Abstract
Background
Insomnia is a highly prevalent symptom occurred during and post-chemotherapy. Acupuncture may have beneficial effects in the management of chemotherapy-associated insomnia. This study was conducted to determine the efficacy and safety of acupuncture in improving chemotherapy-associated insomnia in breast cancer patients.
Methods
This assessor-participant blinded, randomized, sham-controlled trial was conducted from November 2019 to January 2022 (follow-up completed July 2022). Participants were referred by oncologists from two Hong Kong hospitals. Assessments and interventions were conducted at the outpatient clinic of School of Chinese Medicine, the University of Hong Kong. The 138 breast cancer patients with chemotherapy-associated insomnia were randomly assigned to receive either 15 sessions of active acupuncture regimen by combining needling into body acupoints and acupressure on auricular acupoints or sham acupuncture control (69 each) for 18 weeks, followed by 24 weeks of follow-up. The primary outcome was measured using Insomnia Severity Index (ISI). Secondary outcomes included the Pittsburgh Sleep Quality Index, Actiwatch and sleep diary for sleep parameters, depression and anxiety, fatigue and pain, and quality of life.
Results
There were 87.7% (121/138) participants who completed the primary endpoint (week-6). The active acupuncture regimen was not superior to the sham control in reducing ISI score from baseline to 6 weeks (mean difference: − 0.4, 95% CI − 1.8–1.1; P = 0.609), but produced short-term treatment and long-term follow-up better outcomes in improving sleep onset latency, total sleep time, sleep efficiency, anxiety, depression, and quality of life. Participants of the active acupuncture group had a pronouncedly higher cessation rate of sleeping medications than the sham control (56.5% vs. 14.3%, P = 0.011). All treatment-related adverse events were mild. No participants discontinued treatments due to adverse events.
Conclusion
The active acupuncture regimen could be considered as an effective option for the management of chemotherapy-associated insomnia. It also could serve as a tapering approach to reduce and even replace the use of sleeping medications in breast cancer patients.
Trial registration Clinicaltrials.gov: NCT04144309. Registered 30 October 2019.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13058-023-01645-0.
Keywords: Chemotherapy-associated insomnia, Breast cancer, Acupuncture, Cessation rate of sleeping medications
Background
Breast cancer patients are at significantly greater risk of developing insomnia than those with other malignancies and the general population [1, 2]. Individuals under chemotherapy experienced higher incidence and more severe levels of insomnia than those who received other cancer treatments such as surgery and radiotherapy [2–4]. More than 1/4 of breast cancer patients developed new-onset insomnia during chemotherapy, and more likely to persist after the completion of cancer treatments than those without chemotherapy [5, 6]. The development of chemotherapy-associated insomnia in breast cancer patients is associated with multiple factors which may trigger and even aggravate insomnia, mainly including psychological stress due to cancer diagnosis and treatments, direct effects of medications and chemotherapy, reduction in physical activity, disruption in circadian, and somatic-cognitive-cortical hyperarousal [7–10]. The presence of insomnia not only increases risks of psychiatric and physical comorbidities [11, 12], but also reduces patients’ willingness to complete cancer treatments and decreases quality of life. Although pharmacological therapy is the mainstay of the management of insomnia in cancer patients, it is generally recommended for short-term use, as sedative-hypnotic medications often cause undesirable side effects, particularly dependence and tolerance [13]. Cognitive behavioral therapy may be efficacious for improving sleep quality in breast cancer women [14], but intensive labour requirements and high treatment costs have limited the access to this non-pharmacological therapy [15, 16].
Over the past two decades, acupuncture has gained increasing popularity in the treatment of insomnia, cancer and cancer-related symptoms [17–19]. Numerous clinical practice guidelines have recommended acupuncture as an effective option for chemotherapy-induced side effects [17, 20]. There have been a large number of clinical trials that demonstrated promising efficacy of acupuncture for various types of insomnia [21, 22]. However, the effectiveness of acupuncture for chemotherapy-associated insomnia remains for further evaluation. Our recent pilot-controlled trial has shown the potential benefits of the combination of needling into body acupoints and auricular acupressure in 30 breast cancer patients who underwent chemotherapy and experienced insomnia [23]. We therefore hypothesized that the combination acupuncture regimen could produce better outcomes than sham regimen in improving chemotherapy-associated insomnia. To test this hypothesis, an assessor-participant blinded, randomized, sham-controlled trial was designed to compare the efficacy and safety of the two regimens in breast cancer patients with chemotherapy-associated insomnia.
Methods
Design and settings
This was an assessor-participant-data analyst blinded, randomized, sham-controlled trial. The trial was approved by Institutional Review Board of the University of Hong Kong (HKU)/Hospital Authority Hong Kong (HK) West Cluster (UW 19-045) and Research Ethics Committee of HK Sanatorium & Hospital (REC-2019-14). The trial was registered in www.clinicaltrials.gov (NCT04144309). The study was conducted in accordance with Standards for Reporting Interventions in Clinical Trials of Acupuncture and Consolidated Standards of Reporting Trials statement [24, 25].
Recruitment and treatment occurred between November 2019 and January 2022; follow-up assessments completed in July 2022. Participants were referred by oncologists from HK Queen Mary Hospital and HK Sanatorium & Hospital. Multiple promotions were carried out to facilitate recruitment, including advertising on newspapers and Facebook. Participants gave voluntary, written, informed consent after study procedures were fully explained. Assessments and interventions were conducted at the outpatient clinic of School of Chinese Medicine, HKU.
Participants
Patients were eligible if they were: (i) females aged 18–75; (ii) had a diagnosis of stage I–IV breast cancer; (iii) underwent or had completed chemotherapy no more than 6 months; (iv) insomnia occurred at least 3 nights/week and lasted at least one month, with the fulfilment of the diagnostic criteria for brief insomnia disorder of the Diagnostic and Statistical Manual of Mental Disorders (5th Edition); and (v) the severity of insomnia reached at least 10 points of Insomnia Severity Index (ISI) over the past 2 weeks.
Patients were excluded if they had: (i) other sleep disorders (e.g., obstructive sleep apnoea), irregular sleep pattern, or shift work; (ii) severe hearing, visual or language defects; (iii) severe hematological dysfunction (e.g., haemoglobin < 8 g/dL, platelet count < 60,000/μL, absolute neutrophil count < 1000/μL); (iv) pacemakers or other electronic implants that might interfere with electroacupuncture; (v) had acupuncture treatment in the past 3 months; or (vi) participated in other clinical trials in the past 3 months.
Randomization
Permuted block randomization (block sizes of 2/4/6) was used. After baseline assessments, participants were randomly assigned to two groups at a ratio of 1:1 according to the randomization sequence. The randomization sequence was generated by an independent biostatistician using Microsoft Excel prior to study initiation. Individual randomization code was sealed in sequentially numbered opaque envelopes and opened by acupuncturist after participants completed baseline assessments.
Blinding and allocation concealment
Investigators and research staffs who performed screening, assessments, and data entry/re-entry/analysis were blinded to group allocation. Participants were informed that they would have same chance of allocating to either group and would be blinded to allocation. Treatment was delivered individually in a separate room to avoid communications among participants about treatment experience. Eye mask was used to block participant’s vision during treatment so that she was unaware of acupuncture procedure. Acupuncturists were instructed not to acquire participants’ information except group allocation. Interactions between acupuncturists and participants were kept to a minimum to avoid accidental disclosure of group allocation.
Intervention
Participants continued routine care and symptom management during study. Acupuncture treatment was carried out by registered Chinese Medicine Practitioners (CMPs) had at least 5-year clinical practice experience and completed training workshop prior to study initiation. The training workshop included introduction of treatment protocol, standard procedures of active and sham acupuncture, and conversation skills with participants. The treatment protocol [26] was developed based on neuroanatomic mechanisms [27, 28], CMPs’ experience and previous clinical trials [21, 29]. Participants received a total of 15 sessions of treatments, with 12 sessions given twice weekly as intensive treatment over the first 6 consecutive weeks, 3 sessions given once every 4 weeks from 7 to 18 weeks to maintain treatment effects, and follow-up from 19 to 42 weeks.
Active acupuncture regimen
Active acupuncture regimen consisted of electroacupuncture on body acupoints and auricular acupressure. Six fixed acupoints (EX-HN1, GV20, GV24, PC6, KI3 and SP6) were utilized for treating insomnia [30–33]. Four additional acupoints were selected based on comorbid symptoms (Table 1). Locations/therapeutic effects of acupoints are summarized in Additional file 1: eTable S1A. Details procedures are listed in Additional file 1: eTable S1B. Auricular acupressure was conducted by embedding black, hard Vaccaria seeds on surface of bilateral 3 auricular points (Heart, Shenmen and Sympathetic).
Table 1.
Recommendation of additional acupoints based on comorbid symptoms
| Comorbid symptoms | Recommended acupoints | |
|---|---|---|
| Dizziness | EX-HN3 (Yintang) | ST36 (Zusanli) |
| Headache | EX-HN3 (Yintang) | LI4 (Hegu) |
| Fatigue | ST36 (Zusanli) | CV4 (Guanyuan) |
| Hot flushes | KI3 (Taixi) | CV4 (Guanyuan) |
| depression/anxiety | EX-HN3 (Yintang) | LR3 (Taichong) |
| Nausea/vomiting | ST36 (Zusanli) | LI4 (Hegu) |
| Loss of appetite | ST36 (Zusanli) | LI4 (Hegu) |
| Diarrhea/constipation | ST25 (Tianshu) | ST36 (Zusanli) |
Sham acupuncture regimen
Sham points that are located at 1–2 cm adjacent to meridian-based acupoints were used for sham electroacupuncture [34]. Streitberger’s non-invasive retractable needles were compressed via guiding tubes onto point skin [35]. For sham auricular acupressure, 3 sham points in helix region (HX7, HX8, HX9) that are located remotely from inner ear area and their effects are not indicated for insomnia were selected [36]. Soft stem piths of Medulla Junci were cut and dyed in black were used to mimic auricular acupressure [37].
Concomitant use of psychotropic medications
Psychotropic medications were allowed to be prescribed at the discretion of psychiatrists and general physicians during study. The proportion of participants who were prescribed with sleeping medications (sedatives, hypnotics and anxiolytics), dosage and weekly frequency of use were recorded. The dosage used was converted as diazepam equivalent dosage. Four mean dosages were obtained by averaging across a 2 week duration [38]: prior to entry, post-treatment 6 weeks and 18 weeks, and prior to the end of follow-up. Cessation rate of sleeping medications was calculated.
Assessments
Primary outcome
Details of outcomes have been reported previously [26]. The primary outcome was defined as the change in ISI score at 6 weeks of treatment from baseline. ISI is a 7-item self-rating questionnaire that has good internal consistency and construct validity in differentiating insomnia severity in cancer patients [39]. ISI score ranges from 0 to 28. The higher score represents more severe insomnia, with 10 points indicative of subthreshold level of insomnia [40, 41].
Secondary outcomes
Secondary outcomes included sleeping measures, depression and anxiety, fatigue and pain, and quality of life. Actiwatch [42] (Spectrum Plus, Philips Respironics, USA) and Sleep Diary [43] were used to record sleep onset latency (SOL), wake time after sleep onset (WASO), total sleep time (TST), and sleep efficiency (SE). Participants were required to record for 2 sessions of 7 consecutive nights starting at baseline and starting after 6 week of treatment. Pittsburgh Sleep Quality Index (PSQI) was a 19-item questionnaire used to assess sleep dysfunction, with higher score indicating poorer sleep quality [44, 45].
The severity of depression/anxiety was measured using Hospital Anxiety and Depression Scale (HADS) which is a 14-item questionnaire with two subscales to evaluate the severity of depressive and anxiety symptoms [46, 47]. Fatigue and pain symptoms were measured using Brief Fatigue Inventory (BFI) and Brief Pain Inventory-Short Form (BPI-SF), respectively. BFI is a 9-item self-rating scale designed to evaluate the severity and impact of cancer-related fatigue on daily functioning, with higher score corresponding to greater level of fatigue [48]. BPI-SF is a self-administered questionnaire developed to evaluate pain severity and pain interference on daily function over the past 24 h [49, 50]. Quality of life was measured using Functional Assessment of Cancer Therapy-Breast Cancer (FACT-B). FACT-B is a 37-item self-reported instrument devised to assess multidimensional health-related quality of life in breast cancer patients [51], with higher score indicating better quality of life [52]. Questionnaires were completed at baseline, week-3, 6, 10, 14, 18, 30 and 42.
Adverse events
Adverse events (AEs) were recorded during study. Whether an AE related to treatment was determined by acupuncturists. Serious AEs were immediately reported to principal investigator and Ethics Committee within 24 h of the occurrence.
Credibility, expectancy, adherence, and successiveness of blinding
The credibility of treatment was assessed using the 4-item, 6-point Credibility Rating Scale [53]. Expectancy for acupuncture outcomes was assessed using the 4-item 5-point Acupuncture Expectancy Scale [54]. Greater scores of both scales represent stronger confidence and higher expectancy to treatment. Adherence was assessed by counting the number of completed treatment sessions. Participants were asked to guess which type of acupuncture they had received after the third treatment [55]. James’ and Bang’s blinding index were calculated to evaluate successiveness of blinding [56].
Data security and monitoring
All data were secured in compliance with HK Personal Data (Privacy) Ordinance (CAP 486). A data and safety monitoring board (DSMB) was established [57] and comprised of an independent biostatistician, an oncologist and a psychiatrist. They were not involved in the conduct of the trial. DSMB meetings were held prior to the initiation of recruitment and after the final analysis. To prevent loss of follow-up, text messages were sent or phone calls were made to individual participants a day before scheduled visits. The research team held weekly meetings to troubleshoot issues pertaining to patient recruitment and retention.
Statistical analysis
Estimation of sample size
There was no study has previously been conducted in the female with breast cancer experiencing chemotherapy-associated insomnia. The sample size was estimated based on anticipated changes of ISI score. Based on previous trials among non-cancer population with primary insomnia [21, 58], in which the reduction of ISI score was between 2.3 and 5.0, with median value of 2.5 and pooled standard deviation of 4.7. The study was assumed to detect a 2.5 difference between groups in reduced ISI score, with a 95% level of significance and an 80% power. A total of 138 subjects (69/group) were needed in the consideration of 20% dropout rate.
Data analysis
The efficacy was analyzed in the intention-to-treat population, defined as participants who completed baseline assessments. For missing data, the multiple imputation method was used under the missing-at-random (MAR) assumption. The sensitivity of MAR assumption of missing data was tested with a pattern-mixture model [59], in which the robustness of the analyzed results of primary outcome was examined. The primary outcome and continuous secondary outcomes were compared using a mixed-effect model adjusted with baseline values, with time, group, and interaction between time and group as the fixed effects, and individual subject as the random effect.
Categorical variables, including categorical baseline variables, cessation rate of sleeping medications and incidence of AEs, were analyzed using Chi-square or Fisher’s exact tests. Unpaired t-test or Mann–Whitney U test was used to compare continuous variables between groups, including baseline variables, changes in Actiwatch-/diary-recorded variables, credibility score and medication dosage. Statistical analyses were performed with SPSS version 26.0 (IBM Corporation, Armonk, NY) and SAS version 9.4 (SAS Inc.). Hypothesis testing was carried out at 5% (2-sided) significance level.
Results
Participant characteristics
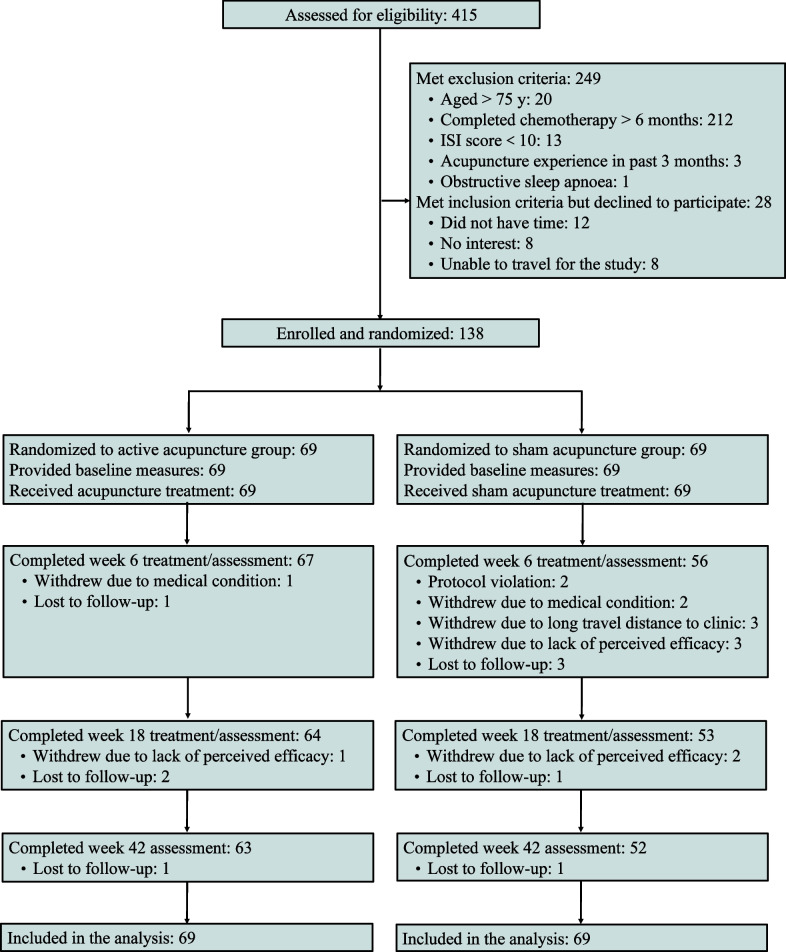
Of 415 patients screened, 166 eligible, but 28 declined to participate (Fig. 1). The remaining 138 were randomly assigned to the active and sham acupuncture groups (69/group). The proportion of participants who completed at least 12 treatments was 97.1% (67/69) in the active acupuncture group and 81.2% (56/69) in the sham control group. The discontinuation rate was 10.9% (15/138) over the first 6 weeks and 16.7% (23/138) over the whole trial. The overall discontinuation rate of the active acupuncture was pronouncedly lower than that of the sham control [8.7% (6/69) vs. 24.6% (17/69), P = 0.012].
Fig. 1.
Flow diagram of progress of recruitment, treatment and follow-up
Demographic and baseline variables were similar between groups. All participants were Asian women with mean (SD) age of 52.2 (9.0) years. The mean ISI score was 17.1 (4.4) points, with the duration of insomnia of 10.0 (6.7) months. There were 32.6% (45/138) participants who were taking sleeping medications at entry (Table 2).
Table 2.
Participants characteristics
| Characteristic | Active acupuncture (n = 69) | Sham acupuncture (n = 69) | Total (n = 138) | P value* |
|---|---|---|---|---|
| Age, years | 51.7 ± 9.6 | 52.7 ± 8.3 | 52.2 ± 9.0 | 0.540 |
| Body mass index, kg/m2 | 22.8 ± 2.9 | 24.0 ± 16.9 | 23.4 ± 12.1 | 0.581 |
| Marital status | 0.586 | |||
| Married or living with partner | 48 (69.6) | 45 (65.2) | 93 (67.4) | |
| Single, separated, divorced, or widowed | 21 (30.4) | 24 (34.8) | 45 (32.6) | |
| Educational attainment | 0.959 | |||
| Primary or below | 7 (10.1) | 8 (11.6) | 15 (10.9) | |
| Secondary | 33 (47.8) | 33 (47.8) | 66 (47.8) | |
| Post-secondary or above | 29 (42.0) | 28 (40.6) | 57 (41.3) | |
| Household monthly income (HK$) | 0.111 | |||
| < 20,000 | 36 (52.2) | 39 (56.5) | 75 (54.3) | |
| 20,000–50,000 | 23 (33.3) | 13 (18.8) | 36 (26.1) | |
| > 50,000 | 10 (14.5) | 15 (21.7) | 25 (18.1) | |
| No answer | 0 (0.0) | 2 (2.9) | 2 (1.4) | |
| Occupation | 0.384 | |||
| Professional and associate professional | 22 (31.9) | 20 (29.0) | 42 (30.4) | |
| Skilled and semi-skilled worker | 3 (4.3) | 3 (4.3) | 6 (4.3) | |
| Unskilled worker | 19 (27.5) | 12 (17.4) | 31 (22.5) | |
| Retired/unemployed/housework | 25 (36.2) | 34 (49.3) | 59 (42.8) | |
| Menopausal status at entry | 0.390 | |||
| Premenopausal | 4 (5.8) | 1 (1.4) | 5 (3.6) | |
| Perimenopausal | 13 (18.8) | 13 (18.8) | 26 (18.8) | |
| Postmenopausal | 52 (75.4) | 55 (79.7) | 107 (77.5) | |
| Breast cancer stage | 0.479 | |||
| I | 13 (18.8) | 19 (27.5) | 32 (23.2) | |
| II | 29 (42.0) | 26 (37.7) | 55 (39.9) | |
| III | 15 (21.7) | 17 (24.6) | 32 (23.2) | |
| IV | 10 (14.5) | 7 (10.1) | 17 (12.3) | |
| No answer | 2 (2.9) | 0 (0.0) | 2 (1.4) | |
| Prior surgery | 61 (88.4) | 64 (92.8) | 125 (90.6) | 0.382 |
| Prior radiotherapy | 37 (53.6) | 40 (58.0) | 77 (55.8) | 0.607 |
| Prior hormonal therapy | 34 (49.3) | 38 (55.1) | 72 (52.2) | 0.495 |
| Adjuvant Chemotherapy | 51 (73.9) | 57 (82.6) | 108 (78.3) | 0.417 |
| Under or post-chemotherapy at entry | 0.687 | |||
| Under | 15 (21.7) | 17 (24.6) | 32 (23.2) | |
| Post | 54 (78.3) | 52 (75.4) | 72 (76.8) | |
| Chemotherapy regimens | 0.583 | |||
| AC/TC | 14 (20.3) | 20 (29.0) | 34 (24.6) | |
| TAC | 6 (8.7) | 10 (14.5) | 16 (11.6) | |
| AC/EC + T/P | 13 (18.8) | 9 (13.0) | 22 (15.9) | |
| FEC + T | 8 (11.6) | 5 (7.2) | 13 (9.4) | |
| Carboplatin-containing | 17 (24.6) | 14 (20.3) | 31 (22.5) | |
| Others | 11 (15.9) | 11 (15.9) | 22 (15.9) | |
| Insomnia mean duration, monthsa | 9.0 (3.0, 13.5) | 10.0 (5.5, 15.0) | 9.0 (4.8, 14.0) | 0.141 |
| Sleep aids, prior 2 weeks | ||||
| Sleep medications | 26 (37.7) | 19 (27.5) | 45 (32.6) | 0.204 |
| Chinese herbal medicine | 16 (23.2) | 24 (34.8) | 40 (29.0) | 0.133 |
| Prior acupuncture | 50 (72.5) | 46 (66.7) | 96 (69.6) | 0.459 |
| ISI | 17.4 ± 4.3 | 16.7 ± 4.5 | 17.1 ± 4.4 | 0.358 |
| PSQI | 13.6 ± 3.4 | 12.7 ± 3.3 | 13.1 ± 3.4 | 0.100 |
| Actiwatch | ||||
| SOL, min | 14.2 ± 14.5 | 14.2 ± 12.3 | 14.2 ± 13.4 | 0.976 |
| WASO, min | 112.6 ± 40.2 | 112.5 ± 38.4 | 112.5 ± 39.2 | 0.991 |
| TST, min | 359.1 ± 51.1 | 357.7 ± 51.3 | 358.4 ± 51.0 | 0.878 |
| SE, % | 72.8 ± 7.9 | 72.9 ± 7.5 | 72.8 ± 7.7 | 0.938 |
| Sleep diary | ||||
| SOL, min | 51.5 ± 38.6 | 47.8 ± 35.7 | 49.6 ± 37.1 | 0.558 |
| WASO, min | 58.0 ± 44.0 | 56.2 ± 47.1 | 57.1 ± 45.4 | 0.820 |
| TST, min | 319.4 ± 85.2 | 331.1 ± 96.8 | 325.3 ± 91.1 | 0.453 |
| SE, % | 64.8 ± 15.4 | 68.2 ± 17.9 | 66.5 ± 16.8 | 0.231 |
| HADS | ||||
| Anxiety | 9.2 ± 3.9 | 8.6 ± 3.2 | 8.9 ± 3.6 | 0.316 |
| Depression | 8.4 ± 4.1 | 8.1 ± 3.8 | 8.3 ± 3.9 | 0.682 |
| BFI | 5.6 ± 2.1 | 5.6 ± 2.1 | 5.6 ± 2.1 | 0.939 |
| BPI-SF | ||||
| Pain severity | 3.4 ± 2.9 | 4.3 ± 2.4 | 3.9 ± 2.7 | 0.054 |
| Pain interference | 3.3 ± 3.1 | 3.9 ± 2.7 | 3.6 ± 2.9 | 0.203 |
| FACT-B | 80.3 ± 21.3 | 82.5 ± 19.7 | 81.4 ± 20.4 | 0.536 |
| AES | 14.8 ± 3.4 | 14.8 ± 3.3 | 14.8 ± 3.3 | 0.980 |
Data are presented as mean ± standard deviation, number (%), or median (interquartile range)
AC/TC Adriamycin and cyclophosphamide, or taxotere and cyclophosphamide; AC/EC Adriamycin and cyclophosphamide, or epirubicin and cyclophosphamide; BFI Brief fatigue inventory; BPI-SF Brief pain inventory-short form; FEC + T Fluorouracil, epirubicin and cyclophosphamide, plus taxotere; ISI Insomnia severity index; PSQI Pittsburgh sleep quality index; SOL Sleep onset latency; TAC Taxotere, adriamycin, and cyclophosphamide; T/P Taxotere or paclitaxel; WASO Wake after sleep onset; TST Total sleep time; SE Sleep efficiency; HADS Hospital anxiety and depression scale; FACT-B Functional assessment of cancer therapy-breast cancer; AES Acupuncture expectancy scale
aDuration of insomnia was reported by the participant and verified by assessors before enrollment
*Comparison between acupuncture group and sham acupuncture group by or Fisher’s exact test, unpaired t-test, or Mann–Whitney U test
Cessation rate and sleeping medications used
The active acupuncture group had a higher cessation rate of sleeping medication during 18–20 weeks than the sham control (56.5% vs. 14.3%, P = 0.011). Dosage of sleeping medications and weekly frequency of use were not significantly different between groups (Fig. 2, Additional file 1: eTable S2A). Characteristics of participants use sedatives, hypnotics, anxiolytics in two groups were similar (Fig. 2, Additional file 1: eTable S2B).
Fig. 2.
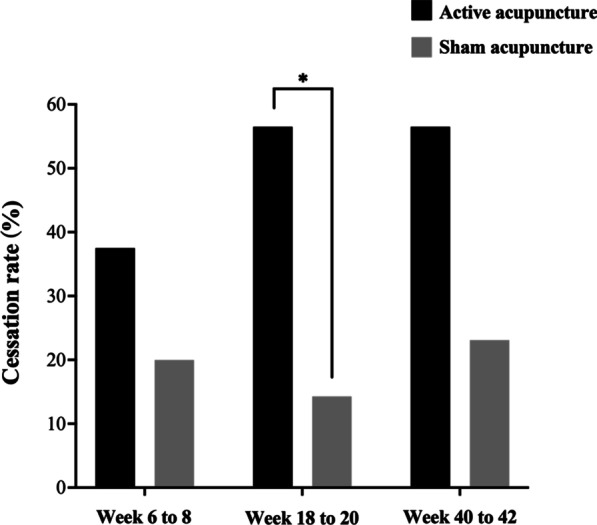
Cessation rate over time. *P < 0.05, comparison between groups by test
Primary outcome
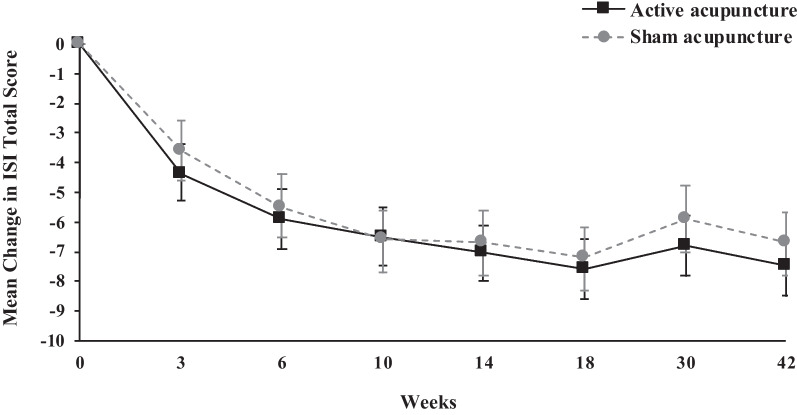
The ISI-measured severity of insomnia strikingly reduced from baseline over the 42 week course in two groups (all Ps < 0.001), but the magnitude of ISI score reduction was not significantly different between groups at any measurement points, including at 6 weeks with a 0.4 difference between the two groups (95% CI − 1.8–1.1; P = 0.609 (Table 3, Fig. 3). The tipping-point sensitivity analysis of shift ISI score showed that the non-differences were not reversed under missing at random with ISI score shifted from the minimum of 7 to the maximum of 28 (Additional file 1: eTable S3). Under the adjustment for baseline expectancy for treatment outcomes, the results did not show deviations (Additional file 1: eTable S4).
Table 3.
Changes in outcomes from baseline by groups of sham-controlled study
| Outcomes | Change from baseline | Between-group difference | P valuea | |
|---|---|---|---|---|
| Active acupuncture (n = 69) | Sham acupuncture (n = 69) | Active acupuncture versus Sham acupuncture | ||
| Primary outcome | ||||
| ISI† | ||||
| Week-3 | − 4.4 (− 5.4 to − 3.5)# | − 3.6 (− 4.6 to − 2.6)# | − 0.8 (− 2.2 to 0.6) | 0.265 |
| Week-6 | − 5.9 (− 6.9 to − 4.9)# | − 5.5 (− 6.6 to − 4.5)# | − 0.4 (− 1.8 to 1.1) | 0.609 |
| Week-10 | − 6.5 (− 7.5 to − 5.5)# | − 6.6 (− 7.6 to − 5.5)# | 0.1 (− 1.4 to 1.6) | 0.894 |
| Week-14 | − 7.0 (− 7.9 to − 6.0)# | − 6.7 (− 7.8 to − 5.6)# | − 0.2 (− 1.7 to 1.2) | 0.736 |
| Week-18 | − 7.6 (− 8.6 to − 6.6)# | − 7.2 (− 8.2 to − 6.1)# | − 0.5 (− 1.9 to 1.0) | 0.541 |
| Week-30 | − 6.8 (− 7.8 to − 5.8)# | − 5.9 (− 7.0 to − 4.8)# | − 0.9 (− 2.3 to 0.6) | 0.249 |
| Week-42 | − 7.5 (− 8.5 to − 6.5)# | − 6.7 (− 7.7 to − 5.6)# | − 0.8 (− 2.3 to 0.6) | 0.265 |
| Secondary outcomes | ||||
| Actiwatch, week-6 | ||||
| SOL, min† | − 7.5 (− 9.8 to − 5.3)# | 1.5 (− 0.9 to 3.9) | − 9.0 (− 12.3 to − 5.7) | < .001 |
| WASO, min† | − 7.2 (− 12.1 to − 2.4)# | − 8.2 (− 13.5 to − 2.9)# | 1.0 (− 6.2 to 8.2) | 0.784 |
| TST, min‡ | 2.7 (− 4.2 to 9.6) | 2.3 (− 5.3 to 9.9) | 0.4 (− 9.8 to 10.6) | 0.943 |
| SE, % ‡ | 2.8 (1.8 to 3.8)# | 1.3 (0.3 to 2.4) | 1.5 (0.0 to 2.9) | 0.049 |
| Sleep diary, week-6 | ||||
| SOL, min† | − 11.4 (− 18.9 to − 3.9)# | − 3.3 (− 11.1 to 4.6) | − 8.1 (− 16.1 to − 0.2) | 0.044 |
| WASO, min† | − 9.2 (− 16.2 to 2.2) | − 13.5 (− 21.2 to − 5.8)# | 4.3 (− 6.1 to 14.8) | 0.413 |
| TST, min‡ | 42.8 (32.2 to 53.3)# | 13.5 (1.9 to 25.1) | 29.2 (13.5 to 44.9) | < .001 |
| SE, % ‡ | 8.6 (6.5 to 10.6)# | 4.1 (1.8 to 6.4)# | 4.4 (1.3 to 7.5) | 0.005 |
| PSQI† | ||||
| Week-3 | − 2.3 (− 3.0 to − 1.5)# | − 2.1 (− 2.9 to − 1.4)# | − 0.1 (− 1.2 to 0.9) | 0.808 |
| Week-6 | − 3.4 (− 4.2 to − 2.7)# | − 3.3 (− 4.2 to − 2.5)# | − 0.1 (− 1.2 to 1.0) | 0.894 |
| Week-10 | − 4.1 (− 4.8 to − 3.3)# | − 3.9 (− 4.7 to − 3.1)# | − 0.2 (− 1.3 to 0.9) | 0.742 |
| Week-14 | − 4.6 (− 5.3 to − 3.8)# | − 3.7 (− 4.5 to − 2.9)# | − 0.9 (− 2.0 to 0.2) | 0.123 |
| Week-18 | − 5.0 (− 5.7 to − 4.2)# | − 4.5 (− 5.3 to − 3.7)# | − 0.5 (− 1.6 to 0.7) | 0.408 |
| Week-30 | − 4.9 (− 5.6 to − 4.1)# | − 4.0 (− 4.9 to − 3.2)# | − 0.8 (− 2.0 to 0.3) | 0.148 |
| Week-42 | − 5.2 (− 6.0 to − 4.4)# | − 4.2 (− 5.0 to − 3.3)# | − 1.0 (− 2.1 to 0.1) | 0.084 |
| HADS-Anxiety† | ||||
| Week-3 | − 1.0 (− 1.6 to − 0.4)# | − 0.5 (− 1.1 to 0.1) | − 0.5 (− 1.3 to 0.3) | 0.250 |
| Week-6 | − 2.0 (− 2.5 to − 1.4)# | − 1.2 (− 1.9 to − 0.6)# | − 0.7 (− 1.6 to 0.1) | 0.098 |
| Week-10 | − 2.1 (− 2.7 to − 1.5)# | − 1.0 (− 1.7 to − 0.4)# | − 1.1 (− 1.9 to − 0.2) | 0.016 |
| Week-14 | − 1.9 (− 2.5 to − 1.3)# | − 1.3 (− 2.0 to − 0.7)# | − 0.6 (− 1.4 to 0.3) | 0.213 |
| Week-18 | − 2.3 (− 2.9 to − 1.7)# | − 1.8 (− 2.4 to − 1.1)# | − 0.5 (− 1.4 to 0.4) | 0.256 |
| Week-30 | − 2.1 (− 2.7 to − 1.5)# | − 1.8 (− 2.5 to − 1.2)# | − 0.2 (− 1.1 to 0.6) | 0.593 |
| Week-42 | − 2.6 (− 3.2 to − 2.0)# | − 1.0 (− 1.7 to − 0.4)# | − 1.6 (− 2.4 to − 0.7) | 0.001 |
| HADS-Depression† | ||||
| Week-3 | − 0.7 (− 1.4 to − 0.1)# | − 0.8 (− 1.5 to − 0.1)# | − 0.1 (− 0.9 to 1.0) | 0.889 |
| Week-6 | − 1.3 (− 1.9 to − 0.6)# | − 1.5 (− 2.2 to − 0.8)# | 0.2 (− 0.7 to 1.2) | 0.613 |
| Week-10 | − 2.0 (− 2.6 to − 1.3)# | − 1.5 (− 2.2 to − 0.8)# | − 0.5 (− 1.4 to 0.5) | 0.354 |
| Week-14 | − 2.1 (− 2.8 to − 1.5)# | − 1.7 (− 2.4 to − 1.0)# | − 0.4 (− 1.4 to 0.5) | 0.382 |
| Week-18 | − 2.2 (− 2.9 to − 1.6)# | − 2.2 (− 2.9 to − 1.5)# | − 0.0 (− 1.0 to 0.9) | 0.932 |
| Week-30 | − 2.1 (− 2.8 to − 1.5)# | − 1.5 (− 2.3 to − 0.8)# | − 0.6 (− 1.6 to 0.4) | 0.227 |
| Week-42 | − 3.2 (− 3.9 to − 2.5)# | − 1.3 (− 2.0 to − 0.6)# | − 1.9 (− 2.9 to − 0.9) | < .001 |
| BFI† | ||||
| Week-3 | − 1.0 (− 1.5 to − 0.6)# | − 1.1 (− 1.5 to − 0.6)# | 0.0 (− 0.6 to 0.7) | 0.917 |
| Week-6 | − 1.7 (− 2.2 to − 1.3)# | − 1.7 (− 2.2 to − 1.2)# | − 0.0 (− 0.7 to 0.6) | 0.907 |
| Week-10 | − 2.1 (− 2.6 to − 1.7)# | − 1.6 (− 2.1 to − 1.1)# | − 0.5 (− 1.2 to 0.1) | 0.117 |
| Week-14 | − 2.5 (− 2.9 to − 2.0)# | − 2.1 (− 2.6 to − 1.6)# | − 0.4 (− 1.0 to 0.3) | 0.261 |
| Week-18 | − 2.6 (− 3.1 to − 2.2)# | − 2.2 (− 2.7 to − 1.7)# | − 0.4 (− 1.1 to 0.2) | 0.189 |
| Week-30 | − 2.4 (− 2.9 to − 2.0)# | − 1.9 (− 2.4 to − 1.4)# | − 0.5 (− 1.2 to 0.1) | 0.110 |
| Week-42 | − 2.7 (− 3.2 to − 2.2)# | − 2.1 (− 2.6 to − 1.6)# | − 0.5 (− 1.2 to 0.2) | 0.134 |
| BPI-SF pain severity† | ||||
| Week-3 | − 0.7 (− 1.2 to − 0.2)# | − 0.5 (− 1.0 to 0.0)# | 0.2 (− 0.9 to 0.5) | 0.578 |
| Week-6 | − 0.6 (− 1.1 to − 0.1) | − 0.6 (− 1.2 to − 0.1)# | 0.0 (− 0.7 to 0.8) | 0.914 |
| Week-10 | − 1.0 (− 1.5 to − 0.5)# | − 1.2 (− 1.7 to − 0.6)# | 0.2 (− 0.6 to 0.9) | 0.642 |
| Week-14 | − 1.0 (− 1.4 to − 0.5)# | − 1.0 (− 1.5 to − 0.5)# | 0.0 (− 0.7 to 0.8) | 0.917 |
| Week-18 | − 0.7 (− 1.2 to − 0.2) | − 1.2 (− 1.7 to − 0.6)# | 0.4 (− 0.3 to 1.2) | 0.232 |
| Week-30 | − 1.0 (− 1.5 to − 0.5)# | − 1.2 (− 1.8 to − 0.7)# | 0.3 (− 0.5 to 1.0) | 0.470 |
| Week-42 | − 1.1 (− 1.6 to − 0.6)# | − 0.7 (− 1.2 to − 0.2)# | 0.4 (− 1.1 to 0.4) | 0.285 |
| BPI-SF pain interference† | ||||
| Week-3 | − 0.8 (− 1.3 to − 0.3)# | − 0.5 (− 1.0 to 0.0)# | − 0.3 (− 1.0 to 0.4) | 0.375 |
| Week-6 | − 0.9 (− 1.4 to − 0.4) | − 0.8 (− 1.3 to − 0.3)# | − 0.1 (− 0.8 to 0.6) | 0.817 |
| Week-10 | − 1.2 (− 1.7 to − 0.7)# | − 1.0 (− 1.5 to − 0.5)# | − 0.2 (− 0.9 to 0.5) | 0.528 |
| Week-14 | − 1.5 (− 2.0 to − 1.0)# | − 1.3 (− 1.8 to − 0.8)# | − 0.2 (− 0.9 to 0.5) | 0.530 |
| Week-18 | − 1.2 (− 1.7 to − 0.7)# | − 1.5 (− 2.1 to − 1.0)# | 0.4 (− 0.4 to 1.1) | 0.331 |
| Week-30 | − 1.2 (− 1.7 to − 0.7)# | − 1.6 (− 2.1 to − 1.1)# | 0.4 (− 0.3 to 1.1) | 0.300 |
| Week-42 | − 1.6 (− 2.1 to − 1.1)# | − 1.2 (− 1.7 to − 0.6)# | − 0.5 (− 1.2 to 0.3) | 0.210 |
| FACT-B‡ | ||||
| Week-3 | 6.3 (3.3 to 9.4)# | 6.8 (3.6 to 10.0)# | − 0.4 (− 4.9 to 4.0) | 0.842 |
| Week-6 | 10.6 (7.5 to 13.7)# | 9.8 (6.5 to 13.1)# | 0.8 (− 3.8 to 5.3) | 0.742 |
| Week-10 | 14.2 (11.1 to 17.3)# | 10.5 (7.1 to 13.9)# | 3.7 (− 0.9 to 8.3) | 0.118 |
| Week-14 | 14.7 (11.6 to 17.9)# | 9.4 (6.0 to 12.8)# | 5.3 (0.7 to 9.9) | 0.024 |
| Week-18 | 15.8 (12.6 to 18.9)# | 12.3 (8.9 to 15.7)# | 3.5 (− 1.2 to 8.1) | 0.141 |
| Week-30 | 14.4 (11.3 to 17.6)# | 12.6 (9.2 to 16.0)# | 1.8 (− 2.8 to 6.5) | 0.441 |
| Week-42 | 19.1 (15.9 to 22.3)# | 10.1 (6.6 to 13.5)# | 9.0 (4.3 to 13.7) | < .001 |
Data are presented as mean (95% confidence interval)
BFI Brief fatigue inventory; BPI-SF Brief pain inventory-short form; ISI Insomnia severity index; PSQI Pittsburgh sleep quality index; SOL Sleep-onset latency; WASO Wake after sleep onset; TST Total sleep time; SE Sleep efficiency; HADS Hospital anxiety and depression scale; FACT-B Functional assessment of cancer therapy-breast cancer
†A greater negative value represents improvement in symptoms
‡A greater positive value represents improvement in symptoms
#P < 0.05, calculated using a mixed-effects model with baseline adjustment to illustrate pre- and post-treatment within-group differences
aP value was calculated using a mixed-effects model with baseline adjustment to illustrate between-group differences
Fig. 3.
Mean change of ISI total score over time. Error bar represents 95% confidence interval
Secondary outcomes
Secondary outcomes are summarized in Table 3. Within-group comparisons revealed that both groups had significant improvements from baseline on sleep quality, wake time after sleep onset, anxiety, depression, fatigue, pain, and quality of life (all Ps < 0.05). At 6 weeks, the active acupuncture was more effective than sham control in shortening sleep onset latency recorded with Actiwatch (P < 0.001) and diary (P = 0.044), increasing diary-recorded total sleep time (P < 0.001), Actiwatch (P = 0.049) and diary-measured (P = 0.005) sleep efficiency. Changes in PSQI did not differ between two groups over the whole study. The active acupuncture group had significantly greater effects than the sham control in reducing severity of HADS-measured anxiety at 10 weeks (P = 0.016) and both anxiety and depression at 42 weeks (P ≤ 0.001), and improving FACT-B-measured quality of life at 14 (P = 0.024) and 42 weeks (P < 0.001).
Adverse events
Treatment-related AEs were mild (Additional file 1: eTable S5). Two non-treatment-related serious AEs (pneumonia) were reported in the active acupuncture group. The most common AE observed in the active acupuncture group was bruising [13.0% (6/69)]. There were 4 sham acupuncture-treated participants who reported auricular skin allergic reaction. No participants discontinued treatments due to AEs.
Credibility for treatment and blinding design
Credibility score for treatment was not significantly different between groups (Additional file 1: eTable S6). James’ blinding index was 0.72 (95% CI 0.65–0.78; P = 1.000), and Bang’s blinding index was 0.13 (− 0.01–0.27; P = 0.038) for the active acupuncture and 0.09 (− 0.09–0.27; P = 0.150) for the sham control, indicating high successiveness of blinding (Additional file 1: eTables S7A and S7B).
Discussion
The main purpose of this trial was to determine whether the active acupuncture regimen could produce superior efficacy over the sham control in improving chemotherapy-associated insomnia in breast cancer patients. Following 12 sessions of treatment, participants on the active acupuncture regimen showed significantly greater improvements than sham control in sleep onset latency, total sleep time and sleep efficiency, which were examined daily with the objective assessment Actiwatch and subjective assessment diary. Likewise, it is evidenced that active acupuncture was markedly associated with improvements in objective sleep parameters, including increases in total sleep time and sleep efficiency [60]. The present study showed that two groups did not differ in changes in ISI and PSQI scores, which were measured biweekly and monthly, respectively. The reasons for this discrepancy are twofold: firstly, the short-term efficacy of acupuncture in alleviating insomnia may be more apparent than long-term efficacy. Previous studies have demonstrated robust short-term effects of acupuncture for insomnia in breast cancer survivors and adult women [61, 62]. Secondly, daily-based sleep parameters from Actiwatch/diary seemed to be more sensitive in detecting improvements of insomnia than those assessed biweekly/monthly.
Participants on the active acupuncture regimen achieved better treatment outcomes than those on the sham control in reducing comorbid anxiety and depression and improving quality of life during treatment and follow-up. Similar results have been observed in recent trial that demonstrated the superior efficacy of active acupuncture regimen in improving chemotherapy-induced cognitive decline, distress caused psychological and somatic symptoms and social functions in breast cancer patients [29]. It is also consistent with numerous studies that confirm the effectiveness of acupuncture for anxiety and depressive disorders [63, 64]. Additionally, this study displayed that the active acupuncture group had a much lower discontinuation rate compared to the sham control. One common reason for discontinuation was lack of perceived efficacy. The current results affirm the notion that active acupuncture regimen is not only effective in improving insomnia and psychiatric disorders occurred during and post-chemotherapy in breast cancer patients, but such superior efficacy also could be well sustained over a follow-up period.
Moreover, in a follow-up period of 18–20 weeks, a much greater proportion of the participants in the active acupuncture group stopped taking sleeping medications than the sham control group. Our previous study, nonetheless, displayed no difference in cessation rate in long-term benzodiazepine users between electroacupuncture and non-invasive placebo control [65]. This seemed mainly due to the fact that occurrence of insomnia in this study was directly associated with chemotherapy, whereas insomnia in previous study was comorbid with psychiatric disorders [65]. It is therefore suggested that the active acupuncture regimen could serve as a tapering approach to reduce and even replace the use of sleeping medications in breast cancer patients.
Similar to previous studies [63, 66], this study presented that acupuncture had a high safety profile with well tolerability. There were only few mild adverse events reported from both groups and no participants discontinued treatments due to adverse events. In this study, while acupuncture therapy earned high trustworthiness from most participants, there was no difference between groups in credibility scores. The primary outcome was not deviated under adjustment for baseline expectancy, indicating that treatment outcomes were not confounded with participants’ expectancy.
Several possible mechanisms have been proposed to understand the effect of acupuncture for cancer-related insomnia. First, acupuncture can regulate cerebral neurotransmitters associated with sleep regulation, such as gamma aminobutyric acid, 5-hydroxytryptamine, dopamine, noradrenaline, acetylcholine, histamine and orexin [67, 68]. Second, acupuncture can reduce heart rate variability, blood pressure, and sympathetic nerve activity, which are frequently dysregulated in individuals with insomnia [69]. Acupuncture may counterbalance the over-excited sympathetic system, thus relieving insomnia symptoms. Third, the anti-inflammation of acupuncture may contribute to its effect for chemotherapy-associated insomnia [70]. Finally, acupuncture relieves physical (i.e., fatigue, pain, hot flushes, neurotoxicity) and psychological (i.e., depression, anxiety) comorbidities related to cancer and cancer treatment, and hence to improve sleep quality [71]. More research to investigate the mechanisms of acupuncture for cancer-related insomnia are warranted.
The efficacy of treatments might be affected by the precipitating or perpetuating factors of chemotherapy-associated insomnia [7, 8]. Acupuncture has been reported to generate an 8.3-point reduction in ISI score among cancer survivors who have completed active treatments [33], and about 6-point reduction among breast cancer patients undergo or post-chemotherapy in our present and previous trial [23]. Similarly, behavioral therapy produced a 10.9-point reduction in ISI score among cancer survivors [33], and around 6-point reduction in patients with breast cancer undergoing chemotherapy [72]. Although variations in treatment procedures and participant characteristics might partially explain the differences in efficacy of acupuncture or behavioral therapy among population with different cancer trajectory phases, the multiple physical and mental challenges faced by patients undergoing chemotherapy must be taken into account when comparing the results of these studies.
The sham control produced greater improvement in ISI score after 6-week of intervention than other sham-controlled acupuncture trials [21, 58], consequently led to the non-significant between-group difference and smaller effect size. The heterogeneous effects of sham acupuncture in different studies were associated with multiple factors. Firstly, the greater effect of sham acupuncture in the present study might be associated with longer treatment duration, more treatment sessions, and spontaneous relief of chemotherapy-related symptoms following the completion of chemotherapy. Secondly, 70% of participants had prior acupuncture experience, indicating participants had high expectancy in the effectiveness of treatment, thereby might overoptimize treatment responses. High expectancy might be related to high response rate in sham control which makes it more difficult to detect additional “specific” effects of active acupuncture over placebo [73]. Therefore, the tentatively non-inferiority effect of sham acupuncture seen in this study should be treated with caution.
One should consider the following limitations of this study. Firstly, although Streitberger’s retractable placebo needles have been widely used to differentiate specific effects of inserted acupuncture from non-inserted needles, it still could produce widespread modulatory effects at multiple levels of central nervous system by exciting mechanoreceptors underneath skin via pressure from non-inserted needles [28]. Numerous neuroimaging studies have demonstrated that there were no differences between “real” and “sham” acupuncture in neuromodulation of functional brain networks [28, 74]. Caution should be exercised in interpreting the tentatively non-inferiority effect of the sham acupuncture in this study. Recently, we have introduced minimum acupuncture stimulation as control, in which acupoints used are unrelated the treated condition, number of acupoints used and stimulation intensity are kept to a minimum at which patients were still aware of receiving active acupuncture [29, 75]. Secondly, the active acupuncture regimen is a combination of needling into body acupoints and acupressure on auricular points. It is unclear whether beneficial effects of such combination were generated from two individual acupuncture modes in a synergistic manner. Finally, only Actiwatch used as objective measure and discrepancies between Actiwatch and sleep diary were observed. Additional objective measures, e.g., polysomnography, should be considered reciprocally to validate the results from Actiwatch and clinical instruments. It also could help gain a better insight into brain mechanisms of the therapeutic effects of acupuncture for insomnia.
Conclusion
The active acupuncture regimen produced better outcomes than the sham control in improving sleep onset latency, total sleep time and sleep efficiency in breast cancer women with chemotherapy-associated insomnia, although there was no significant difference in reducing insomnia severity. Active acupuncture had significantly greater effects in improving anxiety, depression and quality of life. Participants of the active acupuncture group showed a markedly higher cessation rate of sleeping medications. Active acupuncture regimen could be considered as an effective option for chemotherapy-associated insomnia and a tapering approach to reduce and even replace use of sleeping medications in breast cancer patients.
Supplementary Information
Additional file 1: eTable S1A. Location and traditional Chinese medicine (TCM)-based therapeutic effects of the acupoints used in the trial. eTable S1B. Recommendation of additional acupoints based on common comorbid symptoms. eTable S1C. Detailed treatment procedures of active and sham acupuncture regimen. eTable S2A. Cessation rate, dose and weekly frequency of use of sleeping medications. eTable S2B. Characteristics of participants use sedatives, hypnotics, anxiolytics. eTable S3. Tipping-point sensitivity analysis for shift ISI score. eTable S4. Expectancy for treatment outcomes between groups. eTable S5. Adverse events related to treatment. eTable S6. Credibility toward treatment between the two groups. eTable S7A. Assessment of successiveness of blinding. eTable S7B. Results of blinding assessment.
Acknowledgements
We acknowledge the DSMB members, Dr. Chi Ho Chung (The Jockey Club School of Public Health and Primary Care Faculty of Medicine, the Chinese University of Hong Kong), Dr. Tai Chung Lam (Department of Clinical Oncology, HKU), and Dr. Jihui Zhang (Department of Psychiatry, The Chinese University of Hong Kong) for reviewing study design and monitoring trial progress. We thank all participants for their valuable support to this trial. We thank Ms. Lo Lo Yam, Miss Wai Nga Chung and Miss Yuting Ying for their assistance in performing treatments, and all research staffs for their dedication to the research process.
Abbreviations
- AE
Adverse event
- AC
Adriamycin and cyclophosphamide
- AC/EC
Adriamycin and cyclophosphamide, or epirubicin and cyclophosphamide
- AES
Acupuncture expectancy scale
- BFI
Brief fatigue inventory
- BPI-SF
Brief pain inventory-short form
- CMPs
Chinese medicine practitioners
- DSMB
Data and safety monitoring board
- FAC
Fluorouracil, adriamycin, and cyclophosphamide
- FACT-B
Functional assessment of cancer therapy-breast cancer
- FEC + T
Fluorouracil, epirubicin and cyclophosphamide, plus taxotere
- HADS
Hospital anxiety and depression scale
- HK
Hong Kong
- HKU
University of Hong Kong
- ISI
Insomnia severity index
- MAR
Missing-at-random
- SE
Sleep efficiency
- SOL
Sleep onset latency
- T/P
Taxotere or paclitaxel
- TAC
Taxotere, adriamycin, and cyclophosphamide
- TC
Taxotere and cyclophosphamide
- TST
Total sleep time
- WASO
Wake after sleep onset
Author contributions
JLZ drafted the manuscript. ZJZ, LXL, JLZ, HYC, THS, TYC, KFC, and WFY participated in study design. THS and TYC referred participants. ZSQ conducted data analysis. ZJZ revised the manuscript. JLZ and SCY carried out treatments. PYC performed assessments. SFX, YH, CYC provided critical comments. All authors approved the final manuscript.
Funding
This trial was funded by Health and Medical Research Fund, Government of the Hong Kong Special Administrative Region of China (Ref no: 16172761). The funding body was not involved in study design and conduct, data collection, management, analysis and interpretation.
Availability of data and materials
The data underlying this article will be shared on reasonable request addressed to zhangzj@hku.hk.
Declarations
Ethics approval and consent to participate
The trial was approved by Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (UW 19-045) and Research Ethics Committee of Hong Kong Sanatorium & Hospital (REC-2019-14). The research was carried out in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declared no potential competing interests with respect to the research, authorship, and publication of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lixing Lao, Email: llao@vuim.edu.
Zhang-Jin Zhang, Email: zhangzj@hku.hk.
References
- 1.Palesh O, Roscoe J, Mustian K, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. J Clin Oncol. 2010;28(2):292. doi: 10.1200/JCO.2009.22.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davidson JR, MacLean AW, Brundage MD, Schulze K. Sleep disturbance in cancer patients. Soc Sci Med. 2002;54(9):1309–1321. doi: 10.1016/S0277-9536(01)00043-0. [DOI] [PubMed] [Google Scholar]
- 3.Savard J, Ivers H, Savard MH, Morin CM. Cancer treatments and their side effects are associated with aggravation of insomnia: results of a longitudinal study. Cancer. 2015;121(10):1703–1711. doi: 10.1002/cncr.29244. [DOI] [PubMed] [Google Scholar]
- 4.Palesh O, Peppone L, Innominato PF, et al. Prevalence, putative mechanisms, and current management of sleep problems during chemotherapy for cancer. Nat Sci Sleep. 2012;4:151–162. doi: 10.2147/NSS.S18895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleming L, Randell K, Stewart E, et al. Insomnia in breast cancer: a prospective observational study. Sleep. 2019;42(3):zsy245. doi: 10.1093/sleep/zsy245. [DOI] [PubMed] [Google Scholar]
- 6.Hoang HTX, Molassiotis A, Chan CW, Nguyen TH, Liep NV. New-onset insomnia among cancer patients undergoing chemotherapy: prevalence, risk factors, and its correlation with other symptoms. Sleep Breath. 2020;24(1):241–251. doi: 10.1007/s11325-019-01839-x. [DOI] [PubMed] [Google Scholar]
- 7.Savard J, Simard S, Blanchet J, Ivers H, Morin CM. Prevalence, clinical characteristics, and risk factors for insomnia in the context of breast cancer. Sleep. 2001;24(5):583–590. doi: 10.1093/sleep/24.5.583. [DOI] [PubMed] [Google Scholar]
- 8.Souza RCS, Dos Santos MR, das Chagas Valota IA, Sousa CS, Costa Calache ALS. Factors associated with sleep quality during chemotherapy: an integrative review. Nurs Open. 2020;7(5):1274–1284. doi: 10.1002/nop2.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riley KE, Garland SN, Mao JJ, et al. Hyperarousal and Insomnia in survivors of cancer. Int J Behav Med. 2021;28(6):683–691. doi: 10.1007/s12529-021-09962-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ball LJ, Palesh O, Kriegsfeld LJ. The pathophysiologic role of disrupted circadian and neuroendocrine rhythms in breast carcinogenesis. Endocr Rev. 2016;37(5):450–466. doi: 10.1210/er.2015-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haque R, Hsu JW, Avila C, Olmstead R, Carroll JE, Irwin MR. Insomnia and susceptibility to depressive symptoms and fatigue in diverse breast cancer survivors. J Womens Health. 2021;30(11):1604–1615. doi: 10.1089/jwh.2019.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleming L, Gillespie S, Espie CA. The development and impact of insomnia on cancer survivors: a qualitative analysis. Psychooncology. 2010;19(9):991–996. doi: 10.1002/pon.1652. [DOI] [PubMed] [Google Scholar]
- 13.Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19(3):895–908. doi: 10.1200/JCO.2001.19.3.895. [DOI] [PubMed] [Google Scholar]
- 14.Ma Y, Hall DL, Ngo LH, Liu Q, Bain PA, Yeh GY. Efficacy of cognitive behavioral therapy for insomnia in breast cancer: a meta-analysis. Sleep Med Rev. 2021;55:101376. doi: 10.1016/j.smrv.2020.101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edinger JD, Sampson WS. A primary care “friendly” cognitive behavioral insomnia therapy. Sleep. 2003;26(2):177–182. doi: 10.1093/sleep/26.2.177. [DOI] [PubMed] [Google Scholar]
- 16.Koffel E, Bramoweth AD, Ulmer CS. Increasing access to and utilization of cognitive behavioral therapy for insomnia (CBT-I): a narrative review. J Gen Intern Med. 2018;33(6):955–962. doi: 10.1007/s11606-018-4390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mao JJ, Pillai GG, Andrade CJ, et al. Integrative oncology: Addressing the global challenges of cancer prevention and treatment. CA A Cancer J Clin. 2022;72(2):144–164. doi: 10.3322/caac.21706. [DOI] [PubMed] [Google Scholar]
- 18.Mao JJ, Ismaila N, Bao T, et al. Integrative medicine for pain management in oncology: society for integrative oncology–ASCO guideline. J Clin Oncol. 2022;40(34):3998–4024. doi: 10.1200/JCO.22.01357. [DOI] [PubMed] [Google Scholar]
- 19.Deng GE, Rausch SM, Jones LW, et al. Complementary therapies and integrative medicine in lung cancer: diagnosis and management of lung cancer: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5):e420S–e436S. doi: 10.1378/chest.12-2364. [DOI] [PubMed] [Google Scholar]
- 20.Lyman GH, Greenlee H, Bohlke K, et al. Integrative therapies during and after breast cancer treatment: ASCO endorsement of the SIO clinical practice guideline. J Clin Oncol. 2018;36(25):2647–2655. doi: 10.1200/JCO.2018.79.2721. [DOI] [PubMed] [Google Scholar]
- 21.Yin X, Gou M, Xu J, et al. Efficacy and safety of acupuncture treatment on primary insomnia: a randomized controlled trial. Sleep Med. 2017;37:193–200. doi: 10.1016/j.sleep.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Bergdahl L, Broman JE, Berman AH, Haglund K, von Knorring L, Markström A. Sleep patterns in a randomized controlled trial of auricular acupuncture and cognitive behavioral therapy for insomnia. Complement Ther Clin Pract. 2017;28:220–226. doi: 10.1016/j.ctcp.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Qin Z, So TH, et al. Electroacupuncture plus auricular acupressure for chemotherapy-associated insomnia in breast cancer patients: a pilot randomized controlled trial. Integr Cancer Ther. 2021;20:15347354211019103. doi: 10.1177/15347354211019103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacPherson H, Altman DG, Hammerschlag R, et al. Revised standards for reporting interventions in clinical trials of acupuncture (STRICTA): extending the CONSORT statement. J Altern Complement Med. 2010;16(10):ST-1–ST-14. doi: 10.1089/acm.2010.1610. [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Schulz KF, Altman D, Gropu C The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA. 2001;285(15):1987–1991. doi: 10.1001/jama.285.15.1987. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Yang M, So TH, et al. Electroacupuncture plus auricular acupressure on chemotherapy-related insomnia in patients with breast cancer (EACRI): study protocol for a randomized sham-controlled trial. Integr Cancer Ther. 2021;20:15347354211058695. doi: 10.1177/15347354211058695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z-J, Ng R, Man SC, et al. Dense cranial electroacupuncture stimulation for major depressive disorder—a single-blind, randomized, controlled study. PLoS ONE. 2012;7(1):e29651. doi: 10.1371/journal.pone.0029651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Z-J, Wang X-M, McAlonan GM. Neural acupuncture unit: a new concept for interpreting effects and mechanisms of acupuncture. Evid-Based Complement Altern Med. 2012;2012:429412. doi: 10.1155/2012/429412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Z-J, Man S-C, Yam L-L, et al. Electroacupuncture trigeminal nerve stimulation plus body acupuncture for chemotherapy-induced cognitive impairment in breast cancer patients: an assessor-participant blinded, randomized controlled trial. Brain Behav Immun. 2020;88:88–96. doi: 10.1016/j.bbi.2020.04.035. [DOI] [PubMed] [Google Scholar]
- 30.Yeung W-F, Chung K-F, Leung Y-K, Zhang S-P, Law AC. Traditional needle acupuncture treatment for insomnia: a systematic review of randomized controlled trials. Sleep Med. 2009;10(7):694–704. doi: 10.1016/j.sleep.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 31.Yang M, Liou KT, Garland SN, et al. Acupuncture versus cognitive behavioral therapy for pain among cancer survivors with insomnia: an exploratory analysis of a randomized clinical trial. NPJ Breast Cancer. 2021;7(1):148. doi: 10.1038/s41523-021-00355-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liou KT, Root JC, Garland SN, et al. Effects of acupuncture versus cognitive behavioral therapy on cognitive function in cancer survivors with insomnia: a secondary analysis of a randomized clinical trial. Cancer. 2020;126(13):3042–3052. doi: 10.1002/cncr.32847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garland SN, Xie SX, DuHamel K, et al. Acupuncture versus cognitive behavioral therapy for insomnia in cancer survivors: a randomized clinical trial. J Natl Cancer Inst. 2019;111(12):1323–1331. doi: 10.1093/jnci/djz050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang XG, Ying L, Tian XP, Liang FR. Comments on selection of non-acupoints beyond meridians in studies of acupuncture and moxibustion. J Tradit Chin Med. 2010;30(4):309–313. doi: 10.1016/S0254-6272(10)60063-5. [DOI] [PubMed] [Google Scholar]
- 35.Streitberger K, Kleinhenz J. Introducing a placebo needle into acupuncture research. The Lancet. 1998;352(9125):364–365. doi: 10.1016/S0140-6736(97)10471-8. [DOI] [PubMed] [Google Scholar]
- 36.Zou C, Yang L, Wu Y, et al. Auricular acupressure on specific points for hemodialysis patients with insomnia: a pilot randomized controlled trial. PLoS ONE. 2015;10(4):e0122724. doi: 10.1371/journal.pone.0122724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suen LK, Wong TK, Leung AW. Effectiveness of auricular therapy on sleep promotion in the elderly. Am J Chin Med. 2002;30(04):429–449. doi: 10.1142/S0192415X0200051X. [DOI] [PubMed] [Google Scholar]
- 38.Zitman FG, Couvée JE. Chronic benzodiazepine use in general practice patients with depression: an evaluation of controlled treatment and taper-off: report on behalf of the dutch chronic benzodiazepine working group. Br J Psychiatr. 2001;178(4):317–324. doi: 10.1192/bjp.178.4.317. [DOI] [PubMed] [Google Scholar]
- 39.Savard MH, Savard J, Simard S, Ivers H. Empirical validation of the Insomnia Severity Index in cancer patients. Psychooncology. 2005;14(6):429–441. doi: 10.1002/pon.860. [DOI] [PubMed] [Google Scholar]
- 40.Savard J, Savard M-H, Ivers H. Moderators of treatment effects of a video-based cognitive-behavioral therapy for insomnia comorbid with cancer. Behav Sleep Med. 2018;16(3):294–309. doi: 10.1080/15402002.2016.1210148. [DOI] [PubMed] [Google Scholar]
- 41.Morin CM, Belleville G, Bélanger L, Ivers H. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiss AR, Johnson NL, Berger NA, Redline S. Validity of activity-based devices to estimate sleep. J Clin Sleep Med. 2010;6(4):336–342. doi: 10.5664/jcsm.27874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carney CE, Buysse DJ, Ancoli-Israel S, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35(2):287–302. doi: 10.5665/sleep.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatr Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 45.Ho RT, Fong TC. Factor structure of the Chinese version of the Pittsburgh sleep quality index in breast cancer patients. Sleep Med. 2014;15(5):565–569. doi: 10.1016/j.sleep.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 46.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the hospital anxiety and depression scale: an updated literature review. J Psychosom Res. 2002;52(2):69–77. doi: 10.1016/S0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 47.Leung C, Wing Y, Kwong P, Shum ALK. Validation of the Chinese-Cantonese version of the Hospital Anxiety and Depression Scale and comparison with the Hamilton Rating Scale of Depression. Acta Psychiatr Scand. 1999;100(6):456–461. doi: 10.1111/j.1600-0447.1999.tb10897.x. [DOI] [PubMed] [Google Scholar]
- 48.Wang XS, Hao X-S, Wang Y, et al. Validation study of the Chinese version of the brief fatigue inventory (BFI-C) J Pain Symptom Manag. 2004;27(4):322–332. doi: 10.1016/j.jpainsymman.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 49.Mendoza T, Mayne T, Rublee D, Cleeland C. Reliability and validity of a modified brief pain inventory short form in patients with osteoarthritis. Eur J Pain. 2006;10(4):353–361. doi: 10.1016/j.ejpain.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 50.Dhingra L, Lam K, Homel P, et al. Pain in underserved community-dwelling Chinese American cancer patients: demographic and medical correlates. Oncologist. 2011;16(4):523. doi: 10.1634/theoncologist.2010-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wan C, Zhang D, Yang Z, et al. Validation of the simplified Chinese version of the FACT-B for measuring quality of life for patients with breast cancer. Breast Cancer Res Treat. 2007;106(3):413–418. doi: 10.1007/s10549-007-9511-1. [DOI] [PubMed] [Google Scholar]
- 52.Ng R, Lee CF, Wong NS, et al. Measurement properties of the English and Chinese versions of the functional assessment of cancer therapy—breast (FACT-B) in Asian breast cancer patients. Breast Cancer Res Treat. 2012;131(2):619–625. doi: 10.1007/s10549-011-1764-z. [DOI] [PubMed] [Google Scholar]
- 53.Vincent C. Credibility assessment in trials of acupuncture. Complement Med Res. 1990;4(1):8–11. [Google Scholar]
- 54.Mao JJ, Armstrong K, Farrar JT, Bowman MA. Acupuncture expectancy scale: development and preliminary validation in China. Explore. 2007;3(4):372–377. doi: 10.1016/j.explore.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lao L, Bergman S, Hamilton GR, Langenberg P, Berman B. Evaluation of acupuncture for pain control after oral surgery: a placebo-controlled trial. Arch Otolaryngol Head Neck Surg. 1999;125(5):567–572. doi: 10.1001/archotol.125.5.567. [DOI] [PubMed] [Google Scholar]
- 56.Bang H. Random guess and wishful thinking are the best blinding scenarios. Contemp Clin Trials Commun. 2016;3:117–121. doi: 10.1016/j.conctc.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Health NI. Data and safety monitoring board (DSMB) guidelines. Betheseda, Maryland: National Institutes of Health; 2018. [Google Scholar]
- 58.Yeung W-F, Chung K-F, Zhang S-P, Yap T-G, Law AC. Electroacupuncture for primary insomnia: a randomized controlled trial. Sleep. 2009;32(8):1039–1047. doi: 10.1093/sleep/32.8.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Little RJ, D'Agostino R, Cohen ML, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med. 2012;367(14):1355–1360. doi: 10.1056/NEJMsr1203730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao F-Y, Fu Q-Q, Kennedy GA, et al. Can acupuncture improve objective sleep indices in patients with primary insomnia? A systematic review and meta-analysis. Sleep Med. 2021;80:244–259. doi: 10.1016/j.sleep.2021.01.053. [DOI] [PubMed] [Google Scholar]
- 61.Wang X-Y, Yuan S-H, Yang H-Y, et al. Abdominal acupuncture for insomnia in women: a randomized controlled clinical trial. Acupunct Electrother Res. 2008;33(1–2):33–41. doi: 10.3727/036012908803861203. [DOI] [PubMed] [Google Scholar]
- 62.Höxtermann MD, Buner K, Haller H, et al. Efficacy and safety of auricular acupuncture for the treatment of insomnia in breast cancer survivors: a randomized controlled trial. Cancers. 2021;13(16):4082. doi: 10.3390/cancers13164082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang X-Y, Yang N-B, Huang F-F, Ren S, Li Z-J. Effectiveness of acupuncture on anxiety disorder: a systematic review and meta-analysis of randomised controlled trials. Ann Gen Psychiatr. 2021;20(1):1–14. doi: 10.1186/s12991-021-00327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Z-J, Chen H-Y, Yip K-C, Ng R, Wong VT. The effectiveness and safety of acupuncture therapy in depressive disorders: systematic review and meta-analysis. J Affect Disord. 2010;124(1–2):9–21. doi: 10.1016/j.jad.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 65.Yeung W-F, Chung K-F, Zhang Z-J, et al. Electroacupuncture for tapering off long-term benzodiazepine use: a randomized controlled trial. J Psychiatr Res. 2019;109:59–67. doi: 10.1016/j.jpsychires.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 66.Zhang J, Zhang Z, Huang S, et al. Acupuncture for cancer-related insomnia: a systematic review and meta-analysis. Phytomedicine. 2022;102:154160. doi: 10.1016/j.phymed.2022.154160. [DOI] [PubMed] [Google Scholar]
- 67.Franco-Santana LE, Torres-Castillo S, González-Trujano ME, González-Ramírez M. Stimulation of the Po-shen and Shen-hun scalp-acupuncture bands modifies levels of inhibitory and excitatory amino acids in the immature rat brain. Neurochem Int. 2013;63(4):275–282. doi: 10.1016/j.neuint.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 68.Si L, Wang Y, Wuyun G, Bao L, Agula B. The effect of Mongolian medical acupuncture on cytokines and neurotransmitters in the brain tissue of insomniac rats. Eur J Integr Med. 2015;7(5):492–498. doi: 10.1016/j.eujim.2015.05.008. [DOI] [Google Scholar]
- 69.De Zambotti M, Covassin N, De Min TG, Sarlo M, Stegagno L. Sleep onset and cardiovascular activity in primary insomnia. J Sleep Res. 2011;20(2):318–325. doi: 10.1111/j.1365-2869.2010.00871.x. [DOI] [PubMed] [Google Scholar]
- 70.Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: Do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011;29(26):3517. doi: 10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Greenlee H, DuPont-Reyes MJ, Balneaves LG, et al. Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA A Cancer J Clin. 2017;67(3):194–232. doi: 10.3322/caac.21397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Palesh O, Solomon N, Hofmeister E, et al. A novel approach to management of sleep-associated problems in patients with breast cancer (MOSAIC) during chemotherapy: a pilot study. Sleep. 2020;43(10):zsaa070. doi: 10.1093/sleep/zsaa070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Linde K, Witt CM, Streng A, et al. The impact of patient expectations on outcomes in four randomized controlled trials of acupuncture in patients with chronic pain. Pain. 2007;128(3):264–271. doi: 10.1016/j.pain.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 74.Dhond RP, Kettner N, Napadow V. Neuroimaging acupuncture effects in the human brain. J Altern Complement Med. 2007;13(6):603–616. doi: 10.1089/acm.2007.7040. [DOI] [PubMed] [Google Scholar]
- 75.Zhang ZJ, Zhao H, Jin GX, et al. Assessor- and participant-blinded, randomized controlled trial of dense cranial electroacupuncture stimulation plus body acupuncture for neuropsychiatric sequelae of stroke. Psychiatr Clin Neurosci. 2020;74(3):183–190. doi: 10.1111/pcn.12959. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: eTable S1A. Location and traditional Chinese medicine (TCM)-based therapeutic effects of the acupoints used in the trial. eTable S1B. Recommendation of additional acupoints based on common comorbid symptoms. eTable S1C. Detailed treatment procedures of active and sham acupuncture regimen. eTable S2A. Cessation rate, dose and weekly frequency of use of sleeping medications. eTable S2B. Characteristics of participants use sedatives, hypnotics, anxiolytics. eTable S3. Tipping-point sensitivity analysis for shift ISI score. eTable S4. Expectancy for treatment outcomes between groups. eTable S5. Adverse events related to treatment. eTable S6. Credibility toward treatment between the two groups. eTable S7A. Assessment of successiveness of blinding. eTable S7B. Results of blinding assessment.
Data Availability Statement
The data underlying this article will be shared on reasonable request addressed to zhangzj@hku.hk.