ABSTRACT
Therapeutic EUS has witnessed exponential growth in the last decade, but it has been considered investigational until recently. An increasing body of good-quality evidence is now demonstrating clear advantages over established alternatives, adding therapeutic EUS to management algorithms of complex hepato-pancreato-biliary (HPB) and gastrointestinal (GI) conditions. In this review, the available evidence and clinical role of therapeutic EUS in established and evolving applications will be discussed. A Graphical Summary for each scenario will provide (1) technical steps, (2) anatomical sketch, (3) best-supporting evidence, and (4) role in changing current and future GI practice. Therapeutic EUS has accepted well-established applications such as drainage of symptomatic peripancreatic fluid collections, biliary drainage in failed endoscopic retrograde cholangiopancreatography, and treatment of acute cholecystitis in unfit-for-surgery patients. In addition, good-quality evidence on several emerging indications (e.g., treatment of gastric outlet obstruction, local ablation of pancreatic solid lesions, etc.) is promising. Specific emphasis will be given to how these technical innovations have changed management paradigms and algorithms and expanded the possibilities of gastroenterologists to provide therapeutic solutions to old and emerging clinical needs. Therapeutic EUS is cementing its role in everyday practice, radically changing the treatment of different HPB diseases and other conditions (e.g., GI obstruction). The development of dedicated accessories and increased training opportunities will expand the ability of gastroenterologists to deliver highly effective yet minimally invasive therapies, potentially translating into a better quality of life, especially for oncological and fragile patients.
Keywords: choledochoduodenostomy, gallbladder drainage, gastrojejunostomy, hepaticogastrostomy, radiofrequency ablation
INTRODUCTION
Since its development, EUS became an established irreplaceable diagnostic modality, allowing visualization of previously inaccessible anatomical regions with the capability to obtain tissue for diagnosis. Optimization of imaging quality, Doppler, and real-time elastography brought organs surrounding the gastrointestinal (GI) tract within reach. This coupled with the development of linear echo-endoscopes with large therapeutic channels permitted access to fluid collections adjacent to the GI tract, ductal, and even vascular structures using needles and guidewires with millimetric precision under real-time guidance. Case studies and series began to appear from innovators, who explored the therapeutic opportunities that EUS could provide, especially in the palliative setting of inoperable patients. New therapeutic EUS applications followed as dedicated accessories were developed that aided in “simplifying” these techniques. Therapeutic EUS was born.
Until recently, it was difficult to translate the benefits of EUS and its rightful place in therapeutic algorithms, due to the lack of high-quality randomized studies. However, over the last years, good-quality evidence documented the added value of therapeutic EUS over established therapies and cemented its role in patients’ management, at least in tertiary centers with available expertise.
The aim of this manuscript is to provide a state-of-the-art overview of how EUS transformed clinical care from a diagnostic tool toward a range of therapeutic interventions in the management of complex hepato-pancreato-biliary (HPB) diseases.
METHODS
A literature search was performed for available evidence regarding therapeutic EUS up to May 2021.
In this narrative review, available evidence and clinical role of therapeutic EUS in established and evolving applications are discussed proceeding as follows: (i) technical summary, (ii) best available evidence, and (iii) how it is changing current paradigms.
A Graphical Summary is included for each scenario, depicting technical steps, anatomical sketch, best-supporting evidence, and role in changing GI practice.
General technical principles of therapeutic EUS
Therapeutic EUS procedures are performed using linear echo-endoscopes with a large working channel, under CO2 insufflation, under deep conscious sedation or anesthesia. The principle of therapeutic EUS is to obtain an access, usually by creating a fistula/connection between the GI tract and a target organ/cavity. The general technique involves creating a (1) EUS-guided access to the target structure using a 19G needle followed by (2) guidewire insertion and creation of a fistulous tract using a cystotome, needle knife, or dilation balloon. The access is then stabilized with a stent (plastic or metal). These procedures often require the careful exchanges of devices while maintaining the access, and it is a shared opinion among interventional endosonographers that tools aimed at minimizing steps and catheter exchanges would lead to a reduced margin of error. One example of significant procedural simplification was the development of electrocautery-enhanced lumen-apposing metal stents (ec-LAMSs). Indeed, the cautery-enhanced tip allows direct penetration into the target structure without need for needle/guidewire exchanges, followed by stent deployment in one free-hand step. The dumbbell-shaped, fully covered, self-expandable stent design permits the creation of a stable connection between two luminal walls, usually turning into a mature anastomosis within 2 weeks. This specific design prevents perforation, leak, bleeding, and stent migration. In addition, partially covered stents were developed to simplify hepaticogastrostomy (HG), and ablation devices specifically designed to be used under EUS guidance were developed and clinically validated.
EUS-guided fluid collection drainage
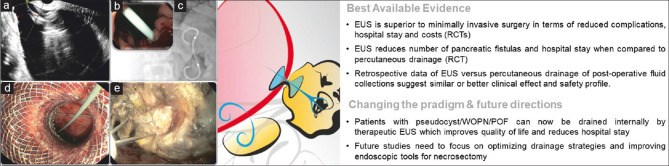
The first pancreatic pseudocyst was drained transmurally under EUS guidance in 1996.[1] This technique was further refined and is now considered standard of care for the treatment of symptomatic pseudocysts and walled-off pancreatic necrosis (WON) avoiding blood vessels and securing controlled placement of stents.[2,3] The minimally invasive procedure also allows internal drainage of the collection, sparing the need for percutaneous drains while minimizing the risk of pancreatic fistulas. Indwelling double-pigtail plastic stents (DPPSs) can successfully treat disconnected pancreatic tail syndrome, prevent pseudocyst recurrence, and diminish the need for pancreatic resections. In case LAMS is placed into a WON, direct endoscopic access and necrosectomy can be performed[4] [Figure 1].
Figure 1.
EUS-guided pancreatic fluid collection drainage: (a) endosonographic appearance of a pancreatic pseudocyst; (b and c) endoscopic (b) and fluoroscopic (c) view of double-pigtail plastic stents utilized for drainage; (d) endoscopic view of the proximal flange of a LAMS; (e) through-the-LAMS direct endoscopic necrosectomy using a tripod grasping forceps. RCT: Randomized controlled trial; WOPN: Walled-off peripancreatic necrosis; POF: Postoperative fluid collections; LAMS: Lumen-apposing metal stent
Core papers from the Dutch Pancreatic Group changed the management of infected pancreatic necrosis. They first reported in the PANTER trial that in patients with infected WON, a minimally invasive step-up approach with a percutaneous drain first was superior to primary surgical intervention.[5] A long-term follow-up of the same patients revealed reduced mortality or major complications, reduced incisional hernias, and reduced pancreatic exocrine and endocrine insufficiency in patients of the step-up versus open necrosectomy group, without any increased risk of re-interventions or recurrences.[6] A subsequent multicenter randomized controlled trial (RCT) (MISER trial) focused on patients who already failed percutaneous therapy: Here, the endoscopic step-up approach (EUS-guided drainage with or without necrosectomy) was found to be significantly superior over surgery in terms of major adverse events (AEs), costs, and quality of life.[7] A similar PENGUIN trial showed the superiority of endoscopic versus surgical necrosectomy in terms of reduced major complications (especially in terms of multiorgan failure and pancreatic fistulas) or death.[8] Finally, in another multicenter RCT (TENSION trial), the endoscopic step-up approach did not show superiority compared to step-up therapy with percutaneous drainage with or without video-assisted retroperitoneal debridement in reducing AEs or death but shortened total hospital stay and reduced pancreatic fistulas.[9] Based on the abovementioned results, EUS-guided drainage, ± endoscopic necrosectomy, is now considered the first step in the management of infected or symptomatic WON.
In drainage of symptomatic or infected pancreatic collections, both DPPSs and LAMSs may be utilized,[10,11,12] but some questions are still open. LAMS has the theoretical advantage of a larger access to the cavity, allowing direct endoscopic necrosectomy. However, despite different retrospective data showed a higher clinical efficacy,[13,14,15] in a recent RCT, LAMS was found not to be superior to DPPS with regard to clinical success or total number of procedures, with a significantly higher occurrence of stent-related AEs in the LAMS group (32.3% vs. 6.9%, P = 0.01),[16] thus demanding a better definition of the best candidates to one approach or the other and a better-standardized revision policy.[17] A reasonable algorithm may involve LAMS drainage for WON with a significant amount of necrosis, whereas pseudocysts or “clear” collections can be adequately addressed through DPPS.
Apart from the rare occurrence of perforation, the most common AE after EUS-guided fluid collection drainage is stent-related bleeding (≈5%), either during drainage or subsequent necrosectomy. These AEs have been attributed to stent-related trauma and secondary pseudoaneurysms, especially as the cavity collapses.[5] The placement of a coaxial DPPS and early-scheduled LAMS removal have been suggested to reduce this risk.[18,19] In case pancreatic homogeneously fluid collections with pancreatic duct rupture, long-term indwelling atraumatic DPPSs are thought to represent a protection against recurrence.[20]
Finally, retrospective evidence on the efficacy of EUS-guided drainage of postsurgical collections is increasingly showing high technical and clinical success, and this procedure has already become the standard of care for this indication in many high-volume cancer centers, allowing internal drainage of collections and early hospital discharge.[21]
EUS-guided biliary drainage
ERCP is currently the gold standard when biliary drainage is required but may fail in up to 10% of cases.[22] Reasons for failure include altered anatomy, tight strictures, and tumor infiltration, which may preclude selective cannulation of the major papilla or prevent stent insertion.[22] In these patients, percutaneous transhepatic biliary drainage (PTBD) has been utilized as a “salvage” solution for failed ERCP. The procedure comprises the need to cross both parietal and visceral peritoneum to access a dilated bile duct and can be associated with major AEs and a reduced quality of life when an external drainage is left in place.[23,24]
EUS allows access to the biliary tree, from the duodenum to the common bile duct (CBD) and from the proximal stomach to liver segments 2–3. Through both routes, a guidewire can be advanced beyond a stenosis and across the papilla into the duodenum to perform a rendezvous procedure or for antegrade placement of a metal stent across a stricture. Alternatively, the procedure can end with transmural stenting, i.e., EUS-guided choledochoduodenostomy (EUS-CD) or EUS-HG.
The superiority of EUS-BD with respect to PTBD in the case of failed ERCP was reported in a meta-analysis,[25] containing data from three small RCTs,[25,26,27] showing higher clinical success with reduced AEs and need for re-interventions in the EUS group. Heterogeneity on how these procedures are performed, the lack of dedicated consumables, and scarcity of specific training may be some of the reasons why EUS-BD procedures are still perceived as investigational and confined to specialized academic centers.
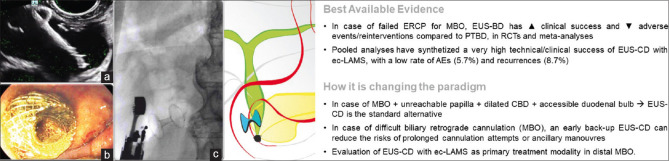
When the CBD is adequately dilated (at least 12 mm, but many authors will consider safe a diameter over 15 mm[28]), EUS-CD can be accomplished using small-caliber ec-LAMS in a few minutes, theoretically without fluoroscopy[29] [Figure 2]. For these reasons, EUS-CD is nowadays more widely available and accepted after ERCP failure. Given the high technical success (95%)[30] and low rate of AEs (5%),[29] EUS-CD was compared to ERCP as the primary drainage strategy in malignant distal biliary obstruction. Results from the largest RCT in the pre-LAMS era reported EUS-BD to be associated with lower AEs, re-interventions, and higher rate of stent patency.[31] Additional randomized studies are ongoing and will evaluate whether EUS-CD with ec-LAMS should replace ERCP as primary treatment modality in malignant distal biliary obstruction.[32,33,34]
Figure 2.
EUS-guided choledochoduodenostomy: (a) endosonographic appearance of the released distal flange of a LAMS inside a dilated common bile duct; (b) endoscopic view of the proximal flange of the LAMS in the duodenal bulb; (c) fluoroscopic view of the released LAMS with aerobilia inside the biliary tree. MBO: Malignant biliary obstruction; EUS-BD: EUS-guided biliary drainage; PTBD: Percutaneous transhepatic biliary drainage; RCT: Randomized clinical trial; AEs: Adverse events; EUS-CD: EUS-guided choledochoduodenostomy; ec-LAMS: Electrocautery-enhanced LAMS; LAMS: Lumen-apposing metal stent
In addition, recent reports suggest that EUS-CD with ec-LAMS may be considered safe even in patients that are deemed potential surgical candidates.[35,36]
When the biliary tree is not enough dilated or the placement of a metal stent is not preferred, the endoscopist might attempt an EUS-guided rendezvous (either extrahepatic from the bulb or intrahepatic from the stomach), at the price of some technical challenges due to the need of guidewire manipulation through the papilla (and potential shearing over the needle), and endoscope exchange for a final retrograde cannulation.
EUS-HG refers to the placement of a covered or partially covered metal stent between a dilated segmental intrahepatic duct and the stomach to treat distal or hilar malignant biliary obstruction as an alternative to PTBD when ERCP fails or is impossible [Figure 3]. RCTs of EUS-BD versus PTBD[26,27,37] showed that this procedure is a valuable alternative to PTBD and allows the HPB physician to individualize biliary drainage by considering anatomy (such as disconnected left and right biliary systems) and residual segmental liver volume in a personalized treatment strategy. Until recently, biliary drainage procedures were lumped together making it difficult to understand the benefit of this approach in hilar strictures[38,39] or postsurgical anatomy.[39,40,41]
Figure 3.
EUS-guided hepaticogastrostomy: (a) EUS-guided puncture of a dilated intrahepatic biliary branch with a 19G needle; (b) contrast injection showing a dilated left intrahepatic biliary tree; (c) endoscopic view of the transgastric (covered) portion of the stent once released. MBO: Malignant biliary obstruction; EUS-BD: EUS-guided biliary drainage; PTBD: Percutaneous transhepatic biliary drainage; RCT: Randomized clinical trial; LAMS: Lumen-apposing metal stent; EUS-HG: EUS-guided hepaticogastrostomy
Therapeutic EUS is rapidly changing GI practice allowing optimal biliary drainage during the same procedure, following failed ERCP, precluding the need for additional anesthesia or other interventions. This, together with targeted selective drainage based on anatomical considerations and tumor biology, significantly impacted the management of malignant biliary diseases.
While the risk of pancreatitis might be lower with EUS-BD than ERCP, procedure-related AEs might happen in ≈15% of procedures.[19] In procedures requiring device exchanges (such as EUS-HG), self-limiting bile leak and capnoperitoneum may occur despite successful completion, which are usually treated conservatively. Conversely, more severe AEs may happen when failing to complete stent release after accessing a dilated biliary tree, as this may result in cholangitis, bile leak, and/or perforation of the gastric/enteric wall, thus requiring urgent percutaneous relief of biliary obstruction or even rescue surgery.[19] These specific risks seem significantly lower (≈6%) with EUS-CD performed with LAMS due to the single-step biliary access and stent release.[29]
EUS-guided gallbladder drainage
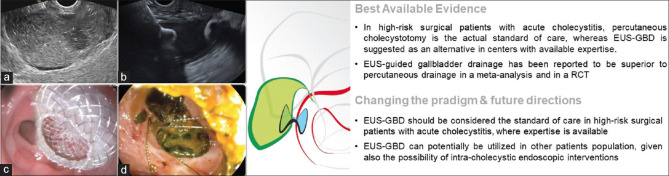
The gold standard treatment for acute cholecystitis (AC) is laparoscopic cholecystectomy (LC). In high surgical risk patients, the Tokyo guidelines 2018 recommend Percutaneous transhepatic gallbladder drainage (PT-GBD) as primary treatment, with EUS-GBD an alternative in centers with available expertise.[42] Subsequently, a meta-analysis including only three retrospective studies using LAMSs showed EUS-GBD to be superior to PT-GBD in terms of length of hospital stay, unplanned readmission, and need for re-interventions.[43] This superiority was additionally demonstrated in a recent RCT: EUS-GBD led to significantly reduced 30-day and 1-year AEs, re-intervention rates, unplanned readmissions, and recurrent cholecystitis compared to PT-GBD, with comparable efficacy and mortality rates.[44] The superiority of EUS-GBD over PT-GBD strongly supports this therapy as a definitive treatment for AC in patients unfit for cholecystectomy [Figure 4].
Figure 4.
EUS-guided gallbladder drainage: (a) endosonographic visualization of a hydropic gallbladder, full of corpuscular material with thickened walls; (b) endosonographic appearance of the released distal flange of a LAMS inside the gallbladder; (c) endoscopic view of the proximal flange of the LAMS in the stomach, with drainage of pus; (d) Through-the-LAMS endoscopic direct cholecystoscopy and stones removal by a basket. EUS-GBD: EUS-guided gallbladder drainage; RCT: Randomized clinical trial; LAMS: Lumen-apposing metal stent
In another provocative study, the current dogma was challenged: after propensity score matching, 30 high-risk surgical patients undergoing EUS-GBD were compared to 30 LC patients. No differences in technical and clinical success, length of hospital stay, 30-day AEs, rates of recurrent biliary events, re-interventions, unplanned readmissions, or mortality were found between the two groups.[45] This study, in addition to another study showing that giant residual gallbladder stones could be successfully treated by laser lithotripsy through the LAMSs,[46] suggests that patient population(s) other than those with AC may benefit from EUS-GBD.[47,48] This is particularly true, given aging populations, with increased numbers of fragile individuals with multiple comorbidities with gallstone disease requiring surgery. These individuals are more prone to surgically related AEs:[49,50,51] a meta-analysis including 326,517 patients undergoing elective LC demonstrated that increasing age was associated with significantly higher AEs (OR: 2.46) and rate of conversion to open cholecystectomy (OR: 1.84).[52] In these fragile patient populations, some individuals may benefit from alternative approaches, such as EUS-GBD.
In EUS-GBD, apart from the risks deriving from technical failures, postprocedural dysfunction due to stent obstruction (<4%) is regarded as the most common AE, although occurring less frequently than in the context of percutaneous cholecystostomies.[44] Scheduled endoscopic stone clearance (cholecystolithotomy) and LAMS exchange for DPPSs are thought to potentially reduce long-term AC recurrence and stent-related trauma, but additional prospective data are needed.[48]
EUS-guided pancreatic duct drainage
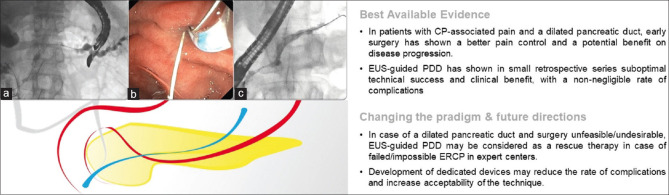
Many questions remain regarding the role of surgery or endoscopy in the management of refractory pain associated with chronic pancreatitis.[3] Consensus exists that ductal hypertension, due to strictures or stones, may generate chronic pain and form the basis of any decompression therapy, either surgical or endoscopic in its management.[53]
The ability to provide ductal decompression requires endoscopic access to the main pancreatic duct (MPD) which may be particularly challenging due to stones, strictures or in postsurgical anatomy. If ERCP fails or when the pancreaticojejunostomy anastomosis is narrowed causing dilation of the MPD, EUS may be utilized to provide salvage therapy.[54,55] EUS-guided pancreatic duct drainage (EUS-PDD) necessitates cannulation of the dilated MPD from the stomach or duodenal bulb with a 19G needle to advance a guidewire across the papilla into the accessible small bowel, or past an anastomotic stricture [Figure 5]. If the guidewire is accessible, the EUS endoscope is exchanged for a duodenoscope to allow standard retrograde pancreatic duct therapy. In case the guidewire cannot be manipulated into the small bowel, a fistulous tract is created using a balloon dilator or cystotome to allow direct transmural placement of a stent through the stomach into the MPD (EUS-guided pancreaticogastrostomy).
Figure 5.
EUS-guided pancreatic duct drainage: (a) EUS-guided transgastric access of a dilated MPD with guidewire manipulated through the papilla inside the duodenum; (b) endoscopic retrograde MPD cannulation parallel to the antegrade guidewire; (c) final retrograde cannulation of the MPD after EUS-guided rendezvous. CP: Chronic pancreatitis; EUS-PDD: EUS-guided pancreatic duct drainage; MPD: Main pancreatic duct
EUS-PDD is challenging even in expert hands with an important risk of complications.[56,57,58,59] Should there be a need to create a fistulous tract for transluminal stenting, the complication rate (mainly pancreatitis and pancreatic collections) may reach 30%.[54] For these reasons, EUS-PDD is currently limited to expert tertiary centers where it remains an important salvage therapy when ERCP drainage fails.
EUS-guided gastrojejunostomy
Since its introduction, more than 600 cases of EUS-guided gastrojejunostomy (EUS-GJ) with placement of a LAMS have been published.[60,61,62,63,64,65] Besides successful applications of this technique in afferent loop syndrome and biliary access in surgically altered anatomy,[66,67,68] its effectiveness has predominantly been illustrated in the management of gastric outlet obstruction (GOO). Although LAMSs have been approved for drainage of pancreatic fluid collections or the biliary tract, their use to create a gastrojejunostomy is still off-label. Various technical approaches of EUS-GJ have been described, with the direct method or free-hand technique being preferred for its ease of use and simplicity.[69,70,71] Although techniques varied, especially in early studies, several systematic reviews have now reported clinical efficacy ranging between 90% and 95%[72,73,74] [Figure 6].
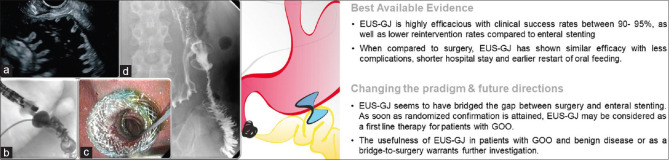
Figure 6.
EUS-guided gastrojejunostomy: (a) EUS-guided transgastric visualization of a dilated fluid-filled jejunal loop; (b) correctly placed ec-LAMS, with contrast medium flowing from the nasojejunal tube inside the stomach through the LAMS; (c) endoscopic view of the proximal flange of the LAMS, with direct visualization of the jejunal loop through the stent; (d) postprocedural gastrointestinal series showing contrast flow through the stent inside the jejunum. EUS-GJ: EUS-guided gastrojejunostomy; GOO: Gastric outlet obstruction. EUS-GJ: EUS-guided gastrojejunostomy; LAMS: Lumen-apposing metal stent; ec-LAMS: Electrocautery-enhanced LAMS
With regard to efficacy, EUS-GJ seems to equal surgical gastrojejunostomy (open or laparoscopic), associated with fewer AEs, earlier restart of oral feeding, and shorter hospital stays.[61,65,75] Endoscopic enteral stenting (ES) for GOO has been around two decades longer and has shown similar advantages when compared to surgical gastroenterostomy but at the cost of more recurrent obstructive symptoms and need for re-interventions.[76,77,78] EUS-GJ seems to have bridged the gap between ES and surgery, showing lower need for re-intervention and similar safety when compared to ES,[79,80,81] while achieving long-term surgical-range efficacy.[61,65,75]
Although until now no randomized data are available, two randomized trials are currently ongoing.[82,83] These studies have been designed to compare EUS-GJ with ES, with primary outcomes being re-intervention rates and recurrent GOO.[82,83] Together with randomized trials comparing EUS-GJ with surgical gastroenterostomy, these efforts will provide a more in-depth evaluation of EUS-GJ clarifying the value and safety of this technique in the management of malignant GOO.
The main risk of EUS-GJ is related to the technical difficulty in penetrating a mobile jejunal loop and subsequently completing the intraluminal release of a large-caliber stent. Stent misdeployments (≈10%) may result in potentially severe AEs, such as GI perforation and intestinal leak; if intraprocedural endoscopic salvage fails, rescue surgical gastrojejunostomy is required.[84]
EUS-guided tumor ablation
The development of specialized EUS-guided radiofrequency ablation (EUS-RFA) needles made it possible to treat focal lesions. This probe, when inserted into a lesion, induces necrosis by delivering high-frequency alternating current. The result of this application can be visualized by EUS as the formation of bubbles followed by the appearance of a hyperechoic zone, both suggestive of a successful ablation[85] [Figure 7]. The desired effect goes beyond cell death and may involve perturbation of the local microenvironment potentially inducing an immunomodulatory response, that may potentially further increase the efficacy of novel target systemic therapies.[85]
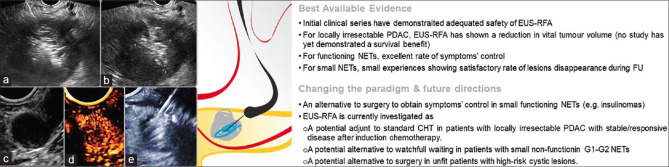
Figure 7.
EUS-RFA: (a) Needle-like EUS-RFA probe inside a pancreatic adenocarcinoma with energy application resulting in the formation of bubbles; (b) endosonographic appearance of a hyperechoic zone inside the hypoechoic pancreatic lesion at the end of the treatment; (c) a 13-mm functioning neuroendocrine tumor of the uncinate process of the pancreas, hyperenhancing at contrast injection (d); (e) EUS-RFA ablation of the same lesion. PDAC: Pancreatic ductal adenocarcinoma; NETs: Neuroendocrine tumors; FU: Follow-up; CHT: Chemotherapy; EUS-RFA: EUS-guided radiofrequency ablation
EUS-RFA demonstrated excellent therapeutic benefit, specifically with regard to symptom control in small functional neuroendocrine tumors (F-NETs), associated with few AEs.[86] For nonfunctional NETs, there are four published series, including a total of 50 patients, with a mean lesion diameter ranging 12.2–20.3 mm, showing a 71%–100% 1-year success rate.[87,88,89,90] The low AEs rate and the evidence that lesion control may extend at least up to 3 years, begs the question whether EUS-RFA may replace surveillance in small (e.g., <20 mm), NF, well-differentiated (Ki67 <5%) NETs in future.[90]
To date, data on EUS-RFA in pancreatic adenocarcinoma regard small series including a total of almost 50 patients,[91,92,93,94,95,96,97] focusing mainly on feasibility and safety. While an objective antitumor response can be detected in most imaging studies, a survival benefit is not yet demonstrated. The theoretical ideal candidate to EUS-RFA is the patient with a locally irresectable pancreatic cancer, with stable disease or partial response after an initial course of induction chemotherapy, since progressive diseases harbor a higher risk of systemic disease burden. A Dutch multicenter trial will elucidate an eventual survival benefit of adding surgically delivered RFA to standard chemotherapy.[98]
An even more speculative question is whether there is an advantage of EUS-guided ablation, in patients unfit for surgery with “high-risk” cysts as an alternative to surveillance or surgery.
EUS-RFA is a promising new tool that must still find its place in the treatment algorithms of focal pancreatic and liver lesions through well-designed clinical studies, in centers with adequate expertise, and after extensive multidisciplinary discussion of available alternatives.
EUS-RFA carries a ≈15% risk of AEs, most of which are mild and represented by postprocedural abdominal pain and acute pancreatitis. To reduce this risk, rectal nonsteroidal anti-inflammatory drugs prophylaxis, similar to ERCP, is usually recommended together with avoiding lesions in proximity of MPD.[85]
EUS-guided endovascular therapy
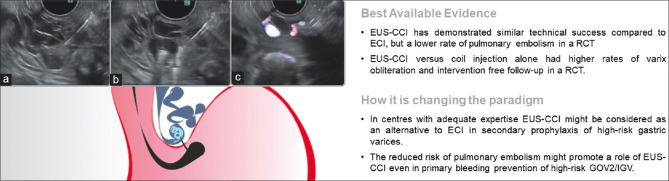
Standard endoscopic cyanoacrylate injection (ECI) comes with a risk of pulmonary glue embolization,[99] making it not the first choice in the context of primary prophylaxis of gastroesophageal varix type 2 (GOV2) and isolated gastric varix (IGV).[100] However, ECI is the recommended therapy for bleeding GOV2 and IGV followed by endoscopic follow-up and/or transjugular intrahepatic portosystemic shunt (TIPS) placement.[101,102] To reduce this risk of pulmonary embolization, EUS-guided coil placement and cyanoacrylate injection (EUS-CCI) has been advocated.[103] The gastrofundal convolute or perforating vessel can typically be identified by EUS from the distal esophagus or cardia[103] and punctured with a 19G or 22G needle for respective 0.035” or 0.018” coil placement [Figure 8]; the selected coil (6–20 mm) should have a diameter up to or larger than the convolute diameter, ensuring fixation within the varix. Depending on the real-time EUS assessment, either another coil is advanced or N-2-butyl-cyanoacrylate or 2-octyl-cyanoacrylate is slowly injected onto the coil “scaffold,” hence reducing the risk of pulmonary embolism. The absence of Doppler flow in the convolute confirms varix obliteration. The largest published experience consists of a retrospective study in 152 patients with GOV2/IGV1 undergoing EUS-CCI for active bleeding and secondary or primary prophylaxis.[104] The technical success rate was >99%, and in the 100 patients with follow-up EUS, 93 (93%) had complete obliteration of the varix. Posttreatment bleeding occurred in 3 of 93 (3%) of these patients. One recent RCT compared EUS-CCI with ECI as primary or secondary prophylaxis for bleeding, showing similar technical success (varix thrombosis at 30 days 73.5% vs. 75%) but a lower rate of pulmonary embolism in EUS-CCI (25% vs. 50%).[105] Another RCT showed that EUS-CCI versus coil injection alone had higher rates of varix obliteration (85% vs. 13%) and intervention-free follow-up (83% vs. 60%, P = 0.010).[106] Moreover, there appears to be a role for EUS-CCI in the management of GOV2/IGV already treated with ECI, in whom TIPS and/or Balloon-occluded Retrograde Transvenous Obliteration is contraindicated (e.g., hepatic encephalopathy) or technically impossible due to extensive portal and splenic vein thrombosis. A recent retrospective analysis also showed that EUS-CCI as primary prophylaxis in patients with “high-risk” gastric varices was technically feasible (100% success and 97% varix obliteration), with low rates of bleeding (2.5%) or complications (5%) during follow-up (mean: 3 years).[107] As such, EUS-CCI appears to be a valuable addition to the arsenal of GI endovascular therapy, but further prospective randomized trials are needed.
Figure 8.
EUS-guided coil placement and -CCI: (a) a gastrofundal convolute is identified by EUS and punctured with a 19G needle; (b) through the needle coil placement + cyanoacrylate injection; (c) absence of Doppler flow in the convolute confirming varix obliteration. ECI: Endoscopic cyanoacrylate injection; GOV2: Gastroesophageal varix, type 2; IGV: Isolated gastric varix; EUS-CCI: EUS-cyanoacrylate injection
Adverse events, informed consent, and medicolegal issues
Most AEs associated with therapeutic EUS are mild and self-limiting. However, its use may be associated with serious AEs including perforation, bleeding, severe pancreatitis and pancreatic collections, bile leak, sepsis, and procedure-related deaths.[19,55] Although severe complications rarely occur in referral centers, it remains imperative that gastroenterologists are adequately trained in their recognition and management. Therapeutic EUS procedures are best performed by endoscopists with adequate training in advanced pancreaticobiliary endoscopy at hospitals where interventional radiologists and surgeons are readily available if needed. As discussed so far, therapeutic endosonographers started to move outside the field of “investigational” procedures, since increasing evidence is translating most of them into standard of care. Much more important, the majority of devices used in these procedures are now regulatory-approved and used on-label. One exception to date is the use of ec-LAMS for EUS-GJ, which is used off-label even if the product itself is frequently used in current clinical practice. Therefore, EUS-GJ should be better performed inside clinical studies, with patients agreeing with specific informed consent, after being presented with all risks and potential alternatives. Most of the patients will anyway agree to the procedure when explained that in the worst clinical scenario, the management of an eventual AE will be to receive surgical gastrojejunostomy, currently suggested as a standard procedure for patients with GOO and an expected long survival.[108,109]
CONCLUSIONS
Therapeutic EUS is a field of endoscopy that witnessed exponential growth in the last decade. It would be important for gastroenterologists and surgeons to understand the added value of therapeutic EUS compared to “established” alternatives. Therapeutic EUS is now becoming standard of care in many different clinical scenarios. An increasing body of evidence suggests its prominent role in everyday practice, radically changing the way we treat HPB diseases and other conditions (e.g., GI obstruction). These results have been obtained through the development of dedicated accessories (e.g., LAMS, dedicated biliary stents, and ablation needles), facilitating safety and ease of use, while allowing superior efficacy compared to previous approaches. For oncological and fragile patients, this can translate into better quality of life, sparing them from permanent external drainages or invasive surgical interventions. Moreover, diseases that were considered to be outside the reach of endoscopy are now becoming the responsibility of the gastroenterologist. This process will need to be accompanied by a cultural dissemination, based on high-quality evidence data, to bring therapeutic EUS to the next level.
Patient anonymity and informed consent
The article includes fully anonymized pictures from patients who have provided specific informed consent.
Financial support and sponsorship
Nil.
Conflicts of interest
Paolo Giorgio Arcidiacono is an Associate Editor of the journal; Alberto Larghi and Mouen Khashab are Editorial Board Members. This article was subject to the journal’s standard procedures, with peer review handled independently of these editors and their research group.
REFERENCES
- 1.Wiersema MJ. Endosonography-guided cystoduodenostomy with a therapeutic ultrasound endoscope. Gastrointest Endosc. 1996;44:614–7. doi: 10.1016/s0016-5107(96)70022-6. [DOI] [PubMed] [Google Scholar]
- 2.Varadarajulu S, Bang JY, Sutton BS, et al. Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology. 2013;145:583–90. doi: 10.1053/j.gastro.2013.05.046. e1. [DOI] [PubMed] [Google Scholar]
- 3.Dumonceau JM, Delhaye M, Tringali A, et al. Endoscopic treatment of chronic pancreatitis:European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2012;44:784–800. doi: 10.1055/s-0032-1309840. [DOI] [PubMed] [Google Scholar]
- 4.Kim YS, Cho JH, Cho DH, et al. Long-term outcomes of direct endoscopic necrosectomy for complicated or symptomatic walled-off necrosis:A Korean multicenter study. Gut Liver. 2021;15:930–9. doi: 10.5009/gnl20304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Santvoort HC, Besselink MG, Bakker OJ, et al. Astep-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362:1491–502. doi: 10.1056/NEJMoa0908821. [DOI] [PubMed] [Google Scholar]
- 6.Hollemans RA, Bakker OJ, Boermeester MA, et al. Superiority of step-up approach vs. open necrosectomy in long-term follow-up of patients with necrotizing pancreatitis. Gastroenterology. 2019;156:1016–26. doi: 10.1053/j.gastro.2018.10.045. [DOI] [PubMed] [Google Scholar]
- 7.Bang JY, Arnoletti JP, Holt BA, et al. An endoscopic transluminal approach, compared with minimally invasive surgery, reduces complications and costs for patients with necrotizing pancreatitis. Gastroenterology. 2019;156:1027–40. doi: 10.1053/j.gastro.2018.11.031. e3. [DOI] [PubMed] [Google Scholar]
- 8.Bakker OJ, Van Santvoort HC, Van Brunschot S, et al. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis:A randomized trial. J Am Med Assoc. 2012;307:1053–61. doi: 10.1001/jama.2012.276. [DOI] [PubMed] [Google Scholar]
- 9.van Brunschot S, van Grinsven J, van Santvoort HC, et al. Endoscopic or surgical step-up approach for infected necrotising pancreatitis:A multicentre randomised trial. Lancet. 2018;391:51–8. doi: 10.1016/S0140-6736(17)32404-2. [DOI] [PubMed] [Google Scholar]
- 10.Park CH, Park SW, Nam E, et al. Comparative efficacy of stents in endoscopic ultrasonography-guided peripancreatic fluid collection drainage:A systematic review and network meta-analysis. J Gastroenterol Hepatol. 2020;35:941–52. doi: 10.1111/jgh.14960. [DOI] [PubMed] [Google Scholar]
- 11.Mohan BP, Jayaraj M, Asokkumar R, et al. Lumen apposing metal stents in drainage of pancreatic walled-off necrosis, are they any better than plastic stents?A systematic review and meta-analysis of studies published since the revised Atlanta classification of pancreatic fluid collections. Endosc Ultrasound. 2019;8:82–90. doi: 10.4103/eus.eus_7_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan S, Zhong C, Ren Y, et al. Are lumen-apposing metal stents more effective than plastic stents for the management of pancreatic fluid collections:An updated systematic review and meta-analysis. Gastroenterol Res Pract. 2020;2020:4952721. doi: 10.1155/2020/4952721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandrasekhara V, Barthet M, Devière J, et al. Safety and efficacy of lumen-apposing metal stents versus plastic stents to treat walled-off pancreatic necrosis:Systematic review and meta-analysis. Endosc Int Open. 2020;8:E1639–53. doi: 10.1055/a-1243-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ge PS, Young JY, Jirapinyo P, et al. Comparative study evaluating lumen apposing metal stents versus double pigtail plastic stents for treatment of walled-off necrosis. Pancreas. 2020;49:236–41. doi: 10.1097/MPA.0000000000001476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guzmán-Calderón E, Chacaltana A, Díaz R, et al. Head-to-head comparison between endoscopic ultrasound guided lumen apposing metal stent and plastic stents for the treatment of pancreatic fluid collections:A systematic review and meta-analysis. J Hepatobiliary Pancreat Sci. 2021;29:198–211. doi: 10.1002/jhbp.1008. [DOI] [PubMed] [Google Scholar]
- 16.Bang JY, Navaneethan U, Hasan MK, et al. Non-superiority of lumen-apposing metal stents over plastic stents for drainage of walled-off necrosis in a randomised trial. Gut. 2019;68:1200–9. doi: 10.1136/gutjnl-2017-315335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanella G, Dell'anna G, Arcidiacono PG. Plastic versus metal EUS-guided drainage of pancreatic fluid collections:Do we really know when to use the hard way? Clin Gastroenterol Hepatol. 2022;20:e1507–8. doi: 10.1016/j.cgh.2021.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Puga M, Consiglieri CF, Busquets J, et al. Safety of lumen-apposing stent with or without coaxial plastic stent for endoscopic ultrasound-guided drainage of pancreatic fluid collections:A retrospective study. Endoscopy. 2018;50:1022–6. doi: 10.1055/a-0582-9127. [DOI] [PubMed] [Google Scholar]
- 19.DeWitt JM, Arain M, Chang KJ, et al. AGA white paper:Interventional endoscopic ultrasound:current status and future directions. Clin Gastroenterol Hepatol. 2020;19:24–40. doi: 10.1016/j.cgh.2020.09.029. [DOI] [PubMed] [Google Scholar]
- 20.Gkolfakis P, Bourguignon A, Arvanitakis M, et al. Indwelling double-pigtail plastic stents for treating disconnected pancreatic duct syndrome-associated peripancreatic fluid collections:Long-term safety and efficacy. Endoscopy. 2021;53:1141–9. doi: 10.1055/a-1319-5093. [DOI] [PubMed] [Google Scholar]
- 21.Storm AC, Levy MJ, Kaura K, et al. Acute and early EUS-guided transmural drainage of symptomatic postoperative fluid collections. Gastrointest Endosc. 2020;91:1085–91. doi: 10.1016/j.gie.2019.11.045. e1. [DOI] [PubMed] [Google Scholar]
- 22.Enochsson L, Swahn F, Arnelo U, et al. Nationwide, population-based data from 11,074 ERCP procedures from the Swedish Registry for Gallstone Surgery and ERCP. Gastrointest Endosc. 2010;72:1175–84. doi: 10.1016/j.gie.2010.07.047. [DOI] [PubMed] [Google Scholar]
- 23.Pedersoli F, Schröder A, Zimmermann M, et al. Percutaneous transhepatic biliary drainage (PTBD) in patients with dilated vs. nondilated bile ducts:technical considerations and complications. Eur Radiol. 2021;31:3035–41. doi: 10.1007/s00330-020-07368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Behera RK, Srivastava DN, Kumar P, et al. Right-sided versus left-sided percutaneous transhepatic biliary drainage in the management of malignant biliary obstruction:A randomized controlled study. Abdom Radiol (NY) 2021;46:768–75. doi: 10.1007/s00261-020-02651-y. [DOI] [PubMed] [Google Scholar]
- 25.Sharaiha RZ, Khan MA, Kamal F, et al. Efficacy and safety of EUS-guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails:A systematic review and meta-analysis. Gastrointest Endosc. 2017;85:904–14. doi: 10.1016/j.gie.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 26.Artifon EL, Aparicio D, Paione JB, et al. Biliary drainage in patients with unresectable, malignant obstruction where ERCP fails:Endoscopic ultrasonography-guided choledochoduodenostomy versus percutaneous drainage. J Clin Gastroenterol. 2012;46:768–74. doi: 10.1097/MCG.0b013e31825f264c. [DOI] [PubMed] [Google Scholar]
- 27.Lee TH, Choi JH, Park DH, et al. Similar efficacies of endoscopic ultrasound-guided transmural and percutaneous drainage for malignant distal biliary obstruction. Clin Gastroenterol Hepatol. 2016;14:1011–9. doi: 10.1016/j.cgh.2015.12.032. e3. [DOI] [PubMed] [Google Scholar]
- 28.Jacques J, Privat J, Pinard F, et al. Endoscopic ultrasound-guided choledochoduodenostomy with electrocautery-enhanced lumen-apposing stents:A retrospective analysis. Endoscopy. 2019;51:540–7. doi: 10.1055/a-0735-9137. [DOI] [PubMed] [Google Scholar]
- 29.Krishnamoorthi R, Dasari CS, Thoguluva Chandrasekar V, et al. Effectiveness and safety of EUS-guided choledochoduodenostomy using lumen-apposing metal stents (LAMS):A systematic review and meta-analysis. Surg Endosc. 2020;34:2866–77. doi: 10.1007/s00464-020-07484-w. [DOI] [PubMed] [Google Scholar]
- 30.Amato A, Sinagra E, Celsa C, et al. Efficacy of lumen-apposing metal stents or self-expandable metal stents for endoscopic ultrasound-guided choledochoduodenostomy:A systematic review and meta-analysis. Endoscopy. 2021;53:1037–47. doi: 10.1055/a-1324-7919. [DOI] [PubMed] [Google Scholar]
- 31.Paik WH, Lee TH, Park DH, et al. EUS-guided biliary drainage versus ERCP for the primary palliation of malignant biliary obstruction:A multicenter randomized clinical trial. Am J Gastroenterol. 2018;113:987–97. doi: 10.1038/s41395-018-0122-8. [DOI] [PubMed] [Google Scholar]
- 32.Itonaga M, Kitano M, Yoshikawa T, et al. Comparison of endoscopic ultrasound-guided choledochoduodenostomy and endoscopic retrograde cholangiopancreatography in first-line biliary drainage for malignant distal bile duct obstruction:A multicenter randomized controlled trial. Medicine (Baltimore) 2021;100:e25268. doi: 10.1097/MD.0000000000025268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.EUS – Guided Choledocho-Duodenostomy Versus ERCP With Covered Metallic Stents in Patients with Unresectable Malignant Distal Common Bile Duct Strictures – Full Text View – ClinicalTrials.gov. Im Internet. [[Last accessed on 2020 Nov 29]]. Available from: https://clinicaltrials.gov/ct2/show/NCT03000855 .
- 34.Chen YI, Callichurn K, Chatterjee A, et al. ELEMENT TRIAL:Study protocol for a randomized controlled trial on endoscopic ultrasound-guided biliary drainage of first intent with a lumen-apposing metal stent vs. endoscopic retrograde cholangio-pancreatography in the management of malignant distal biliary obstruction. Trials. 2019;20:696. doi: 10.1186/s13063-019-3918-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaujoux S, Jacques J, Bourdariat R, et al. Pancreaticoduodenectomy following endoscopic ultrasound-guided choledochoduodenostomy with electrocautery-enhanced lumen-apposing stents an ACHBT –SFED study. HPB. 2021;23:154–60. doi: 10.1016/j.hpb.2020.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Fabbri C, Fugazza A, Binda C, et al. Beyond palliation:Using EUS-guided Choledochoduodenostomy with a lumen-apposing metal stent as a bridge to surgery. A case series. J Gastrointest Liver Dis. 2019;28:125–8. doi: 10.15403/jgld.2014.1121.281.eus. [DOI] [PubMed] [Google Scholar]
- 37.Hedjoudje A, Sportes A, Grabar S, et al. Outcomes of endoscopic ultrasound-guided biliary drainage:A systematic review and meta-analysis. United Eur Gastroenterol J. 2019;7:60–8. doi: 10.1177/2050640618808147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kongkam P, Orprayoon T, Boonmee C, et al. ERCP plus endoscopic ultrasound-guided biliary drainage versus percutaneous transhepatic biliary drainage for malignant hilar biliary obstruction:A multicenter observational open-label study. Endoscopy. 2021;53:55–62. doi: 10.1055/a-1195-8197. [DOI] [PubMed] [Google Scholar]
- 39.Vanella G, Bronswijk M, Maleux G, et al. EUS-guided intrahepatic biliary drainage:A large retrospective series and subgroup comparison between percutaneous drainage in hilar stenoses or postsurgical anatomy. Endosc Int Open. 2020;8:E1782–94. doi: 10.1055/a-1264-7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minaga K, Takenaka M, Ogura T, et al. Endoscopic ultrasound-guided biliary drainage for malignant biliary obstruction with surgically altered anatomy:A multicenter prospective registration study. Therap Adv Gastroenterol. 2020;13:1–11. doi: 10.1177/1756284820930964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iwashita T, Uemura S, Mita N, et al. Endoscopic ultrasound guided-antegrade biliary stenting vs. percutaneous transhepatic biliary stenting for unresectable distal malignant biliary obstruction in patients with surgically altered anatomy. J Hepatobiliary Pancreat Sci. 2020;27:968–76. doi: 10.1002/jhbp.823. [DOI] [PubMed] [Google Scholar]
- 42.Mori Y, Itoi T, Baron TH, et al. Tokyo Guidelines 2018:Management strategies for gallbladder drainage in patients with acute cholecystitis (with videos) J Hepatobiliary Pancreat Sci. 2018;25:87–95. doi: 10.1002/jhbp.504. [DOI] [PubMed] [Google Scholar]
- 43.Luk SW, Irani S, Krishnamoorthi R, et al. Endoscopic ultrasound-guided gallbladder drainage versus percutaneous cholecystostomy for high risk surgical patients with acute cholecystitis:A systematic review and meta-analysis. Endoscopy. 2019;51:722–32. doi: 10.1055/a-0929-6603. [DOI] [PubMed] [Google Scholar]
- 44.Teoh AY, Kitano M, Itoi T, et al. Endosonography-guided gallbladder drainage versus percutaneous cholecystostomy in very high-risk surgical patients with acute cholecystitis:An international randomised multicentre controlled superiority trial (DRAC 1) Gut. 2020;69:1085–91. doi: 10.1136/gutjnl-2019-319996. [DOI] [PubMed] [Google Scholar]
- 45.Teoh AY, Leung CH, Tam PT, et al. EUS-guided gallbladder drainage versus laparoscopic cholecystectomy for acute cholecystitis:A propensity score analysis with 1-year follow-up data. Gastrointest Endosc. 2021;93:577–83. doi: 10.1016/j.gie.2020.06.066. [DOI] [PubMed] [Google Scholar]
- 46.Wang W, Liu B, Qi K, et al. Efficacy and safety of endoscopic laser lithotripsy and lithotomy through the lumen-apposing metal stent for giant gallbladder stones. VideoGIE. 2020;5:318–23. doi: 10.1016/j.vgie.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rimbas M, Crinò SF, Rizzatti G, et al. EUS-guided gallbladder drainage:Where will we go next?Gastrointest Endosc . 2021;94:419–22. doi: 10.1016/j.gie.2021.03.933. [DOI] [PubMed] [Google Scholar]
- 48.Vanella G, Dell'Anna G, Bronswijk M, et al. EUS-guided gallbladder drainage and subsequent peroral endoscopic cholecystolithotomy:A tool to reduce chemotherapy discontinuation in neoplastic patients? VideoGIE. 2021;7:120–7. doi: 10.1016/j.vgie.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hewitt J, Carter B, McCarthy K, et al. Frailty predicts mortality in all emergency surgical admissions regardless of age. An observational study. Age Ageing. 2019;48:388–94. doi: 10.1093/ageing/afy217. [DOI] [PubMed] [Google Scholar]
- 50.Parmar KL, Law J, Carter B, et al. Frailty in older patients undergoing emergency laparotomy:Results from the UK observational Emergency Laparotomy and Frailty (ELF) study. Ann Surg. 2021;273:709–18. doi: 10.1097/SLA.0000000000003402. [DOI] [PubMed] [Google Scholar]
- 51.Chhoda A, Mukewar SS, Mahadev S. Managing gallstone disease in the elderly. Clin Geriatr Med. 2021;37:43–69. doi: 10.1016/j.cger.2020.08.005. [DOI] [PubMed] [Google Scholar]
- 52.Kamarajah SK, Karri S, Bundred JR, et al. Perioperative outcomes after laparoscopic cholecystectomy in elderly patients:A systematic review and meta-analysis. Surg Endosc. 2020;34:4727–40. doi: 10.1007/s00464-020-07805-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Löhr JM, Dominguez-Munoz E, Rosendahl J, et al. United European gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU) United European Gastroenterol J. 2017;5:153–99. doi: 10.1177/2050640616684695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeWitt JM, Arain M, Chang KJ, et al. AGA white paper:Interventional endoscopic ultrasound –Current status and future directions. Clin Gastroenterol Hepatol. 2021;19:24–40. doi: 10.1016/j.cgh.2020.09.029. [DOI] [PubMed] [Google Scholar]
- 55.Teoh AY, Dhir V, Kida M, et al. Consensus guidelines on the optimal management in interventional EUS procedures:Results from the Asian EUS group RAND/UCLA expert panel. Gut. 2018;67:1209–28. doi: 10.1136/gutjnl-2017-314341. [DOI] [PubMed] [Google Scholar]
- 56.Tessier G, Bories E, Arvanitakis M, et al. EUS-guided pancreatogastrostomy and pancreatobulbostomy for the treatment of pain in patients with pancreatic ductal dilatation inaccessible for transpapillary endoscopic therapy. Gastrointest Endosc. 2007;65:233–41. doi: 10.1016/j.gie.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 57.Oh D, Park DH, Cho MK, et al. Feasibility and safety of a fully covered self-expandable metal stent with antimigration properties for EUS-guided pancreatic duct drainage:Early and midterm outcomes (with video) Gastrointest Endosc. 2016;83:366–73. doi: 10.1016/j.gie.2015.07.015. e2. [DOI] [PubMed] [Google Scholar]
- 58.Krafft MR, Croglio MP, James TW, et al. Endoscopic endgame for obstructive pancreatopathy:Outcomes of anterograde EUS-guided pancreatic duct drainage. A dual-center study. Gastrointest Endosc. 2020;92:1055–66. doi: 10.1016/j.gie.2020.04.061. [DOI] [PubMed] [Google Scholar]
- 59.Kahaleh M, Hernandez AJ, Tokar J, et al. EUS-guided pancreaticogastrostomy:Analysis of its efficacy to drain inaccessible pancreatic ducts. Gastrointest Endosc. 2007;65:224–30. doi: 10.1016/j.gie.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 60.Tyberg A, Perez-Miranda M, Sanchez-Ocaña R, et al. Endoscopic ultrasound-guided gastrojejunostomy with a lumen-apposing metal stent:A multicenter, international experience. Endosc Int Open. 2016;4:E276–81. doi: 10.1055/s-0042-101789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khashab MA, Bukhari M, Baron TH, et al. International multicenter comparative trial of endoscopic ultrasonography-guided gastroenterostomy versus surgical gastrojejunostomy for the treatment of malignant gastric outlet obstruction. Endosc Int Open. 2017;5:E275–81. doi: 10.1055/s-0043-101695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kerdsirichairat T, Irani S, Yang J, et al. Durability and long-term outcomes of direct EUS-guided gastroenterostomy using lumen-apposing metal stents for gastric outlet obstruction. Endosc Int Open. 2019;7:E144–50. doi: 10.1055/a-0799-9939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Itoi T, Ishii K, Tanaka R, et al. Current status and perspective of endoscopic ultrasonography-guided gastrojejunostomy:Endoscopic ultrasonography-guided double-balloon-occluded gastrojejunostomy (with videos) J Hepatobiliary Pancreat Sci. 2015;22:3–11. doi: 10.1002/jhbp.148. [DOI] [PubMed] [Google Scholar]
- 64.Itoi T, Ishii K, Ikeuchi N, et al. Prospective evaluation of endoscopic ultrasonography-guided double-balloon-occluded gastrojejunostomy bypass (EPASS) for malignant gastric outlet obstruction. Gut. 2016;65:193–5. doi: 10.1136/gutjnl-2015-310348. [DOI] [PubMed] [Google Scholar]
- 65.Bronswijk M, Vanella G, van Malenstein H, et al. Laparoscopic versus EUS-guided gastroenterostomy for gastric outlet obstruction:An international multicenter propensity score-matched comparison (with video) Gastrointest Endosc. 2021;94:526–36. doi: 10.1016/j.gie.2021.04.006. e2. [DOI] [PubMed] [Google Scholar]
- 66.Brewer Gutierrez OI, Irani SS, Ngamruengphong S, et al. Endoscopic ultrasound-guided entero-enterostomy for the treatment of afferent loop syndrome:A multicenter experience. Endoscopy. 2018;50:891–5. doi: 10.1055/s-0044-102254. [DOI] [PubMed] [Google Scholar]
- 67.Ichkhanian Y, Yang J, James TW, et al. EUS-directed transenteric ERCP in non-Roux-en-Y gastric bypass surgical anatomy patients (with video) Gastrointest Endosc. 2020;91:1188–94. doi: 10.1016/j.gie.2019.12.043. e2. [DOI] [PubMed] [Google Scholar]
- 68.De Bie C, Bronswijk M, Vanella G, et al. EUS-guided hepaticogastrostomy for patients with afferent loop syndrome:A comparison with EUS-guided gastroenterostomy or percutaneous drainage. Surg Endosc. 2022;36:2393–400. doi: 10.1007/s00464-021-08520-z. [DOI] [PubMed] [Google Scholar]
- 69.Irani S, Baron TH, Itoi T, et al. Endoscopic gastroenterostomy:Techniques and review. Curr Opin Gastroenterol. 2017;33:320–9. doi: 10.1097/MOG.0000000000000389. [DOI] [PubMed] [Google Scholar]
- 70.Chen YI, Kunda R, Storm AC, et al. EUS-guided gastroenterostomy:A multicenter study comparing the direct and balloon-assisted techniques. Gastrointest Endosc. 2018;87:1215–21. doi: 10.1016/j.gie.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 71.Bronswijk M, Vanella G, Petrone MC, et al. EUS-guided gastroenterostomy:Less is more!The wireless EUS-guided gastroenterostomy simplified technique. VideoGIE. 2020;5:442. doi: 10.1016/j.vgie.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Antonelli G, Kovacevic B, Karstensen JG, et al. Endoscopic ultrasound-guided gastro-enteric anastomosis:A systematic review and meta-analysis. Dig Liver Dis. 2020;52:1294–301. doi: 10.1016/j.dld.2020.04.021. [DOI] [PubMed] [Google Scholar]
- 73.McCarty TR, Garg R, Thompson CC, et al. Efficacy and safety of EUS-guided gastroenterostomy for benign and malignant gastric outlet obstruction:A systematic review and meta-analysis. Endosc Int Open. 2019;7:E1474–82. doi: 10.1055/a-0996-8178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iqbal U, Khara HS, Hu Y, et al. EUS-guided gastroenterostomy for the management of gastric outlet obstruction:A systematic review and meta-analysis. Endosc Ultrasound. 2020;9:16–23. doi: 10.4103/eus.eus_70_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perez-Miranda M, Tyberg A, Poletto D, et al. EUS-guided gastrojejunostomy versus laparoscopic gastrojejunostomy:An international collaborative study. J Clin Gastroenterol. 2017;51:896–9. doi: 10.1097/MCG.0000000000000887. [DOI] [PubMed] [Google Scholar]
- 76.Jeurnink SM, van Eijck CH, Steyerberg EW, et al. Stent versus gastrojejunostomy for the palliation of gastric outlet obstruction:A systematic review. BMC Gastroenterol. 2007;7:18. doi: 10.1186/1471-230X-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Khashab M, Alawad AS, Shin EJ, et al. Enteral stenting versus gastrojejunostomy for palliation of malignant gastric outlet obstruction. Surg Endosc. 2013;27:2068–75. doi: 10.1007/s00464-012-2712-7. [DOI] [PubMed] [Google Scholar]
- 78.Jeurnink SM, Steyerberg EW, van Hooft JE, et al. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study):A multicenter randomized trial. Gastrointest Endosc. 2010;71:490–9. doi: 10.1016/j.gie.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 79.Chandan S, Khan SR, Mohan BP, et al. EUS-guided gastroenterostomy versus enteral stenting for gastric outlet obstruction:Systematic review and meta-analysis. Endosc Int Open. 2021;9:E496–504. doi: 10.1055/a-1341-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ge PS, Young JY, Dong W, et al. EUS-guided gastroenterostomy versus enteral stent placement for palliation of malignant gastric outlet obstruction. Surg Endosc. 2019;33:3404–11. doi: 10.1007/s00464-018-06636-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen YI, Itoi T, Baron TH, et al. EUS-guided gastroenterostomy is comparable to enteral stenting with fewer re-interventions in malignant gastric outlet obstruction. Surg Endosc. 2017;31:2946–52. doi: 10.1007/s00464-016-5311-1. [DOI] [PubMed] [Google Scholar]
- 82.EPASS versus Uncovered Duodenal Stent for Unresectable Malignant Gastric Outlet Obstruction. Full Text View – ClinicalTrials.gov. Im Internet. [[Last accessed on 2021 Apr 25]]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03823690?cond=Gastric+Outlet+Obstruction&draw=2&rank=2 .
- 83.EUS-GE vs. ES for Palliation of Gastric Outlet Obstruction – Full Text View – ClinicalTrials.gov. Im Internet. [[Last accessed on 2021 Apr 25]]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03259763?cond=Gastric+Outlet+Obstruction&draw=2&rank=1 .
- 84.Ghandour B, Bejjani M, Irani SS, et al. Classification, outcomes, and management of misdeployed stents during EUS-guided gastroenterostomy. Gastrointest Endosc. 2022;95:80–9. doi: 10.1016/j.gie.2021.07.023. [DOI] [PubMed] [Google Scholar]
- 85.Vanella G, Capurso G, Arcidiacono PG. Endosonography-guided radiofrequency ablation in pancreatic diseases:Time to fill the gap between evidence and enthusiasm. J Clin Gastroenterol. 2020;54:591–601. doi: 10.1097/MCG.0000000000001370. [DOI] [PubMed] [Google Scholar]
- 86.Rimbaş M, Rizzatti G, Larghi A. EUS-guided ablation of pancreatic neoplasms. Minerva Gastroenterol. 2022;68:186–201. doi: 10.23736/S2724-5985.21.02866-7. [DOI] [PubMed] [Google Scholar]
- 87.Choi JH, Seo DW, Song TJ, et al. Endoscopic ultrasound-guided radiofrequency ablation for management of benign solid pancreatic tumors. Endoscopy. 2018;50:1099–104. doi: 10.1055/a-0583-8387. [DOI] [PubMed] [Google Scholar]
- 88.Barthet M, Giovannini M, Lesavre N, et al. Endoscopic ultrasound-guided radiofrequency ablation for pancreatic neuroendocrine tumors and pancreatic cystic neoplasms:A prospective multicenter study. Endoscopy. 2019;51:836–42. doi: 10.1055/a-0824-7067. [DOI] [PubMed] [Google Scholar]
- 89.Oleinikov K, Dancour A, Epshtein J, et al. Endoscopic ultrasound-guided radiofrequency ablation:A new therapeutic approach for pancreatic neuroendocrine tumors. J Clin Endocrinol Metab. 2019;104:2637–47. doi: 10.1210/jc.2019-00282. [DOI] [PubMed] [Google Scholar]
- 90.Imperatore N, de Nucci G, Mandelli ED, et al. Endoscopic ultrasound-guided radiofrequency ablation of pancreatic neuroendocrine tumors:A systematic review of the literature. Endosc Int Open. 2020;8:E1759–64. doi: 10.1055/a-1261-9605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Song TJ, Seo DW, Lakhtakia S, et al. Initial experience of EUS-guided radiofrequency ablation of unresectable pancreatic cancer. Gastrointest Endosc. 2016;83:440–3. doi: 10.1016/j.gie.2015.08.048. [DOI] [PubMed] [Google Scholar]
- 92.Scopelliti F, Pea A, Conigliaro R, et al. Technique, safety, and feasibility of EUS-guided radiofrequency ablation in unresectable pancreatic cancer. Surg Endosc. 2018;32:4022–8. doi: 10.1007/s00464-018-6217-x. [DOI] [PubMed] [Google Scholar]
- 93.Crinò SF, D'Onofrio M, Bernardoni L, et al. EUS-guided radiofrequency ablation (EUS-RFA) of solid pancreatic neoplasm using an 18-gauge needle electrode:Feasibility, safety, and technical success. J Gastrointestin Liver Dis. 2018;27:67–72. doi: 10.15403/jgld.2014.1121.271.eus. [DOI] [PubMed] [Google Scholar]
- 94.Wang D, Jin Z, Lei W, et al. Mo1524 endoscopic ultrasound guided radiofrequency ablation for the treatment of advanced pancreatic carcinoma. Gastrointest Endosc. 2013;77:AB414. [Google Scholar]
- 95.Yang J, Zhang X. Tu1357 feasibility and safety of EUS-guided radiofrequency ablation in unresectable pancreatic cancer. Gastrointest Endosc. 2019;89:AB588–9. [Google Scholar]
- 96.Arcidiacono PG, Carrara S, Reni M, et al. Feasibility and safety of EUS-guided cryothermal ablation in patients with locally advanced pancreatic cancer. Gastrointest Endosc. 2012;76:1142–51. doi: 10.1016/j.gie.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 97.Dhaliwal A, Kolli S, Dhindsa BS, et al. Efficacy of EUS-RFA in pancreatic tumors:Is it ready for prime time?A systematic review and meta-analysis. Endosc Int Open. 2020;8:E1243–51. doi: 10.1055/a-1221-5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pancreatic Locally Advanced Irresectable Cancer Ablation (PELICAN). Im Internet. [[Last accessed on 2019 Aug 10]]. Available from: https://clinicaltrials.gov/ct2/show/NCT03690323 .
- 99.Soehendra N, Grimm H, Nam Ch V, et al. N-butyl-2-cyanoacrylate:A supplement to endoscopic sclerotherapy. Endoscopy. 1987;19:221–4. doi: 10.1055/s-2007-1018288. [DOI] [PubMed] [Google Scholar]
- 100.Mishra SR, Sharma BC, Kumar A, et al. Primary prophylaxis of gastric variceal bleeding comparing cyanoacrylate injection and beta-blockers:A randomized controlled trial. J Hepatol. 2011;54:1161–7. doi: 10.1016/j.jhep.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 101.De Franchis R, Abraldes JG, Bajaj J, et al. Expanding consensus in portal hypertension Report of the Baveno VI Consensus Workshop:Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–52. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 102.Garcia-Tsao G, Abraldes JG, Berzigotti A, et al. Portal hypertensive bleeding in cirrhosis:Risk stratification, diagnosis, and management:2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310–35. doi: 10.1002/hep.28906. [DOI] [PubMed] [Google Scholar]
- 103.Binmoeller KF, Weilert F, Shah JN, et al. EUS-guided transesophageal treatment of gastric fundal varices with combined coiling and cyanoacrylate glue injection (with videos) Gastrointest Endosc. 2011;74:1019–25. doi: 10.1016/j.gie.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 104.Bhat YM, Weilert F, Fredrick RT, et al. EUS-guided treatment of gastric fundal varices with combined injection of coils and cyanoacrylate glue:A large U. S. experience over 6 years (with video) Gastrointest Endosc. 2016;83:1164–72. doi: 10.1016/j.gie.2015.09.040. [DOI] [PubMed] [Google Scholar]
- 105.Lôbo MR, Chaves DM, DE Moura DT, et al. Safety and efficacy of EUS-guided coil plus cyanoacrylate versus conventional cyanoacrylate technique in the treatment of gastric varices:A randomized controlled trial. Arq Gastroenterol. 2019;56:99–105. doi: 10.1590/S0004-2803.201900000-08. [DOI] [PubMed] [Google Scholar]
- 106.Robles-Medranda C, Oleas R, Valero M, et al. Endoscopic ultrasonography-guided deployment of embolization coils and cyanoacrylate injection in gastric varices versus coiling alone:A randomized trial. Endoscopy. 2020;52:268–75. doi: 10.1055/a-1123-9054. [DOI] [PubMed] [Google Scholar]
- 107.Kouanda A, Binmoeller K, Hamerski C, et al. Safety and efficacy of EUS-guided coil and glue injection for the primary prophylaxis of gastric variceal hemorrhage. Gastrointest Endosc. 2021;94:291–6. doi: 10.1016/j.gie.2021.01.025. [DOI] [PubMed] [Google Scholar]
- 108.Ahmed O, Lee JH, Thompson CC, et al. AGA clinical practice update on the optimal management of the malignant alimentary tract obstruction:Expert review. Clin Gastroenterol Hepatol. 2021;19:1780–8. doi: 10.1016/j.cgh.2021.03.046. [DOI] [PubMed] [Google Scholar]
- 109.Jue TL, Storm AC, Naveed M, et al. ASGE guideline on the role of endoscopy in the management of benign and malignant gastroduodenal obstruction. Gastrointest Endosc. 2021;93:309–22. doi: 10.1016/j.gie.2020.07.063. e4. [DOI] [PubMed] [Google Scholar]