of Community-Based Organizations in Reducing New HIV Infections Among Black Men Who Have Sex with Men. J Racial Ethn Health Disparities. 2020;7(2):193–201. doi: 10.1007/s40615-019-00691-9. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2018). Diagnoses of HIV infection in the United States and dependent areas, 2017 pdf icon[PDF – 6 MB]. HIV Surveillance Report 2018;29. Available at: https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2017-vol-29.pdf. [Accessed 3 December 2020]. [Google Scholar]
- Centers for Disease Control and Prevention (2019). HIV and African Americans. Avaliable at: https://www.cdc.gov/hiv/group/racialethnic/africanamericans/index.html. [Accessed 3 December 2020].
- Centers for Disease Control and Prevention (2020a). HIV in the Southern United States. Available at: https://www.cdc.gov/hiv/pdf/policies/cdc-hiv-in-the-south-issue-brief.pdf. [Accessed 3 December 2020].
- Centers for Disease Control and Prevention (2020b). Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas—2015 pdf icon[PDF – 2 MB]. HIV Surveillance Supplemental Report 2017;22(2). Available at: https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-22-2.pdf. [Accessed 3 December 2020]. [Google Scholar]
- Centers for Disease Control and Prevention (2020c). Diagnoses of HIV infection among adults and adolescents in metropolitan statistical areas—United States and Puerto Rico, 2018. HIV Surveillance Data Tables 2020;1(No. 3). Available at: http://www.cdc.gov/hiv/library/reports/surveillance-data-tables/vol-1-no-3/index.html. Published August 2020. [Accessed 3 December 2020]. [Google Scholar]
- Centers for Disease Control and Prevention. HIV Surveillance Report (2018) (Preliminary); vol. 30. Available at: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published November 2019. [Accessed 3 December 2020]. [Google Scholar]
- Centers for Disease Control and Prevention. Healthy People (2020). https://www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-health/interventions-resources/poverty Accessed 7/17/2021
- Chan PA, Mena L, Patel R, Oldenburg CE, Beauchamps L, Perez-Brumer AG, Parker S, Mayer KH, Mimiaga MJ, Nunn A (2016). Retention in care outcomes for HIV pre-exposure prophylaxis implementation programmes among men who have sex with men in three US cities. J Int AIDS Soc. 2016;19(1):20903. Published 2016 Jun 13. doi: 10.7448/IAS.19.1.20903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M et al. , (2013). Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083–2090. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- Christine PJ, Auchincloss AH, Bertoni AG, Carnethon MR, Sanchez BN, Moore K, Adar SD, Horwich TB, Watson KE, Diez Roux AV (2015). Longitudinal Associations Between Neighborhood Physical and Social Environments and Incident Type 2 Diabetes Mellitus: The Multi-Ethnic Study of Atherosclerosis (MESA) JAMA Intern Med. 2015;175:1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D, Spear S, Scribner R, Kissinger P, Mason K, Wildgen J (2000). “Broken windows” and the risk of gonorrhea. Am J Public Health. 2000;90(2):230–236. doi: 10.2105/ajph.90.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes J, Castro MC (2020). Spatial analysis of COVID-19 clusters and contextual factors in New York City. Spat Spatiotemporal Epidemiol. 2020;34:100355. doi: 10.1016/j.sste.2020.100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai M, Field N, Grant R, McCormack S (2017). Recent advances in pre-exposure prophylaxis for HIV. BMJ. 2017 Dec 11;359:j5011. doi: 10.1136/bmj.j5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez Roux AV, Mujahid MS, Hirsch JA, Moore K, Moore LV (2016). The Impact of Neighborhoods on CV Risk. Glob Heart. 2016;11:353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez Roux AV (2001). Investigating neighborhood and area effects on health. Am J Public Health. 2001;91(11):1783–1789. doi: 10.2105/ajph.91.11.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux Diez, 2001; Williams DR, Collins C. (2001). Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001;116(5):404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckathorn DD (1997). Respondent-Driven Sampling: A New Approach to the Study of Hidden Populations, Social Problems, Volume 44, Issue 2, 1 May 1997, Pages 174–199, 10.2307/309694. [DOI] [Google Scholar]
- Duncan DT, Kawachi I (2018). Neighborhoods and Health: Oxford University Press. [Google Scholar]
- Fallon SA, Park JN, Ogbue CP, Flynn C, German D (2017). Awareness and Acceptability of Pre-exposure HIV Prophylaxis Among Men Who have Sex with Men in Baltimore. AIDS Behav. 2017 May;21(5):1268–1277. doi: 10.1007/s10461-016-1619-z. [DOI] [PubMed] [Google Scholar]
- Finlayson T, Cha S, Xia M, Trujillo L, Denson D, Prejean J et al. , (2019). Changes in HIV Preexposure Prophylaxis Awareness and Use Among Men Who Have Sex with Men - 20 Urban Areas, 2014 and 2017. MMWR Morb Mortal Wkly Rep. 2019;68(27):597–603. Published 2019 Jul 12. doi: 10.15585/mmwr.mm6827a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006. May;96(5):826–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golrokhi R, Farhoudi B, Taj L, Pahlavian FG, Mazaheri-Tehrani E, Cossarizza A, SeyedAlinaghi S, Mohraz M, & Voltarelli FA (2018). HIV Prevalence and Correlations in Prisons in Different Regions of the World: A Review Article. The open AIDS journal, 12, 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L et al. (2010). Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Resources and Services Administration, Ryan White HIV/AIDS Program, National HIV/AIDS Strategy (2021) https://hab.hrsa.gov/about-ryan-white-hivaids-program/national-hivaids-strategy-updated-2020. Accessed 6/16/21 [Google Scholar]
- Hess KL, Hu X, Lansky A, Mermin J, Hall HI (2017). Lifetime risk of a diagnosis of HIV infection in the United States. Ann Epidemiol. 2017;27(4):238–243. doi: 10.1016/j.annepidem.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billy John O. G., Brewster K, & Grady W (1994). Contextual Effects on the Sexual Behavior of Adolescent Women. Journal of Marriage and Family, 56(2), 387–404. doi: 10.2307/353107. [DOI] [Google Scholar]
- Johnson MO, Rose CD, Dilworth SE, Neilands TB (2012). Advances in the conceptualization and measurement of Health Care Empowerment: development and validation of the Health Care Empowerment inventory. PLoS One. 2012;7(9):e45692. doi: 10.1371/journal.pone.0045692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby JB, Kaneda T (2005). Neighborhood socioeconomic disadvantage and access to health care. J Health Soc Behav. 2005 Mar;46(1):15–31. doi: 10.1177/002214650504600103. [DOI] [PubMed] [Google Scholar]
- Krause N. (1995). Negative interaction and satisfaction with social support among older adults. Journal of Gerontology: Psychological Sciences, 50B, 59–73. [DOI] [PubMed] [Google Scholar]
- Krieger N. Theories for social epidemiology in the twenty-first century—an ecosocial perspective. In: Hofrichter R, editor. Health and Social Justice—Politics, Ideology and Inequity in the Distribution of Disease. Jossey-Bass; San Francisco: 2003. [Google Scholar]
- Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian S. (2005). Painting a truer picture of US socioeconomic and racial/ethnic health inequalities: the Public Health Disparities Geocoding Project. Am J Public Health. 2005;95(2):312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N, Williams DR, Moss NE (1997). Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341–78. doi: 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]
- Krüsi A, Wood E, Montaner J, Kerr T. Social and structural determinants of HAART access and adherence among injection drug users. Int J Drug Policy. 2010. Jan;21(1):4–9. [DOI] [PubMed] [Google Scholar]
- Lavrakas PJ (2008). Encyclopedia of survey research methods (Vols. 1–0). Thousand Oaks, CA: Sage Publications, Inc. doi: 10.4135/9781412963947. [DOI] [Google Scholar]
- Liu AY, Cohen SE, Vittinghoff E, et al. (2016). Preexposure Prophylaxis for HIV Infection Integrated With Municipal- and Community-Based Sexual Health Services. JAMA Intern Med. 2016;176(1):75–84. doi: 10.1001/jamainternmed.2015.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millett GA, Jones AT, Benkeser D, Baral S, Mercer L, Beyrer C, Honermann B, Lankiewicz E, Mena L, Crowley JS, Sherwood J, Sullivan PS. (2020). Assessing differential impacts of COVID-19 on black communities. Ann Epidemiol. 2020;47:37–44. doi: 10.1016/j.annepidem.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milloy MJ, Kerr T, Buxton J, Rhodes T, Krusi A, Guillemi S, Hogg R, Montaner J, Wood E. (2012). Social and environmental predictors of plasma HIV RNA rebound among injection drug users treated with antiretroviral therapy. Journal of Acquired Immune Deficiency Syndromes (1999). 2012 Apr;59(4):393–399. DOI: 10.1097/qai.0b013e3182433288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mississippi State Department of Health STD/HIV Office (2016). 2014 Annual HIV Summary For Mississippi September 1, 2016. Jackson MS. Available at: https://msdh.ms.gov/msdhsite/_static/resources/6921.pdf. [Accessed 3 December 2020]. [Google Scholar]
- Morenoff JD, House JS, Hansen BB, Williams DR, Kaplan GA, Hunte HE. (2007). Understanding social disparities in hypertension prevalence, awareness, treatment, and control: the role of neighborhood context. Soc Sci Med. 2007;65:1853–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palepu A, Milloy MJ, Kerr T, Zhang R, & Wood E (2011). Homelessness and adherence to antiretroviral therapy among a cohort of HIV-infected injection drug users. Journal of urban health : bulletin of the New York Academy of Medicine, 88(3), 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker CM, Garcia J, Philbin MM, Wilson PA, Parker RG, Hirsch JS (2017). Social risk, stigma and space: key concepts for understanding HIV vulnerability among black men who have sex with men in New York City. Cult Health Sex. 2017;19(3):323–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT, Rendina HJ, Lassiter JM, Whitfield TH, Starks TJ, Grov C.(2017). Uptake of HIV Pre-Exposure Prophylaxis (PrEP) in a National Cohort of Gay and Bisexual Men in the United States. J Acquir Immune Defic Syndr. 2017;74(3):285–292. doi: 10.1097/QAI.0000000000001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett KE, Pearl M. (2001). Multilevel analyses of neighbourhood socioeconomic context and health outcomes: a critical review. J Epidemiol Community Health. 2001;55(2):111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poteat T (2021). Navigating the Storm: How to Apply Intersectionality to Public Health in Times of Crisis. American Journal of Public Health, 11, no. 1 (January 1, 2021): pp. 91–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price-Haywood EG, Burton J, Fort D, Seoane L (2020). Hospitalization and Mortality among Black Patients and White Patients with Covid-19. N Engl J Med. 2020;382(26):2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransome Y, Bogart LM, Kawachi I, Kaplan A, Mayer KH, Ojikutu B (2020). Area-level HIV risk and socioeconomic factors associated with willingness to use PrEP among Black people in the U.S. South. Ann Epidemiol. 2020;42:33–41. doi: 10.1016/j.annepidem.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal DW, Eldridge GD, Zack B, Sosman J (2010). HIV testing and treatment with correctional populations: people, not prisoners. J Health Care Poor Underserved. 2010;21(3):977–985. doi: 10.1353/hpu.0.0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons RL, Lei MK, Beach SRH, Barr AB, Simons LG, Gibbons FX, Philibert RA (2018). Discrimination, segregation, and chronic inflammation: Testing the weathering explanation for the poor health of Black Americans. Dev Psychol. 2018;54(10):1993–2006. doi: 10.1037/dev0000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M. Introduction to syndemics: A critical systems approach to public and community health. John Wiley & Sons; 2009. [Google Scholar]
- Snowden JM, Chen YH, McFarland W, Raymond HF (2014). Prevalence and characteristics of users of pre-exposure prophylaxis (PrEP) among men who have sex with men, San Francisco, 2014 in a cross-sectional survey: implications for disparities. Sex Transm Infect. 2017;93(1):52–55. doi: 10.1136/sextrans-2015-052382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommet N, & Morselli D (2017). Keep calm and learn multilevel logistic modeling: A simplified three-step procedure using stata, R, Mplus, and SPSS. International Review of Social Psychology, 30, 203–218. [Google Scholar]
- Stall R, Mills TC, Williamson J, Hart T, Greenwood G, Paul J et al. (2003). Association of co-occurring psychosocial health problems and increased vulnerability to HIV/AIDS among urban men who have sex with men. Am J Public Health. 2003;93(6):939–942. doi: 10.2105/ajph.93.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss BB, Greene GJ, Phillips G 2nd, Bhatia R, Madkins K, Parsons JT, Mustanski B. (2017). “Exploring Patterns of Awareness and Use of HIV Pre-Exposure Prophylaxis Among Young Men Who Have Sex with Men.” AIDS and behavior vol. 21,5 (2017): 1288–1298. doi: 10.1007/s10461-016-1480-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Office of National AIDS Policy (ONAP) (2020). The White House. National HIV/AIDS Strategy for the United States-Updated 2020. Available at: https://files.hiv.gov/s3fs-public/nhas-update.pdf. [Accessed 3 December 2020].
- The Sentencing Project (2020). Trends in U.S. Corrections Available at: https://www.sentencingproject.org/wp-content/uploads/2020/08/Trends-in-US-Corrections.pdf. [Accessed 3 December 2020].
- Thigpen MC, Kebaabetswe PM, Paxton LA, Dawn DK, Rose CE, Segolodi T et al. , (2012). Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5):423–434. doi: 10.1056/NEJMoa1110711]. [DOI] [PubMed] [Google Scholar]
- Truong KD, Ma S. (2006). A systematic review of relations between neighborhoods and mental health. J Ment Health Policy Econ. 2006;9:137–154. [PubMed] [Google Scholar]
- Turan JM, Elafros MA, Logie CH, Swagata B, Turan B, Crockett KB et al. , (2019) Challenges and opportunities in examining and addressing intersectional stigma and health. BMC Med 17, 7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health & Human Services, Office of Minority Health, Profile/African Americans. (2021). https://www.minorityhealth.hhs.gov/omh/browse.aspx?lvl=3&lvlid=61 accessed 6/13/21
- Williams DR, Lawrence JA, Davis BA (2019). Racism and Health: Evidence and Needed Research. Annu Rev Public Health. 2019;40:105–125. doi: 10.1146/annurev-publhealth-040218-043750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Neighbors HW, & Jackson JS (2003). Racial/ethnic discrimination and health: Findings from community studies. American Journal of Public Health, 93, 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR & Mohammed SA (2013). Racism and health I: Pathways and scientific evidence. American Behavioral Scientist, 57, 1152–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2020). HIV/AIDS- People in Prison and Other Closed Settings, Available at: https://www.who.int/hiv/topics/prisons/about/en/. [Accessed 3 December 2020].


