Abstract
Objective:
To identify risk factors for suicidal ideation (SI) following mild traumatic brain injury (mTBI).
Setting:
Eleven US level 1 trauma centers.
Participants:
A total of 1158 emergency department patients with mTBI (Glasgow Coma Scale score=13–15) enrolled in the Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) study.
Design:
Prospective observational study; weights-adjusted multivariable logistic regression models (n’s = 727–883) estimated associations of baseline factors and post-TBI symptoms with SI at 2 weeks and 3, 6, and 12months postinjury.
Main Measures:
PatientHealth Questionnaire, Rivermead Post-Concussion Symptoms Questionnaire.
Results:
Preinjury psychiatric history predicted SI at all follow-ups (adjusted odds ratios [AORs] = 2.26–6.33, P values <.05) and history of prior TBI predicted SI at 2 weeks (AOR = 2.36, 95% confidence interval [CI] = 1.16–4.81, P = .018), 3 months (AOR = 2.62, 95% CI = 1.33–5.16, P = .005), and 6 months postinjury (AOR = 2.54, 95% CI=1.19–5.42, P=.016). Adjusting for these baseline factors, post-TBI symptoms were strongly associated with SI at concurrent (AORs = 1.91–2.88 per standard deviation unit increase in Rivermead Post-Concussion Symptoms Questionnaire score; P values <.0005) and subsequent follow-up visits (AORs = 1.68–2.53; P values <.005). Most of the associations between post-TBI symptoms and SI were statistically explained by co-occurring depression.
Conclusion:
Screening for psychiatric and prior TBI history may help identify patients at risk for SI following mTBI. Awareness of the strong associations of post-TBI symptoms with SI may facilitate interventions to prevent suicide-related outcomes in patients with mTBI.
Keywords: concussion, depression, postconcussive symptoms, suicidal ideation, traumatic brain injury
TRAUMATIC BRAIN INJURY (TBI) causes more than 2.5 million emergency department (ED) visits annually in the United States.1 The majority of TBIs involve transient disruptions in mental status and are classified as mild.2 However, even mild TBI may result in persistent symptoms and long-term problems with social or occupational functioning.3–5
Among the most concerning observations regarding long-term TBI sequelae are those involving suicide-related outcomes.6–8 Associations between TBI and suicide mortality have been found in general population,9–11 military,12,13 and clinical samples,14 and a recent meta-analysis suggests that even mild TBI is associated with a 2-fold increased risk of suicide death.15 Risks of suicidal ideation (SI) and suicide attempts also appear elevated among individuals with mild TBI.15,16
Despite mounting evidence of an association between mild TBI and suicidality, little is known about factors that modify risk of suicidal thoughts and behaviors during the months following injury. Better understanding of this topic could improve care of patients with mild TBI and inform prevention efforts. Some findings point to sociodemographic characteristics,17–19 prior TBI,20–22 mental disorders,6,18,19,23 and substance abuse23 as factors that might affect risk of suicidality after mild TBI. However, evidence largely derives from cross-sectional investigations and studies that included patients with moderate-to-severe TBI. Links between injury-related factors (eg, cause of mild TBI) and other mental health outcomes such as posttraumatic stress disorder24 have been reported; thus, the relationship of injury-related variables to SI merits further investigation. Finally, although postconcussive/post-TBI symptoms correlate strongly with mental disorder symptoms,25,26 few studies have examined the relationship between post-TBI symptom severity and suicidality among individuals with mild TBI.19,27
To address these gaps in the literature, we analyzed data from Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI), a prospective multisite study of ED patients with TBI. To identify risk factors for SI, we evaluated the joint associations of baseline demographic, injury-related, and clinical characteristics with odds of SI at 2 weeks and 3, 6, and 12 months postinjury. In subsequent models that adjusted for baseline factors, we examined whether post-TBI symptoms were associated with odds of SI at follow-up, and whether any associations between post-TBI symptoms and SI were explained by co-occurring depression.
METHODS
Participants/overview
TRACK-TBI (ClinicalTrials.gov NCT02119182) is a prospective, observational study of patients with TBI who presented to EDs of 11 US level 1 trauma centers. To be eligible, patients must have arrived at the ED within 24 hours of injury, reported or displayed evidence of alterations of consciousness or amnesia, and had a head trauma warranting clinical evaluation with a noncontrast head computed tomography (CT) scan ordered by the evaluating physician. The most common reasons for exclusion from TRACK-TBI were unlikely to follow up (eg, low interest in participation, nonresident, homeless; 27.7%), significant medical history that would interfere with follow-up (15.1%), presented more than 24 hours after injury (13.6%), “other” (10.9%), and CT not obtained (9.0%). The “other” category included unable to obtain informed consent, refusal to consent, failure to meet American Congress of Rehabilitation Medicine criteria for TBI, and uncategorized. Less frequent reasons for exclusion (23.7% total) included debilitating mental or neurological disorders that would interfere with informed consent or follow-up/outcome assessment, significant polytrauma that would interfere with follow-up/outcome assessment, language other than English (or Spanish at sites enrolling Spanish speakers), magnetic resonance imaging incompatibility, being a prisoner or in custody, current participation in an interventional trial, being on a mandated psychiatric hold, and pregnancy.
Baseline assessment was conducted in person shortly after ED evaluation. Follow-ups occurred at 2 weeks and 3, 6, and 12 months postinjury. Follow-ups were in person, except for the 3-month visit, which was conducted via telephone. Other follow-ups were occasionally conducted by phone if an in-person visit was not feasible. All patients or their legal representatives gave written informed consent to participate, and study protocols were approved by the institutional review boards at all study sites.
The eligible sample for this study comprised 1158 patients aged 17 years and older (patients whose age was Protected Health Information [n = 2] had age imputed as 90 years) who enrolled in TRACK-TBI between February 26, 2014, and May 4, 2016, and had mild TBI defined as a Glasgow Coma Scale28 score of 13 to 15 upon ED arrival. Because of attrition/missing data, sample sizes ranged from 727 to 883 for the models of SI at 2 weeks and 3, 6, and 12 months.
Measures
Suicidal ideation
Suicidal ideation was measured using item 9 of the Patient Health Questionnaire (PHQ-9),29 a well-validated measure of depression. The PHQ-9 instructions ask respondents to rate how often they have been bothered by depressive symptoms during the past 2 weeks (0 = not at all, 1 = several days, 2 = more than half the days, or 3 = nearly every day). Item 9 evaluates thoughts that you would be better off dead, or of hurting yourself. Patients who rated this item as greater than 0 were classified as having SI.
Baseline characteristics
Demographic information collected at baseline included age, sex, race, ethnicity, and years of education. Evaluation of prior TBI was based on The Ohio State University TBI Identification Method, a validated semistructured assessment of TBI history.30 Respondents were instructed to consider injuries to your head or neck that you may have had at any time in your life while answering a series of yes/no questions about whether they had experienced such injuries related to moving vehicle accidents, falls/being hit by something, sports participation, and other circumstances. Each reported head/neck injury was then queried to assess cause, age, disposition, and whether loss of consciousness (LOC), alteration of consciousness (AOC), or posttraumatic amnesia (PTA) had occurred. We derived a dichotomous variable representing any lifetime history of prior TBI. Respondents who reported 1 or more head/neck injuries that resulted in LOC, AOC, or PTA were classified as having prior TBI.
The baseline interview also contained 3 items assessing whether patients had ever received inpatient treatment, outpatient treatment/counseling, or pharmacotherapy for mental health problems. Patients who responded positively to any of these items were classified as having a preinjury psychiatric history. The Alcohol Use Disorders Identification Test—Consumption scale (AUDIT-C) was used to assess hazardous alcohol use, defined as AUDIT-C of 4 or more for males and AUDIT-C of 3 or more for females.31 We categorized participants as having past year drug use based on a yes/no item that assessed use of illicit/recreational drugs or misuse of prescription drugs in the preceding year (any versus none).
Medical records and information from the baseline interview were used to determine cause of injury, neuroimaging results (positive or negative findings referable to trauma on head CT), ED disposition, and presence of LOC and PTA. Per a prior TRACK-TBI study,24 injury causes (eg, motor vehicle collision, fall, assault) were classified into 2 categories for analysis (accidental or violent cause). The CT scans were read by a board-certified neuroradiologist and results were categorized as positive (evidence of intracranial injury) versus negative (no intracranial injury). Patient triage disposition was categorized as discharge from the ED, hospitalization outside of the intensive care unit (ward), or hospitalization in the intensive care unit. The rate of missing data for LOC (6%) and PTA (10%) would have significantly reduced sample sizes for the models of SI; we decided to omit these variables from the models based on analyses showing that the bivariate associations of LOC and PTA with SI at all follow-ups were nonsignificant (see Supplemental Digital Content 1, available at: http://links.lww.com/JHTR/A355).
Post-TBI and depressive symptoms at follow-up
The Rivermead Post-Concussion Symptoms Questionnaire (RPQ)32 contains 16 items evaluating somatic, cognitive, and emotional symptoms that may occur following TBI. The version used in TRACK-TBI asked respondents to rate symptom severity during the last 7 days. The RPQ items are rated as 0 (not experienced at all), 1 (no more of a problem than preinjury), 2 (mild problem), 3 (moderate problem), or 4 (severe problem); however, ratings of 1 are recoded as 0, given that a rating of 1 indicates stability of a preexisting symptom.32 The most comprehensive psychometric study of the RPQ to date33 suggests that the scale is largely unidimensional, justifying use of total scores. The RPQ total scores were derived by summing the recoded item ratings (theoretical range = 0–64). We present raw RPQ scores in descriptive analyses to convey absolute levels of post-TBI symptom severity. For the logistic regression analysis, we standardized the RPQ scores (M = 0, SD = 1). This approach yields more interpretable odds ratios that represent the change in odds of SI associated with a standard deviation increase in RPQ score.
Because our measure of SI derived from the PHQ-9, we used another measure, the depression subscale of the Brief Symptom Inventory (BSI-18),34 to quantify severity of depressive symptoms at follow-up. The BSI-18 asks respondents to rate how much emotional and somatic symptoms have bothered them during the past 7 days (0 = not at all to 4 = very much). We derived a modified BSI-18 depression (BSI-D) subscale score by calculating the sum of all BSI-D items except for item 17 that assesses SI (theoretical range 0–20). Modified BSI-D scores were standardized (M = 0, SD = 1) prior to regression analysis to facilitate interpretation of coefficients.
Data analysis
To adjust for missing data at follow-up visits, we applied propensity weights using generalized boosted regression models.35 Weights-adjusted multivariable logistic regression models were fit to evaluate the associations of baseline variables with SI at 2 weeks and 3, 6, and 12 months. Subsequent models that adjusted for the baseline variables examined whether post-TBI symptoms were associated with SI at the same follow-up visit and at the subsequent follow-up visit and whether any associations between post-TBI symptoms and SI were explained by depression. Variance inflation factors were examined for all models to rule out multicollinearity among the independent variables, and Hosmer-Lemeshow goodness-of-fit tests were examined to rule out poor-fitting models. Design-based Wald χ2 tests were used to examine multivariable significance. R version 3.5.1 was used to conduct all analyses.36 Two-tailed P < .05 was considered statistically significant.
RESULTS
Sample characteristics at baseline
The majority of the analysis sample was male (65.1%) and white (77.0%), with 17.2% identifying as black and 5.8% specifying other race. Average age was 40.5 years (SD = 17.2) and average education level was 13.5 years (SD = 2.9). Approximately one-third (31.7%) reported prior TBI, two-fifths (39.8%) screened positive for hazardous alcohol use, one-fourth (24.6%) reported past-year drug use, and one-fifth (21.2%) had a psychiatric history. In terms of injury characteristics, the vast majority of mild TBIs were due to accidents (94.0%), with just 6.0% attributable to violent causes (eg, assault). Most CT results were negative (69.3%). About two-thirds of mild TBI patients had ward (42.0%) or intensive care unit (24.3%) admissions, with the remainder discharged from the ED (33.8%).
Baseline characteristics stratified by SI status at each follow-up visit are shown in Supplemental Digital Content 1, available at: http://links.lww.com/JHTR/A355. Bivariate Fisher exact tests showed that preinjury psychiatric history (P values < .01), prior TBI (P values < .01), and past-year drug use (P values < .05) were more common in those with SI at each follow-up.
Sample characteristics at follow-up
Weighted point prevalence of SI on the PHQ-9 was 5.4%, 5.3%, 4.3%, and 4.0% at 2 weeks and 3, 6, and 12 months, respectively. Of the 1009 mild TBI patients with PHQ-9 data from 1 or more follow-ups, 103 (10.2%) reported SI during at least 1 follow-up. Among the 630 mild TBI patients with PHQ-9 data from all 4 follow-ups, the most common patterns of SI endorsement were SI at 3 months only (n = 14), SI at 6 months only (n = 11), SI at 12 months only (n = 11), SI at 2 weeks only (n = 10), and SI at all 4 visits (n = 9).
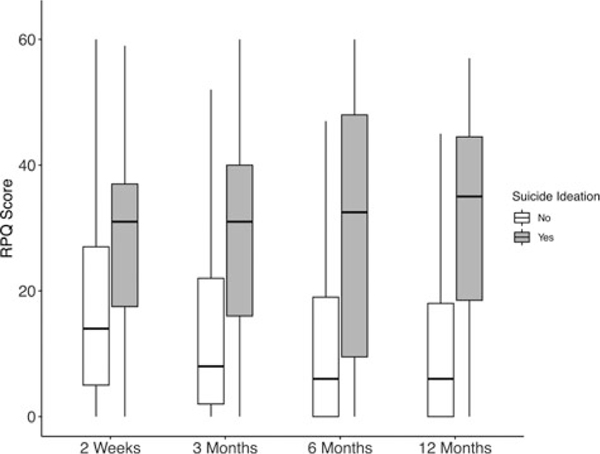
Mean RPQ score at follow-up ranged from 18.3 (SD = 15.0) at 2 weeks to 12.2 (SD = 14.3) at 12 months, and mean BSI-D score ranged from 3.8 (SD = 4.5) at 2 weeks to 2.8 (SD = 4.2) at 12 months (see Supplemental Digital Content 1, available at: http://links.lww.com/JHTR/A355). Post-TBI and depressive symptoms were strongly correlated at all time points (r values = 0.61–0.68, P values < .001), and Wilcoxon rank sum tests indicated that patients with SI reported substantially more post-TBI and depressive symptoms at all follow-ups (P values < .0001; see Figure 1 and Supplemental Digital Content 1, available at: http://links.lww.com/JHTR/A355).
Figure 1.
Rivermead Post-Concussion Symptoms Questionnaire scores at 2 weeks, 3 months, 6 months, and 12 months postinjury by suicidal ideation (yes/no) on item 9 of the Patient Health Questionnaire. RPQ indicates Rivermead Post-Concussion Symptoms Questionnaire.
Baseline predictors of SI at follow-up
The first set of multivariable logistic regression models estimated associations of baseline factors with SI at each follow-up visit (see Table 1). Psychiatric history was associated with increased odds of SI at all follow-up visits (2 weeks: adjusted odds ratio [AOR] = 2.26, 95% confidence interval [CI] = 1.01–5.05, P = .046; 3 months: AOR = 3.07, 95% CI = 1.47–6.42, P = .003; 6 months: AOR = 4.55, 95% CI = 1.90–10.86, P = .001; and 12 months: AOR = 6.33, 95% CI = 2.68–14.95, P < .0005). Prior TBI predicted SI at 2 weeks (AOR = 2.36, 95% CI = 1.16–4.81, P = .018), 3 months (AOR = 2.62, 95% CI = 1.33–5.16, p = .005), and 6 months (AOR = 2.54, 95% CI = 1.19–5.42, p = .016), but its association with SI at 12 months was nonsignificant (P = .10). Patients who identified as black or other race had higher odds of SI than white patients (AOR = 2.68, 95% CI = 1.26–5.73, P = .011) and Hispanic patients had higher odds of SI than non-Hispanic patients (AOR = 3.05, 95% CI = 1.29–7.25, P = .011) at the 3-month follow-up only. Other baseline variables did not predict SI in any of the multivariable models.
TABLE 1.
Associations of baseline demographic, injury-related, and clinical characteristics with suicidal ideation at follow-up among patients with mild traumatic brain injurya
| Association with suicidal ideation |
||||||||
|---|---|---|---|---|---|---|---|---|
| 2 wk (n = 883) |
3 mo (n = 830) |
6 mo (n = 788) |
12 mo (n = 727) |
|||||
| Baseline factors | AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | AOR | 95% CI |
|
| ||||||||
| Age, y | 0.98 | 0.96–1.00 | 0.99 | 0.97–1.01 | 1.00 | 0.98–1.02 | 0.99 | 0.97–1.01 |
| Female sex (reference: male) | 1.03 | 0.49–2.18 | 1.31 | 0.65–2.62 | 0.66 | 0.28–1.52 | 0.65 | 0.28–1.55 |
| Black/other raceb (reference: white) | 1.32 | 0.59–2.96 | 2.68 | 1.26–5.73 | 1.78 | 0.74–4.26 | 1.49 | 0.61–3.66 |
| Hispanic ethnicity (reference: non-Hispanic) | 1.67 | 0.64–4.35 | 3.05 | 1.29–7.25 | 1.63 | 0.53–5.03 | 0.88 | 0.21–3.72 |
| Education, y | 0.97 | 0.86–1.10 | 0.97 | 0.88–1.08 | 1.10 | 0.96–1.25 | 0.94 | 0.83–1.08 |
| Violent injury causec (reference: accidental) | … | … | 0.69 | 0.20–2.46 | 0.37 | 0.05–2.81 | 1.46 | 0.39–5.47 |
| Positive CT result (reference: negative result) | 0.44 | 0.17–1.13 | 0.81 | 0.32–2.10 | 0.49 | 0.16–1.53 | 0.91 | 0.36–2.29 |
| Ward admission (reference: ED discharge) | 1.76 | 0.85–3.62 | 0.71 | 0.34–1.49 | 0.87 | 0.40–1.91 | 0.75 | 0.32–1.78 |
| ICU admission (reference: ED discharge) | 1.64 | 0.60–4.50 | 0.70 | 0.22–2.18 | 1.06 | 0.29–3.88 | 1.42 | 0.50–4.06 |
| Psychiatric history (reference: no history) | 2.26 | 1.01–5.05 | 3.07 | 1.47–6.42 | 4.55 | 1.90–10.86 | 6.33 | 2.68–14.95 |
| Prior TBI (reference: no prior TBI) | 2.36 | 1.16–4.81 | 2.62 | 1.33–5.16 | 2.54 | 1.19–5.42 | 1.93 | 0.87–4.24 |
| Hazardous alcohol used (reference: no) | 0.67 | 0.34–1.31 | 0.99 | 0.51–1.95 | 0.80 | 0.36–1.77 | 0.87 | 0.41–1.82 |
| Past year drug use (reference: none) | 1.38 | 0.69–2.75 | 1.30 | 0.66–2.54 | 1.51 | 0.69–3.29 | 1.61 | 0.68–3.85 |
| Hosmer-Lemeshow goodness of fite, χ 2, P | 4.91 | P = .77 | 8.91 | P = .35 | 11.09 | P = .20 | 7.25 | P = .51 |
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; CT, computed tomographic; ED, emergency department; ICU, intensive care unit; TBI, traumatic brain injury.
The table displays results of 4 separate multivariable logistic regression models that evaluated associations of baseline variables with reporting suicidal ideation on the Patient Health Questionnaire at 2 weeks and at 3, 6, and 12 months following mild TBI. Values in boldface indicate statistical significance (P < .05).
Because of low representation of some racial groups, race was dichotomized (white versus black or other) prior to the regression analysis.
Injury cause was removed from the model of 2-week suicidal ideation because 1 group had zero outcome events, preventing accurate estimation of the odds ratio and 95% confidence interval. Injury cause was not associated with suicidal ideation at 2 weeks postinjury in a bivariate model (P > .10).
Defined as a total score of 4 or more (for males) and 3 or more (for females) on the Alcohol Use Disorders Identification Test—Consumption Scale (AUDIT-C).
For Hosmer-Lemeshow goodness-of-fit tests, 8 df with number of groups (g = 10) were used.
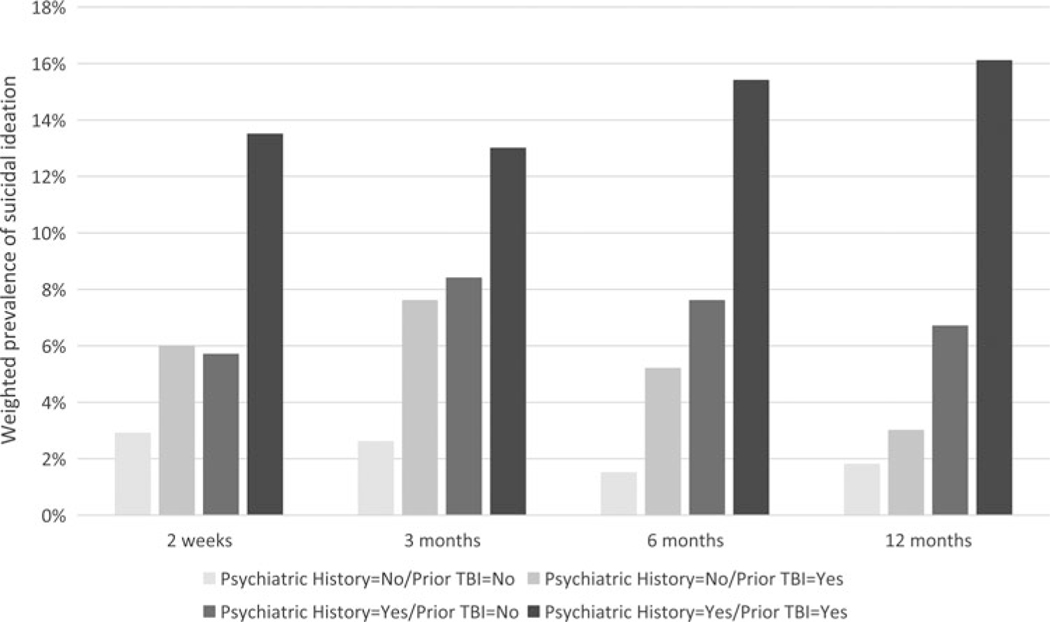
Given the robust effects of psychiatric history and prior TBI on SI, post hoc models that included psychiatric history prior × TBI interactions were fit to explore possible synergistic effects of these factors. The interaction terms were nonsignificant in all models (P values > .40), suggesting that psychiatric history and prior TBI had additive as opposed to synergistic effects. The prevalence of SI at follow-up for patients with psychiatric history, prior TBI, both risk factors, and neither risk factor reflects their additive effects (see Figure 2).
Figure 2.
Weighted prevalence of suicidal ideation among emergency department patients with mild TBI by preinjury psychiatric history and history of prior TBI. Suicidal ideation during the preceding 2 weeks was assessed using item 9 of the Patient Health Questionnaire at 2 weeks, 3 months, 6 months, and 12 months postinjury. TBI indicates traumatic brain injury.
Post-TBI symptoms and SI
Adjusting for all baseline variables, post-TBI symptoms at each visit were strongly associated with SI at the same follow-up visit (2 weeks: AOR = 1.91 per standard deviation unit increase in RPQ score, 95% CI = 1.48–2.45; 3 months: AOR = 2.23, 95% CI = 1.74–2.86; 6 months: AOR = 2.47, 95% CI = 1.75–3.49; and 12 months: AOR = 2.88, 95% CI = 2.09–3.97, P values <.0005). The modified BSI-D score was added to these models to evaluate whether the cross-sectional associations of post-TBI symptoms with SI were statistically explained by co-occurring depression. As expected, depression was strongly associated with SI at each follow-up (AORs = 3.20–5.00 per standard deviation unit increase in modified BSI-D score; P values <. 0005). Adjustment for concurrent depression resulted in marked attenuation of the associations of post-TBI symptoms with SI (AORs = 0.74–1.15 per standard deviation unit increase in RPQ score; all P values > .20).
We also evaluated whether post-TBI symptom severity contributed to prediction of SI at the subsequent follow-up visit. Adjusting for all baseline variables, RPQ score at 2 weeks predicted SI at 3 months (AOR = 1.68, 95% CI = 1.22–2.32, P = .001), RPQ score at 3 months predicted SI at 6 months (AOR = 1.80, 95% CI = 1.29–2.51, P = .001), and RPQ score at 6 months predicted SI at 12 months (AOR = 2.53, 95% CI = 1.75–3.65, P < .0005). When controls for SI at the prior visit were applied (eg, SI at 2 weeks was added to the model of SI at 3 months), the effects of the temporally prior RPQ score on SI at 3 months (AOR = 1.37, 95% CI = 0.92–2.06, P = .13) and SI at 6 months (AOR = 1.37, 95% CI = 0.85–2.21, P = .20) became nonsignificant. However, the effect of RPQ score at 6 months on SI at 12 months remained significant after adjusting for SI at 6 months (AOR = 1.76, 95% CI = 1.22–2.54, P = .002), as well as after adjusting for both SI and BSI-D score at 6 months (AOR = 1.83, 95% CI = 1.17–2.87, P = .008).
DISCUSSION
In a cohort of patients with mild TBI presenting to level 1 trauma centers, preinjury psychiatric history and prior TBI were the strongest baseline predictors of SI during the year after injury. Patients reporting greater post-TBI symptoms at follow-up also reported more SI, which appeared largely explained by the higher burden of depression these patients experienced. Yet, evidence also suggested that post-TBI symptoms at 6 months contributed independently to risk of SI at 12 months, beyond the effects of prior SI and depression. These findings emphasize the importance of screening for mental health problems and prior TBI in the ED and of assessment of depression and SI among patients with persistent post-TBI symptoms.
Robust associations between preinjury psychiatric history and postinjury SI were expected, given that a range of mental disorders are strongly associated with suicidality.37 Previous prospective studies of patients with complicated mild to severe TBI,18 and of patients attending follow-up visits several months after mild TBI,19 also found that preinjury psychiatric status (eg, mood disorders, prior suicide attempt) predicted postinjury SI. Our results build on that prior evidence and reinforce the importance of assessing preinjury psychiatric status even among patients whose TBIs are considered mild.
A more striking finding was that prior TBI was associated with substantially increased risk of SI at 2 weeks, 3 months, and 6 months postinjury, even after adjusting for psychiatric history. To our knowledge, this study is the first to show that prior TBI is prospectively associated with SI among individuals presenting with TBI of any severity. This finding converges with evidence from cross-sectional studies of military samples, which have observed higher rates of SI among current and former service members with multiple TBIs.20–22 Notably, a previous TRACK-TBI analysis of risk factors for posttraumatic stress disorder and depression following mild TBI24 found that prior TBI was not independently associated with risk of depression and was a substantially weaker (though significant) predictor of posttraumatic stress disorder. This raises the possibility that TBI recurrence may play a larger role in the development or persistence of SI following TBI than in development/persistence of other mental health problems. It has been proposed (and some evidence suggests)38 that cumulative neurological effects of multiple TBIs could result in more pronounced impairments in problem solving, emotion regulation, and social functioning, which in turn could increase risk of suicidal thoughts and behaviors.22 Future studies should investigate whether these proposed mechanisms explain associations between repetitive TBI and suicide-related outcomes. It is also possible that certain other risk factors (eg, impulsivity) increase vulnerability to both repetitive TBI and suicidality.
Another clinically important finding was that post-TBI symptom severity was strongly associated with SI at the same visit and at the subsequent visit. Further analysis suggested that the cross-sectional associations between post-TBI symptoms and SI were statistically explained by co-occurring depression, while the prospective associations between post-TBI symptoms and SI were largely explained by prior SI. However, RPQ score at 6 months predicted SI at 12 months, even after controlling for SI and depression at 6 months. Patients who continue to report high levels of post-TBI symptoms 6 months after mild TBI may be appropriate candidates for interventions to mitigate risk of developing depression and SI.
Complaints of persistent post-TBI symptoms or elevated scores on measures such as the RPQ should prompt assessment of depression in general and suicidal thoughts in particular, so that appropriate suicide risk assessment and intervention can be undertaken. A range of evidence-based pharmacological and behavioral treatments for depression are available.39 It is imperative to facilitate patient engagement with such resources, when indicated, in order to alleviate depressive symptoms, reduce risk of suicidal behaviors, and promote overall recovery after mild TBI. Educating physicians with respect to recognition and treatment of depression may help prevent suicidal behaviors.40
None of the injury-related variables considered in the analysis (eg, cause of mild TBI, CT results) were associated with SI in the multivariable models. Several studies that included individuals with more severe TBI also failed to find associations of head injury characteristics with suicidal thoughts and behaviors.8,20,41 Patients who identified their race as black or other, or their ethnicity as Hispanic, displayed increased risk of SI at 3 months postinjury; other than this, demographic factors did not predict SI in the mild TBI cohort. In general, a focus on evaluating preinjury psychiatric history, prior TBI history, and post-TBI symptom severity appears warranted for purposes of quantifying risk of SI following mild TBI. Mild TBI patients who report a history of both psychiatric treatment and prior TBI may be at dramatically increased risk (eg, an estimated 8 times increased risk of SI at 3 months postinjury, relative to patients with neither risk factor).
Prevalence of past year SI in the US adult population has been estimated at 3% to 4%.42,43 Past year SI was not evaluated in TRACK-TBI; however, we can infer that the past year rate was elevated in the mild TBI cohort relative to the general population, given that roughly 10% of the study sample reported past 2-week SI on the PHQ-9 during 1 or more follow-ups. On the other hand, the TRACK-TBI mild TBI cohort displayed somewhat lower prevalence of SI than patients admitted to a level 1 trauma center for complicated mild (Glasgow Coma Scale score of 13–15 with radiological evidence of intracranial injury) to severe TBI.18 Point prevalence of SI on the PHQ-9 in that study ranged from 7% to 10% during the year following injury (vs 4%−5% in this sample), with 25% of the cohort reporting suicidal thoughts at 1 or more follow-ups. The most obvious cross-study difference is the inclusion of patients with moderate to severe TBI in the earlier study, which may explain the higher rate of SI observed therein. Emergency department patients with mild TBI who attended follow-ups at a multidisciplinary trauma clinic also displayed somewhat higher prevalence of SI than was observed in this study (eg, 8.2% vs 4.3% at 6 months postinjury).19 Patients with more persistent post-TBI symptoms may have been more likely to attend follow-up visits19; if so, the higher rate of SI observed in that sample would be consistent with the strong association of post-TBI symptoms and SI found in this study.
Strengths of this study include the prospective design, recruitment of a large sample from trauma centers across the United States, and consideration of a wide range of variables in relation to postinjury SI. One limitation is that variables such as SI, psychiatric history, and prior TBI were based on self-report data, which are vulnerable to response bias. We do not presume that our outcome measure captured all cases of SI during the year following mild TBI, as the 2-week time frame for evaluation of SI on the PHQ-9 did not cover the full interval between follow-ups, except in the case of the 2-week visit. In addition, single-item measures are less likely to provide full coverage of the construct of interest and are generally less reliable than multi-item measures. In clinical settings, multimodal assessment (eg, self-report screening as well as clinician assessment) is strongly recommended, particularly for individuals with multiple risk factors for SI.
Certain exclusion criteria (eg, debilitating mental disorders; being on a mandated psychiatric hold) presumably resulted in exclusion of some individuals at high risk of suicidality, although these were relatively infrequent reasons for exclusion from TRACK-TBI. Results may not generalize to patients whose initial triage does not include referral for head CT, or to individuals who sustain mild TBI but do not present to the ED for evaluation. We did not consider the possible effect of treatments for physical or mental health problems on risk of SI. In addition, we lacked information regarding preinjury SI, which precluded analysis of the extent to which baseline variables predicted new-onset versus persistence of preexisting SI. These remain important areas for future study.
It is important to note that although SI can be a precursor to suicidal behavior, most individuals who think about suicide do not subsequently attempt or complete suicide.44 Moreover, some risk factors for suicidal thoughts differ from risk factors for suicide attempts and mortality.45 The current results do not necessarily imply that individuals with mild TBI who report preinjury mental health problems, prior TBI, or more severe post-TBI symptoms are at an increased risk of suicide attempt or death.
CONCLUSION
Emergency department patients with mild TBI who reported psychiatric or prior TBI history at baseline displayed increased risk of SI during the year following injury. In addition, post-TBI symptoms were strongly associated with SI, due in large part to the greater burden of depression among patients with more post-TBI symptoms. Our findings highlight the importance of evaluating psychiatric and prior TBI history among patients with mild TBI, and of assessing for SI when these risk factors are present. Moreover, assessment of suicidal thoughts should occur when mild TBI patients present with persistent post-TBI symptoms, so that appropriate risk assessment and intervention can be initiated. Further investigation is needed to evaluate associations of specific characteristics of TBI history with risk of SI (eg, number and/or severity of prior TBIs), potential mechanisms underlying the associations of repetitive TBI and post-TBI symptoms with suicidal thoughts, and efficacy of interventions for mental health sequelae of mild TBI.
Supplementary Material
Acknowledgments
The authors thank Jason Barber, MS, of University of Washington, Seattle, for assistance with data extraction. The authors are also grateful to Amy J. Markowitz, JD, University of California, San Francisco, for providing expert editorial assistance. No compensation was received for this work.
This research was supported by the National Institutes of Health (grant U01NS086090) and US Department of Defense (grant W81XWH-14-2-0176). Abbott Laboratories provided funding for add-in TRACK-TBI clinical studies. One Mind provided funding for TRACK-TBI patients’ stipends and support to clinical sites.
Dr Nelson reported grants from Department of Defense (DoD) during the conduct of the study; grants from National Institutes of Health (NIH), grants from Medical College of Wisconsin Center for Patient Care and Outcomes Research, grants from Advancing a Healthier Wisconsin, and grants from the DoD outside the submitted work. Dr Temkin reported grants from NIH-National Institute of Neurological Disorders and Stroke (NINDS) during the conduct of the study. Dr McCrea reported grants from the NIH and DoD during the conduct of the study. Dr Yuh reported grants from the University of California, San Francisco, during the conduct of the study; in addition, Dr Yuh had a patent for USPTO No. 62/269,778 pending. Dr Giacino reported grants from the NIH-NINDS during the conduct of the study. Dr Manley reported grants from the NINDS during the conduct of the study; research funding from the US Department of Energy, grants from the DoD, research funding from Abbott Laboratories, grants from the National Football League Scientific Advisory Board, and research funding from One Mind outside the submitted work; in addition, Dr Manley had a patent for Interpretation and Quantification of Emergency Features on Head Computed Tomography issued. He served for 2 seasons as an unaffiliated neurologic consultant for home games of the Oakland Raiders; he was compensated $1500 per game for 6 games during the 2017 season but received no compensation for this work during the 2018 season. Dr Stein reported personal fees from Aptinyx, Bionomics, Janssen, and Neurocrine, as well as personal fees and stock options from Oxeia Biopharmaceuticals outside the submitted work. Dr Adeoye reported grants from the NIH/NINDS during the conduct of the study. Dr Boase reported grants from TRACK-TBI Grant during the conduct of the study. Dr Bodien reported grants from Spaulding Rehabilitation Hospital during the conduct of the study. Dr Corrigan reported grants from University of California, San Francisco, during the conduct of the study. Dr Diaz-Arrastia reported personal fees and research funding from Neural Analytics Inc and travel reimbursement from Brain Box Solutions, Inc outside the submitted work. Dr Duhaime reported grants from the NIH during the conduct of the study. Dr Feeser reported grants from Virginia Commonwealth University during the conduct of the study. Dr Ferguson reported grants from the NIH/NINDS, the DoD, Veterans Affairs, Craig H. Neilsen Foundation, Wings for Life Foundation, and the Department of Energy during the conduct of the study. Dr Goldman reported grants from the NINDS and USC Schaeffer Center during the conduct of the study; personal fees from Amgen, Avanir Pharmaceuticals, Acadia Pharmaceuticals, Aspen Health Strategy Group, and Celgene outside the submitted work. Dr Gopinath reported grants from the NIH and DoD during the conduct of the study. Dr Kreitzer reported personal fees from Portola outside the submitted work. Dr Lindsell reported grants from the NIH during the conduct of the study. Dr Machamer reported grants from the NIH during the conduct of the study. Dr Madden reported grants from the NIH TRACK-TBI Study and One Mind for research during the conduct of the study Dr McAllister reported grants from the UCSF from the NIH and the National Collegiate Athletic Association and the DoD during the conduct of the study. Dr Merchant and Dr Valadka are TRACK-TBI investigators at Virginia Commonwealth University. Dr Mukherjee reported grants from GE Healthcare and nonfinancial support from GE-NFL Head Health Initiative outside the submitted work; in addition, Dr Mukherjee had a patent for USPTO No. 62/269,778 pending. Dr Robertson reported grants from the NIH and the DoD during the conduct of the study. Dr Rosand reported grants from the NIH during the conduct of the study and personal fees from Boehringer Ingelheim and New Beta Innovations outside the submitted work. Dr Sander reported grants from the NIH during the conduct of the study. Dr Vespa reported grants from the NIH during the conduct of the study. Dr Zafonte received royalties from Oakstone for an educational CD (Physical Medicine and Rehabilitation: a Comprehensive Review) and Demos publishing for serving as coeditor of Brain Injury Medicine. Dr Zafonte serves or served on the scientific advisory boards of Myomo, Oxeia Biopharma, Biodirection, and Elminda. He also evaluates patients in the MGH Brain and Body-TRUST Program, which is funded by the National Football League Players Association. Dr Zafonte had served on the Mackey White Committee. No other disclosures were reported.
The TRACK-TBI Investigators are as follows:
Opeolu Adeoye, MD, University of Cincinnati; Neeraj Badjatia, MD, University of Maryland; Kim Boase, University of Washington; Yelena Bodien, PhD, Massachusetts General Hospital; M. Ross Bullock, MD, PhD, University of Miami; Randall Chesnut, MD, University of Washington; John D. Corrigan, PhD, ABPP, The Ohio State University; Karen Crawford, University of Southern California; Ramon Diaz-Arrastia, MD, PhD, University of Pennsylvania; Ann-Christine Duhaime, MD, Massachusetts General Hospital; Richard Ellenbogen, MD, University of Washington; V. Ramana Feeser, MD, Virginia Commonwealth University; Adam R. Ferguson, PhD, University of California, San Francisco; Brandon Foreman, MD, University of Cincinnati; Raquel Gardner, University of California, San Francisco; Etienne Gaudette, PhD, University of Southern California; Dana Goldman, PhD, University of Southern California; Luis Gonzalez, TIRR Memorial Hermann; Shankar Gopinath, MD, Baylor College of Medicine; Rao Gullapalli, PhD, University of Maryland; J. Claude Hemphill, MD, University of California, San Francisco; Gillian Hotz, PhD, University of Miami; Frederick K. Korley, MD, PhD, University of Michigan; Joel Kramer, PsyD, University of California, San Francisco; Natalie Kreitzer, MD, University of Cincinnati; Harvey Levin, MD, Baylor College of Medicine; Chris Lindsell, PhD, Vanderbilt University; Joan Machamer, MA, University of Washington; Christopher Madden, MD, UT Southwestern; Alastair Martin, PhD, University of California, San Francisco; Thomas McAllister, MD, Indiana University; Randall Merchant, PhD, Virginia Commonwealth University; Pratik Mukherjee, MD, PhD, University of California, San Francisco; Laura B. Ngwenya, MD, PhD, University of Cincinnati; David Okonkwo, MD, PhD, University of Pittsburgh; Eva Palacios, PhD, University of California, San Francisco; Daniel Perl, MD, Uniformed Services University; Ava Puccio, PhD, University of Pittsburgh; Miri Rabinowitz, PhD, University of Pittsburgh; Claudia Robertson, MD, Baylor College of Medicine; Jonathan Rosand, MD, MSc, Massachusetts General Hospital; Angelle Sander, PhD, Baylor College of Medicine; Gabriella Satris, University of California, San Francisco; David Schnyer, PhD, UT Austin; Seth Seabury, PhD, University of Southern California; Sabrina Taylor, PhD, University of California, San Francisco; Arthur Toga, PhD, University of Southern California; Alex Valadka, MD, Virginia Commonwealth University; Mary Vassar, RN MS, University of California, San Francisco; Paul Vespa, MD, University of California, Los Angeles; Kevin Wang, PhD, University of Florida; John K. Yue, MD, University of California, San Francisco; and Ross Zafonte, Harvard Medical School.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.headtraumarehab.com).
The other authors declare no conflicts of interest.
REFERENCES
- 1.Centers for Disease Control and Prevention. TBI-related emergency department (ED) visits. https://www.cdc.gov/traumaticbraininjury/data/tbi-edhd.html. Updated March 29, 2019. Accessed August 28, 2019.
- 2.Centers for Disease Control and Prevention. Report to Congress on Traumatic Brain Injury in the United States: Epidemiology and Rehabilitation. Atlanta, GA: National Center for Injury Prevention and Control, Division of Unintentional Injury Prevention; 2015. [Google Scholar]
- 3.Ponsford J, Cameron P, Fitzgerald M, Grant M, Mikocka-Walus A. Long-term outcomes after uncomplicated mild traumatic brain injury: a comparison with trauma controls. J Neurotrauma. 2011;28(6):937–946. [DOI] [PubMed] [Google Scholar]
- 4.McMahon P, Hricik A, Yue JK, et al. Symptomatology and functional outcome in mild traumatic brain injury: results from the prospective TRACK-TBI study. J Neurotrauma. 2014;31(1): 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson LD, Temkin NR, Dikmen S, et al. Recovery after mild traumatic brain injury in patients presenting to US level I trauma centers: a Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) Study. JAMA Neurol. 2019; 76(9):1049–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher LB, Pedrelli P, Iverson GL, et al. Prevalence of suicidal behaviour following traumatic brain injury: longitudinal follow-up data from the NIDRR Traumatic Brain Injury Model Systems. Brain Inj. 2016;30(11):1311–1318. [DOI] [PubMed] [Google Scholar]
- 7.Simpson G, Tate R. Suicidality in people surviving a traumatic brain injury: prevalence, risk factors and implications for clinical management. Brain Inj. 2007;21(13–14):1335–1351. [DOI] [PubMed] [Google Scholar]
- 8.Simpson G, Tate R. Suicidality after traumatic brain injury: demographic, injury and clinical correlates. Psychol Med. 2002;32(4):687–697. [DOI] [PubMed] [Google Scholar]
- 9.Ahmedani BK, Peterson EL, Hu Y, et al. Major physical health conditions and risk of suicide. Am J Prev Med. 2017;53(3):308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madsen T, Erlangsen A, Orlovska S, Mofaddy R, Nordentoft M, Benros ME. Association between traumatic brain injury and risk of suicide. JAMA. 2018;320(6):580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fazel S, Wolf A, Pillas D, Lichtenstein P, Langstrom N. Suicide, fatal injuries, and other causes of premature mortality in patients with traumatic brain injury: a 41-year Swedish population study. JAMA Psychiatry. 2014;71(3):326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips CJ, LeardMann CA, Vyas KJ, Crum-Cianflone NF, White MR. Risk factors associated with suicide completions among US enlisted marines. Am J Epidemiol. 2017;186(6):668–678. [DOI] [PubMed] [Google Scholar]
- 13.Brenner LA, Ignacio RV, Blow FC. Suicide and traumatic brain injury among individuals seeking Veterans Health Administration services. J Head Trauma Rehabil. 2011;26(4):257–264. [DOI] [PubMed] [Google Scholar]
- 14.Boggs JM, Beck A, Hubley S, et al. General medical, mental health, and demographic risk factors associated with suicide by firearm compared with other means. Psychiatr Serv. 2018;69(6):677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fralick M, Sy E, Hassan A, Burke MJ, Mostofsky E, Karsies T. Association of concussion with the risk of suicide: a systematic review and meta-analysis. JAMA Neurol. 2019;76(2):144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bryant RA, O’Donnell ML, Forbes D, McFarlane AC, Silove D, Creamer M. The course of suicide risk following traumatic injury. J Clin Psychiatry. 2016;77(5):648–653. [DOI] [PubMed] [Google Scholar]
- 17.Wisco BE, Marx BP, Holowka DW, et al. Traumatic brain injury, PTSD, and current suicidal ideation among Iraq and Afghanistan U.S. veterans. J Trauma Stress. 2014;27(2):244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackelprang JL, Bombardier CH, Fann JR, Temkin NR, Barber JK, Dikmen SS. Rates and predictors of suicidal ideation during the first year after traumatic brain injury. Am J Public Health. 2014;104(7):e100–e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bethune A, da Costa L, van Niftrik CHB, Feinstein A. Suicidal ideation after mild traumatic brain injury: a consecutive Canadian sample. Arch Suicide Res. 2017;21(3):392–402. [DOI] [PubMed] [Google Scholar]
- 20.Shura RD, Nazem S, Miskey HM, et al. Relationship between traumatic brain injury history and recent suicidal ideation in Iraq/Afghanistan-era veterans. Psychol Serv. 2019;16(2):312–320. [DOI] [PubMed] [Google Scholar]
- 21.Lindquist LK, Love HC, Elbogen EB. Traumatic brain injury in Iraq and Afghanistan veterans: new results from a national random sample study. J Neuropsychiatry Clin Neurosci. 2017;29(3):254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bryan CJ, Clemans TA. Repetitive traumatic brain injury, psychological symptoms, and suicide risk in a clinical sample of deployed military personnel. JAMA Psychiatry. 2013;70(7):686–691. [DOI] [PubMed] [Google Scholar]
- 23.Tsaousides T, Cantor JB, Gordon WA. Suicidal ideation following traumatic brain injury: prevalence rates and correlates in adults living in the community. J Head Trauma Rehabil. 2011;26(4):265–275. [DOI] [PubMed] [Google Scholar]
- 24.Stein MB, Jain S, Giacino JT, et al. Risk of posttraumatic stress disorder and major depression in civilian patients after mild traumatic brain injury: a TRACK-TBI Study. JAMA Psychiatry. 2019;76(3):249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herrmann N, Rapoport MJ, Rajaram RD, et al. Factor analysis of the Rivermead Post-Concussion Symptoms Questionnaire in mild to moderate traumatic brain injury patients. J Neuropsychiatry Clin Neurosci. 2009;21(2):181–188. [DOI] [PubMed] [Google Scholar]
- 26.Agtarap S, Campbell-Sills L, Thomas ML, Kessler RC, Ursano RJ, Stein MB. Postconcussive, posttraumatic stress and depressive symptoms in recently deployed U.S. Army soldiers with traumatic brain injury. Psychol Assess. 2019;31(11):1340–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell-Sills L, Stein MB, Liu H, et al. Associations of lifetime traumatic brain injury characteristics with prospective suicide attempt among deployed US Army soldiers. J Head Trauma Rehabil. 2020;35(1):14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teasdale G, Jennett B. Assessment and prognosis of coma after head injury. Acta Neurochir (Wien). 1976;34(1–4):45–55. [DOI] [PubMed] [Google Scholar]
- 29.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corrigan JD, Bogner J. Initial reliability and validity of the Ohio State University TBI Identification Method. J Head Trauma Rehabil. 2007;22(6):318–329. [DOI] [PubMed] [Google Scholar]
- 31.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789–1795. [DOI] [PubMed] [Google Scholar]
- 32.King NS, Crawford S, Wenden FJ, Moss NE, Wade DT. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol. 1995;242(9):587–592. [DOI] [PubMed] [Google Scholar]
- 33.Agtarap S, Kramer MD, Campbell-Sills L, et al. Invariance of the bifactor structure of mild traumatic brain injury (mTBI) symptoms on the Rivermead Postconcussion Symptoms Questionnaire across time, demographic characteristics, and clinical groups: A TRACK-TBI Study [published online ahead of print April 24, 2020]. Assessment. doi: 10.1177/1073191120913941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Derogatis L BSI-18: Brief Symptom Inventory 18 Administration, Scoring, and Procedures Manual. Minneapolis, MN: NCS Pearson Inc; 2001. [Google Scholar]
- 35.McCaffrey DF, Ridgeway G, Morral AR. Propensity score estimation with boosted regression for evaluating causal effects in observational studies. Psychol Methods. 2004;9(4):403–425. [DOI] [PubMed] [Google Scholar]
- 36.R: A language and environment for statistical computing [computer program]. Vienna, Austria: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 37.Klonsky ED, May AM, Saffer BY. Suicide, suicide attempts, and suicidal ideation. Annu Rev Clin Psychol. 2016;12:307–330. [DOI] [PubMed] [Google Scholar]
- 38.Nolan A, Hennessy E, Krukowski K, et al. Repeated mild head injury leads to wide-ranging deficits in higher-order cognitive functions associated with the prefrontal cortex. J Neurotrauma. 2018;35(20):2425–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qaseem A, Barry MJ, Kansagara D, Clinical guidelines committee of the American College of Physicians. Nonpharmacologic versus pharmacologic treatment of adult patients with major depressive disorder: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;164(5):350–359. [DOI] [PubMed] [Google Scholar]
- 40.Mann JJ, Apter A, Bertolote J, et al. Suicide prevention strategies: a systematic review. JAMA. 2005;294(16):2064–2074. [DOI] [PubMed] [Google Scholar]
- 41.Kesinger MR, Juengst SB, Bertisch H, et al. Acute trauma factor associations with suicidality across the first 5 years after traumatic brain injury. Arch Phys Med Rehabil. 2016;97(8):1301–1308. [DOI] [PubMed] [Google Scholar]
- 42.Kessler RC, Berglund P, Borges G, Nock M, Wang PS. Trends in suicide ideation, plans, gestures, and attempts in the United States, 1990–1992 to 2001–2003. JAMA. 2005;293(20):2487–2495. [DOI] [PubMed] [Google Scholar]
- 43.Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results From the 2018 National Survey on Drug Use and Health (HHS Publication No. PEP19–5068, NSDUH Series H-54). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2019. [Google Scholar]
- 44.Glenn CR, Nock MK. Improving the short-term prediction of suicidal behavior. Am J Prev Med. 2014;47(3 suppl 2):S176–S180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nock MK, Borges G, Bromet EJ, Cha CB, Kessler RC, Lee S. Suicide and suicidal behavior. Epidemiol Rev. 2008;30:133–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.