Abstract
The study of the molecular mechanisms underlying the action of immunomodulatory drugs is important for substantiating their therapeutic effect. In the present work, spontaneous and TNFα-induced secretion of IL-1α and IL-8 pro-inflammatory cytokines, as well as the level of the ICAM-1 adhesion molecule in EA.hy 926 endothelial cell culture and peripheral blood mononuclear cells of healthy donors, is studied using an in vitro model of inflammation in the presence of α-glutamyl–tryptophan (α-Glu–Trp) and Cytovir-3. The aim was to evaluate cellular mechanisms mediating the immunomodulatory effect of α-Glu–Trp and Cytovir-3 drugs. It was shown that α-Glu–Trp reduced TNFα-induced IL-1α production and increased TNFα-stimulated level of the ICAM-1 surface molecule of endothelial cells. At the same time, the drug reduced secretion of the IL-8 cytokine induced by TNFα and increased the spontaneous level of ICAM-1 in mononuclear cells. Cytovir-3 had an activating effect on EA.hy 926 endothelial cells and human peripheral blood mononuclear leukocytes. In its presence, there was an increase in the spontaneous secretion of IL-8 by endothelial and mononuclear cells. In addition, Cytovir-3 increased the level of TNFα-induced ICAM-1 on endothelial cells and increased the spontaneous level of this surface molecule on mononuclear cells. Suppression of stimulated production of pro-inflammatory cytokines under the action of α-Glu–Trp both separately and as a part of Cytovir-3 may determine its anti-inflammatory properties. However, an increased level of the surface ICAM-1 molecule indicates mechanisms that enhance the functional activity of these cells, which is equally important for the implementation of an effective immune response to infection and repair of damaged tissues during inflammatory response.
Keywords: α-glutamyl–tryptophan, Cytovir-3, EA.hy 926 endothelial cells, human peripheral blood mononuclear leukocytes, ICAM-1, cytokine secretion, ARVI
INTRODUCTION
The high variability of the antigenic composition of the influenza virus, as well as a significant number of ARVI pathogens, poses major challenges for development of effective drugs to treat these infections (Smirnov et al. 2020; Malainou and Herold, 2019; Zinina et al., 2021). Relatedly, the prevention and treatment of SARS is still an important problem that needs to be resolved.
α-Glutamyl–tryptophan (α-Glu–Trp) is a synthetic peptide immunomodulator that is widely used as a drug for the treatment of immunodeficiency. It is known that the drug has a modulating effect on metabolic processes in cells; stimulates the functional activity of cells of the immune system; has an antioxidant effect; stimulates tissue regeneration; accelerates wound healing; activates the functions of connective tissue cells, endotheliocytes, macrophages, and leukocytes in the lesion; and inhibits the production of histamine and serotonin during inflammation (Smirnov et al., 2021; Khavinson et al., 2021). Based on these properties, the pharmaceutical company JSC Cytomed (St. Petersburg, Russia) created the drug Cytovir-3 for the treatment and prevention of influenza and ARVI in adults and children. The drug is a combination of three medicinal substances: bendazole hydrochloride (Dibazol), α-Glu–Trp–sodium (Timogen® sodium), and ascorbic acid.
Cytovir-3 is an agent in etiotropic and immunostimulating therapy and has an antiviral effect against influenza A and B and other viruses that cause acute respiratory viral diseases. The drug can be used both for emergency prevention and treatment, including COVID-19 coronavirus infection (Shipitsyn et al., 2010; Khavinson et al., 2020; Golovacheva et al., 2021; Smirnov et al., 2021). The therapeutic effect of the drug is due to the combined action of its constituent substances. It has now been proven that bendazole and its derivatives can be agonists of TLR-3, TLR-8, and RLR receptors that recognize patterns associated with pathogenic microorganisms (Beesu et al., 2014, 2016). The interaction of benzimidazole analogs with these molecules inhibits the production of pro-inflammatory cytokines (Ullah et al., 2022; Eskandari et al., 2022). Ascorbic acid is a substance with a proven antioxidant and anti-inflammatory effect (Smirnov et al., 2020; Oudemans-Straaten et al., 2014).
The α-Glu–Trp test substance presumably has an immunomodulatory effect in vivo by modulating the expression of genes of pro-inflammatory factors that are under control of the NF-kB transcription factor.
The aim of this work was to study the cellular mechanisms mediating the immunomodulatory effect of α-Glu–Trp. We examined in vitro possible mechanisms of the implementation of the pharmacological effect of α-Glu–Trp and the drug Cytovir-3, which contains α-Glu–Trp, in EA.hy 926 and peripheral blood mononuclear leukocytes. The TNFα pro-inflammatory cytokine, which has a wide range of activities, was used to model cell activation. To assess the immunomodulating effect of drugs, we studied the secretion of the IL-1α and IL-8 pro-inflammatory cytokines, which regulate the development of inflammation during infection. Additionally, surface expression of the inducible ICAM-1 molecule was analyzed.
The selection of the cellular model of research was due to the fact that leukocytes are the main regulatory and effector elements in the development of inflammation and immune response. One of the main functions of endothelial cells is to regulate the leukocyte from the bloodstream to tissues due to the secretion of cytokines and expression of adhesion molecules, which contributes to repair processes. Leukocytes and endothelial cells are the targets of various pro-inflammatory factors (cytokines, components of microorganisms, etc.) and express receptors for these factors on their surface (Moskalets, 2018; Chong et al., 2021).
TNFRI receptors are present on almost all cell types, while TNFRII receptors are present predominantly on cells of the immune system. The binding of TNFα to receptors activates, in particular, NF-kB transcription factor, i.e., to the classical pathway of induction of pro-inflammatory cytokines. The expression of all the studied factors is under the control of the NF-kB transcription factor, and, therefore, the obtained data allow us to indirectly assess the possibility of modulating NF-kB activation under the influence of the studied substances (Markey et al., 2015; Zhang et al., 2020).
IL-1α and IL-1β are traditionally classified as IL‑1 because these cytokines interact with the same receptor cells and their effects are indistinguishable. IL-1α is produced by macrophages, dendritic cells, endothelial and stromal cells, and B-lymphocytes. Macrophages are important to control the immune reaction, switching the stages of the immune response, and implementing effective phagocytosis. The receptors of the TLR family cause the activation of classical pro-inflammatory signaling cascades, which induces the expression of the main inflammatory cytokines: IL-1β and IL-12 interleukins, tumor necrosis factor α (TNFα), and a number of major chemokines, including IL-8. The active immune response of macrophages is maintained by type-1 T helpers (Th1) (Guo et al., 2012; Beesu et al., 2014; Kaneko et al., 2019).
Secreted IL-1α is processed by extracellular proteases to form an active cytokine. Processing of IL-1β takes place inside the cell with the participation of caspase-1. Caspase-1 activation occurs within the inflammasome, and this requires binding of intracellular NLR receptors to PAMP. IL-1 receptors are spontaneously expressed on many cell types. The major targets of this cytokine are endothelial cells and leukocytes, which induce the expression of more than 100 genes. The main effects of IL-1 are the activation and migration of leukocytes (Markey et al., 2015; Chong et al., 2021).
IL-8 is a pro-inflammatory chemokine produced by myeloid and endothelial cells after their activation. The stimulus for the IL-8 synthesis is the binding of TLR to agonists (microbial and viral products) or the action of pro-inflammatory cytokines (IL-1, TNFα, etc.). IL-8 belongs to the chemokine family and plays a critical role in the activation and recruitment of neutrophils to the inflammatory focus of the upper respiratory tract. An important effect of IL-8 is its angiogenic effect, both in the development of inflammation and in wound healing (Guo et al., 2012; Khalil et al., 2021; Matsushima et al., 2022).
ICAM-1 is an adhesion molecule that has a low spontaneous level of expression in cells. The level of the surface molecule increases under the influence of pro-inflammatory cytokines, which are components of microorganisms, as well as various stress factors on cells (Moskalets, 2018; Bui et al., 2020). The molecule provides intercellular interactions in conditions of inflammation. On endothelial cells, ICAM-1 is a counterligand for leukocyte integrin receptors and ensures their migration into tissues under conditions of inflammation. It is a protective factor in the fight against infectious agents. ICAM-1, which is expressed on mononuclear phagocytes, is involved in the formation of the immunological synapse and antigen presentation, which can be used in the design of new vaccines or immunomodulatory drugs (Guo, 2012; Soema et al., 2015; Khavinson et al., 2021).
MATERIALS AND METHODS
Cells
Endothelial cells of the EA.hy 926 permanent cell line were obtained from the University of North Carolina (United States), and mononuclear leukocytes of peripheral blood of healthy donors were received from the bank of the Blood Transfusion Department, First Pavlov State Medical University, St. Petersburg.
Cell Culture
EA.hy 926 endothelial cells were cultured in complete culture medium containing DMEM/F12 and 10% fetal calf serum (ICN, United States) and 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mM glutamine (Flow Laboratories, United Kingdom). To disintegrate the monolayer, 0.25% EDTA solution (Biolot, Russia) was used. Cells were subcultured every 3–4 days and splat at a ratio of 1 : 3.
Granulocyte and mononuclear leukocyte fractions were isolated from heparinized blood by cell sedimentation in ficoll–verografin with a density gradient of 1.077 g/cm3 (Biolot, Russia). Whole blood was diluted twofold with buffered physiological saline (pH 7.2); 8 mL of diluted blood was layered on 4 mL of ficoll–verografin solution and centrifuged in a horizontal rotor at 400 g for 30 min. Mononuclear leukocytes were taken from the interphase ring formed at the interface between the ficoll–verografin mixture and diluted blood and washed three times with the same buffer at pH 7.2. Cell viability was determined by staining with 0.2% Trypan Blue solution. Cells were counted in a Goryaev’s chamber. Mononuclear leucocytes were resuspended in RPMI-1640 medium containing 10% inactivated fetal calf serum (ICN, United States), 50 μg/mL gentamicin sulfate (Samson, Russia), and 2 mM L-glutamine (ICN, United States).
Drugs
The effects of the following drugs were tested: α‑Glu–Trp–sodium and Cytovir-3, this being a mixture of three active components—α-Glu–Trp–sodium, bendazole–hydrochloride (MBNPK Cytomed, Russia), and ascorbic acid. The preparations were diluted in complete DMEM/F12 (for endothelial cells) or RPMI-1640 (for mononuclear leukocytes) culture medium (Biolot, Russia).
For the most adequate assessment of effective dosages of the drugs, their minimum toxic dose was preliminarily determined by dilution in complete culture medium. The concentration range of drugs that are not toxic to cells were estimated with routine cytotoxic tests. Cytovir-3 toxicity to suspension cells was examined with flow cytometry using staining with propidium iodide DNA-binding dye (Sigma-Aldrich, United States) to stain dead cells. The toxicity of Cytovir-3 to endothelial cells was studied by a colorimetric method, staining the cell monolayer with crystal violet. The toxicity α-Glu–Trp preparation for mononuclear leukocytes was evaluated of the basis of the activity of mitochondrial dehydrogenases with the MTT test (Van Meerloo et al., 2011). Based on the results of the studies, concentrations of the α-Glu–Trp preparation of 100.0, 10.0, and 1.0 μg/mL and of the Cytovir-3 preparation of 10.0, 1.0, and 0.1 ng/mL were chosen for further experiments.
Drug Treatment
To test the effect of the drugs on spontaneous and induced secretion of IL-1α, IL-8, and IFNα cytokines, EA.hy 926 endothelial cells were seeded in wells of a 24-well plate at a concentration of 106 cells/mL of culture medium containing 10% fetal calf serum. The cells were cultivated in a CO2 incubator for 4 h in the presence or absence of 50 U/mL TNFα pro-inflammatory cytokine (Refnolin, recombinant TNFα; Sanitas, Lithuania; specific activity of the drug: 1 U corresponds to 0.06 ng). Next, a culture medium containing the test preparations in three concentrations that was nontoxic for the cells selected in the preliminary experiment was added to the plate wells and cultivated for 24 h. The supernatants were, then, harvested and stored at –20°C for subsequent enzyme immunoassay (ELISA).
Peripheral blood mononuclear cells from healthy donors were resuspended in complete RPMI-1640 culture medium and added to the wells of a 24-well plate at a concentration of 106 cells/mL. Subsequent procedures were the same as those described above for EA.hy 926 endothelial cells.
Enzyme Immunoassay
The secretion of cytokines by cells was assessed by ELISA using commercial test systems (Cytokine, Russia) according to the manufacturer’s recommendations. The optical density in the wells was measured with a Bio-Rad Model 680 spectrophotometer (Bio-Rad, United States).
Flow Cytometry
To assess the spontaneous and induced level of the surface ICAM-1 molecules, suspensions of EA.hy 926 endothelial cells and peripheral blood mononuclear cells after stimulation, as in the case of cytokines, were transferred to test tubes for flow cytometric analysis and stained using monoclonal antibodies against ICAM-1 that were labeled with FITC (Beckman Coulter, United States) according to the manufacturer’s recommendations. Samples were analyzed on a NAVIOS flow cytometer (Beckman Coulter, United States). The level of the surface molecule of ICAM-1 was assessed by quantitative determination of the mean fluorescence intensity.
Statistical Treatment
Mean values and standard deviation from three independent experiments were determined using Microsoft Office Excel, 2010. Student’s t-test was used to assess statistical significance. p < 0.05 was considered to be statistically significant.
RESULTS
Production of IL-8 by EA.hy 926 Endothelial Cells
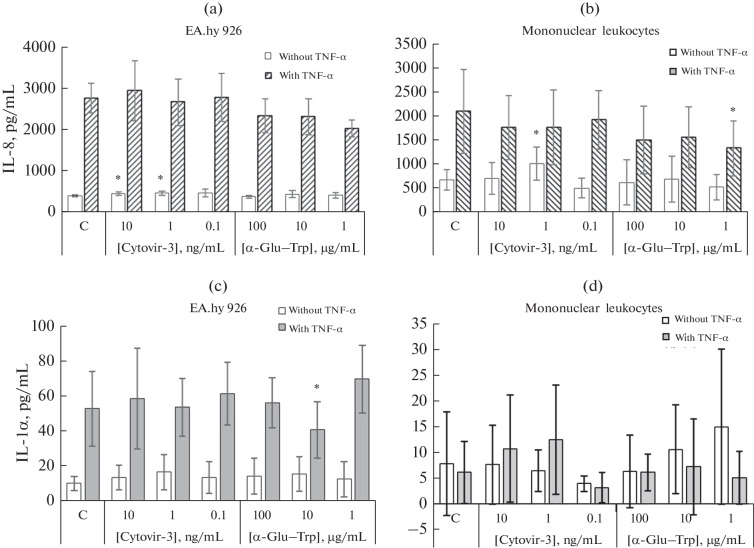
EA.hy 926 endothelial cells had a high spontaneous level of IL-8 secretion, which increased by more than fivefold in the presence of TNFα (Fig. 1a). The α‑Glu–Trp preparation did not statistically significantly change the spontaneous and TNFα-enhanced secretion of IL-8 by EA.hy 926 cells. However, a dose-dependent decline in the induced production of this cytokine was observed (at an α-Glu–Trp concentration of 1 µg/mL, the decrease was 26.37%, p > 0.05) (Fig. 1a).
Fig. 1.
Effect of Cytovir-3 and α-Glu–Trp drugs on the secretion of (a, b) IL-8 and (c, d) IL-1α by (a, c) EA.hy 926 endothelial cells and (b, d) mononuclear (MN) leukocytes of donor peripheral blood. The cells were cultured for 4 h in the presence or absence of TNFα pro-inflammatory cytokine and, then, with Cytovir-3 and α-Glu–Trp at various concentrations or without the drug (C, control) in the complete RPMI-1640 culture medium for 24 h. IL-8 and IL-1α were assessed by ELISA in spontaneous and induced TNFα-induced supernatants of cell samples. Data are presented as mean values and standard deviations. * Differences are significant compared with the control group at p < 0.05.
Production of IL-8 by Mononuclear Cells
Mononuclear leukocytes had a high spontaneous level of IL-8 synthesis, which increased by more than three times under the influence of TNFα (Fig. 1b). When cultivating in the presence of Cytovir-3 at a concentration of 1 ng/mL, spontaneous secretion of IL-8 increased by 49.46% (p < 0.05) was noted, which, however, decreased after induction with TNFα. α‑Glu–Trp alone had no effect on the spontaneous secretion of IL-8; however, at a concentration of 1 µg/mL, it statistically significantly suppressed—by 36.28% (p < 0.05)—the cytokine secretion induced in the presence of TNFα (Fig. 1b).
Production of IL-1α by EA.hy 926 Endothelial Cells
The spontaneous level of IL-1α secretion in endothelial cell culture was low, but it significantly increased under the influence of TNFα (Fig. 1c). Cytovir-3 had no effect on spontaneous and TNFα-induced IL-1α production by these cells. α-Glu–Trp also had no effect on the spontaneous secretion of this cytokine; however, at a concentration of 10 μg/mL, it reduced the secretion enhanced by TNFα by 22.79% (p < 0.05). At a concentration of 1 μg/mL, it increased it by 31.64% (p > 0.05), which was not statistically significant with a large scatter of values (Fig. 1c).
Production of IL-1α by Mononuclear Cells
A low level of spontaneous IL-1α secretion was registered in the culture of mononuclear leukocytes. It changed insignificantly in the presence of TNFα. Cultivation with Cytovir-3 and α-Glu–Trp did not have a significant effect on the spontaneous level of IL-1α secretion by mononuclear leukocytes; however, there was a trend towards a decrease in the level of IL-1α induced by TNFα (Fig. 1d).
ICAM-1 Expression on EA.hy 926 Endothelial Cells
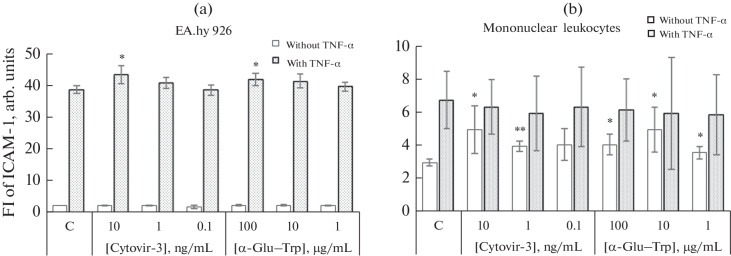
EA.hy 926 endothelial cells had a low spontaneous level of the ICAM-1 adhesion molecule. It increased when cells were cultured in the presence of TNFα (Fig. 2a). Cytovir-3 did not affect the spontaneous level of ICAM-1 in endothelial cells, but, at a concentration of 10 ng/mL, it statistically significantly increased the level of the molecule induced by TNFα by 12.14% (p < 0.05). Cultivation with α-Glu–Trp also had no effect on the spontaneous level of ICAM-1, but, at a concentration of 100 µg/mL, it increased the mean fluorescence intensity of this molecule induced by TNFα by 8.27% (p < 0.05) (Fig. 2a).
Fig. 2.
Effect of Cytovir-3 and α-Glu–Trp drugs on the level of the ICAM-1 surface molecule of (a) EA.hy 926 endothelial cells and (b) mononuclear (MN) leukocytes of peripheral blood donors. Cells were cultured for 4 h in the presence or absence of the pro-inflammatory cytokine TNFα and, then, with Cytovir-3 and α-Glu–Trp at various concentrations or without the drug (C, control) in complete RPMI-1640 culture medium for 24 h. The fluorescence intensity values (FI) of FITC-labeled ICAM-1 was assessed by flow cytometry in spontaneous and TNFα-induced cell samples. Mean values and their standard deviations are shown. * Differences are significant compared with the control group at p < 0.05. ** Differences are significant compared with the control group at p < 0.01.
ICAM-1 Expression on Mononuclear Cells
Mononuclear leukocytes also had low spontaneous levels of ICAM-1. However, the level of this molecule induced by TNFα was significantly lower than in endothelial cells (Fig. 2b). Cytovir-3 at concentrations of 10 and 1 ng/mL increased the spontaneous level of ICAM-1 on cells by 66.67% (p < 0.05) and 33.33% (p < 0.01), respectively. The α-Glu–Trp preparation at all concentrations (100, 10, and 1 µg/mL) increased the spontaneous mean fluorescence intensity of mononuclear cells by 36.67, 66.67, and 20.00%, respectively (p < 0.05). Neither Cytovir-3 nor α-Glu–Trp significantly affected the level of the surface ICAM-1 molecule after TNFα stimulation (Fig. 2b).
DISCUSSION
Thus, the data obtained on the model of endothelial EA.hy 926 cells and mononuclear leukocytes showed similar multidirectional effects of the studied drugs on cells of different origin.
When endothelial cells were cultivated in the presence of α-Glu–Trp at a concentration of 10 μg/mL, the production of IL-1α induced by TNFα decreased, while at the level of the surface ICAM-1 molecule stimulated by this pro-inflammatory cytokine increased a concentration of 100 μg/mL.
The effect of α-Glu–Trp on mononuclear leukocytes was also multidirectional. In its presence at concentration of 1 μg/mL, significantly decreased TNFα-induced IL-8 production was registered. At all three concentrations (100, 10, and 1 µg/mL), the drug increased the spontaneous level of the surface ICAM-1 molecule, increasing the mean fluorescence intensity by 1–2 arb. units. Suppression of the production of pro-inflammatory cytokines by α-Glu–Trp may be responsible for its anti-inflammatory properties, but its ability to increase the level of the ICAM-1 molecule indicates the ability of α-Glu–Trp to activate cells (Bui et al., 2020; Moser et al., 2021). Taking it into account that the effect of the α-Glu–Trp on the studied cell functions was multidirectional, it seems unlikely that the drug can directly affect the level of phosphorylation of the NF-kB factor (Chong et al., 2021).
Experiments have shown that Cytovir-3 had a similar activating effect on EA.hy 926 endothelial cells and human peripheral-blood mononuclear leukocytes at various concentrations. The detected stimulating effect of Cytovir-3 may also be associated with the effect of bendazole in the composition of the drug. It has been shown that the drug itself and its derivatives can be agonists of receptors that recognize the conservative structures of microorganisms TLR and RLR. Activation of intracellular MAP kinases (mitogen-activated protein kinases) and NF-kB signaling pathways mediated by these interactions increases the cellular expression of activation markers such as ICAM-1, but decreases the production of pro-inflammatory cytokines induced by various factors (Chong et al., 2021; Eskandari et al., 2022).
In conclusion, the revealed effects of α-Glu–Trp and Cytovir-3 indicate that they have an anti-inflammatory effect due to the suppression of stimulated production of pro-inflammatory cytokines. Their ability to activate cells by increasing the level of the surface ICAM-1 molecule makes it possible to implement protective mechanisms in the fight against infection of various etiology, as well as improving the repair of tissues damaged by the inflammatory process.
AUTHOR CONTRIBUTIONS
E.A. Starikova and T.A. Kudryavtseva: research goals, experiment design; E.A Starikova: experiment design; T.A. Kudryavtseva, E.A. Starikova, E.G. Golovacheva, and V.A. Apryatina: analysis of the results; E.G. Golovacheva and V.A. Apryatina: manuscript writing.
FUNDING
This work was carried out as part of research work at Institute of Experimental Medicine, Northwest Branch, Russian Academy of Medical Sciences, on the order with the and financial support of AO MBNPK Cytomed.
COMPLIANCE WITH ETHICAL STANDARDS
The authors declare that they have no conflicts of interest. Experiments involving animals or human beings have not been performed.
Footnotes
Abbreviations: ELISA—enzyme immunoassay; α-Glu–Trp—α-glutamyl–tryptophan.
Translated by I. Fridlyanskaya
REFERENCES
- 1.Beesu M., Malladi S.S., Fox L.M., Jones C.D., Dixit A., David S.A. Human Toll-like receptor 8-selective agonistic activities in 1-alkyl-1H-benzimidazol-2-amines. J. Med. Chem. 2014;57:7325. doi: 10.1021/jm500701q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beesu M., Caruso G., Salyer A.C., Shukla N.M., Khetani K.K., Smith L.J., Fox L.M., Tanji H., Ohto U., Shimizu T., David S.A. Identification of a human toll-like receptor (TLR) 8-specific agonist and a functional pan-tlr inhibitor in 2-aminoimidazoles. J. Med. Chem. 2016;59:3311. doi: 10.1021/acs.jmedchem.6b00023. [DOI] [PubMed] [Google Scholar]
- 3.Bui T.M., Wiesolek H.L., Sumagin R. ICAM-1: a master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J. Leukoc. Biol. 2020;108:787–799. doi: 10.1002/JLB.2MR0220-549R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chong D.L.W., Rebeyrol C., José R.J., Williams A.E., Brown J.S., Scotton C.J., Porter J.C. ICAM-1 and ICAM-2 are differentially expressed and up-regulated on inflamed pulmonary epithelium, but neither ICAM-2 nor LFA-1: ICAM-1 are required for neutrophil migration into the airways in vivo. Front. Immunol. 2021;12:691. doi: 10.3389/fimmu.2021.691957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eskandari M., Asgharzadeh F., Askarnia-Faal M.M., Naimi H., Avan A., Ahadi M., Vossoughinia H., Gharib M., Soleimani A., Naghibzadeh N., Ferns G., Ryzhikov M., Khazaei M., Hassanian S.M. Mebendazole, an anti-helminth drug, suppresses inflammation, oxidative stress and injury in a mouse model of ulcerative colitis. Sci. Rep. 2022;12:10249. doi: 10.1038/s41598-022-14420-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiore-Gartland A., Panoskaltsis-Mortari A., Agan A.A., Mistry A.J., Thomas P.G., Matthay M.A. Front. Immunol. 2017;8:1423. doi: 10.3389/fimmu.2017.01423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golovacheva E.G., Afanasyeva O.I., Goncharova E.S., Bykovskaya A.G., Davletgareeva D.V., Apryati-na V.A. Possibilities of therapeutic correction of ENT pathology associated with COVID-19 in children on an outpatient basis. Vestn. Otorinolaringol. 2021;86:69. doi: 10.17116/otorino20218606169. [DOI] [PubMed] [Google Scholar]
- 8.Guo F., Mead J., Aliya N., Wang L., Cuconati A., Wei L., Li K.M., Block T., Guo J.-T., Chang J. RO 90-7501 enhances TLR3 and RLR agonist induced antiviral response. PLoS One. 2012;7:e42583. doi: 10.1371/journal.pone.0042583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaneko N., Kurata M., Yamamoto T., Morikawa S., Masumoto J. The role of interleukin-1 in general pathology. Inflamm. Regener. 2019;39:12. doi: 10.1186/s41232-019-0101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khalil B.A., Elemam N.M., Maghazachi A.A. Chemokines and chemokine receptors during COVID-19 infection. Comput. Struct. Biotechnol. J. 2021;19:976. doi: 10.1016/j.csbj.2021.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khavinson, V.Kh., Korneenkov, A.A., Popovich, I.G., Gudkov, A.V., and Kuznik, B.I., Meta-analysis of the immunomodulatory activity of the medicinal peptide drug timalin, Sovrem. Probl. Zdravookhr. Med. Stat., 2020, no. 4, p. 108. 10.24411/2312-2935-2020-00100
- 12.Khavinson V.H., Linkova N., Chalisova N.I., Ivko O.M. Timalin: application for immunocorrection and molecular aspects of biological activity. Usp. Sovrem. Biol. 2021;141:25. doi: 10.31857/S0042132420060046. [DOI] [Google Scholar]
- 13.Malainou C., Herold S. Influenza. Internist (Berl.) 2019;60:1127. doi: 10.1007/s00108-019-00670-6. [DOI] [PubMed] [Google Scholar]
- 14.Markey, K.A., Gartlan, K.H., Kuns, R.D., MacDonald, K.P., and Hill, G.R., Imaging the immunological synapse between dendritic cells and T cells, J. Immunol. Methods, 2015, vol. 423, p. 40. https://doi.org/10.1016 / j.jim.2015.04.029 [DOI] [PubMed]
- 15.Matsushima K., Yang D., Oppenheim J.J. Interleukin-8: an evolving chemokine. Cytokine. 2022;153:155828. doi: 10.1016/j.cyto.2022.155828. [DOI] [PubMed] [Google Scholar]
- 16.Moser T., Hoepner L., Schwenker K., Seiberl M., Feige J., Akgün K., Haschke-Becher E., Ziemssen T., Sellner J. Cladribine alters immune cell surface molecules for adhesion and costimulation: further insights to the mode of action in multiple sclerosis. Cells. 2021;10:3116. doi: 10.3390/cells10113116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moskalets O.V. Molecules of cellular adhesion ICAM-1 and VCAM-1 in infectious pathology. Tikhookean. Med. Zh. 2018;2:21. doi: 10.17238/PmJ1609-1175.2018.2.21-25. [DOI] [Google Scholar]
- 18.Oudemans-van Straaten H.M., Spoelstra-de Man A.M.E., de Waard M.C. Vitamin C revisited. Crit. Care. 2014;18:460. doi: 10.1186/s13054-014-0460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shipitsyn, K.S., Ogarkov, P.I., Smirnov, V.S., Zhogolev, S.D., and Zhogolev, K.D., Prevention of acute respiratory viral infections and pneumonia in an organized team, Epidemiol. Infekts. Bol., 2010, no. 1, p. 57. 10.17816/EID40479
- 20.Smirnov, V.S., Zarubaev, V.V., and Petlenko, S.V., Biologiya vozbuditelei i kontrol’ grippa i ORVI (Biology of Pathogens and Control of Influenza and ARVI), St. Petersburg: Gippokrat, 2020.
- 21.Smirnov V.S., Leneva I.A., Kudryavtseva T.A., Fayzuloev E.B., Zaplutanov V.A., Petlenko S.V., Kartasho-va N.P., Gracheva A.V., Korchevaya E.R. Possibilities of suppressing the cytopathogenic effect of SARS-CoV‑2 coronavirus according to the results of the antiviral activity of Cytovir®-3 in in vitro study. Antibiot. Khimioter. 2021;66:4. doi: 10.37489/0235-2990-2021-66-5-6-4-10. [DOI] [Google Scholar]
- 22.Soema P.C., Kompier R., Amorij J.P., Kersten G.F. Current and next generation influenza vaccines: formulation and production strategies. Eur. J. Pharm. Biopharm. 2015;94:251. doi: 10.1016/j.ejpb.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 23.Ullah A., Al Kury L.T., Althobaiti Y.S., Ali T., Shah F.A. Benzimidazole derivatives as new potential NLRP3 inflammasome inhibitors that provide neuroprotection in a rodent model of neurodegeneration and memory impairment. J. Inflamm. Res. 2022;15:3873. doi: 10.2147/JIR.S351913. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Van Meerloo J., Kaspers G.J.L., Cloos J. Cell sensitivity assays: the MTT assay. Methods Mol. Biol. 2011;731:237. doi: 10.1007/978-1-61779-080-5_20. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y., Liu H., Tang W., Qiu Q., Peng J. Resveratrol prevents TNF-α-induced VCAM-1 and ICAM-1 upregulation in endothelial progenitor cells via reduction of NF-κB activation. J. Int. Med. Res. 2020;48:300060520945131. doi: 10.1177/0300060520945131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zinina E.P., Tsarenko S.V., Logunov D.Yu., Tukhvatullin A.I., Babayants A.V., Abramov A.A. The role of pro-inflammatory and anti-inflammatory cytokines in bacterial pneumonia. Literature review, Vestn. Intens. Ter. A.I. Saltanova. 2021;1:77. doi: 10.21320/1818-474X-2021-1-77-89. [DOI] [Google Scholar]