Abstract
The world continues to contend with COVID-19, fueled by the emergence of viral variants. At the same time, a subset of convalescent individuals continues to experience persistent and prolonged sequelae, known as long COVID. Clinical, autopsy, animal and in vitro studies all reveal endothelial injury in acute COVID-19 and convalescent patients. Endothelial dysfunction is now recognized as a central factor in COVID-19 progression and long COVID development. Different organs contain different types of endothelia, each with specific features, forming different endothelial barriers and executing different physiological functions. Endothelial injury results in contraction of cell margins (increased permeability), shedding of glycocalyx, extension of phosphatidylserine-rich filopods, and barrier damage. During acute SARS-CoV-2 infection, damaged endothelial cells promote diffuse microthrombi and destroy the endothelial (including blood–air, blood–brain, glomerular filtration and intestinal–blood) barriers, leading to multiple organ dysfunction. During the convalescence period, a subset of patients is unable to fully recover due to persistent endothelial dysfunction, contributing to long COVID. There is still an important knowledge gap between endothelial barrier damage in different organs and COVID-19 sequelae. In this article, we mainly focus on these endothelial barriers and their contribution to long COVID.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10456-023-09878-5.
Keywords: Long COVID, Endothelial barrier, Vaccination, Antithrombotic therapy
Introduction
As of March 3, 2023, more than 758 million confirmed cases of COVID-19 have been reported to WHO [1]. Although most patients return to their baseline state of health after infection, a subset of patients have ongoing health problems, known as post-acute sequelae of COVID-19 or long COVID. The WHO defines long COVID as the continuation or development of new symptoms 3 months after the initial SARS-CoV-2 infection, with these symptoms lasting for at least 2 months without other explanation [2]. The persistent symptoms can occur even after an oligosymptomatic infection [3]. These symptoms include fatigue, shortness of breath, dyspnea, chest tightness, cough, arthralgia, headache and cognitive dysfunction. It is estimated that long COVID affects 10–30% of patients and can persist > 1 year [4, 5]. The exact mechanisms responsible for long COVID remain unknown. Endothelial cells (ECs) play an indispensable role in hemostasis, immune reaction and angiogenesis. They can also form different barrier structures and execute different physiological functions, such as organ protection (blood–brain barrier), gas exchange (blood–air barrier), nutrient absorption (intestinal–blood barrier), and excretion of harmful substances (glomerular filtration barrier) [6]. Endothelial dysfunction is described in COVID-19 and convalescent patients [7–9]. However, relatively little is known about the role of endothelial barriers in different organs in COVID-19. In this article, we discuss the injury of these endothelial barriers during acute infection and their possible contribution to long COVID.
Endothelial dysfunction during SARS-CoV-2 infection
ECs express ACE2, which allows SARS-CoV-2 to infect these cells. Early autopsy results have revealed viral inclusions in apoptotic ECs and microvascular endotheliitis in the lung, kidney, small bowel and heart [10, 11]. Subsequent animal and in vitro experiments find that SARS-CoV-2 can directly infect and replicate in ECs [12, 13] and that spike protein directly damages ECs through downregulation of ACE2 [14]. The concurrent release of inflammatory cytokines (such as IL-6, IL-1, TNF) can also activate ECs. Studies reveal that plasma from COVID-19 patients can directly cause EC damage in correlation with the levels of inflammation [12, 15]. Additionally, inflammation and coagulation are closely linked with endothelial response. Activated platelets, increased thrombin and neutrophil extracellular traps amplify endotheliopathy, inducing a proinflammatory and prothrombotic endothelium [7, 16].
Activated complement can also damage ECs. Complement has been found deposited in the damaged microvasculature [17]. After infection, the spike and nucleocapsid proteins are recognized by lectin, while virus-specific antibodies interact with the classical pathways to activate complement [18, 19]. Furthermore, competition of virus with C3b-regulatory factor H for binding to heparan sulfate removes the inhibitory effects of factor H on C3, sustaining activation of the alternative pathway. After activation, C3a and C5a bind to C3aR and C5R on ECs, increasing vascular permeability and inducing inflammatory responses [18]. More importantly, the formation of membrane attack complex (C5b-9) directly damages ECs and leads to apoptosis. Hematologic data reveal higher levels of FVIII, vWF, P-selectin, PAI-1, TFPI and syndecan-1 in COVID-19 [20, 21], consistent with endothelial injury. Collectively, these factors result in endothelial barrier damage, phosphatidylserine exposure, glycocalyx shedding, microparticles and Weibel–Palade bodies release, and up-regulation of adhesion factors [22, 23].
Persistent endothelial dysfunction in convalescent patients
During the convalescence period, a subset of COVID-19 patients is unable to fully recover and develop long COVID. In these patients, elevated levels of D-dimer, FVIII, thrombin, vWF, ICAM-1 and IL-6 persist, even 1 year after recovery [8, 9]. Moreover, persistent endothelial glycocalyx damage, higher levels of circulating ECs, and impaired vascular and myocardial function are also observed in these patients [24–26], supporting the hypothesis of ongoing endothelial dysfunction. Putative mechanisms include persistent viral presence, inflammation, and immune dysregulation. One study of B cell responses to SARS-CoV-2 showed continuous evolution and somatic hypermutation as late as 6 months after infection, and that the viral RNA (3/14 patients) and nucleocapsid protein (5/14) were detectable in intestinal biopsies 4 months after mild COVID-19 [27]. Another study found persistent viral RNA (32/46 patients) and antigen (nucleocapsid and envelope proteins; 24/46) in intestinal biopsies of inflammatory bowel disease patients 7 month after mild COVID-19 [28]. Moreover, persistent nucleocapsid protein was also detected in liver, gallbladder and lymph node [29]. Combined with the cases of recurrence after recovery [30], these data may indicate that a reservoir of the coronavirus lingers after acute infection [31]. Recent observational study found that vaccination can lessen or alleviate long COVID symptoms [32], suggesting that vaccine-induced adaptive immunity reduced persistent viruses. Even if these viruses cannot replicate, the viral debris (nucleic acids or proteins) may continue to damage EC, induce cytokines and activate immune responses. Consistent with this, the phenomenon of “COVID toes” reveal that the viral debris, spike proteins, can result in vasoconstriction, perivascular and periadnexal inflammation, and ultimately pandemic-associated pernio [33]. Correlation analysis showed that long COVID was associated with non-RBD IgG, but not RBD IgG, supporting the role of the viral debris [34].
Although persistent virus could induce inflammation and immune dysregulation, only some patients with long COVID have detectable viral loads. In most convalescent patients, increased inflammation and immune dysregulation may be due to delay or defects in the resolution of tissue damage after acute infection [35]. Numerous studies have shown elevated cytokines (such as IL-1, IL-6, IFNβ, IFNλ1), activated myeloid and cytotoxic T cells in the plasma and tissues of discharged patients, which are strongly associated with long COVID [31, 36–39]. Another study found that elevated cytokines were positively correlated with circulating ECs, and that these cytotoxic T cells highly expressed some receptors that may potentially interact with activated ECs [24]. The authors proposed that persistent cytokine release and cytotoxic T cells activates ECs in convalescent COVID-19 patients. Additionally, sustained microcirculation damage in patients with long COVID has been observed using sublingual and retinal microscopy [40, 41]. The roles of virus, inflammation and immune dysregulation in endothelial injury still needs further confirmation.
Early features of endothelial dysfunction—phosphatidylserine exposure, glycocalyx shedding and increased permeability
Phosphatidylserine exposure
Phosphatidylserine (PS) is the most abundant negatively charged phospholipid in mammalian cells and is usually confined to the inner leaflet of the cell membrane [42]. This asymmetry is maintained through ATP-dependent inward transport of PS by flippases and outward transport of other phospholipids by floppases (Suppl. Fig. left). Upon stimulation, transiently increased calcium inhibits ATP-dependent transport and stimulates the nonselective lipid transporter scramblase (ATP-independent), resulting in PS exposure on the outer membrane. Any stimulation which induces cell activation, injury, or death results in cell PS exposure and release of PS-rich microparticles [42]. Specific to ECs, injury causes contraction of cell margins (increased permeability) followed by the extension of PS+ filopods (Fig. 1a–c) [21, 43]. PS exposure and increased permeability are early features of endothelial dysfunction. A recent in vitro study showed that treatment of cultured lung microvasculature EC with serum from COVID-19 patients of any disease severity increased endothelial permeability [12]. However, only serum from patients with moderate or critical disease induced VEGF, IL-6 secretion, and only direct viral infection can induce PAI-1 or IL-8 secretion. Exposed PS provides binding sites for tenase and prothrombinase complexes, promoting Xa and thrombin formation [44]. Additionally, PS activates tissue factor on ECs, triggering the extrinsic coagulation pathway (Suppl. Fig. right). This leads to diffuse fibrin deposits (microthrombi) along the PS+ filopodia [21]. This pathway explains the increased thrombosis that has been observed in COVID-19 [45] and convalescent patients [46].
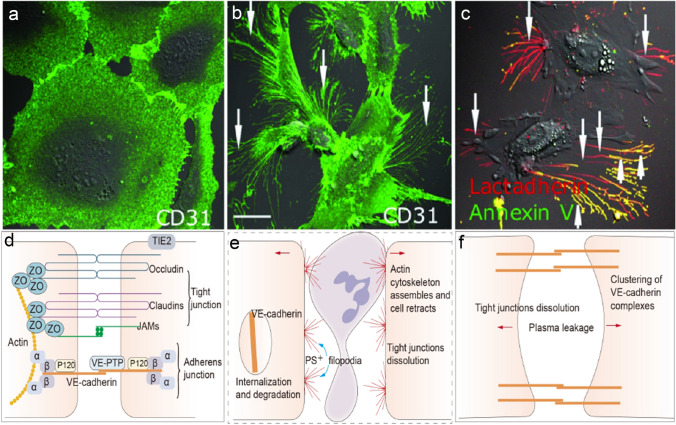
Fig. 1.
Endothelial barrier injury and phosphatidylserine (PS) exposure. Human umbilical vein endothelial cells (ECs) were incubated with (b, c) or without (a) isolated extracellular chromatin for 24 h (our previous study [43]). ECs were stained with CD31-Alexa Fluor 488 or with Alexa 647-lactadherin and FITC-annexin V (detecting PS). Stimulation with extracellular chromatin led to the retraction of cell margins (increased permeability) and extension of filopods (b, arrows) and PS exposure on the filopods (c, arrows). The inset bar represents 5 mm in (a–c). d Endothelial barrier is primarily composed of tight and adherens junctions. Tight junctions include claudins, occludins, junctional adhesion molecule (JAMs), and zonula occludens (ZO) proteins. Adherens junctions contain vascular endothelial cadherin (VE-cadherin), p120-catentin, α-catenin, β-catenin and vascular endothelial-protein tyrosine phosphatase (VE-PTP). ZO and α-catenin linked to the actin cytoskeleton, and VE-cadherin mediates cell-cell contact through its extracellular domain. Tie2 and vascular endothelial-protein tyrosine phosphatase (VE-PTP) prevent VE-cadherin from phosphorylation, stabilizing it. e Most stimulation (e.g. VEGF, LPS, cytokines, extracellular chromatin) can disrupt tight junctions and induce VE-cadherin internalization and degradation. Subsequently, actin cytoskeleton assemble and cells retract, increasing permeability. f Histamine induces tight junction dissolution, rapid redistribution of cortical actin and clustering of junctional complexes, resulting in small gaps between ECs
Glycocalyx shedding
The vascular endothelial surface is coated by glycocalyx, a ubiquitous gel-like layer composed of proteoglycans and glycosaminoglycans [23, 47, 48]. The endothelial glycocalyx is an important part of the vascular barrier, which modulates leukocyte–endothelial interactions, thrombus formation and vascular permeability. Besides these functions, the glycocalyx also mechanotransduces shear forces from flowing blood to activate cytoprotective intracellular signaling. Glycocalyx damage is another early feature of endothelial dysfunction. Multiple factors including hypervolemia, hyperglycemia and inflammation are toxic to the glycocalyx, leading to glycocalyx shedding [47, 48]. The destruction of the glycocalyx can result in capillary leakage, edema, accelerated inflammation, platelet aggregation, hypercoagulation and a loss of vascular responsiveness. In COVID-19, damaged glycocalyx has been observed in patients with acute infection and convalescence [26, 49, 50]. A prospective study showed that individuals without pre-existing conditions had elevated syndecan-1 levels 88 days after a mild SARS-CoV-2 infection (without hospitalization), indicating that even mild SARS-CoV-2 infection can result in a persistent endothelial injury [49]. Moreover, several other studies found persistent glycocalyx impairment in post-COVID patients as long as 12 months after infection [26, 51]. Meanwhile, Osiaevi et al. showed completely restored glycocalyx dimensions in patient with long COVID about 1.5 years after infection [40]. Although restored glycocalyx levels indicate endothelial recovery, these patients still have persistent capillary rarefication which is closely associated with long COVID symptoms.
Endothelial barrier damage
The endothelial barrier is comprised of cells connected by intercellular junctions such as tight junctions and adherens junctions (Fig. 1d) [6]. Tight junctions are composed of transmembrane claudins and occludins, with cytoplasmic zonula occludens (ZO) proteins that anchor tight junctions to the actin cytoskeleton. Adherens junctions contain vascular endothelial cadherin (VE-cadherin), which mediates cell-cell contact through its extracellular domain. p120-catentin binds to and stabilizes VE-cadherin, which is linked to the actin cytoskeleton via α-catenin. Tie2 (activated by angiopoietin-1) and vascular endothelial-protein tyrosine phosphatase (VE-PTP) prevent VE-cadherin from phosphorylation, stabilizing it. The endothelial barrier can open through two mechanisms: (1) disruption of endothelial junctions by dissociation of junctional complexes or endocytosis of junctional proteins (such as: VEGF, LPS, cytokines; Fig. 1e); (2) generation of small gaps by clustering of junctional complexes and formation of focal adherens junctions (such as: histamine; Fig. 1f). COVID-19 patients have decreased the levels of claudins, occludins and VE-cadherin, resulting in increased endothelial permeability [12, 15, 52]. Damaged blood–air [38], blood–brain [39, 53], glomerular filtration [54], and intestinal–blood [55] barriers have been described. In the following sections, we will discuss the damages of these barriers and their contribution to long COVID.
Blood–air barrier
The blood–air barrier (thickness 0.1 μm) is responsible for gas exchange and consists of a layer of alveolar epithelium (Type I and II) and endothelium separated by a thin basement membrane [56]. In COVID-19, virus infection and subsequent release of cytokines destroy the endothelial glycocalyx and barrier, resulting in leakage of plasma and formation of hyaline membrane in alveoli [57]. Subsequently, rapidly activated ECs (PS exposure) promote diffuse pulmonary microthrombi beyond the damaged alveoli (autopsy results reveal arteriole thrombi in both damaged and more preserved lung parenchyma [58]), reducing perfusion of the corresponding normal alveolar tissue. Additionally, sublingual microscopy finds an up to 90% reduction of small capillaries (diameter 4–6 μm), suggesting a systematic reduction of small capillaries [50]. Consistently, semiquantitative CT analysis shows that patients have a substantial reduction of pulmonary blood volume in small vessels (cross-sectional area < 5 mm2) and a substantial increase in large ones (cross-sectional area ≧ 5 mm2) [59]. Aberrant angiogenesis (e.g. intussusceptive angiogenesis [10] and intrapersonal bronchopulmonary anastomoses [60]) and diffuse pulmonary microthrombi may contribute to these anomalies in the distribution of blood volume. Both these alveolar and capillary abnormalities are involved in the development of hypoxemia during acute infection.
After virus clearance, alveolar and endothelial cells proliferate to repair damaged cells and barriers, restoring normal alveolar function [57]. However, not all COVID-19 patients return to normal lung function. Abnormal lung function (decreased dynamic lung volumes and diffusing capacity) and CT (ground glass shadows, consolidation and fibrosis) have been described in convalescent patients [61, 62]. A recent study analyzing radiological and histological results finds three clusters of discharged patients with persistent respiratory and/or systemic symptoms [62]. In “Cluster 2” patients, CT shows residual ground glass, sometimes consolidations, and no fibrotic distortion (Fig. 2). The corresponding histological findings reveal abundant infiltration of lymphocytes (T and B) infiltration. An immuno-proteomic study confirmed the increased presence of BAL cytotoxic lymphocytes in patients with long COVID, which were associated with epithelial injury, apoptosis, and tissue repair [38]. Besides these findings, correlation analyses has shown that BAL cytotoxic T/B cells were associated with lower diffusing capacity, FVC and FEV1 [38, 63]. These data suggest that cytotoxic lymphocytes contribute to the damage of ECs and endothelial barrier, promoting microthrombi, leaking the plasma into alveoli and decreasing alveolar function.
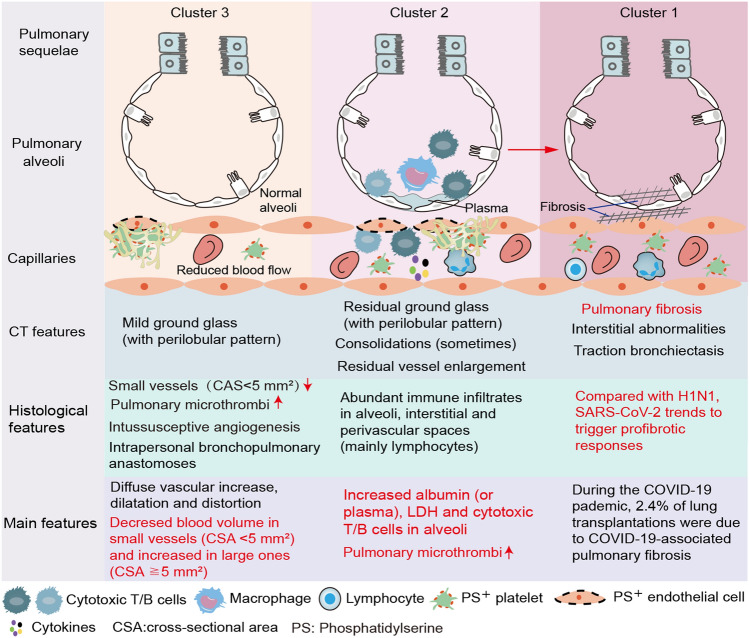
Fig. 2.
Abnormal blood–air barrier contributes to the long-term lung sequelae. During acute infection, small vessel loss, intussusceptive angiogenesis, intrapulmonary bronchopulmonary anastomoses and diffuse pulmonary microthrombi reduces blood flow in small vessels (cross-sectional area < 5 mm2) and increases in large vessels (cross-sectional area ≧ 5 mm2). After discharge, these vascular changes persist and decrease (normal) alveolar perfusion in a subset of patients (Cluster 3). For convalescent patients, persistent inflammation and immune dysregulation (e.g. cytotoxic lymphocyte) damaged alveolar epithelial and endothelial cells, resulting in leakage of plasma into the alveoli (Cluster 2). SARS-CoV-2 trends to trigger profibrotic responses (Cluster 1). Besides, damaged alveoli (Cluster 2) with persistent capillary microthrombi may eventually develop into alveolar fibrosis
“Cluster 1” patients present with pulmonary fibrosis, which is another cause of long-term lung sequela. A recent meta-analysis showed that fibrotic sequelae occurred in 29% of COVID-19 patients who underwent CT after a median 3-month follow-up [64]. Compared with H1N1, SARS-CoV-2 tends to trigger profibrotic macrophage responses and pronounced fibroproliferative acute respiratory distress syndrome [65]. In extreme cases, it can quickly lead to pulmonary fibrosis, causing irreversible damage and requiring lung transplantation. In a retrospective study including 3,039 patients with lung transplantation during the pandemic, 2.4% were associated with COVID-19 pulmonary fibrosis [66]. Pulmonary fibrosis can also be induced by severe or persistent alveolar injury. Persistent capillary microthrombi inhibits the restoration of blood–air barrier (“Cluster 2” patients), which may eventually progress to pulmonary fibrosis (irreversible).
“Cluster 3” patients are characterized by diffuse vascular increase, dilatation and distortion in an otherwise normal parenchyma with mild peripheral ground glass. These features are residual pulmonary vascular changes after infection resolution. Aberrant angiogenesis (e.g. intussusceptive angiogenesis and intrapulmonary bronchopulmonary anastomoses) do not appear to recover by hospital discharge. Recent study finds a persistent capillary rarefication under sublingual microscopy in patients with long COVID even 18 months after infection [40]. Additionally, pulmonary microthrombi persist in these convalescent patients, although to a lesser extent than in the acute phase. Higher levels of microclots [67] and platelet activation [68] have been described in patients with long COVID compared to individuals not experiencing ongoing symptoms. Relatedly, Werlein et al. recently found a large number of microthrombi in heart samples using scanning electron microscope, which light microscopy failed to detect [69]. Ventilation perfusion single-photon emission computed tomography detects pulmonary perfusion deficits 6 weeks after acute COVID-19 illness, supporting the hypothesis of persistent lung microthrombi [70]. All of these factors reduce alveolar perfusion of normal parenchyma resulting in mild ventilation-perfusion mismatch and persistent respiratory symptoms.
Blood–brain barrier (BBB)
The BBB consists of capillary and postcapillary venule (endothelial barrier), pericytes, basement membrane, and astrocyte endfeet (Fig. 3) [71, 72]. Pericytes are embedded in the endothelial basement membrane and sealed by astrocyte endfeet. The endothelial basement and astrocyte endfeet fuse at the capillary BBB, while form an enclosed space (perivascular space) at the postcapillary venules, in which a few antigen presenting cells (APCs) reside. The capillary BBB is the site of controlled transport of fluids and solutes into the central nervous system (CNS), whereas extravasation of immune cells into the CNS parenchyma occurs at the level of postcapillary venules. During acute infection, COVID-19 patients usually develop neuropsychiatric symptoms, including anosmia, stroke, delirium, primary psychiatric syndromes, encephalopathy, and peripheral nerve syndromes [73, 74]. Neuropsychiatric sequelae are often reported after discharge [74], and are strongly associated with decreased self-care ability [75]. Autopsy results reveal brain hypoxic/ischaemic changes, microhemorrhages, neuroinflammation and sparse virus (limited in ECs) [76, 77].
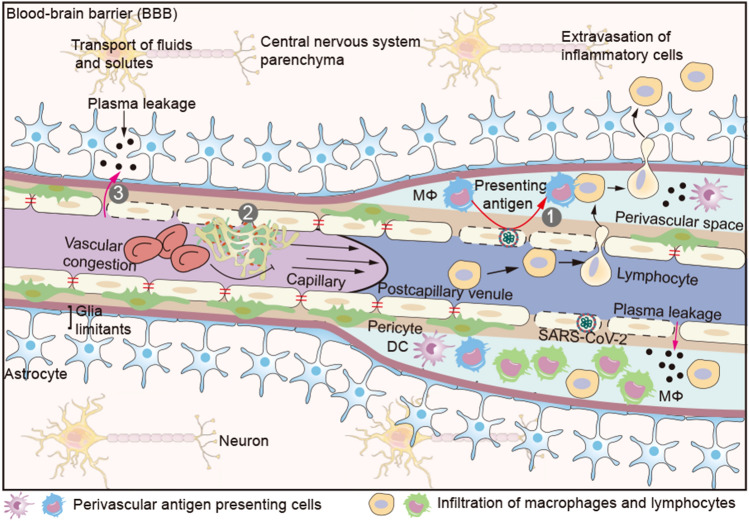
Fig. 3.
Blood–brain barrier (BBB). The BBB consists primarily of capillary and postcapillary venule, pericyte, basement and astrocyte end-feet. The endothelial basement and astrocyte end-feet form an enclosed space (perivascular space) at the postcapillary venules, in which a few antigen presenting cells (APCs) reside. Extravasation of immune cells into the brain parenchyma occurs at the level of postcapillary venules. Activated immune cells can only cross the endothelial barrier, but not the astrocyte end-feet. Only after a second recognition of their cognate antigen on perivascular APCs, these activated lymphocytes can enter brain parenchyma. (1) Perivascular APCs (macrophage or DC) infiltrate (SARS-CoV-2) infected ECs and present cognate antigens to activated lymphocytes for entry into the brain parenchyma. (2) Damaged cerebrovascular ECs promote (micro) thrombi and aggravate brain hypoxia. (3) Increased vascular pressure due to microthrombi pushes plasma into the brain parenchyma at the site of endothelial barrier injury. Serum proteins such as fibrin (ogen), thrombin, and plasmin (ogen) can activate microglia enhancing neurons death. DC dendritic cell; Mφ macrophage
Hypoxia-related neurological sequelae
Hypoxic damage is the most common pathological change in the brain, including neuronal shrinkage, neuronal loss, and reactive astrocytosis [76]. SARS-CoV-2 disrupts the blood–gas barrier, leading to long-time hypoxemia. Meanwhile, endothelial dysfunction promotes a higher risk of cerebral thrombosis, which further aggravates brain hypoxia [78]. A recent large meta-analysis showed a higher rate of ischemic stroke (OR: 3.58 [1.43–8.92]) in COVID-19 patients compared with noninfected contemporary or historical controls [79]. The brain is a highly metabolic organ, and even short-term minor hypoxemia can cause neurological symptoms. Prolonged and severe hypoxemia may result in severe neurological symptoms that often delay recovery. A recent multicenter study showed that survivors of severe COVID-19 commonly recovered consciousness weeks after cessation of mechanical ventilation [80]. These extended recovery periods were strongly associated with the severity and duration of hypoxemia. Similarly, some psychotic abnormalities, especially accompanied with delirium or confusion, may persist after the infection resolves. More importantly, persist microthrombi in brain cause persistent neurological symptoms after discharge.
Secondary antigen and neuroinflammation
Neuroinflammation is a common pathological change in the brain during COVID-19 infection, including T cell infiltration, activation of microglia and microglial nodules [39, 76]. Under normal physiological conditions, naive lymphocytes cannot cross the endothelial barrier. Even activated lymphocytes can only pass through the endothelial barrier, but not the astrocyte end-feet [72]. These features of the BBB effectively isolate normal brain tissue from abnormal systemic immune responses. Only after a second recognition of their cognate antigen on perivascular APCs can these activated lymphocytes enter CNS parenchyma [72]. SARS-CoV-2 is detected in cerebrovascular ECs, suggesting they can precisely provide the secondary antigen (Fig. 3). Consistent with this, deep spatial analysis of postmortem brain tissue identified neuroinflammation, SARS-CoV-2 in ECs and accumulation of macrophages (APCs) in the perivascular space, all of which were closely interacting with activated lymphocytes (mainly CD8+ T) [39]. After entering the CNS parenchyma, cytotoxic CD8+ T activate microglia, promote microglial nodules formation, and damage nerve cells, leading to persistent neuropsychiatric symptoms.
BBB leakage
Micro-hemorrhage, another common pathological change in the brain, indicates loss of cerebrovascular integrity or BBB leakage. In a histopathological study, fibrinogen was detected in the parenchyma even with relatively intact vasculature (10/13 dead patients) [53], supporting BBB leakage. Other larger plasma proteins (e.g. IgM) were also observed in the parenchyma [77]. In a non-human primate model of SARS-CoV-2 infection, the authors found extensive micro-hemorrhages in the brain, in the absence of surrounding CD61+ platelet aggregation, suggesting RBC leakage from damaged BBB [81]. SARS-CoV-2 spike protein or elevated cytokines can diminish the tight junctions and increase endothelial permeability of BBB [82, 83]. The mechanism by which the plasma or RBCs across the astrocyte end-feet is still unclear. Reactive astrocytes surrounding the perivascular space were observed in postmortem brain tissue, which may indicate the presence of damaged astrocyte end-feet [53, 77]. Histological analysis revealed that fibrinogen was detect leaking from congested blood vessels [53]. This may indicate that increased vascular pressure due to microthrombi pushed plasma into the brain parenchyma (Fig. 3). Serum proteins such as fibrin(ogen), thrombin, and plasmin(ogen) can activate microglia, enhancing neuronal death [71]. Additionally, ruptured RBCs can release neurotoxic hemoglobin and free iron (Fe2+), and induce generation of reactive oxygen species, which are all toxic for neural tissue. Since these leaks take time to cause neurological damage, they are more likely to be associated with delayed neuropsychiatric symptoms.
BBB transport systems
In addition to the barrier function, the BBB also has a critical role in CNS homeostasis, nutrition, and brain–body communication [71]. Brain endothelial transport systems regulate molecular exchanges between blood-and-brain and brain-and-blood. They include carrier-mediated transport (nutrients, T3/T4 thyroid, etc.), receptor-mediated transport (insulin, amyloid-β, α-synuclein), active efflux (drugs, xenobiotics, drug conjugates, and nucleosides) and ion transport [71]. These transport systems can also carry cytokines (e.g. IL-1, IL-6, TNF-α, CCL2) through the BBB, affecting brain function [83]. IL-1α, for instance, crosses the BBB at the posterior division of the septum, inducing cognitive dysfunction [84]. TNF-α crossing the BBB acts on microglia to induce apoptosis of dopaminergic cells in the substantia nigra [85]. These cytokines are elevated in patients with long COVID [35, 36]. Recent studies found a strong correlation between persistent CNS (especially psychiatric) symptoms and elevated cytokines [36, 86].
Endothelial dysfunction can affect the BBB transport systems, which is also associated with some CNS diseases. Alzheimer’s disease, for instance, is a common neurodegenerative disease characterized by dementia and deposition of amyloid-β and tau in brain regions that execute learning and memory. Impaired efflux and increased influx of amyloid-β (receptor-mediated transport) across the BBB are implicated in disease pathogenesis [71, 87]. Similarly, Parkinson’s disease is caused by the deposition of α-synuclein in the dopaminergic region [71]. COVID-19 associated Alzheimer’s and Parkinson’s diseases have been reported [73]. These diseases may be partly related to the accelerated BBB breakdown and disturbance of the endothelial transport system after infection. Whether disturbances of other endothelial transports, such as active efflux (of drugs), are associated with neuropsychiatric symptoms deserves further study.
Glomerular filtration barrier
The glomerular filtration barrier (GFB) consists of three layers: endothelium with glycocalyx, basement membrane and podocytes (Fig. 4a) [54, 56, 88]. Glomerular ECs form the initial barrier to filtration. These cells possess fenestrae (diameter: 60–80 nm), which is covered with a thin diaphragm that modulates the sieving properties. ECs actively synthesize glycocalyx, which provides a filtration barrier with charge selectively. Podocytes form primary, secondary and tertiary (foot) processes, which attach to the basement membrane and shape a second barrier [54, 56]. Podocytes can also regulate glomerular EC growth, survival, differentiation and permeability by releasing VEGF A [88]. In COVID-19, approximately 28% of hospitalized patients develop acute kidney injury (AKI) [89]. The main features are elevated creatinine, decreased eGFR, proteinuria, hematuria, as well as protein (erythrocyte) casts [90, 91]. Renal biopsy and autopsy results primarily reveal varying degrees of acute tubular injury, with occasional glomerular injury [91–93]. Additionally, discharged patients also exhibit higher risks of AKI, eGFR decline, and major adverse kidney events [94]. More importantly, hospitalized and discharged COVID-19 patients with renal dysfunction often experience a sustained eGFR decline, suggesting persistent glomerular damage. GFB plays an important role in the acute tubular injury and persistent glomerular damage.
Fig. 4.
Glomerular filtration barrier (GFB) and sustained kidney damage. c, d Under normal physiological conditions, the GFB filter 30% of blood flow to form protopuria, and 65–70% of water is reabsorbed at proximal tubules, which markedly increase the viscosity of efferent arterioles and tubule capillaries. Congested RBCs push other blood cells to the edge of the bloodstream. b After SARS-CoV-2 infection, cytokine storm activates massive blood cells/ECs, leading to uncontrolled phosphatidylserine (PS) exposure. At the renal tubules, these excess PS (mainly PS+ PLT and MPs) are crowded at the edge of the bloodstream (or closed to the damaged ECs), which discriminatorily increase peritubule thrombosis and tubule injury. a Simultaneously, damage of glomerular ECs results in loss of glycocalyx, decreased fenestrae, barrier opening (leakage of plasma or erythrocytes), cells swelling and microthrombi. SARS-CoV-2, cannot cross the endothelial fenestrations, infects podocytes either after infecting ECs or by crossing the damaged endothelial barrier. Damaged podocytes lose foot processes and reduce the release of VEGF A, further aggravating the injury of ECs. Persistent inflammation and immune dysregulation in convalescent patients maintain and exacerbate these injuries, leading to persistently reduced eGFR. c, e Severe glomerular endothelial injury can result in the accumulation of plasma in Bowman’s capsule, increasing the blood viscosity of glomerular capillaries and efferent arterioles. Leakage of plasma or erythrocyte in initial urine eventually forms proteinuria or hematuria. Additionally, diffuse microthrombi can result in stagnation of blood flow in part of glomeruli and subsequent retention of initial urine. The leaking plasma protein (erythrocytes) in initial urine ultimately forms protein (erythrocyte) casts after concentration and acidification in renal tubules (e). HCT hematocrit; PS phosphatidylserine; MPs microparticles; PLT platelets
GFB filters approximately 30% of the blood volume into the renal capsule to form initial urine, which markedly increases the viscosity of efferent arterioles (Fig. 4c) [56]. Efferent arterioles leave glomeruli to form peritubular plexus of capillaries. Later, 65–70% of the water in initial urine is reabsorbed in proximal tubules, gradually decreasing the viscosity of the fluid contained in capillaries behind the proximal tubules (Fig. 4d) [54, 56, 88]. As a result, there is physiologic hyperviscosity between efferent arterioles and proximal tubules. In the hyperviscosity blood, congested RBCs push platelets towards the vessel walls increasing the risk of thrombosis. During SARS-CoV-2 infection, cytokine storm results in the mass activation of blood cells and ECs, leading to uncontrolled PS exposure, termed PS storm. At the renal tubules, this excess PS (mainly PS-rich platelets and microparticles) are crowded at the edge of the bloodstream (or close to damaged ECs), which discriminatorily increases peritubule thrombosis and subsequent renal tubule injury (Fig. 4b). Consistent with this, clinical data show that AKI is the most common extra-pulmonary organ injury in COVID-19 [75]. Acute tubular injury reduces the reabsorption capacity of renal tubules, resulting in selective proteinuria.
Simultaneously, viral infection [11], elevated cytokines and activated complement also damage glomerular ECs, resulting in shedding of glycocalyx, increasing endothelial permeability and decreasing EC fenestrations [23, 88] (Fig. 4a). Gene analysis finds decreased levels of VE-Cadherin, claudin-1, claudin-5, occludin and Tie mRNA in renal ECs [93]. Increased endothelial permeability leads to plasma (erythrocyte) leakage and formation of non-selective proteinuria (hematuria). Severe endothelial injury can result in the accumulation of plasma in Bowman’s capsule, worsening the blood viscosity of glomerular capillaries and efferent arterioles (Fig. 4c). PS exposure on ECs promotes microthrombi, reducing renal perfusion. A dual-energy computed tomography study showed that half of mild-to-moderate COVID-19 patients had kidney perfusion abnormalities [95]. Abnormal renal perfusion decreases eGFR and increases plasma creatinine levels. Additionally, diffuse microthrombi can result in arrest of blood flow in parts of the glomeruli. Due to the lack of continuous formation of initial urine, the leaking plasma protein (erythrocytes) stagnates in the kidney, and subsequently forms protein (erythrocyte) casts in renal tubules or collecting tubes after concentration and acidification (Fig. 4e). Especially in COVID-19 patients with rhabdomyolysis [91], released intracellular substances severely damage ECs and promote diffuse microthrombi, blocking numbers glomeruli. The increased myoglobin in the plasma leaks and stagnates in the kidney, eventually forming myoglobin casts in the renal tubules or collecting tubes.
Although SARS-CoV-2 cannot cross the endothelial fenestrations, early results have shown that it has a renal tropism and can directly infect podocytes [96]. SARS-CoV-2 may infect podocytes either after infecting ECs or by crossing the damaged endothelial barrier (Fig. 4a). Damaged podocytes lose foot processes and reduce the release of VEGF A, further aggravating the injury of glomerular ECs [54]. During acute infection, disease progression is driven primarily by hypoxia, elevated cytokines, endothelial damage and thrombosis. Thus, AKI mainly presents as tubular injury due to ischemia and hypoxia. After discharge, hypoxic and ischemic factors are significantly alleviated, kidney injury mainly presents as GBF injury (sustained eGFR decline) due to persistent endothelial dysfunction.
Intestinal–blood barrier
The intestinal–blood barrier consists of intestinal microbiota, epithelial barrier, immune system in the lamina propria, and the capillary endothelial barrier (Fig. 5a) [97]. The epithelial barrier protects the host from microbial infection and, at the same time, allows the host to tolerate the microbiota. Specialized enterocytes (such as goblet and Paneth cells, which respectively produce mucus and secrete antimicrobial peptides) provide additional support for the maintenance of the intestinal barrier. The immune system in the lamina propria senses the intestinal microbiota or their metabolic products and translates the signals into host physiological responses and contributes to shaping the systemic immune system [98]. ECs in the intestine capillary are fenestrated and can completely or partly prevent the translocation of microorganisms or their metabolic products. In addition, enteric pericytes and glial cells, closely associated with ECs, contribute to preserve intestinal epithelial and endothelial barrier integrity.
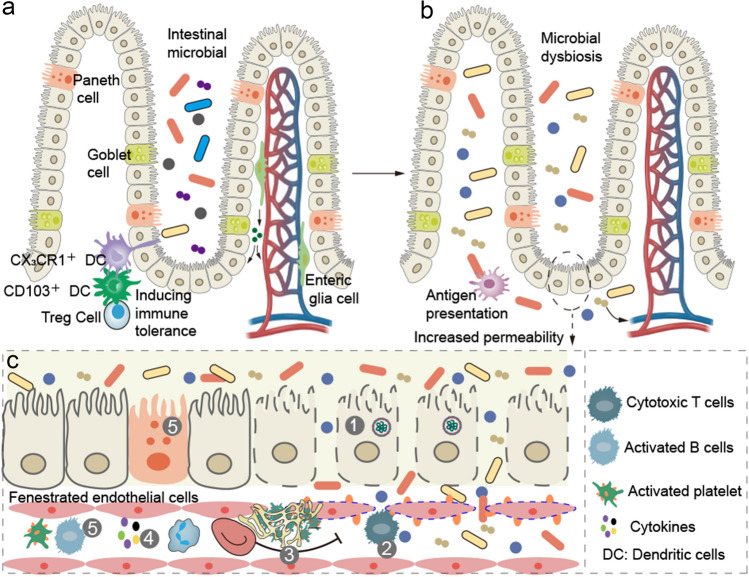
Fig. 5.
Intestinal–blood barrier and gastrointestinal sequelae. a Intestinal–blood barrier consists of intestinal microbiota, epithelial barrier, immune system in the lamina propria and capillary endothelial barrier. Epithelial barrier protects the host from the microbial infection and, at the same time, allow the host to tolerate the microbiota. Goblet and Paneth cells respectively produce mucus and secrete antimicrobial peptides, protecting the intestinal barrier. Dendritic cells in the lamina propria senses the intestinal microbiota or their metabolic products and to induce immune tolerance. Pericytes and enteric glial cells, closely associated with fenestrated endothelial cells, contribute to preserve intestinal epithelial and endothelial barrier integrity. b SARS-CoV-2 infection increases the intestinal permeability and alters intestinal microbiota. Dendritic cells sense these microbiota and present antigens. c Accumulation of B and Paneth cells suppresses intestinal inflammation and promotes epithelial barrier repair in COVID-19. Persistent viral presence, inflammation and immune dysregulation (cytotoxic T cells) in convalescent patients maintain intestinal endothelial injury and microthrombi, which not only delay accumulation of B and Paneth cells, but also impede epithelial and endothelial repairs
In COVID-19, 48–54% of patients have intestinal infection, which can increase the intestinal permeability and alter intestinal microbiota (Fig. 5b) [99, 100]. Clinical data has shown that most gastrointestinal symptoms (GIS) are self-limiting (the duration of GIS: 4 [2–7] days) [101]. A recent longitudinal analysis in a SARS-CoV-2-infected rhesus macaque model revealed that intestinal enterocytes were degraded at 3 dpi but recovered rapidly by 7 and 10 dpi. Results further showed that B and Paneth cell accumulation in intestinal tissue at 3–10 dpi suppressed intestinal inflammation and promoted epithelial barrier repair [102]. This self-repair of epithelial barrier partly explains the self-limiting nature of GIS. Meanwhile, a subset of patients develops severe GIS, including weight loss, abdominal pain, intestinal necrosis and dissemination of intestinal flora (or sepsis) [55, 103]. These severe GIS are strongly associated with intestinal thrombi [55]. Damaged ECs promote intestinal thrombosis, resulting in intestinal ischemia, dysfunction (e.g. malabsorption), necrosis, barrier injury and eventually resection. Persistent microthrombi not only delays accumulation of B and Paneth cells, but also directly inhibits epithelial and endothelial repairs, impeding intestinal–blood barrier recovery and leading to gastrointestinal sequelae. As shown in Fig. 5c, persistent virus presence, inflammation and immune dysregulation (e.g. cytotoxic T cells) all contribute to intestinal endothelial dysfunction (or microthrombi) in convalescent patients. Beyond that, reduced capillaries due to abnormal angiogenesis may also be involved in gastrointestinal symptoms in acute and convalescent patients, though direct evidence for this is still lacking.
Damaged intestinal–blood barrier is always accompanied by intestinal microbial dysbiosis (reduced bacteria diversity and richness, increased opportunistic pathogens, and lowered beneficial bacterium), which is associated with multisystem sequelae [104]. An observational study found that persistent respiratory symptoms were correlated with opportunistic gut pathogens, neuropsychiatric symptoms and fatigue with nosocomial gut pathogens, and hair loss with butyrate-producing species [105]. Results also showed a positive correlation between 6-min walking distances and some microflora products (such as several short-chain fatty acids). Another study revealed that intestinal microbial alterations were associated with persistent low-grade inflammation and respiratory dysfunction (lower diffusing capacity) at the 3-month follow up [106]. A recent case reported that modulating intestinal microbiota (a high-fiber formula) can alleviate severe long COVID symptoms, including severe “loss of appetite”, nausea, palpitation and anxiety [107]. In addition, the liver function, serum lipid profile, insulin level, and leptin level were also improved. Overall, intestinal microbial dysbiosis plays an important role in multisystem sequelae for some patients.
Treatment
Vaccination
Vaccination is the leading strategy to combat the COVID-19 pandemic, by reducing the risk of infection, severe disease, and mortality. Decreased infection rates and disease severity mean a lower burden of long COVID for the medical system. Additionally, vaccination can decrease long COVID symptoms. A large prospective study showed that being fully vaccinated halved the long-duration (28 days) symptoms of COVID-19 patients [108]. A recent observational study revealed that a first vaccine dose was associated with a 12.8% decrease (− 18.6 to − 6.6%) in the odds of long COVID, a second dose with another 8.8% decrease (− 14.1 to − 3.1%), and continued improvement with subsequent doses [32]. However, it is important to emphasize that one dose of the vaccine may not provide sustained protection. Another article studied the relationship between vaccination and long COVID in outpatients and found that the number of vaccine doses was associated with lower long COVID prevalence (41.8% [37.0−46.7%] in unvaccinated patients, 30.0% [6.7−65.2%] with 1 dose, 17.4% [7.8−31.4%] with 2 doses, and 16.0% [11.8−21.0%] with 3 doses) [109]. Additionally, vaccination has a similar protective effect in patients with breakthrough infection. A recent observational study revealed that patients with breakthrough infection had lower mortality and a decreased chance of long COVID compared to unvaccinated ones [110]. In summary, vaccination is the first choice for reducing the prevalence of long COVID.
Antithrombotic therapy
It is becoming clear that vaccines can’t completely prevent long COVID. A recent observational study found that patients with breakthrough infection had a higher risk of long COVID than individuals without COVID-19 or influenza patients [110]. Another report showed that the prevalence of post-COVID symptoms 4 weeks after Omicron and Delta breakthrough infections was 4.5% and 10.8%, respectively [111]. These findings emphasize the need for continued optimization of strategies for primary prevention of long COVID. The degree of endothelial injury in patients with long COVID is strongly associated with the degree of injury during acute infection [112]. Patients with serious COVID-19 are more likely to develop long COVID [113]. Early antithrombotic therapy can prevent EC injury and disease progression, thus potentially reducing the risk of long COVID. In addition, recent results found a positive correlation between the presence of microthrombi and the occurrence of intussusceptive angiogenesis in COVID-19 [69]. Antithrombotic therapy may therefor decrease abnormal angiogenesis. Beyond that, in the recently published results of the REMAP-CAP Randomized Clinical Trial (RCT), although antiplatelet agents did not improve the 21-day organ support-free days of critically ill COVID-19 patients, they showed a 92.3% probability of improved 90-day survival, 95.0% probability of improved 180-day survival, and a 97.4% probability of improved health-related quality of life in survivors at 6 months [114]. Similarly, in the OVID RCT, enoxaparin didn’t improve the hospitalization and all-cause death within 30 day in outpatients but decreased their 90-day cardiovascular events [115]. These results support that early antithrombotic therapy has a long-term benefit in COVID-19, even in the absence of short-term efficacy.
An earlier retrospective study showed that post-discharge thromboprophylaxis (mostly at prophylactic doses) was associated with a 46% decrease in major thromboembolism or all-cause mortality within 90 days [116]. Additionally, a recent retrospective study revealed that post-discharge therapy using prophylactic DOAC or dipyridamole improved long-term (393 ± 87 days) cardiovascular events, cardiovascular mortality and all-cause mortality in COVID-19 survivors [117]. Moreover, a RCT study further confirmed the benefits of extended thromboprophylaxis (rivaroxaban, 10 mg/days, 35 days) in discharged COVID-19 patients with higher thrombotic risk factors [118]. These data indicate that extended antithrombotic therapy decrease long-term sequelae. More importantly, a recent prospective study showed that sulodexide (250 LRU bid; 21 days) significantly improved endothelial dysfunction and alleviated chest pain and palpitations in patients with long COVID (median time between COVID-19 infection and inclusion: 71 days) [119]. In another article, resolution of symptoms was seen in all 24 patients with long COVID receiving triple antithrombotic drugs [120]. Overall, early antithrombotic therapy may be another option for reducing long COVID.
Metabolic risk factors
Metabolic risk factors, such as hypertension, dyslipidaemia, obesity and diabetes, can damage ECs and are associated with severe COVID-19, higher mortality and long COVID [36, 121, 122]. A recent study showed that COVID-19 ICU survivors with severe obesity experienced more long-term physical and mental symptoms [121]. Another paper found that type 2 diabetes was associated with persistent cough, fatigue and other symptoms [122]. It is clear evidence that management of these risk factors can lower endothelial injury and cytokines levels, reducing cardiovascular, cerebrovascular and renal complications. These benefits may analogously apply to patients with long COVID. Although direct evidence is still lacking, some researchers believe that controlling these risk factors can reduce long COVID [123, 124].
Omicron infection and long COVID
The Omicron variant was first detected in late 2021, and has since swept the globe, becoming the predominant pandemic virus. Although Omicron is less virulent, it is more transmissible, increasing the concern about long COVID [125, 126]. A recent report showed a reduction in odds of long COVID with the Omicron variant versus the Delta variant, but a higher absolute number of people experiencing long COVID at a given time [110]. Another study found that, compared to Delta, Omicron may have similar risk of sub-acute (30–89 days) and lower chronic (≧ 90 days) post-covid symptoms [127]. More importantly, a recent communication revealed that out of 428 individuals hospitalized for Omicron, 76% had persistent symptoms 4 to 12 weeks after their infection [128]. Thus, it is still necessary to actively prevent the occurrence of long COVID for Omicron infection.
Although there are currently no definitely effective drugs for long COVID, some treatments have been proposed to reduce the risk. Vaccination and antithrombotic therapy [129] are two important candidate treatments. First, vaccination should be actively promoted. Recent studies reveal that at least three doses of vaccination are needed to effectively prevent Omicron infection [130, 131]. Data also shows that vaccinated individuals have lower risk of long COVID after Omicron infection than those who are unvaccinated [132]. Second, antithrombotic therapy (prophylactic dose) is recommended for mild patients with thrombotic risk factors (e.g. obesity, hyperglycemia, diabetes, history of thrombus, active cancer, age ≧ 70 years, respiratory/cardiac/renal/liver failure). Thromboelastographic results revealed that the clot parameters in patients with mild Omicron infection were higher than health controls, and lower than patients with Beta and Delta infections [133]. For these Omicron patients with thrombotic risk factors, antithrombotic therapy is needed to reduce the long-term sequelae (e.g. cardiovascular events [115]). However, for the younger patients, recent data has shown that they had preserved vascular health and cardiac autonomic function [134], and antithrombotic therapy may be not recommended for these patients. Third, early antithrombotic therapy is essential for all hospitalized patients with Omicron infections (without contraindications). Therapeutic dose of anticoagulant therapy can prevent COVID-19 progression, reduce ICU admission, mortality and risk of long COVID. Lastly, improving endothelial function (e.g. sulodexide) and reducing microthrombi (e.g. rivaroxaban, aspirin) can lessen or alleviate long COVID symptoms [119, 120].
Conclusion
ECs and endothelial barriers, which form the inner lining of blood vessels, display remarkable heterogeneity in structure and function to maintain normal physiological functions in different organs. In COVID-19, disseminated virus, elevated cytokines and activated complement damage ECs, increasing endothelial permeability, reducing and/or remodeling microcapillaries, and affecting functions throughout the body. After recovery from infection, a subset of patients have persistent endothelial dysfunction and capillary rarefication, presenting with multisystem sequelae (long COVID). Early endothelial dysfunction mainly includes PS exposure, glycocalyx shedding and barrier injury, which lead to leakage of plasma and the formation of microthrombi, and affect normal physiological function of the lung, brain, kidneys and intestinal tract. Prevention of endothelial injury can prevent COVID-19 progression, and reduce ICU admission, mortality and long COVID.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
JS and XW designed the research, analyzed the data, and wrote the primary manuscript. MX, HJ and CW contributed to literature retrieval, data acquisition and analysis of data. VAN analyzed the data and revised the primary manuscript. JS and VAN discussed and confirmed the final manuscript. Other authors read and approved the final version.
Declarations
Competing interest
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization (2023) https://covid19.who.int/
- 2.World Health Organization (2021) A clinical case definition of post COVID-19 condition by a Delphi consensus. https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 [DOI] [PMC free article] [PubMed]
- 3.Blomberg B, Mohn KG, Brokstad KA, Zhou F, Linchausen DW, Hansen BA, Lartey S, Onyango TB, Kuwelker K, Sævik M, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021;27(9):1607–1613. doi: 10.1038/s41591-021-01433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tran VT, Porcher R, Pane I, Ravaud P. Course of post COVID-19 disease symptoms over time in the ComPaRe long COVID prospective e-cohort. Nat Commun. 2022;13:1812. doi: 10.1038/s41467-022-29513-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2022;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wettschureck N, Strilic B, Offermanns S. Passing the vascular barrier: endothelial signaling processes controlling extravasation. Physiol Rev. 2019;99(3):1467–1525. doi: 10.1152/physrev.00037.2018. [DOI] [PubMed] [Google Scholar]
- 7.Bonaventura A, Vecchié A, Dagna L, Martinod K, Dixon DL, Van Tassell BW, Dentali F, Montecucco F, Massberg S, Levi M, Abbate A. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. 2021;21(5):319–329. doi: 10.1038/s41577-021-00536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fogarty H, Townsend L, Morrin H, Ahmad A, Comerford C, Karampini E, Englert H, Byrne M, Bergin C, O’Sullivan JM, et al. Persistent endotheliopathy in the pathogenesis of Long COVID syndrome. J Thromb Haemost. 2021;19:2546–2553. doi: 10.1111/jth.15490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan BE, Wong SW, Sum CLL, Lim GH, Leung BP, Tan CW, Ramanathan K, Dalan R, Cheung C, Lim XR, et al. Hypercoagulability, endotheliopathy, and inflammation approximating 1 year after recovery: assessing the long-term outcomes in COVID-19 patients. Am J Hematol. 2022;97(7):915–923. doi: 10.1002/ajh.26575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joffre J, Rodriguez L, Matthay ZA, Lloyd E, Fields AT, Bainton RJ, Kurien P, Sil A, Calfee CS, Woodruff PG, et al. COVID-19-associated lung microvascular endotheliopathy: a “from the bench” perspective. Am J Respir Crit Care Med. 2022;206(8):961–972. doi: 10.1164/rccm.202107-1774OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu F, Han K, Blair R, Kenst K, Qin Z, Upcin B, Wörsdörfer P, Midkiff CC, Mudd J, Belyaeva E, et al. SARS-CoV-2 infects endothelial cells in vivo and in vitro. Front Cell Infect Microbiol. 2021;11:701278. doi: 10.3389/fcimb.2021.701278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lei Y, Zhang J, Schiavon CR, He M, Chen L, Shen H, Zhang Y, Yin Q, Cho Y, Andrade L, et al. SARS-CoV-2 spike protein impairs endothelial function via downregulation of ACE2. Circ Res. 2021;128:1323–1326. doi: 10.1161/CIRCRESAHA.121.318902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michalick L, Weidenfeld S, Grimmer B, Fatykhova D, Solymosi PD, Behrens F, Dohmen M, Brack MC, Schulz S, Thomasch E, et al. Plasma mediators in patients with severe COVID-19 cause lung endothelial barrier failure. Eur Respir J. 2021;57:2002384. doi: 10.1183/13993003.02384-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barrett TJ, Cornwell M, Myndzar K, Rolling CC, Xia Y, Drenkova K, Biebuyck A, Fields AT, Tawil M, Luttrell-Williams E, et al. Platelets amplify endotheliopathy in COVID-19. Sci Adv. 2021;7:eabh2434. doi: 10.1126/sciadv.abh2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noris M, Benigni A, Remuzzi G. The case of complement activation in COVID-19 multiorgan impact. Kidney Int. 2020;98(2):314–322. doi: 10.1016/j.kint.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halawa S, Pullamsetti SS, Bangham CRM, Stenmark KR, Dorfmüller P, Frid MG, Butrous G, Morrell NW, de Jesus Perez VA, Stuart DI, et al. Potential long-term effects of SARS-CoV-2 infection on the pulmonary vasculature: a global perspective. Nat Rev Cardiol. 2022;19:314–331. doi: 10.1038/s41569-021-00640-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Afzali B, Noris M, Lambrecht BN, Kemper C. The state of complement in COVID-19. Nat Rev Immunol. 2022;22(2):77–84. doi: 10.1038/s41577-021-00665-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dupont A, Rauch A, Staessens S, Moussa M, Rosa M, Corseaux D, Jeanpierre E, Goutay J, Caplan M, Varlet P, et al. Vascular endothelial damage in the pathogenesis of organ injury in severe COVID-19. Arterioscler Thromb Vasc Biol. 2021;41:1760–1773. doi: 10.1161/ATVBAHA.120.315595. [DOI] [PubMed] [Google Scholar]
- 21.Goshua G, Pine AB, Meizlish ML, Chang CH, Zhang H, Bahel P, Baluha A, Bar N, Bona RD, Burns AJ, et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7(8):e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He Z, Si Y, Jiang T, Ma R, Zhang Y, Cao M, Li T, Yao Z, Zhao L, Fang S, et al. Phosphotidylserine exposure and neutrophil extracellular traps enhance procoagulant activity in patients with inflammatory bowel disease. Thromb Haemost. 2016;115:738–751. doi: 10.1160/TH15-09-0710. [DOI] [PubMed] [Google Scholar]
- 23.Flaumenhaft R, Enjyoji K, Schmaier AA. Vasculopathy in COVID-19. Blood. 2022;140(3):222–235. doi: 10.1182/blood.2021012250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chioh FW, Fong SW, Young BE, Wu KX, Siau A, Krishnan S, Chan YH, Carissimo G, Teo LL, Gao F, et al. Convalescent COVID-19 patients are susceptible to endothelial dysfunction due to persistent immune activation. Elife. 2021;10:e64909. doi: 10.7554/eLife.64909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zanoli L, Gaudio A, Mikhailidis DP, Katsiki N, Castellino N, Lo Cicero L, Geraci G, Sessa C, Fiorito L, Marino F, et al. Vascular dysfunction of COVID-19 is partially reverted in the long-term. Circ Res. 2022;130(9):1276–1285. doi: 10.1161/CIRCRESAHA.121.320460. [DOI] [PubMed] [Google Scholar]
- 26.Lambadiari V, Mitrakou A, Kountouri A, Thymis J, Katogiannis K, Korakas E, Varlamos C, Andreadou I, Tsoumani M, Triantafyllidi H, et al. Association of COVID-19 with impaired endothelial glycocalyx, vascular function and myocardial deformation 4 months after infection. Eur J Heart Fail. 2021;23(11):1916–1926. doi: 10.1002/ejhf.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaebler C, Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Tokuyama M, Cho A, Jankovic M, Schaefer-Babajew D, Oliveira TY, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591(7851):639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zollner A, Koch R, Jukic A, Pfister A, Meyer M, Rössler A, Kimpel J, Adolph TE, Tilg H, et al. Post-acute COVID-19 is characterized by gut viral antigen persistence in inflammatory bowel diseases. Gastroenterology. 2022;163(2):495–506. doi: 10.1053/j.gastro.2022.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheung CCL, Goh D, Lim X, Tien TZ, Lim JCT, Lee JN, Tan B, Tay ZEA, Wan WY, Chen EX, et al. Residual SARS-CoV-2 viral antigens detected in GI and hepatic tissues from five recovered patients with COVID-19. Gut. 2022;71(1):226–229. doi: 10.1136/gutjnl-2021-324280. [DOI] [PubMed] [Google Scholar]
- 30.Hu F, Chen F, Ou Z, Fan Q, Tan X, Wang Y, Pan Y, Ke B, Li L, Guan Y, et al. A compromised specific humoral immune response against the SARS-CoV-2 receptor-binding domain is related to viral persistence and periodic shedding in the gastrointestinal tract. Cell Mol immunol. 2020;17(11):1119–1125. doi: 10.1038/s41423-020-00550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neurath MF, Überla K, Ng SC. Gut as viral reservoir: lessons from gut viromes, HIV and COVID-19. Gut. 2021;70(9):1605–1608. doi: 10.1136/gutjnl-2021-324622. [DOI] [PubMed] [Google Scholar]
- 32.Ayoubkhani D, Bermingham C, Pouwels KB, Glickman M, Nafilyan V, Zaccardi F, Khunti K, Alwan NA, Walker AS. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ. 2022;377:e069676. doi: 10.1136/bmj-2021-069676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arkin LM, Moon JJ, Tran JM, Asgari S, O’Farrelly C, Casanova JL, Cowen EW, Mays JW, Singh AM, Drolet BA, COVID Human Genetic Effort From your nose to your toes: a review of severe acute respiratory syndrome coronavirus 2 pandemic-associated pernio. J Invest Dermatol. 2021;141(12):2791–2796. doi: 10.1016/j.jid.2021.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peghin M, De Martino M, Palese A, Gerussi V, Bontempo G, Graziano E, Visintini E, D’Elia D, Dellai F, Marrella F, et al. Post-COVID-19 syndrome and humoral response association after 1 year in vaccinated and unvaccinated patients. Clin Microbiol Infect. 2022;28(8):1140–1148. doi: 10.1016/j.cmi.2022.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehandru S, Merad M. Pathological sequelae of long-haul COVID. Nat Immunol. 2022;23(2):194–202. doi: 10.1038/s41590-021-01104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.PHOSP-COVID Collaborative Group Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: a prospective observational study. Lancet Respir Med. 2022;10(8):761–775. doi: 10.1016/S2213-2600(22)00127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phetsouphanh C, Darley DR, Wilson DB, Howe A, Munier CML, Patel SK, Juno JA, Burrell LM, Kent SJ, Dore GJ, et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol. 2022;23(2):210–216. doi: 10.1038/s41590-021-01113-x. [DOI] [PubMed] [Google Scholar]
- 38.Vijayakumar B, Boustani K, Ogger PP, Papadaki A, Tonkin J, Orton CM, Ghai P, Suveizdyte K, Hewitt RJ, Desai SR, et al. Immuno-proteomic profiling reveals aberrant immune cell regulation in the airways of individuals with ongoing post-COVID-19 respiratory disease. Immunity. 2022;55(3):542–556e5. doi: 10.1016/j.immuni.2022.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwabenland M, Salié H, Tanevski J, Killmer S, Lago MS, Schlaak AE, Mayer L, Matschke J, Püschel K, Fitzek A, et al. Deep spatial profiling of human COVID-19 brains reveals neuroinflammation with distinct microanatomical microglia-T-cell interactions. Immunity. 2021;54(7):1594–1610e11. doi: 10.1016/j.immuni.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osiaevi I, Schulze A, Evers G, Harmening K, Vink H, Kümpers P, Mohr M, Rovas A. Persistent capillary rarefication in long COVID syndrome. Angiogenesis. 2023;26(1):53–61. doi: 10.1007/s10456-022-09850-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szewczykowski C, Mardin C, Lucio M, Wallukat G, Hoffmanns J, Schröder T, Raith F, Rogge L, Heltmann F, Moritz M, et al. Long COVID: association of functional autoantibodies against G-protein-coupled receptors with an impaired retinal microcirculation. Int J Mol Sci. 2022;23(13):7209. doi: 10.3390/ijms23137209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Versteeg HH, Heemskerk JW, Levi M, Reitsma PH. New fundamentals in hemostasis. Physiol Rev. 2013;93:327–358. doi: 10.1152/physrev.00016.2011. [DOI] [PubMed] [Google Scholar]
- 43.Cao M, Li T, He Z, Wang L, Yang X, Kou Y, Zou L, Dong X, Novakovic VA, Bi Y, Kou J, et al. Promyelocytic extracellular chromatin exacerbates coagulation and fibrinolysis in acute promyelocytic leukemia. Blood. 2017;129(13):1855–1864. doi: 10.1182/blood-2016-09-739334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi J, Gilbert GE. Lactadherin inhibits enzyme complexes of blood coagulation by completing for phospholipid binding sites. Blood. 2003;101:2628–2636. doi: 10.1182/blood-2002-07-1951. [DOI] [PubMed] [Google Scholar]
- 45.Thomas M, Scully M. Clinical features of thrombosis and bleeding in COVID-19. Blood. 2022;385:1680–1689. doi: 10.1182/blood.2021012247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katsoularis I, Fonseca-Rodríguez O, Farrington P, Jerndal H, Lundevaller EH, Sund M, Lindmark K, Fors Connolly AM. Risks of deep vein thrombosis, pulmonary embolism, and bleeding after covid-19: nationwide self-controlled cases series and matched cohort study. BMJ. 2022;377:e069590. doi: 10.1056/NEJMoa2109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iba T, Levy JH. Derangement of the endothelial glycocalyx in sepsis. J Thromb Haemost. 2019;17(2):283–294. doi: 10.1111/jth.14371. [DOI] [PubMed] [Google Scholar]
- 48.Smadja DM, Mentzer SJ, Fontenay M, Laffan MA, Ackermann M, Helms J, Jonigk D, Chocron R, Pier GB, Gendron N, et al. COVID-19 is a systemic vascular hemopathy: insight for mechanistic and clinical aspects. Angiogenesis. 2021;24:755–788. doi: 10.1007/s10456-021-09805-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vollenberg R, Tepasse PR, Ochs K, Floer M, Strauss M, Rennebaum F, Kabar I, Rovas A, Nowacki T. Indications of persistent glycocalyx damage in convalescent COVID-19 patients: a prospective multicenter study and hypothesis. Virus. 2021;13(11):2324. doi: 10.3390/v13112324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rovas A, Osiaevi I, Buscher K, Sackarnd J, Tepasse PR, Fobker M, Kühn J, Braune S, Göbel U, Thölking G, et al. Microvascular dysfunction in COVID-19: the MYSTIC study. Angiogenesis. 2021;24(1):145–157. doi: 10.1007/s10456-020-09753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ikonomidis I, Lambadiari V, Mitrakou A, Kountouri A, Katogiannis K, Thymis J, Korakas E, Pavlidis G, Kazakou P, Panagopoulos G, et al. Myocardial work and vascular dysfunction are partially improved at 12 months after COVID-19 infection. Eur J Heart Fail. 2022;24(4):727–729. doi: 10.1002/ejhf.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D’Agnillo F, Walters KA, Xiao Y, Sheng ZM, Scherler K, Park J, Gygli S, Rosas LA, Sadtler K, Kalish H, et al. Lung epithelial and endothelial damage, loss of tissue repair, inhibition of fibrinolysis, and cellular senescence in fatal COVID-19. Sci Transl Med. 2021;13(620):eabj7790. doi: 10.1126/scitranslmed.abj7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee MH, Perl DP, Nair G, Li W, Maric D, Murray H, Dodd SJ, Koretsky AP, Watts JA, Cheung V, et al. Microvascular injury in the brains of patients with Covid-19. N Engl J Med. 2021;384:481–483. doi: 10.1056/NEJMc2033369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daehn IS, Duffield JS. The glomerular filtration barrier: a structural target for novel kidney therapies. Nat Rev Drug Discov. 2021;20:770–788. doi: 10.1038/s41573-021-00242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu X, Jing H, Wang C, Wang Y, Zuo N, Jiang T, Novakovic VA, Shi J. Intestinal damage in COVID-19: SARS-CoV-2 infection and intestinal thrombosis. Front Microbiol. 2022;13:860931. doi: 10.3389/fmicb.2022.860931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Cir Res. 2007;100(2):174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 57.Lamers MM, Haagmans BL. SARS-CoV-2 pathogenesis. Nat Rew Microbiol. 2022;20(5):270–284. doi: 10.1038/s41579-022-00713-0. [DOI] [PubMed] [Google Scholar]
- 58.Dolhnikoff M, Duarte-Neto AN, de Almeida Monteiro RA, da Silva LFF, de Oliveira EP, Saldiva PHN, Mauad T, Negri EM. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J Thromb Haemost. 2020;18:1517–1519. doi: 10.1111/jth.14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morris MF, Pershad Y, Kang P, Ridenour L, Lavon B, Lanclus M, Godon R, De Backer J, Glassberg MK. Altered pulmonary blood volume distribution as a biomarker for predicting outcomes in COVID-19 disease. Eur Respir J. 2021;58:2004133. doi: 10.1183/13993003.04133-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ackermann M, Tafforeau P, Wagner WL, Walsh CL, Werlein C, Kühnel MP, Länger FP, Disney C, Bodey AJ, Bellier A, et al. The bronchial circulation in COVID-19 pneumonia. Am J Respir Crit Care Med. 2022;205(1):121–125. doi: 10.1164/rccm.202103-0594IM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sonnweber T, Sahanic S, Pizzini A, Luger A, Schwabl C, Sonnweber B, Kurz K, Koppelstätter S, Haschka D, Petzer V, et al. Cardiopulmonary recovery after COVID-19: an observational prospective multicentre trial. Eur Respir J. 2021;57(4):2003481. doi: 10.1183/13993003.03481-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ravaglia C, Doglioni C, Chilosi M, Piciucchi S, Dubini A, Rossi G, Pedica F, Puglisi S, Donati L, Tomassetti S, Poletti V. Clinical, radiological, and pathological findings in patients with persistent lung disease following SARS-CoV-2 infection. Eur Respir J. 2022;60(4):2102411. doi: 10.1183/13993003.02411-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheon IS, Li C, Son YM, Goplen NP, Wu Y, Cassmann T, Wang Z, Wei X, Tang J, Li Y, et al. Immune signatures underlying post-acute COVID-19 lung sequelae. Sci Immunol. 2021;6(65):eabk1741. doi: 10.1126/sciimmunol.abk1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fabbri L, Moss S, Khan FA, Chi W, Xia J, Robinson K, Smyth AR, Jenkins G, Stewart I. Parenchymal lung abnormalities following hospitalisation for COVID-19 and viral pneumonitis: a systematic review and meta-analysis. Thorax. 2022;78(2):191–201. doi: 10.1136/thoraxjnl-2021-218275. [DOI] [PubMed] [Google Scholar]
- 65.Wendisch D, Dietrich O, Mari T, von Stillfried S, Ibarra IL, Mittermaier M, Mache C, Chua RL, Knoll R, Timm S, et al. SARS-CoV-2 infection triggers profibrotic macrophage responses and lung fibrosis. Cell. 2021;184(26):6243–6261e27. doi: 10.1016/j.cell.2021.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roach A, Chikwe J, Catarino P, Rampolla R, Noble PW, Megna D, Chen Q, Emerson D, Egorova N, Keshavjee S, Kirklin JK. Lung transplantation for Covid-19-related respiratory failure in the United States. N Engl J Med. 2022;386(12):1187–1188. doi: 10.1056/NEJMc2117024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pretorius E, Venter C, Laubscher GJ, Kotze MJ, Oladejo SO, Watson LR, Rajaratnam K, Watson BW, Kell DB. Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with long COVID/Post-Acute sequelae of COVID-19 (PASC) Cardiovasc Diabetol. 2022;21(1):148. doi: 10.1186/s12933-022-01579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martins-Gonçalves R, Campos MM, Palhinha L, Azevedo-Quintanilha IG, Abud Mendes M, Ramos Temerozo J, Toledo-Mendes J, Rosado-de-Castro PH, Bozza FA, Souza Rodrigues R, et al. Persisting platelet activation and hyperactivity in COVID-19 survivors. Circ Res. 2022;131(11):944–947. doi: 10.1161/CIRCRESAHA.122.321659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Werlein C, Ackermann M, Stark H, Shah HR, Tzankov A, Haslbauer JD, von Stillfried S, Bülow RD, El-Armouche A, Kuenzel S, et al. Inflammation and vascular remodeling in COVID-19 hearts. Angiogenesis. 2022 doi: 10.1007/s10456-022-09860-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dhawan RT, Gopalan D, Howard L, Vicente A, Park M, Manalan K, Wallner I, Marsden P, Dave S, Branley H, Russell G, Dharmarajah N, Kon OM. Beyond the clot: perfusion imaging of the pulmonary vasculature after COVID-19. Lancet Respir Med. 2021;9(1):107–116. doi: 10.1016/S2213-2600(20)30407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood–brain barrier: from physiology to disease and back. Physiol Rev. 2019;99(1):21–78. doi: 10.1152/physrev.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Engelhardt B, Vajkoczy P, Weller RO. The movers and shapers in immune privilege of the CNS. Nat Immunol. 2017;18(2):123–131. doi: 10.1038/ni.3666. [DOI] [PubMed] [Google Scholar]
- 73.Xu E, Xie Y, Al-Aly Z. Long-term neurologic outcomes of COVID-19. Nat Med. 2022;28(11):2406–2415. doi: 10.1038/s41591-022-02001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spudich S, Nath A. Nervous system consequences of COVID-19. Science. 2022;375(6578):267–269. doi: 10.1126/science.abm2052. [DOI] [PubMed] [Google Scholar]
- 75.Drake TM, Riad AM, Fairfield CJ, Egan C, Knight SR, Pius R, Hardwick HE, Norman L, Shaw CA, McLean KA, et al. Characterisation of in-hospital complications associated with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol UK: a prospective, multicentre cohort study. Lancet. 2021;398(10296):223–237. doi: 10.1016/S0140-6736(21)00799-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thakur KT, Miller EH, Glendinning MD, Al-Dalahmah O, Banu MA, Boehme AK, Boubour AL, Bruce SS, Chong AM, Claassen J, et al. COVID-19 neuropathology at Columbia University Irving Medical Center/New York Presbyterian Hospital. Brain. 2021;144(9):2696–2708. doi: 10.1093/brain/awab148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee MH, Perl DP, Steiner J, Pasternack N, Li W, Maric D, Safavi F, Horkayne-Szakaly I, Jones R, Stram MN, et al. Neurovascular injury with complement activation and inflammation in COVID-19. Brain. 2022;145(7):2555–2568. doi: 10.1093/brain/awac151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Michele M, Kahan J, Berto I, Schiavo OG, Iacobucci M, Toni D, Merkler AE. Cerebrovascular complications of COVID-19 and COVID-19 vaccination. Cir Res. 2022;130(8):1187–1203. doi: 10.1161/CIRCRESAHA.122.319954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Katsanos AH, Palaiodimou L, Zand R, Yaghi S, Kamel H, Navi BB, Turc G, Romoli M, Sharma VK, Mavridis D, et al. The impact of SARS-CoV-2 on stroke epidemiology and care: a meta-analysis. Ann Neurol. 2021;89(3):380–388. doi: 10.1002/ana.25967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Waldrop G, Safavynia SA, Barra ME, Agarwal S, Berlin DA, Boehme AK, Brodie D, Choi JM, Doyle K, Fins JJ, et al. Prolonged unconsciousness is common in COVID-19 and associated with hypoxemia. Ann Neurol. 2022;91(6):740–755. doi: 10.1002/ana.26342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rutkai I, Mayer MG, Hellmers LM, Ning B, Huang Z, Monjure CJ, Coyne C, Silvestri R, Golden N, Hensley K, et al. Neuropathology and virus in brain of SARS-CoV-2 infected non-human primates. Nat Commun. 2022;13(1):1745. doi: 10.1038/s41467-022-29440-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Buzhdygan TP, DeOre BJ, Baldwin-Leclair A, Bullock TA, McGary HM, Khan JA, Razmpour R, Hale JF, Galie PA, Potula R, et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood–brain barrier. Neurobiol Dis. 2020;146:105131. doi: 10.1016/j.nbd.2020.105131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Erickson MA, Banks WA. Neuroimmune axes of the blood–brain barriers and blood–brain interfaces: bases for physiological regulation, disease states, and pharmacological interventions. Pharmacol Rev. 2018;70(2):278–314. doi: 10.1124/pr.117.014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Banks WA, Farr SA, La Scola ME, Morley JE. Intravenous human interleukin-1alpha impairs memory processing in mice: dependence on blood–brain barrier transport into posterior division of the septum. J Pharmacol Exp Ther. 2001;299(2):536–541. [PubMed] [Google Scholar]
- 85.Joshi G, Aluise CD, Cole MP, Sultana R, Pierce WM, Vore M, St Clair DK, Butterfield DA. Alterations in brain antioxidant enzymes and redox proteomic identification of oxidized brain proteins induced by the anti-cancer drug adriamycin: implications for oxidative stress-mediated chemobrain. Neuroscience. 2010;166(3):796–807. doi: 10.1016/j.neuroscience.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Busatto GF, de Araujo AL, Castaldelli-Maia JM, Damiano RF, Imamura M, Guedes BF, Pinna FR, Sawamura MVY, Mancini MC, da Silva KR, et al. Post-acute sequelae of SARS-CoV-2 infection: relationship of central nervous system manifestations with physical disability and systemic inflammation. Psychol Med. 2022;52(12):2387–2398. doi: 10.1017/S0033291722001374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ferrari C, Sorbi S. The complexity of Alzheimer’s disease: an evolving puzzle. Physiol Rev. 2021;101(3):1047–1081. doi: 10.1152/physrev.00015.2020. [DOI] [PubMed] [Google Scholar]
- 88.Jourde-Chiche N, Fakhouri F, Dou L, Bellien J, Burtey S, Frimat M, Jarrot PA, Kaplanski G, Le Quintrec M, Pernin V, et al. Endothelium structure and function in kidney health and disease. Nat Rev Nephrol. 2019;15(2):87–108. doi: 10.1038/s41581-018-0098-z. [DOI] [PubMed] [Google Scholar]
- 89.Yende S, Parikh CR. Long COVID and kidney disease. Nat Rev Nephrol. 2021;17(12):792–793. doi: 10.1038/s41581-021-00487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Legrand M, Bell S, Forni L, Joannidis M, Koyner JL, Liu K, Cantaluppi V. Pathophysiology of COVID-19-associated acute kidney injury. Nat Rev Nephrol. 2021;17(11):751–764. doi: 10.1038/s41581-021-00452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sharma P, Uppal NN, Wanchoo R, Shah HH, Yang Y, Parikh R, Khanin Y, Madireddy V, Larsen CP, Jhaveri KD, et al. COVID-19-associated kidney injury: a case series of kidney biopsy findings. J Am Soc Nephrol. 2020;31(9):1948–1958. doi: 10.1681/ASN.2020050699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, Yi F, Yang HC, Fogo AB, Nie X, Zhang C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Volbeda M, Jou-Valencia D, van den Heuvel MC, Knoester M, Zwiers PJ, Pillay J, Berger SP, van der Voort PHJ, Zijlstra JG, van Meurs M, Moser J. Comparison of renal histopathology and gene expression profiles between severe COVID-19 and bacterial sepsis in critically ill patients. Crit Care. 2021;25(1):202. doi: 10.1186/s13054-021-03631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bowe B, Xie Y, Xu E, Al-Aly Z. Kidney outcomes in long COVID. J Am Soc Nephrol. 2021;32(11):2851–2862. doi: 10.1681/ASN.2021060734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Idilman IS, Telli Dizman G, Ardali Duzgun S, Irmak I, Karcaaltincaba M, Inkaya AC, Demirkazik F, Durhan G, Gulsun Akpinar M, Ariyurek OM, et al. Lung and kidney perfusion deficits diagnosed by dual-energy computed tomography in patients with COVID-19-related systemic microangiopathy. Eur Radiol. 2021;31(2):1090–1099. doi: 10.1007/s00330-020-07155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, Chilla S, Heinemann A, Wanner N, Liu S, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383(6):590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Spadoni I, Fornasa G, Rescigno M. Organ-specific protection mediated by cooperation between vascular and epithelial barriers. Nat Rev Immunol. 2017;17(12):761–773. doi: 10.1038/nri.2017.100. [DOI] [PubMed] [Google Scholar]
- 98.Thaiss CA, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature. 2016;535(7610):65–74. doi: 10.1038/nature18847. [DOI] [PubMed] [Google Scholar]
- 99.Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R, Ng YY, Chu MY, Chung TWH, Tam AR, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in faecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159:81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zuo T, Zhang F, Lui GCY, Yeoh YK, Li AYL, Zhan H, Wan Y, Chung ACK, Cheung CP, Chen N, et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159(3):944–955. doi: 10.1053/j.gastro.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sultan S, Altayar O, Siddique SM, Davitkov P, Feuerstein JD, Lim JK, et al. AGA institute rapid review of the gastrointestinal and liver manifestations of COVID-19, meta-analysis of international data, and recommendations for the consultative management of patients with COVID-19. Gastroenterology. 2020;159(1):320–334e27. doi: 10.1053/j.gastro.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zheng H, Chen Y, Li J, Li H, Zhao X, Li J, Yang F, Li Y, Liu C, Qin L, et al. Longitudinal analyses reveal distinct immune response landscapes in lung and intestinal tissues from SARS-CoV-2-infected rhesus macaques. Cell Rep. 2022;39(8):110864. doi: 10.1016/j.celrep.2022.111014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Norsa L, Bonaffini PA, Indriolo A, Valle C, Sonzogni A, Sironi S. Poor outcome of intestinal ischemic manifestations of COVID-19. Gastroenterology. 2020;159(4):1595–1597e1. doi: 10.1053/j.gastro.2020.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lau RI, Zhang F, Liu Q, Su Q, Chan FKL, Ng SC. Gut microbiota in COVID-19: key microbial changes, potential mechanisms and clinical applications. Nat Rev Gastroenterol Hepatol. 2022 doi: 10.1038/s41575-022-00698-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu Q, Mak JWY, Su Q, Yeoh YK, Lui GC, Ng SSS, Zhang F, Li AYL, Lu W, Hui DS, et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut. 2022;71(3):544–552. doi: 10.1136/gutjnl-2021-325989. [DOI] [PubMed] [Google Scholar]
- 106.Vestad B, Ueland T, Lerum TV, Dahl TB, Holm K, Barratt-Due A, Kåsine T, Dyrhol-Riise AM, Stiksrud B, Tonby K, et al. Respiratory dysfunction three months after severe COVID-19 is associated with gut microbiota alterations. J Intern Med. 2022;291(6):801–812. doi: 10.1111/joim.13458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang Y, Wu G, Zhao L, Wang W. Nutritional modulation of gut microbiota alleviates severe gastrointestinal symptoms in a patient with post-acute COVID-19 syndrome. mBio. 2022;13(2):e0380121. doi: 10.1128/mbio.03801-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Antonelli M, Penfold RS, Merino J, Sudre CH, Molteni E, Berry S, Canas LS, Graham MS, Klaser K, Modat M, et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis. 2022;22(1):43–55. doi: 10.1016/S1473-3099(21)00460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Azzolini E, Levi R, Sarti R, Pozzi C, Mollura M, Mantovani A, Rescigno M. Association between BNT162b2 vaccination and long COVID after infections not requiring hospitalization in health care workers. JAMA. 2022;328(7):676–678. doi: 10.1001/jama.2022.11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022;28(7):1461–1467. doi: 10.1038/s41591-022-01840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Antonelli M, Pujol JC, Spector TD, Ourselin S, Steves CJ. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet. 2022;399(10343):2263–2264. doi: 10.1016/S0140-6736(22)00941-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Oikonomou E, Souvaliotis N, Lampsas S, Siasos G, Poulakou G, Theofilis P, Papaioannou TG, Haidich AB, Tsaousi G, Ntousopoulos V, et al. Endothelial dysfunction in acute and long standing COVID-19: a prospective cohort study. Vascul Pharmacol. 2022;144:106975. doi: 10.1016/j.vph.2022.106975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xie Y, Bowe B, Al-Aly Z. Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status. Nat Commun. 2021;12(1):6571. doi: 10.1038/s41467-021-26513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Writing Committee for the REMAP- CAP. Investigators, Higgins AM, Berry LR, Lorenzi E, Murthy S, McQuilten Z, Mouncey PR, Al-Beidh F, Annane D, Arabi YM, et al. Long-term (180-Day) outcomes in critically ill patients with COVID-19 in the REMAP-CAP randomized clinical trial. JAMA. 2023;329(1):39–51. doi: 10.1001/jama.2022.23257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Voci D, Götschi A, Held U, Bingisser R, Colucci G, Duerschmied D, Fumagalli RM, Gerber B, Hasse B, Keller D, et al. Enoxaparin for outpatients with COVID-19: 90-day results from the randomised, open-label, parallel-group, multinational, phase III OVID trial. Thromb Res. 2023;221:157–163. doi: 10.1016/j.thromres.2022.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Giannis D, Allen SL, Tsang J, Flint S, Pinhasov T, Williams S, Tan G, Thakur R, Leung C, Snyder M, et al. Postdischarge thromboembolic outcomes and mortality of hospitalized patients with COVID-19: the CORE-19 registry. Blood. 2021;137(20):2838–2847. doi: 10.1182/blood.2020010529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Motloch LJ, Jirak P, Mirna M, Fiedler L, Davtyan PA, Lakman IA, Gareeva DF, Tyurin AV, Gumerov RM, et al. Early antithrombotic post-discharge therapy using prophylactic DOAC or dipyridamole improves long-term survival and cardiovascular outcomes in hospitalized COVID-19 survivors. Front Cardiovasc Med. 2022;9:916156. doi: 10.3389/fcvm.2022.916156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ramacciotti E, Barile Agati L, Calderaro D, Aguiar VCR, Spyropoulos AC, de Oliveira CCC, et al. Rivaroxaban versus no anticoagulation for post-discharge thromboprophylaxis after hospitalisation for COVID-19 (MICHELLE): an open-label, multicentre, randomised, controlled trial. Lancet. 2022;399(10319):50–59. doi: 10.1016/S0140-6736(21)02392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Charfeddine S, Ibnhadjamor H, Jdidi J, Torjmen S, Kraiem S, Bahloul A, Makni A, Kallel N, Moussa N, Boudaya M, et al. Sulodexide significantly improves endothelial dysfunction and alleviates chest pain and palpitations in patients with long-COVID-19: insights from TUN-EndCOV Study. Front Cardiovasc Med. 2022;9:866113. doi: 10.3389/fcvm.2022.866113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pretorius E, Venter C, Laubsher GJ, Kotze MJ, Moremi K, Oladejo S, et al. Combined triple treatment of fibrin amyloid microclots and platelet pathology in individuals with long COVID/post-acute sequelae of COVID-19 (PASC) can resolve their persistent symptoms. Res Square. 2022;21(1):148. doi: 10.21203/rs.3.rs-1205453/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kooistra E, Heesakkers H, Pickkers P, Zegers M, van den Boogaard M. Long-term impairments are most pronounced in critically ill patients with COVID-19 with severe obesity. Am J Respir Crit Care Med. 2022;206(8):1037–1039. doi: 10.1164/rccm.202202-0376LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Su Y, Yuan D, Chen DG, Ng RH, Wang K, Choi J, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185(5):881–895. doi: 10.1016/j.cell.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Florencio LL, Fernández-de-Las-Peñas C. Long COVID: systemic inflammation and obesity as therapeutic targets. Lancet Respir Med. 2022;10(8):726–727. doi: 10.1016/S2213-2600(22)00159-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Khunti K, Davies MJ, Kosiborod MN, Nauck MA. Long COVID-metabolic risk factors and novel therapeutic management. Nat Rev Endocrinol. 2021;17(7):379–380. doi: 10.1038/s41574-021-00495-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Armando F, Beythien G, Kaiser FK, Allnoch L, Heydemann L, Rosiak M, Becker S, Gonzalez-Hernandez M, Lamers MM, Haagmans BL, et al. SARS-CoV-2 omicron variant causes mild pathology in the upper and lower respiratory tract of hamsters. Nat Commun. 2022;12(1):3519. doi: 10.1038/s41467-022-31200-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mahase E. Covid-19: hospital admission 50–70% less likely with omicron than delta, but transmission a major concern. BMJ. 2021;375:N3151. doi: 10.1136/bmj.n3151. [DOI] [PubMed] [Google Scholar]
- 127.Magnusson K, Kristoffersen DT, Dell’Isola A, Kiadaliri A, Turkiewicz A, Runhaar J, Bierma-Zeinstra S, Englund M, Magnus PM, Kinge JM. Post-covid medical complaints following infection with SARS-CoV-2 omicron vs delta variants. Nat Commun. 2022;13(1):7363. doi: 10.1038/s41467-022-35240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.European Congress of Microbiology & Infectious Diseases (2022) Different SARS-CoV-2 variants may give rise to different long COVID symptoms, study suggests. https://www.eurekalert.org/news-releases/947495
- 129.Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21(3):133–146. doi: 10.1038/s41579-022-00846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tsang NNY, So HC, Cowling BJ, Leung GM, Ip DKM. Effectiveness of BNT162b2 and CoronaVac COVID-19 vaccination against asymptomatic and symptomatic infection of SARS-CoV-2 omicron BA.2 in Hong Kong: a prospective cohort study. Lancet Infect Dis. 2022 doi: 10.1016/S1473-3099(22)00732-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Woodbridge Y, Amit S, Huppert A, Kopelman NM. Viral load dynamics of SARS-CoV-2 delta and omicron variants following multiple vaccine doses and previous infection. Nat Commun. 2022;13(1):6706. doi: 10.1038/s41467-022-33096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nehme M, Vetter P, Chappuis F, Kaiser L, Guessous I, CoviCare Study Team Prevalence of post-COV condition 12 weeks after omicron infection compared to negative controls and association with vaccination status. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac947. [DOI] [PubMed] [Google Scholar]
- 133.Grobbelaar LM, Kruger A, Venter C, Burger EM, Laubscher GJ, Maponga TG, Kotze MJ, Kwaan HC, Miller JB, Fulkerson D, et al. Relative hypercoagulopathy of the SARS-CoV-2 beta and delta variants when compared to the less severe omicron variants is related to TEG parameters, the extent of fibrin amyloid microclots, and the severity of clinical illness. Semin Thromb Hemost. 2022;48(7):858–868. doi: 10.1055/s-0042-1756306. [DOI] [PubMed] [Google Scholar]
- 134.Skow RJ, Nandadeva D, Grotle AK, Stephens BY, Wright AN, Fadel PJ. Impact of breakthrough COVID-19 cases during the omicron wave on vascular health and cardiac autonomic function in young adults. Am J Physiol Heart Circ Physiol. 2022;323(1):H59–H64. doi: 10.1152/ajpheart.00189.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.