Abstract
Background:
Alcohol-associated liver disease (ALD) is a major chronic liver disease around the world without successful treatment. Acute alcoholic hepatitis is one of the most severe forms of ALD with high mortality, which is often associated with binge drinking. Alcohol drinking dysregulates lipid metabolism, increases adipose tissue lipolysis, and induces liver steatosis and adipose tissue atrophy. Increasing evidence implicates that crosstalk of liver and adipose tissue in the pathogenesis of ALD. Mechanistic target of rapamycin (mTOR) is a phosphatidylinositol 3-kinase (PI3K)-like serine/threonine protein kinase that regulates lipid metabolism, cell proliferation and autophagy. However, the role of mTOR in regulating adipose-liver crosstalk in binge drinking-induced organ damage remains unclear.
Methods:
We generated liver-specific and adipocyte-specific regulatory-associated protein of mTOR (Rptor) knockout (RptorLKO and RptorAKO) as well as Mtor knockout (MtorLKO and MtorAKO) mice, by crossing Rptorflox and Mtorflox mice with albumin Cre or adiponectin Cre mice, respectively. In addition, we generated liver and adipocyte double deletion of Rptor or Mtor (MtorLAKO and RptorLAKO) mice. The knockout mice with their matched wild-type littermates (RptorWT and MtorWT) were subjected to acute gavage of 7 g/kg ethanol.
Results:
Mice with adipocyte deletion of Rptor or Mtor developed hepatomegaly and adipose tissue atrophy. Alcohol gavage increased liver injury, hepatic steatosis and inflammation in mouse livers as demonstrated by elevated serum alanine aminotransferase activities, increased hepatic levels of triglyceride and increased hepatic numbers of CD68 positive macrophages in mouse livers after alcohol gavage. Liver injury was further exacerbated by deletion of adipocyte Rptor or Mtor. Serum adipokine array analysis revealed that increased levels of pro-inflammatory cytokines IL-6 and TNFα as well as chemokine MCP-1 following acute alcohol gavage in wild-type mice, which were further increased in adipocyte-specific Mtor or Rptor knockout mice. Conversely, levels of anti-inflammatory cytokine IL-10 decreased in adipocyte-specific Mtor or Rptor knockout mice. The levels of circulating fibroblast growth factor 21 (FGF21) increased whereas levels of circulating adiponectin and fetuin A decreased in wild-type mice after alcohol gavage. Intriguingly, adipocyte-specific Mtor or Rptor knockout mice already had decreased basal level of FGF21 which increased by alcohol gavage. Moreover, adipocyte-specific Mtor or Rptor knockout mice already had increased basal level of adiponectin and decreased fetuin A which were not further changed by alcohol gavage.
Conclusions:
Adipocyte but not hepatocyte ablation of Mtor pathway contributes to acute alcohol-induced liver injury with increased inflammation. Our results demonstrate the critical role of adipocyte mTOR in regulating the adipose-liver crosstalk in ALD.
Keywords: Alcohol-associated liver disease (ALD), Liver injury, Mechanistic target of rapamycin (mTOR), Adipose atrophy, Adipokine, Liver-adipose tissue crosstalk
1. Introduction
Alcohol-associated liver disease (ALD) is a major chronic liver disease around the world without successful treatment. The pathogenesis of ALD is characterized by simple steatosis, which can progress to more severe alcoholic hepatitis, fibrosis, cirrhosis, and eventually hepatocellular carcinoma.1–3 Acute alcoholic hepatitis is one of the most severe forms of ALD with high mortality, which is often associated with binge drinking.1 Alcohol consumption can lead to the damage of multiple organs, including the liver, pancreas, lung, kidney, adipose, and brain.4 Chronic alcohol consumption dysregulates lipid metabolism, increases adipose tissue lipolysis, and induces liver steatosis and adipose tissue atrophy. This suggests a critical role of liver-adipose axis in the pathogenesis of ALD.5,6 Moreover, chronic ethanol increases cytochrome P450 2E1 and aldehyde dehydrogenase (ALDH) activity in adipose tissues, which contributes to adipose tissue inflammation and the clearance of toxic metabolites in adipose tissue.7,8 Binge drinking induces hepatic steatosis, however, studies on the effect of binge drinking on adipose tissue are scarce.9
Mechanistic target of rapamycin (mTOR) is a phosphatidylinositol 3-kinase (PI3K)-like serine/threonine protein kinase that regulates lipid metabolism, cell proliferation and autophagy.5,10,11 mTOR is the catalytic core subunit of two distinct multiprotein complexes: mTOR complex 1 (mTORC1) and mTORC2. mTORC1 and mTORC2 share several subunits: mTOR, DEP domain-containing mTOR-interacting protein (DEPTOR) and mammalian lethal with SEC13 protein 8 (mLST8). These two complexes also have distinct components: regulatory-associated protein of mTOR (RPTOR) and protein kinase B (AKT)/PKB substrate 40 kDa (PRAS40) in mTORC1 and Raptor-independent companion of mTOR (RICTOR), protein observed with Rictor-1 and Rictor-2 (PROTOR 1/2) and mammalian stress-activated protein kinase-interacting protein (mSin1) in mTORC2. Pharmacological and genetic activation of mTOR promotes lipogenesis and suppresses lipolysis.10,11 Chronic alcohol consumption altered mTOR signaling in adipose tissues and livers as well as lead to adipose atrophy and liver injury.5,12 Adipocyte deletion of Rptor exacerbated adipose atrophy and liver injury in chronic alcohol-fed mice, suggesting adipocyte mTOR may play a critical role in liver-adipose tissue crosstalk in chronic alcohol-induced organ damage.5 Our previous study showed that acute alcohol binge drinking also altered hepatic mTOR signaling.13 However, the tissue-specific role of mTOR in regulating liver-adipose tissue crosstalk in binge drinking-induced organ damage remains unclear.
In this study, we investigated the role of mTOR in regulating liver-adipose tissue crosstalk in an alcohol binge drinking model using mice with hepatic and/or adipocyte deletion of Rptor or Mtor.
2. Materials and methods
2.1. Ethical approval
All procedures were approved by the Institutional Animal Care and Use Committee of the University of Kansas Medical Center (21–05-175).
2.2. Animals
Mtorflox (#011009), Rptorflox (#013188), Albumin-cre (#003574) and Adipoq-cre (#010803) mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). To generate liver-specific Mtor knockout (KO) (MtorLKO) or Rptor KO (RptorLKO) mice, Mtorflox or Rptorflox mice were crossed with Albumin-cre mice. To generate adipose-specific Mtor KO (MtorAKO) or Rptor KO (RptorAKO) mice, Mtorflox or Rptorflox mice were crossed with Adipoq-cre mice. To generate liver- and adipose-specific Mtor DKO (MtorLAKO) or Rptor DKO (RptorLAKO) mice, MtorLKO mice were crossed with MtorAKO mice or RptorLKO mice were crossed with RptorAKO mice. Cre negative mice were used as wild-type control (MtorWT and RptorWT). For the acute ALD model, 8–10-week-old male mice were treated with 7 g/kg ethanol by oral gavage and control group mice were gavaged with maltose for 6 h. Mice were specific pathogen free and maintained in a barrier rodent facility under standard experimental conditions.
2.3. Hepatic triglyceride (TG) and cholesterol analysis
Hepatic lipid extraction was performed as described previously.14 Frozen liver tissues (20–50 mg) were grinded into powder using a mortar and pestle followed by chloroform-methanol extraction. TG and cholesterol analysis of lipid extracts was performed using GPO-Triglyceride Reagent Set and Cholesterol liquid reagent (#T7532 and #C7510 respectively, Pointe Scientific) following the manufacturer’s instruction.
2.4. Serum biochemistry analyses
Blood samples were collected by cardiac puncture and sera were obtained by centrifugation. Serum alanine aminotransferase (ALT) activity (#A7526, Pointe Scientific), TG and cholesterol were measured by using commercially available kits following the manufacturer’s instruction as described previously.15
2.5. Histology and immunohistochemistry analyses
Livers and adipose tissues were fixed in 10% neutral formalin followed by paraffin embedding. Paraffin-embedded liver sections (5 μm) were stained with hematoxylin and eosin (H&E) for pathological evaluation. Immunostaining for F4/80 (#14–4801-82, Thermo Fisher) and CD68 (#137002, Biolegend) positive macrophages were performed as described previously.16
2.6. Protein extract and immunoblot analyses
Total liver proteins were extracted using radio-immunoprecipitation assay (RIPA) buffer (1% Igepal CA-630, 0.5% sodium deoxycholate, 0.1% sodium dodecyl (lauryl) sulfate (SDS) in phosphate buffered saline) supplemented with protease inhibitor cocktail (Bimake). Protein (30 μg) was separated on a SDS-PAGE gel and transferred to a PVDF membrane. Membranes were probed with appropriate primary and secondary antibodies and visualized with SuperSignal plus chemiluminescent substrate (Thermo Fisher Scientific). Densitometry analysis was performed with Image J and normalized to β-actin. Primary antibodies anti-FGF21 (# ab64857, Abcam) and anti-β-actin (#A-5441, Sigma) were used in this study.
2.7. Serum adipokine array analysis
Equal amount of serum from each treatment group was pooled and 80 μL of pooled sera were subjected to adipokine array analysis using Proteome Profiler Mouse Adipokine Array Kit (#ARY013, R&D Systems) following the manufacture’s instruction.
2.8. Statistical analysis
All graphs and data were generated and analyzed using Graphpad Prism 8. All experimental data are expressed as the mean ± standard error of the mean (SEM) and subjected to one-way analysis of variance (ANOVA) with Turkey post hoc test (multi-group comparisons). P-value <0.05 was considered as significant.
3. Results
3.1. Acute alcohol exposure leads to steatosis and liver injury and are exacerbated by adipocyte deletion of Mtor in mice
Our previous study showed that alcohol exposure altered mTOR signaling in the liver and adipose tissue.5,13 To examine the effect of the mTOR pathway in the liver and adipose tissue of acute alcohol-treated mice, we established Mtor wild-type (MtorWT), liver-specific Mtor KO (MtorLKO), adipocyte-specific Mtor KO (MtorAKO) as well as liver- and adipocyte-specific Mtor KO (MtorLAKO) mice. The mice were treated with alcohol binge for 6 h. All of the mice developed steatosis as shown by H&E staining with increased hepatic triglyceride (TG), but not cholesterol in mouse livers after acute alcohol exposure (Fig. 1A and B). Levels of hepatic TG in MtorLKO mice were comparable with MtorWT mice. Intriguingly, TG levels further increased in alcohol-treated MtorAKO and MtorLAKO mouse livers but were similar in MtorAKO and MtorLAKO mice (Fig. 1A and B). Moreover, MtorAKO and MtorLAKO mice developed hepatomegaly with or without alcohol gavage (Fig. 1C). Similar to the steatosis data, acute alcohol exposure induced liver injury with increased serum alanine aminotransferase (ALT) activity in MtorWT and MtorLKO mice, which further increased in MtorAKO and MtorLAKO mice (Fig. 1D). All these data suggested that acute alcohol exposure leads to steatosis and liver injury which are exacerbated by the deletion of adipocyte but not hepatic Mtor.
Fig. 1. Acute alcohol exposure leads to steatosis and liver injury and MtorAKO and MtorLAKO mice have exacerbated liver injury.
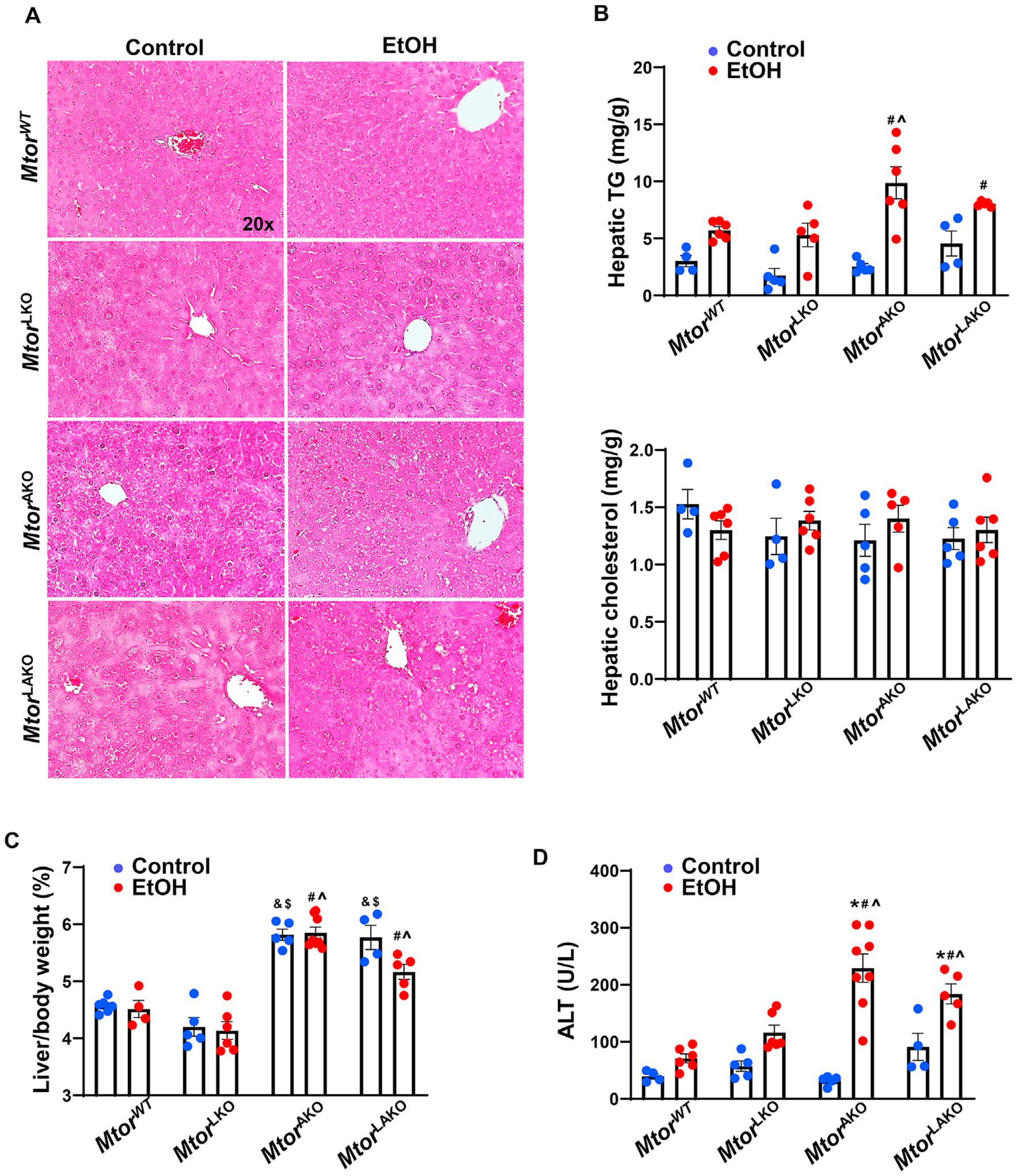
Mtor wild-type (MtorWT), liver-specific Mtor KO (MtorLKO), adipocyte-specific Mtor KO (MtorAKO) as well as liver- and adipocyte-specific Mtor KO (MtorLAKO) mice were generated by crossing floxed Mtor mice with albumin Cre or adiponectin Cre mice. The mice were treated with ethanol 7 g/kg or equal amount water by oral gavage for 6 h. (A) Representative images of H&E staining of the livers. (B) Hepatic TG and cholesterol were quantified. (C) Liver/body weight ratio was quantified. (D) Serum ALT activities were measured. Data are expressed as means ± SEM (n = 4 to 8) and subjected to one-way ANOVA with Turkey post hoc test. *P < 0.05 EtOH vs. control; #P < 0.05 vs. WT EtOH; ^P < 0.05 vs. LKO EtOH; &P < 0.05 vs. WT control; $P < 0.05 vs. LKO control. Original magnification, × 20. Abbreviations: ALT, alanine aminotransferase; EtOH, ethanol; H&E, hematoxylin and eosin; KO, knockout; mTOR, mechanistic target of rapamycin; TG, triglyceride; WT, wild-type.
3.2. Adipocyte deletion of Mtor but not acute alcohol binge leads to adipose atrophy in mice
We did not find significant changes in the serum levels of TG and cholesterol in MtorWT and MtorLKO mice with or without alcohol gavage. While no significant differences in serum levels of TG and cholesterol were found in MtorWT and MtorAKO or MtorLAKO mice gavaged with maltose, serum levels of cholesterol were significantly increased in MtorLAKO mice in alcohol gavaged mice compared with their respective maltose gavaged mice (Fig. 2A). White adipose tissue (WAT) and brown adipose tissue (BAT) mass slightly increased but not significantly in MtorWT mice after alcohol gavage (Fig. 2B). However, compared to MtorWT control mice, MtorAKO and MtorLAKO control mice had approximately 20–25% decreased epididymal WAT (eWAT), 40–50% decreased inguinal WAT (iWAT), and 50–60% decreased interscapular BAT (iBAT) mass. iBAT further decreased by 32% in MtorAKO mice after alcohol gavage compared to their control mice. eWAT and iBAT decreased by 25% and 45% in MtorLAKO mice after alcohol gavage compared to their control mice (Fig. 2B). The sizes of eWAT adipocytes were similar in MtorWT and MtorLKO mice with or without alcohol (Fig. 2C and D). Although the adipose mass was smaller in MtorAKO and MtorLAKO mice, the sizes of eWAT adipocytes of MtorAKO and MtorLAKO mice were similar to MtorWT and MtorLKO mice with or without alcohol gavage (Fig. 2C and D). Together, these data indicate that loss of adipocyte Mtor but not acute alcohol consumption may lead to adipose atrophy in mice.
Fig. 2. Adipocyte deletion of Mtor leads to adipose atrophy in mice.
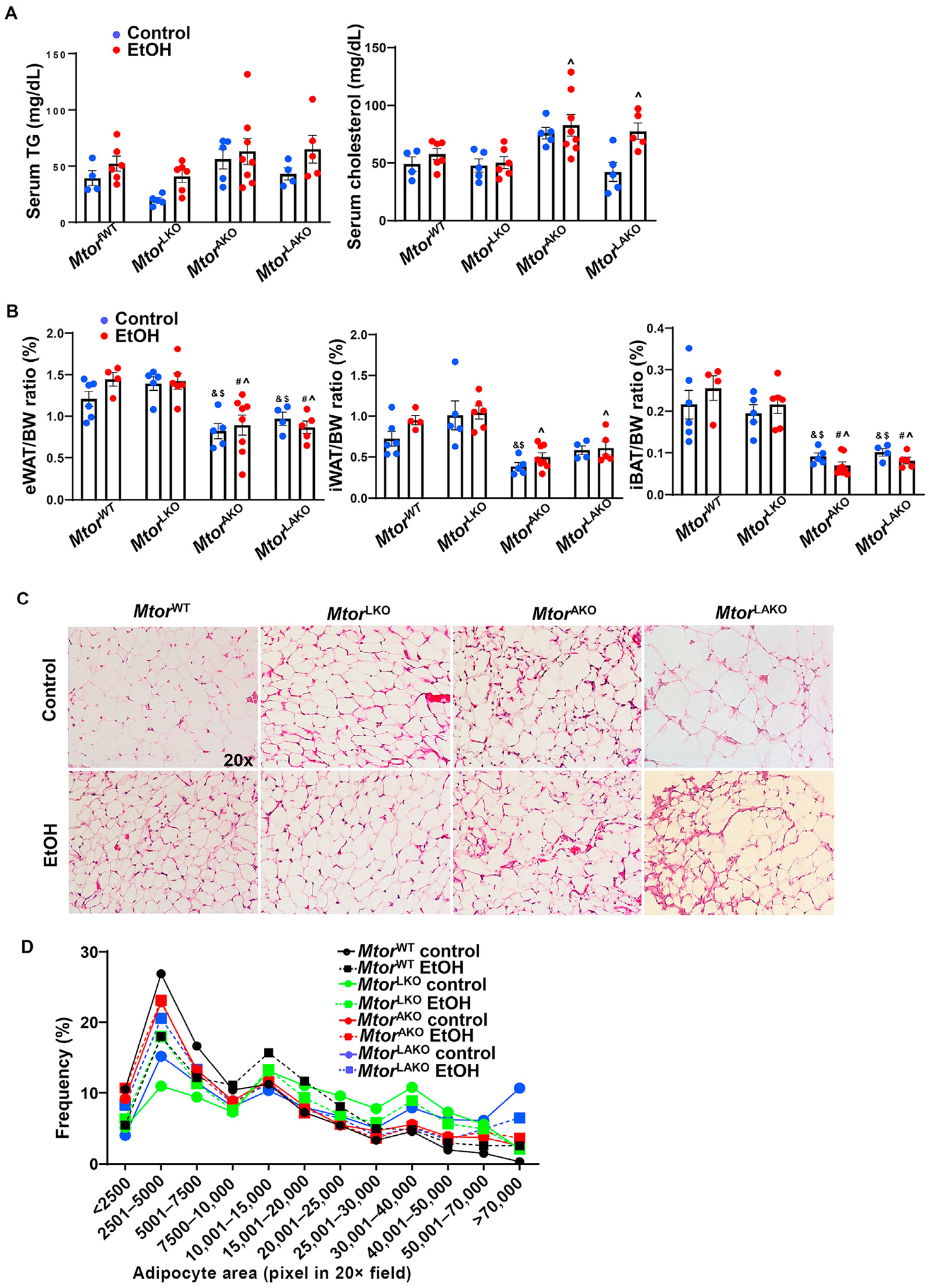
Mice were treated as in Fig. 1. (A) TG and cholesterol levels were measured. (B) Adipose tissue/BW ratio was quantified. (C) Representative images of H&E staining of eWAT. (D) Distribution of adipocyte area of eWAT was analyzed. Around 1191–2294 adipocytes from 2 to 3 mice in each group were quantified. Data are expressed as means ± SEM (n = 4 to 8, A, B) and subjected to one-way ANOVA with Turkey post hoc test. #P < 0.05 vs. WT EtOH; ^P < 0.05 vs. LKO EtOH; &P < 0.05 vs. WT control; $P < 0.05 vs. LKO control. Original magnification, × 20. Abbreviations: BW, body weight; EtOH, ethanol; eWAT, epididymal white adipose tissue; H&E, hematoxylin and eosin; iBAT, interscapular brown adipose tissue; iWAT, inguinal white adipose tissue; KO, knockout; mTOR, mechanistic target of rapamycin; TG, triglyceride; WT, wild-type.
3.3. The effects of adipocyte-deletion of Mtor on acute alcohol gavaged-induced inflammation and changes of adipokines and cytokines in mice
As we found that adipocyte but not hepatic deletion of Mtor exacerbated acute alcohol-induced steatosis and liver injury in mice, we next tried to determine the underlying mechanisms. As shown in Fig. 3A and B, there was no significant induction of macrophage marker F4/80 in MtorWT and MtorLAKO mouse livers in response to alcohol. However, F4/80 increased in MtorLKO mouse livers and decreased in MtorAKO mouse livers after acute alcohol binge. In contrast to F4/80 staining data, alcohol increased CD68 staining in all groups (Fig. 3C and D).
Fig. 3. Acute alcohol exposure increases inflammation and altered adipokines in MtorAKO mice.
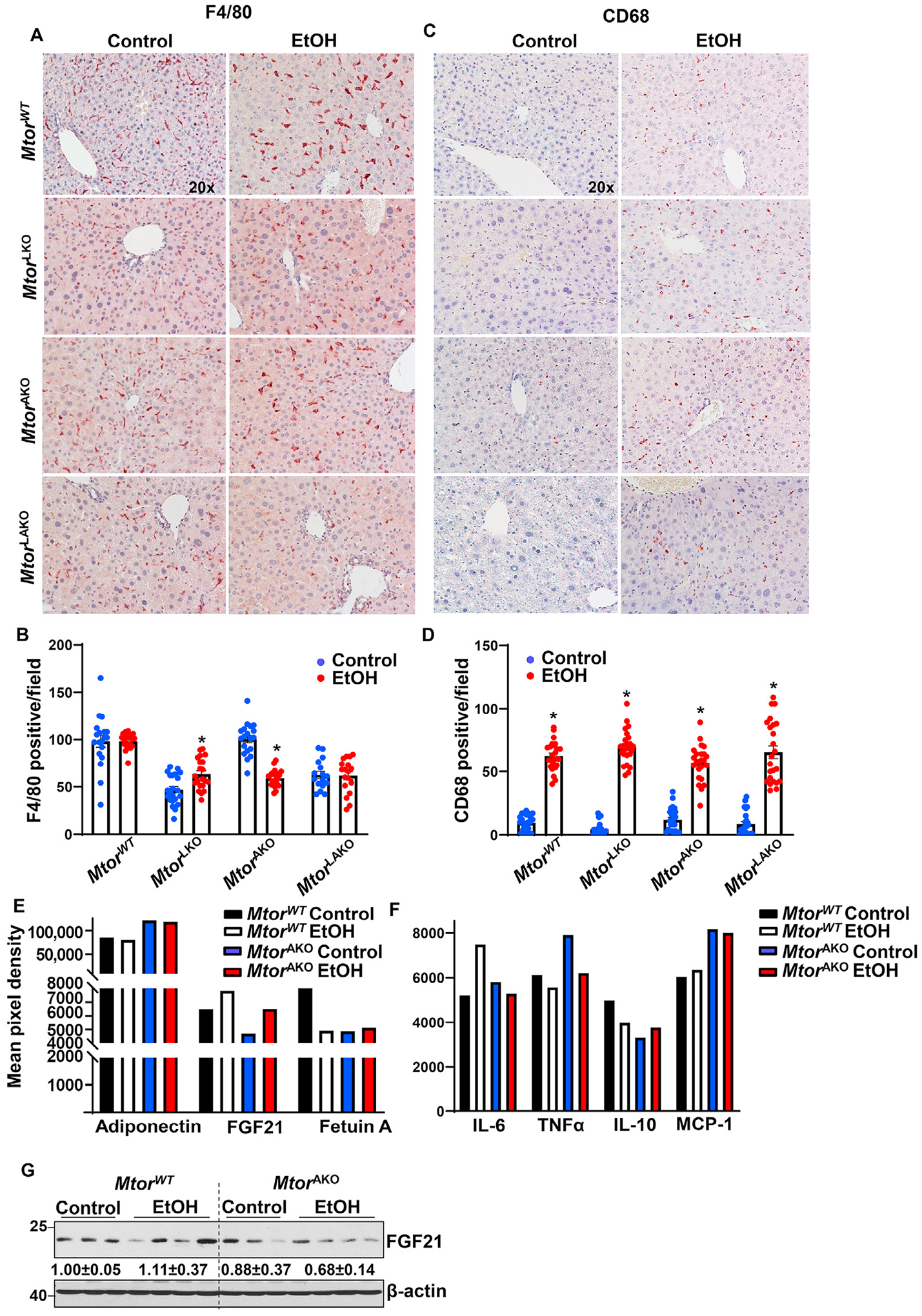
Mice were treated as in Fig. 1. (A) F4/80 was stained in the livers by immunohistochemistry staining and (B) F4/80 positive macrophages were quantified. (C) CD68 was stained in the livers by immunohistochemistry staining and (D) CD68 positive macrophages were quantified. (E, F) Equal amount of serum from the same group was pooled. Circulating adipokines (E) and inflammatory mediators (F) were analyzed by Proteome Profiler Mouse Adipokine Array Kit. Data are expressed as means ± SEM and subjected to one-way ANOVA with Turkey post hoc test. n = 3 (A–D); n = 5 to 8 (E, F). *P < 0.05 EtOH vs. control. (G) Hepatic FGF21 levels in mice were measured by immunoblot analysis. Original magnification, × 20. Abbreviations: EtOH, ethanol; FGF21, fibroblast growth factor 21; IL-6, interleukin-6; IL-10, interleukin-10; KO, knockout; MCP-1, monocyte chemoattractant protein-1; mTOR, mechanistic target of rapamycin; TNFα, tumor necrosis factor alpha; TG, triglyceride; WT, wild-type.
To further characterize the mechanism by which adipocyte deletion of Mtor contributes to ALD, adipokine array was performed in MtorWT and MtorAKO mouse sera. Adiponectin, one of the major adipokines, regulates lipid and glucose metabolism, promotes insulin sensitivity, and plays a protective role in various metabolic scenarios.17 MtorAKO mice had higher adiponectin compared to MtorWT mice after maltose gavage, but there were no significant changes after alcohol gavage in both MtorAKO mice and MtorWT mice (Fig. 3E). The levels of serum FGF21 were lower in MtorAKO mice in the control group but increased after alcohol gavage (Fig. 3E). The levels of circulating fetuin A, another adipokine and hepatokine that inhibits adiponectin, were lower in MtorWT mice in response to alcohol (Fig. 3E). The levels of circulating fetuin A in MtorAKO mice were lower compared with MtorWT mice regardless of alcohol gavage. On the other hand, alcohol gavage increased levels of pro-inflammatory cytokines and chemokine interleukin-6 (IL-6). It did not affect the levels of serum tumor necrosis factor alpha (TNFα) and monocyte chemoattractant protein-1 (MCP-1) but decreased anti-inflammatory cytokine interleukin-10 (IL-10) in MtorWT mice (Fig. 3F). Adipocyte deletion of Mtor increased levels of TNFα, MCP1 and IL-6 with or without alcohol gavage (Fig. 3F). Hepatic FGF21 levels increased in MtorWT mice by alcohol (Fig. 3G). Hepatic FGF21 levels were lower in MtorAKO control mice and further decreased by alcohol (Fig. 3G). Taken together, acute alcohol binge increases hepatic inflammation and the secretion of FGF21 but decreases the secretion of fetuin A in WT mice. Loss of adipose Mtor decreases the secretion of fetuin A but increases serum levels of TNFα and MCP-1, which are not affected by alcohol binge drinking.
3.4. Loss of adipocyte but not hepatic Rptor exacerbates acute alcohol gavage-induced liver injury
As mTOR exists in both mTORC1 and mTORC2, to determine whether the disruption of mTORC1 or mTORC2 would contribute to the detrimental effect on ALD, we established Rptor wild-type (RptorWT), liver-specific Rptor KO (RptorLKO), adipocyte-specific Rptor KO (RptorAKO) as well as liver- and adipocyte-specific Rptor KO (RptorLAKO) mice, which would only disrupt mTORC1 without affecting mTORC2. Similar to mTOR deletion mice, deletion of either adipocyte or liver Raptor did not affect hepatic steatosis after acute alcohol gavage as demonstrated by the accumulation of lipid droplets shown by H&E staining and hepatic TG but not cholesterol contents in mouse livers (Fig. 4A and B). RptorAKO and RptorLAKO mice also developed hepatomegaly with or without alcohol gavage (Fig. 4C). Acute alcohol increased liver injury with elevated serum ALT activity in RptorWT mice, which was further increased in RptorAKO and RptorLAKO mice but not RptorLKO mice (Fig. 4D).
Fig. 4. Acute alcohol exposure leads to steatosis and liver injury and RptorAKO and RptorLAKO mice have exacerbated liver injury.
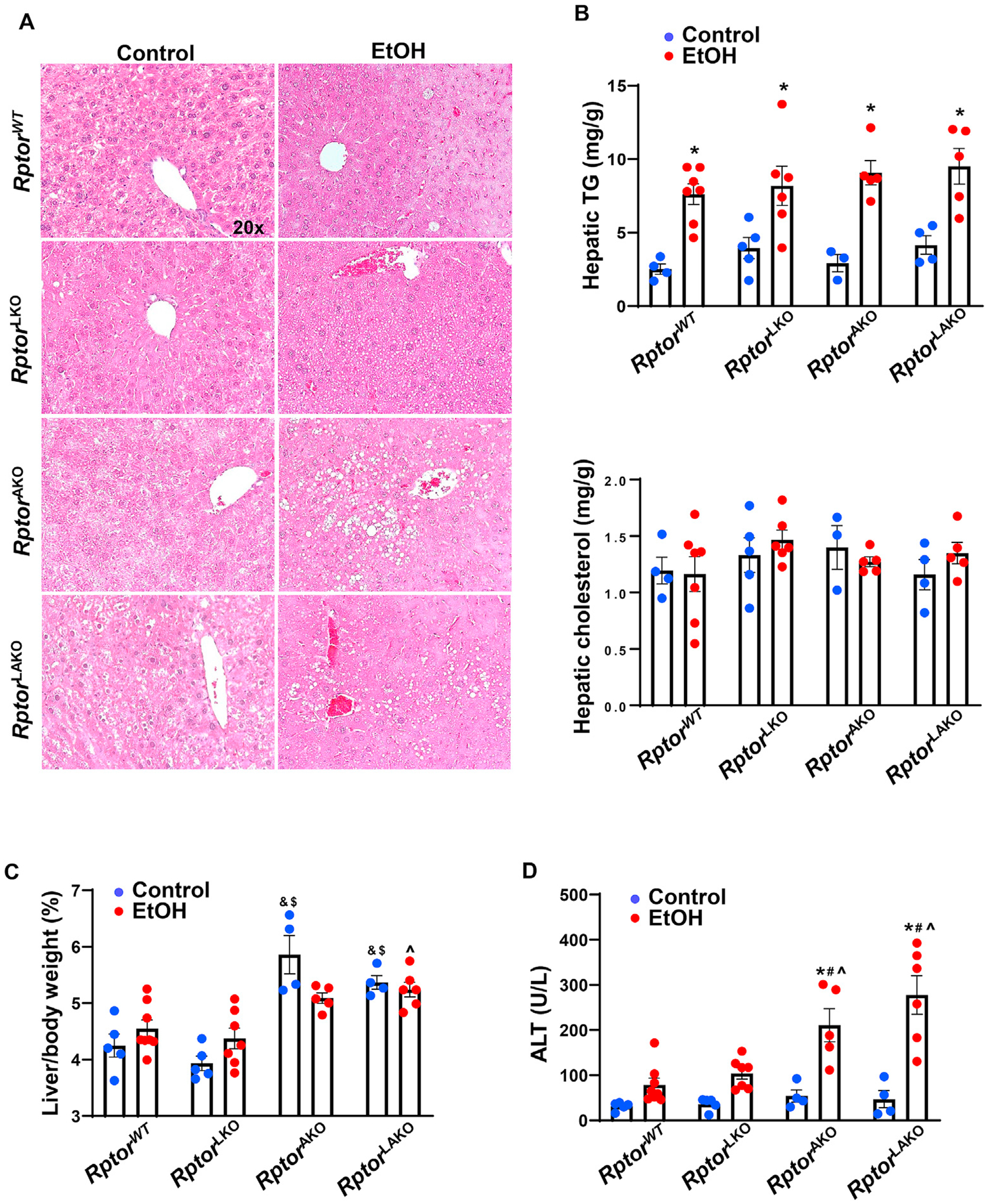
Rptor wild-type (RptorWT), liver-specific Rptor KO (RptorLKO), adipocyte-specific Rptor KO (RptorAKO) as well as liver- and adipocyte-specific Rptor KO (RptorLAKO) mice were generated by crossing floxed Rptor mice with albumin Cre or adiponectin Cre mice. The mice were treated with ethanol 7 g/kg or equal amount of water by oral gavage for 6 h. (A) Representative images of H&E staining of the livers. (B) Hepatic TG and cholesterol were quantified. (C) Liver/body weight ratio was quantified. (D) Serum ALT activities were measured. Data are expressed as means ± SEM (n = 4 to 7) and subjected to one-way ANOVA with Turkey post hoc test. *P < 0.05 EtOH vs. control; #P < 0.05 vs. WT EtOH; ^P < 0.05 vs. LKO EtOH; &P < 0.05 vs. WT control; $P < 0.05 vs. LKO control. Original magnification, × 20. Abbreviations: ALT, alanine aminotransferase; EtOH, ethanol; H&E, hematoxylin and eosin; KO, knockout; Rptor, regulatory-associated protein of mTOR; TG, triglyceride; WT, wild-type.
3.5. Adipocyte deletion of Rptor leads to adipose atrophy in mice
Acute alcohol binge slightly increased levels of serum TG in RptorWT mice (Fig. 5A). However, RptorAKO and RptorLAKO mice had much higher serum levels of TG compared to RptorWT and RptorLKO mice after alcohol gavage (Fig. 5A). RptorAKO and RptorLAKO mice had approximately 50% decreased eWAT, 40–60% decreased iWAT and 40–50% decreased iBAT mass compared to RptorWT mice (Fig. 5B). Alcohol gavage did not add more synergistic effects on adipose mass except that alcohol further decreased eWAT mass in RptorAKO and RptorLAKO mice compared to RptorWT mice (Fig. 5B). The sizes of eWAT adipocytes and fat mass were similar in RptorWT and RptorLKO mice with or without alcohol gavage (Fig. 5C and D). Interestingly, the average sizes of eWAT adipocytes were around 20% smaller in RptorAKO and RptorLAKO control mice compared to RptorWT and RptorLKO mice. Notably, the sizes of adipocytes in eWAT of RptorAKO and RptorLAKO mice were heterogeneous with increased number of smaller or larger adipocytes than RptorWT adipocytes, which was different from the adipocyte deletion of Mtor in mice (Fig. 5C and D). In addition to the differences of basal adipocyte size, alcohol gavage further increased the number of smaller or larger adipocytes compared to their control littermates gavaged with maltose (Fig. 5C and D). These data suggested that adipocyte deletion of Rptor leads to epididymal adipose atrophy, which is further exacerbated by alcohol in mice.
Fig. 5. Adipocyte deletion of Rptor leads to adipose atrophy and is further altered by alcohol in mice.
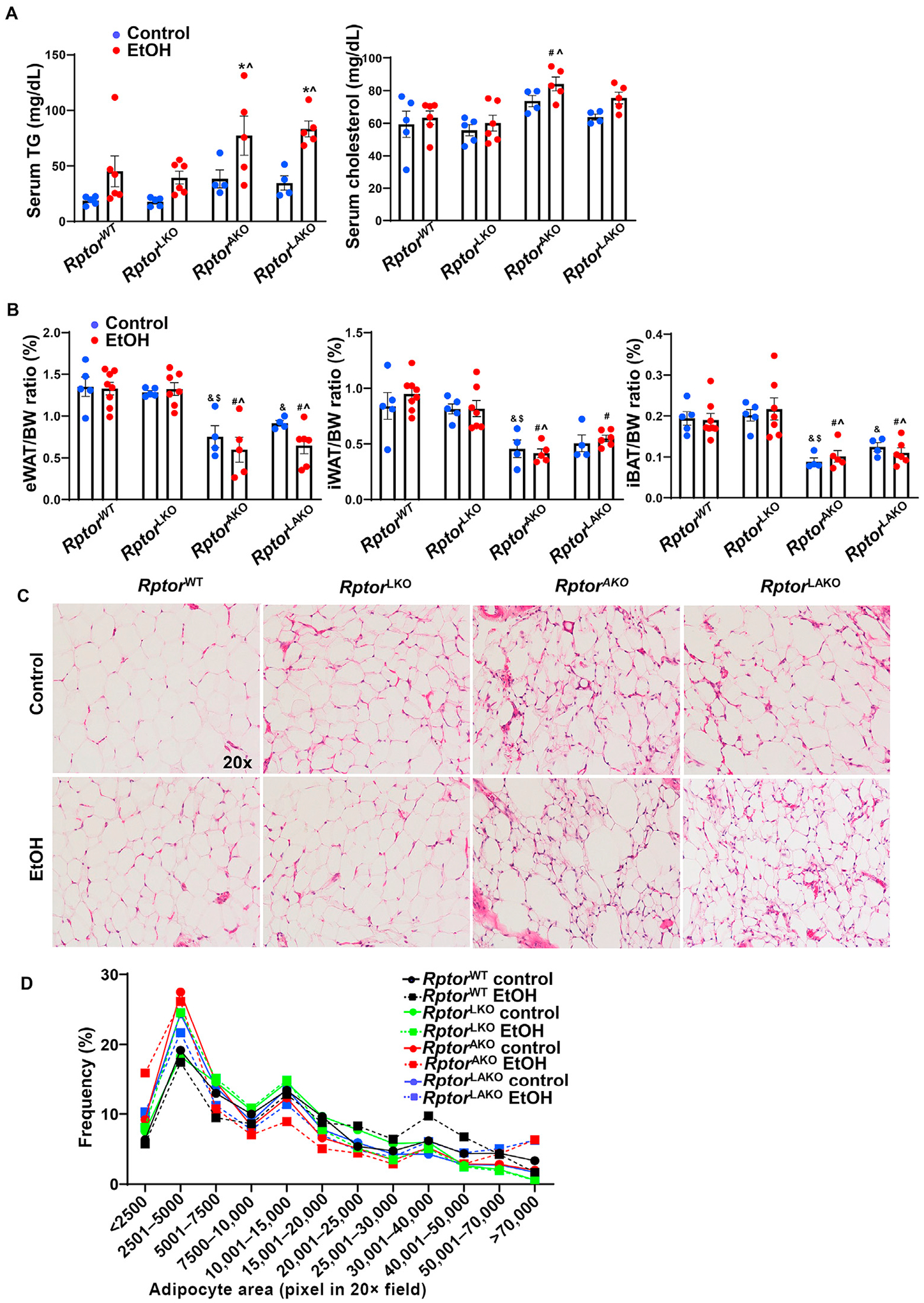
Mice were treated as in Fig. 4. (A) Serum triglyceride (TG) and cholesterol levels were measured. (B) Adipose tissue/BW ratio was quantified. (C) Representative images of H&E staining of eWAT. (D) Distribution of adipocyte area of eWAT was analyzed. Around 1783–3207 adipocytes from 2 to 4 mice in each group were quantified. Data are expressed as means ± SEM (n = 4 to 7, A, B) and subjected to one-way ANOVA with Turkey post hoc test. *P < 0.05 EtOH vs. control; #P < 0.05 vs. WT EtOH; ^P < 0.05 vs. LKO EtOH; &P < 0.05 vs. WT control; $P < 0.05 vs. LKO control. Original magnification, × 20. Abbreviations: BW, body weight; EtOH, ethanol; eWAT, epididymal white adipose tissue; H&E, hematoxylin and eosin; iBAT, interscapular brown adipose tissue; iWAT, inguinal white adipose tissue; KO, knockout; Rptor, regulatory-associated protein of mTOR; TG, triglyceride; WT, wild-type.
3.6. The effects of adipocyte deletion of Rptor on acute alcohol gavage-induced inflammation and changes of serum adipokines and cytokines in mice
We found that the number of F4/80 positive cells decreased in RptorWT and RptorLAKO mice but did not change in alcohol gavaged RptorAKO and RptorLKO mice compared to maltose gavaged mice (Fig. 6A and B). However, alcohol gavage significantly increased the number of CD68 positive cells in all the genotypes except RptorLAKO mice (Fig. 6C and D). Notably, the number of CD68 positive cells was already significantly higher in RptorAKO and RptorLAKO mice only received maltose compared to RptorWT mice, suggesting possible increased basal level inflammation in RptorAKO and RptorLAKO mice.
Fig. 6. Acute alcohol exposure increases inflammation and altered adipokines in RptorAKO mice.
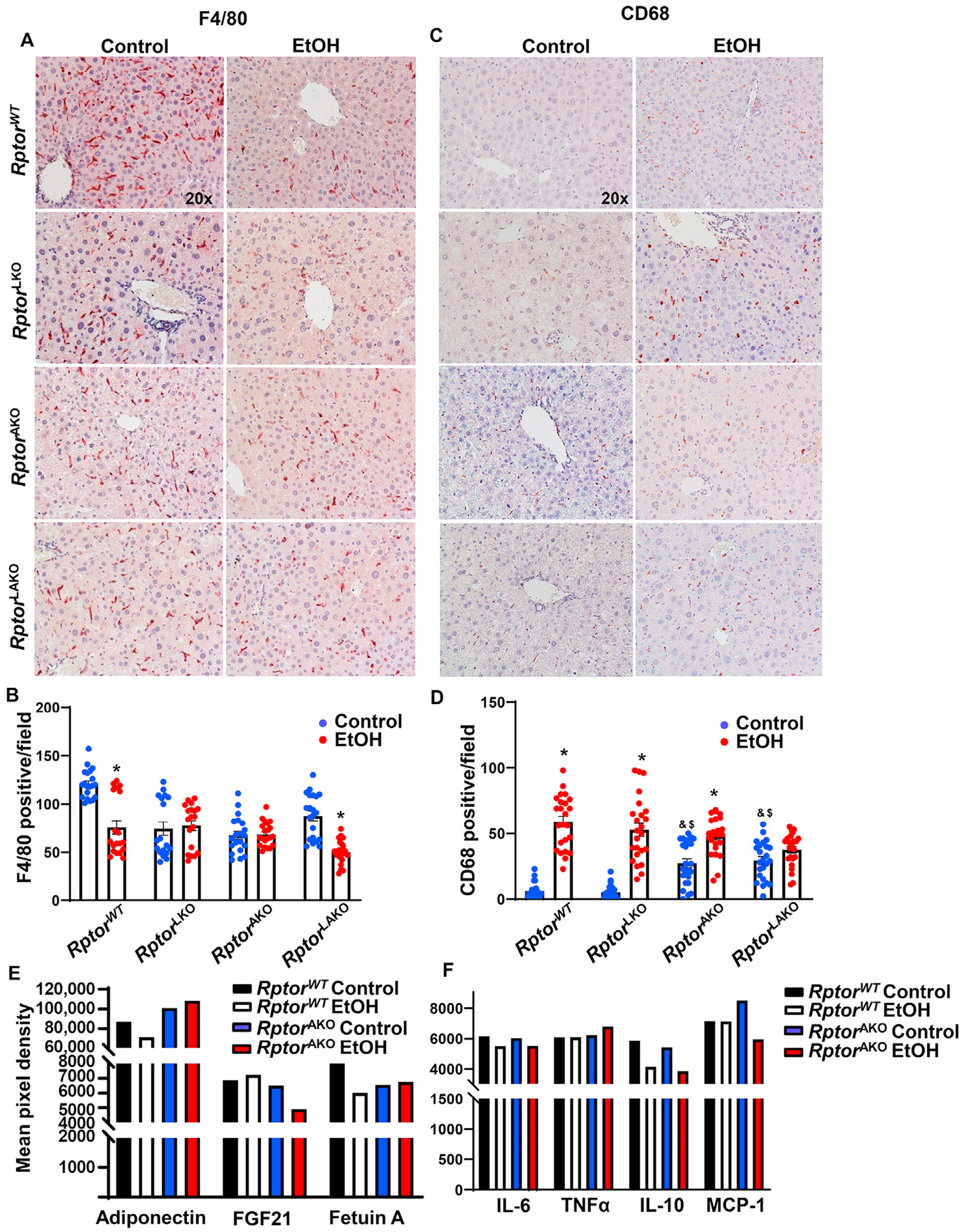
Mice were treated as in Fig. 4. (A) F4/80 was stained in the livers by immunohistochemistry staining and (B) F4/80 positive macrophages were quantified. (C) CD68 was stained in the livers by immunohistochemistry staining and (D) CD68 positive macrophages were quantified. (E, F) Equal amount of serum from the same group was pooled. Circulating adipokines (E) and inflammatory mediators (F) were analyzed by Proteome Profiler Mouse Adipokine Array Kit. Data are expressed as means ± SEM and subjected to one-way ANOVA with Turkey post hoc test. n = 3 (A–D); n = 4–7 (E–F). *P < 0.05 EtOH vs. control; #P < 0.05 vs. WT EtOH; ^P < 0.05 vs. LKO EtOH; &P < 0.05 vs. WT control; $P < 0.05 vs. LKO control. Original magnification, × 20. Abbreviations: EtOH, ethanol; FGF21, fibroblast growth factor 21; IL-6, interleukin-6; IL-10, interleukin-10; KO, knockout; MCP-1, monocyte chemoattractant protein-1; Rptor, regulatory-associated protein of mTOR; TNFα, tumor necrosis factor alpha; TG, triglyceride; WT, wild-type.
Adipokine array analysis showed that RptorAKO mice had higher levels of serum adiponectin but lower serum levels of FGF21 and fetuin A levels compared to RptorWT mice regardless of alcohol gavage (Fig. 6E). Serum FGF21 further decreased in RptorAKO mice after alcohol gavage (Fig. 6E). The serum levels of TNFα and IL-6 did not change with or without alcohol gavage in RptorWT and RptorAKO mice. Alcohol gavage decreased the levels of serum IL-10 in both RptorWT and RptorAKO mice. The levels of serum MCP-1 were slightly higher in RptorAKO mice but were decreased by alcohol gavage compared to RptorWT with or without alcohol gavage (Fig. 6F). Together, these data indicates that acute alcohol consumption may increase hepatic inflammation and alter the circulating hepatokines and cytokines, which are partially affected by the deletion of adipocyte Rptor.
4. Discussion
ALD is a burgeoning health problem worldwide without effective treatment. In this study, we characterized the effects of acute alcohol exposure on mTOR signaling in mouse livers and adipose tissues as well as liver-adipose tissue crosstalk in alcohol-induced steatosis and liver injury. Acute alcohol exposure induced hepatic steatosis and liver injury in mice as well as increased circulating FGF21, unchanged or slightly decreased adiponectin and decreased fetuin A. Hepatic deletion of Mtor or Rptor did not alter steatosis or liver injury. Adipocyte deletion of Mtor or Rptor significantly decreased adipose mass and further exacerbated alcohol-induced liver injury as well as decreased FGF21 and fetuin A but increased adiponectin. Double deletion of Mtor or Rptor in the liver and adipocyte had similar phenotype as single adipocyte deletion.
Alcohol exposure including both acute and chronic induces hepatic steatosis.12,13 Our present study confirmed that acute alcohol binge led to steatosis. mTOR is a nutrition sensor that regulates lipid metabolism, cell proliferation and autophagy.5,10,11 Acute alcohol binge inhibits mTOR activation, activates autophagy and ameliorates alcohol-induced liver injury in the livers.13 Inhibition of autophagy by the deletion of forkhead box O3 (FoxO3), a transcription factor which regulates autophagy, enhanced acute alcohol-induced steatosis and liver injury.9 However, the role of mTOR in acute alcohol binge is still unclear. In our present study, we found that genetic deletion of mTOR or Rptor in the liver did not protect against alcohol-induced steatosis and liver injury. Our current data suggested that the inhibition of mTOR in the liver is not sufficient to ameliorate alcohol-induced liver injury in an acute alcohol binge model. In contrast to the acute alcohol binge model, chronic alcohol feeding activates mTOR signaling which contributes to ALD.12,18 Chao et al.12 found that the activation of mTOR by alcohol-decreased transcription factor EB (TFEB) nuclear translocation and resulted in insufficient autophagy. Overexpression of TFEB or inhibition of mTOR by torin 1, an mTOR inhibitor, protected alcohol-induced steatosis and liver injury. On the other hand, Chen et al.18 reported that the activation of mTOR by alcohol-decreased DEPTOR, an mTORC1 negative regulator, and increased transcriptional activity of the lipogenic transcription factor sterol regulatory element-binding protein-1 (SREBP-1) and lipogenesis in the livers. Overexpression of DEPTOR ameliorated ALD.18 The exact role of the mTOR pathway in ALD still needs to be further characterized. Interestingly, our study further demonstrated that mice with adipocyte deletion of Mtor or Rptor exacerbated acute alcohol-induced liver injury. Loss of Mtor or Rptor in the liver combined with adipocytes had similar liver injury to adipocyte deletion of Mtor or Rptor. All these results suggested adipocyte mTOR signaling but not hepatic mTOR contributes to liver injury in mice in response to acute alcohol exposure in mice. Future studies are needed to further investigate the different mTOR response to acute or chronic alcohol exposure.
Genetic deletion of Rptor/mTORC1 in adipose tissue has smaller adipose tissue mass. The heterogeneous adipocyte size which is either larger or smaller than normal is likely due to increased lipolysis and decreased lipogenesis.5,19,20 Genetic deletion of Rictor/mTORC2 in adipose tissue has normal adipose tissue mass and distribution as well as normal adipocyte size.21 Ablation of mTOR in adipose which loses both mTORC1 and mTORC2 has a similar adipose phenotype to Rptor adipose deletion.22 Consistent with these previous studies, our study also demonstrated that adipocyte deletion of Mtor or Rptor decreased adipose tissue mass at basal level. Adipocyte deletion of Rptor but not Mtor also altered adipocyte sizes. Malnutrition is a major complication of ALD patients with clinical findings such as muscle wasting/skeletal muscle loss, lean body mass, and loss of subcutaneous fat.23,24 Malnutrition is also associated with the severity of ALD. Adipocyte-deletion of Mtor/Rptor mice had significantly decreased lean body mass and loss of adipose tissues which resembled some features with human ALD. Adipokines are important for adipose-liver and adipose-muscle crosstalk in ALD. However, whether loss of adipocyte Mtor/Rptor affects muscle strength needs to be further investigated.
How does ablation of mTOR signaling in adipose tissue impact alcohol-induced liver injury in an acute alcohol binge model? There is growing evidence to suggest that adipose tissue is a key regulator of metabolism other than an inert energy storage organ.6 It is well known that adipose tissue is an endocrine organ that actively synthesizes and secretes proteins (i.e., adipokines) and other inflammatory mediators (i.e., TNFα, IL-6) into the circulation to regulate the function of other tissues/organs in a paracrine manner. Adiponectin, one of the most abundant adipokines in adipose tissue, has an anti-steatotic effect on hepatocytes by enhancing free fatty acid oxidation, reducing free fatty acid influx and de novo lipogenesis in ALD.17 However, its response to alcohol exposure is still controversial. While most human studies demonstrated that adiponectin increased in human serum with alcohol consumption, many animal studies showed decreased adiponectin with alcohol consumption.25–28 Consistent with most of the previous studies, alcohol decreased circulating adiponectin in WT mice. Adipocyte deletion of Mtor or Rptor increased circulating adiponectin in mice. Fetuin A, a classic hepatokine, which has also recently become considered as an adipokine, is negatively correlated with adiponectin and promotes steatosis and fibrosis in metabolic liver diseases.29 The role of fetuin A in ALD is not well studied. Only one study has shown that moderate alcohol consumption lead to a decreased in serum fetuin A among women.30 Similar findings in alcohol binge mouse models showed that circulating fetuin A decreased. Our results suggested that increased adiponectin and decreased fetuin A are adaptive responses to alcohol. Circulating FGF21, another important adipokine and hepatokine and potential mediator of the liver-adipose tissue axis, increased in response to alcohol. The basal level of FGF21 was already low in mice with adipocyte deletion of Mtor or Rptor which further decreased in adipocyte deletion of Rptor by alcohol. These results were consistent with previous reported study that alcohol binge drinking increased circulating FGF21 in human and mice.31 Previous studies demonstrated that FGF21 has hepatoprotective effect in liver injury. FGF21 null mice developed increased alcohol-induced liver injury with increased inflammation, steatosis and fibrosis when supplied alcohol in drinking water, and show higher mortality than their wild-type counterparts when fed with Lieber-DeCarli diet.31,32 In addition to ALD model, FGF21 null mice exacerbated acetaminophen hepatoxicity and mortality.33 Decreased circulating FGF21 in adipocyte deletion of Mtor or Rptor mice may contribute to liver injury by alcohol binge. Previous study also demonstrated that FGF21 induces expression and secretion of adiponectin in adipocytes, and adiponectin is obligatory for FGF21 function in regulating glucose and lipid metabolism.34 In this study, circulating FGF21 reduced but adiponectin increased in MtorAKO or RptorAKO control mice (Figs. 3E and 6E). Furthermore, alcohol-induced circulating FGF21 but not adiponectin (Figs. 3E and 6E). All these data suggested that adiponectin secretion could be independent of FGF21. Future studies are needed to further dissect how alcohol alters adiponectin, fetuin A and FGF21 in either acute or chronic alcohol exposure and how altered basal levels of adiponectin, fetuin A and FGF21 affect the liver response to alcohol.
In human studies, it had been reported that women are enhanced vulnerability to develop alcohol-related diseases probably due to decreased gastric alcohol dehydrogenase activity which results in higher blood alcohol levels.35,36 However, gender differences of ALD in mice were controversial. One study demonstrated that female mice were susceptible to alcohol-induce liver injury, the other one found that there were no gender differences in mouse model.37,38 Overall, male mice were used in most ALD studies. However, gender differences in ALD mouse model need to be explored.
In conclusion, acute alcohol binge leads to steatosis and liver injury which is exacerbated by adipocyte deletion of Mtor or Rptor but not hepatic deletion of Mtor or Rptor. Decreased circulating FGF21 in adipocyte-specific Mtor or Rptor KO mice may contribute to elevated liver injury in response to acute alcohol. Targeting adipose mTOR signaling and adipocyte lipolysis could be a potential approach for improving ALD.
Acknowledgements
The research was supported in part by the USA NIDDK DK129234, NIAAA AA026904, and NIGMS P20GM144269 and P30GM118247 (to H.-M. Ni).
Footnotes
Declaration of competing interest
The authors declare that they have no conflict of interest.
References
- 1.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams JA, Manley S, Ding WX. New advances in molecular mechanisms and emerging therapeutic targets in alcoholic liver diseases. World J Gastroenterol. 2014;20:12908–12933. 10.3748/wjg.v20.i36.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagy LE, Ding WX, Cresci G, Saikia P, Shah VH. Linking pathogenic mechanisms of alcoholic liver disease with clinical phenotypes. Gastroenterology. 2016;150: 1756–1768. 10.1053/j.gastro.2016.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Wang S, Ni HM, Huang H, Ding WX. Autophagy in alcohol-induced multiorgan injury: mechanisms and potential therapeutic targets. Biomed Res Int. 2014, 498491. 10.1155/2014/498491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Chao X, Wang S, Williams JA, Ni HM, Ding WX. Role of mechanistic target of rapamycin and autophagy in alcohol-induced adipose atrophy and liver injury. Am J Pathol. 2020;190:158–175. 10.1016/j.ajpath.2019.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steiner JL, Lang CH. Alcohol, adipose tissue and lipid dysregulation. Biomolecules. 2017;7:16. 10.3390/biom7010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sebastian BM, Roychowdhury S, Tang H, et al. Identification of a cytochrome P4502E1/Bid/C1q-dependent axis mediating inflammation in adipose tissue after chronic ethanol feeding to mice. J Biol Chem. 2011;286:35989–35997. 10.1074/jbc.M111.254201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang W, Zhong W, Sun X, et al. Visceral white adipose tissue is susceptible to alcohol-induced lipodystrophy in rats: role of acetaldehyde. Alcohol Clin Exp Res. 2015;39:416–423. 10.1111/acer.12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ni HM, Du K, You M, Ding WX. Critical role of FoxO3a in alcohol-induced autophagy and hepatotoxicity. Am J Pathol. 2013;183:1815–1825. 10.1016/j.ajpath.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamming DW, Sabatini DM. A central role for mTOR in lipid homeostasis. Cell Metab. 2013;18:465–469. 10.1016/j.cmet.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simcox J, Lamming DW. The central moTOR of metabolism. Dev Cell. 2022;57: 691–706. 10.1016/j.devcel.2022.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chao X, Wang S, Zhao K, et al. Impaired TFEB-mediated lysosome biogenesis and autophagy promote chronic ethanol-induced liver injury and steatosis in mice. Gastroenterology. 2018;155:865–879. 10.1053/j.gastro.2018.05.027. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding WX, Li M, Chen X, et al. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology. 2010;139:1740–1752. 10.1053/j.gastro.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang X, Fulte S, Deng F, et al. Lack of VMP1 impairs hepatic lipoprotein secretion and promotes nonalcoholic steatohepatitis. J Hepatol. 2022;77: 619–631. 10.1016/j.jhep.2022.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ni HM, Chao X, Kaseff J, et al. Receptor-interacting serine/threonine-protein kinase 3 (RIPK3)-mixed lineage kinase domain-like protein (MLKL)-mediated necroptosis contributes to ischemia-reperfusion injury of steatotic livers. Am J Pathol. 2019;189:1363–1374. 10.1016/j.ajpath.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ni HM, McGill MR, Chao X, et al. Removal of acetaminophen protein adducts by autophagy protects against acetaminophen-induced liver injury in mice. J Hepatol. 2016;65:354–362. 10.1016/j.jhep.2016.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marra F, Bertolani C. Adipokines in liver diseases. Hepatology. 2009;50: 957–969. 10.1002/hep.23046. [DOI] [PubMed] [Google Scholar]
- 18.Chen H, Shen F, Sherban A, et al. DEP domain-containing mTOR-interacting protein suppresses lipogenesis and ameliorates hepatic steatosis and acute-on-chronic liver injury in alcoholic liver disease. Hepatology. 2018;68:496–514. 10.1002/hep.29849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee PL, Jung SM, Guertin DA. The complex roles of mechanistic target of rapamycin in adipocytes and beyond. Trends Endocrinol Metab. 2017;28: 319–339. 10.1016/j.tem.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paolella LM, Mukherjee S, Tran CM, et al. mTORC1 restrains adipocyte lipolysis to prevent systemic hyperlipidemia. Mol Metab. 2020;32:136–147. 10.1016/j.molmet.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang Y, Wallace M, Sanchez-Gurmaches J, et al. Adipose tissue mTORC2 regulates ChREBP-driven de novo lipogenesis and hepatic glucose metabolism. Nat Commun. 2016;7, 11365. 10.1038/ncomms11365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shan T, Zhang P, Jiang Q, Xiong Y, Wang Y, Kuang S. Adipocyte-specific deletion of mTOR inhibits adipose tissue development and causes insulin resistance in mice. Diabetologia. 2016;59:1995–2004. 10.1007/s00125-016-4006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dasarathy S. Nutrition and alcoholic liver disease: effects of alcoholism on nutrition, effects of nutrition on alcoholic liver disease, and nutritional therapies for alcoholic liver disease. Clin Liver Dis. 2016;20:535–550. 10.1016/j.cld.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McClain CJ, Barve SS, Barve A, Marsano L. Alcoholic liver disease and malnutrition. Alcohol Clin Exp Res. 2011;35:815–820. 10.1111/j.1530-0277.2010.01405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bell S, Britton A. The role of alcohol consumption in regulating circulating levels of adiponectin: a prospective cohort study. J Clin Endocrinol Metab. 2015;100:2763–2768. 10.1210/jc.2015-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hillemacher T, Weinland C, Heberlein A, et al. Increased levels of adiponectin and resistin in alcohol dependence–possible link to craving. Drug Alcohol Depend. 2009;99:333–337. 10.1016/j.drugalcdep.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 27.Song Z, Zhou Z, Deaciuc I, Chen T, McClain CJ. Inhibition of adiponectin production by homocysteine: a potential mechanism for alcoholic liver disease. Hepatology. 2008;47:867–879. 10.1002/hep.22074. [DOI] [PubMed] [Google Scholar]
- 28.Shen Z, Liang X, Rogers CQ, Rideout D, You M. Involvement of adiponectin-SIRT1-AMPK signaling in the protective action of rosiglitazone against alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol. 2010;298: G364–G374. 10.1152/ajpgi.00456.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khadir A, Kavalakatt S, Madhu D, et al. Fetuin-A levels are increased in the adipose tissue of diabetic obese humans but not in circulation. Lipids Health Dis. 2018;17:291. 10.1186/s12944-018-0919-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ley SH, Sun Q, Jimenez MC, et al. Association between alcohol consumption and plasma fetuin-A and its contribution to incident type 2 diabetes in women. Diabetologia. 2014;57:93–101. 10.1007/s00125-013-3077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desai BN, Singhal G, Watanable M, et al. Fibroblast growth factor 21 (FGF21) is robustly induced by ethanol and has a protective role in ethanol associated liver injury. Mol Metab. 2017;6:1395–1406. 10.1016/j.molmet.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Zhao C, Xiao J, et al. Fibroblast growth factor 21 deficiency exacerbates chronic alcohol-induced hepatic steatosis and injury. Sci Rep. 2016;6, 31026. 10.1038/srep31026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye D, Wang Y, Li H, et al. Fibroblast growth factor 21 protects against acetaminophen-induced hepatotoxicity by potentiating peroxisome proliferator-activated receptor coactivator protein-1alpha-mediated antioxidant capacity in mice. Hepatology. 2014;60:977–989. 10.1002/hep.27060. [DOI] [PubMed] [Google Scholar]
- 34.Lin Z, Tian H, Lam KS, et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 2013;17:779–789. 10.1016/j.cmet.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Baraona E, Abittan CS, Dohmen K, et al. Gender differences in pharmacokinetics of alcohol. Alcohol Clin Exp Res. 2001;25:502–507. [PubMed] [Google Scholar]
- 36.Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med. 1990;322:95–99. 10.1056/NEJM199001113220205. [DOI] [PubMed] [Google Scholar]
- 37.Fulham MA, Mandrekar P. Sexual dimorphism in alcohol induced adipose inflammation relates to liver injury. PLoS One. 2016;11, e0164225. 10.1371/journal.pone.0164225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inamine T, Yang AM, Wang L, Lee KC, Lloente C, Schnable B. Genetic loss of immunoglobulin A does not influence development of alcoholic steatohepatitis in mice. Alcohol Clin Exp Res. 2016;40:2604–2613. 10.1111/acer.13239. [DOI] [PMC free article] [PubMed] [Google Scholar]


