ABSTRACT
Backgrounds and Objectives:
Although pancreatic cancer (PC) has an extremely poor prognosis, the 5-year survival rate of patients with pancreatic high-grade precancerous lesion without invasive carcinoma (PHP) is favorable. PHP diagnosis and identification of patients requiring intervention are needed. We aimed to validate a modified PC detection scoring system regarding its detection ability for PHP and PC in the general population.
Subjects and Methods:
We modified an existing PC detection scoring system that incorporates low-grade risk (LGR) factors (family history, presence of diabetes mellitus [DM] or worsening DM, heavy drinking, smoking, stomach symptoms, weight loss, and pancreatic enzyme) and high-grade risk (HGR) factors (new-onset DM, familial PC, jaundice, tumor biomarkers, chronic pancreatitis, intraductal papillary mucinous neoplasm, cysts, hereditary PC syndrome, and hereditary pancreatitis). Each factor was scored as one point; LGR score ≥3 points and/or HGR score ≥1 point (positive scores) were indicative of PC. The newly modified scoring system incorporated main pancreatic duct dilation as an HGR factor. The PHP diagnosis rate using this scoring system combined with EUS was prospectively analyzed.
Results:
Among 544 patients with positive scores, 10 had PHP. The diagnosis rates were 1.8% for PHP and 4.2% for invasive PC. Although the number of LGR and HGR factors tended to increase with PC progression, none of the individual factors were significantly different between patients with PHP and those without lesions.
Conclusion:
The newly modified scoring system evaluating multiple factors associated with PC could potentially identify patients with higher risk of PHP or PC.
Keywords: EUS, intraductal papillary mucinous neoplasm, pancreatic cancer, pancreatic intraepithelial neoplasia, screening
INTRODUCTION
Pancreatic cancer (PC) is associated with an extremely poor prognosis, with an overall 5-year survival rate of <10%.[1] In contrast, the 5-year survival rate of patients with Union for International Cancer Control stage 0 PC (in situ), defined as a pancreatic high-grade precancerous lesion (PHP) in the absence of invasive carcinoma,[2,3] is 85.8%. Therefore, early diagnosis is essential;[4] however, the incidence of PHP among all patients with PC is only 1.7%.[4] Surveillance efforts for this disease in asymptomatic high-risk patients with PC-associated gene mutations or familial PC are gradually increasing worldwide.[5,6,7,8,9,10] However, only 5%–10% of patients with PC have familial risks, whereas 3%–5% have inherited genetic syndromes.[11] Therefore, a method for reliable PC surveillance of the general population that is not limited to those with familial and genetic risks is required. The Japan Pancreas Society determined risk factors and clinical symptoms of PC and recommended active screening examinations of the pancreas for eligible patients in its clinical guidelines.[12] Nonetheless, a systematic selection method for the general population whose pancreas requires an intervention has not been established.
In recent years, a new simple scoring system for diagnosing early-stage PC in the general population was developed; PC-related symptoms and risk factors are classified as low-grade risk (LGR) and high-grade risk (HGR) factors.[13] This system can be easily utilized in daily clinical practice and provides the opportunity for initial pancreatic intervention. Notably, PCs with a diameter of ≤20 mm were detectable using the scoring system combined with EUS, with a relatively high detection ability (sensitivity, 100%; specificity, 64.4%).[13] In this prospective single-center study, this scoring system was slightly modified to improve its applicability and effectiveness; we aimed to validate this system in terms of its ability to detect PHP and PC in the general population.
SUBJECTS AND METHODS
Modified scoring system and EUS examination
Previously reported risk factors and characteristic symptoms of PC were classified as LGR and HGR factors according to their degree of relevance to PC.[11,12,13,14] LGR factors included a sporadic family history of PC (nonfamilial PC) in a relation, diabetes mellitus (DM), heavy drinking (≥37.5 g of ethanol/day), current smoking and/or a smoking history, obesity (body mass index ≥25 kg/m2 or being declared as obese by a doctor), stomach symptoms or back pain, relatively abrupt weight loss (≥2 kg within 3 months), DM worsening without a conspicuous cause, and elevated concentration of pancreatic enzyme (amylase).[11,12,13,14,15] HGR factors included new-onset DM (within 2 years), familial PC, jaundice (bilirubin level ≥2.0 mg/dL and increased direct bilirubin level), increased levels of tumor markers (carbohydrate antigen 19–9 and/or carcinoembryonic antigen), recognized medical history of chronic pancreatitis, medical history of intraductal papillary mucinous neoplasm (IPMN), medical history of pancreatic cysts, hereditary PC syndrome, and hereditary pancreatitis.[11,12,13,14,15,16] IPMN was defined as a pancreatic cyst with a clear connection to the pancreatic duct; all other cysts were defined as pancreatic cysts. Each factor was counted as a single point toward the risk score; as we previously reported, the sensitivity and specificity of LGR score ≥3 points (LGR score positive) and/or HGR score ≥1 point (HGR score positive) for small-diameter PCs were 100% and 64.4%, respectively.[13] This scoring system was slightly modified to include a medical history of main pancreatic duct (MPD) dilation (≥2.5 mm in diameter) as an HGR factor, as this was reported to be strongly relevant to PC diagnosis (particularly early-stage disease).[13,17,18,19] Based on a previous study,[13] patients with LGR score ≥3 points and/or HGR score ≥1 point were defined as having positive scores [Figure 1].
Figure 1.
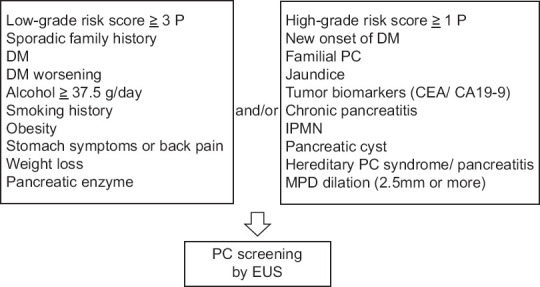
Modified scoring system for the early diagnosis of pancreatic cancer CA19-9, carbohydrate antigen 19–9; CEA, carcinoembryonic antigen; DM, diabetes mellitus; IPMN, intraductal papillary mucinous neoplasm; MPD, main pancreatic duct; PC, pancreatic cancer
All factors related to the updated scoring system were evaluated using questionnaires and blood tests performed before the examination. Nontumorous imaging abnormalities that had already been detected by other radiological modalities before the initial EUS examination (such as pancreatic cysts and MPD dilation) were only evaluated as HGR factors. All screening procedures were performed using a convex-type EUS (UCT-260; Olympus Medical Systems, Tokyo, Japan) combined with an Aloka ultrasound processor (Prosound F75; Aloka, Tokyo, Japan) by two expert endoscopists who have performed over 1500 EUS examinations and have more than 8 years of experience in EUS-based diagnoses of early PC.
Screening strategy and study outcomes
Patients who visited the Department of Gastroenterology at our hospital between March 2018 and July 2019 with any clinical symptoms or risk factors, agreed to participate in the study, and were screened by their initial EUS examination were enrolled. Patients with any pancreatic diseases undergoing repeated imaging examination, including EUS, in the surveillance program were excluded. We also excluded patients who were strongly suggested to have pancreatic tumors using other imaging modalities, such as magnetic resonance imaging (MRI) and computed tomography (CT). Patients with chronic pancreatitis, IPMN, and MPD dilation should originally be incorporated into the surveillance program for more intensive evaluation. Of note, chronic pancreatitis, IPMN, and MPD dilation, which were considered in the scoring system in this study, were first pointed out right before the initial EUS intervention. Therefore, these factors were incorporated beforehand into the surveillance program and were evaluated as the information to give us an opportunity for initial EUS intervention.
Among patients who were initially examined by EUS, those with positive scores were analyzed and classified into four groups, namely, those with PHP; those with any resectable lesions (PHP and resectable PC [resectable lesions]); those with lesions of any type (PHP, resectable PC, and unresectable PC [all lesions]); and those without any of these lesions (without lesions). High-grade pancreatic intraepithelial neoplasia (PanIN), PanIN-3, and IPMN with high-grade dysplasia (high-grade IPMN) are considered PHP with a high risk of progression to invasive carcinoma. Therefore, these lesions requiring surgical management were categorized as PHP in this study.[2,3]
The primary endpoint was the diagnosis rate of PHP without an invasive carcinoma among patients with positive scores. In contrast, the secondary endpoint was the diagnosis rate of invasive PC. In addition, patients with positive scores were examined, and the effectiveness of the LGR/HGR factors and scoring system was analyzed in patients with PHP and PC, compared with those without PHP and PC. Pancreatic cysts, IPMN, MPD dilation, and chronic pancreatitis detected by initial EUS intervention were analyzed to determine whether they were correlated with the presence of PHP and PC. The clinical and imaging characteristics of PHP were also carefully analyzed.
This study was designed and conducted in accordance with the Declaration of Helsinki at our hospital; the study protocol was registered in the University Hospital Medical Information Network Clinical Trials Registry after receiving approval from our Institutional Review Board (No. 139). Informed consent was obtained from each patient before enrollment.
Surgical indication and histopathological diagnosis
When the presence of PC and PHP was strongly suspected based on initial EUS examination findings, we performed additional evaluations with other imaging modalities (such as MRI and CT). We also performed fine-needle aspiration during EUS to identify mass-forming PC, as well as pancreatic juice cytology evaluation during endoscopic retrograde pancreatography (ERP) for nonmass-forming PHP, for preoperative histopathological diagnosis.[17,20] The imaging and cytological findings were reviewed by expert endoscopists, surgeons, radiologists, and pathologists, who together determined the surgical indication for pancreatic resection.
Pancreatic surgical specimens were reassessed by a single pathologist with special expertise in pancreatic pathology. All histological sections were carefully reviewed, and particular attention was paid to the main and branch pancreatic ducts, retention cysts around the dilated MPD, and stenosis for diagnosis. In particular, high-grade PanIN and IPMN were carefully diagnosed. In patients with unresectable PC, the tumor histology was determined using EUS-fine-needle aspiration and biopsy specimens.
Statistical analyses
Continuous variables are expressed as means ± standard deviations depending on the normality of the distribution. Logistic regression analyses were performed on covariates that could potentially influence the detection of PHP and PC. Factors that were significant in the univariate analysis were subjected to multivariate logistic regression analyses; statistical significance was set at P < 0.05. All data analyses were conducted using SPSS (version 28.0; IBM Corp, Armonk, NY, USA).
RESULTS
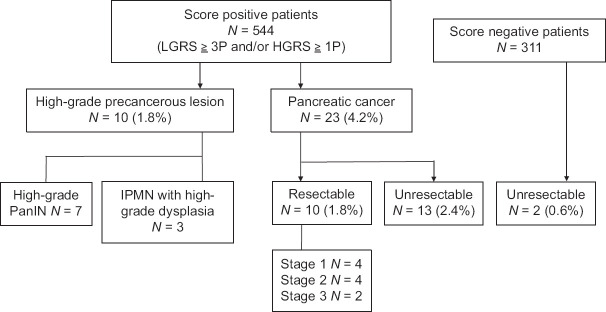
During the study period, 544 patients with positive scores according to our modified system were prospectively enrolled and surveyed using data from their initial EUS. Surgical resections were performed in 22 patients with suspicious PHP or PC lesions. Two patients with low-grade PanIN were categorized as patients without PHP and PC. Finally, the diagnoses of ten patients with PHP (seven with high-grade PanIN and three with high-grade IPMN) and ten patients with resectable PC were histopathologically confirmed. The overall diagnosis rates when using our scoring system in combination with EUS were 1.8% for PHP, 1.8% for resectable PC, 4.2% for all PCs (including ten resectable PCs and 13 unresectable PCs), and 6.1% for all lesions. Furthermore, 311 patients with negative scores according to our system were screened during the same period, and two (0.6%) patients had unresectable PC [Figure 2]. No adverse events due to the EUS surveillance occurred.
Figure 2.
The number of enrolled patients with positive scores and the diagnosis rate for high-grade pancreatic precancerous lesion and invasive pancreatic cancer HGR, high-grade risk; IPMN: Intraductal papillary mucinous neoplasm; LGR: Low-grade risk; PanIN, pancreatic intraepithelial neoplasia
In the diagnosis of patients with PHP, resectable lesions, and all lesions, the significant individual factors and scores were analyzed and compared to those of patients without lesions. Regarding the LGR factor and score analysis, none of the factors were significantly different between patients with PHP and those without pancreatic lesions. Elevated serum pancreatic enzyme was significant in patients with resectable lesions. Stomach symptoms, back pain, weight loss, average LGR score, or LGR score positive were significant in patients with all lesions, and weight loss was the most significant factor in patients with all lesions in the multivariate logistic analysis [Table 1].
Table 1.
Low-grade risk factors associated with the detection of high-grade precancerous or cancerous pancreatic lesions in score positive patients
| Without lesions (n=511) | PHP (n=10) | P (PHP vs. without lesions) | Resectable lesions (n=20) | P (resectable lesions vs. without lesions) | All lesions (n=33) | P (all lesions vs. without lesions) (univariate/multivariate logistic analysis) | |
|---|---|---|---|---|---|---|---|
| Mean age±SD, years | 67.9±12.2 | 70.4±7.1 | 0.526 | 70.6±8.8 | 0.336 | 71.9±9.3 | 0.070 |
| Male sex, n (%) | 312 (61.1) | 8 (80.0) | 0.239 | 16 (80.0) | 0.098 | 21 (63.6) | 0.087 |
| LGRF n (%) | |||||||
| Sporadic family history | 73 (14.3) | 2 (20.0) | 0.613 | 4 (20.0) | 0.479 | 4 (12.1) | 0.730 |
| DM | 185 (36.2) | 3 (30.0) | 0.687 | 6 (30.0) | 0.572 | 11 (33.3) | 0.739 |
| DM worsening | 54 (10.6) | 0 | 0.997 | 1 (5.0) | 0.435 | 3 (9.1) | 0.789 |
| Heavy drinking | 199 (38.9) | 7 (70.0) | 0.062 | 11 (55.0) | 0.156 | 14 (42.4) | 0.692 |
| Smoking history | 153 (29.9) | 4 (40.0) | 0.496 | 10 (50.0) | 0.063 | 13 (39.4) | 0.256 |
| Obesity | 143 (30.0) | 3 (30.0) | 0.888 | 4 (20.0) | 0.437 | 8 (24.2) | 0.642 |
| Stomach symptoms or back pain | 140 (27.4) | 3 (30.0) | 0.855 | 7 (35.0) | 0.458 | 17 (51.5) | 0.004*/0.060 |
| Weight loss | 101 (19.8) | 0 | 0.997 | 7 (35.0) | 0.105 | 18 (54.5) | <0.001*/0.001* |
| Pancreatic enzyme | 63 (12.3) | 3 (30.0) | 0.113 | 6 (30.0) | 0.028* | 8 (24.2) | 0.055 |
| Score valuation | |||||||
| Mean LGRS±SD, point | 2.2±1.5 | 2.5±1.0 | 0.459 | 2.8±1.1 | 0.056 | 2.9±1.0 | 0.004*/0.505 |
| LGRS positive, n (%) | 230 (45.0) | 6 (60.0) | 0.353 | 13 (65.0) | 0.086 | 24 (72.7) | 0.003*/0.222 |
*P<0.05 compared to patients without pancreatic high-grade precancerous lesion and PC. DM: Diabetes mellitus; LGRF: Low-grade risk factors; LGRS: Low-grade risk score; PC: Pancreatic cancer; PHP: Pancreatic high-grade precancerous lesion; SD: Standard deviation
For the HGR factor and score analysis, none of the factors were significantly different between patients with PHP and those without pancreatic lesions. The HGR score was significant in patients with resectable lesions. New-onset DM, jaundice, elevated serum tumor biomarkers, HGR score, and elevated serum tumor biomarkers were the significant factors in patients of all groups, and tumor biomarker was the most significant factor in the multivariate logistic analysis [Table 2]. Sensitivity for PHP patient detection via LGR score positive, HGR score positive, and both scores positive were 60%, 80%, and 100%, respectively.
Table 2.
High-grade risk factors associated with the detection of high-grade precancerous or cancerous pancreatic lesions in score positive patients
| Without lesions (n=511) | PHP (n=10) | P (PHP vs. without lesions) | Resectable lesions (n=20) | P (resectable lesions vs. without lesions) | All lesions (n=33) | P (all lesions vs. without lesions) (univariate/multivariate logistic analysis) | |
|---|---|---|---|---|---|---|---|
| HGRF factor, n (%) | |||||||
| Familial PC | 6 (1.2) | 0 | 0.999 | 0 | 0.999 | 0 | 0.999 |
| New-onset DM | 39 (7.6) | 1 (10.0) | 0.781 | 3 (15.0) | 0.242 | 6 (18.2) | 0.040*/0.268 |
| Jaundice | 13 (2.5) | 0 | 0.999 | 2 (10.0) | 0.069 | 4 (12.1) | 0.006*/0.441 |
| Tumor biomarkers | 131 (25.6) | 2 (20.0) | 0.687 | 9 (45.0) | 0.061 | 22 (66.7) | <0.001*/0.003* |
| Chronic pancreatitis | 22 (4.3) | 0 | 0.998 | 0 | 0.998 | 0 | 0.998 |
| Medical history of IPMN | 76 (14.9) | 2 (20.0) | 0.654 | 4 (20.0) | 0.532 | 4 (12.1) | 0.666 |
| Medical history of pancreatic cyst | 109 (21.3) | 3 (30.0) | 0.512 | 4 (20.0) | 0.887 | 4 (12.1) | 0.214 |
| Hereditary PC syndrome/pancreatitis | 0 | 0 | NA | 0 | NA | 0 | NA |
| MPD dilation | 41 (8.0) | 2 (20.0) | 0.192 | 4 (20.0) | 0.071 | 5 (15.2) | 0.162 |
| Score valuation | |||||||
| Mean HGRS±SD, point | 0.9±0.6 | 1.0±0.5 | 0.417 | 1.3±0.9 | 0.001* | 1.4±0.8 | <0.001*/0.355 |
| HGRS≥1 point, n (%) | 377 (73.8) | 9 (90.0) | 0.272 | 18 (90.0) | 0.122 | 31 (93.9) | 0.020*/0.692 |
*P<0.05 compared to patients without pancreatic high-grade precancerous lesion and PC. DM: Diabetes mellitus; HGRF: High-grade risk factors; HGRS: High-grade risk score; IPMN: Intraductal papillary mucinous neoplasm; MPD: Main pancreatic duct; NA: No assessment; PC: Pancreatic cancer; PHP: Pancreatic high-grade precancerous lesion; SD: Standard deviation
In terms of initial EUS findings, IPMN was significantly associated with the diagnosis of PHP. Moreover, MPD dilation was significantly associated with the detection of PHP, resectable lesions, and all lesions [Table 3].
Table 3.
Initial EUS findings significantly accompanied by high-grade precancerous or cancerous pancreatic lesions
| Without lesions (n=511), n (%) | PHP (n=10), n (%) | P (PHP vs. without lesions), n (%) | Resectable lesions (n=20), n (%) | P (resectable lesions vs. without lesions), n (%) | All lesions (n=33), n (%) | P (all lesions vs. Without lesions), n (%) | |
|---|---|---|---|---|---|---|---|
| Pancreatic cyst | 90 (17.6) | 2 (20.0) | 0.845 | 2 (10.0) | 0.386 | 2 (6.0) | 0.105 |
| IPMN | 148 (28.9) | 6 (60.0) | 0.046* | 8 (40.0) | 0.292 | 8 (24.2) | 0.562 |
| MPD dilation | 87 (17.0) | 9 (90.0) | 0.008* | 15 (75.0) | 0.005* | 22 (66.7) | 0.001* |
| Chronic pancreatitis | 19 (3.7) | 1 (10.0) | 0.328 | 2 (10.0) | 0.176 | 3 (9.0) | 0.143 |
*P<0.05 compared to patients without pancreatic high-grade precancerous lesion and PC. IPMN: Intraductal papillary mucinous neoplasm; MPD: Main pancreatic duct; PC: Pancreatic cancer; PHP: Pancreatic high-grade precancerous lesion
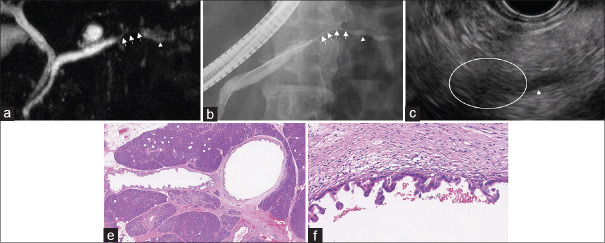
The clinical characteristics of patients with PHP are presented in Table 4. MPD dilation was detected in 90% of the patients (9/10), with a median MPD diameter of 5 mm (range: 2–10 mm). The frequency of stenosis among patients with high-grade PanIN was 57% (4/7), and that of retention cyst and/or concomitant IPMN was 71% (5/7). The imaging and histological findings of a representative high-grade PanIN are shown in Figure 3. In this patient, EUS revealed hypoechoic areas surrounding the MPD stenosis with downstream MPD dilation. MPD stenosis accompanying distal MPD dilation was also detected using MRI and ERP. Scattered high-grade PanIN in the main and branch pancreatic ducts was histologically confirmed; additionally, high-grade PanIN showed inflammatory cell infiltration and fibrosis surrounding the lesion. Furthermore, two patients with high-grade IPMN showed enhancement of a mural nodule (5 and 7 mm), and one patient with high-grade IPMN was diagnosed with main duct type IPMN without a mural nodule.
Table 4.
Clinical characteristics of patients with high-grade pancreatic precancerous lesions
| Age/sex | Location | LGRFLGRS (points) | HGRFHGRS (points) | Image findings | ||
|---|---|---|---|---|---|---|
|
| ||||||
| MPD dilation (mm) | Cyst or IPMN | Histological diagnosis | ||||
| 58/male | Body | Alcohol, smoking, obesity 3 | Cyst 1 | 2.0 | (−) | High-grade PanIN |
| 71/female | Tail | Alcohol, abdominal pain 2 | MPD dilation 1 | 10.0 | (+) | High-grade PanIN |
| 73/male | Tail | DM, alcohol, abdominal pain 3 | None | 3.0 | (+) | High-grade PanIN |
| 70/male | Tail | DM, alcohol, smoking 3 | New-onset DM 1 | 4.0 | (+) | High-grade PanIN |
| 62/male | Head | Alcohol, smoking, pancreatic enzyme 3 | None | 4.5 | (−) | High-grade PanIN |
| 72/female | Body | Obesity 1 | Cyst 1 | 3.0 | (+) | High-grade PanIN |
| 72/male | Body | Family history 1 | Cyst 1 | 2.5 | (+) | High-grade PanIN |
| 66/male | Head | Smoking, abdominal pain, pancreatic enzyme 3 | IPMN 1 | 9.0 | (+) | IPMN with high-grade dysplasia |
| 82/male | Body | Family history, alcohol, obesity 3 | IPMN 1 | 4.5 | (+) | IPMN with high-grade dysplasia |
| 78/male | Body | DM, alcohol 2P | MPD dilation 1P | 10 | (+) | IPMN with high-grade dysplasia |
DM: Diabetes mellitus; HGRF: High-grade risk factor; HGRS: High-grade risk score; IPMN: Intraductal papillary mucinous neoplasm; LGRF: Low-grade risk factor; LGRS: Low-grade risk score; MN: Mural nodule; MPD: Main pancreatic duct; PanIN: Pancreatic intraepithelial neoplasia
Figure 3.
A 70-year-old man with high-grade pancreatic intraepithelial neoplasia (a and b) MRCP, ERP showed pancreatic duct stenosis (arrow) and upstream dilation (arrow head). (c) EUS showed hypoechoic areas surrounding the stenosis (white circle). (e and f) Hematoxylin and eosin; magnification, ×12.5 and ×200, MRCP: Magnetic resonance cholangio pancreatography, ERP: Endoscopic retrograde pancreatography
DISCUSSION
With our modified scoring system, the PHP diagnosis rate was 1.8%, whereas the invasive PC diagnosis rate was 4.3%. The incidence of resectable PCs among all of those detected was relatively high (43.4%); in comparison, <20% of patients with invasive PCs were reported to be eligible for surgical resection.[21] In addition, none of the LGR/HGR factors were significantly different between patients with PHP and those without lesions, whereas elevated concentration of pancreatic enzyme and weight loss were only significantly different between patients with resectable lesions and those with all lesions (PHP and PC) and without PHP and PC, respectively. This suggests that PHP and early-stage PC could be detected by evaluating not only a single specific factor but also multiple factors (positive scores).
In previous studies on patients with certain genetic or strong familial risk of PC, the PHP detection rate ranged from 0.5% to 1.6%.[6,7,8,9] Moreover, a previous literature review reported a detection rate of 0.74% for both PHP and invasive PC,[22] whereas a recently published meta-analysis reported a detection rate of 0.9% for PHP and T1N0M0 pancreatic adenocarcinoma.[23] A relatively large number of subjects with PHP and resectable PC were identified using our modified scoring system compared to screening based on strong familial and genetic risks only. Therefore, a method for the identification of patients who should be surveyed for PC is required; nonetheless, some obstacles remain, given the difficulty in diagnosis and the low incidence of PC (12.9 cases/100,000 person-years).[5] The American Medical Association recommends PC screening only for patients with certain genetic or familial risk of this disease and does not recommend it for the asymptomatic general population with other risk factors[5] because too few cases of PC have been detected in patients at increased familial and genetic risks (1.6%).[10] However, the detection rate of PHP was not reported in previous systematic reviews.
Only 25% of patients with PHP and early-stage PCs have symptoms,[17] which renders early diagnosis difficult. In this study, only three patients with PHP had symptoms, whereas the remaining seven asymptomatic patients were diagnosed based on several factors that provided positive scores rather than genetic or familial risk. To detect PHP and early-stage PCs, careful attention should be paid to the multiple risk factors and clinical symptoms. In this study, LGR score positive provided the chance of finding PHP unexpectedly (sensitivity of 60%), and HGR score positive also provided more chance of PHP detection (sensitivity of 80%). The HGR factors included chronic pancreatitis, IPMN, and MPD dilation, and these lesions should be managed under intensive surveillance. Our present study results reveal that HGR score considering these lesions is informative and provides an opportunity for initial EUS intervention for PHP detection. Of note, LGR and HGR score may need to be assessed separately according to the strength of the risk. However, relatively many patients with PHP and PC were identified in this study according to the initial EUS intervention based on LGR and HGR score analyses.
The number of LGR and HGR factors tended to increase with the progression of PC (from none in patients with PHP to two in those with resectable or all lesions). Consequently, patients with all lesions tended to have significant individual symptoms or risk factors. Therefore, they may be identified efficiently using individual factors. In contrast, patients with early-stage PC, especially PHP, may not be efficiently detected using individual factors only since they may not possess significant individual factors. This study showed that PHP and early-stage PC could be detected by efficient evaluation of the multiple factors.
If the recently published recommendation[5,10] were adopted, PC detection in the asymptomatic general population with risk factors other than familial and genetic risks may be impossible. Several studies on PC screening focused not only on familial risk but also on other factors reportedly associated with relatively high diagnosis rates for PHP and early-stage PC.[13,24] Therefore, PC screening should be established for symptomatic and asymptomatic patients with risk factors.
High-grade PanIN lesions are usually diagnosed in resected pancreatic specimens that also harbor invasive carcinoma.[2,20,25] High-grade PanIN without invasive carcinoma cannot be detected by standard clinical and radiological approaches,[20,26] whereas the detection of minor changes, such as MPD dilation and retention cysts, is useful for detecting curable and noninvasive high-grade neoplasms.[6,20] Microscopic intraepithelial changes in high-grade PanINs could not be readily detected via imaging modalities;[2,20] therefore, indirect imaging findings, such as MPD dilation, stenosis, and retention cysts, play an important role in the detection of high-grade PanIN.[17,18,19,20,27] An enhanced mural nodule in IPMN is also important for the diagnosis of high-grade IPMN.[11] In this study, MPD dilation was found in most patients with PHP, which was consistent with previous studies that showed that MPD dilation was a useful indicator of early-stage PC.[13,17,18,19,20,27] The incorporation of MPD dilation as an HGR factor was, therefore, justified; hence, the existence of high-grade PanIN should be suspected when MPD dilation is detected on imaging.
Approaches toward detecting curable diseases, such as PHP, are crucial for improving the outcomes of patients.[4,17,26] Some studies found that patients with PHP had long survival times that surpassed those of patients with other PC stages,[17,28] thereby indicating that the detection and treatment of PHP should contribute to improving the prognosis of PC. The development of tumor cells in the pancreas takes an average of 12 years, with low-grade PanIN proceeding to high-grade during the final years; subclones with metastatic capacity appear and escape to distant organs over an average of 7 years once high-grade PanIN begins to infiltrate.[29] The window for diagnosing PC before it reaches metastatic potential is prohibitively small.
A strength of our study was that EUS using our modified scoring system produced a relatively high detection rate for PHP and invasive PC. Moreover, we demonstrated the importance of EUS intervention for PHP and resectable PC with our scoring system not only in patients with genetic or familial risks but also in those with several other risk factors and clinical symptoms. However, this study also had some limitations. Our modified scoring system did not assign a weight to each risk factor or symptom in terms of relative association with PC; the accuracy of the scoring system ought to increase if these factors are weighted. The efficacy of surveillance using our system with respect to reducing the morbidity and mortality rates in PC patients with positive scores should be investigated, and the benefits and drawbacks of surgical interventions should be evaluated.[5,10] Furthermore, changes in the quality of life due to screening interventions should be assessed.[30] In addition, some lesions may potentially be missed by EUS and could subsequently progress to invasive cancer. Therefore, patients evaluated using our modified scoring system should continue to be followed. Finally, selection bias of the screened patients may have been present; hence, further confirmation via a prospective comparative multicenter study with long-term observation is necessary for improving the versatility of the data.
CONCLUSION
Our modified scoring system for evaluating multiple risk factors and clinical symptoms offered a high detection rate for PHP and invasive PC, suggesting that it may be an important tool in the early detection of such lesions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Ren B, Liu X, Suriawinata AA. Pancreatic ductal adenocarcinoma and its precursor lesions:Histopathology, cytopathology, and molecular pathology. Am J Pathol. 2019;189:9–21. doi: 10.1016/j.ajpath.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Gill AJ, Klimstra DS, Lam AK. In:WHO Classification of Tumours of the Digestive System. 5th ed. England: World Health Organization; 2019. Tumours of the pancreas; pp. 295–340. [Google Scholar]
- 4.Egawa S, Toma H, Ohigashi H, et al. Japan Pancreatic Cancer Registry;30th year anniversary: Japan Pancreas Society. Pancreas. 2012;41:985–92. doi: 10.1097/MPA.0b013e318258055c. [DOI] [PubMed] [Google Scholar]
- 5.Owens DK, Davidson KW, Krist AH, et al. Screening for pancreatic cancer. JAMA. 2019;322:438. doi: 10.1001/jama.2019.10232. [DOI] [PubMed] [Google Scholar]
- 6.Canto MI, Hruban RH, Fishman EK, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012;142:796–804. doi: 10.1053/j.gastro.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basturk O, Hong SM, Wood LD, et al. Arevised classification system and recommendations from the Baltimore consensus meeting for neoplastic precursor lesions in the pancreas. Am J Surg Pathol. 2015;39:1730–41. doi: 10.1097/PAS.0000000000000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Potjer TP, Schot I, Langer P, et al. Variation in precursor lesions of pancreatic cancer among high-risk groups. Clin Cancer Res. 2013;19:442–9. doi: 10.1158/1078-0432.CCR-12-2730. [DOI] [PubMed] [Google Scholar]
- 9.Vasen H, Ibrahim I, Ponce CG, et al. Benefit of surveillance for pancreatic cancer in high-risk individuals:Outcome of long-term prospective follow-up studies from three European expert centers. J Clin Oncol. 2016;34:2010–9. doi: 10.1200/JCO.2015.64.0730. [DOI] [PubMed] [Google Scholar]
- 10.Henrikson NB, Aiello Bowles EJ, Blasi PR, et al. Screening for pancreatic cancer:Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;322:445–54. doi: 10.1001/jama.2019.6190. [DOI] [PubMed] [Google Scholar]
- 11.Singhi AD, Koay EJ, Chari ST, et al. Early detection of pancreatic cancer:Opportunities and challenges. Gastroenterology. 2019;156:2024–40. doi: 10.1053/j.gastro.2019.01.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamaguchi K, Okusaka T, Shimizu K, et al. Clinical practice guidelines for pancreatic cancer 2016 from the Japan Pancreas Society:A synopsis. Pancreas. 2017;46:595–604. doi: 10.1097/MPA.0000000000000816. [DOI] [PubMed] [Google Scholar]
- 13.Sagami R, Nishikiori H, Anami K, et al. Utility of endoscopic ultrasonography screening for small pancreatic cancer and proposal for a new scoring system for screening. Pancreas. 2018;47:257–64. doi: 10.1097/MPA.0000000000000988. [DOI] [PubMed] [Google Scholar]
- 14.Wolfgang CL, Herman JM, Laheru DA, et al. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63:318–48. doi: 10.3322/caac.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma C, Eltawil KM, Renfrew PD, et al. Advances in diagnosis, treatment and palliation of pancreatic carcinoma:1990-2010. World J Gastroenterol. 2011;17:867–97. doi: 10.3748/wjg.v17.i7.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holly EA, Chaliha I, Bracci PM, et al. Signs and symptoms of pancreatic cancer:A population-based case-control study in the San Francisco Bay area. Clin Gastroenterol Hepatol. 2004;2:510–7. doi: 10.1016/s1542-3565(04)00171-5. [DOI] [PubMed] [Google Scholar]
- 17.Kanno A, Masamune A, Hanada K, et al. Multicenter study of early pancreatic cancer in Japan. Pancreatology. 2018;18:61–7. doi: 10.1016/j.pan.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Izumi Y, Hanada K, Okazaki A, et al. Endoscopic ultrasound findings and pathological features of pancreatic carcinoma in situ . Endosc Int Open. 2019;7:E585–93. doi: 10.1055/a-0839-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canto MI, Goggins M, Yeo CJ, et al. Screening for pancreatic neoplasia in high-risk individuals:An EUS-based approach. Clin Gastroenterol Hepatol. 2004;2:606–21. doi: 10.1016/s1542-3565(04)00244-7. [DOI] [PubMed] [Google Scholar]
- 20.Sagami R, Yamao K, Nakahodo J, et al. Pre-operative imaging and pathological diagnosis of localized high-grade pancreatic intra-epithelial neoplasia without invasive carcinoma. Cancers (Basel) 2021;13:945. doi: 10.3390/cancers13050945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleeff J, Korc M, Apte M, et al. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022. doi: 10.1038/nrdp.2016.22. [DOI] [PubMed] [Google Scholar]
- 22.Corral JE, Mareth KF, Riegert-Johnson DL, et al. Diagnostic yield from screening asymptomatic individuals at high risk for pancreatic cancer:A meta-analysis of cohort studies. Clin Gastroenterol Hepatol. 2019;17:41–53. doi: 10.1016/j.cgh.2018.04.065. [DOI] [PubMed] [Google Scholar]
- 23.Kogekar N, Diaz KE, Weinberg AD, et al. Surveillance of high-risk individuals for pancreatic cancer with EUS and MRI:A meta-analysis. Pancreatology. 2020;20:1739–46. doi: 10.1016/j.pan.2020.10.025. [DOI] [PubMed] [Google Scholar]
- 24.Hanada K, Okazaki A, Hirano N, et al. Diagnostic strategies for early pancreatic cancer. J Gastroenterol. 2015;50:147–54. doi: 10.1007/s00535-014-1026-z. [DOI] [PubMed] [Google Scholar]
- 25.Murphy SJ, Hart SN, Lima JF, et al. Genetic alterations associated with progression from pancreatic intraepithelial neoplasia to invasive pancreatic tumor. Gastroenterology. 2013;145:1098–109. doi: 10.1053/j.gastro.2013.07.049. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lennon AM, Wolfgang CL, Canto MI, et al. The early detection of pancreatic cancer:What will it take to diagnose and treat curable pancreatic neoplasia? Cancer Res. 2014;74:3381–9. doi: 10.1158/0008-5472.CAN-14-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakahodo J, Kikuyama M, Nojiri S, et al. Focal parenchymal atrophy of pancreas:An important sign of underlying high-grade pancreatic intraepithelial neoplasia without invasive carcinoma, i.e., carcinoma in situ. Pancreatology. 2020;20:1689–97. doi: 10.1016/j.pan.2020.09.020. [DOI] [PubMed] [Google Scholar]
- 28.Canto MI, Almario JA, Schulick RD, et al. Risk of neoplastic progression in individuals at high risk for pancreatic cancer undergoing long-term surveillance. Gastroenterology. 2018;155:740–51. doi: 10.1053/j.gastro.2018.05.035. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–7. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cazacu IM, Luzuriaga Chavez AA, Mendoza TR, et al. Quality of life impact of EUS in patients at risk for developing pancreatic cancer. Endosc Ultrasound. 2020;9:53–8. doi: 10.4103/eus.eus_56_19. [DOI] [PMC free article] [PubMed] [Google Scholar]