Elevated [CO2] affects stomatal characteristics differently in wheat elite cultivars and wild accessions, resulting in faster kinetics in wild relatives, a trait useful to enhanced photosynthesis and water use.
Keywords: Bread wheat, net CO2 assimilation rate (A), stomatal density, stomatal conductance (gs), Triticum aestivum L, wheat relatives (Aegilops tauschii, Triticum turgidum ssp. dicoccoides, Triticum turgidum ssp. dicoccon)
Abstract
The ability of plants to respond to changes in the environment is crucial to their survival and reproductive success. The impact of increasing the atmospheric CO2 concentration (a[CO2]), mediated by behavioral and developmental responses of stomata, on crop performance remains a concern under all climate change scenarios, with potential impacts on future food security. To identify possible beneficial traits that could be exploited for future breeding, phenotypic variation in morphological traits including stomatal size and density, as well as physiological responses and, critically, the effect of growth [CO2] on these traits, was assessed in six wheat relative accessions (including Aegilops tauschii, Triticum turgidum ssp. Dicoccoides, and T. turgidum ssp. dicoccon) and five elite bread wheat T. aestivum cultivars. Exploiting a range of different species and ploidy, we identified key differences in photosynthetic capacity between elite hexaploid wheat and wheat relatives. We also report differences in the speed of stomatal responses which were found to be faster in wheat relatives than in elite cultivars, a trait that could be useful for enhanced photosynthetic carbon gain and water use efficiency. Furthermore, these traits do not all appear to be influenced by elevated [CO2], and determining the underlying genetics will be critical for future breeding programmes.
Introduction
Prior to the industrial revolution, the atmospheric CO2 concentration (a[CO2]) was maintained at a value close to 280 ppm for ~1000 preceding years (Tans and Keeling, 2016). Subsequently, anthropogenic CO2 emissions, primarily through the burning of fossil fuels, have increased the present day atmospheric CO2 concentration to 419 ppm (NOAA, 2022). With the current increases in CO2 emissions associated with modern day activities, the Intergovernmental Panel on Climate Change projections include scenarios of [CO2] doubling from current levels by the end of the century (IPCC, 2021), which to date has resulted in a rise in global temperature (of ~1.1 °C, World Meteorological Organization, 2022) and is predicted to rise with further increases in [CO2] (Stockwell et al., 2021). Elevated [CO2] generally increases leaf photosynthetic rates in a range of C3 crops from potatoes (Lawson et al., 2001) to soybean (Rogers et al., 2004), through increased substrate for Rubisco (the enzyme involved in the first major step of carbon fixation) and the suppression of photorespiration. A recent review of 18 C3 crops grown using free-air CO2 enrichment (FACE) technology with elevated CO2 of 200 ppm above ambient [CO2] reported that most species exhibited increased yields by ~18% (Ainsworth and Long, 2021). However, the same study also highlighted that yield increases were not consistent across species or cultivars, and yield benefits with elevated [CO2] were correlated with sink strength (Ainsworth and Long, 2021).
As stomatal conductance (gs) regulates gas exchange between the leaf interior and the external environment, stomatal responses to changing climatic conditions are critical in determining CO2 supply for photosynthesis (A) and water loss through transpiration (Lawson et al., 1998; Morison et al., 2008). Transpirational water loss also plays a key role in nutrient uptake from the plant roots as well as evaporative cooling of the leaf tissue and the maintenance of optimal leaf temperatures for photosynthesis (Raven, 1977, 2002; Hetherington and Woodward, 2003; Peterson et al., 2010; McAusland et al., 2016; Murray et al., 2016; Lawson and Vialet-Chabrand, 2019). Therefore, stomatal dynamics will have a pivotal role in determining C3 crop productivity in future climates (Lawson et al., 2010, 2012).
g s is determined by anatomical features as well as functional aspects of the guard cells, both of which are influenced by growth [CO2] (and temperature) (Woodward, 1987; Matthews and Lawson, 2019; Stevens et al., 2021). Both stomatal anatomy and behavior are modified by elevated [CO2], with most species responding by decreasing stomatal density (Woodward, 1987; Poole et al., 1996) and reducing aperture (see review by Stevens et al., 2021). Reducing stomatal aperture under elevated [CO2] greatly increases intrinsic water use efficiency (WUEi; A/gs) with potential benefits for plant growth (Leakey et al., 2009; Sreeharsha et al., 2015). On the other hand, reductions in gs can negatively impact on photosynthesis through diffusional constraints, as well increases in leaf temperature (Matthews and Lawson, 2019).
The number and size of stomata on the leaf determine the maximum potential stomatal conductance (gsmax; Lawson and Morison 2004; Lawson et al., 2010; McElwain et al., 2016), whilst pore aperture/behavior regulates the short-time scale dynamics of gs and gas exchange (Dow et al., 2014; Takahashi et al., 2015). The majority of studies that have explored variation in gs or the influence of growth conditions on anatomy and function have examined steady-state conditions (Schlüter et al., 2003; Doheny-Adams et al., 2012; Tanaka et al., 2013); however, recent studies have illustrated the significant impact of dynamic gs responses on A (Sakoda et al., 2020, 2022; Vialet-Chabrand and Lawson, 2020) and WUEi (Papanatsiou et al., 2019; Acevedo-Siaca et al., 2021; Pignon et al., 2021). Generally, slow stomatal opening limits the CO2 assimilation rate, reducing the speed of photosynthetic induction (Long et al., 2022), whilst slow closure erodes WUEi (Lawson and Blatt, 2014; McAusland et al., 2016; Qu et al., 2016, 2020; Lawson and Vialet-Chabrand, 2019). The rapidity of stomatal responses to changing climatic conditions is also critically important for maintaining optimal leaf temperature (Matthews and Lawson, 2019; Stevens et al. 2021) and for photosynthesis and plant productivity (Moore et al., 2021). Dynamic responses have been linked to both morphological and physiological variation in stomata (Drake et al., 2013; Lawson and Blatt, 2014; Zhang et al., 2019); however, few studies have explored the impact of changing climate conditions such as growth [CO2] on morphophysiological characteristics. Consequently, natural variation in rapidity of stomatal conductance between cultivars and species, as well as the influence of changing climatic conditions on these traits, could provide currently unexploited targets for improving crop productivity in future climates (Lawson et al., 2012; Faralli et al., 2019; Faralli and Lawson, 2020).
Here we explored the impact of elevated CO2 concentration (e[CO2]) of 800 ppm, approximately double that of the current atmospheric [CO2] (a[CO2]), on the physiology and growth of 11 different wheat progenitor and elite cultivar accessions. Wheat (Triticum aestivum L.) is a principal global food grain source, grown on more land area than any other commercial crop. In addition, it is one of the largest traded primary crop commodities, along with maize and rice (FAO, 2014). Globally, wheat provides >20% of the calories consumed by the human population (Braun and Atlin, 2010; Lobell et al., 2011). Modern wheat is a hexaploid species containing three sets of chromosomes (A, B, and D subgenomes). These subgenomes originated from three different diploid grass species and combined during two hybridization events (Kerber and Rowland, 1974; Faris, 2014; Marcussen et al., 2014). Initially diploid wheat Triticum urartu (subgenome AA ancestor) hybridized with the B genome ancestor Aegilops speltoides ssp. ligustica (Huang et al., 2002; Dvorak and Akhunov, 2005; Peng et al., 2011) to produce wild emmer wheat Triticum turgidum ssp. dicoccoides (genome AABB). In the second event, T. turgidum ssp. dicoccoides hybridized with the wild goat grass Aegilops tauschii to produce the modern hexaploid Triticum aestivum ssp. aestivum (AABBDD; Huang et al., 2002; Charmet, 2011; Faris, 2014). In this study, phenotypic variation in morphological traits including stomatal size and density, as well as physiological responses and, critically, the effect of growth [CO2] on these traits, was assessed in six wheat relative (WR) accessions (including the species Aegilops tauschii, T. turgidum ssp. dicoccoides, and T. turgidum ssp. dicoccon) and five elite wheat T. aestivum cultivars (Claire, Rialto, Robigus, Soissons, and Xi19) to identify possible beneficial traits that could be exploited for future breeding.
Materials and methods
Plant growth conditions
Triticum and Aegilops species (listed in Table 1) were germinated in a greenhouse compartment (at BASF, Ghent, Belgium) with supplementary lighting (Master Greenpower CGT 400 W E40 HPS lights) to ensure a typical summer day length of 15.30 h. At 14 d post-emergence, plants were vernalized in a controlled environment (custom-made growth chamber, BASF, Ghent, Belgium) for 10 weeks at 4 °C, with 75 μmol m–2 s–1 PPFD, over a 10 h photoperiod using an in-house-produced 60/40 peat-based sowing and cutting soil (including NPK Compound Fertilizer 12-14-24 (0.8 kg m–3). Plants were then transferred into 4 liter pots using a peat-based, boron-free potting soil [including NPK Compound Fertilizer 12-14-24 (2 kg m–3)] and grown in two separate growth environments, one at current (2018) atmospheric [CO2] (408 ppm CO2) and a second at an elevated [CO2] of 800 ppm. Both growth chambers had a light intensity (at pot height) of 800 ± 20 µmol m–2 s–1 with a 2:1 high pressure sodium:metal halide lighting mix (Master Greenpower CGT 400 W E40 and Powerstar HQI-BT 400 W/D PRO 400 W Daylight E40, respectively) for a 15 h light/9 h dark photoperiod. With the exception of [CO2], both growth environments were set to identical conditions: air temperature controlled to 20 °C and 18 °C (±1 °C) day and night, respectively, and relative humidity maintained at a constant 65%. Plants were well watered using a drip irrigation system to the roots. All wheat measurements were taken from the flag leaf, at Zadoks growth stage 49 (GS 49, first awns/scurs visible) to GS 59 (ear emergence complete) (Zadoks et al., 1974). Six repetitions of each measurement were completed per accession unless stated below.
Table 1.
Species investigated, including ploidy and common name
| Species/cultivar abbreviation | Species | Common name | Ploidy |
|---|---|---|---|
| Claire | Triticum aestivum | Common or bread wheat | Hexaploid |
| Rialto | Triticum aestivum | Common or bread wheat | Hexaploid |
| Robigus | Triticum aestivum | Common or bread wheat | Hexaploid |
| Soissons | Triticum aestivum | Common or bread wheat | Hexaploid |
| Xi19 | Triticum aestivum | Common or bread wheat | Hexaploid |
| TRI 11502 | Triticum dicoccoides | Wild emmer | Tetraploid |
| TRI 3432 | Triticum dicoccon | Emmer | Tetraploid |
| IG 48509 | Aegilops tauchii | Goat grass or rough-spike hard grass | Diploid |
| IG48514 | Aegilops tauchii | Goat grass or rough-spike hard grass | Diploid |
| KU2018 | Aegilops tauchii | Goat grass or rough-spike hard grass | Diploid |
| KU 2036 | Aegilops tauchii | Goat grass or rough-spike hard grass | Diploid |
Species abbreviation is how the species is referred to in the text. All seeds were provided from the NIAB collection.
Leaf anatomical measurements
Measurements of stomatal density and size
Stomatal density (SD) was measured from impressions taken from both the adaxial (upper) and abaxial (lower) leaf surface using silicone impression material (Xantopren, Heraeus, Germany) following the methods of Weyers and Johansen (1985) using six leaves per species/cultivar, measured at the middle of the leaf lamina. SD, guard cell length (GCL; used as a proxy for stomatal size), and pore length (PL) were all measured via light microscopy (Olympus BX60, Essex, UK). Total magnification was 100-fold for SD measurements and 400-fold for GCL and PL measurements.
Anatomical maximum stomatal conductance (gsmax: mol m–2 s–1) was calculated from the measurements of SD and stomatal dimensions (Equation 1) following the equations of Franks and Farquhar (2001):
| (1) |
Where d is the diffusivity of water in air (m2 s–1, at 22 °C), v is the molar volume of air (m3 mol–1, at 22 °C), and pore depth (l; μm) was equal to guard cell width at the centre of the stoma represented as half the GCL. The mean maximum stomatal pore area (amax; μm2) was calculated assuming stomatal pores were elliptical with the major axis equal to pore length and the minor axis equal to half pore length (see McElwain et al., 2015).
Leaf thickness
Leaf thickness (LT) measurements were taken using the MultispeQ v1.0 instrument (Michigan State University, MI, USA) (Kuhlgert et al., 2016). The device was calibrated using 0.18 mm thick filter paper (Whatman 1001-110, Maidstone, Kent, UK). A mean leaf thickness was calculated from three repeat measurements per leaf from three separate leaves per species/cultivar.
Dry weight and leaf area
Leaf area was measured using a bench-top area meter (LI-3100C, Li-Cor, Lincoln, NE, USA) where the mean leaf area was calculated from three repeat measurements per leaf from three separate leaves per species/cultivar. Leaves were then placed in paper bags and dried at 60 °C to constant weight and measured using a four-digit balance (Kern, Northamptonshire, UK).
Leaf gas exchange
Stomatal conductance to water vapor (gs) and the rate of photosynthetic CO2 assimilation (A) were measured using a portable gas exchange system (Li-Cor 6400XT, Li-Cor) with an integrated light source (Li6400-40, Li-Cor), consisting of blue and red light-emitting diodes. Leaf temperature and VPD were controlled to 22 °C and 1 ± 0.2 kPa, respectively, throughout the measurements. Gas exchange measurements had a constant flow rate set at 300 μmol s–1, with cuvette conditions maintained at a CO2 concentration of 400 μmol mol–1 (for both plant growth CO2 treatments). Gas exchange analysis was completed within the first 7 h of the photoperiod, to minimize any diurnal effects on stomatal opening and photosynthetic activation. All measurements were conducted on the mid-point of fully expanded flag leaves, before anthesis (GS 49–59) (Zadoks et al., 1974). Intrinsic water use efficiency was calculated as WUEi=A/gs. Between five and seven repetitions of each measurement were completed per accession for gas exchange data.
PPFD step measurements
To measure the response of A and gs to a single step increase in PPFD, leaves were equilibrated at a PPFD of 100 μmol m–2 s–1 until both A and gs were at steady state (defined as <2% change in rate over 5 min). Measurements were made at 30 s intervals, for 10 min at 100 μmol m–2 s–1, after which PPFD was increased in a single step to 1000 μmol m–2 s–1 and recorded for a further 60 min. Leaf temperature (Ti), VPD, and [CO2] were all maintained at 22 °C, 1 ± 0.2 kPa, and 400 µmol mol–1, respectively, throughout the measurement. These data were used to model the response of A, gs, and WUEi to changes in PPFD.
Intracellular CO 2 response curves (A/C i)
A/Ci response curves [net CO2 assimilation rate (A) to intercellular CO2 concentration (Ci)] were measured at 1500 μmol m–2 s–1 PPFD. Photosynthesis was initially stabilized for a minimum of 15 min at 400 μmol mol–1, then decreased and measured at 250, 150, 100, and 50 μmol mol–1 before returning to the initial value of 400 μmol mol–1, and increased to 550, 700, 900, 1100, 1300, and 1500 μmol mol–1. Photosynthesis was measured at each [CO2] after ~3 min. Leaf temperature and VPD were controlled to 22 °C and 1 ± 0.5 kPa, respectively.
Modeling gas exchange parameters
The maximum velocity of Rubisco for carboxylation (Vcmax) and the maximum rate of electron transport demand for ribulose bisphosphate dehydrogenase (RuBP) regeneration (Jmax) were calculated from the A/Ci response using equations from von Caemmerer and Farquhar (1981), as described by Sharkey et al. (2007) using the Rubisco kinetic constants for wheat (Carmo-Silva et al., 2010). The response of gs to the step change in PPFD was analyzed following the method described in McAusland et al. (2016). In summary, the optimum function in R (www.r-project.org; version 3.5.3), a model representing gs as a function of time, was fitted on each observed response as shown in Equation 2:
| (2) |
The model uses a sigmoidal equation rather than an exponential slope, with an initial time lag (the time before gs starts to increase, λ, min), a time constant (the time taken to reach 63% of the variation, k, min), an initial value (r0, mol m−2 s−1), and a steady-state target [the value when the plateau is reached (gsmax, mol m−2 s−1]. The time was set to 0 when PPFD was increased from 100 μmol m−2 s−1 to 1000 μmol m−2 s−1 (Vialet-Chabrand et al., 2013).
Statistical analysis
All statistical analyses were conducted using R software (www.r-project.org; version 3.5.3). For SD, GCL, and gsmax, a Shapiro–Wilk test was used to test for normality and a Levene’s test of homogeneity was used to determine if samples had equal variance. A log transformation was applied when data were not normally distributed (P<0.05, Shapiro–Wilk test) to achieve normality and meet modeling assumptions of an ANOVA. Single factor differences were analyzed using t-tests with a Bonferroni–Hochberg end correction or a one-way ANOVA, as described in the figure legends. When more than one factor existed, a two-way ANOVA was applied with an interaction between the two factors, and, if a significant difference was found (P<0.05), a Tukey post-hoc test was performed.
Results
Stomatal anatomy
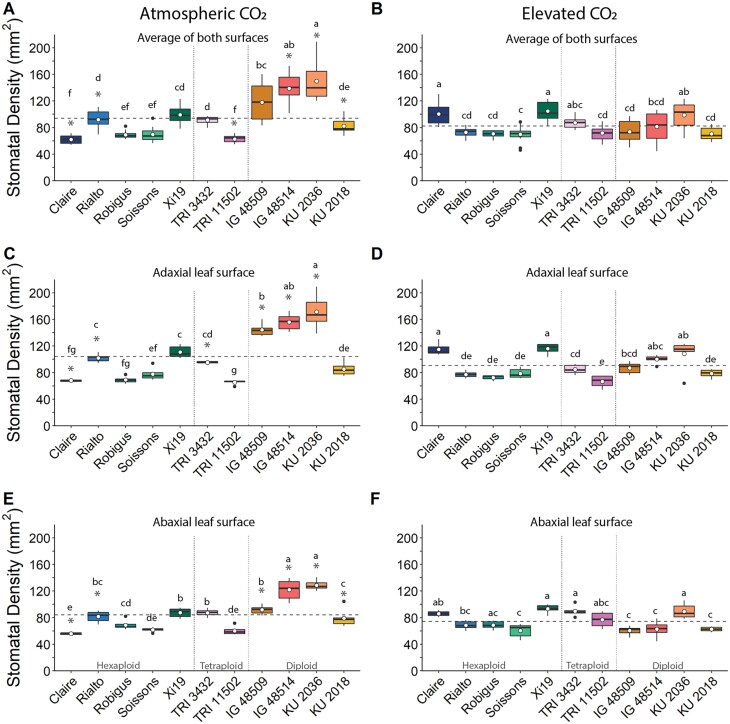
Stomatal anatomy including SD and GCL was measured in five elite T. aestivum cultivars (Claire, Rialto, Robigus, Soissons, and Xi19; all hexaploid) and six WRs (four diploid lines IG 48509, IG 48514, KU 2018, and KU 2036; and two tetraploid lines TRI 3432 and TRI 11502), grown at two [CO2], atmospheric (a[CO2]) at ~408 ppm and elevated (e[CO2]) at ~800 ppm. Significant (P<0.05) variation in combined (adaxial+abaxial) leaf SD was found between species grown at a[CO2] (Fig. 1A) with the hexaploid cultivars ranging from ~0 mm2 to ~100 mm2 and the wheat relatives showing a larger range of ~60 mm2 to ~160 mm2, with a +60% difference between the lowest and the highest mean SDs. When grown at e[CO2] (Fig. 1B), less variation between and within species was observed. The majority of WRs showed a decrease in SD, with the exception of the T. dicoccoides accession TRI 11502 in which SD increased. No consistent pattern of change was observed for the elite hexaploid cultivars, with two cultivars showing no change in SD, while Rialto decreased, and Claire increased SD (P<0.05). SD was higher on the adaxial (upper) leaf surface (Fig. 1C) compared with the abaxial (lower) (Fig. 1E) surface (P<0.05) and SD on the adaxial surface was influenced to a greater extent by e[CO2] (Fig. 1D) and accounted for a greater proportion of changes in total leaf SD compared with the abaxial surface, and this was particularly evident in the WRs. Overall, there was no relationship between SD in plants grown a[CO2] and e[CO2] (Supplementary Fig. S1A). However, those species showing a change in SD with e[CO2] on the adaxial surface also had significantly altered SD on the abaxial surface, albeit of a smaller magnitude (Fig 1F). These data suggest that the majority of the combined (adaxial+abaxial) SD is determined by adaxial density (Fig. 1). Although there was no consistent species response of GCL to growth at e[CO2] (Supplementary Fig. S2), a significant (P=0.0055) positive correlation was observed between GCL from plants grown at ambient and elevated [CO2] (Supplementary Fig. S1B). The smallest GCL was observed for Ae. tauschii accession KU 2036 at ~29 μm at a[CO2] and ~35 μm at e[CO2], while the largest GCL was found on the bread wheat cultivars Xi19 at ~47 μm at a[CO2] and Robigus at ~45 μm at e[CO2]. When species were separated by ploidy, diploid species tended to respond to e[CO2] by increasing GCL; however, this was not always significant (Supplementary Fig. S2). Tetraploids had a tendency to decrease in GCL, but no specific trends were observed for hexaploids. Unlike the case for SD, it appears that average leaf GCL was determined by both the adaxial and abaxial leaf surfaces, as similar responses to e[CO2] were observed on both, and together reflected the observed differences in combined (adaxial+abaxial) leaf averages (Supplementary Fig. S2).
Fig. 1.
Mean (white dot) and variation (box and whisker plots displaying distribution of biological replicates) of flag leaf stomatal density (mm2), calculated from the average of both leaf surfaces (A and B), the adaxial leaf surface (C and D), and the abaxial leaf surface (E and F) for 11 wheat species grown at atmospheric CO2 (~408 ppm; A, C, and E) and elevated CO2 (~800 ppm; B, D, and F). Different letters represent statistically significant differences (P<0.05) between species means using the results of a Tukey test following a two-way ANOVA. A dashed line represents mean stomatal density of all wheat lines for the specific CO2 treatment and leaf surface. Dotted lines separate wheat by ploidy. To test the effect of growth at elevated [CO2] on stomatal density, a t-test with a Bonferroni–Hochberg end correction (n=6) was used to compare stomatal density means of individual wheat lines, with gray asterisks indicating significant differences (P<0.05).
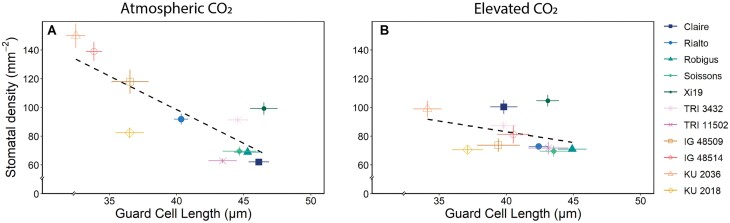
A negative correlation between SD and GCL was evident for wheat grown at ambient [CO2] (P≤0.001; Fig. 2A), demonstrating a relationship between decreasing SD and increasing stomatal size, driven mostly by the change in the four diploid accessions. Aegilops tauschii accession KU 2036 had the highest SD and smallest GCL, and the accession with the lowest SD mean (cv. Claire) had one of the largest GCLs. Although a similar trend of decreasing GCL with increasing SD was observed for the species and cultivars when grown under e[CO2], this relationship was not significant (Fig. 2B). This is most likely to be attributable to the reduced range in SD under e[CO2], particularly for the four Ae. tauschii accessions (Fig. 1B).
Fig. 2.
Correlation between total stomatal density (mm2) and total guard cell length (µm) for each species, calculated for the average of both leaf surfaces, for 11 wheat species grown at atmospheric CO2 (~408 ppm; A) and elevated CO2 (~800 ppm; B). The black dotted line represents the trend in the data between the two variables. Atmospheric CO2 correlation= –0.552 (P=6.79e-12) and elevated CO2 correlation=0.0625 (P=0.483) using a Pearson’s correlation test. Error bars represent the SE (n=12).
Anatomical potential maximum rate of g s
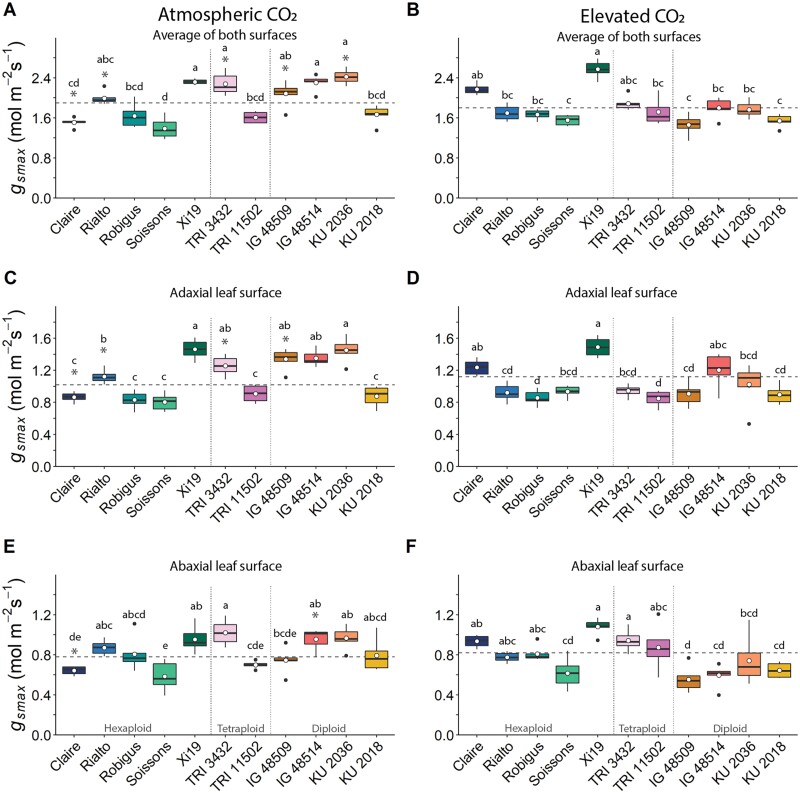
SD and measurements of stomatal size (GCL) were used to calculate the maximum anatomical stomatal conductance (gsmax), assuming fully open pores. At a[CO2], a considerable range of gsmax values were determined between accessions (Fig. 3A), with cv. Soissons displaying the lowest and Ae. tauschii accession KU 2036 the highest values (an increase of ~41%, driven predominantly by the differences in SD; Fig. 1). Higher gsmax values were observed on the adaxial leaf surface irrespective of growth [CO2] (Fig. 3C, D), with typical values >1.0 mol m–2 s–1, whereas gsmax values on the abaxial surface (except e[CO2]-grown Xi19) were <1.0 mol m–2 s–1 (Fig. 3E, F). There was considerable variation in the response of gsmax when grown under e[CO2] compared with a[CO2] (Fig. 3B). These differences appear to be driven mostly by the changes in SD (Fig. 1), but not exclusively as the CO2 response patterns between SD (Fig. 1) and gsmax (Fig. 3) were not identical. Similar to the patterns described for SD, there was a tendency for reduction in gsmax with e[CO2] driven mostly by adaxial gsmax. However, interestingly, not all of the changes in SD translated into changes in gsmax, strongly indicating a role for changes in GCL with e[CO2] to compensate for changes in density, maintaining a similar gsmax (Lawson and Morison, 2004; Harrison et al., 2020; Wall et al., 2022); for example, the diploid and tetraploid species have similar gsmax to that of the hexaploid species even though SD is much higher in the diploid species.
Fig. 3.
Mean (white dot) and variation (box and whisker plots displaying distribution of biological replicates) of flag leaf gsmax (mol m–1 s–1), calculated from the average of both leaf surfaces (A and B), the adaxial leaf surface (C and D), and the abaxial leaf surface (E and F) for 11 wheat species grown at atmospheric CO2 (~408 ppm; A, C, and E) and elevated CO2 (~800 ppm; B, D, and F). Different letters within each graph represent statistically significant differences (P<0.05) between means using the results of a Tukey test following a two-way ANOVA. The dashed line represents the mean gsmax of specific CO2 treatment and leaf surface. Dotted lines separate wheat by ploidy. To test the effect of growth at elevated [CO2] on gsmax, a t-test with a Bonferroni–Hochberg end correction (n=6) was used to compare individual wheat line means, with gray asterisks indicating significant differences (P<0.05).
Leaf gas exchange
Response of gsand A to a step change in PPFD
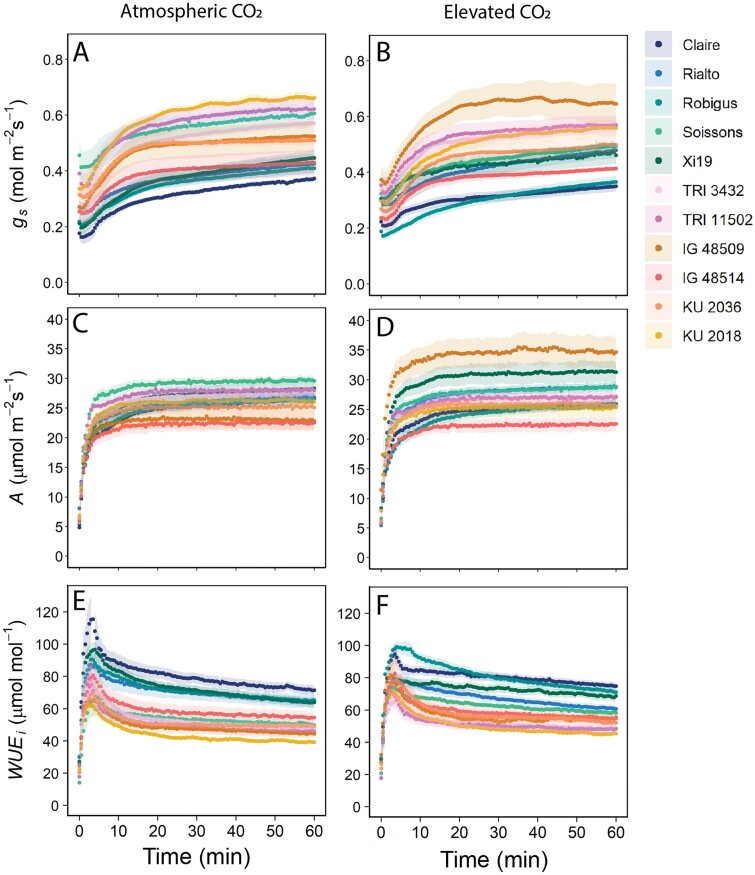
The effect of e[CO2] on stomatal behavior/kinetics was assessed by measurements of gs and A following a step increase in PPFD (Fig. 4). As expected, all species and cultivars exhibited an increase in gs and A with increasing irradiance. In general, A rapidly increased compared with gs when light was increased (Fig. 4A–D), and this resulted in the maximum WUEi value being reached within a few minutes of the change in PPFD (Fig. 4E, F). Further increases in gs with time drove a continuous decrease in WUEi, and this trend continued after A had reached a maximum steady state. Considerable variation in A, gs, and WUEi was observed in plants grown under both [CO2] treatments, although the variation was more apparent in growth at e[CO2], particularly for A and gs (Fig. 4A–D).
Fig. 4.
Temporal response of stomatal conductance (gs; A and B), net CO2 assimilation (A; C and D), and intrinsic water use efficiency (WUEi; E and F), to a step increase in light intensity (from 100 μmol m–1 s–1 to 1000 μmol m–1 s–1 PPFD for 60 min) for 11 wheat species grown at atmospheric CO2 (~408 ppm) and elevated CO2 (~800 ppm). Gas exchange parameters (gs and A) were recorded at 30 s intervals, and leaf temperature and VPD were maintained at 22 °C, and 1 ± 0.2 kPa, respectively. Error ribbons represent the mean ±SE (n=5–7).
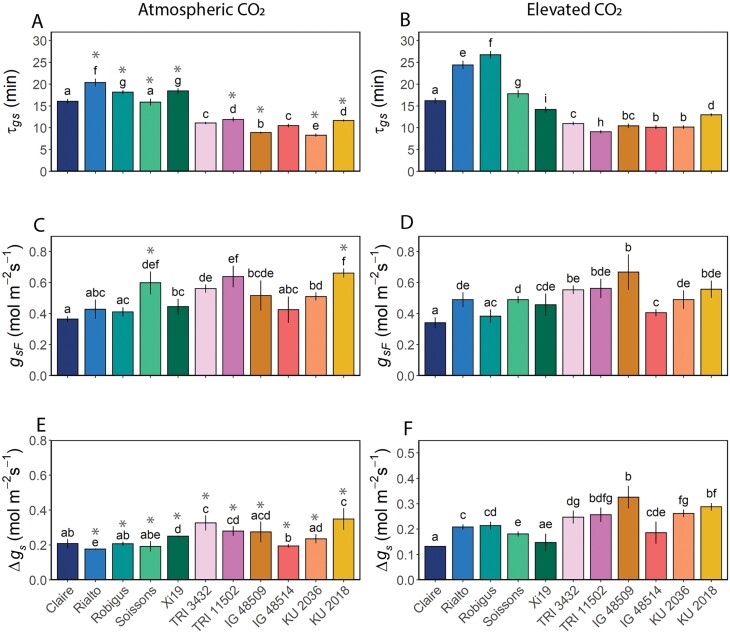
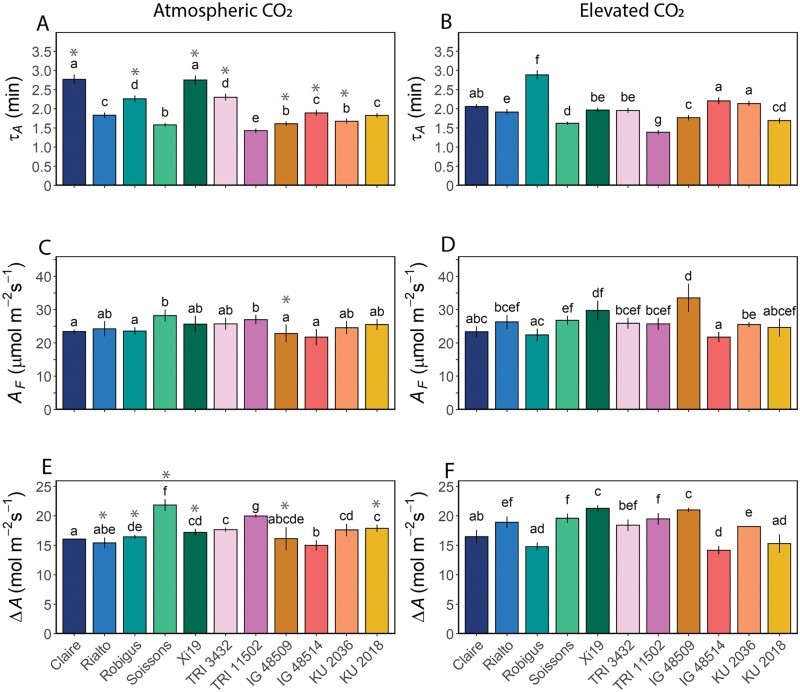
The time constant to reach 63% of the final value for gs (τgs) as an indicator of the rapidity (Fig. 5) was significantly greater (P<0.05) in the hexaploid wheat compared with other species, regardless of growth [CO2] (Fig. 5A, B). Hexaploid lines averaged 20 min to reach maximum gs while the other species averaged 10 min. In general, e[CO2] increased the time constant (indicating slower stomatal responses) in most species with the exception of cv. Claire and T. dicoccon accession TRI 3432 which showed no significant differences, and T. dicoccoides accession TRI 11502 and cv. Xi19 which were significantly faster with e[CO2] (Fig. 5B). Interestingly, there was a significant positive correlation (P<0.05) between τgs in plants grown at ambient [CO2] and at e[CO2], indicating that speed was inherent with limited influence of growth environment (Supplementary Fig. S3). However, the speed of the gs response did not influence the overall final gs (gsF) achieved (Fig. 5C–F), with no correlation observed between the two. Growth at e[CO2] did not influence gsF values, with no differences observed between most accessions, with the exception of cv. Soissons and KU 2018 (Fig. 5C, D). On the other hand, the magnitude of change in gs (Δgs) decreased in almost all species and cultivars with growth at e[CO2], with the exception of the hexaploid cv. Rialto and Robigus and the Ae. tauschii accession IG 48509, and this was related to the speed of response, with slow responding accessions (mostly the hexaploids) having a lower Δgs compared with the fast responders in which Δgs was greater (Fig. 5E), although this correlation was only significant (at P=0.0107) when plants were grown under e[CO2] and not significant at ambient a[CO2] (Supplementary Fig. S4A, B). The fact that there was no effect of e[CO2] on gsF indicates that minimum gs must have been higher with growth at high [CO2]. The more rapid gs responses did not, however, impact on τA (Fig. 6), with no clear relationship between the two parameters. Δgs was positively correlated with gsF at both a[CO2] and e[CO2] (Supplementary Fig. S4C, D). Under both ambient and elevated growth [CO2] conditions, the greater the gsF, the higher the AF achieved (Supplementary Fig. S4E, F), and under ambient but not elevated [CO2] this was also correlated with a greater change in A (ΔA) (Supplementary Fig. S4G, H), suggesting diffusion constraints by gs on the kinetic responses of A. The final value of A (AF, Fig. 6) at 1000 μmol m–2 s–1 PPFD was similar across the different accessions, ~25 μmol m–2 s-1 for both CO2 growth treatments, although values for two accessions were significantly (P<0.05) higher when grown at e[CO2], hexaploid cv. Xi19, ~32 μmol m–2 s–1 and Ae. tauschii accession IG 48509, ~35 μmol m–2 s–1, and, not unexpectedly, this was highly positively correlated with the ΔA (Supplementary Fig. S4I, J). The change in A (ΔA) was similar across the different species, showing a typical increase of 15 μmol m–2 s–1 with a few exceptions being higher at 20 μmol m–2 s–1 (Fig. 6E, F). However, there was no relationship between τA and these values in plants grown under e[CO2] (Fig. 6), but the speed of the A response was negatively correlated with gsF, suggesting possible differences in the induction of photosynthesis due to both stomatal and biochemical constraints (Supplementary Fig. S4K, L).
Fig. 5.
Time constant for stomatal opening [τgs (min); A and B], final stomatal conductance value [gsF (mol m–2 s–1); B and D] after a step increase in light intensity from 100 μmol m–2 s–1 to 1000 μmol m–2 s–1 PPFD, and the difference in gs [Δgs (mol m–2 s–1)] between 100 μmol m–2 s–1 and 1000 μmol m–2 s–1 PPFD (E and F). The 11 wheat species were grown at both at atmospheric [CO2] (~408 ppm; A, C, and E) and elevated [CO2] (~800 ppm; B, D, and F). Error bars represent 95% confidence intervals using the results of a Tukey test following a two-way ANOVA. To test the effect of growth at elevated [CO2], a t-test with a Bonferroni–Hochberg end correction (n=5–7) was used to compare individual wheat line means, with gray asterisks indicating significant differences (P<0.05).
Fig. 6.
Time constant for light-saturated carbon assimilation [τA (min); A and B), final light-saturated carbon assimilation rate [AF (µmol m–2 s–1); B and D] after a step increase in light intensity from 100 μmol m–2 s–1 to 1000 μmol m–2 s–1 PPFD, and the difference in A [ΔA (µmol m–2 s–1)] between 100 μmol m–2 s–1 and 1000 μmol m–2 s–1 PPFD (E and F). The 11 wheat species were grown at both at atmospheric [CO2] (~408 ppm; A, C, and E) and elevated [CO2] (~800 ppm; B, D, and F). Error bars represent 95% confidence intervals using the results of a Tukey test following a two-way ANOVA. To test the effect of growth at elevated [CO2], a t-test with a Bonferroni–Hochberg end correction (n=5–7) was used to compare individual wheat line means, with gray asterisks indicating significant differences (P<0.05).
A/C i response analysis
In order to assess changes to photosynthetic capacity, the response of assimilation rate (A) as a function of internal [CO2] (Ci; Supplementary Fig. S5) was determined on the flag leaf on plants grown in the two [CO2] environments. All accessions exhibited the expected increase in A with increased Ci before reaching a plateau. Accessions grown at ambient [CO2] displayed significant variation in their responses (Supplementary Fig. S5A). In general, hexaploid accessions had the highest assimilation rates, and greater Vcmax, Jmax, and Amax values at both ambient and e[CO2] (Supplementary Fig. S6) whilst those of the tetraploid and diploid species were lower, indicating a reduced photosynthetic capacity. Growth under e[CO2] had no significant influence on photosynthetic capacity, in any of the species.
Plant growth
Multiple leaf growth parameters were measured including flag leaf area (LA; Supplementary Fig. S7), DW (Supplementary Fig. S8), and leaf thickness (LT; Supplementary Fig. S8). In general, all hexaploid wheat accessions had a greater LA (Supplementary Fig. S7) than other species, except for the tetraploid T. dicoccon accession TRI 3432 when grown at a[CO2]. A similar trend followed for e[CO2]-grown wheat, although there was less variation between species. No significant differences were observed between accessions from the same species from a[CO2] to e[CO2] except for Ae. tauschii accession IG 48509 in which LA increased. DW (Supplementary Fig. S8) followed the same trends as LA. In general, there was a trend for hexaploid and tetraploid species having thicker leaves than the diploid species at both CO2 growth treatments (Supplementary Fig. S9), the exception being cv. Soissons in which LT was reduced at e[CO2]. These data suggest that the diploid species had smaller thinner leaves compared with the hexaploid wheat species.
Discussion
The global human population is expected to reach >9.5 billion by 2050, putting increasing pressure on breeders and crop scientists to improve yields to ensure sufficient food (Asseng et al., 2020). However, with the continued increases in global [CO2], along with predicted changes to climate, it is vital that crop improvement programs consider the impact of these changes on crop performance and identify valuable physiological resilience traits (and the underlying genetics) that maintain productivity in a diverse range of environmental conditions. Genetic engineering approaches have demonstrated that enhancing photosynthetic capacity and stomatal behavior can successfully deliver crops with greater yield and resource use efficiency (Ruiz-Vera et al., 2017; López-Calcagno et al., 2020; De Souza et al., 2022). However, another powerful approach is exploiting natural variation in various physiological traits including photosynthesis (Driever et al., 2014; Carmo-Silva et al., 2017; Faralli and Lawson, 2019) and stomatal dynamics (Faralli et al., 2019, 2022; Sakoda et al., 2022). Exploiting variation in current elite bread wheat germplasm (e.g. Driever et al., 2014; Faralli et al., 2019) as well as crop relatives (McAusland et al., 2020; Sharwood et al., 2022) offers significant potential to identify novel allelic variation (Sakoda et al., 2022; Sharwood et al., 2022; Yin et al., 2022). Here we have explored the impact of growth [CO2] on variation in photosynthesis, stomatal anatomy, and stomatal kinetics in several elite wheat cultivars and their tetraploid and diploid relatives.
It is well documented that significant variation in stomatal anatomy exists between and within species, spatially within leaves (Ticha, 1982; Smith et al., 1989; Willmer and Fricker, 1996; Weyers and Lawson, 1997; Weyers et al., 1997) and on different leaf surfaces (Wall et al., 2022), all of which are influenced by the growth environment (Poole et al., 1996; Croxdale, 2000; Lawson et al., 2002). Stomatal density is one of the most plastic traits and is affected by a great number of environmental parameters (Matthews and Lawson, 2019; Stevens et al., 2021). Increasing growth [CO2] most commonly decreases SD in the majority of plant species investigated (Woodward, 1987), but not all (Lodge et al., 2001), and the degree of change is not the same even within cultivars of the same species (Dusenge et al., 2019). Not unexpectedly, in this study we observed significant variation across and between species and cultivars. The highest SDs were observed in the tetraploid relatives, with some individuals having double that of some elite varieties. A possible explanation for the high SD in the WRs is the smaller leaf area in these species (Supplementary Fig. S7). Therefore, expansion or differentiation of the epidermal cells in the elite cultivars would reduce SD (Lawson et al., 2002). Interestingly, growth at e[CO2] generally reduced SD in diploid species, but not in the elite cultivars (except Rialto), and therefore no relationship between SD in plants grown under the two [CO2] was observed (Supplementary Fig. S1). Furthermore, variation within and between cultivars was generally reduced at e[CO2], although the underlying cause of the reduced variation is currently unknown. However, as these plants were grown in controlled environments, and the only changing variable was [CO2] (with all other parameters kept constant), it is possible that plants grown at a[CO2] were subjected to greater variation in [CO2] (due to photosynthetic draw down), and that the plants were more sensitive and responsive to this variation. For example, the a[CO2] growth chambers ware maintained at 400 ppm; however, photosynthetic CO2 fixation would result in short-term dynamic draw down of [CO2] to ~320 ppm, whilst the same draw down in e[CO2] would result in variation only between 700 ppm and 800 ppm, and plants would be less sensitive to these changes (Franks et al., 2012) as these levels will saturate photosynthesis (Supplementary Figs S2, S3).
The SD variation and response to [CO2] were mainly the result of anatomical changes on the adaxial leaf surface, suggesting two important points. Firstly, the receptors or signaling pathways responsible for detecting and responding to growth at e[CO2] which drive changes in stomatal patterning are complex and either they reside separately on the two surfaces (and are not mesophyll driven) or there is limited surface to surface communication. Secondly, stomata on the adaxial surface play a more prominent role in gaseous exchange than those on the abaxial surface. This agrees with the recent work by Wall et al. (2022) who demonstrated that adaxial stomata make the greatest contribution to leaf gas exchange in amphistomatous bread wheat. Tsutsumi et al. (2014) reported that elevated [CO2] decreased leaf size in rice, and this was accompanied by a decrease in epidermal cell numbers on the adaxial surface, but a reduction in cell size on the abaxial surface, thus providing a possible explanation for the differences observed between surfaces in different cultivars. GCL (as an indicator of stomatal size) was generally lower in the WRs compared with the elite cultivars, and together with SD was used to determine the maximum potential gs (gsmax) for the accessions investigated. As above, the variation in gsmax was driven mostly by SD (and at the leaf level due to differences on the adaxial surface), but not entirely, with GCL clearly having a secondary role, as has previously been shown (Lawson and Morison, 2004). These findings indicate that there are some compensatory processes between SD and GCL (or size) to maintain a level of gsmax across species (Lawson and Morison, 2004; Bussis et al., 2006; McElwain et al., 2016). The strong negative correlation observed between SD and stomatal size in plants grown at ambient [CO2] agrees with several reports that have shown that lowered SD results in increased size (Franks and Farquhar, 2007). What is particularly interesting is that this size–density relationship was lost in plants grown at e[CO2], due in part to the decrease in SD variation with growth at e[CO2], and the fact that no relationship between SD at the two growth [CO2] were observed; however, GCL was positively correlated between plants grown in the two environments. The anatomical constraints of gsmax can translate into species-specific differences in operational or functional gs (McElwain et al., 2016), often with implications for carbon gain and water use efficiency—particularly in dynamic environments (Vialet-Chabrand et al., 2016; Lawson and Vialet Chabrand, 2019). Dynamic stomatal responses and the speeds of stomatal responses to changing environmental cues have recently received considerable attention for optimizing A relative to water loss and WUEi (Farquhar and Sharkey, 1982; Mansfield et al., 1990; Lawson et al., 2010; Buckley and Mott, 2013; Lawson and Blatt, 2014; Buckley, 2017; Vialet-Chabrand et al., 2017; Matthews et al., 2018; Papanatsiou et al., 2019; Yamori et al., 2020). Stomatal conductance, although closely correlated with A, is an order of magnitude slower to respond to these changes than photosynthetic responses and can therefore lead to a disconnect between A and gs, as slow stomatal opening can limit CO2 uptake whilst slow closure can erode water use efficiency (Drake et al., 2013; Lawson and Vialet-Chabrand, 2019; Vialet-Chabrand and Lawson, 2019). Exploiting variation in kinetic stomatal responses has been proposed as a possible route to increase the speed of stomatal responses to be more in tune with photosynthetic demands for CO2 (Lawson et al., 2018). We know that stomatal kinetics depend on species (McAusland et al., 2016), cultivar (McAusland et al., 2020; Stevens et al., 2021), environmental conditions (Ainsworth and Long, 2005, 2021; De Souza et al., 2020), and time of day (Matthews et al., 2017). Here the kinetic responses of gs to increasing PPFD were up to 50% slower in the elite cultivars compared with the diploid and tetraploid WRs, and growth at e[CO2] decreased the speed even further. This agrees with previous reports that gs responses are slower in species with a lower density of larger guard cells (e.g. Elliott-Kingston et al., 2016) as we have observed here in the WRs, and growth under elevated [CO2] could amplify this, dampening the gs response (Knapp et al., 1994). Surprisingly, these differences did not directly translate into differences in final gs values at high PPFD, most probably due to greater variation in τgs than gsF. However, slow gs responses did result in a lower ∆gs under e[CO2], implying that stomatal speed influences overall gs behavior at elevated but not a[CO2]. This is most likely to be due to a greater variation in gs in plants grown under e[CO2]. ∆gs was positively correlated with gsF (Supplementary Fig. S4), further supporting the idea that stomatal kinetics influence overall gs behavior and final values achieved. Such a relationship has previously been shown for tobacco, with the greater the change the higher the gs value achieved (von Caemmerer et al., 2004). Together, these findings indicate that both anatomical and biochemical/physiological components determine the speed of gs responses (Lawson and Blatt, 2014) and that both the rapidity in stomatal responses and the magnitude of change influence gs values. Growth under e[CO2] reduced the magnitude of change in gs following the step increase in PPFD; however, it is clear that this was driven by differences in minimum gs and not the maximum achieved (gsF). This could be due to differences in SD with growth under e[CO2] or that guard cell sensitivity to [CO2] was reduced under these conditions, ultimately increasing gs at low light (Hetherington and Woodward, 2003; Chater et al., 2015). The final gs values (gsF) positively correlated with AF at ambient and elevated [CO2] (Supplementary Fig. S4), clearly demonstrating a diffusional constraint on photosynthetic induction rates, and highlights the importance of stomatal behavior in carbon assimilation (Lawson et al., 2012; De Souza et al., 2020; Long et al., 2022). gsF also positively correlated with ∆A, providing further support for a diffusional constraint on A. The fact that a similar relationship was not observed in plants grown at e[CO2] is most probably due to gsF not being influenced by growth at e[CO2] and therefore decreased gs control on CO2 diffusion and A. The negatively correlation between gsF and τA (at both growth [CO2]) illustrates the importance of gs in photosynthetic induction (Lawson et al., 2010; Long et al., 2022). Furthermore, the tight correlation between ∆A and AF (Supplementary Fig. S4) suggests that photosynthetic capacity at low PPFD was less variable than at high PPFD, and the final A reached depends on the magnitude and kinetic changes in A, that are driven by both stomatal and biochemical traits (Lawson et al., 2012).
The kinetic responses revealed more variation between accessions in both A and gs at e[CO2] compared with ambient; however, the two compensated for changes in one relative to the other to maintain a similar WUEi to plants grown in ambient conditions. This demonstrates the importance of measuring both physiological components that make up WUEi as well as the attributing anatomical features (Lawson et al., 2010). The strong correlation between the speed of gs at ambient [CO2] and e[CO2] indicates that the rapidity of gs depends on stomatal anatomy and biochemistry, and not only differences in photosynthetic biochemistry. This is also supported by the lack of any influence that growth [CO2] had on Vcmax, Amax, and Jmax (Supplementary Fig. S6). Therefore, stomatal speed is an inherent trait within these species and cultivars, supporting the notion that such a phenotype could be a key trait which could be incorporated for future breeding programmes.
In conclusion, this study has demonstrated that there is significant variation between species and cultivars in stomatal anatomy and function as well as photosynthetic capacity, and that growth at e[CO2] does not necessarily impact on all of them, or in the same way. Current hexaploid bread wheat has a number of desirable traits, such as larger leaves and higher photosynthetic capacity, lower SD with a small Δgs (and therefore potential water saving capacity) compared with their WRs. Furthermore, SD in these species was not influenced by growth at e[CO2]. It is possible that these traits have been unintentionally selected for during the breeding process. However, the WRs have much faster stomatal kinetics compared with modern wheat species, and although here this did not directly translate into improved A (as in previous studies) it was directly related to gsF which correlated significantly with Amax (Supplementary Fig. S4), suggesting some reduced stomatal diffusional constraints on A in cultivars with greater Δgs. Such phenotyping traits could also be beneficial for increased WUEi as well as maintaining optimal leaf temperatures, highlighting the potential to exploit natural variation in different species, WRs, and elite crop varieties to develop idiotypes to maintain productivity in future climates.
Supplementary data
The following supplementary data are available at JXB online.
Fig. S1. Correlation between SD and GCL of 11 wheat species grown at a[CO2] and e[CO2].
Fig. S2. Variation of flag leaf guard cell length of both abaxial and adaxial leaf surfaces for 11 wheat species grown at a[CO2] and e[CO2].
Fig. S3. Correlations between the time constant for stomatal opening of wheat species grown at a[CO2] and e[CO2].
Fig. S4. Correlation between kinetic parameters for 11 wheat species grown at a[CO2] and e[CO2].
Fig. S5. The response of net CO2 assimilation to intercellular [CO2] under saturating PPFD for 11 wheat species grown at a[CO2] and e[CO2].
Fig. S6. Photosynthetic capacity including the maximum RuBP-saturated rate of carboxylation, the maximum RuBP-saturated rate of carboxylation, and the light- and CO2-saturated rate of photosynthesis for 11 wheat species grown at a[CO2] and e[CO2].
Fig. S7. Variation of flag leaf area for 11 wheat species grown at a[CO2] and e[CO2].
Fig. S8. Variation of flag leaf dry weight for 11 wheat species grown at a[CO2] and e[CO2].
Fig. S9. Variation of flag leaf thickness for 11 wheat species grown at a[CO2] and e[CO2].
Acknowledgements
We would like to acknowledge the team of from BASF, Belgium for assistance and support with growing plants and data collection.
Contributor Information
Shellie Wall, School of Life Sciences, University of Essex, Colchester CO4 3SQ, UK.
James Cockram, NIAB, 93 Lawrence Weaver Road, Cambridge CB3 0LE, UK.
Silvere Vialet-Chabrand, School of Life Sciences, University of Essex, Colchester CO4 3SQ, UK.
Jeroen Van Rie, BASF Belgium Coordination Center CommV-Innovation Center Gent, Technologiepark-Zwijnaarde 101, 9052 Gent, Belgium.
Alexander Gallé, BASF Belgium Coordination Center CommV-Innovation Center Gent, Technologiepark-Zwijnaarde 101, 9052 Gent, Belgium.
Tracy Lawson, School of Life Sciences, University of Essex, Colchester CO4 3SQ, UK.
John Lunn, MPI of Molecular Plant Physiology, Germany.
Author contributions
SW and TL: designing the experiments and writing the manuscript; SW: performing all experiments and data acquisition; SW, SVC, and TL: data analysis; SVC: modeling and analyzing the induction data. All authors contributed to editing the manuscript.
Conflict of interest
No conflict of interest declared.
Funding
SW was supported by a Biotechnology and Biological Sciences Research Council (BBSRC) industrial studentship (1775930) awarded to BASF (JVR), Essex (TL), and NAIB (JC). SV-C was supported by the Global Challenges Research Fund as part of TIGR2ESS: Transforming India’s Green Revolution by Research and Empowerment for Sustainable Food Supplies (BB/P027970/1) awarded to TL. TL also acknowledges funding support through the BBSRC IWYP Programme (BB/S005080/1).
Data availability
The data that support the findings of this study are openly available from this link: http://researchdata.essex.ac.uk/165/
References
- Acevedo-Siaca LG, Dionora J, Laza R, Paul Quick W, Long SP.. 2021. Dynamics of photosynthetic induction and relaxation within the canopy of rice and two wild relatives. Food and Energy Security 10, e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth EA, Long SP.. 2005. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytologist 165, 351–371. doi: 10.1111/j.1469-8137.2004.01224.x. [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Long SP.. 2021. 30 years of free-air carbon dioxide enrichment (FACE): what have we learned about future crop productivity and its potential for adaptation? Global Change Biology 27, 27–49. doi: 10.1111/gcb.15375. [DOI] [PubMed] [Google Scholar]
- Asseng S, Guarin JR, Raman M, Monje O, Kiss G, Despommier DD, Meggers FM, Gauthier PPG.. 2020. Wheat yield potential in controlled-environment vertical farms. Proceedings of the National Academy of Sciences, USA 117, 19131–19135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun HJ, Atlin GPT.. 2010. Multi-location testing as a tool to identify plant response to global climate change. In: Reynolds MP, ed. Climate change and crop production. Wallingford, UK: CABI Publishing,115–138. [Google Scholar]
- Buckley TN. 2017. Modeling stomatal conductance. Plant Physiology 174, 572–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley TN, Mott KA.. 2013. Modelling stomatal conductance in response to environmental factors. Plant, Cell & Environment 36, 1691–1699. [DOI] [PubMed] [Google Scholar]
- Bussis D, von Groll U, Fisahn J, Altmann T.. 2006. Stomatal aperture can compensate altered stomatal density in Arabidopsis thaliana at growth light conditions. Functional Plant Biology 33, 1037–1043. [DOI] [PubMed] [Google Scholar]
- Carmo-Silva E, Andralojc PJ, Scales JC, Driever SM, Mead A, Lawson T, Raines CA, Parry MAJ.. 2017. Phenotyping of field-grown wheat in the UK highlights contribution of light response of photosynthesis and flag leaf longevity to grain yield. Journal of Experimental Botany 68, 3473–3486. doi: 10.1093/jxb/erx169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Silva AE, Keys AJ, Andralojc PJ, Powers SJ, Arrabaça, MCParry, MAJ. 2010. Rubisco activities, properties, and regulation in three different C4 grasses under drought. Journal of Experimental Botany 61, 2355–2366. doi: 10.1093/JXB/ERQ071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charmet G. 2011. Wheat domestication: lessons for the future. Comptes Rendus Biologies 334, 212–220. [DOI] [PubMed] [Google Scholar]
- Chater C, Peng K, Movahedi M, et al. 2015. Elevated CO2-induced responses in stomata require ABA and ABA signaling. Current Biology 25, 2709–2716. doi: 10.1016/j.cub.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxdale JL. 2000. Stomatal patterning in angiosperms. American Journal of Botany 87, 1069–1080. [PubMed] [Google Scholar]
- De Souza AP, Burgess SJ, Doran L, Hansen J, Manukyan L, Maryn N, Gotarkar D, Leonelli L, Niyogi KK, Long SP.. 2022. Soybean photosynthesis and crop yield are improved by accelerating recovery from photoprotection. Science 377, 851–854. [DOI] [PubMed] [Google Scholar]
- De Souza AP, Wang Y, Orr DJ, Carmo-Silva E, Long SP.. 2020. Photosynthesis across African cassava germplasm is limited by Rubisco and mesophyll conductance at steady state, but by stomatal conductance in fluctuating light. New Phytologist 225, 2498–2512. doi: 10.1111/NPH.16142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doheny-Adams T, Hunt L, Franks PJ, Beerling DJ, Gray JE.. 2012. Genetic manipulation of stomatal density influences stomatal size, plant growth and tolerance to restricted water supply across a growth carbon dioxide gradient. Philosophical Transactions of the Royal Society B: Biological Sciences 367, 547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow GJ, Berry JA, Bergmann DC.. 2014. The physiological importance of developmental mechanisms that enforce proper stomatal spacing in Arabidopsis thaliana. New Phytologist 201, 1205–1217. doi: 10.1111/nph.12586. [DOI] [PubMed] [Google Scholar]
- Drake PL, Froend RH, Franks PJ.. 2013. Smaller, faster stomata: scaling of stomatal size, rate of response, and stomatal conductance. Journal of Experimental Botany 64, 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driever SM, Lawson T, Andralojc PJ, Raines CA, Parry MAJJ.. 2014. Natural variation in photosynthetic capacity, growth, and yield in 64 field-grown wheat genotypes. Journal of Experimental Botany 65, 4959–4973. doi: 10.1093/jxb/eru253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusenge ME, Duarte AG, Way DA.. 2019. Plant carbon metabolism and climate change: elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytologist 221, 32–49. doi: 10.1111/NPH.15283. [DOI] [PubMed] [Google Scholar]
- Dvorak J, Akhunov ED.. 2005. Tempos of gene locus deletions and duplications and their relationship to recombination rate during diploid and polyploid evolution in the Aegilops–Triticum alliance. Genetics 171, 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott-Kingston C, Haworth M, Yearsley JM, Batke SP, Lawson T, McElwain JC.. 2016. Does size matter? Atmospheric CO2 may be a stronger driver of stomatal closing rate than stomatal size in taxa that diversified under low CO2. Frontiers in Plant Science 7, 1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO. 2014. Wheat—the largest primary commodity. Rome: Food and Agriculture Organization. [Google Scholar]
- Faralli M, Bontempo L, BianchediPL, Moser C, Bertamini M, Lawson T, Camin F, Stefanini M, Varotto C.. 2022. Natural variation in stomatal dynamics drives divergence in heat stress tolerance and contributes to the seasonal intrinsic water-use efficiency in Vitis vinifera (subsp. sativa and sylvestris). Journal of Experimental Botany 73, 617–648. [DOI] [PubMed] [Google Scholar]
- Faralli M, Cockram J, Ober E, Wall S, Galle A, Van Rie J, Raines C, Lawson C.. 2019. Genotypic, developmental and environmental effects on the rapidity of gs in wheat: impacts on carbon gain and water-use efficiency. Frontiers in Plant Science 10, 492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faralli M, Lawson T.. 2020. Natural genetic variation in photosynthesis: an untapped resource to increase crop yield potential? The Plant Journal 101, 518–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faris JD. 2014. Wheat domestication: key to agricultural revolutions past and future. In: Tuberosa R, Graner A, Frison E, eds. Genomics of plant genetic resources., pp. 439–464. Springer, Dordrecht, the Netherlands. [Google Scholar]
- Farquhar GD, Sharkey TD.. 1982. Stomatal conductance and photosynthesis. Annual Review of Plant Physiology 33, 317–345. [Google Scholar]
- Franks PJ, Farquhar GD.. 2001. The effect of exogenous abscisic acid on stomatal development, stomatal mechanics, and leaf gas exchange in Tradescantia virginiana. Plant Physiology 125, 935–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks P, Farqhuar GD.. 2007. The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiology 143, 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks PJ, Freckleton RP, Beaulieu JM, Leitch IJ, Beerling DJ.. 2012. Megacycles of atmospheric carbon dioxide concentration correlate with fossil plant genome size. Philosophical Transactions of the Royal Society B: Biological Sciences 367, 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison EL, Arce Cubas L, Gray JE, Hepworth C.. 2020. The influence of stomatal morphology and distribution on photosynthetic gas exchange. The Plant Journal 101, 768–779. doi: 10.1111/tpj.14560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington AM, Woodward FI.. 2003. The role of stomata in sensing and driving environmental change. Nature 424, 901–908. [DOI] [PubMed] [Google Scholar]
- Huang S, Sirikhachornkit A, Su X, Faris J, Gill B, Haselkorn R, Gornicki P.. 2002. Genes encoding plastid acetyl-CoA carboxylase and 3-phosphoglycerate kinase of the Triticum/Aegilops complex and the evolutionary history of polyploid wheat. Proceedings of the National Academy of Sciences, USA 99, 8133–8138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC. 2021. Climate Change 2021: the physical science basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Kerber ER, Rowland GG.. 1974. Origin of the free threshing character in hexaploid wheat. Canadian Journal of Genetics and Cytology 16, 145–154. [Google Scholar]
- Knapp AK, Fahnestock JT, Owensby CE.. 1994. Elevated atmospheric CO2 alters stomatal responses to variable sunlight in a C4 grass. Plant, Cell & Environment 17, 189–195. [Google Scholar]
- Kuhlgert S, Austic G, Zegarac R, et al. 2016. MultispeQ Beta: a tool for large-scale plant phenotyping connected to the open photosynQ network. Royal Society Open Science 3, 160592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson T, Blatt M.. 2014. Stomatal size, speed and responsiveness impact on photosynthesis and water use efficiency. Plant Physiology 164, 1556–1570. doi: 10.1104/pp.114.237107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson T, Craigon J, Black CR, Colls JJ, Tullock AM, Landon G.. 2001. Effects of elevated carbon dioxide and ozone on the growth and yield of potatoes (Solanum tuberosum) grown in open-top chambers. Environmental Pollution 111, 479–491. doi: 10.1016/S0269-7491(00)00080-4. [DOI] [PubMed] [Google Scholar]
- Lawson T, Craigon J, Black CR, Colls JJ, Landon G, Weyers JDB.. 2002. Impact of elevated CO2 and O3 on gas exchange parameters and epidermal characteristics in potato (Solanum tuberosum L.). Journal of Experimental Botany 53, 737–746. doi: 10.1016/s0269-7491(00)00080-4. [DOI] [PubMed] [Google Scholar]
- Lawson T, Kramer DM, Raines CA.. 2012. Improving yield by exploiting mechanisms underlying natural variation of photosynthesis. Current Opinions in Plant Biotechnology 23, 215–220. doi: 10.1016/j.copbio.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Lawson T, Morison JI.. 2004. Stomatal function and physiology. In: Hemsley AR, Poole I, eds. The evolution of plant physiology: from whole plants to ecosystem. Cambridge, UK: Elsevier Academic Press, 217–242. [Google Scholar]
- Lawson T, Terashima I, Fujita T, Wang Y.. 2018. Co-ordination between photosynthesis and stomatal behaviour. In: Sharkey TD, Govindje, eds. The leaf: a platform for performing photosynthesis and feeding the plant. Cham: Springer, 141–161. [Google Scholar]
- Lawson T, Vialet-Chabrand S.. 2019. Speedy stomata, photosynthesis and plant water use efficiency. New Phytologist 221, 93–98. doi: 10.1111/nph.15330. [DOI] [PubMed] [Google Scholar]
- Lawson T, von Caemmerer S, Baroli I.. 2010. Photosynthesis and stomatal behaviour. Progress in Botany 72. doi: 10.1007/978-3-642-13145-5_11. [DOI] [Google Scholar]
- Lawson T, Weyers JDB, A’brook R.. 1998. The nature of heterogeneity in stomatal behaviour of Phaseolus vulgaris L. primary leaves. Journal of Experimental Botany 49, 1387–1395. doi: 10.1093/jxb/49.325.1387. [DOI] [Google Scholar]
- Leakey ADB, Ainsworth EA, Bernacchi CJ, Rogers A, Long SP, Ort DR.. 2009. Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. Journal of Experimental Botany 60, 2859–2876. doi: 10.1093/jxb/erp096. [DOI] [PubMed] [Google Scholar]
- Lobell DB, Schlenker W, Costa-Roberts J.. 2011. Climate trends and global crop production since 1980. Science 333, 616–620. [DOI] [PubMed] [Google Scholar]
- Lodge RJ, Dijkstra P, Drake BG, Morison JIL.. 2001. Stomatal acclimation to increased CO2 concentration in a Florida scrub oak species Quercus myrtifolia Willd. Plant, Cell & Environment 24, 77–88. doi: 10.1046/j.1365-3040.2001.00659.x. [DOI] [Google Scholar]
- Long SP, Taylor SH, Burgess SJ, Carmo-Silva E, Lawson T, De Souza A, Leonelli L, Wang Y.. 2022. Into the shadows and back into sunlight: photosynthesis in fluctuating light. Annual Review of Plant Biology 73, 617–648. [DOI] [PubMed] [Google Scholar]
- López-Calcagno PE, Brown KL, Simkin AJ, Fisk SJ, Vialet-Chabrand S, Lawson T, Raines CA.. 2020. Stimulating photosynthetic processes increases productivity and water-use efficiency in the field. Nature Plants 6, 1054–1063. doi: 10.1038/s41477-020-0740-1. [DOI] [PubMed] [Google Scholar]
- Mansfield TA, Hetherington AM, Atkinson CJ.. 1990. Some current aspects of stomatal physiology. Annual Review of Plant Physiology and Plant Molecular Biology 41, 55–75. [Google Scholar]
- Marcussen T, Sandve SR, Heier L, et al. 2014. Ancient hybridizations among the ancestral genomes of bread wheat. Science 345, 1250092. [DOI] [PubMed] [Google Scholar]
- Matthews JA, Lawson T.. 2019. Climate change and stomatal physiology. Annual Plant Reviews 2, doi: 10.1002/9781119312994.apr0667 [DOI] [Google Scholar]
- Matthews JA, Vialet-Chabrand SR, Lawson T.. 2017. Diurnal variation in gas exchange: the balance between carbon fixation and water loss. Plant Physiology 174, 614–623. doi: 10.1104/pp.17.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews JS, Vialet-Chabrand SR, Lawson T.. 2018. Acclimation to fluctuating light impacts the rapidity and diurnal rhythm of stomatal conductance. Plant Physiology 176, 1939–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAusland L, Vialet-Chabrand S, Davey P, Baker NR, Brendel O, Lawson T.. 2016. Effects of kinetics of light-induced stomatal responses on photosynthesis and water-use efficiency. New Phytologist 211, 1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAusland L, Vialet-Chabrand S, Jauregui I, et al. 2020. Variation in key leaf photosynthetic traits across wheat wild relatives is accession-dependent not species-dependent. New Phytologist 228, 1767–1780. [DOI] [PubMed] [Google Scholar]
- McElwain JC, Montañez IP, White JD, Wilson JP.. 2016. Was atmospheric CO2 capped at 1000 ppm over the past 300 million years? Palaeogeography, Palaeoclimatology, Palaeoecology 441, 653–658. [Google Scholar]
- McElwain JC, Yiotis C, Lawson T.. 2015. Using modern plant trait relationships between observed and theoretical maximum stomatal conductance and vein density to examine patterns of plant macroevolution. New Phytologist 209, 94–103. doi: 10.1111/nph.13579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CA, Meacham-Hensold K, Lemonnier P, Slattery RA, Benjamin C, Bernacchi C, Lawson T, Cavanagh AP.. 2021. The effect of increasing temperature on crop photosynthesis: from enzymes to ecosystems. Journal of Experimental Botany 72, 2822–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morison JIL, Baker N, Mullineaux P, Davies W.. 2008. Improving water use in crop production. Philosophical Transactions of the Royal Society B: Biological Sciences 363, 639–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RR, Emblow MSM, Hetherington AM, Foster GD.. 2016. Plant virus infections control stomatal development. Scientific Reports 6, 34507. doi: 10.1038/srep34507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOAA. 2022. Climate change: atmospheric carbon dioxide. Earth System Research Laboratory. Available at: www.esrl.noaa.gov. Accessed: 20/07/2022. [Google Scholar]
- Papanatsiou M, Petersen J, Henderson L, Wang Y, Christie JM, Blatt MR.. 2019. Optogenetic manipulation of stomatal kinetics improves carbon assimilation, water use, and growth. Science 363, 1456–1459. [DOI] [PubMed] [Google Scholar]
- Peng JH, Dongfa S, Nevo SE, Peng JH, Sun D, Nevo E.. 2011. Domestication evolution, genetics and genomics in wheat. Molecular Breeding 28, 281–301. [Google Scholar]
- Peterson KM, Rychel AL, Torii KU.. 2010. Out of the mouths of plants: the molecular basis of the evolution and diversity of stomatal development. The Plant Cell 22, 296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignon CP, Leakey ADB, Long SP, Kromdijk J.. 2021. Drivers of natural variation in water-use efficiency under fluctuating light are promising targets for improvement in Sorghum. Frontiers in Plant Science 12, 627432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole I, Weyers JDB, Lawson T, Raven JA.. 1996. Variations in stomatal density and index: implications for palaeoclimatic reconstructions. Plant, Cell & Environment 19, 705–712. [Google Scholar]
- Qu M, Essemine J, Xu J, et al. 2020. Alterations in stomatal response to fluctuating light increase biomass and yield of rice under drought conditions. The Plant Journal 104, 1334–1347. [DOI] [PubMed] [Google Scholar]
- Qu M, Hamdani S, Li W, et al. 2016. Rapid stomatal response to fluctuating light: an under-explored mechanism to improve drought tolerance in rice. Functional Plant Biology 43, 727. [DOI] [PubMed] [Google Scholar]
- Raven JA. 1977. The evolution of vascular land plants in relation to supracellular transport processes. Advances in Botanical Research 5, 153–219. [Google Scholar]
- Raven JA. 2002. Selection pressures on stomatal evolution. New Phytologist 153, 371–386. [DOI] [PubMed] [Google Scholar]
- Rogers A, Allen DJ, Davey PA, et al. 2004. Leaf photosynthesis and carbohydrate dynamics of soybeans grown throughout their life-cycle under free-air carbon dioxide enrichment. Plant, Cell & Environment 27, 449–458. doi: 10.1111/J.1365-3040.2004.01163.X. [DOI] [Google Scholar]
- Ruiz-Vera UM, De Souza AP, Long SP, Ort DR.. 2017. The role of sink strength and nitrogen availability in the downregulation of photosynthetic capacity in field grown Nicotiana tabacum L. at elevated CO2 concentration. Frontiers in Plant Science 8, 998. doi: 10.3389/FPLS.2017.00998/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakoda K, Adachi S, Yamori W, Tanaka Y.. 2022. Towards improved dynamic photosynthesis in C3 crops by utilizing natural genetic variation. Journal of Experimental Botany 73, 3109–3121. doi: 10.1093/jxb/erac100. [DOI] [PubMed] [Google Scholar]
- Sakoda K, Yamori W, Shimada T, Sugano SS, Hara-Nishimura I, Tanaka Y.. 2020. Higher stomatal density improves photosynthetic induction and biomass production in Arabidopsis under fluctuating light. Frontiers in Plant Science 11, 589603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlüter U, Muschak M, Berger D, Altmann T.. 2003. Photosynthetic performance of an Arabidopsis mutant with elevated stomatal density (sdd1-1) under different light regimes. Journal of Experimental Botany 54, 867–874. [DOI] [PubMed] [Google Scholar]
- Sharkey DT, Bernacchi CI, Farquhar GD, Singsaas EL.. 2007. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant, Cell & Environment 30, 1035–1040. [DOI] [PubMed] [Google Scholar]
- Sharwood RE, Quick WP, Sargent D, Estavillo GM, Silva-Perez V, Furbank RT.. 2022. Mining for allelic gold: finding genetic variation in photosynthetic traits in crops and wild relatives. Journal of Experimental Botany 73, 3085–3108. doi: 10.1093/jxb/erac081. [DOI] [PubMed] [Google Scholar]
- Smith S, Weyers JDB, Berry WG.. 1989. Variation in stomatal characteristics over the lower surface of Commelina communis leaves. Plant, Cell & Environment 12, 653–659. [Google Scholar]
- Sreeharsha RV, Sekhar KM, Reddy AR.. 2015. Delayed flowering is associated with lack of photosynthetic acclimation in Pigeon pea (Cajanus cajan L) grown under elevated CO2. Plant Science 231, 82–93. [DOI] [PubMed] [Google Scholar]
- Stevens J, Faralli M, Wall S, Stamford JD, Lawson T.. 2021. Stomatal responses to climate change. In: Becklin K, Ward K, Way DA, eds. Photosynthesis, respiration and climate change. Cham: Springer, 17–47. [Google Scholar]
- Stockwell C, Geiges A, Ramalope D, Gidden M, Hare B, de Villafranca Casa MJ, Moisio M, Hans F, Fekete H.. 2021. Glasgow’s 2030 credibility gap: net zero’s lip service to climate action. https://climateactiontracker.org/publications/glasgows-2030-credibility-gap-net-zeros-lip-service-to-climate-action/
- Takahashi S, Monda K, Negi J, Konishi F, Ishikawa S, Hashimoto-Sugimoto M, Goto N, Iba K.. 2015. Natural variation in stomatal responses to environmental changes among Arabidopsis thaliana ecotypes. PLoS One 10, e0117449. doi: 10.1371/journal.pone.0117449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Sugano SS, Shimada T, Hara-Nishimura I.. 2013. Enhancement of leaf photosynthetic capacity through increased stomatal density in Arabidopsis. New Phytologist 198, 757–764. [DOI] [PubMed] [Google Scholar]
- Tans P, Keeling R.. 2016. Trends in atmospheric carbon dioxide. NOAA. Available online at: http://www.esrl.noaa.gov/gmd/ccgg/trends. Accessed: 20/ July 2022.
- Ticha I. 1982. Photosynthetic characteristics during ontogenesis of leaves. 7. Stomata density and sizes. Photosynthetica 16, 375–471. [Google Scholar]
- Tsutsumi K, Konno M, Miyazawa SI, Miyao M.. 2014. Sites of action of elevated CO2 on leaf development in rice: discrimination between the effects of elevated CO2 and nitrogen deficiency. Plant and Cell Physiology 55, 258–268. doi: 10.1093/jxb/erac447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialet-Chabrand S, Dreyer E, Brendel O.. 2013. Performance of a new dynamic model for predicting diurnal time courses of stomatal conductance at the leaf level. Plant, Cell & Environment 36, 1529–1546. [DOI] [PubMed] [Google Scholar]
- Vialet-Chabrand S, Lawson T.. 2019. Dynamic leaf energy balance: deriving stomatal conductance from thermal imaging in a dynamic environment. Journal of Experimental Botany 70, 2839–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialet-Chabrand S, Lawson T.. 2020. Thermography methods to assess stomatal behaviour in a dynamic environment. Journal of Experimental Botany 71, 2329–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialet-Chabrand S, Matthews JSA, Brendel O, Blatt M, Wang Y, Hills A, Griffiths H, Rogers S, Lawson T.. 2016. Modelling water use efficiency in a dynamic environment: an example using Arabidopsis thaliana. Plant Science 251, 65–74. doi: 10.1016/j.plantsci.2016.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialet-Chabrand SR, Matthews JSA, McAusland L, Blatt M, Griffiths H, Lawson T.. 2017. Temporal dynamics of stomatal behaviour: modelling, and implications for photosynthesis and water use. Plant Physiology 174, 603–613. doi: 10.1104/pp.17.00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Caemmerer S, Farquhar GD.. 1981. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153, 376–387. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Lawson T, Oxborough K, Baker NR, Andrews TJ, Raines CA.. 2004. Stomatal conductance does not correlate with photosynthetic capacity in transgenic tobacco with reduced amounts of Rubisco. Journal of Experimental Botany 55, 1157–1166. [DOI] [PubMed] [Google Scholar]
- Wall S, Vialet-Chabrand S, Davey P, Van Rie J, Galle A, Cockram J, Lawson T.. 2022. Stomata on the abaxial and adaxial leaf surface contribute differently to leaf gas exchange and photosynthesis in wheat. New Phytologist 235, 1743–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyers JDB, Johansen LG.. 1985. Accurate estimation of stomatal aperture from silicone rubber impressions. New Phytologist 101, 109–115. [DOI] [PubMed] [Google Scholar]
- Weyers JDB, Lawson T.. 1997. Heterogeneity in stomatal characteristics. Advances in Botanical Research 26, 317–352. [Google Scholar]
- Weyers JDB, Lawson T, Peng ZY.. 1997. Variation in stomatal characteristics at the whole-leaf level. In: Van Gardingen PR, Foody GM, Curran PJ, eds. Scaling-up from cell to landscape. Cambridge, UK: Cambridge University Press, 129–149. [Google Scholar]
- Willmer C, Fricker M.. 1996. Stomata. Dordrecht: Springer Netherlands. [Google Scholar]
- Woodward FI. 1987. Stomatal numbers are sensitive to increases in CO2 from pre-industrial levels. Nature 327, 617–618. [Google Scholar]
- World Meteorological Organization. 2022. State of the global climate 2021. https://library.wmo.int/index.php?lvl=notice_displayandid=21880#.Yw9oPnbMKUl [Google Scholar]
- Yamori W, Kusumi K, Iba K, Terashima I.. 2020. Increased stomatal conductance induces rapid changes to photosynthetic rate in response to naturally fluctuating light conditions in rice. Plant, Cell & Environment 43, 1230–1240. [DOI] [PubMed] [Google Scholar]
- Yin X, Gu J, Dingkuhn M, Struik PC.. 2022. A model-guided holistic review of exploiting natural variation of photosynthesis traits in crop improvement. Journal of Experimental Botany 73, 3173–3188. doi: 10.1093/jxb/erac109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadoks JC, Chang TT, Konzak CF.. 1974. A decimal code for the growth stages of cereals. Weed Research 14, 415–421. [Google Scholar]
- Zhang Q, Peng S, Li Y.. 2019. Increase rate of light-induced stomatal conductance is related to stomatal size in the genus Oryza. Journal of Experimental Botany 70, 5259–5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are openly available from this link: http://researchdata.essex.ac.uk/165/